Wed, Dec 31, 2025
Volume 10, Issue 4 (Autumn 2024)
Caspian J Neurol Sci 2024, 10(4): 312-324 |
Back to browse issues page
Ethics code: IR.UMSU.AEC.1401.014
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Abbaszadeh M E, Pourheydar B, Farjah G. Effects of Crocin, Azithromycin, and Their Co-administration on Sciatic Nerve Injury in Rats. Caspian J Neurol Sci 2024; 10 (4) :312-324
URL: http://cjns.gums.ac.ir/article-1-697-en.html
URL: http://cjns.gums.ac.ir/article-1-697-en.html
1- Department of Anatomical Sciences, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran.
2- Department of Anatomical Sciences, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran. ,pourheydar.b@umsu.ac.ir
2- Department of Anatomical Sciences, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran. ,
Full-Text [PDF 2275 kb]
(397 Downloads)
| Abstract (HTML) (1393 Views)
Full-Text: (643 Views)
Introduction
Although peripheral nerve injury does not pose a direct threat to life, it results in substantial impairments that impact the everyday functioning of individuals and have significant social and economic ramifications. The majority of these wounds result from mechanical trauma, which could permanently harm the nerves and cause severe chronic pain, loss of sensation, loss of motor function, or paralysis [1, 2]. Despite the peripheral nerves’ ability to regenerate, this capability is inadequate in guaranteeing complete patient recovery, preservation of functional integrity, and enhancement of overall quality of life. When choosing treatments, it is important to consider many factors, including the degree, size, and location of the damage, the age of the patient, and the time elapsed between the injury and therapeutic intervention. Therefore, the selection of appropriate methods has significant importance in the process of peripheral nerve healing [3]. Following peripheral nerve loss, there is a subsequent occurrence of ischemia and inflammatory processes, which give rise to neurological impairment. Several therapeutic interventions may be used to alleviate the effects of these processes. Numerous pharmaceuticals, metabolites, and chemical compounds have undergone extensive evaluation to reduce the ensuing consequences of peripheral nerve injury. Furthermore, ongoing research efforts are actively pursued in this domain [4].
Crocin (CR), a water-soluble carotenoid with high antioxidant activity, is a significant component of saffron [5, 6, 7]. Multiple studies have shown that CR has various therapeutic benefits, including antioxidant, anti-apoptotic, anti-inflammatory, and antihypertensive properties [8, 9, 10, 11]. Moreover, recent studies have demonstrated the ability of CR to reduce neuroinflammation and neurodegeneration in autoimmune encephalomyelitis by retaining the density of myelin and axons in the spinal cord [8]. Additionally, CR has been demonstrated to accelerate functional recovery, decrease oxidative stress and repair myeline sheath after sciatic nerve crush injury in rats [6].
Azithromycin (AZ), an antibiotic approved by the Food and Drug Administration (FDA), is often used to treat several illnesses due to its notable anti-inflammatory and immunomodulatory properties [12, 13]. The administration of AZ has been shown to inhibit the signaling pathways of signal transducer and activator of transcription 1 and nuclear factor-kappa B (NF-kB) in macrophages, leading to the manifestation of anti-inflammatory properties [14]. During this particular phase, the administration of AZ effectively hinders the activation of pro-inflammatory macrophages (M1) while concurrently promoting the activation of anti-inflammatory macrophages (M2) [15, 16]. Moreover, recent studies verify in favor of the use of AZ as a therapeutic intervention for neurological disorders in neonates, including but not limited to stroke, retinal ischemia, muscular-spinal atrophy, and hypoxic-ischemic brain damage [13, 17]. A further investigation revealed that the 7 days administration of AZ can accelerate regeneration of the sciatic nerve, improve motor and sensory function recovery, and upregulate the expression of nerve growth factor and brain-derived neurotrophic factor genes after sciatic nerve crush injury in rats [18].
This research aimed to investigate and compare the effect of CR and AZ and their co-administration on functional, histological, and biochemical alterations after sciatic nerve crush injury. The purpose was to determine the most advantageous technique for treating sciatic nerve injury in rats.
Materials and Methods
Animals and experimental design
A total of 40 adult albino rats, weighing 230 to 250 g, were acquired from the Department of Pharmacology at Urmia University of Medical Sciences. The rats were housed in standard laboratory settings, which included a light-dark cycle of 12 hours each, a temperature-controlled environment ranging from 23 °C to 25 °C, and ad libitum access to food and water. The experimental protocols adhered to the guidelines outlined in the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
Five groups of rats were randomly formed (8 animals per group). Group 1, or the control group, consisted of rats without sciatic nerve crush injury induction and received normal saline and distilled water as vehicles daily for seven days. Group 2, or the lesion group, underwent a surgically-induced sciatic nerve crush injury; they received normal saline and distilled water as vehicles daily for seven days. Group 3, or the CR group, after sciatic nerve crush injury induction, received CR (soluble in normal saline, 50 mg/kg, IP) daily for seven days [19]. Group 4, or the AZ group, after sciatic nerve crush injury induction, received AZ (soluble in distilled water, 160 mg/kg orally) daily for seven days [20]. Group 5, or the AZ+CR group, after inducing sciatic nerve crush injury, received 50 mg/kg CR and 160 mg/kg AZ daily for seven days.
Surgery
The rats were sedated by intraperitoneal administration of ketamine (80 mg/kg) and xylazine (10 mg/kg). The surgical area was shaved entirely and sterilized using betadine solution, spanning from the femoral head’s inferior aspect to the knee joint’s superior aspect. A small incision about 2–3 cm was made on the skin in the desired area. After the separation of the skin from the underlying tissues and the displacement of the muscles, the sciatic nerve became discernible deep in this region. Subsequently, a compression maneuver was performed on the sciatic nerve with medium surgical hemostatic forceps for 60 seconds at a location situated 1 cm proximal to the trifurcation site. The nerve compression was standardized across all rats using a single pair of locking forceps. After compression, the nerve was repositioned to its original anatomical location, the incision margins were sutured, and the wound was thoroughly cleansed. The animals were provided with appropriate thermal conditions during and after the treatment until they regained consciousness. Following the rats’ consciousness restoration, they were transferred to individual enclosures and subjected to standard environmental settings, including regulated light exposure, temperature, and humidity levels [6].
Sciatic functional index
On the 14th, 28th and 42nd postoperative days, the sciatic functional index (SFI) was used to assess functional recovery. For this experiment, the rat’s hind feet were soaked in ink and permitted to walk through a corridor covered with white paper. The SFI was then determined using the Equation 1:
1. SFI=-38.8[EPL-NPL/NPL]+109.5[ETS-NTS/NTS]+13.3[EIT-NIT/NIT]–8.8
In Equation 1, PL indicates the sole length, TS is the distance between toes 1 and 5, IT is the distance between toes 2 and 4, E denotes the experimental side, and N is the healthy side. All measurements were taken in mm. An SFI score of 0 was considered normal leg movement, while -100 indicated significant impairment [21].
Hot plate test
According to some research [22], the hot plate test was utilized to assess the thermal threshold. The test was conducted on the 42nd day after surgery using digital hot plate equipment. During the experiment, the gadget’s temperature was adjusted to 56 °C, after which the rats were placed on the hot plate. The response time measurement included recording the duration, in seconds, that the rats needed to withdraw their injured leg from the screen. The process was conducted three times for each animal, with a time interval of 10 minutes between each repetition. To minimize the risk of harm to the rat’s foot, the duration of exposure on the hot plate did not exceed 12 seconds.
Mechanical withdrawal thresholds
Von Frey filaments and a mesh apparatus were used to evaluate the responses of animals to mechanical stimulation to measure the presence of mechanical allodynia. The Von Frey hair consists of a handle and a slender polyethylene thread with different thicknesses, resulting in a range of forces exerted on the animal’s feet, ranging from 8 to 300 g. The mesh structure consists of a metallic net supported by four bases, allowing unrestricted access from the lower side. On the 42nd postoperative day, the rats were positioned on the mesh substrate to conduct this experiment. Subsequently, their injured hind paws were subjected to stimulation originating from the mid-plantar surface, using a variety of filaments that varied in pressure intensity, ranging from low to high. Each stimulation lasted for a duration of 6 to 8 seconds. The filament with the lowest pressure eliciting the animal’s paw withdrawal reaction was defined as the stimulation threshold [23].
Biochemical analysis of malondialdehyde (MDA) and total antioxidant capacity (TAC)
On the 42nd day of the study, the animals were administered ketamine (80 mg/kg) and xylazine (10 mg/kg) to induce sedation. Subsequently, 5 mL of blood was collected directly from their cardiac region and immediately transferred to test tubes that were stored on ice. The sample underwent centrifugation at a speed of 3000 rpm for 10 min. Following the separation of the serum, it was then held at a temperature of -70 °C until the levels of MDA and TAC were assessed. The MDA test was performed to evaluate the extent of lipid peroxidation, a key indicator of oxidative stress in the tissue samples. Lipid peroxidation results from the oxidative degradation of lipids, which can lead to cellular damage, and is commonly assessed using the thiobarbituric acid (TBA) reactive substances assay.
TBA reaction technique
The MDA levels were measured using the TBA reaction technique, following the method described by Ohkawa et al. [24]. This method involves the reaction of MDA, a product of lipid peroxidation, with TBA to form a pink chromogenic complex.
Colorimetric analysis
The resulting chromogenic compound exhibits maximum absorbance at a wavelength of 535 nm. This colorimetric analysis allows quantifying MDA concentration based on its light absorption properties.
MDA standard curve
A standard curve was constructed using tetraethoxypropane (TEP) as the MDA standard. This curve is used to determine the concentration of MDA in the samples. TEP is employed due to its known reactivity with TBA, which allows for accurate calibration and quantification.
Quantification
The concentration of MDA in the samples is expressed in µmol/L. The absorbance readings of the samples are compared against the standard curve to determine the MDA levels. The TAC test was performed using the TAC kit from Randox (Crumlin, County Antrim, UK). This assay is designed to measure the overall antioxidant capability of a sample by assessing its ability to scavenge free radicals. The quantification of caspase-3-positive cells was performed inside a tissue sample by counting the number of such cells present in 1 mm2. This measurement was then used to compare and analyze the differences between the different experimental groups. In addition, software-based methods were used to investigate the cellular distribution [25].
Reaction principle
The assay employs the compound 3-ethylbenzothiazoline-6-sulfonate (ABTS), which forms a radical cation known as ABTS in the presence of peroxidase and hydrogen peroxide (H2O2)⁺. The radical cation exhibits a blue-green hue, which indicates the radicals’ presence.
Formation of ABTS+
During the reaction, ABTS reacts with H₂O₂ in the presence of peroxidase to generate the ABTS⁺ radical cation. The intensity of the blue-green color correlates with the concentration of ABTS⁺, a measure of oxidative stress.
Colorimetric detection
The color change is measured using a spectrophotometer at a wavelength of 600 nm. The intensity of the blue-green hue detected at this wavelength is directly proportional to the concentration of ABTS⁺ and inversely proportional to the antioxidant activity of the sample.
Antioxidant capacity determination
The antioxidant capacity of the sample is determined by comparing its ability to inhibit the formation of ABTS⁺ to that of a standard with known antioxidant properties. The greater the inhibition of the ABTS⁺ formation, the higher the antioxidant capacity of the sample.
Histopathological evaluation
On the 42nd day of the study, following the administration of sedatives to the animals and subsequent blood collection for biochemical analysis, a 1 cm segment from the central portion of the sciatic nerve was taken inside the area of injury and preserved using paraformaldehyde. Following a 48 h period, the tissue underwent processing. Subsequently, 5 µ slices were cut from the tissue and subjected to staining with Luxol fast blue. For each tissue sample, 10 random fields of view were selected, and histological evaluation (myelinated fibers diameter, myelin sheath diameter, and number of myelinated fibers) was then conducted with a light microscope set at a magnification of 400× and ImageJ software, version 1.52.
The Luxol fast blue staining technique was used to stain the myelin sheath in tissue slices of the sciatic nerve, resulting in a blue coloration. The morphometrics of the sciatic nerve, including the number of myelinated filaments, axon diameter and myelin sheath thickness, were evaluated using ImageJ software, version 1.52 in this study.
Immunohistochemical staining for apoptosis
The tissue segment slides were subjected to a temperature of 60 °C for about 25 min using a hot air oven (Venticell, MMM, Einrichtungen, Germany). The tissue slices underwent deparaffinization in xylene twice, with each instance lasting for 5 minutes. Subsequently, rehydration was carried out by subjecting the tissue slices to an alcohol gradient, starting with a concentration of 90% and gradually decreasing to 80%, 70% and finally 50%. The antigen retrieval technique was conducted using a solution containing sodium citrate at a concentration of 10 mM. The immunohistochemical staining procedure was performed according to the instructions provided by the manufacturers, Biocare and ScyTek, based in the United States. The endogenous peroxidase activity was suppressed by immersing the sample in a peroxidase-blocking solution containing 0.03% hydrogen peroxide with sodium acid for 5 minutes. The tissue slices underwent a gentle rinsing using phosphate-buffered saline (PBS, pH 7.2) before being subjected to overnight incubation at -4°C with primary antibodies targeting caspase-3 (1:500). Before immersion in a buffer solution, the sections were subjected to a gentle washing process using washing buffer (PBS, pH 7.2). Subsequently, the slides were placed into a humidified, which contained an anti-microbial substance along with an appropriate amount of streptavidin-HRP (streptavidin chemically linked to horseradish peroxidase) in phosphate-buffered saline (PBS). The slides were incubated for 15 minutes. The tissue sections were cleaned in a washing buffer solution and then immersed in a buffer bath. The tissue slices were subjected to the addition of a DAB chromogen, which underwent incubation for 5 more minutes. Subsequently, the sections were subjected to a counterstaining process using hematoxylin for 20 seconds. Next, the sections were immersed in a solution of mild ammonia (0.037 mL) for 10 seconds, then rinsed with distilled water and subsequent covering [26].
Statistical analysis
SPSS software, version 16 was utilized for statistical analysis. We assessed the distribution of the data using the Kolmogorov-Smirnov test. The results indicated that the data were normally distributed. To evaluate the significant differences between groups, statistical analysis was performed using a one-way analysis of variance (ANOVA). A Bonferroni post hoc analysis was used to compare all groups together. All data were presented in Mean±SD, with P≤0.05 regarded as statistically significant.
Results
SFI results
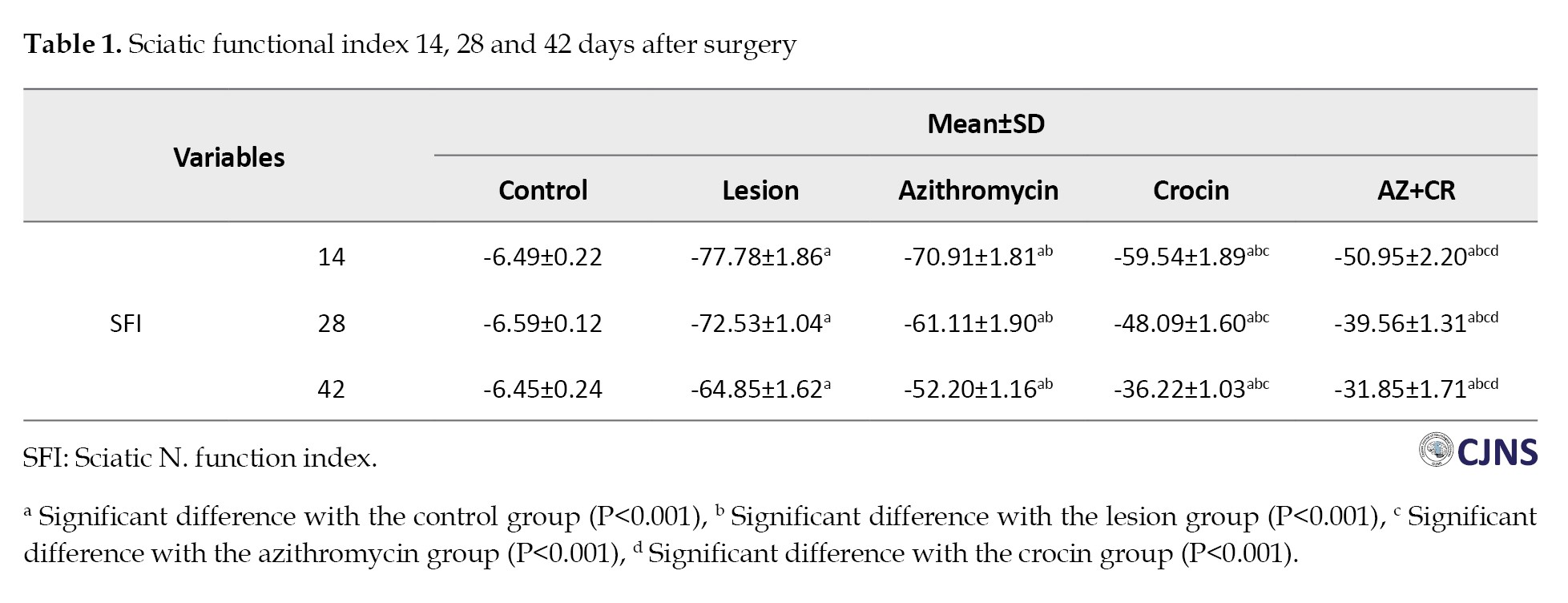
We analyzed the SFI values of rats on days 14, 28 and 42 after surgery. The lesion group had the lowest mean SFI and severe leg weakness. However, the CR, AZ and AZ+CR groups showed higher SFI values than the lesion group (P≤0.001), and all three treatment groups showed improved motor function. The AZ+CR group had a better effect on sciatic nerve motor recovery than the CR and AZ groups (P≤0.001). The CR group had higher SFI levels than the AZ group at all three time points (day 14, day 28 and day 42) (P≤0.001), indicating that CR had a better effect on SFI values than AZ. The control group had the highest SFI level in all phases (Table 1).
Hot plate results
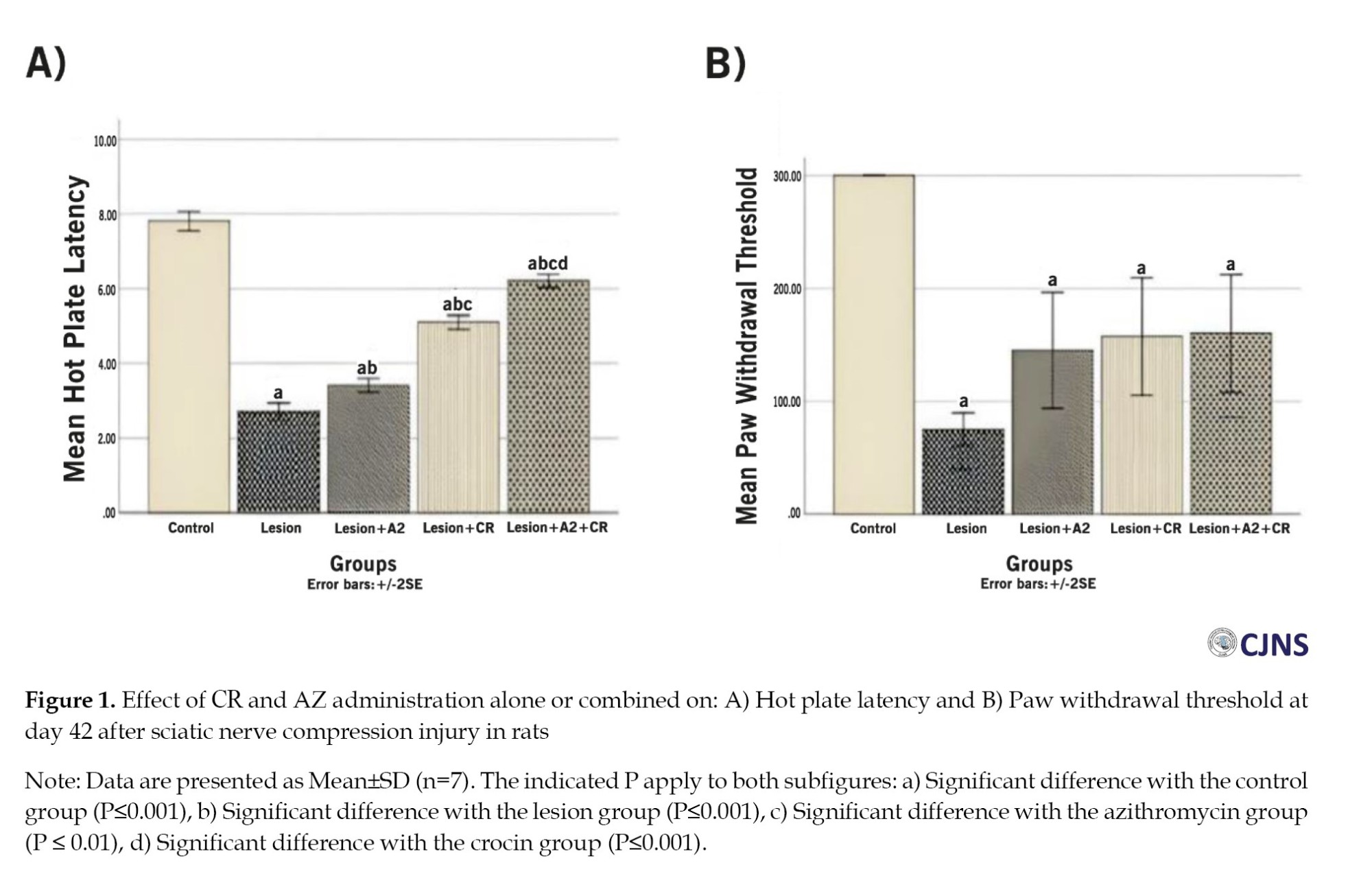
Forty-two days after surgery, the control group showed the longest reaction time to thermal stimulation (7.81±0.36 s), which decreased in the lesion group (2.71±0.32 s) (P≤0.001), indicating thermal hyperalgesia. The AZ, CR and AZ+CR groups had significantly higher reaction times (3.41±0.25, 5.10±0.26 and 6.21±0.25 s, respectively) (P<0.001), indicating an increase in the response threshold and reducing thermal hyperalgesia. The AZ+CR group showed a higher increase (P≤0.001), and CR was more effective than AZ in reducing thermal hyperalgesia in the response threshold to thermal stimulation (P≤0.001) (Figure 1A).
Mechanical withdrawal threshold results
The results of the investigation revealed a significant decrease in the paw withdrawal threshold among the lesion group (75±20.70 g) compared to the control group (300±0 g) (P<0.001), suggesting the presence of mechanical allodynia after sciatic nerve damage. The mechanical withdrawal threshold exhibited a greater value in the treatment groups AZ (145±72.31 g), CR (157.50±73.63 g) and AZ+CR (160±74.07 g) compared to the lesion group; however, this difference did not reach statistical significance (P=0.203 compare to AZ group, P=0.070 compare to CR group and P=0.056 compare to AZ+CR). The combined CR and AZ treatment demonstrated better results than the administration of AZ alone (Figure 1B).
Biochemical analysis results
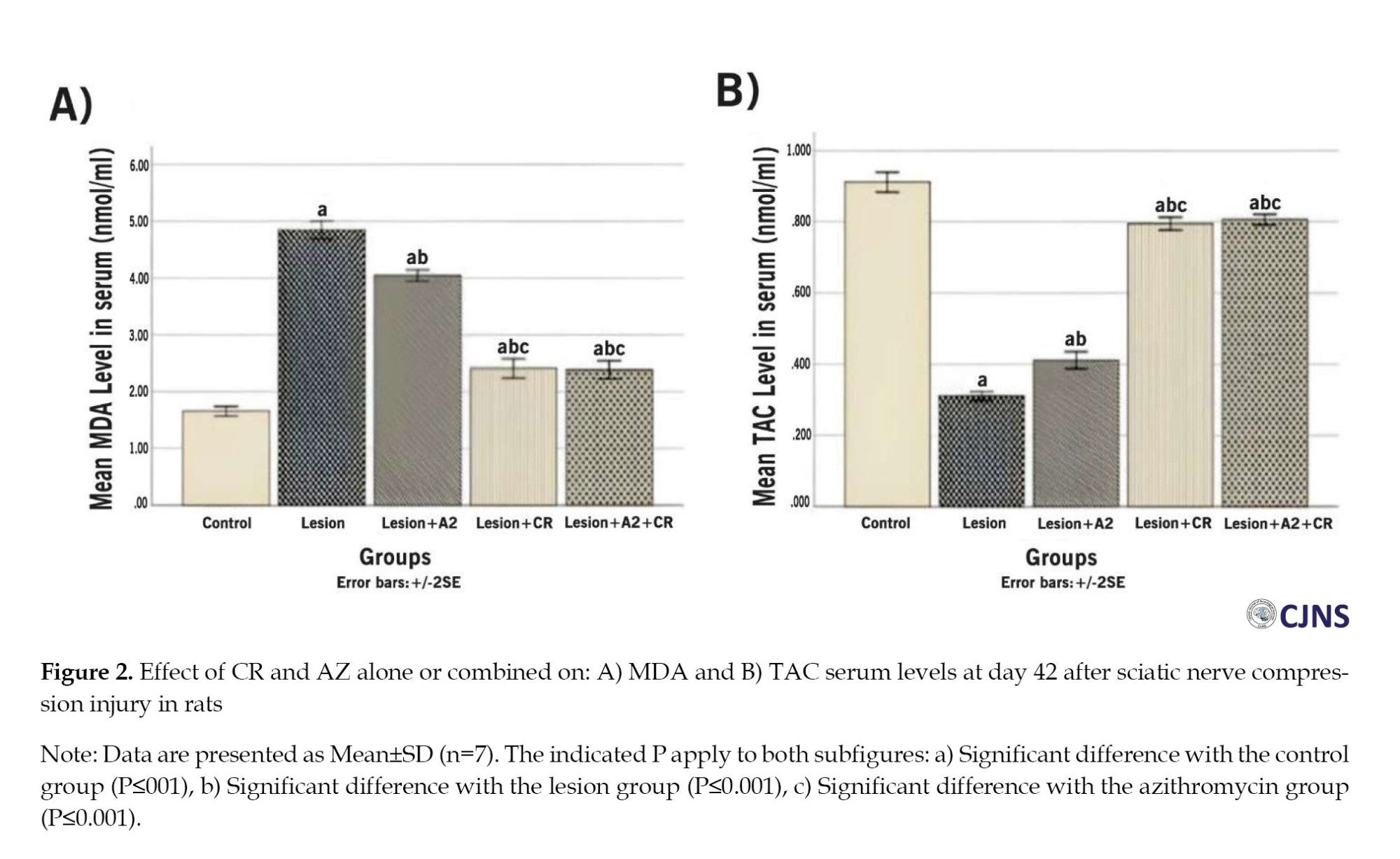
Forty-two days after surgery, the control group had the lowest average serum MDA levels (1.66±0.12 nmol/mL). In contrast, the lesion group had significantly higher mean MDA levels (4.84±0.22 nmol/mL) (P≤0.001), indicating increased lipid peroxidation. However, the AZ, CR and AZ+CR treatment groups had lower MDA levels (4.04±0.14, 2.41±0.24 and 2.38±0.23 nmol/mL, respectively) than the lesion group (P≤0.001), indicating effective therapeutic approaches to reducing lipid peroxidation. The effect was more robust in the AZ+CR groups, with lower and significant mean serum MDA levels (P≤0.001). The difference in mean serum MDA between the CR and AZ+CR groups was not statistically significant (P>0.999), but these two groups had significant differences with the AZ group (P≤0.001) (Figure 2. A). The results showed that serum TAC levels were lowest in the lesion group (0.31±0.02 nmol/mL) Forty-two days after surgery. However, the AZ, CR and AZ+CR treatment groups had significantly higher mean serum TAC levels (0.41±0.03, 0.8±0.03 and 0.81±0.02 nmol/mL, respectively) than the lesion group (P≤0.001). The CR and AZ+CR groups had no significant difference in serum TAC levels (P>0.999); these two groups had a significant difference with the AZ group (P≤0.001), while the control group had the highest mean serum TAC level (0.91±0.04 nmol/ml) and significantly differed from other groups (P≤0.001) (Figure 2B).
Histopathological results
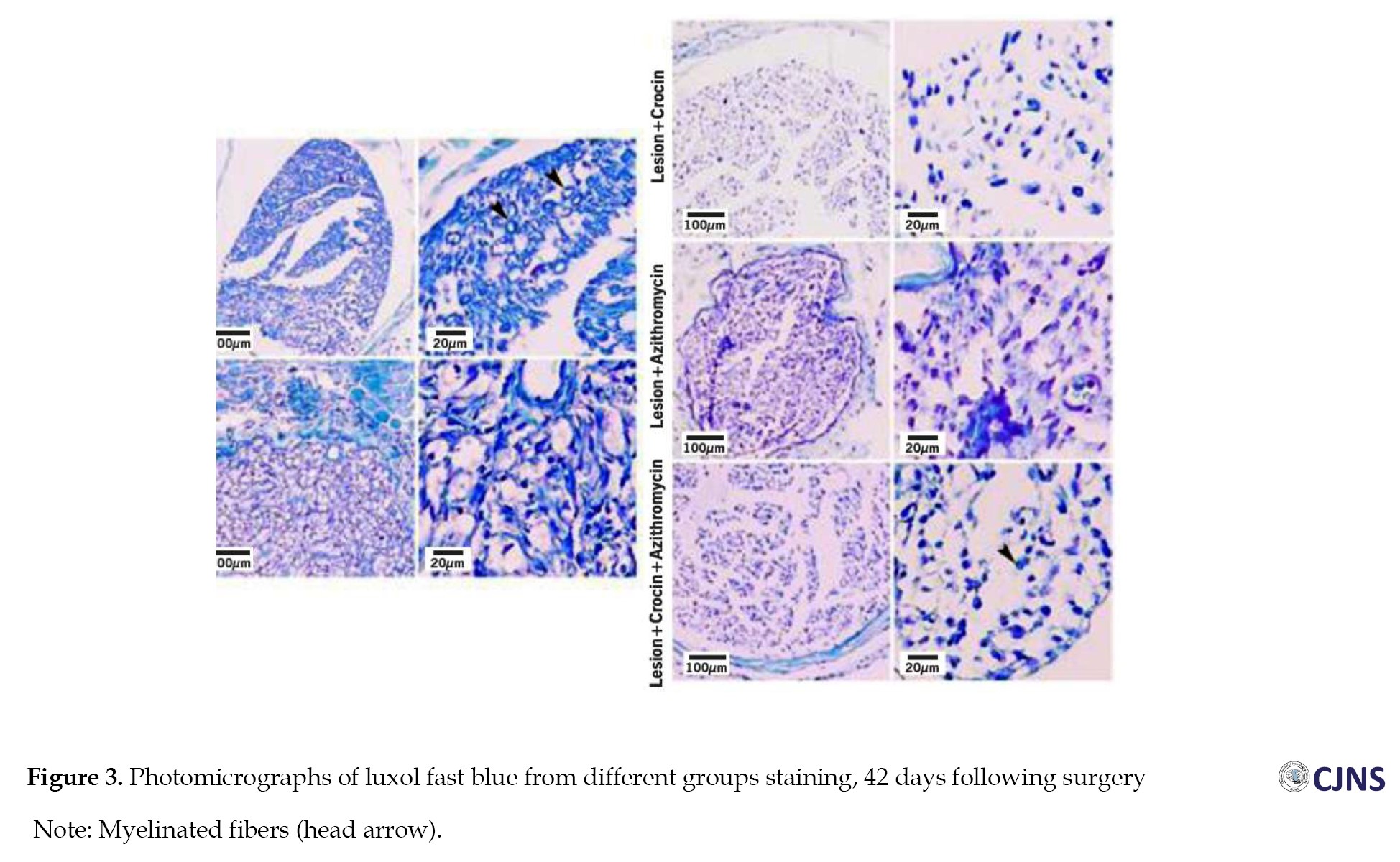
Figure 3 shows tissue sections stained with Luxol fast blue in different groups. Based on these sections, variables of myelinated fiber diameter, myelin sheath diameter, and myelinated fiber number were measured. The results of these three variables are as follows:
The study found that the lesion group had the lowest mean of myelinated fibers diameter (6.28±0.17 µm) on the 42nd day after surgery, which increased significantly in the AZ, CR, and AZ+CR treatment groups (7±0.15, 8.65±0.11 and 8.76±0.22 µm, respectively) (P≤0.001). The CR and AZ+CR groups had a higher mean of myelinated fibers diameter than the AZ group (P≤0.001), but no significant difference was found between these two groups (P=1). The control group had the highest mean of myelinated fibers diameter (9.12±0.22 µm) and was significantly different from other groups (P≤0.05) (Table 2).
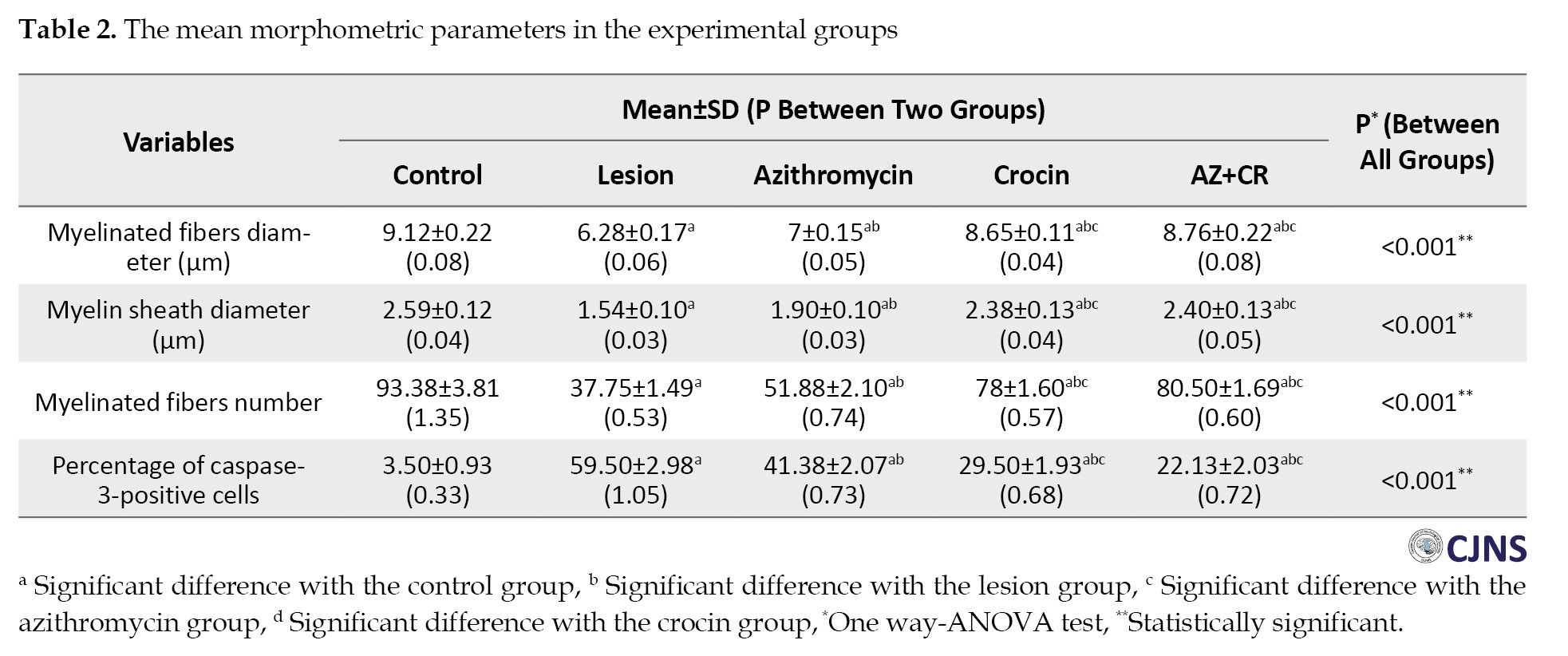
The research investigation revealed a significant difference in the average diameter of the myelin sheath between the AZ, CR and AZ+CR groups (1.90±0.10, 2.38±0.13 and 2.40±0.13 µm, respectively) compared to the lesion group (1.54±0.10 µm) (P≤0.001). There was no statistically significant difference between the groups receiving CR and AZ+CR. However, an important difference was observed between these two groups and the group receiving AZ alone. The mean diameter of the myelin sheath in the control group (2.59±0.12 µm) exhibited a statistically significant difference (P≤0.05) (Table 2).
The control group had the highest mean number of myelinated fibers (93.38±3.81), but after sciatic nerve injury, the number decreased significantly (37.75±1.49) (P≤0.001). However, the AZ, CR and AZ+CR treatment groups had significantly higher myelinated fibers (51.88±2.10, 78±1.60 and 80.50±1.69, respectively) than the lesion group (P≤0.001). No significant difference was observed between the CR and AZ+CR groups (P=0.371), but there was a significant difference between these two groups and the AZ group (P≤0.001) (Table 2).
Based on these results, it can be concluded that demyelination caused by sciatic nerve damage significantly improved in the treatment groups. Among these treatment groups, CR and AZ+CR treatments showed better results in remyelination.
Immunohistochemical results
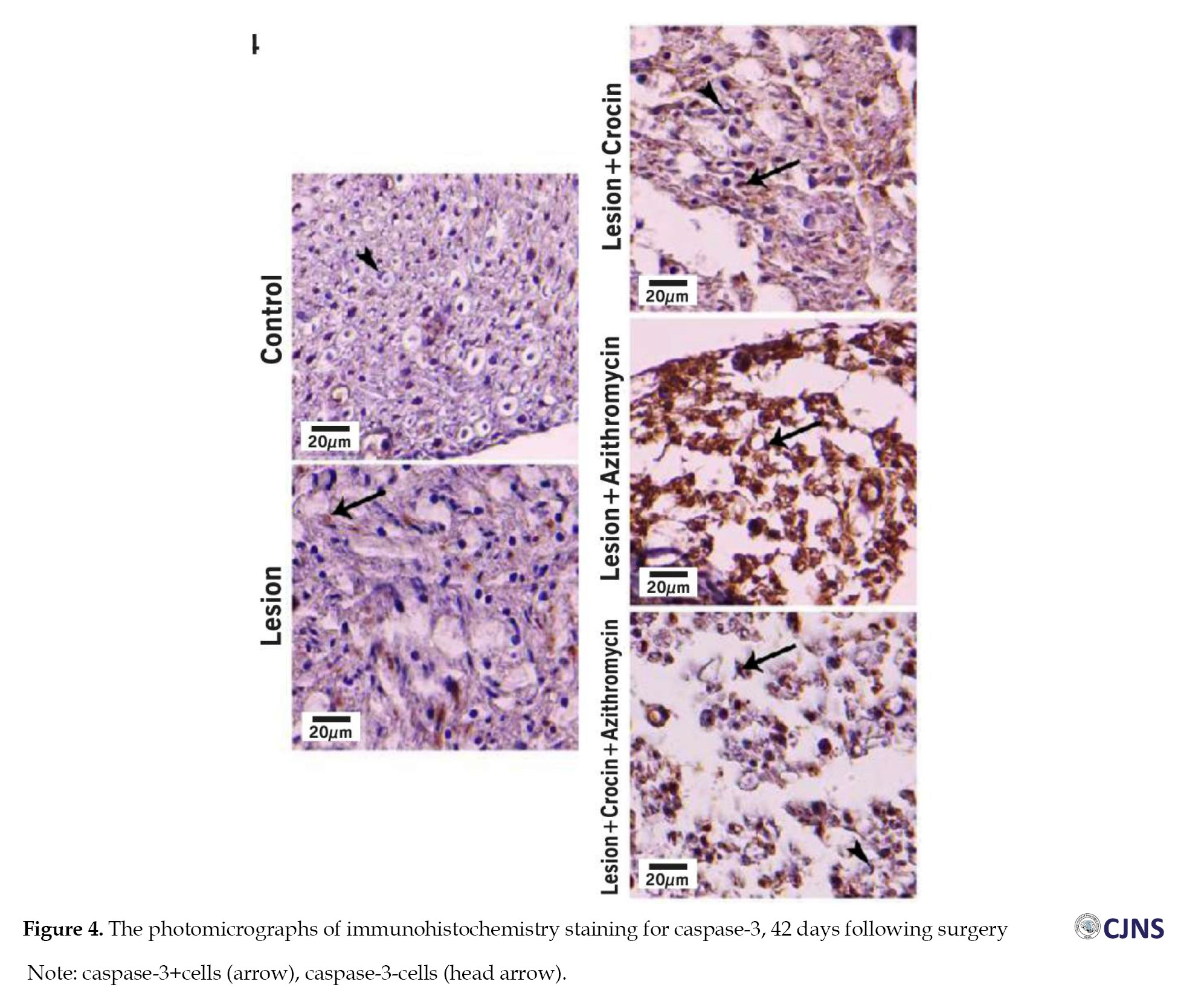
Figure 4 shows caspase-3-stained slides. The study found that the lesion group had a higher percentage of caspase-3-positive cells (59.50±2.98%), indicating increased nerve tissue cell apoptosis after sciatic nerve injury. However, the AZ, CR and AZ+CR groups had lower apoptosis rates (41.38±2.07%, 29.50±1.93% and 22.13±2.03%, respectively) than the lesion group (P≤0.001). The AZ+CR treatment method was better than CR and AZ alone in reducing apoptosis (P≤0.001). CR also performed better than AZ in preventing apoptosis (P≤0.001). The control group had the lowest percentage of caspase-3-positive cells (3.50±0.93%), with a significant difference from the other groups (P≤0.001) (Table 2).
Discussion
Peripheral nerve damage may lead to long-term tissue atrophy and dysfunction of organs, resulting in substantial financial burdens for both people and societies [27, 28]. Pharmacological interventions have emerged as a promising treatment modality for peripheral nerve crush injuries, with the identification of efficacious drugs that might potentially enhance nerve regeneration being recognized as an essential therapeutic need [29]. Consequently, given the characteristics of AZ and CR, the present study investigated the effects of individual and combined drug administrations on sciatic nerve crush injuries.
According to our findings, AZ can improve sciatic nerve motor and sensory function, reduce oxidative stress and apoptosis in nerve tissue cells, and expedite remyelination. These findings were congruent with the findings of Ferdowsi et al. who found that a 150 mg/kg AZ injection improved sciatic nerve mobility and sensation, expedited remyelination, and raised neurotrophic factors [18]. CR, like AZ, had a significant difference with the lesion group in motor, sensory, oxidative stress, apoptosis, and myelin sheath histopathologic tests in our study and caused the sciatic nerve lesion to improve in the measured parameters. Tamaddonfard et al. found that CR injection at doses of 5, 20 and 80 mg/kg enhanced sciatic nerve motor function, reduced oxidative stress, and accelerated remyelination in their investigation [6]. However, based on our findings, CR was more effective than AZ in mending sciatic nerve injuries, and CR showed a significant difference in the tests done.
The neuroprotective advantages of AZ may be attributed to its immunomodulatory effects, which include the reduction of inflammatory cytokines, restriction of neutrophil infiltration, and promotion of macrophage polarization towards the M2 phenotype [30]. Macrophages are essential contributors to peripheral nerve regeneration, as they are responsible for the clearance of waste products, the production of growth factors, and the restoration of the extracellular matrix [31, 32]. Nevertheless, activating pro-inflammatory macrophages, also known as M1 macrophages, results in the generation of oxidative stress and the synthesis of pro-inflammatory cytokines. This cascade of events ultimately leads to neurotoxicity and the subsequent loss of axons. In contrast, M2 macrophages, also known as anti-inflammatory macrophages, can promote angiogenesis, cell replacement, and extracellular matrix repair, ultimately resulting in the regeneration of nerve tissue [33, 34]. In a study conducted by Zhang et al. (2015), the effects of AZ on spinal cord injury were investigated. The study conducted by the researchers found that administering AZ augments the population of anti-inflammatory macrophages while simultaneously reducing the activation of pro-inflammatory macrophages. This outcome significantly impacted the preservation of nerve tissue and subsequent improvement in motor function recovery [35].
The primary preventive advantages of CR may be attributed to its antioxidant, anti-inflammatory, anti-apoptotic, and immune-modulating properties [36-38]. CR can potentially provide neuroprotection via its ability to modulate inflammation, oxidative stress, and apoptosis [36, 39, 40]. CR has been shown to possess potential anti-inflammatory properties. It can inhibit inflammation in neurodegenerative processes by modulating the NF-kB signaling pathway and promoting the activation of macrophages in neuronal and glial cells [41, 42]. Moreover, studies have shown that CR has neuroprotective properties via the inhibition of enzymes, including cyclooxygenase (COX)-2, as well as inflammatory pathways such as mitogen-activated kinases and adenosine monophosphate- and 5’-adenosine monophosphate-activated protein kinases. Additionally, it hinders the synthesis of pro-inflammatory cytokines, including tumor necrosis factor (TNF)α, interleukin (IL)-1, IL-2, IL-6, IL-8, and IL-12 [37, 43]. CR has been shown to have anti-inflammatory and neuroprotective properties in damaged nerve cells via the inhibition of nitric oxide (NO) production and the downregulation of inducible NO synthase (iNOS) expression [37]. CR has been shown to have anti-inflammatory properties by inhibiting the generation of TNF, hence exerting an influence on neuroinflammation [37, 44].
In contrast, CR treatment reduces apoptotic markers in instances of neuronal injury, notably in neurodegenerative conditions [36]. The anti-apoptotic effects of CR have been shown in the brain via the downregulation of Bax and upregulation of Bcl-2 levels [45, 46]. CR has been shown to potentially hinder the caspase 3-mediated cleavages that produce Bax and nuclear condensation in several neurodegenerative disorders [45, 47]. CR potentially inhibits cell death in the apoptotic process associated with neurodegeneration [47, 48]. The neuroprotective activity of CR is strongly associated with its antioxidant capacity [46]. CR can scavenge free radicals, hence providing cellular protection against oxidative stress. Possible processes in this context include identifying changes in enzyme activity and translation levels within the cellular redox system. Enzymes such as glutathione peroxidase (GPx), glutathione-S-transferase (GST), catalase (CAT) and superoxide dismutase (SOD) have been implicated in these changes. In contrast, the endoplasmic reticulum is significantly associated with cellular oxidative stress. Evidence suggests a correlation between alterations in the mRNA expression level of endoplasmic reticulum stressors and the development of certain stress-related disorders, including Alzheimer disease and cancer. CR is believed to be an oxidative stress inhibitor that activates the endoplasmic reticulum by producing specific genes via mRNA. This activation reduces ER stress, as seen by decreased levels of MDA and NO in neuronal cell disorders [43].
The measurement of MDA was included in the study to assess lipid peroxidation as an indicator of oxidative stress. MDA levels directly measure oxidative damage, a useful marker for understanding the extent of lipid peroxidation in the experimental model. While our study did not include measurements of GSH and SOD, we focused on MDA to provide insight into oxidative stress and its potential impact on the experimental outcomes. MDA is a crucial indicator of oxidative damage, and its levels can offer valuable information about the overall oxidative status.
Based on these factors, the stronger efficacy of CR in ameliorating sciatic nerve damage, as compared to AZ, may be attributed to its wider range of actions and greater diversity. The neuroprotective effects of AZ may be attributed exclusively to its immunomodulatory and anti-inflammatory capabilities. In contrast, CR has notable antioxidant and anti-apoptotic qualities and anti-inflammatory properties. In contrast, the concurrent administration of the two drugs mentioned above exhibited results comparable to those of the CR group across most of the assessed parameters. The proposition that the two therapies may have synergistic benefits was met with some degree of skepticism.
This study performed a series of experiments to assess the motor and sensory functionality of the sciatic nerve, investigate the antioxidant properties, examine alterations in the myelin sheath, and quantify the occurrence of nerve tissue apoptosis. The objective was to get a more comprehensive understanding of the effects of CR and AZ on the rehabilitation of sciatic nerve injuries. Nevertheless, a thorough understanding of the observed results in the AZ+CR group and the lack of a synergistic effect between CR and AZ necessitate molecular studies.
Our study assessed the effects of CR and AZ on sciatic nerve damage in terms of motor, sensory, oxidative stress, apoptosis, and histopathological aspects; however, molecular testing, particularly neurotrophic factor assessment, would have been preferable. It is also possible that the study’s findings would have differed if more animals or a longer period had been used. We could not have done these tests because of time, budget, and moral limitations.
Conclusion
In conclusion, our study’s results indicate that administering CR and AZ via a 7 day injection regimen in rats may expedite the recovery of sciatic nerve injuries. Notably, CR exhibits superior therapeutic properties compared to AZ. The potential reason for this disparity might be attributed to the multifaceted pharmacological activities of CR, which include anti-inflammatory, antioxidant, and anti-apoptotic properties. In contrast, AZ primarily exhibits anti-inflammatory and immune-modulating effects. Also, the combined administration of AZ and CR did not provide statistically significant differences in most parameters compared to the group receiving just CR. Consequently, the proposed synergistic effect of these two drugs was called into doubt. Further investigation in molecular research will be necessary for further studies to ascertain the etiology of this particular medical problem.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. This study did not develop new unique reagents. No custom code was used in this study. No large datasets were generated. The published article includes all the analyses generated during this study.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the local Ethics Committee of Urmia University of Medical Sciences (Code: IR.UMSU.AEC.1401.014).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Although peripheral nerve injury does not pose a direct threat to life, it results in substantial impairments that impact the everyday functioning of individuals and have significant social and economic ramifications. The majority of these wounds result from mechanical trauma, which could permanently harm the nerves and cause severe chronic pain, loss of sensation, loss of motor function, or paralysis [1, 2]. Despite the peripheral nerves’ ability to regenerate, this capability is inadequate in guaranteeing complete patient recovery, preservation of functional integrity, and enhancement of overall quality of life. When choosing treatments, it is important to consider many factors, including the degree, size, and location of the damage, the age of the patient, and the time elapsed between the injury and therapeutic intervention. Therefore, the selection of appropriate methods has significant importance in the process of peripheral nerve healing [3]. Following peripheral nerve loss, there is a subsequent occurrence of ischemia and inflammatory processes, which give rise to neurological impairment. Several therapeutic interventions may be used to alleviate the effects of these processes. Numerous pharmaceuticals, metabolites, and chemical compounds have undergone extensive evaluation to reduce the ensuing consequences of peripheral nerve injury. Furthermore, ongoing research efforts are actively pursued in this domain [4].
Crocin (CR), a water-soluble carotenoid with high antioxidant activity, is a significant component of saffron [5, 6, 7]. Multiple studies have shown that CR has various therapeutic benefits, including antioxidant, anti-apoptotic, anti-inflammatory, and antihypertensive properties [8, 9, 10, 11]. Moreover, recent studies have demonstrated the ability of CR to reduce neuroinflammation and neurodegeneration in autoimmune encephalomyelitis by retaining the density of myelin and axons in the spinal cord [8]. Additionally, CR has been demonstrated to accelerate functional recovery, decrease oxidative stress and repair myeline sheath after sciatic nerve crush injury in rats [6].
Azithromycin (AZ), an antibiotic approved by the Food and Drug Administration (FDA), is often used to treat several illnesses due to its notable anti-inflammatory and immunomodulatory properties [12, 13]. The administration of AZ has been shown to inhibit the signaling pathways of signal transducer and activator of transcription 1 and nuclear factor-kappa B (NF-kB) in macrophages, leading to the manifestation of anti-inflammatory properties [14]. During this particular phase, the administration of AZ effectively hinders the activation of pro-inflammatory macrophages (M1) while concurrently promoting the activation of anti-inflammatory macrophages (M2) [15, 16]. Moreover, recent studies verify in favor of the use of AZ as a therapeutic intervention for neurological disorders in neonates, including but not limited to stroke, retinal ischemia, muscular-spinal atrophy, and hypoxic-ischemic brain damage [13, 17]. A further investigation revealed that the 7 days administration of AZ can accelerate regeneration of the sciatic nerve, improve motor and sensory function recovery, and upregulate the expression of nerve growth factor and brain-derived neurotrophic factor genes after sciatic nerve crush injury in rats [18].
This research aimed to investigate and compare the effect of CR and AZ and their co-administration on functional, histological, and biochemical alterations after sciatic nerve crush injury. The purpose was to determine the most advantageous technique for treating sciatic nerve injury in rats.
Materials and Methods
Animals and experimental design
A total of 40 adult albino rats, weighing 230 to 250 g, were acquired from the Department of Pharmacology at Urmia University of Medical Sciences. The rats were housed in standard laboratory settings, which included a light-dark cycle of 12 hours each, a temperature-controlled environment ranging from 23 °C to 25 °C, and ad libitum access to food and water. The experimental protocols adhered to the guidelines outlined in the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
Five groups of rats were randomly formed (8 animals per group). Group 1, or the control group, consisted of rats without sciatic nerve crush injury induction and received normal saline and distilled water as vehicles daily for seven days. Group 2, or the lesion group, underwent a surgically-induced sciatic nerve crush injury; they received normal saline and distilled water as vehicles daily for seven days. Group 3, or the CR group, after sciatic nerve crush injury induction, received CR (soluble in normal saline, 50 mg/kg, IP) daily for seven days [19]. Group 4, or the AZ group, after sciatic nerve crush injury induction, received AZ (soluble in distilled water, 160 mg/kg orally) daily for seven days [20]. Group 5, or the AZ+CR group, after inducing sciatic nerve crush injury, received 50 mg/kg CR and 160 mg/kg AZ daily for seven days.
Surgery
The rats were sedated by intraperitoneal administration of ketamine (80 mg/kg) and xylazine (10 mg/kg). The surgical area was shaved entirely and sterilized using betadine solution, spanning from the femoral head’s inferior aspect to the knee joint’s superior aspect. A small incision about 2–3 cm was made on the skin in the desired area. After the separation of the skin from the underlying tissues and the displacement of the muscles, the sciatic nerve became discernible deep in this region. Subsequently, a compression maneuver was performed on the sciatic nerve with medium surgical hemostatic forceps for 60 seconds at a location situated 1 cm proximal to the trifurcation site. The nerve compression was standardized across all rats using a single pair of locking forceps. After compression, the nerve was repositioned to its original anatomical location, the incision margins were sutured, and the wound was thoroughly cleansed. The animals were provided with appropriate thermal conditions during and after the treatment until they regained consciousness. Following the rats’ consciousness restoration, they were transferred to individual enclosures and subjected to standard environmental settings, including regulated light exposure, temperature, and humidity levels [6].
Sciatic functional index
On the 14th, 28th and 42nd postoperative days, the sciatic functional index (SFI) was used to assess functional recovery. For this experiment, the rat’s hind feet were soaked in ink and permitted to walk through a corridor covered with white paper. The SFI was then determined using the Equation 1:
1. SFI=-38.8[EPL-NPL/NPL]+109.5[ETS-NTS/NTS]+13.3[EIT-NIT/NIT]–8.8
In Equation 1, PL indicates the sole length, TS is the distance between toes 1 and 5, IT is the distance between toes 2 and 4, E denotes the experimental side, and N is the healthy side. All measurements were taken in mm. An SFI score of 0 was considered normal leg movement, while -100 indicated significant impairment [21].
Hot plate test
According to some research [22], the hot plate test was utilized to assess the thermal threshold. The test was conducted on the 42nd day after surgery using digital hot plate equipment. During the experiment, the gadget’s temperature was adjusted to 56 °C, after which the rats were placed on the hot plate. The response time measurement included recording the duration, in seconds, that the rats needed to withdraw their injured leg from the screen. The process was conducted three times for each animal, with a time interval of 10 minutes between each repetition. To minimize the risk of harm to the rat’s foot, the duration of exposure on the hot plate did not exceed 12 seconds.
Mechanical withdrawal thresholds
Von Frey filaments and a mesh apparatus were used to evaluate the responses of animals to mechanical stimulation to measure the presence of mechanical allodynia. The Von Frey hair consists of a handle and a slender polyethylene thread with different thicknesses, resulting in a range of forces exerted on the animal’s feet, ranging from 8 to 300 g. The mesh structure consists of a metallic net supported by four bases, allowing unrestricted access from the lower side. On the 42nd postoperative day, the rats were positioned on the mesh substrate to conduct this experiment. Subsequently, their injured hind paws were subjected to stimulation originating from the mid-plantar surface, using a variety of filaments that varied in pressure intensity, ranging from low to high. Each stimulation lasted for a duration of 6 to 8 seconds. The filament with the lowest pressure eliciting the animal’s paw withdrawal reaction was defined as the stimulation threshold [23].
Biochemical analysis of malondialdehyde (MDA) and total antioxidant capacity (TAC)
On the 42nd day of the study, the animals were administered ketamine (80 mg/kg) and xylazine (10 mg/kg) to induce sedation. Subsequently, 5 mL of blood was collected directly from their cardiac region and immediately transferred to test tubes that were stored on ice. The sample underwent centrifugation at a speed of 3000 rpm for 10 min. Following the separation of the serum, it was then held at a temperature of -70 °C until the levels of MDA and TAC were assessed. The MDA test was performed to evaluate the extent of lipid peroxidation, a key indicator of oxidative stress in the tissue samples. Lipid peroxidation results from the oxidative degradation of lipids, which can lead to cellular damage, and is commonly assessed using the thiobarbituric acid (TBA) reactive substances assay.
TBA reaction technique
The MDA levels were measured using the TBA reaction technique, following the method described by Ohkawa et al. [24]. This method involves the reaction of MDA, a product of lipid peroxidation, with TBA to form a pink chromogenic complex.
Colorimetric analysis
The resulting chromogenic compound exhibits maximum absorbance at a wavelength of 535 nm. This colorimetric analysis allows quantifying MDA concentration based on its light absorption properties.
MDA standard curve
A standard curve was constructed using tetraethoxypropane (TEP) as the MDA standard. This curve is used to determine the concentration of MDA in the samples. TEP is employed due to its known reactivity with TBA, which allows for accurate calibration and quantification.
Quantification
The concentration of MDA in the samples is expressed in µmol/L. The absorbance readings of the samples are compared against the standard curve to determine the MDA levels. The TAC test was performed using the TAC kit from Randox (Crumlin, County Antrim, UK). This assay is designed to measure the overall antioxidant capability of a sample by assessing its ability to scavenge free radicals. The quantification of caspase-3-positive cells was performed inside a tissue sample by counting the number of such cells present in 1 mm2. This measurement was then used to compare and analyze the differences between the different experimental groups. In addition, software-based methods were used to investigate the cellular distribution [25].
Reaction principle
The assay employs the compound 3-ethylbenzothiazoline-6-sulfonate (ABTS), which forms a radical cation known as ABTS in the presence of peroxidase and hydrogen peroxide (H2O2)⁺. The radical cation exhibits a blue-green hue, which indicates the radicals’ presence.
Formation of ABTS+
During the reaction, ABTS reacts with H₂O₂ in the presence of peroxidase to generate the ABTS⁺ radical cation. The intensity of the blue-green color correlates with the concentration of ABTS⁺, a measure of oxidative stress.
Colorimetric detection
The color change is measured using a spectrophotometer at a wavelength of 600 nm. The intensity of the blue-green hue detected at this wavelength is directly proportional to the concentration of ABTS⁺ and inversely proportional to the antioxidant activity of the sample.
Antioxidant capacity determination
The antioxidant capacity of the sample is determined by comparing its ability to inhibit the formation of ABTS⁺ to that of a standard with known antioxidant properties. The greater the inhibition of the ABTS⁺ formation, the higher the antioxidant capacity of the sample.
Histopathological evaluation
On the 42nd day of the study, following the administration of sedatives to the animals and subsequent blood collection for biochemical analysis, a 1 cm segment from the central portion of the sciatic nerve was taken inside the area of injury and preserved using paraformaldehyde. Following a 48 h period, the tissue underwent processing. Subsequently, 5 µ slices were cut from the tissue and subjected to staining with Luxol fast blue. For each tissue sample, 10 random fields of view were selected, and histological evaluation (myelinated fibers diameter, myelin sheath diameter, and number of myelinated fibers) was then conducted with a light microscope set at a magnification of 400× and ImageJ software, version 1.52.
The Luxol fast blue staining technique was used to stain the myelin sheath in tissue slices of the sciatic nerve, resulting in a blue coloration. The morphometrics of the sciatic nerve, including the number of myelinated filaments, axon diameter and myelin sheath thickness, were evaluated using ImageJ software, version 1.52 in this study.
Immunohistochemical staining for apoptosis
The tissue segment slides were subjected to a temperature of 60 °C for about 25 min using a hot air oven (Venticell, MMM, Einrichtungen, Germany). The tissue slices underwent deparaffinization in xylene twice, with each instance lasting for 5 minutes. Subsequently, rehydration was carried out by subjecting the tissue slices to an alcohol gradient, starting with a concentration of 90% and gradually decreasing to 80%, 70% and finally 50%. The antigen retrieval technique was conducted using a solution containing sodium citrate at a concentration of 10 mM. The immunohistochemical staining procedure was performed according to the instructions provided by the manufacturers, Biocare and ScyTek, based in the United States. The endogenous peroxidase activity was suppressed by immersing the sample in a peroxidase-blocking solution containing 0.03% hydrogen peroxide with sodium acid for 5 minutes. The tissue slices underwent a gentle rinsing using phosphate-buffered saline (PBS, pH 7.2) before being subjected to overnight incubation at -4°C with primary antibodies targeting caspase-3 (1:500). Before immersion in a buffer solution, the sections were subjected to a gentle washing process using washing buffer (PBS, pH 7.2). Subsequently, the slides were placed into a humidified, which contained an anti-microbial substance along with an appropriate amount of streptavidin-HRP (streptavidin chemically linked to horseradish peroxidase) in phosphate-buffered saline (PBS). The slides were incubated for 15 minutes. The tissue sections were cleaned in a washing buffer solution and then immersed in a buffer bath. The tissue slices were subjected to the addition of a DAB chromogen, which underwent incubation for 5 more minutes. Subsequently, the sections were subjected to a counterstaining process using hematoxylin for 20 seconds. Next, the sections were immersed in a solution of mild ammonia (0.037 mL) for 10 seconds, then rinsed with distilled water and subsequent covering [26].
Statistical analysis
SPSS software, version 16 was utilized for statistical analysis. We assessed the distribution of the data using the Kolmogorov-Smirnov test. The results indicated that the data were normally distributed. To evaluate the significant differences between groups, statistical analysis was performed using a one-way analysis of variance (ANOVA). A Bonferroni post hoc analysis was used to compare all groups together. All data were presented in Mean±SD, with P≤0.05 regarded as statistically significant.
Results
SFI results
We analyzed the SFI values of rats on days 14, 28 and 42 after surgery. The lesion group had the lowest mean SFI and severe leg weakness. However, the CR, AZ and AZ+CR groups showed higher SFI values than the lesion group (P≤0.001), and all three treatment groups showed improved motor function. The AZ+CR group had a better effect on sciatic nerve motor recovery than the CR and AZ groups (P≤0.001). The CR group had higher SFI levels than the AZ group at all three time points (day 14, day 28 and day 42) (P≤0.001), indicating that CR had a better effect on SFI values than AZ. The control group had the highest SFI level in all phases (Table 1).

Hot plate results
Forty-two days after surgery, the control group showed the longest reaction time to thermal stimulation (7.81±0.36 s), which decreased in the lesion group (2.71±0.32 s) (P≤0.001), indicating thermal hyperalgesia. The AZ, CR and AZ+CR groups had significantly higher reaction times (3.41±0.25, 5.10±0.26 and 6.21±0.25 s, respectively) (P<0.001), indicating an increase in the response threshold and reducing thermal hyperalgesia. The AZ+CR group showed a higher increase (P≤0.001), and CR was more effective than AZ in reducing thermal hyperalgesia in the response threshold to thermal stimulation (P≤0.001) (Figure 1A).
Mechanical withdrawal threshold results
The results of the investigation revealed a significant decrease in the paw withdrawal threshold among the lesion group (75±20.70 g) compared to the control group (300±0 g) (P<0.001), suggesting the presence of mechanical allodynia after sciatic nerve damage. The mechanical withdrawal threshold exhibited a greater value in the treatment groups AZ (145±72.31 g), CR (157.50±73.63 g) and AZ+CR (160±74.07 g) compared to the lesion group; however, this difference did not reach statistical significance (P=0.203 compare to AZ group, P=0.070 compare to CR group and P=0.056 compare to AZ+CR). The combined CR and AZ treatment demonstrated better results than the administration of AZ alone (Figure 1B).
Biochemical analysis results
Forty-two days after surgery, the control group had the lowest average serum MDA levels (1.66±0.12 nmol/mL). In contrast, the lesion group had significantly higher mean MDA levels (4.84±0.22 nmol/mL) (P≤0.001), indicating increased lipid peroxidation. However, the AZ, CR and AZ+CR treatment groups had lower MDA levels (4.04±0.14, 2.41±0.24 and 2.38±0.23 nmol/mL, respectively) than the lesion group (P≤0.001), indicating effective therapeutic approaches to reducing lipid peroxidation. The effect was more robust in the AZ+CR groups, with lower and significant mean serum MDA levels (P≤0.001). The difference in mean serum MDA between the CR and AZ+CR groups was not statistically significant (P>0.999), but these two groups had significant differences with the AZ group (P≤0.001) (Figure 2. A). The results showed that serum TAC levels were lowest in the lesion group (0.31±0.02 nmol/mL) Forty-two days after surgery. However, the AZ, CR and AZ+CR treatment groups had significantly higher mean serum TAC levels (0.41±0.03, 0.8±0.03 and 0.81±0.02 nmol/mL, respectively) than the lesion group (P≤0.001). The CR and AZ+CR groups had no significant difference in serum TAC levels (P>0.999); these two groups had a significant difference with the AZ group (P≤0.001), while the control group had the highest mean serum TAC level (0.91±0.04 nmol/ml) and significantly differed from other groups (P≤0.001) (Figure 2B).
Histopathological results
Figure 3 shows tissue sections stained with Luxol fast blue in different groups. Based on these sections, variables of myelinated fiber diameter, myelin sheath diameter, and myelinated fiber number were measured. The results of these three variables are as follows:
The study found that the lesion group had the lowest mean of myelinated fibers diameter (6.28±0.17 µm) on the 42nd day after surgery, which increased significantly in the AZ, CR, and AZ+CR treatment groups (7±0.15, 8.65±0.11 and 8.76±0.22 µm, respectively) (P≤0.001). The CR and AZ+CR groups had a higher mean of myelinated fibers diameter than the AZ group (P≤0.001), but no significant difference was found between these two groups (P=1). The control group had the highest mean of myelinated fibers diameter (9.12±0.22 µm) and was significantly different from other groups (P≤0.05) (Table 2).

The research investigation revealed a significant difference in the average diameter of the myelin sheath between the AZ, CR and AZ+CR groups (1.90±0.10, 2.38±0.13 and 2.40±0.13 µm, respectively) compared to the lesion group (1.54±0.10 µm) (P≤0.001). There was no statistically significant difference between the groups receiving CR and AZ+CR. However, an important difference was observed between these two groups and the group receiving AZ alone. The mean diameter of the myelin sheath in the control group (2.59±0.12 µm) exhibited a statistically significant difference (P≤0.05) (Table 2).
The control group had the highest mean number of myelinated fibers (93.38±3.81), but after sciatic nerve injury, the number decreased significantly (37.75±1.49) (P≤0.001). However, the AZ, CR and AZ+CR treatment groups had significantly higher myelinated fibers (51.88±2.10, 78±1.60 and 80.50±1.69, respectively) than the lesion group (P≤0.001). No significant difference was observed between the CR and AZ+CR groups (P=0.371), but there was a significant difference between these two groups and the AZ group (P≤0.001) (Table 2).
Based on these results, it can be concluded that demyelination caused by sciatic nerve damage significantly improved in the treatment groups. Among these treatment groups, CR and AZ+CR treatments showed better results in remyelination.
Immunohistochemical results
Figure 4 shows caspase-3-stained slides. The study found that the lesion group had a higher percentage of caspase-3-positive cells (59.50±2.98%), indicating increased nerve tissue cell apoptosis after sciatic nerve injury. However, the AZ, CR and AZ+CR groups had lower apoptosis rates (41.38±2.07%, 29.50±1.93% and 22.13±2.03%, respectively) than the lesion group (P≤0.001). The AZ+CR treatment method was better than CR and AZ alone in reducing apoptosis (P≤0.001). CR also performed better than AZ in preventing apoptosis (P≤0.001). The control group had the lowest percentage of caspase-3-positive cells (3.50±0.93%), with a significant difference from the other groups (P≤0.001) (Table 2).
Discussion
Peripheral nerve damage may lead to long-term tissue atrophy and dysfunction of organs, resulting in substantial financial burdens for both people and societies [27, 28]. Pharmacological interventions have emerged as a promising treatment modality for peripheral nerve crush injuries, with the identification of efficacious drugs that might potentially enhance nerve regeneration being recognized as an essential therapeutic need [29]. Consequently, given the characteristics of AZ and CR, the present study investigated the effects of individual and combined drug administrations on sciatic nerve crush injuries.
According to our findings, AZ can improve sciatic nerve motor and sensory function, reduce oxidative stress and apoptosis in nerve tissue cells, and expedite remyelination. These findings were congruent with the findings of Ferdowsi et al. who found that a 150 mg/kg AZ injection improved sciatic nerve mobility and sensation, expedited remyelination, and raised neurotrophic factors [18]. CR, like AZ, had a significant difference with the lesion group in motor, sensory, oxidative stress, apoptosis, and myelin sheath histopathologic tests in our study and caused the sciatic nerve lesion to improve in the measured parameters. Tamaddonfard et al. found that CR injection at doses of 5, 20 and 80 mg/kg enhanced sciatic nerve motor function, reduced oxidative stress, and accelerated remyelination in their investigation [6]. However, based on our findings, CR was more effective than AZ in mending sciatic nerve injuries, and CR showed a significant difference in the tests done.
The neuroprotective advantages of AZ may be attributed to its immunomodulatory effects, which include the reduction of inflammatory cytokines, restriction of neutrophil infiltration, and promotion of macrophage polarization towards the M2 phenotype [30]. Macrophages are essential contributors to peripheral nerve regeneration, as they are responsible for the clearance of waste products, the production of growth factors, and the restoration of the extracellular matrix [31, 32]. Nevertheless, activating pro-inflammatory macrophages, also known as M1 macrophages, results in the generation of oxidative stress and the synthesis of pro-inflammatory cytokines. This cascade of events ultimately leads to neurotoxicity and the subsequent loss of axons. In contrast, M2 macrophages, also known as anti-inflammatory macrophages, can promote angiogenesis, cell replacement, and extracellular matrix repair, ultimately resulting in the regeneration of nerve tissue [33, 34]. In a study conducted by Zhang et al. (2015), the effects of AZ on spinal cord injury were investigated. The study conducted by the researchers found that administering AZ augments the population of anti-inflammatory macrophages while simultaneously reducing the activation of pro-inflammatory macrophages. This outcome significantly impacted the preservation of nerve tissue and subsequent improvement in motor function recovery [35].
The primary preventive advantages of CR may be attributed to its antioxidant, anti-inflammatory, anti-apoptotic, and immune-modulating properties [36-38]. CR can potentially provide neuroprotection via its ability to modulate inflammation, oxidative stress, and apoptosis [36, 39, 40]. CR has been shown to possess potential anti-inflammatory properties. It can inhibit inflammation in neurodegenerative processes by modulating the NF-kB signaling pathway and promoting the activation of macrophages in neuronal and glial cells [41, 42]. Moreover, studies have shown that CR has neuroprotective properties via the inhibition of enzymes, including cyclooxygenase (COX)-2, as well as inflammatory pathways such as mitogen-activated kinases and adenosine monophosphate- and 5’-adenosine monophosphate-activated protein kinases. Additionally, it hinders the synthesis of pro-inflammatory cytokines, including tumor necrosis factor (TNF)α, interleukin (IL)-1, IL-2, IL-6, IL-8, and IL-12 [37, 43]. CR has been shown to have anti-inflammatory and neuroprotective properties in damaged nerve cells via the inhibition of nitric oxide (NO) production and the downregulation of inducible NO synthase (iNOS) expression [37]. CR has been shown to have anti-inflammatory properties by inhibiting the generation of TNF, hence exerting an influence on neuroinflammation [37, 44].
In contrast, CR treatment reduces apoptotic markers in instances of neuronal injury, notably in neurodegenerative conditions [36]. The anti-apoptotic effects of CR have been shown in the brain via the downregulation of Bax and upregulation of Bcl-2 levels [45, 46]. CR has been shown to potentially hinder the caspase 3-mediated cleavages that produce Bax and nuclear condensation in several neurodegenerative disorders [45, 47]. CR potentially inhibits cell death in the apoptotic process associated with neurodegeneration [47, 48]. The neuroprotective activity of CR is strongly associated with its antioxidant capacity [46]. CR can scavenge free radicals, hence providing cellular protection against oxidative stress. Possible processes in this context include identifying changes in enzyme activity and translation levels within the cellular redox system. Enzymes such as glutathione peroxidase (GPx), glutathione-S-transferase (GST), catalase (CAT) and superoxide dismutase (SOD) have been implicated in these changes. In contrast, the endoplasmic reticulum is significantly associated with cellular oxidative stress. Evidence suggests a correlation between alterations in the mRNA expression level of endoplasmic reticulum stressors and the development of certain stress-related disorders, including Alzheimer disease and cancer. CR is believed to be an oxidative stress inhibitor that activates the endoplasmic reticulum by producing specific genes via mRNA. This activation reduces ER stress, as seen by decreased levels of MDA and NO in neuronal cell disorders [43].
The measurement of MDA was included in the study to assess lipid peroxidation as an indicator of oxidative stress. MDA levels directly measure oxidative damage, a useful marker for understanding the extent of lipid peroxidation in the experimental model. While our study did not include measurements of GSH and SOD, we focused on MDA to provide insight into oxidative stress and its potential impact on the experimental outcomes. MDA is a crucial indicator of oxidative damage, and its levels can offer valuable information about the overall oxidative status.
Based on these factors, the stronger efficacy of CR in ameliorating sciatic nerve damage, as compared to AZ, may be attributed to its wider range of actions and greater diversity. The neuroprotective effects of AZ may be attributed exclusively to its immunomodulatory and anti-inflammatory capabilities. In contrast, CR has notable antioxidant and anti-apoptotic qualities and anti-inflammatory properties. In contrast, the concurrent administration of the two drugs mentioned above exhibited results comparable to those of the CR group across most of the assessed parameters. The proposition that the two therapies may have synergistic benefits was met with some degree of skepticism.
This study performed a series of experiments to assess the motor and sensory functionality of the sciatic nerve, investigate the antioxidant properties, examine alterations in the myelin sheath, and quantify the occurrence of nerve tissue apoptosis. The objective was to get a more comprehensive understanding of the effects of CR and AZ on the rehabilitation of sciatic nerve injuries. Nevertheless, a thorough understanding of the observed results in the AZ+CR group and the lack of a synergistic effect between CR and AZ necessitate molecular studies.
Our study assessed the effects of CR and AZ on sciatic nerve damage in terms of motor, sensory, oxidative stress, apoptosis, and histopathological aspects; however, molecular testing, particularly neurotrophic factor assessment, would have been preferable. It is also possible that the study’s findings would have differed if more animals or a longer period had been used. We could not have done these tests because of time, budget, and moral limitations.
Conclusion
In conclusion, our study’s results indicate that administering CR and AZ via a 7 day injection regimen in rats may expedite the recovery of sciatic nerve injuries. Notably, CR exhibits superior therapeutic properties compared to AZ. The potential reason for this disparity might be attributed to the multifaceted pharmacological activities of CR, which include anti-inflammatory, antioxidant, and anti-apoptotic properties. In contrast, AZ primarily exhibits anti-inflammatory and immune-modulating effects. Also, the combined administration of AZ and CR did not provide statistically significant differences in most parameters compared to the group receiving just CR. Consequently, the proposed synergistic effect of these two drugs was called into doubt. Further investigation in molecular research will be necessary for further studies to ascertain the etiology of this particular medical problem.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. This study did not develop new unique reagents. No custom code was used in this study. No large datasets were generated. The published article includes all the analyses generated during this study.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the local Ethics Committee of Urmia University of Medical Sciences (Code: IR.UMSU.AEC.1401.014).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
- Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: From Augustus Waller's observations to neuroinflammation. J Peripher Nerv Syst. 2002; 7(1):13-27. [DOI:10.1046/j.1529-8027.2002.02002.x] [PMID]
- Emel E, Ergün SS, Kotan D, Gürsoy EB, Parman Y, Zengin A, et al. Effects of insulin-like growth factor-I and platelet-rich plasma on sciatic nerve crush injury in a rat model. J Neurosurg. 2011; 114(2):522-8. [DOI:10.3171/2010.9.JNS091928] [PMID]
- Devesa P, Gelabert M, Gonźlez-Mosquera T, Gallego R, Relova JL, Devesa J, et al. Growth hormone treatment enhances the functional recovery of sciatic nerves after transection and repair. Muscle Nerve. 2012; 45(3):385-92. [DOI:10.1002/mus.22303] [PMID]
- Yüce S, Cemal Gökçe E, Işkdemir A, Koç ER, Cemil DB, Gökçe A, et al. An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Ann Plast Surg. 2015; 74(6):684-92. [DOI:10.1097/SAP.0000000000000026] [PMID]
- Gholamnezhad Z, Koushyar H, Byrami G, Boskabady MH. The extract of crocus sativus and its constituent safranal, affect serum levels of endothelin and total protein in sensitized guinea pigs. Iran J Basic Med Sci. 2013; 16(9):1022-6. [PMID] [PMCID]
- Tamaddonfard E, Farshid AA, Ahmadian E, Hamidhoseyni A. Crocin enhanced functional recovery after sciatic nerve crush injury in rats. Iran J Basic Med Sci. 2013; 16(1):83-90. [PMID] [PMCID]
- Tamaddonfard E, Farshid AA, Asri-Rezaee S, Javadi S, Khosravi V, Rahman B, et al. Crocin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2013;16(1):91.
- Deslauriers AM, Afkhami-Goli A, Paul AM, Bhat RK, Acharjee S, Ellestad KK, et al. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J Immunol. 2011; 187(9):4788-99. [DOI:10.4049/jimmunol.1004111] [PMID]
- Mehri S, Abnous K, Mousavi SH, Shariaty VM, Hosseinzadeh H. Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell Mol Neurobiol. 2012; 32(2):227-35. [DOI:10.1007/s10571-011-9752-8] [PMID]
- Razavi M, Hosseinzadeh H, Abnous K, Motamedshariaty VS, Imenshahidi M. Crocin restores hypotensive effect of subchronic administration of diazinon in rats. Iran J Basic Med Sci. 2013; 16(1):64. [DOI:10.22038/ijbms.2013.250]
- Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, Hosseinzadeh H. Effects of chronic crocin treatment on desoxycorticosterone acetate (doca)-salt hypertensive rats. Iran J Basic Med Sci. 2014; 17(1):9. [DOI:10.22038/ijbms.2014.2149]
- Murphy BS, Sundareshan V, Cory TJ, Hayes D Jr, Anstead MI, Feola DJ. Azithromycin alters macrophage phenotype. J Antimicrob Chemother. 2008; 61(3):554-60. [DOI:10.1093/jac/dkn007] [PMID]
- Amantea D, Certo M, Petrelli F, Bagetta G. Neuroprotective properties of a macrolide antibiotic in a mouse model of middle cerebral artery occlusion: Characterization of the immunomodulatory effects and validation of the efficacy of intravenous administration. Assay Drug Dev Technol. 2016; 14(5):298-307. [DOI:10.1089/adt.2016.728] [PMID] [PMCID]
- Haydar D, Cory TJ, Birket SE, Murphy BS, Pennypacker KR, Sinai AP, et al. Azithromycin polarizes macrophages to an M2 phenotype via inhibition of the STAT1 and NF-κB signaling pathways. J Immunol. 2019; 203(4):1021-30. [DOI:10.4049/jimmunol.1801228] [PMID] [PMCID]
- Gensel JC, Kopper TJ, Zhang B, Orr MB, Bailey WM. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci Rep. 2017; 7:40144. [DOI:10.1038/srep40144] [PMID] [PMCID]
- Zhang B, Kopper TJ, Liu X, Cui Z, Van Lanen SG, Gensel JC. Macrolide derivatives reduce proinflammatory macrophage activation and macrophage-mediated neurotoxicity. CNS Neurosci Ther. 2019; 25(5):591-600. [DOI:10.1111/cns.13092] [PMID] [PMCID]
- Osman EY, Washington CW, Simon ME, Megiddo D, Greif H, Lorson CL. Analysis of azithromycin monohydrate as a single or a combinatorial therapy in a mouse model of severe spinal muscular Atrophy. J Neuromuscul Dis. 2017; 4(3):237-49. [DOI:10.3233/JND-170230] [PMID]
- Ferdowsi S, Abdolmaleki A, Asadi A, Zahri S. Effect of azithromycin on sciatic nerve injury in the wistar rats. Neurochem Res. 2023; 48(1):161-71. [DOI:10.1007/s11064-022-03721-x] [PMID]
- Karami M, Bathaie SZ, Tiraihi T, Habibi Rezaie M, Arab-Kheradmand J, Faghihzadeh S. [The effect of crocin and its mechanism of action on chronic pain induced by spinal cord contusion in a rat model (Persian)]. Pathobiol Res. 2013; 16(1):63-73. [Link]
- Kopper TJ, McFarlane KE, Bailey WM, Orr MB, Zhang B, Gensel JC. Delayed azithromycin treatment improves recovery after mouse spinal cord injury. Front Cell Neurosci. 2019; 13:490. [DOI:10.3389/fncel.2019.00490] [PMID] [PMCID]
- Nobakhti-Afshar A, Najafpour A, Mohammadi R, Zarei L. Assessment of neuroprotective effects of local administration of 17- beta- estradiol on peripheral nerve regeneration in ovariectomized female rats. Bull Emerg Trauma. 2016; 4(3):141-9. [PMID] [PMCID]
- Ma J, Liu J, Yu H, Wang Q, Chen Y, Xiang L. Curcumin promotes nerve regeneration and functional recovery in rat model of nerve crush injury. Neurosci Lett. 2013; 547:26-31. [DOI:10.1016/j.neulet.2013.04.054] [PMID]
- Cobianchi S, de Cruz J, Navarro X. Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain. Exp Neurol. 2014; 255:1-11. [DOI:10.1016/j.expneurol.2014.02.008] [PMID]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95(2):351-8. [DOI:10.1016/0003-2697(79)90738-3] [PMID]
- Ghyasi R, Mohaddes G, Naderi R. Combination effect of voluntary exercise and garlic (Allium sativum) on oxidative stress, cholesterol level and histopathology of heart tissue in type 1 diabetic rats. J Cardiovasc Thorac Res. 2019; 11(1):61-67. [DOI:10.15171/jcvtr.2019.10] [PMID] [PMCID]
- Mosadegh M, Hasanzadeh S, Razi M. Nicotine-induced damages in testicular tissue of rats; evidences for bcl-2, p53 and caspase-3 expression. Iran J Basic Med Sci. 2017; 20(2):199-208. [DOI:10.22038/ijbms.2017.8249] [PMID]
- Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg Focus. 2004; 16(5):E1. [DOI:10.3171/foc.2004.16.5.2] [PMID]
- Johnson EO, Zoubos AB, Soucacos PN. Regeneration and repair of peripheral nerves. Injury. 2005; 36(Suppl 4):S24-9. [DOI:10.1016/j.injury.2005.10.012] [PMID]
- Feng X, Yuan W. Dexamethasone enhanced functional recovery after sciatic nerve crush injury in rats. Biomed Res Int. 2015; 2015:627923. [DOI:10.1155/2015/627923] [PMID] [PMCID]
- Amantea D, Certo M, Petrelli F, Tassorelli C, Micieli G, Corasaniti MT, et al. Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards M2 phenotype. Exp Neurol. 2016; 275(Pt 1):116-25. [DOI:10.1016/j.expneurol.2015.10.012] [PMID]
- Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012; 33(34):8793-801. [DOI:10.1016/j.biomaterials.2012.08.050] [PMID] [PMCID]
- Chen P, Piao X, Bonaldo P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015; 130(5):605-18. [DOI:10.1007/s00401-015-1482-4] [PMID]
- Zigmond RE, Echevarria FD. Macrophage biology in the peripheral nervous system after injury. Prog Neurobiol. 2019; 173:102-21. [DOI:10.1016/j.pneurobio.2018.12.001] [PMID] [PMCID]
- Rios R, Jablonka-Shariff A, Broberg C, Snyder-Warwick AK. Macrophage roles in peripheral nervous system injury and pathology: Allies in neuromuscular junction recovery. Mol Cell Neurosci. 2021; 111:103590. [DOI:10.1016/j.mcn.2021.103590] [PMID] [PMCID]
- Zhang B, Bailey WM, Kopper TJ, Orr MB, Feola DJ, Gensel JC. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J Neuroinflammation. 2015; 12:218. [DOI:10.1186/s12974-015-0440-3] [PMID] [PMCID]
- Soeda S, Ochiai T, Shimeno H, Saito H, Abe K, Tanaka H, et al. Pharmacological activities of crocin in saffron. Journal of Natural Medicines. 2007; 61:102-11. [DOI:10.1007/s11418-006-0120-9]
- Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010; 648(1-3):110-6. [DOI:10.1016/j.ejphar.2010.09.003] [PMID]
- Sarshoori JR, Asadi MH, Mohammadi MT. Neuroprotective effects of crocin on the histopathological alterations following brain ischemia-reperfusion injury in rat. Iran J Basic Med Sci. 2014; 17(11):895-902. [PMID] [PMCID]
- Qi Y, Chen L, Zhang L, Liu WB, Chen XY, Yang XG. Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp Eye Res. 2013; 107:44-51. [DOI:10.1016/j.exer.2012.11.011] [PMID]
- Wang K, Zhang L, Rao W, Su N, Hui H, Wang L, et al. Neuroprotective effects of crocin against traumatic brain injury in mice: Involvement of notch signaling pathway. Neurosci Lett. 2015; 591:53-8. [DOI:10.1016/j.neulet.2015.02.016] [PMID]
- Li S, Liu X, Lei J, Yang J, Tian P, Gao Y. Crocin protects podocytes against oxidative stress and inflammation induced by high glucose through inhibition of NF-κB. Cell Physiol Biochem. 2017; 42(4):1481-92. [DOI:10.1159/000470650] [PMID]
- Mazumder AG, Sharma P, Patial V, Singh D. Crocin attenuates kindling development and associated cognitive impairments in mice via inhibiting reactive oxygen species-mediated NF-κB activation. Basic Clin Pharmacol Toxicol. 2017; 120(5):426-33. [DOI:10.1111/bcpt.12694] [PMID]
- Korani S, Korani M, Sathyapalan T, Sahebkar A. Therapeutic effects of crocin in autoimmune diseases: A review. Biofactors. 2019; 45(6):835-43. [DOI:10.1002/biof.1557] [PMID]
- Rajaei Z, Hosseini M, Alaei H. Effects of crocin on brain oxidative damage and aversive memory in a 6-OHDA model of Parkinson's disease. Arq Neuropsiquiatr. 2016; 74(9):723-9. [DOI:10.1590/0004-282X20160131] [PMID]
- Shafahi M, Vaezi G, Shajiee H, Sharafi S, Khaksari M. Crocin inhibits apoptosis and astrogliosis of hippocampus neurons against methamphetamine neurotoxicity via antioxidant and anti-inflammatory mechanisms. Neurochem Res. 2018; 43(12):2252-9. [DOI:10.1007/s11064-018-2644-2] [PMID]
- Mozaffari S, Ramezany Yasuj S, Motaghinejad M, Motevalian M, Kheiri R. Crocin acting as a neuroprotective agent against methamphetamine-induced neurodegeneration via CREB-BDNF signaling pathway. Iran J Pharm Res. 2019; 18(2):745-58. [DOI:10.22037/ijpr.2019.2393] [PMID]
- Ochiai T, Ohno S, Soeda S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of alpha-tocopherol. Neurosci Lett. 2004 362(1):61-4. [DOI:10.1016/j.neulet.2004.02.067] [PMID]
- Hosseinzadeh H, Abootorabi A, Sadeghnia HR. Protective effect of Crocus sativus stigma extract and crocin (trans-crocin 4) on methyl methanesulfonate-induced DNA damage in mice organs. DNA Cell Biol. 2008; 27(12):657-64. [DOI:10.1089/dna.2008.0767] [PMID]
Type of Study: Research |
Subject:
General
Received: 2024/02/18 | Accepted: 2024/08/16 | Published: 2024/10/1
Received: 2024/02/18 | Accepted: 2024/08/16 | Published: 2024/10/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |