Fri, Jan 2, 2026
Volume 11, Issue 1 (Winter 2025)
Caspian J Neurol Sci 2025, 11(1): 17-27 |
Back to browse issues page
Ethics code: IR.GUMS.REC.1401.380
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
AmirAshjei Asalemi K, Saberi A, Ghayeghran A, Fallah Arzpeyma S, Eskandarieh S, Sahraian M A, et al . Clinical and Imaging Features of Familial and Sporadic Multiple Sclerosis. Caspian J Neurol Sci 2025; 11 (1) :17-27
URL: http://cjns.gums.ac.ir/article-1-748-en.html
URL: http://cjns.gums.ac.ir/article-1-748-en.html
Kamal AmirAshjei Asalemi1 

 , Alia Saberi2
, Alia Saberi2 

 , Amirreza Ghayeghran2
, Amirreza Ghayeghran2 

 , Sima Fallah Arzpeyma3
, Sima Fallah Arzpeyma3 

 , Sharareh Eskandarieh4
, Sharareh Eskandarieh4 

 , Mohammad Ali Sahraian4
, Mohammad Ali Sahraian4 

 , Hamidreza Hatamian2
, Hamidreza Hatamian2 

 , Kasra Sarlak1
, Kasra Sarlak1 

 , Negin Ashoori5
, Negin Ashoori5 

 , Nima Broomand Lomer *6
, Nima Broomand Lomer *6 




 , Alia Saberi2
, Alia Saberi2 

 , Amirreza Ghayeghran2
, Amirreza Ghayeghran2 

 , Sima Fallah Arzpeyma3
, Sima Fallah Arzpeyma3 

 , Sharareh Eskandarieh4
, Sharareh Eskandarieh4 

 , Mohammad Ali Sahraian4
, Mohammad Ali Sahraian4 

 , Hamidreza Hatamian2
, Hamidreza Hatamian2 

 , Kasra Sarlak1
, Kasra Sarlak1 

 , Negin Ashoori5
, Negin Ashoori5 

 , Nima Broomand Lomer *6
, Nima Broomand Lomer *6 


1- Department of Neurology, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
2- Neurosciences Research Center, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
3- Department of Radiology, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
4- Neuroscience Institute, Multiple Sclerosis Research Center, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran.
5- Department of Neurology, Faculty of Medicine, Ardabil Branch, Islamic Azad University, Ardabil, Iran.
6- Department of Neurology, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran. ,nima.broomand@gmail.com
2- Neurosciences Research Center, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
3- Department of Radiology, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
4- Neuroscience Institute, Multiple Sclerosis Research Center, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran.
5- Department of Neurology, Faculty of Medicine, Ardabil Branch, Islamic Azad University, Ardabil, Iran.
6- Department of Neurology, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran. ,
Keywords: Multiple sclerosis, Magnetic resonance imaging, Relapsing-Remitting, Disability evaluation
Full-Text [PDF 1931 kb]
(436 Downloads)
| Abstract (HTML) (992 Views)
Full-Text: (342 Views)
Introduction
Multiple sclerosis (MS) is a chronic and demyelinating neurodegenerative disease that affects the central nervous system (CNS) and leads to significant disabilities in young adults [1, 2]. Typically, patients undergo a relapsing-remitting course, followed by a progressive phase many years later [3]. Clinical manifestations include motor, sensory, visual and autonomic impairment alongside several other symptoms [3-5]. Magnetic resonance imaging (MRI) is the primary paraclinical diagnostic modality and can show the demyelinated lesions known as plaques [4].
Although the exact cause of MS is unknown, an intricate gene-environment interaction plays a significant role in its pathogenesis [6, 7]. In addition to numerous environmental factors such as Epstein–Barr virus (EBV) infection, smoking, low sunlight exposure, vitamin D deficiency and obesity that are strongly suspected to be associated with MS development [8, 9], a genetic component undoubtedly contributes to the disease. This assumption can be supported by the familial aggregation of MS, varying prevalence rates among different races, and a higher likelihood of affecting monozygotic twins. While a simple inheritance pattern is not expected in MS, human genome studies have identified the considerable role of HLA-DR and 233 significant genetic associations with MS susceptibility [10, 11].
While sporadic MS (SMS) was initially considered the only existing form of the disease, familial MS (FMS) was later presented in 1933 [12]. FMS is characterized by the presence of at least one family member of first-, second-, third-degree, or other relatives of the patient diagnosed with MS [13]. The prevalence of MS varies based on ethnicity and residential region and has globally increased to 2.8 million in 2020, which is 30% higher than that in 2013 [14, 15]. Most MS cases are sporadic, and FMS constitutes nearly 12.6% of the MS population [13].
According to the limited number of studies comparing SMS and FMS, findings suggest a likely difference in the age of onset (AAO) and CNS lesion distribution between these two disease categories [2, 16-18]. However, the precise impact of heredity on the clinical features, development, and prognosis of MS is still unclear [19]. Expanding our knowledge about the unique characteristics of the familial form of MS enlightens the significance of genetic and environmental factors in this disease and improves the diagnostic and therapeutic methods. This study was designed to compare familial and SMS cases to comprehensively understand their variations and implications.
Materials and Methods
Study design
This analytical study employed a cross-sectional design to investigate patients with clinically definite MS registered in the nationwide MS registry of Iran (NMSRI)-Guilan. A definite MS diagnosis was made by an expert neurologist based on the 2017 revised McDonald criteria [20]. The data were collected by a medical student who retrieved information from the medical records of individuals with MS in this registry system. This dataset encompassed an extensive array of demographic and clinical data, including age, gender, onset age, disease clinical course (such as relapsing‐remitting MS, primary progressive MS, secondary progressive MS and clinically isolated syndrome), disease duration, number of attacks (defined by neurological symptoms that have persisted for longer than 24 hours within the past 12 months), medical comorbidities, first presentation of the disease, FMS history, utilization of disease-modifying therapies (DMTs), expanded disability status scale (EDSS) score and the most recent brain MRI. If data records were inadequate, patients were contacted via telephone for additional information and if not feasible, they were invited by medical experts for an appointment to provide additional information through physical examination or a review of their medical records.
Study participants
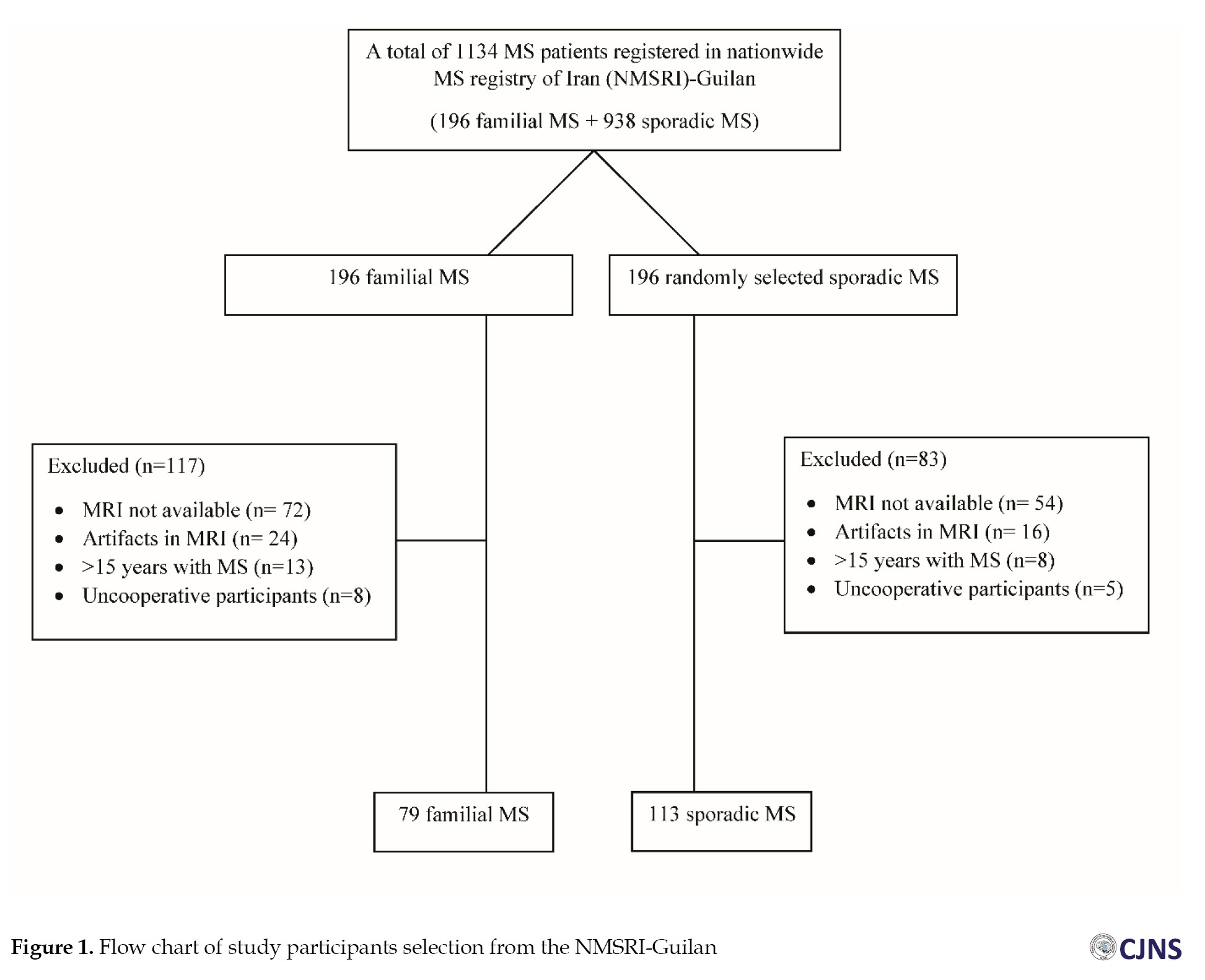
As of early 2023, there were 1134 registered MS patients in NMSRI-Guilan, with 196 patients (17.2%) diagnosed with FMS. Initially, we assessed all FMS patients (n=196) along with an equal number of randomly chosen SMS patients (n=196). After a comprehensive review of their medical records, 117 FMS and 83 SMS were excluded due to the unavailability of their MRI scans, severe artifacts, disease duration exceeding 15 years, and insufficient patient cooperation. Consequently, 79 FMS and 113 SMS were included in the study. The patient selection process is shown in Figure 1. The inclusion criteria were as follows: Patients definitively diagnosed with MS according to the 2017 revised McDonald criteria [20] and less than 15 years after the onset of their disease. The exclusion criteria were as follows: inadequate data in medical records, unrecoverable or incomplete MRI sequences or presence of severe artifacts, presence of other neurological disorders impeding MS diagnosis, and a duration of more than 15 years since the onset of their disease.
Patient classification
Sporadic cases of MS pertain to individuals who are the only members in their family diagnosed with the disease. Conversely, at least one first to third-degree relative was also diagnosed with MS in FMS cases. First-degree relatives include parents and siblings, second-degree relatives include uncles and aunts, while third-degree relatives include cousins and others.
Magnetic resonance imaging acquisition and analysis
Subjects in both groups underwent a 1.5 T brain MRI scan using an 18-channel MRI scanner (Magnetom Avanto; Siemens Medical Solutions, Erlangen, Germany). The imaging protocol comprised T1-weighted images (with echo time [TE]=12 ms and repetition time [TR]=664 ms). Thin-slice images (1 mm) were acquired in axial, sagittal and coronal views. During post-processing analysis, an expert radiologist, utilizing OsiriX software, version 12.0 [21], evaluated the characteristics of brain lesions. The assessment included the location of lesions (periventricular, callosal, cervical, infratentorial, juxtacortical, or cortical), the number of lesions, the area of the smallest and largest lesion (mm2) and the presence of black holes. The imaging data were analyzed concerning the number of lesions, explicitly focusing on the axial view and T2-weighted and FLAIR MRI images. Additionally, the location of the lesions was categorized, and the presence or absence of black holes was determined by examining the T1-weighted view.
Statistical analysis
We employed the Mean±SD or the median and interquartile range to depict the quantitative data based on the distribution’s normality. Additionally, categorical data was delineated using numerical representations and percentages. For inferential analysis, we initially assessed the normality of quantitative variables using visual methods, specifically the Q-Q chart, skewness, kurtosis, and the Shapiro-Wilk test. To compare quantitative variables across two groups, the independent t-test was utilized if the data exhibited a normal distribution; otherwise, the Mann-Whitney U test was applied. The chi-squared and Fisher exact tests were employed for categorical variables. All analyses were conducted using SPSS software, Version 26.0. The effect size of significant correlations was determined using Spearman’s correlation coefficient. Subsequently, significant correlations underwent further evaluation through backward multivariate regression analysis.
Results
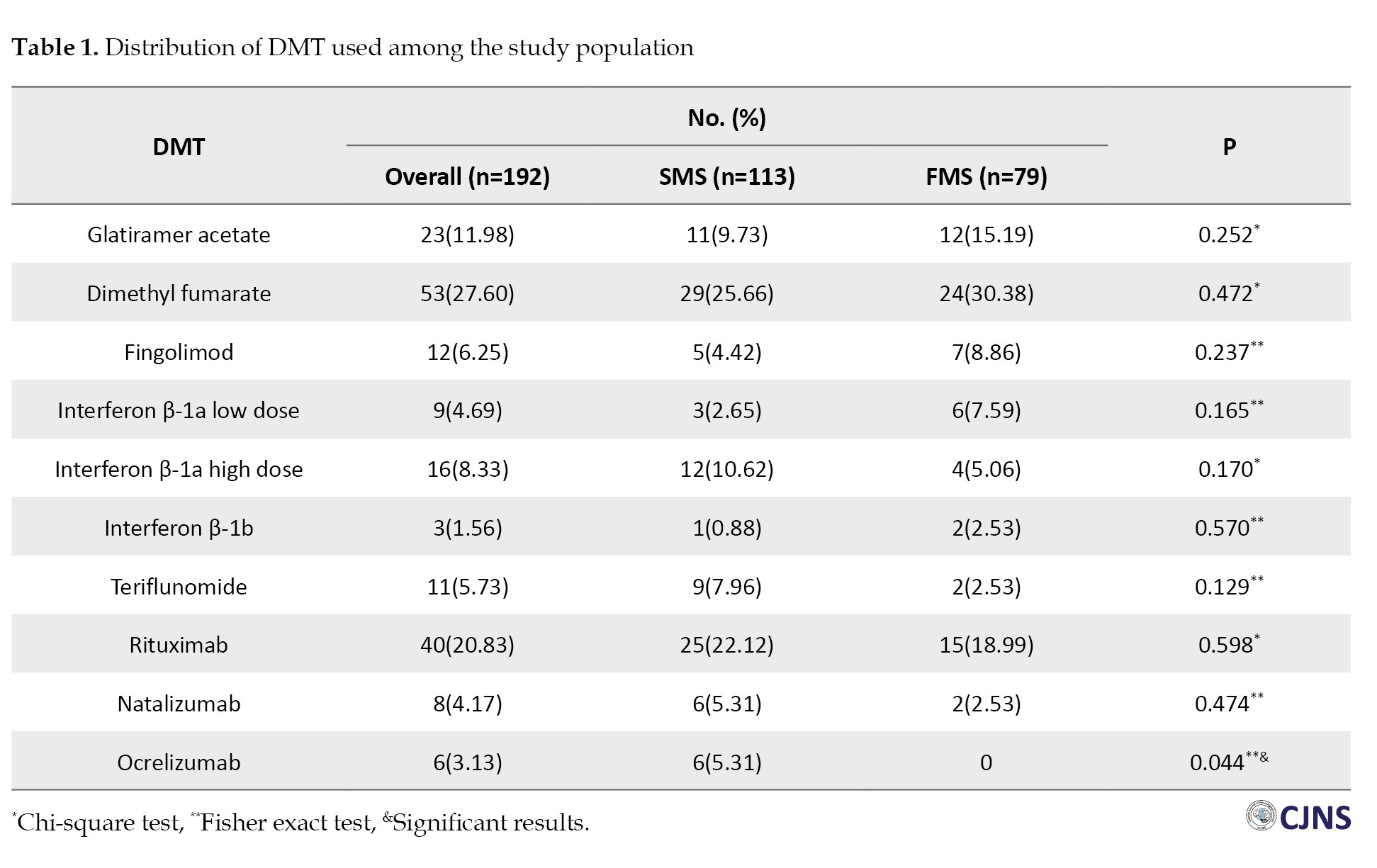
This study assessed 192 MS patients consisting of 79 FMS (41.1%) and 113 SMS (58.9%) patients. Patients were approximately matched in terms of DMTs utilization, except for ocrelizumab, which exhibited a statistically significant elevation in the SMS group (Table 1).
While the average age of patients between FMS and SMS exhibited a statistically borderline difference (FMS: 34.82±9.40; SMS: 37.71±10.83, P=0.057), a notable statistically significant difference was observed in the onset age between the two groups (FMS: 28.92±9.46; SMS: 31.48±9.55, P=0.034). Moreover, our investigation revealed no statistically significant differences between the two groups concerning gender distribution (P=0.604), disease duration (P=0.738), number of attacks (P=0.42) and medical comorbidities (P=0.237). Table 2 represents the study participants’ demographic and clinical characteristics.
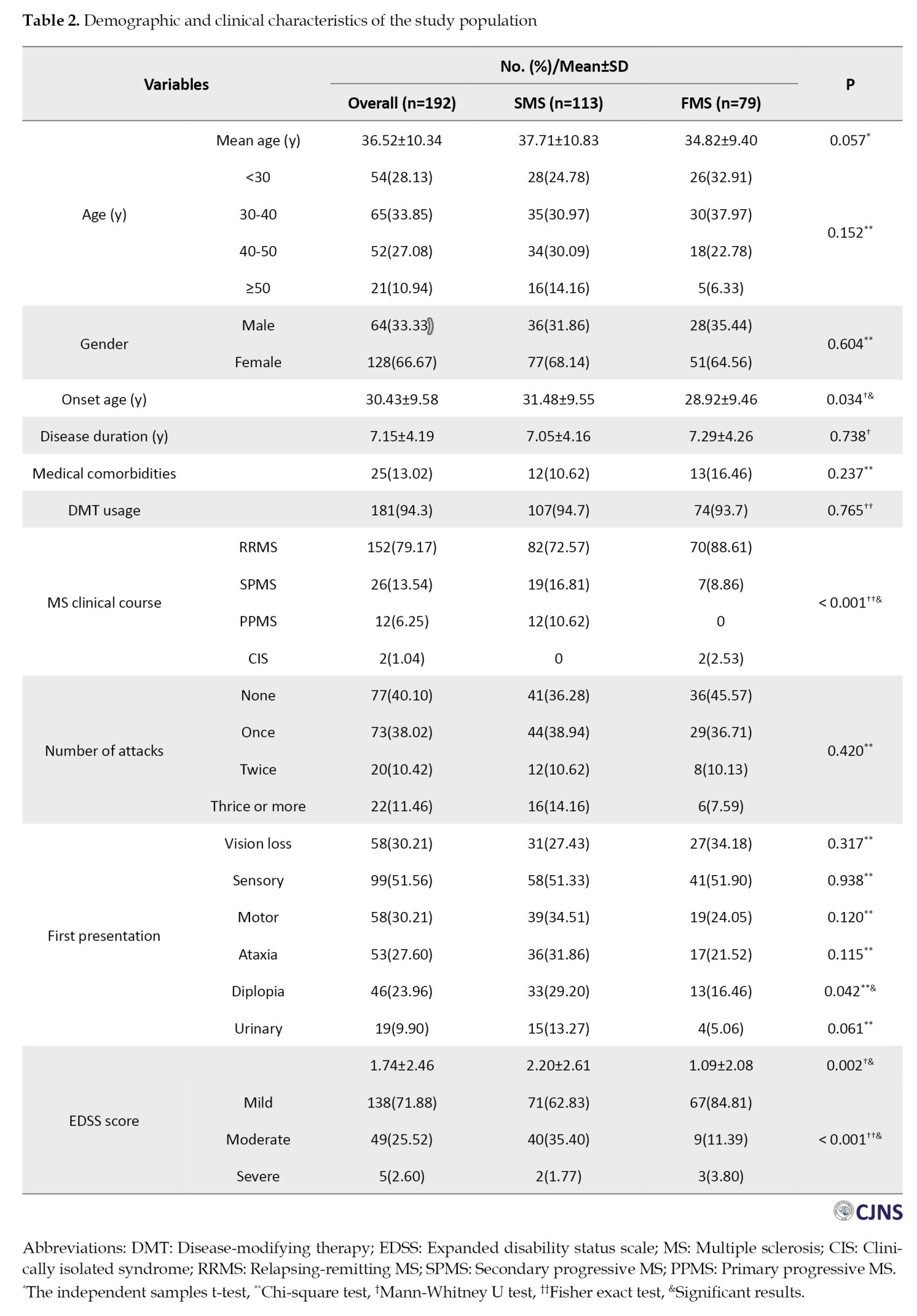
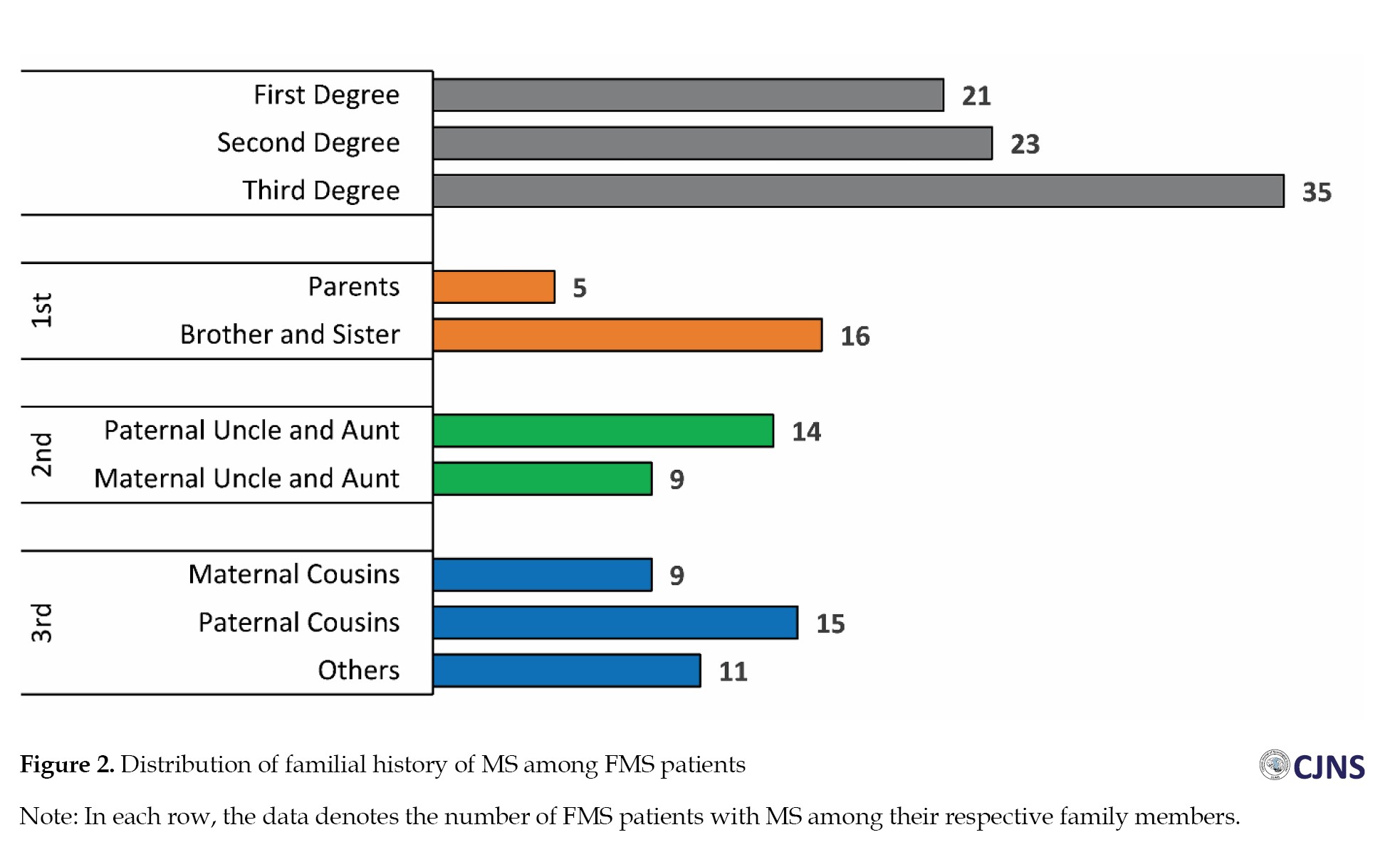
Within the FMS group, 21 individuals (26.5%) exhibited a familial predisposition to MS among their first-degree relatives, 23(29.1%) among second-degree relatives, and 35(44.4%) among third-degree relatives (Figure 2). When comparing the initial disease presentations between the two groups, sensory symptoms emerged as the most common (51.3% in SMS vs 51.9% in FMS, P=0.938). Moreover, among all the other primary manifestations, only diplopia was significantly higher in the SMS group (29.2% in SMS vs 16.4% in FMS, P=0.042) (Table 2).
In comparing imaging findings between the two groups, the SMS group exhibited a significantly higher number of lesions (31.59±25.36, range: 4-170, P=0.017) in contrast to the FMS group (22.83±17.17, range: 2-67, P=0.017). Furthermore, a statistically significant difference was observed in the average size of the smallest lesions (FMS: 8.12±8.58, SMS: 4.65±2.57 mm2, P<0.001). However, there was no statistically significant difference found in the average size of the largest lesions (FMS: 98.57±80.97, SMS: 100.84±105.72 mm2, P=0.555) or in the incidence of black holes (SMS: 25.3% vs FMS: 23.8%, P=0.822). Imaging findings of study participants can be found in Table 3.
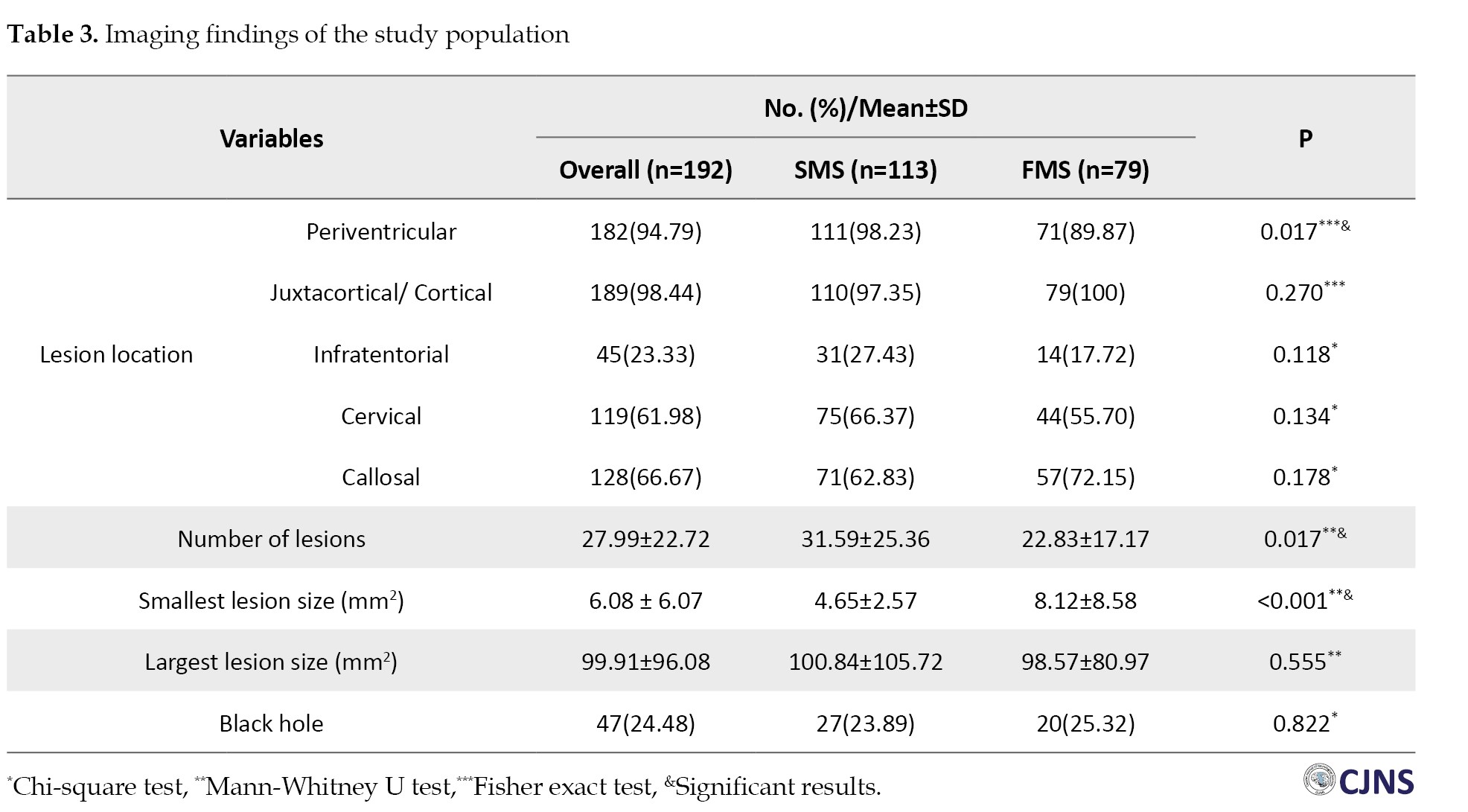
The frequency of lesions in the periventricular region was significantly higher in the SMS group (98.2%) compared to the FMS group (89.8%, P=0.017). However, no statistically significant difference was observed in juxtacortical/cortical (P=0.27), infratentorial (P=0.118), cervical (P=0.134) and callosal (P=0.178) regions between the two groups (Table 3).
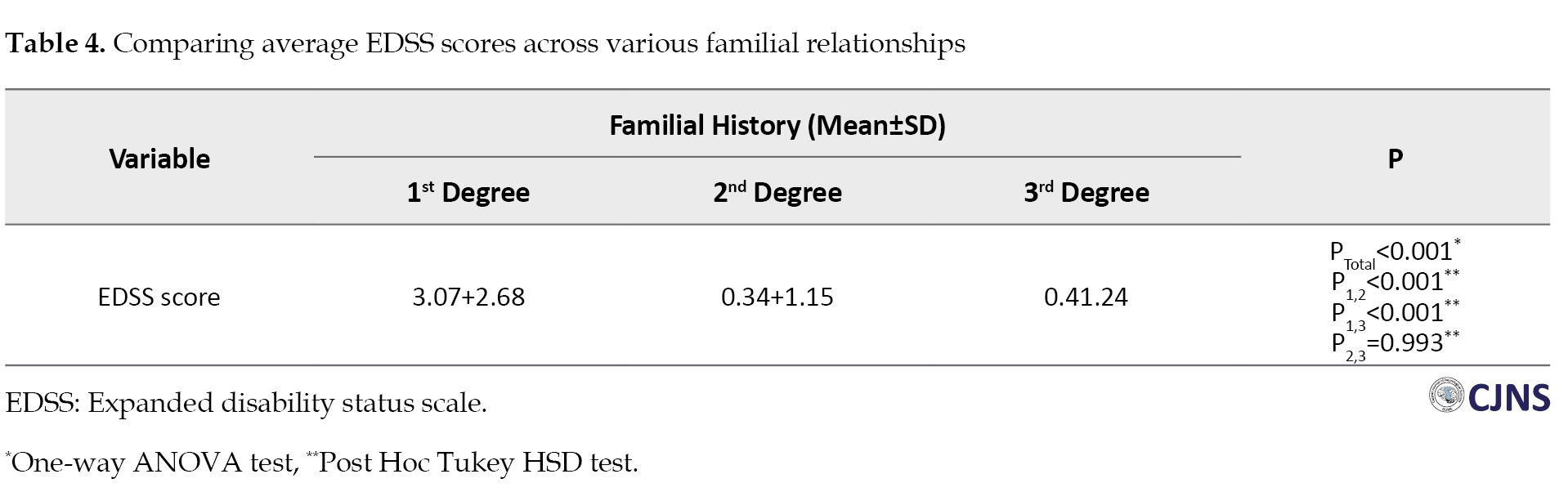
The average score of EDSS was significantly higher in the SMS group (2.20±2.61) compared to the FMS group (1.09±2.08, P=0.002). Interestingly, most patients in both groups showed mild EDSS scores (SMS: 62.8% vs FMS: 84.8%) (Table 2). Moreover, when comparing EDSS scores among different degrees of relatives, those in the first degree exhibited significantly higher scores (3.07±2.68) than second-degree (0.34±1.15) and third-degree (0.4±1.24, P1,2<0.001, P1,3<0.001, P2,3=0.993) relatives (Table 4).
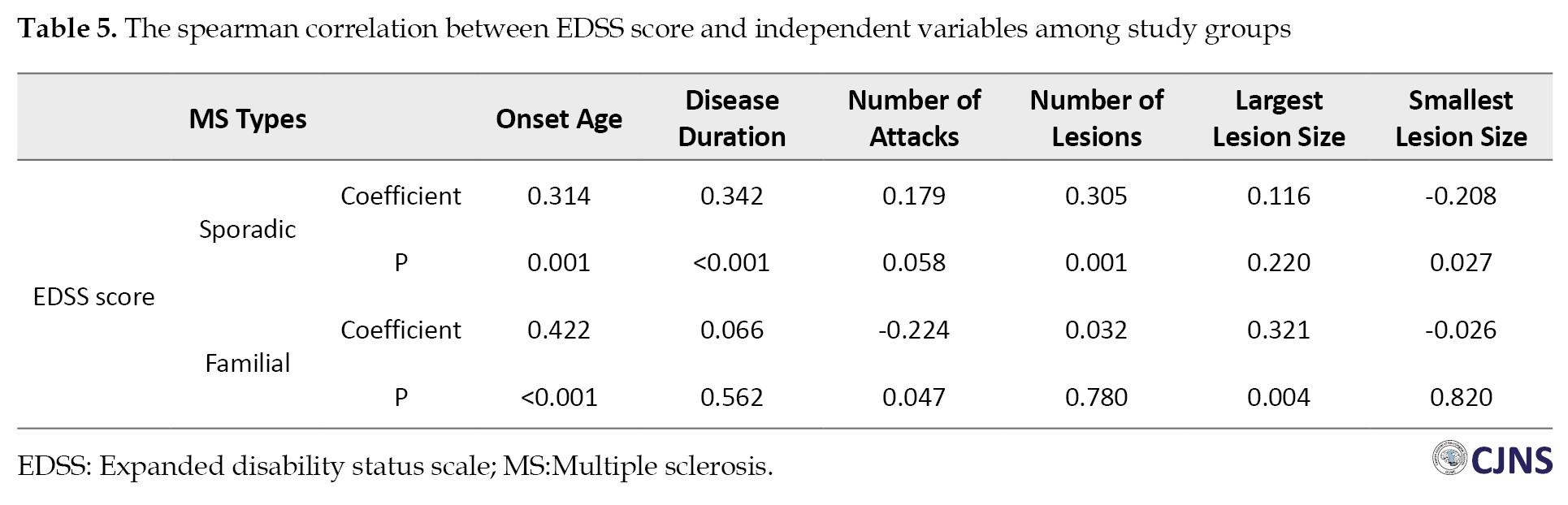
Within the SMS group, a moderate correlation was detected between the EDSS score and various factors, including onset age (r=0.314, P=0.001), disease duration (r=0.342, P<0.001), number of lesions (r=0.305, P=0.001) and the smallest lesion size (r=-0.208, P=0.027). Conversely, in the FMS group, significant correlations were found between the EDSS score and onset age (r=0.422, P<0.001), number of attacks (r=-0.224, P=0.047) and the largest lesion size (r=0.321, P=0.004) (Table 5).
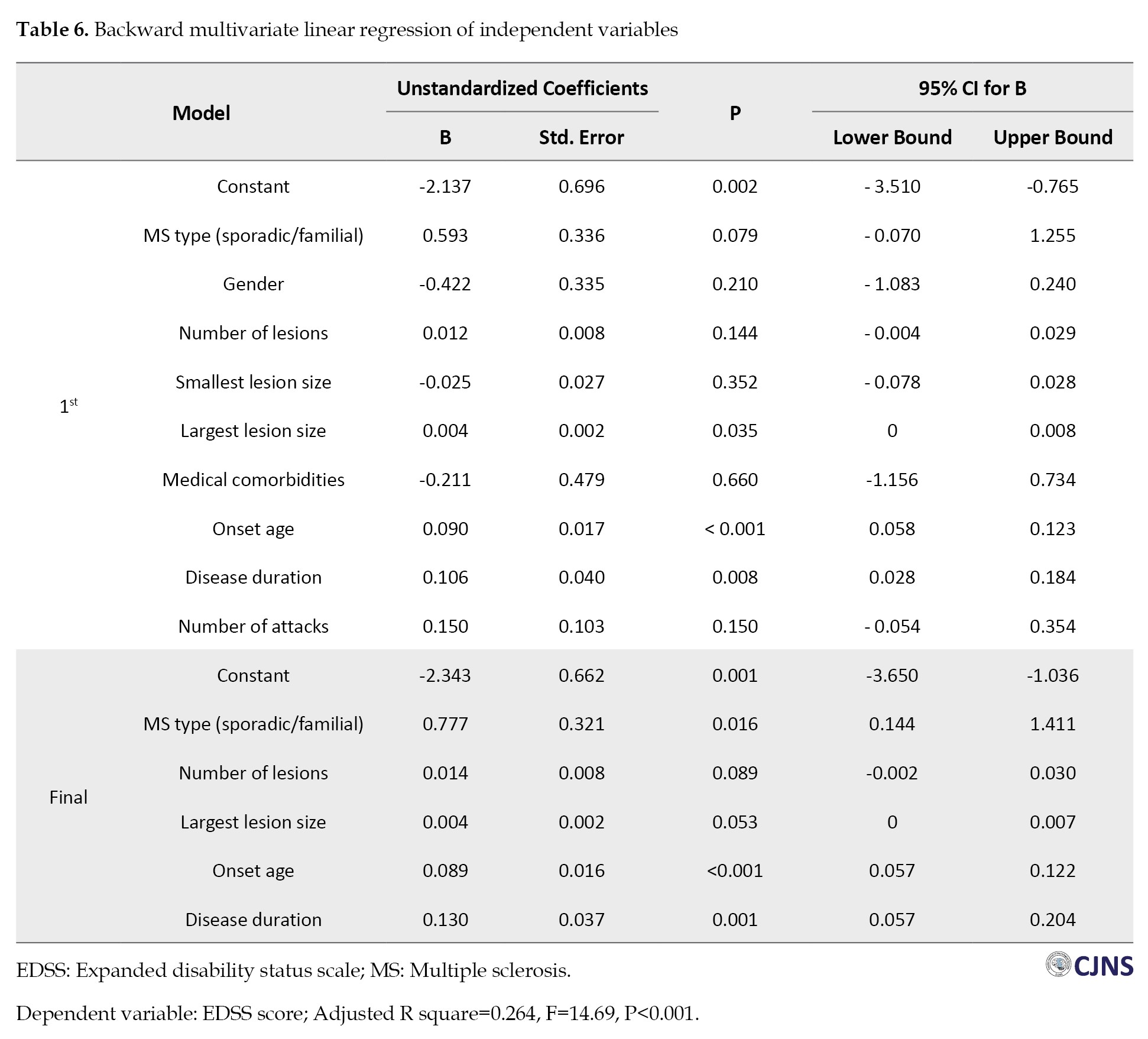
Multivariate linear regression analysis revealed that, collectively, the type of MS (SMS or FMS), number of lesions, largest lesion size, onset age and disease duration accounted for approximately 26.4% of the variability observed in the EDSS score (P<0.001). Notably, the final model underscored significant associations between the type of MS (FMS or SMS) (β=0.78, P=0.016), onset age (β=0.09, P<0.001) and disease duration (β=0.13, P=0.001) with the EDSS score (Table 6).
Discussion
This investigation sought to delineate demographic, clinical, and imaging distinctions among individuals with sporadic and familial forms of MS. The SMS cohort exhibited significantly higher AAO, higher incidence of diplopia as the initial manifestation, higher EDSS scores, higher number of total lesions, higher number of lesions in the periventricular region, and lower average size of the smallest lesions relative to the FMS cohort. Moreover, the EDSS score was higher in the SMS group and demonstrated significant associations with MS subtype (SMS or FMS), onset age and disease duration.
In the present investigation, the FMS group exhibited a lower AAO, aligning with the findings reported by Salehi et al. [22] and Katsavos et al. [16]. Notably, Katsavos et al. discerned a reduced AAO in FMS patients with the affected first-degree relatives. In contrast, those with third-degree relatives showed no such distinction [16]. Conversely, several other investigations failed to observe a significant distinction in the AAO between individuals with FMS and SMS [2, 17, 19, 23-25]. This outcome might be because MS is a complex disease with both genetic and environmental factors contributing to its onset and progression. FMS suggests a stronger genetic component, possibly leading to an earlier disease onset. On the other hand, SMS may have a later onset because it could be more influenced by environmental factors, which may take longer to exert their effects. Furthermore, genetic predisposition in FMS may interact with environmental factors at an earlier age, leading to an earlier onset of the disease.
No significant difference in disease duration was observed between the two groups, aligning with findings from other research studies [2, 17, 19].
Furthermore, our study found no difference in gender distribution between familial and sporadic types of MS. This observation aligns with findings from prior research [2, 16, 17, 26]. It was established that MS results from the interplay between genetic and environmental factors. Although females are relatively more susceptible to MS than males, this holds for both sporadic and familial cases; hence, the gender distribution remains parallel in both scenarios.
Regarding the frequency of attacks, most patients in both groups encountered one or fewer attacks. However, the observed difference between the two groups was not statistically significant, consistent with the findings of Moghadam et al. [2]. In contrast, Andrijauskis et al. [19] reported a higher annual attack rate in FMS compared to SMS, with 1.4 attacks vs 0.8 attacks (P<0.05).
In our investigation, certain patients presented with concurrent medical comorbidities such as hypertension, diabetes, respiratory disorders, thyroid disorders, migraine, and seizures. However, no significant difference was observed between the two study groups, aligning with the conclusions drawn by Moghadam et al. [2] and Mokhtari et al. [25]. Given the complex interplay between genetic, environmental, and lifestyle factors that contribute to the pathogenesis of MS, it is plausible that these same factors may also impact the co-occurrence of other medical comorbidities. Therefore, further investigation is warranted to understand these associations comprehensively.
We observed a higher incidence of diplopia among SMS patients as the first presentation of the disease, consistent with findings by Katsavos et al. [16] and Faraji et al. [23]. Nevertheless, Mokhtari et al. [25] reported no significant difference between the two groups regarding visual symptoms as the initial manifestation of the disease.
In this study, we observed significantly higher EDSS scores in the SMS group, contrary to prior research outcomes. Moghadam et al. [2] and Katsavos et al. [16] reported no substantial difference in EDSS scores between the two groups. In contrast, Andrijauskis et al. [19] and Faraji et al. [23] identified lower EDSS scores in SMS patients. Furthermore, we observed a higher EDSS score in FMS patients with first-degree relatives affected by MS. Conversely, Tipirneni et al. [17] found no significant variations in EDSS scores based on the degrees of familial relationships (first, second and third degrees).
Our investigation revealed that the FMS patients exhibited fewer lesions and a higher average size of the smallest lesion than the SMS group. Nonetheless, there was no statistically significant difference between the two groups’ average size of the largest lesion and the occurrence of black holes. Tipirneni et al. [17] reported a greater average T1-lesion volume in the FMS group.
Furthermore, we observed a notable increase in lesions within the periventricular region among SMS patients, aligning with the results reported by Moghadam et al. [2]. Interestingly, they also noted significantly more lesions within the callosal region among FMS patients. Katsavos et al. [16] reported higher lesions in the subcortical and cervical regions for SMS and FMS patients, respectively. Additionally, Andrijauskis et al. [19] identified a significant rise in lesions within the cerebellum and brainstem, specifically among FMS patients.
Conclusion
In conclusion, FMS exhibits distinct characteristics from SMS across various facets, such as an earlier onset age, a predominance of RRMS phenotype, lower rate of diplopia, lower EDSS scores, lower lesion frequency in the periventricular area, fewer overall lesion numbers, and a higher average size of the smallest lesions.
Study limitations
The present investigation encountered certain limitations. Unfortunately, many patients in NMSRI-Guilan lacked MRI scans, and many available MRIs had severe artifacts, leading to their exclusion and thus reducing the sample size. This limitation raises concerns about the finality of our findings and may affect their generalizability to the broader MS patient population. The cross-sectional nature of our design offers a snapshot of a specific moment, preventing the establishment of definitive cause-and-effect relationships and failing to capture changes over time. Uncontrolled variables, such as lifestyle factors and healthcare accessibility, could introduce confounding elements into our results. Additionally, the reliance on self-reported data to categorize patients as FMS introduces the possibility of bias. Participant responses may be influenced by inaccurate recall or intentional alteration. Lastly, the study’s confinement to a specific geographical location (Guilan Province, Iran) suggests caution in extrapolating the results to populations with distinct cultural, social, or environmental conditions. As a result, there is a need for more extensive multinational investigations that incorporate the assessment of genetic predisposition among participants to ensure the generalizability of the findings.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1401.380). The researchers adhered to the ethical principles of the Declaration of Helsinki. Additionally, signed informed consent was acquired from each participant, along with approval from the chairman of the MS Society of Guilan province for data extraction.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization: Alia Saberi, Amirreza Ghayeghran, and Mohammad Ali Sahraian; Methodology: Sharareh Eskandarieh; Formal analysis: Sharareh Eskandarieh and Nima Broomand Lomer; Supervision: Alia Saberi and Amirreza Ghayeghran; Writing the original draft: Kamal Amir Ashjei Asalemi, Nima Broomand Lomer, Kasra Sarlak, and Negin Ashoori; Investigation: Kamal AmirAshjei Asalemi; Writing, review, editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the Clinical Research Development Unit of Poursina Hospital, Guilan University of Medical Sciences, Guilan, Iran.
References
Multiple sclerosis (MS) is a chronic and demyelinating neurodegenerative disease that affects the central nervous system (CNS) and leads to significant disabilities in young adults [1, 2]. Typically, patients undergo a relapsing-remitting course, followed by a progressive phase many years later [3]. Clinical manifestations include motor, sensory, visual and autonomic impairment alongside several other symptoms [3-5]. Magnetic resonance imaging (MRI) is the primary paraclinical diagnostic modality and can show the demyelinated lesions known as plaques [4].
Although the exact cause of MS is unknown, an intricate gene-environment interaction plays a significant role in its pathogenesis [6, 7]. In addition to numerous environmental factors such as Epstein–Barr virus (EBV) infection, smoking, low sunlight exposure, vitamin D deficiency and obesity that are strongly suspected to be associated with MS development [8, 9], a genetic component undoubtedly contributes to the disease. This assumption can be supported by the familial aggregation of MS, varying prevalence rates among different races, and a higher likelihood of affecting monozygotic twins. While a simple inheritance pattern is not expected in MS, human genome studies have identified the considerable role of HLA-DR and 233 significant genetic associations with MS susceptibility [10, 11].
While sporadic MS (SMS) was initially considered the only existing form of the disease, familial MS (FMS) was later presented in 1933 [12]. FMS is characterized by the presence of at least one family member of first-, second-, third-degree, or other relatives of the patient diagnosed with MS [13]. The prevalence of MS varies based on ethnicity and residential region and has globally increased to 2.8 million in 2020, which is 30% higher than that in 2013 [14, 15]. Most MS cases are sporadic, and FMS constitutes nearly 12.6% of the MS population [13].
According to the limited number of studies comparing SMS and FMS, findings suggest a likely difference in the age of onset (AAO) and CNS lesion distribution between these two disease categories [2, 16-18]. However, the precise impact of heredity on the clinical features, development, and prognosis of MS is still unclear [19]. Expanding our knowledge about the unique characteristics of the familial form of MS enlightens the significance of genetic and environmental factors in this disease and improves the diagnostic and therapeutic methods. This study was designed to compare familial and SMS cases to comprehensively understand their variations and implications.
Materials and Methods
Study design
This analytical study employed a cross-sectional design to investigate patients with clinically definite MS registered in the nationwide MS registry of Iran (NMSRI)-Guilan. A definite MS diagnosis was made by an expert neurologist based on the 2017 revised McDonald criteria [20]. The data were collected by a medical student who retrieved information from the medical records of individuals with MS in this registry system. This dataset encompassed an extensive array of demographic and clinical data, including age, gender, onset age, disease clinical course (such as relapsing‐remitting MS, primary progressive MS, secondary progressive MS and clinically isolated syndrome), disease duration, number of attacks (defined by neurological symptoms that have persisted for longer than 24 hours within the past 12 months), medical comorbidities, first presentation of the disease, FMS history, utilization of disease-modifying therapies (DMTs), expanded disability status scale (EDSS) score and the most recent brain MRI. If data records were inadequate, patients were contacted via telephone for additional information and if not feasible, they were invited by medical experts for an appointment to provide additional information through physical examination or a review of their medical records.
Study participants
As of early 2023, there were 1134 registered MS patients in NMSRI-Guilan, with 196 patients (17.2%) diagnosed with FMS. Initially, we assessed all FMS patients (n=196) along with an equal number of randomly chosen SMS patients (n=196). After a comprehensive review of their medical records, 117 FMS and 83 SMS were excluded due to the unavailability of their MRI scans, severe artifacts, disease duration exceeding 15 years, and insufficient patient cooperation. Consequently, 79 FMS and 113 SMS were included in the study. The patient selection process is shown in Figure 1. The inclusion criteria were as follows: Patients definitively diagnosed with MS according to the 2017 revised McDonald criteria [20] and less than 15 years after the onset of their disease. The exclusion criteria were as follows: inadequate data in medical records, unrecoverable or incomplete MRI sequences or presence of severe artifacts, presence of other neurological disorders impeding MS diagnosis, and a duration of more than 15 years since the onset of their disease.
Patient classification
Sporadic cases of MS pertain to individuals who are the only members in their family diagnosed with the disease. Conversely, at least one first to third-degree relative was also diagnosed with MS in FMS cases. First-degree relatives include parents and siblings, second-degree relatives include uncles and aunts, while third-degree relatives include cousins and others.
Magnetic resonance imaging acquisition and analysis
Subjects in both groups underwent a 1.5 T brain MRI scan using an 18-channel MRI scanner (Magnetom Avanto; Siemens Medical Solutions, Erlangen, Germany). The imaging protocol comprised T1-weighted images (with echo time [TE]=12 ms and repetition time [TR]=664 ms). Thin-slice images (1 mm) were acquired in axial, sagittal and coronal views. During post-processing analysis, an expert radiologist, utilizing OsiriX software, version 12.0 [21], evaluated the characteristics of brain lesions. The assessment included the location of lesions (periventricular, callosal, cervical, infratentorial, juxtacortical, or cortical), the number of lesions, the area of the smallest and largest lesion (mm2) and the presence of black holes. The imaging data were analyzed concerning the number of lesions, explicitly focusing on the axial view and T2-weighted and FLAIR MRI images. Additionally, the location of the lesions was categorized, and the presence or absence of black holes was determined by examining the T1-weighted view.
Statistical analysis
We employed the Mean±SD or the median and interquartile range to depict the quantitative data based on the distribution’s normality. Additionally, categorical data was delineated using numerical representations and percentages. For inferential analysis, we initially assessed the normality of quantitative variables using visual methods, specifically the Q-Q chart, skewness, kurtosis, and the Shapiro-Wilk test. To compare quantitative variables across two groups, the independent t-test was utilized if the data exhibited a normal distribution; otherwise, the Mann-Whitney U test was applied. The chi-squared and Fisher exact tests were employed for categorical variables. All analyses were conducted using SPSS software, Version 26.0. The effect size of significant correlations was determined using Spearman’s correlation coefficient. Subsequently, significant correlations underwent further evaluation through backward multivariate regression analysis.
Results
This study assessed 192 MS patients consisting of 79 FMS (41.1%) and 113 SMS (58.9%) patients. Patients were approximately matched in terms of DMTs utilization, except for ocrelizumab, which exhibited a statistically significant elevation in the SMS group (Table 1).

While the average age of patients between FMS and SMS exhibited a statistically borderline difference (FMS: 34.82±9.40; SMS: 37.71±10.83, P=0.057), a notable statistically significant difference was observed in the onset age between the two groups (FMS: 28.92±9.46; SMS: 31.48±9.55, P=0.034). Moreover, our investigation revealed no statistically significant differences between the two groups concerning gender distribution (P=0.604), disease duration (P=0.738), number of attacks (P=0.42) and medical comorbidities (P=0.237). Table 2 represents the study participants’ demographic and clinical characteristics.

Within the FMS group, 21 individuals (26.5%) exhibited a familial predisposition to MS among their first-degree relatives, 23(29.1%) among second-degree relatives, and 35(44.4%) among third-degree relatives (Figure 2). When comparing the initial disease presentations between the two groups, sensory symptoms emerged as the most common (51.3% in SMS vs 51.9% in FMS, P=0.938). Moreover, among all the other primary manifestations, only diplopia was significantly higher in the SMS group (29.2% in SMS vs 16.4% in FMS, P=0.042) (Table 2).
In comparing imaging findings between the two groups, the SMS group exhibited a significantly higher number of lesions (31.59±25.36, range: 4-170, P=0.017) in contrast to the FMS group (22.83±17.17, range: 2-67, P=0.017). Furthermore, a statistically significant difference was observed in the average size of the smallest lesions (FMS: 8.12±8.58, SMS: 4.65±2.57 mm2, P<0.001). However, there was no statistically significant difference found in the average size of the largest lesions (FMS: 98.57±80.97, SMS: 100.84±105.72 mm2, P=0.555) or in the incidence of black holes (SMS: 25.3% vs FMS: 23.8%, P=0.822). Imaging findings of study participants can be found in Table 3.

The frequency of lesions in the periventricular region was significantly higher in the SMS group (98.2%) compared to the FMS group (89.8%, P=0.017). However, no statistically significant difference was observed in juxtacortical/cortical (P=0.27), infratentorial (P=0.118), cervical (P=0.134) and callosal (P=0.178) regions between the two groups (Table 3).
The average score of EDSS was significantly higher in the SMS group (2.20±2.61) compared to the FMS group (1.09±2.08, P=0.002). Interestingly, most patients in both groups showed mild EDSS scores (SMS: 62.8% vs FMS: 84.8%) (Table 2). Moreover, when comparing EDSS scores among different degrees of relatives, those in the first degree exhibited significantly higher scores (3.07±2.68) than second-degree (0.34±1.15) and third-degree (0.4±1.24, P1,2<0.001, P1,3<0.001, P2,3=0.993) relatives (Table 4).

Within the SMS group, a moderate correlation was detected between the EDSS score and various factors, including onset age (r=0.314, P=0.001), disease duration (r=0.342, P<0.001), number of lesions (r=0.305, P=0.001) and the smallest lesion size (r=-0.208, P=0.027). Conversely, in the FMS group, significant correlations were found between the EDSS score and onset age (r=0.422, P<0.001), number of attacks (r=-0.224, P=0.047) and the largest lesion size (r=0.321, P=0.004) (Table 5).

Multivariate linear regression analysis revealed that, collectively, the type of MS (SMS or FMS), number of lesions, largest lesion size, onset age and disease duration accounted for approximately 26.4% of the variability observed in the EDSS score (P<0.001). Notably, the final model underscored significant associations between the type of MS (FMS or SMS) (β=0.78, P=0.016), onset age (β=0.09, P<0.001) and disease duration (β=0.13, P=0.001) with the EDSS score (Table 6).

Discussion
This investigation sought to delineate demographic, clinical, and imaging distinctions among individuals with sporadic and familial forms of MS. The SMS cohort exhibited significantly higher AAO, higher incidence of diplopia as the initial manifestation, higher EDSS scores, higher number of total lesions, higher number of lesions in the periventricular region, and lower average size of the smallest lesions relative to the FMS cohort. Moreover, the EDSS score was higher in the SMS group and demonstrated significant associations with MS subtype (SMS or FMS), onset age and disease duration.
In the present investigation, the FMS group exhibited a lower AAO, aligning with the findings reported by Salehi et al. [22] and Katsavos et al. [16]. Notably, Katsavos et al. discerned a reduced AAO in FMS patients with the affected first-degree relatives. In contrast, those with third-degree relatives showed no such distinction [16]. Conversely, several other investigations failed to observe a significant distinction in the AAO between individuals with FMS and SMS [2, 17, 19, 23-25]. This outcome might be because MS is a complex disease with both genetic and environmental factors contributing to its onset and progression. FMS suggests a stronger genetic component, possibly leading to an earlier disease onset. On the other hand, SMS may have a later onset because it could be more influenced by environmental factors, which may take longer to exert their effects. Furthermore, genetic predisposition in FMS may interact with environmental factors at an earlier age, leading to an earlier onset of the disease.
No significant difference in disease duration was observed between the two groups, aligning with findings from other research studies [2, 17, 19].
Furthermore, our study found no difference in gender distribution between familial and sporadic types of MS. This observation aligns with findings from prior research [2, 16, 17, 26]. It was established that MS results from the interplay between genetic and environmental factors. Although females are relatively more susceptible to MS than males, this holds for both sporadic and familial cases; hence, the gender distribution remains parallel in both scenarios.
Regarding the frequency of attacks, most patients in both groups encountered one or fewer attacks. However, the observed difference between the two groups was not statistically significant, consistent with the findings of Moghadam et al. [2]. In contrast, Andrijauskis et al. [19] reported a higher annual attack rate in FMS compared to SMS, with 1.4 attacks vs 0.8 attacks (P<0.05).
In our investigation, certain patients presented with concurrent medical comorbidities such as hypertension, diabetes, respiratory disorders, thyroid disorders, migraine, and seizures. However, no significant difference was observed between the two study groups, aligning with the conclusions drawn by Moghadam et al. [2] and Mokhtari et al. [25]. Given the complex interplay between genetic, environmental, and lifestyle factors that contribute to the pathogenesis of MS, it is plausible that these same factors may also impact the co-occurrence of other medical comorbidities. Therefore, further investigation is warranted to understand these associations comprehensively.
We observed a higher incidence of diplopia among SMS patients as the first presentation of the disease, consistent with findings by Katsavos et al. [16] and Faraji et al. [23]. Nevertheless, Mokhtari et al. [25] reported no significant difference between the two groups regarding visual symptoms as the initial manifestation of the disease.
In this study, we observed significantly higher EDSS scores in the SMS group, contrary to prior research outcomes. Moghadam et al. [2] and Katsavos et al. [16] reported no substantial difference in EDSS scores between the two groups. In contrast, Andrijauskis et al. [19] and Faraji et al. [23] identified lower EDSS scores in SMS patients. Furthermore, we observed a higher EDSS score in FMS patients with first-degree relatives affected by MS. Conversely, Tipirneni et al. [17] found no significant variations in EDSS scores based on the degrees of familial relationships (first, second and third degrees).
Our investigation revealed that the FMS patients exhibited fewer lesions and a higher average size of the smallest lesion than the SMS group. Nonetheless, there was no statistically significant difference between the two groups’ average size of the largest lesion and the occurrence of black holes. Tipirneni et al. [17] reported a greater average T1-lesion volume in the FMS group.
Furthermore, we observed a notable increase in lesions within the periventricular region among SMS patients, aligning with the results reported by Moghadam et al. [2]. Interestingly, they also noted significantly more lesions within the callosal region among FMS patients. Katsavos et al. [16] reported higher lesions in the subcortical and cervical regions for SMS and FMS patients, respectively. Additionally, Andrijauskis et al. [19] identified a significant rise in lesions within the cerebellum and brainstem, specifically among FMS patients.
Conclusion
In conclusion, FMS exhibits distinct characteristics from SMS across various facets, such as an earlier onset age, a predominance of RRMS phenotype, lower rate of diplopia, lower EDSS scores, lower lesion frequency in the periventricular area, fewer overall lesion numbers, and a higher average size of the smallest lesions.
Study limitations
The present investigation encountered certain limitations. Unfortunately, many patients in NMSRI-Guilan lacked MRI scans, and many available MRIs had severe artifacts, leading to their exclusion and thus reducing the sample size. This limitation raises concerns about the finality of our findings and may affect their generalizability to the broader MS patient population. The cross-sectional nature of our design offers a snapshot of a specific moment, preventing the establishment of definitive cause-and-effect relationships and failing to capture changes over time. Uncontrolled variables, such as lifestyle factors and healthcare accessibility, could introduce confounding elements into our results. Additionally, the reliance on self-reported data to categorize patients as FMS introduces the possibility of bias. Participant responses may be influenced by inaccurate recall or intentional alteration. Lastly, the study’s confinement to a specific geographical location (Guilan Province, Iran) suggests caution in extrapolating the results to populations with distinct cultural, social, or environmental conditions. As a result, there is a need for more extensive multinational investigations that incorporate the assessment of genetic predisposition among participants to ensure the generalizability of the findings.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1401.380). The researchers adhered to the ethical principles of the Declaration of Helsinki. Additionally, signed informed consent was acquired from each participant, along with approval from the chairman of the MS Society of Guilan province for data extraction.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization: Alia Saberi, Amirreza Ghayeghran, and Mohammad Ali Sahraian; Methodology: Sharareh Eskandarieh; Formal analysis: Sharareh Eskandarieh and Nima Broomand Lomer; Supervision: Alia Saberi and Amirreza Ghayeghran; Writing the original draft: Kamal Amir Ashjei Asalemi, Nima Broomand Lomer, Kasra Sarlak, and Negin Ashoori; Investigation: Kamal AmirAshjei Asalemi; Writing, review, editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the Clinical Research Development Unit of Poursina Hospital, Guilan University of Medical Sciences, Guilan, Iran.
References
- Ceccarelli A, Mifsud VA, Dogar A. Demographic and clinical characteristics of familial and sporadic multiple sclerosis: A single center exploratory study from Abu Dhabi. J Clin Neurosci. 2020; 76:145-7. [DOI:10.1016/j.jocn.2020.04.007] [PMID]
- Moghadam NB, Ghaffari M, Rashed SS, Valaie N, Hesami O, Niloofar P, et al. MRI but not demographic or clinical characteristics differ between familial and sporadic MS cases. Mult Scler Relat Disord. 2021; 56:103235. [DOI:10.1016/j.msard.2021.103235] [PMID]
- Huang WJ, Chen WW, Zhang X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp Ther Med. 2017; 13(6):3163-6. [DOI:10.3892/etm.2017.4410] [PMID] [PMCID]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008; 372(9648):1502-17. [DOI:10.1016/S0140-6736(08)61620-7] [PMID]
- Saberi A, Ashkan M, Hatamian H, Ashraf A, Homaie Rad E, Bakhshi A, et al. Migraine headache in multiple sclerosis. Is more frequent among MS patients? Rom J Neurol. 2023; 22(1):54-7. [DOI:10.37897/RJN.2023.1.9]
- Ebers GC, Sadovnick AD, Risch NJ. A genetic basis for familial aggregation in multiple sclerosis. Canadian collaborative study group. Nature. 1995; 377(6545):150-1. [DOI:10.1038/377150a0] [PMID]
- Dobson R, Giovannoni G. Multiple sclerosis-A review. Eur J Neurol. 2019; 26(1):27-40. [DOI:10.1111/ene.13819] [PMID]
- Alfredsson L, Olsson T. Lifestyle and environmental factors in multiple sclerosis. Cold Spring Harb Perspect Med. 2019; 9(4):a028944. [DOI:10.1101/cshperspect.a028944] [PMID] [PMCID]
- Nourbakhsh B, Mowry EM. Multiple sclerosis risk factors and pathogenesis. Continuum. 2019; 25(3):596-610. [DOI:10.1212/CON.0000000000000725] [PMID]
- Hollenbach JA, Oksenberg JR. The immunogenetics of multiple sclerosis: A comprehensive review. J Autoimmun. 2015; 64:13-25. [DOI:10.1016/j.jaut.2015.06.010] [PMID] [PMCID]
- International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019; 365(6460):eaav7188. [DOI:10.1126/science.aav7188] [PMID]
- Curtius F, Speer H. Multiple Sklerose und Erbanlage. Z Gesamte Neurol Psychiatr. 1938; 160(1):226-45. [DOI:10.1007/BF02877977]
- Harirchian MH, Fatehi F, Sarraf P, Honarvar NM, Bitarafan S. Worldwide prevalence of familial multiple sclerosis: A systematic review and meta-analysis. Mult Scler Relat Disord. 2018; 20:43-7. [DOI:10.1016/j.msard.2017.12.015] [PMID]
- Yamasaki R, Kira JI. Multiple Sclerosis. Adv Exp Med Biol. 2019;1190:217-47. [DOI:10.1007/978-981-32-9636-7_14] [PMID]
- Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler. 2020; 26(14):1816-21. [DOI:10.1177/1352458520970841] [PMID] [PMCID]
- Katsavos S, Artemiadis A, Davaki P, Stamboulis E, Kilindireas K, Anagnostouli M. Familial multiple sclerosis in Greece: Distinct clinical and imaging characteristics in comparison with the sporadic disease. Clin Neurol Neurosurg. 2018; 173:144-9. [DOI:10.1016/j.clineuro.2018.08.021] [PMID]
- Tipirneni A, Weinstock-Guttman B, Ramanathan M, Abdelrahman N, Hussein S, Hagemeier J, et al. MRI characteristics of familial and sporadic multiple sclerosis patients. Mult Scler. 2013; 19(9):1145-52. [DOI:10.1177/1352458512469697] [PMID]
- Koch M, Zhao Y, Yee I, Guimond C, Kingwell E, Rieckmann P, et al. Disease onset in familial and sporadic primary progressive multiple sclerosis. Mult Scler. 2010; 16(6):694-700. [DOI:10.1177/1352458510367661] [PMID]
- Andrijauskis D, Balnyte R, Keturkaite I, Vaitkus A. Clinical and diagnostic features of patients with familial multiple sclerosis. Med Hypotheses. 2019; 131:109310. [DOI:10.1016/j.mehy.2019.109310] [PMID]
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018; 17(2):162-73. [DOI:10.1016/S1474-4422(17)30470-2] [PMID]
- Rosset A, Spadola L, Ratib O. OsiriX: An open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004; 17(3):205-16. [DOI:10.1007/s10278-004-1014-6] [PMID] [PMCID]
- Salehi Z, Almasi-Hashiani A, Sahraian MA, Eskandarieh S. Epidemiology of familial multiple sclerosis: A population-based study in Tehran during 1999-2018. Mult Scler Relat Disord. 2020; 43:102178. [DOI:10.1016/j.msard.2020.102178] [PMID]
- Faraji F, Mohaghegh P, Talaie A. Epidemiology of familial multiple sclerosis and its comparison to sporadic form in Markazi Province, Iran. Mult Scler Relat Disord. 2022; 68:104231. [DOI:10.1016/j.msard.2022.104231] [PMID]
- Bunul SD. Comparing clinical and radiological features in familial and sporadic multiple sclerosis. Cureus. 2023; 15(9):e44504. [DOI:10.7759/cureus.44504] [PMID] [PMCID]
- Mokhtari S, Houshi S, Mirmosayyeb O, Barzegar M, Afshari-Safavi A, Ghasemi M, et al. Demographic and clinical characteristics of familial and sporadic multiple sclerosis patients. Int J Prev Med. 2023; 14:86. [DOI:10.4103/ijpvm.ijpvm_187_22] [PMID] [PMCID]
- Steenhof M, Stenager E, Nielsen NM, Kyvik K, Möller S, Hertz JM. Familial multiple sclerosis patients have a shorter delay in diagnosis than sporadic cases. Mult Scler Relat Disord. 2019; 32:97-102. [DOI:10.1016/j.msard.2019.04.012] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2024/05/17 | Accepted: 2024/06/18 | Published: 2025/01/1
Received: 2024/05/17 | Accepted: 2024/06/18 | Published: 2025/01/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |