Mon, Dec 29, 2025
Volume 10, Issue 1 (Winter 2024)
Caspian J Neurol Sci 2024, 10(1): 57-67 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nosratiyan M, Farjah G H, Saberi H. The Effect of Low-intensity Remote Ultrasound on Sciatic Nerve Regeneration in Male Rats. Caspian J Neurol Sci 2024; 10 (1) :57-67
URL: http://cjns.gums.ac.ir/article-1-691-en.html
URL: http://cjns.gums.ac.ir/article-1-691-en.html
1- Student Research Committee, Urmia University of Medical Sciences, Urmia, Iran.
2- Department of Anatomy, Neurophysiology Research Center, School of Medicine, Cellular and Molecular Research Institute, Urmia University of Medical Sciences, Urmia, Iran.
3- Department of Medical Physics, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran.
2- Department of Anatomy, Neurophysiology Research Center, School of Medicine, Cellular and Molecular Research Institute, Urmia University of Medical Sciences, Urmia, Iran.
3- Department of Medical Physics, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran.
Full-Text [PDF 2384 kb]
(633 Downloads)
| Abstract (HTML) (1174 Views)
Full-Text: (455 Views)
Introduction
Peripheral nerve injuries are a group of disorders usually caused by accidents, falls, and penetrating injuries [1], which often result in significant functional impairment and permanent disability [2]. Treatment methods for peripheral nerve injuries depend on the anatomical location, degree, and level of injury [3].
When a nerve is crushed, several non-surgical methods, including pharmacological [4], electrical [5], and laser therapies, have been employed to activate myelination and improve nerve function after peripheral nerve injury [6].
Previous studies show that for therapeutic purposes, ultrasound is effective in skeletal muscle [7], tendon, ligament, bone, and soft tissue regeneration [8]. Low-intensity ultrasound is a non-invasive therapeutic approach that facilitates peripheral nerve regeneration following nerve injury [9].
Since ultrasound is done through the contact of a prop (the head of the ultrasound device) with the skin, in case of serious damage to the skin, it is not possible to use ultrasound therapy for deeply damaged tissues. Our previous study showed that remote ischemic preconditioning in the contralateral limb may reduce the complications of reperfusion ischemia in other body organs and tissues [10].
We emitted low-intensity ultrasound waves on the healthy lower limb (remote ultrasound) to examine their effect on the function, tissue, and biochemical parameters of the damaged sciatic nerve. The literature review shows that the present study is the first to investigate remote ultrasound to treat sciatic nerve injury.
Materials and Methods
Animals
Twenty-four Sprague-Dawley male rats (200-220 g) were divided into 3 groups: Sham surgery, control (sciatic crush injury, without ultrasonic treatment), and remote ultrasound (sciatic crush injury, effective ultrasound radiation in the opposite hind limb). The rats had free access food and water under standard conditions (22±2°C; 12 hours of light and 12 hours of darkness).
Surgery procedure
The rats were subjected to general anesthesia by intraperitoneal injection (ketamine 100 and xylazine 15 mg/kg of body weight). The left sciatic nerve was exposed after cutting the posterolateral area of the thigh, followed by blunt dissection between the gluteus maximus and quadriceps muscles. The left sciatic nerve was exposed without crushing the nerve (sham surgery group). In the control and ultrasound groups, the sciatic nerve (on the left) was crushed with the help of fine forceps (30 s), 1 cm proximal to the bifurcation of the nerve, and the injury site was marked with a 6-0 nylon suture. At the end of the surgery, the muscle and skin were sutured [11].
Remote ultrasound irradiation
In the remote ultrasound group (after crushing the sciatic nerve), the right hind limb was shaved. Then it was treated with low-intensity ultrasound using aquasonic gel (probe diameter: 2 cm; duration of irradiation: 10 minutes, 3 times a week for 4 weeks; frequency: 1.1 MHz; intensity: 0.5 W/cm2) [12].
Functional tests
In all animals, the sciatic functional test (SFI) was elevated one day before the operation until the eighth week (days 7, 21, 28, 35, and 56). So that the soles of the animals` hind limbs were covered with black ink. The rats were allowed to walk on the track and leave their hind limb footprints on the white paper, and the SFI was calculated according to the according to the Bain`s formula [13].
Biochemical analysis
Before the surgery, the animals were anesthetized (ketamine 100 mg/kg), then blood samples were taken directly from the heart. To prepare plasma, blood samples were centrifuged following the cold cycle (3500 rpm; 4°C; 10 min). The plasma samples were kept in a freezer (-80°C) until biochemical analysis. The plasma levels of the total antioxidant capacity (TAC) and malondialdehyde (MDA) were evaluated using calorimetric assay kits (Elabscience, Wuhan, China). Using the FRAP (fluorescence recovery after photobleaching) method, the plasma level of TAC was measured at a wavelength of 520 nm. Thiobarbituric acid (TBA) reagent dyed plasma MDA pink and was measured at 532 nm wavelength. The HSP70 of plasma was evaluated by immunoassay (HSP70 high sensitivity EIA kit, ADI-EKS-715, Enzo Life Sciences, Inc.) 4.5 hours after isolation. The absorbance is read at 450 nm [14]. Plasma interleukin-6 (IL-6) was tested by immunoassay (Rat IL-6 EIA kit, ab 100772, Abcam) within 4.5 hours after isolation. The solution’s color changes from blue to yellow, and the absorbance is read at 450 nm [15].
Histological evaluation
On the 28th and 56th days after the operation, the third part of the sciatic nerve (in the place where the nerve was crushed) and also the middle part of the gastrocnemius muscle were removed in all groups. The tissues were fixed in formalin 10%, and transverse sections with a thickness of 5 µm were prepared after the preparation of paraffin blocks. The nerve sections were stained with toluidine blue 1%, and the myelinated axons were counted. By randomly selecting 4 fields from each section, the myelinated fiber’s diameter and the myelin sheath’s thickness were measured with a calibrated eyepiece [16]. In addition, the muscle sections were stained with hematoxylin and eosin (H&E), and 4 microscopic fields from the sections were randomly selected. Then, with a calibrated eyepiece, the diameter of the muscle fibers was measured [17].
Immunohistochemistry
To count the S-100 positive Schwann cells under a light microscope, transverse sections (4 µm) were initially prepared from the nerve tissues. Then, anti-S-100 (Dako, 1:200 dilution) was used as a marker for Schwann cells. After blocking non-specific immunoreactions (according to S-100 staining kit instructions), samples were incubated in S-100 protein antibody solution, then horseradish peroxidase-labeled secondary antibody solution for 15-20 minutes, and finally washed with phosphate-buffered saline [18].
Statistical analysis
The SPSS software, version 16 was used for statistical data analysis (Chicago, IL, USA). After determining the Mean±SEM, the obtained data were analyzed by one-way ANOVA and Tukey’s post hoc test. The significant difference was set at P<0.05.
Results
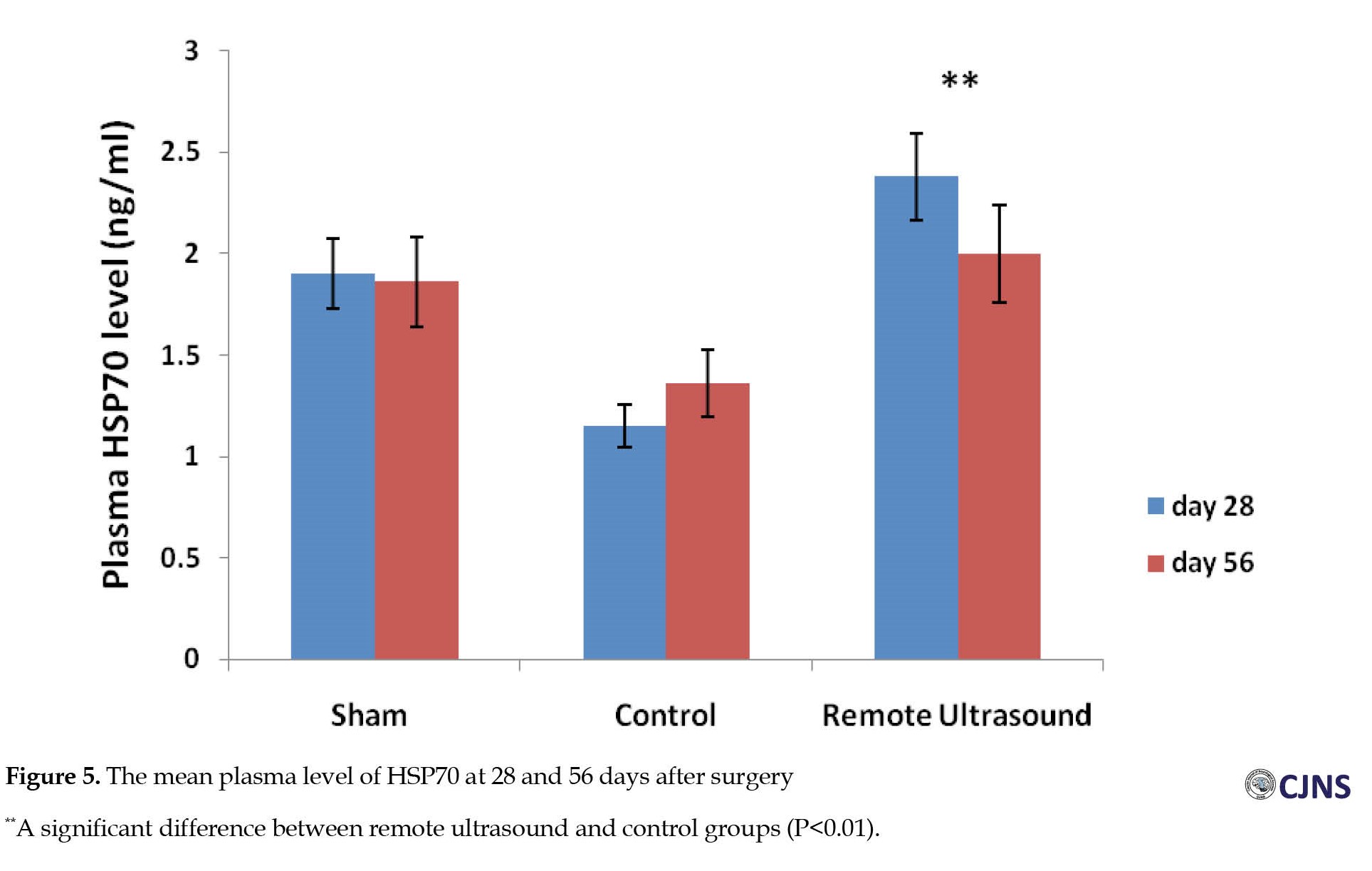
The mean SFI decreased significantly 7 days after operation in the experimental groups. On the 35th, 49th, and 56th postoperative days, the Mean±SEM SFI values for the remote ultrasound group were -18.06±2.3, -12.92±1.87, -11.95±2.91; and -36.61±3.24, -33.57±3.91, -33.42±4.79 for the control group, respectively (P<0.01). No significant difference (P>0.05) was observed between the ultrasound and sham surgery groups in the average SFI on days 35, 49, and 56 after surgery (Figure 1). The Mean±SEM plasma levels of MDA on days 28 and 56 after surgery in the remote ultrasound group (84.46±4.25 and 60.29±4.56 nmol/L, respectively) were significantly (P<0.01) lower than the control group (113.7±8.05 and 133.6±6.29 nmol/L, respectively) (Figure 2). At 28 days after surgery, the Mean±SEM plasma level of TAC in the remote ultrasound group (0.78±0.08 mmol/L) was significantly (P<0.01) greater than the control group (0.43±0.17 mmol/L) (Figure 3). The mean plasma level of IL-6 on days 28 and 56 after surgery in the control group (94.81±9.54 and 79.71±6.31 pg/mL) was significantly (P<0.05) greater than in the remote ultrasound group (45.33±2.05 and 54.21±4.76 pg/mL), respectively (Figure 4). On the days 28 and 56 after surgery, the Mean±SEM plasma level of HSP70 in the remote ultrasound group (2.38±0.04 and 2±0.01 ng/mL, respectively) was significantly (P<0.01) greater than in the control group (1.15±0.02 and 1.36±0.01 ng/mL, respectively). Fifty-six days after surgery, no significant difference (P˃0.05) was observed between the remote ultrasound and sham surgery groups (Figure 5). On day 56 after surgery, the Mean±SEM muscle fiber diameters in the remote ultrasound group (45.76±2.21 µm) compared with the control group (40.46±3.04 µm) showed significant differences (P<0.05) (Figure 6). On the days 28 and 56 after surgery, the Mean±SEM myelinated axon diameters in the remote ultrasound group (10.18±1.88 and 12.98±1.67 µm, respectively) was significantly (P<0.01) greater than the control group (6.28±1.44 and 10.8±2.23 µm, respectively). However, 56 days after surgery, there was no significant difference (P˃0.05) between remote ultrasound and sham surgery (Figure 7). On days 28 and 56 post-operation, the results showed that the mean number of S-100 positive Schwann cells in the remote ultrasound group (6414±218 and 8801±413, respectively) was higher than that in the control group (4553±256 and 6461±311, respectively) (P<0.001) (Figure 8).
Discussion
The results of this study showed that remote ultrasound therapy can enhance nerve regeneration. To the best of our knowledge, this is the first study that evaluates the effect of remote ultrasound on peripheral nerve repair. Although there is extensive information about the effect of ultrasound in treating musculoskeletal diseases compared to peripheral nerve diseases, recent studies show that ultrasound can be useful in repairing damaged peripheral nerves [7, 9].
One of the reasons it may not be possible to use ultrasound to repair peripheral nerves is physical damage where the skin is injured or destroyed. Previous studies showed that remote ischemic preconditioning (RIPC) can reduce ischemia-perfusion injury of other organs [10, 19].
In this study, the mean SFI and the mean muscle fiber diameter of the gastrocnemius muscle in the remote ultrasound group had a significant difference compared to the control group. So far, it has been established that the therapeutic effects of ultrasound occur through thermal and non-thermal (mechanical) changes in the target tissue [20]. Applying ultrasound effectively warm tissues, including the periosteum, collagenous tissues (fascia, ligaments, Joint capsule, and tendons), and muscles [8]. Increasing the temperature of the tissues causes blood vessels to dilate, blood flow to increase, and chronic inflammatory conditions to be relieved [21]. In addition, activation of interstitial and extracellular responses leads to tissue regeneration and angiogenesis [22]. It seems that the beneficial effects of ultrasound are related to the upregulation of anti-inflammatory and pro-inflammatory mediators, such as IL-6 [21]. The results of this study show that the mean plasma level of IL-6 in the control group was significantly higher than the remote ultrasound group. An experimental study shows that blocking of IL-6 and inhibition of the JAK/STAT3 pathway can suppress muscle atrophy [23].
In the present study, a significant increase in the mean myelinated axon diameters in the remote ultrasound group shows that this therapy can enhance the repair of damaged sciatic nerve by reducing neuronal cell apoptosis and inflammatory infiltration by decreasing the plasma level of IL-6 [24]. In addition, the mean plasma level of HSP70 in the remote ultrasound group was higher than the control group. Ultrasound irradiation upregulates HSP70 expression [25], and repeated use of ultrasound for soft tissue treatment causes the synthesis of HSP in skeletal muscles [26]. Peripheral nerve damage, including nerve crush, causes long-term pro-inflammatory responses in the nerve and spinal cord [27]. The anti-inflammatory property of HSP70 has been confirmed in both laboratory and animal models, so using heat shock proteins in treating chronic inflammatory diseases may be effective [28].
Our results showed that the mean plasma level of TAC increased and the plasma level of MDA reduced in the remote ultrasound group compared to the control group. This study showed that at least one part of the effect of remote ultrasound in repairing the crushed sciatic nerve is due to its antioxidant activity. Research shows that ultrasound treatment can produce antioxidant peptides [29].
Schwann cells, known as the glial cells of the peripheral nervous system, are among the most versatile cells of the body’s nervous system. In addition to ensuring neurons’ survival, these cells effectively find the axonal path during peripheral nerve repair [30]. Ultrasonic stimulation may effectively regenerate damaged peripheral nerves by directly stimulating Schwann cells [31]. Our results showed that the number of Schwann cells increased significantly in the remote ultrasound group compared to the control group. One of the advantages of using ultrasound in nerve regeneration is that it is probably used to repair Schwann cells [9]. In addition, low-intensity ultrasound induces nerve regeneration and improves its function by increasing Schwann cell proliferation, migration, and nerve growth factor expression [32]. Low-intensity pulsed ultrasound causes the expression of brain-derived neurotrophic factor (BDNF), which improves both function and histology in sciatic nerve crush injury in rats [33] so that the wet weight of the target muscle was correlated with the level of BDNFmRNA expression in the crushed nerve and ipsilateral dorsal root ganglia [34].
Conclusion
The study results show that in cases where the direct use of ultrasound is not possible due to soft tissue damage, the remote ultrasound method may help treat crushed peripheral nerve injuries. However, further studies are needed to determine the effectiveness and mechanism of action of remote ultrasound. In future studies, it is suggested that the healing process of peripheral nerves be investigated by irradiating remote ultrasound waves with different intensities and frequencies on other body organs.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Urmia University of Medical Sciences [No.: IR.UMSU.REC.1398.171].
Funding
This work was supported by the Student Research Committee of Urmia University of Medical Sciences (Grant No.: 2584).
Authors contributions
Conceptualization, methodology, investigation, writing, and funding acquisition: All authors; Supervision: Gholam Hossein Farjah.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the Student Research Committee, Urmia University of Medical Sciences, for providing research facilities.
References
Peripheral nerve injuries are a group of disorders usually caused by accidents, falls, and penetrating injuries [1], which often result in significant functional impairment and permanent disability [2]. Treatment methods for peripheral nerve injuries depend on the anatomical location, degree, and level of injury [3].
When a nerve is crushed, several non-surgical methods, including pharmacological [4], electrical [5], and laser therapies, have been employed to activate myelination and improve nerve function after peripheral nerve injury [6].
Previous studies show that for therapeutic purposes, ultrasound is effective in skeletal muscle [7], tendon, ligament, bone, and soft tissue regeneration [8]. Low-intensity ultrasound is a non-invasive therapeutic approach that facilitates peripheral nerve regeneration following nerve injury [9].
Since ultrasound is done through the contact of a prop (the head of the ultrasound device) with the skin, in case of serious damage to the skin, it is not possible to use ultrasound therapy for deeply damaged tissues. Our previous study showed that remote ischemic preconditioning in the contralateral limb may reduce the complications of reperfusion ischemia in other body organs and tissues [10].
We emitted low-intensity ultrasound waves on the healthy lower limb (remote ultrasound) to examine their effect on the function, tissue, and biochemical parameters of the damaged sciatic nerve. The literature review shows that the present study is the first to investigate remote ultrasound to treat sciatic nerve injury.
Materials and Methods
Animals
Twenty-four Sprague-Dawley male rats (200-220 g) were divided into 3 groups: Sham surgery, control (sciatic crush injury, without ultrasonic treatment), and remote ultrasound (sciatic crush injury, effective ultrasound radiation in the opposite hind limb). The rats had free access food and water under standard conditions (22±2°C; 12 hours of light and 12 hours of darkness).
Surgery procedure
The rats were subjected to general anesthesia by intraperitoneal injection (ketamine 100 and xylazine 15 mg/kg of body weight). The left sciatic nerve was exposed after cutting the posterolateral area of the thigh, followed by blunt dissection between the gluteus maximus and quadriceps muscles. The left sciatic nerve was exposed without crushing the nerve (sham surgery group). In the control and ultrasound groups, the sciatic nerve (on the left) was crushed with the help of fine forceps (30 s), 1 cm proximal to the bifurcation of the nerve, and the injury site was marked with a 6-0 nylon suture. At the end of the surgery, the muscle and skin were sutured [11].
Remote ultrasound irradiation
In the remote ultrasound group (after crushing the sciatic nerve), the right hind limb was shaved. Then it was treated with low-intensity ultrasound using aquasonic gel (probe diameter: 2 cm; duration of irradiation: 10 minutes, 3 times a week for 4 weeks; frequency: 1.1 MHz; intensity: 0.5 W/cm2) [12].
Functional tests
In all animals, the sciatic functional test (SFI) was elevated one day before the operation until the eighth week (days 7, 21, 28, 35, and 56). So that the soles of the animals` hind limbs were covered with black ink. The rats were allowed to walk on the track and leave their hind limb footprints on the white paper, and the SFI was calculated according to the according to the Bain`s formula [13].
Biochemical analysis
Before the surgery, the animals were anesthetized (ketamine 100 mg/kg), then blood samples were taken directly from the heart. To prepare plasma, blood samples were centrifuged following the cold cycle (3500 rpm; 4°C; 10 min). The plasma samples were kept in a freezer (-80°C) until biochemical analysis. The plasma levels of the total antioxidant capacity (TAC) and malondialdehyde (MDA) were evaluated using calorimetric assay kits (Elabscience, Wuhan, China). Using the FRAP (fluorescence recovery after photobleaching) method, the plasma level of TAC was measured at a wavelength of 520 nm. Thiobarbituric acid (TBA) reagent dyed plasma MDA pink and was measured at 532 nm wavelength. The HSP70 of plasma was evaluated by immunoassay (HSP70 high sensitivity EIA kit, ADI-EKS-715, Enzo Life Sciences, Inc.) 4.5 hours after isolation. The absorbance is read at 450 nm [14]. Plasma interleukin-6 (IL-6) was tested by immunoassay (Rat IL-6 EIA kit, ab 100772, Abcam) within 4.5 hours after isolation. The solution’s color changes from blue to yellow, and the absorbance is read at 450 nm [15].
Histological evaluation
On the 28th and 56th days after the operation, the third part of the sciatic nerve (in the place where the nerve was crushed) and also the middle part of the gastrocnemius muscle were removed in all groups. The tissues were fixed in formalin 10%, and transverse sections with a thickness of 5 µm were prepared after the preparation of paraffin blocks. The nerve sections were stained with toluidine blue 1%, and the myelinated axons were counted. By randomly selecting 4 fields from each section, the myelinated fiber’s diameter and the myelin sheath’s thickness were measured with a calibrated eyepiece [16]. In addition, the muscle sections were stained with hematoxylin and eosin (H&E), and 4 microscopic fields from the sections were randomly selected. Then, with a calibrated eyepiece, the diameter of the muscle fibers was measured [17].
Immunohistochemistry
To count the S-100 positive Schwann cells under a light microscope, transverse sections (4 µm) were initially prepared from the nerve tissues. Then, anti-S-100 (Dako, 1:200 dilution) was used as a marker for Schwann cells. After blocking non-specific immunoreactions (according to S-100 staining kit instructions), samples were incubated in S-100 protein antibody solution, then horseradish peroxidase-labeled secondary antibody solution for 15-20 minutes, and finally washed with phosphate-buffered saline [18].
Statistical analysis
The SPSS software, version 16 was used for statistical data analysis (Chicago, IL, USA). After determining the Mean±SEM, the obtained data were analyzed by one-way ANOVA and Tukey’s post hoc test. The significant difference was set at P<0.05.
Results
The mean SFI decreased significantly 7 days after operation in the experimental groups. On the 35th, 49th, and 56th postoperative days, the Mean±SEM SFI values for the remote ultrasound group were -18.06±2.3, -12.92±1.87, -11.95±2.91; and -36.61±3.24, -33.57±3.91, -33.42±4.79 for the control group, respectively (P<0.01). No significant difference (P>0.05) was observed between the ultrasound and sham surgery groups in the average SFI on days 35, 49, and 56 after surgery (Figure 1). The Mean±SEM plasma levels of MDA on days 28 and 56 after surgery in the remote ultrasound group (84.46±4.25 and 60.29±4.56 nmol/L, respectively) were significantly (P<0.01) lower than the control group (113.7±8.05 and 133.6±6.29 nmol/L, respectively) (Figure 2). At 28 days after surgery, the Mean±SEM plasma level of TAC in the remote ultrasound group (0.78±0.08 mmol/L) was significantly (P<0.01) greater than the control group (0.43±0.17 mmol/L) (Figure 3). The mean plasma level of IL-6 on days 28 and 56 after surgery in the control group (94.81±9.54 and 79.71±6.31 pg/mL) was significantly (P<0.05) greater than in the remote ultrasound group (45.33±2.05 and 54.21±4.76 pg/mL), respectively (Figure 4). On the days 28 and 56 after surgery, the Mean±SEM plasma level of HSP70 in the remote ultrasound group (2.38±0.04 and 2±0.01 ng/mL, respectively) was significantly (P<0.01) greater than in the control group (1.15±0.02 and 1.36±0.01 ng/mL, respectively). Fifty-six days after surgery, no significant difference (P˃0.05) was observed between the remote ultrasound and sham surgery groups (Figure 5). On day 56 after surgery, the Mean±SEM muscle fiber diameters in the remote ultrasound group (45.76±2.21 µm) compared with the control group (40.46±3.04 µm) showed significant differences (P<0.05) (Figure 6). On the days 28 and 56 after surgery, the Mean±SEM myelinated axon diameters in the remote ultrasound group (10.18±1.88 and 12.98±1.67 µm, respectively) was significantly (P<0.01) greater than the control group (6.28±1.44 and 10.8±2.23 µm, respectively). However, 56 days after surgery, there was no significant difference (P˃0.05) between remote ultrasound and sham surgery (Figure 7). On days 28 and 56 post-operation, the results showed that the mean number of S-100 positive Schwann cells in the remote ultrasound group (6414±218 and 8801±413, respectively) was higher than that in the control group (4553±256 and 6461±311, respectively) (P<0.001) (Figure 8).
Discussion
The results of this study showed that remote ultrasound therapy can enhance nerve regeneration. To the best of our knowledge, this is the first study that evaluates the effect of remote ultrasound on peripheral nerve repair. Although there is extensive information about the effect of ultrasound in treating musculoskeletal diseases compared to peripheral nerve diseases, recent studies show that ultrasound can be useful in repairing damaged peripheral nerves [7, 9].
One of the reasons it may not be possible to use ultrasound to repair peripheral nerves is physical damage where the skin is injured or destroyed. Previous studies showed that remote ischemic preconditioning (RIPC) can reduce ischemia-perfusion injury of other organs [10, 19].
In this study, the mean SFI and the mean muscle fiber diameter of the gastrocnemius muscle in the remote ultrasound group had a significant difference compared to the control group. So far, it has been established that the therapeutic effects of ultrasound occur through thermal and non-thermal (mechanical) changes in the target tissue [20]. Applying ultrasound effectively warm tissues, including the periosteum, collagenous tissues (fascia, ligaments, Joint capsule, and tendons), and muscles [8]. Increasing the temperature of the tissues causes blood vessels to dilate, blood flow to increase, and chronic inflammatory conditions to be relieved [21]. In addition, activation of interstitial and extracellular responses leads to tissue regeneration and angiogenesis [22]. It seems that the beneficial effects of ultrasound are related to the upregulation of anti-inflammatory and pro-inflammatory mediators, such as IL-6 [21]. The results of this study show that the mean plasma level of IL-6 in the control group was significantly higher than the remote ultrasound group. An experimental study shows that blocking of IL-6 and inhibition of the JAK/STAT3 pathway can suppress muscle atrophy [23].
In the present study, a significant increase in the mean myelinated axon diameters in the remote ultrasound group shows that this therapy can enhance the repair of damaged sciatic nerve by reducing neuronal cell apoptosis and inflammatory infiltration by decreasing the plasma level of IL-6 [24]. In addition, the mean plasma level of HSP70 in the remote ultrasound group was higher than the control group. Ultrasound irradiation upregulates HSP70 expression [25], and repeated use of ultrasound for soft tissue treatment causes the synthesis of HSP in skeletal muscles [26]. Peripheral nerve damage, including nerve crush, causes long-term pro-inflammatory responses in the nerve and spinal cord [27]. The anti-inflammatory property of HSP70 has been confirmed in both laboratory and animal models, so using heat shock proteins in treating chronic inflammatory diseases may be effective [28].
Our results showed that the mean plasma level of TAC increased and the plasma level of MDA reduced in the remote ultrasound group compared to the control group. This study showed that at least one part of the effect of remote ultrasound in repairing the crushed sciatic nerve is due to its antioxidant activity. Research shows that ultrasound treatment can produce antioxidant peptides [29].
Schwann cells, known as the glial cells of the peripheral nervous system, are among the most versatile cells of the body’s nervous system. In addition to ensuring neurons’ survival, these cells effectively find the axonal path during peripheral nerve repair [30]. Ultrasonic stimulation may effectively regenerate damaged peripheral nerves by directly stimulating Schwann cells [31]. Our results showed that the number of Schwann cells increased significantly in the remote ultrasound group compared to the control group. One of the advantages of using ultrasound in nerve regeneration is that it is probably used to repair Schwann cells [9]. In addition, low-intensity ultrasound induces nerve regeneration and improves its function by increasing Schwann cell proliferation, migration, and nerve growth factor expression [32]. Low-intensity pulsed ultrasound causes the expression of brain-derived neurotrophic factor (BDNF), which improves both function and histology in sciatic nerve crush injury in rats [33] so that the wet weight of the target muscle was correlated with the level of BDNFmRNA expression in the crushed nerve and ipsilateral dorsal root ganglia [34].
Conclusion
The study results show that in cases where the direct use of ultrasound is not possible due to soft tissue damage, the remote ultrasound method may help treat crushed peripheral nerve injuries. However, further studies are needed to determine the effectiveness and mechanism of action of remote ultrasound. In future studies, it is suggested that the healing process of peripheral nerves be investigated by irradiating remote ultrasound waves with different intensities and frequencies on other body organs.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Urmia University of Medical Sciences [No.: IR.UMSU.REC.1398.171].
Funding
This work was supported by the Student Research Committee of Urmia University of Medical Sciences (Grant No.: 2584).
Authors contributions
Conceptualization, methodology, investigation, writing, and funding acquisition: All authors; Supervision: Gholam Hossein Farjah.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the Student Research Committee, Urmia University of Medical Sciences, for providing research facilities.
References
- Kamble N, Shukla D, Bhat D. Peripheral Nerve Injuries: Electrophysiology for the Neurosurgeon. Neurol India. 2019; 67(6):1419-22. [DOI:10.4103/0028-3886.273626] [PMID]
- Modrak M, Talukder MAH, Gurgenashvili K, Noble M, Elfar JC. Peripheral nerve injury and myelination: Potential therapeutic strategies. J Neurosci Res. 2020; 98(5):780-95. [DOI:10.1002/jnr.24538] [PMID]
- Panagopoulos GN, Megaloikonomos PD, Mavrogenis AF. The present and future for peripheral nerve regeneration. Orthopedics. 2016; 40(1):e141–56. [DOI:10.3928/01477447-20161019-01]
- Bolandghamat S, Behnam-Rassouli M. Recent findings on the effects of pharmacological agents on the nerve regeneration after peripheral nerve injury. Curr Neuropharmacol. 2020; 18(11):1154-63. [DOI:10.2174/1570159X18666200507084024] [PMID]
- Juckett L, Saffari TM, Ormseth B, Senger JL, Moore AM. The effect of electrical stimulation on nerve regeneration following peripheral nerve injury. Biomolecules. 2022; 12(12):1856. [DOI:10.3390/biom12121856] [PMID]
- Araujo T, Andreo L, Tobelem DDC, Silva T, Malavazzi TCDS, Martinelli A, et al. Effects of systemic vascular photobiomodulation using LED or laser on sensory-motor recovery following a peripheral nerve injury in Wistar rats. Photochem Photobiol Sci. 2023; 22(3):567-77. [DOI:10.1007/s43630-022-00335-8] [PMID]
- Qin H, Luo Z, Sun Y, He Z, Qi B, Chen Y, et al. Low-intensity pulsed ultrasound promotes skeletal muscle regeneration via modulating the inflammatory immune microenvironment. Int J Biol Sci. 2023; 19(4):1123-45. [DOI:10.7150/ijbs.79685] [PMID]
- Lai WC, Iglesias BC, Mark BJ, Wang D. Low-intensity pulsed ultrasound augments tendon, ligament, and bone-soft tissue healing in preclinical animal models: A systematic review. Arthroscopy. 2021; 37(7):2318-33.e3. [DOI:10.1016/j.arthro.2021.02.019] [PMID]
- Acheta J, Stephens SBZ, Belin S, Poitelon Y. Therapeutic low-intensity ultrasound for peripheral nerve regeneration - A schwann cell perspective. Front Cell Neurosci. 2022; 15:812588. [DOI:10.3389/fncel.2021.812588] [PMID]
- Karimipour M, Farjah GH, Molazadeh F, Ansari M, Pourheidar B. Protective effect of contralateral, ipsilateral, and bilateral remote ischemic preconditioning on spinal cord ischemia reperfusion injury in rats. Turk Neurosurg. 2019; 29(6):933-9. [DOI:10.5137/1019-5149.JTN.26237-19.3] [PMID]
- Farjah, GH, Peirouvi T, Heshmatian B, Yasami M, Dolatkhah MA. The effect of short-term treatments of a gonadotropinreleasing hormone analog (buserelin) on sciatic nerve regeneration. Iran J Vet Res. 2014; 15(2):104-9. [DOI:10.22099/IJVR.2014.2337]
- Jiang W, Wang Y, Tang J, Peng J, Wang Y, Guo Q, et al. Low-intensity pulsed ultrasound treatment improved the rate of autograft peripheral nerve regeneration in rat. Sci Rep. 2016; 6:22773. [DOI:10.1038/srep22773] [PMID]
- Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989; 83(1):129-38. [DOI:10.1097/00006534-198901000-00024] [PMID]
- Wu R, Gao W, Dong Z, Su Y, Ji Y, Liao J, et al. Plasma heat shock protein 70 is associated with the onset of acute myocardial infarction and total occlusion in target vessels. Front Cardiovasc Med. 2021; 8:688702. [DOI:10.3389/fcvm.2021.688702] [PMID]
- Almatroodi SA, Alnuqaydan AM, Babiker AY, Almogbel MA, Khan AA, Husain Rahmani A. 6-gingerol, a bioactive compound of ginger attenuates renal damage in streptozotocin-induced diabetic rats by regulating the oxidative stress and inflammation. Pharmaceutics. 2021; 13(3):317. [DOI:10.3390/pharmaceutics13030317] [PMID]
- Farjah GH, Fazli F. The effect of chick embryo amniotic fluid on sciatic nerve regeneration of rats. Iran J Vet Res. 2015; 16(2):167-71. [PMID]
- Farjah GH, Fazli F, Karimipour M, Pourheidar B, Heshmatiyan B, Pourheidar M. The effect of bone marrow mesenchymal stem cells on recovery of skeletal muscle after neurotization surgery in rat. Iran J Basic Med Sci. 2018; 21(3):236-43. [PMID]
- Farjah GH, Mohammdzadeh S, Zirak Javanmard M. The effect of lycopene in egg shell membrane guidance channel on sciatic nerve regeneration in rats. Iran J Basic Med Sci. 2020; 23(4):527-33. [PMID]
- Liu Y, Li AQ, Ma W, Gao YB, Deng LQ, Zhang C, et al. Limb remote ischemic preconditioning reduces repeated ketamine exposure-induced adverse effects in the developing brain of rats. J Mol Neurosci. 2019; 68(1):58-65. [DOI:10.1007/s12031-019-01282-3] [PMID]
- Kim C, Lim M, Woodworth GF, Arvanitis CD. The roles of thermal and mechanical stress in focused ultrasound-mediated immunomodulation and immunotherapy for central nervous system tumors. J Neurooncol. 2022; 157(2):221-36. [DOI:10.1007/s11060-022-03973-1] [PMID]
- Rodrigues M, Barbosa RI, Neves LMS, Kuriki HU, Gonçalves ECD, Santos ARS, et al. Therapeutic ultrasound ameliorates hyperalgesia and edema on CFA-induced persistent inflammatory response in mice. Braz J Anesthesiol. 2023; 73(5):626-34. [DOI:10.1016/j.bjane.2022.08.004] [PMID]
- Latt LD, Jaffe DE, Tang Y, Taljanovic MS. Evaluation and treatment of chronic plantar fasciitis. Foot Ankle Orthop. 2020; 5(1):2473011419896763. [DOI:10.1177/2473011419896763] [PMID]
- Huang Z, Zhong L, Zhu J, Xu H, Ma W, Zhang L, et al. Inhibition of IL-6/JAK/STAT3 pathway rescues denervation-induced skeletal muscle atrophy. Ann Transl Med. 2020; 8(24):1681. [DOI:10.21037/atm-20-7269] [PMID]
- Zhang D, Jing B, Chen ZN, Li X, Shi HM, Zheng YC, et al. Ferulic acid alleviates sciatica by inhibiting neuroinflammation and promoting nerve repair via the TLR4/NF-κB pathway. CNS Neurosci Ther. 2023; 29(4):1000-11. [DOI:10.1111/cns.14060] [PMID]
- Zhang Z, Ma Y, Guo S, He Y, Bai G, Zhang W. Low-intensity pulsed ultrasound stimulation facilitates in vitro osteogenic differentiation of human adipose-derived stem cells via up-regulation of heat shock protein (HSP)70, HSP90, and bone morphogenetic protein (BMP) signaling pathway. Biosci Rep. 2018; 38(3):BSR20180087. [DOI:10.1042/BSR20180087] [PMID]
- Nussbaum EL, Locke M. Heat shock protein expression in rat skeletal muscle after repeated applications of pulsed and continuous ultrasound. Arch Phys Med Rehabil. 2007; 88(6):785-90. [DOI:10.1016/j.apmr.2007.03.020] [PMID]
- Niehaus JK, Taylor-Blake B, Loo L, Simon JM, Zylka MJ. Spinal macrophages resolve nociceptive hypersensitivity after peripheral injury. Neuron. 2021; 109(8):1274-82.e6. [DOI:10.1016/j.neuron.2021.02.018] [PMID]
- Tukaj S. Immunoregulacyjne właściwości białek Hsp70 [Immunoregulatory properties of Hsp70]. Postepy Hig Med Dosw (Online). 2014; 68:722-7. Polish. [DOI:10.5604/17322693.1107329] [PMID]
- Habinshuti I, Mu TH, Zhang M. Ultrasound microwave-assisted enzymatic production and characterisation of antioxidant peptides from sweet potato protein. Ultrason Sonochem. 2020; 69:105262. [DOI:10.1016/j.ultsonch.2020.105262] [PMID]
- Bosch-Queralt M, Fledrich R, Stassart RM. Schwann cell functions in peripheral nerve development and repair. Neurobiol Dis. 2023; 176:105952. [DOI:10.1016/j.nbd.2022.105952] [PMID]
- Chang CJ, Hsu SH. The effects of low-intensity ultrasound on peripheral nerve regeneration in poly(DL-lactic acid-co-glycolic acid) conduits seeded with Schwann cells. Ultrasound Med Biol. 2004; 30(8):1079-84. [DOI:10.1016/j.ultrasmedbio.2004.06.005] [PMID]
- Li Z, Ye K, Yin Y, Zhou J, Li D, Gan Y, et al. Low-intensity pulsed ultrasound ameliorates erectile dysfunction induced by bilateral cavernous nerve injury through enhancing Schwann cell-mediated cavernous nerve regeneration. Andrology. 2023; 11(6):1188-202. [DOI:10.1111/andr.13406] [PMID]
- Wang T, Ito A, Xu S, Kawai H, Kuroki H, Aoyama T. Low-intensity pulsed ultrasound prompts both functional and histologic improvements while upregulating the brain-derived neurotrophic factor expression after sciatic crush injury in rats. Ultrasound Med Biol. 2021; 47(6):1586-95. [DOI:10.1016/j.ultrasmedbio.2021.02.009] [PMID]
- Ni XJ, Wang XD, Zhao YH, Sun HL, Hu YM, Yao J, et al. The effect of low-intensity ultrasound on brain-derived neurotropic factor expression in a rat sciatic nerve crushed injury model. Ultrasound Med Biol. 2017; 43(2):461-8. [DOI:10.1016/j.ultrasmedbio.2016.09.017] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2024/01/28 | Accepted: 2024/01/20 | Published: 2024/01/20
Received: 2024/01/28 | Accepted: 2024/01/20 | Published: 2024/01/20
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |