Sat, May 18, 2024
Volume 9, Issue 4 (Autumn 2023)
Caspian J Neurol Sci 2023, 9(4): 229-243 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Soleimani R, Jalali M M, Bakhtiari H, Eslamdoust-Siahestalkhi F, Jalali S M. Probiotic Add-on Therapy in the First-episode Schizophrenia: A Randomized Controlled Trial. Caspian J Neurol Sci 2023; 9 (4) :229-243
URL: http://cjns.gums.ac.ir/article-1-665-en.html
URL: http://cjns.gums.ac.ir/article-1-665-en.html
Robabeh Soleimani * 

 1, Mir Mohammad Jalali2
1, Mir Mohammad Jalali2 

 , Hoda Bakhtiari1
, Hoda Bakhtiari1 

 , Fatemeh Eslamdoust-Siahestalkhi1
, Fatemeh Eslamdoust-Siahestalkhi1 

 , Seyede Melika Jalali3
, Seyede Melika Jalali3 




 1, Mir Mohammad Jalali2
1, Mir Mohammad Jalali2 

 , Hoda Bakhtiari1
, Hoda Bakhtiari1 

 , Fatemeh Eslamdoust-Siahestalkhi1
, Fatemeh Eslamdoust-Siahestalkhi1 

 , Seyede Melika Jalali3
, Seyede Melika Jalali3 


1- Department of Psychiatry, Kavosh Cognitive Behavior Sciences and Addiction Research Center, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
2- Department of Otolaryngology Head and Neck Surgery, Otorhinolaryngology Research Center, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
3- Department of Pharmacology, Faculty of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran
2- Department of Otolaryngology Head and Neck Surgery, Otorhinolaryngology Research Center, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
3- Department of Pharmacology, Faculty of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran
Full-Text [PDF 2243 kb]
(140 Downloads)
| Abstract (HTML) (349 Views)
Full-Text: (147 Views)
Introduction
Schizophrenia (SZ) is a chronic and serious psychiatric disorder. Its prevalence in the world population is about 1% [1]. The disease is characterized by positive symptoms, negative symptoms, and cognitive dysfunction, the latter two being particularly resistant to antipsychotics [2]. Cognitive impairment and negative symptoms present challenges in managing SZ [3, 4, 5, 6]. Therefore, it is necessary to regularly evaluate new combinations of nutritional and pharmacological therapy for these symptoms. Then again, antipsychotic medications are associated with cardiometabolic adverse effects, including weight gain, dyslipidemia, insulin resistance or frank type 2 diabetes mellitus, and hypertension [7].
Research into the pathogenesis of schizophrenia has pointed to a potential role for the human microbiome and the gut-brain axis, which involves bidirectional communication between the central and enteric nervous systems [8]. Disturbed gut microbiota is linked to increased systemic inflammation [9], and consequent neural inflammation may be directly related to schizophrenia [10].
Preclinical studies have consistently demonstrated that fecal microbiota transplantation from patients with different psychiatric disorders leads to the development of the behavioral and physiological profiles of the disease in germ-free mice [11-15]. In this regard, 4 studies [16-19] have examined the relationship between schizophrenia and probiotics and found that probiotic supplementation reduced the positive and negative syndrome scale (PANSS) scores but not significantly. On the other hand, evidence suggests that probiotics can reduce constipation [16, 20], weight gain, and metabolic side effects [21] commonly associated with antipsychotic use. Probiotics may improve metabolic parameters through anti-inflammatory and insulin-resistance properties [22]. Dickerson et al. [16] showed that probiotic supplementation for 14 weeks can prevent common physical symptoms in patients with SZ but have no effects on the clinical syndrome scale.
Despite the high prevalence of dyslipidemia in schizophrenia and the increased risk of metabolic complications and cardiovascular disease, the focus in this population is primarily on managing psychotic symptoms, and their physical health is generally overlooked.
To date, few studies have examined the lessening of metabolic impairments in patients with SZ, and the evidence regarding the effects of probiotics on metabolic status is insufficient. Research on schizophrenia needs more randomized clinical trials (RCTs) to provide sufficient proof, draw reasonable conclusions, and consequently determine the effect of probiotic supplements on the clinical symptoms and metabolic status of patients with SZ [23]. This study aimed to assess the impact of probiotic supplementation on improving clinical signs and adverse metabolic effects commonly observed in patients with first-episode schizophrenia receiving antipsychotics.
Materials and Methods
Study patients
From March 2019 to June 2021, a randomized, double-blind, placebo-controlled trial was conducted at Shafa Hospital, Rasht City, Iran. This study included inpatients from the Psychiatric Department who met the eligibility criteria: 1) Aged between 18 and 60 years, 2) First-episode schizophrenia diagnosis according to the DSM-5, and 3) Clinically stable for 4 weeks or more. Clinical stability was defined as 1) No change in oral antipsychotics (olanzapine or risperidone) dosage, 2) Clinical global impressions-improvement of illness (CGI-S) score ≤4, and 3) Positive symptom score ≤4 in brief psychiatric rating scale (BPRS). The exclusion criteria were as follows: 1) Duration of positive symptoms exceeding two years, 2) Diagnosis of serious neurologic or cardiovascular disease, 3) Using any drug for weight loss, 4) A history of substance use or alcohol in the last three months (except nicotine or caffeine), 5) Receiving antibiotics for any reason in the last two weeks, 6) Mental retardation (intelligent quotient of <70), and 7) Pregnancy.
Study design
After enrollment in the clinical study, the patients were treated on an outpatient basis. All patients received probiotic supplements (FamiLact capsule, Zist Takhmir Co., Tehran, Iran) or a placebo once daily. FamiLact capsules (500 mg) contain gram-positive organisms and 38.5 mg Fructooligosaccharides. The microorganisms are 9×109 colony-forming units (CFU)/g of viable, lyophilized Lactobacilli (Lactobacillus Acidophilus, Lactobacillus Casei, Lactobacillus Delbrueckii Subsp. Lactobacillus Bulgaricus, and Lactobacillus Rhamnosus), 1.25×1010 of Bifidobacteria (Bifidobacterium Longum and Bifidobacterium Breve), and 1.5×1010 of Streptococcus Salivarius Subsp. Thermophilus.
Randomization and blinding
Patients who met the inclusion criteria were randomly assigned to probiotic and placebo groups (equal numbers) for 12 weeks. Randomization and blinding were performed by an independent pharmacist using a sealed coded envelope. Block randomization (size 4) was performed using a computer-generated random allocation sequence. The probiotic supplement and placebo were indistinguishable in appearance and content.
Outcome measures
The primary endpoints were assessing clinical symptoms using BPRS and PANSS. Secondary endpoints included the severity of psychiatric symptoms using CGI-S, clinical response, and biometric and biochemical measures. Clinical response was defined as a ≥25% reduction in BPRS score after 3 months. Biometric and biochemical variables included blood pressure (BP), body mass index (BMI), triglycerides (TG), total cholesterol, and fasting blood sugar (FBS). Lipid profiles and FBS were measured using an enzymatic kit (Pars Azmun, Tehran, Iran) with intra-assay CVs of less than 5%. Primary objectives were measured at baseline and then 4, 8, and 12 weeks after the study started. All secondary objectives were assessed at baseline and 12 weeks later.
Sample size
The required sample size was computed using the Stata statistical software, version 14 (StataCorp LP, College Station, TX, USA). Leucht et al. [24] demonstrated a 25% BPRS score reduction as “minimally improved.” According to the included trials in this study, the standard deviation of the BPRS total score at baseline was 12.2. In addition, Leddy-Stacy and Rosenheck [25] suggested the minimum clinically important difference for the PANSS to be between 4.25 and 8.30 total points. From a previous study [18], we estimated the Mean±SD difference between active intervention and placebo group to be 70.9±11.4. Furthermore, we supposed that a 5-point difference in PANSS between active intervention and placebo would be clinically significant. Using analysis of covariance (ANCOVA) with a correlation coefficient of 0.7, 80% power, and 95% confidence at three measurement points, a total of 52 participants would be required. Accounting for a 20% loss, 62 participants (31 per arm) would be sufficient to estimate effect sizes for each of the two primary objectives.
Statistical analyses
We presented quantitative variables with Mean±SD and categorical variables with absolute frequencies and percentages. Data normality was assessed with the Shapiro-Wilk test. Repeated measures of analysis of variance (ANOVA) were used to analyze the effect of probiotics on primary objectives (BPRS and PANSS). Partial Eta squared (η2) was used to calculate effect sizes for significant main effects, with the following standards to determine small (0.01-0.059), medium (0.06-0.139), and large (>0.14) effect sizes.
Secondary analyses were exploratory without formal sample size estimation. The clinical response variable was considered a categorical variable. A chi-squared test was performed to assess the effect of probiotics on the clinical response. The chi-squared test compared the proportion of patients with a CGI-S score of 1 or 2 (very much or much improved). Other secondary objectives were analyzed using analysis of variance/covariance (ANOVA/ANCOVA) after adjusting for the baseline levels of variables. We presented effect sizes as standardized mean differences (Cohen’s d) or risk ratios (RR) with 95% confidence intervals (95% CI).
Data were analyzed according to the intention-to-treat principle using the Stata statistical software. To handle missing data, we used simple mean imputation [26]. Also, we used subgroup analysis, comparing the baseline characteristics between completers and dropouts. Statistical tests were two-tailed. P≤0.05 were considered to indicate statistical significance.
Results
Of 62 enrolled patients, 55(88.7%) completed the study. There were no significant differences in baseline characteristics between 7 patients who dropped out at week 8 or 12 and completers (Supplementary Table 1).
Figure 1 depicts the flow of patients’ enrolment through the study according to the consolidated reporting trial standards (CONSORT) statement. Table 1 presents the patients’ characteristics at baseline.
None of the variables with a P<0.05 were strong confounders for primary and secondary objectives [27, 28].
Primary outcomes
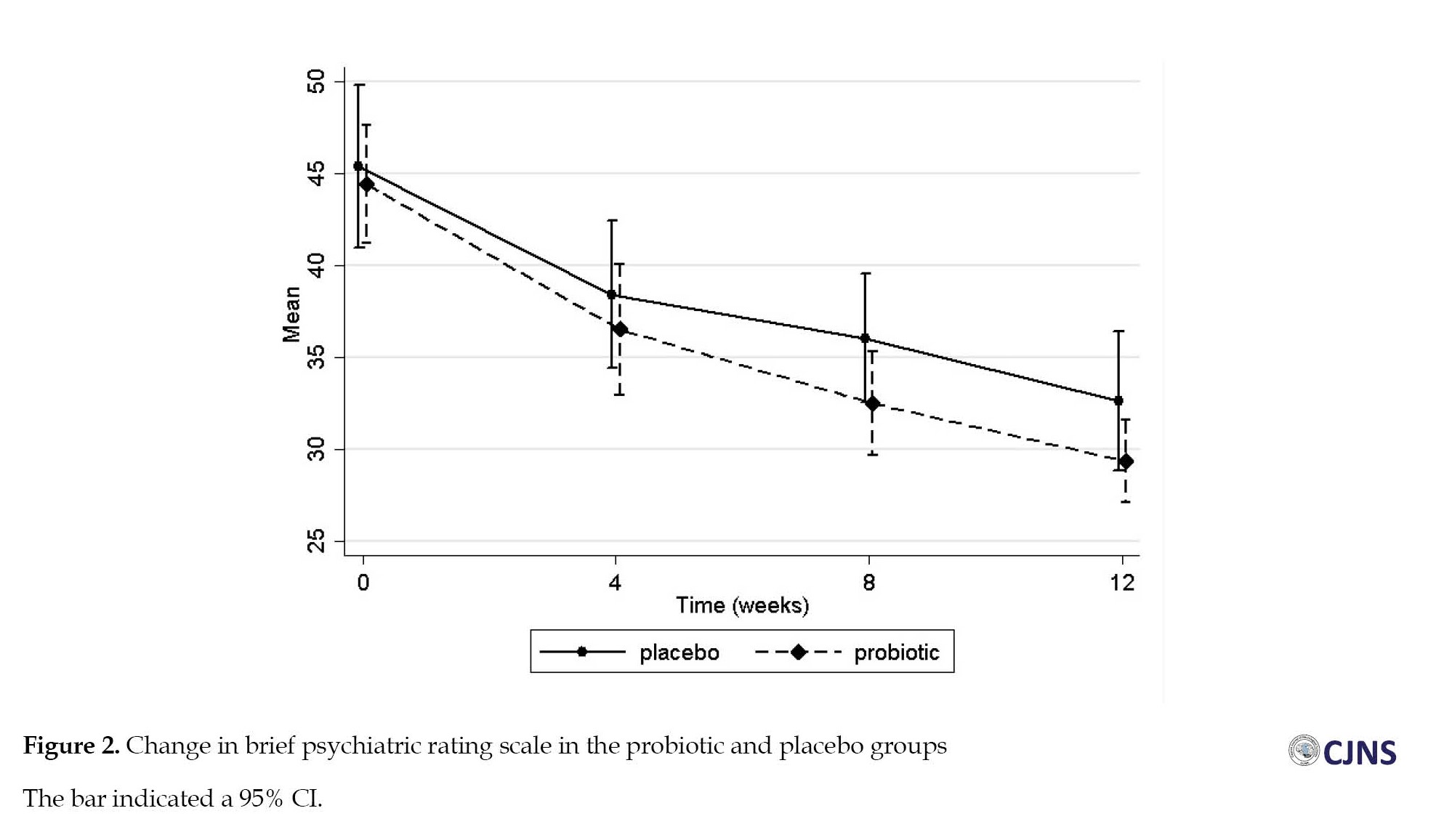
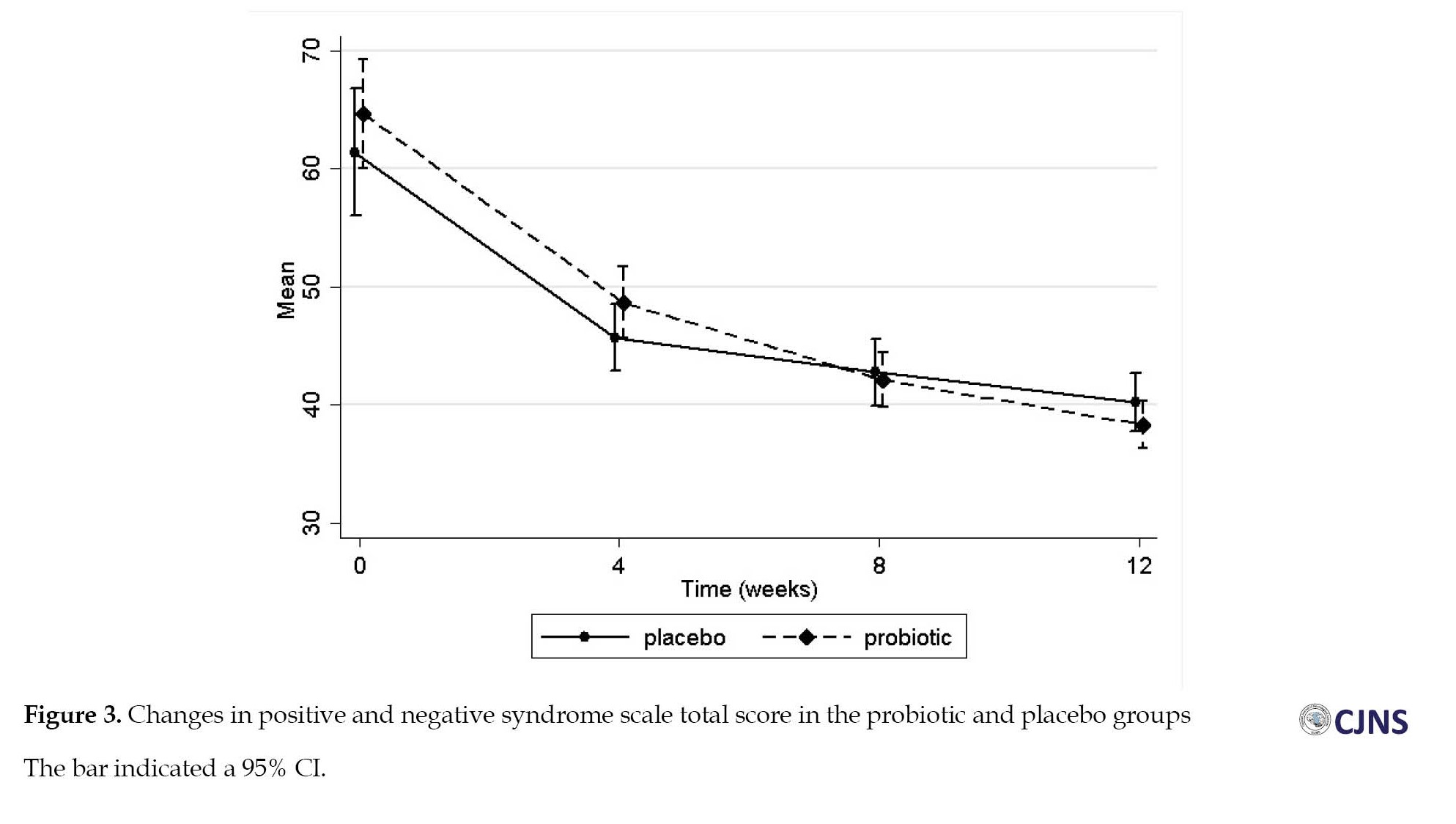
Clinical psychiatric symptoms (BPRS and PANSS) improved in both groups at 12 weeks (Table 2), and repeated measure ANOVA revealed a significant effect of time on these outcomes with medium effect sizes (Figure 2, Figure 3).
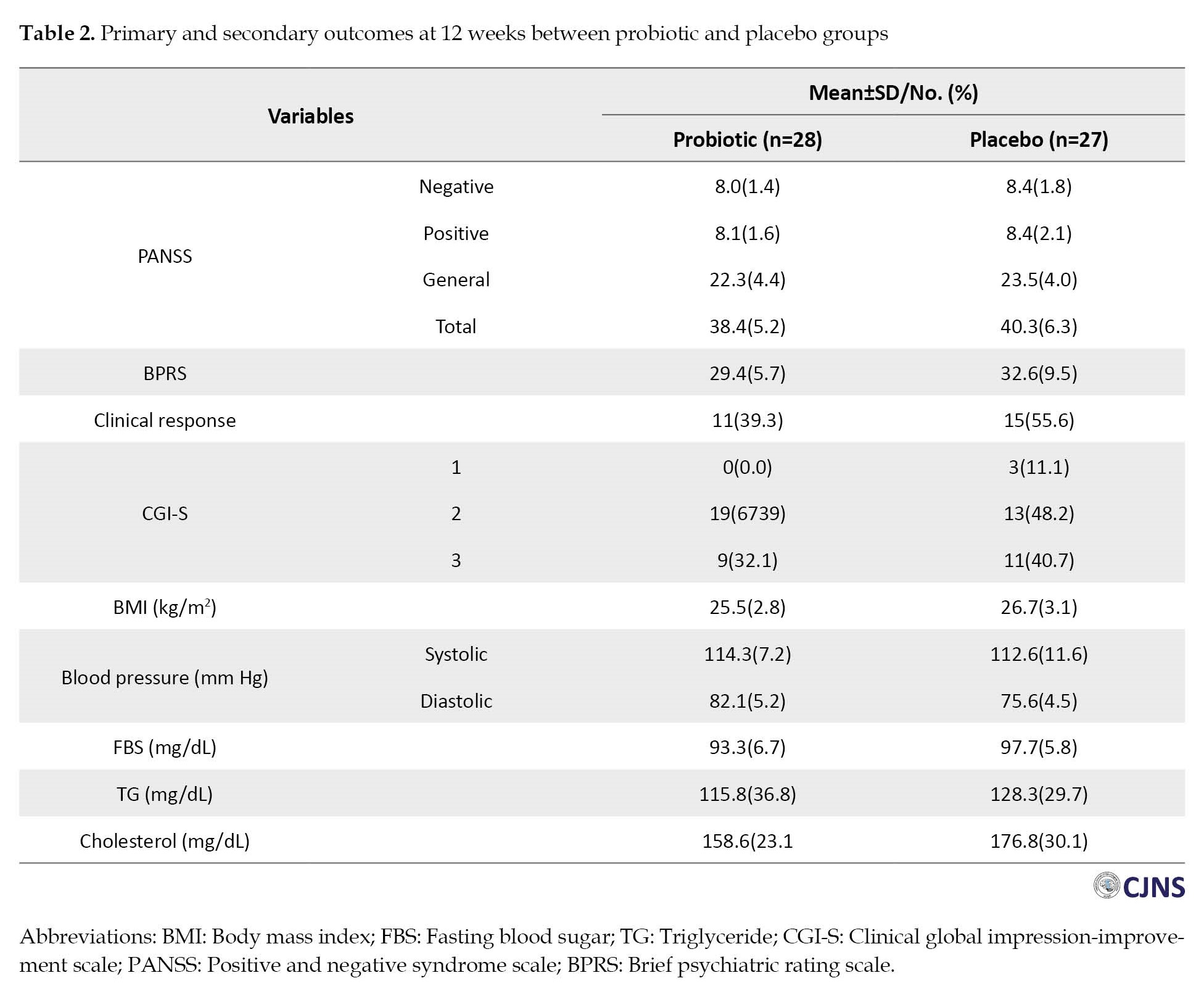 The mean BPRS score was reduced from 44.4 to 29.4 in the probiotic group and 45.4 to 32.6 in the placebo group. However, the results indicated no significant group effect on clinical symptoms (P=0.296).
The mean BPRS score was reduced from 44.4 to 29.4 in the probiotic group and 45.4 to 32.6 in the placebo group. However, the results indicated no significant group effect on clinical symptoms (P=0.296).
The mean total score of PANSS was reduced by 26.3, from 64.7 at the baseline to 38.4, after receiving antipsychotic drugs and probiotics for 12 weeks and by 21.2, from 61.45 at the baseline to 40.3, in the placebo group. Also, we observed no significant difference in PANSS score improvement between the two groups (P=0.995). Similar trends were also observed for all subscales of the PANSS (Supplementary Table 1). Mean differences of primary objectives at varying times and results of repeated measure ANOVA could be observed in Table 3.
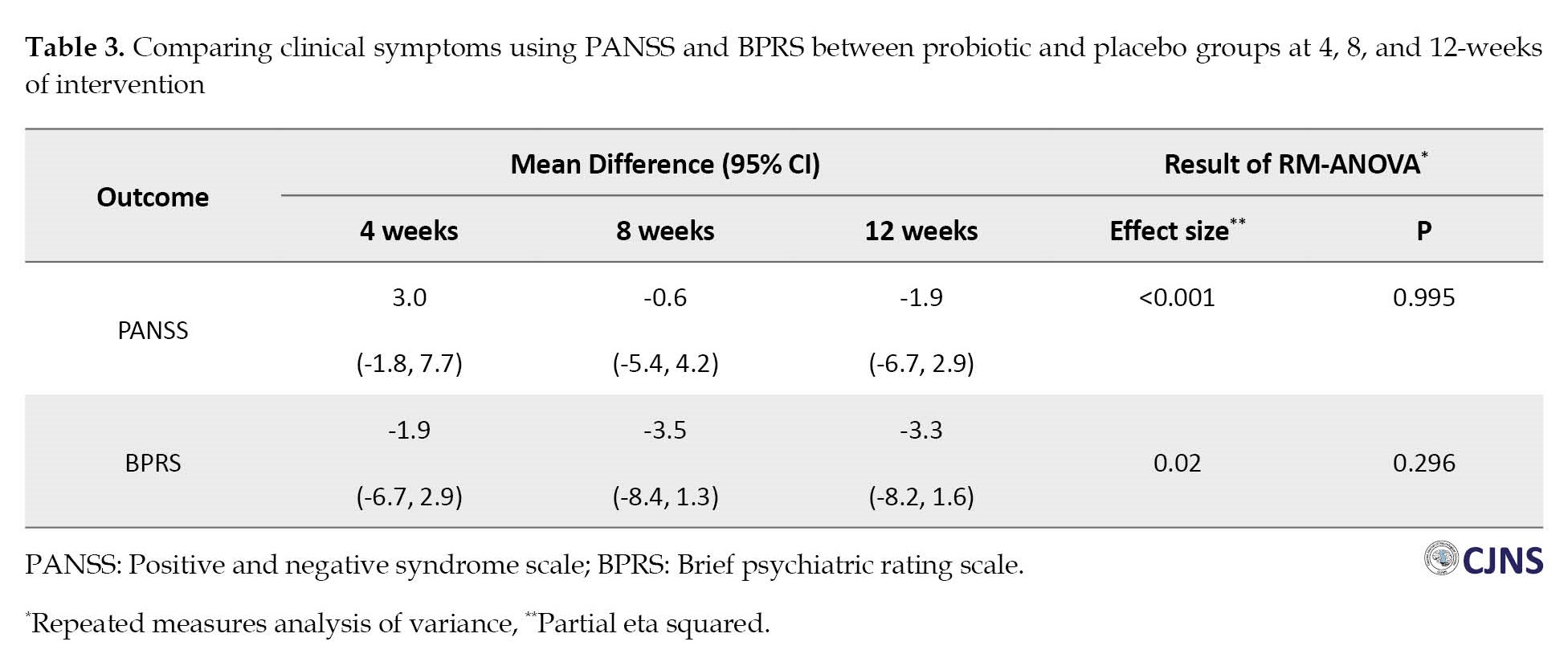
In addition, we calculated effect sizes using Cohen’s d formula to examine the extent of treatment effects within the group. Effect sizes were classified as small (0.20–0.49), medium (0.50–0.79), and large (0.80 and more). Large effect sizes were observed in the BPRS and the PANSS in the probiotic group at 4 weeks (0.87 and 1.52), at 8 weeks (1.47 and 2.30), and at 12 weeks (2.02 and 2.73).
Secondary outcomes
Clinical responses in the probiotic and placebo groups were observed in 11 patients (39.3%) and 15 patients (55.6%), respectively (RR: 0.72; 95% CI, 0.42%-1.24%; X2=1.46; P=0.23). In terms of CGI-S, a score of 1 or 2 was observed in 19 patients (54.3%) in the probiotic group and 16 patients (45.7%) in the placebo group (RR: 1.20; 95% CI, 0.70%-2.06%; X2=0.44; P=0.51). Adjusting for baseline values, the systolic blood pressure and BMI were similar in the two groups. The mean diastolic blood pressure was 82.1(5.2) mm Hg with probiotics and 75.6(4.6) mm Hg with placebo (Cohen’s d: 4.79; 95% CI, 2.78%-6.80%; P<0.001). Interestingly, statistical analyses showed lower levels of all three biochemical variables (FBS, triglyceride, and cholesterol) in the probiotic group compared to the placebo group (P<0.05). The Cohen’s d for these biochemical variables were 0.70, 1.57, and 0.68, respectively. The magnitude of these values was interpreted as a medium-large effect size. Table 4 shows the results of ANOVA/ANCOVA analyses in detail.
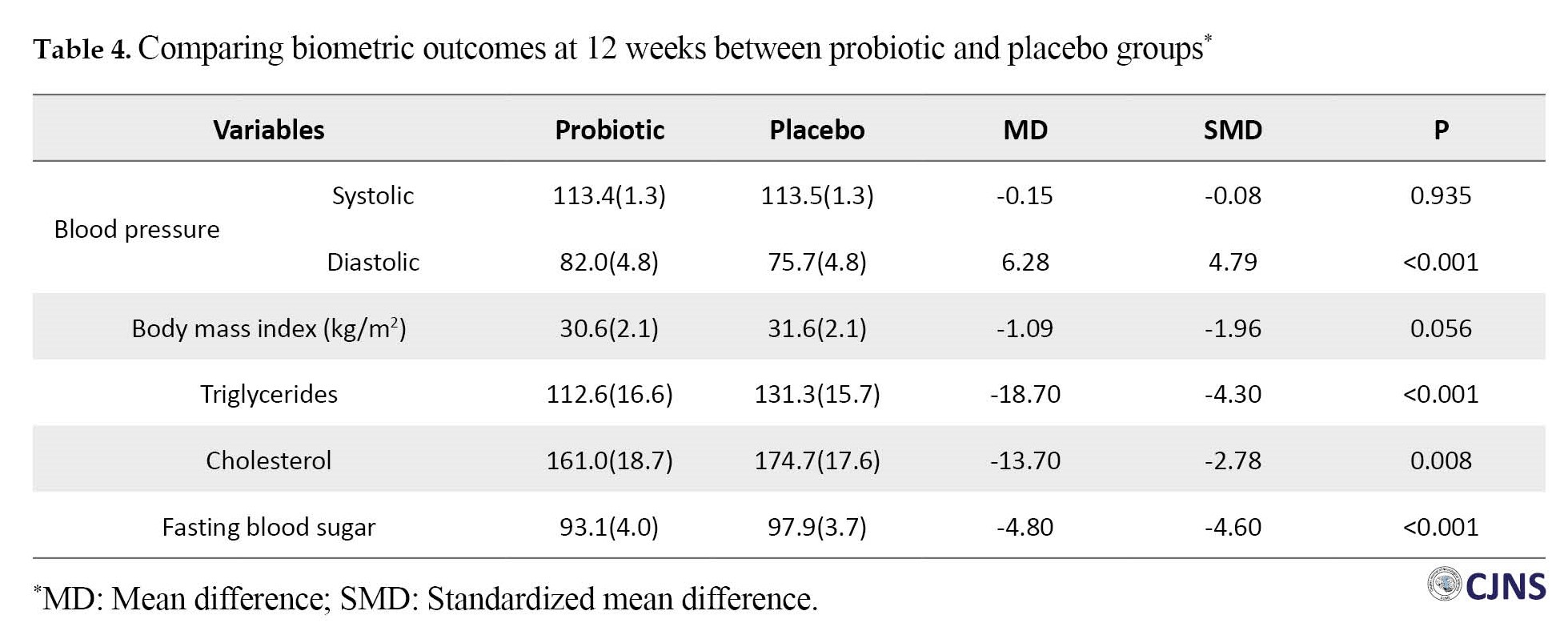
Post-hoc analysis
The Mean±SD weight of patients in the probiotics and placebo groups was 81.9(9.1) kg and 85.1(11.0) kg, respectively. Patients in the probiotic group gained 7.9 kg, while those in the placebo group gained 10.6 kg. The difference in weight between the two groups was not significant (P=0.08). We identified individuals who experienced a weight gain of 7% or more [29]. In the first three months, 62.5% of individuals in the olanzapine group and 70.0% in the risperidone group gained weight by 7% or more from baseline in the probiotic group. However, the corresponding values in the placebo group were 100.0% and 66.7%, respectively.
Discussion
Antipsychotic medication is associated with side effects, including metabolic dysregulation, constipation, and cognitive impairment. Probiotics have been investigated as a potential intervention to prevent or reduce these side effects with several studies reporting promising results. A recent meta-analysis showed the effects of probiotic supplementation in several psychiatric disorders [30]. However, it is uncertain whether probiotics improve clinical symptoms in SZ patients. We could not observe any significant effects of the intervention on BPRS and PANSS (effect size of 0.02 for BPRS and <0.001 for PANSS). This result was consistent with previous studies [16, 31]. Dickerson treated SZ patients with probiotic or placebo supplementation in a randomized controlled trial. The researchers did not observe a significant difference in psychiatric symptom scores between probiotic and placebo-supplemented groups [16]. Similarly, two subsequent studies reported no significant difference in PANSS scores in probiotic supplementation [18, 19].
Contrary to these studies, Ghaderi et al. [17] observed an improvement in general and total PANSS scores in patients with chronic schizophrenia after taking probiotics and vitamin D for 12 weeks. However, it had no effects on negative and positive subscales and BPRS scores. The current disagreements could be explained through different doses of probiotics, various study designs, and participants’ diverse characteristics. We used an add-on design where all patients received standard therapy with either probiotics or placebo. Although this design is common in therapy trials for different diseases, such studies are not directly informative about a drug as monotherapy. Therefore, a larger research may need to be conducted to find a significant difference.
The present trial showed the positive effects of probiotic supplementation on weight, FBS, and lipid profile (metabolic indices). In line with the present study, Ghaderi et al. [17] reported the beneficial effects of vitamin D and probiotics on lipid profiles and glycemic control in chronic schizophrenia. Kang et al. [32] conducted an RCT to assess the efficacy and safety of probiotic supplements in mitigating antipsychotic-induced metabolic disturbance and increased body weight. Drug-naïve first-episode schizophrenia patients were randomly assigned to receive olanzapine plus probiotics or olanzapine alone. After 12 weeks of treatment with the addition of probiotics, the researchers found a nominal level of significant differences in BMI and body weight between treatments. Still, these changes became non-significant after adjusting for increased appetite. Lipid profiles (triglycerides, total cholesterol, high-density lipoprotein, and low-density lipoprotein) significantly increased in both groups (P<0.0001). However, only total cholesterol shows a significant difference between the two treatment groups (P=0.028). Another study [31] compared the effects of probiotics and dietary fiber on weight gain and metabolic disturbances in drug-naïve, first-episode schizophrenia patients receiving olanzapine. After 12 weeks, FBS and lipid profiles (except for HDL-C) showed no significant difference between the two groups. Huang et al. [33] assessed the effects of probiotics and dietary fiber alone or in combination on weight gain due to atypical antipsychotics in patients with schizophrenia or bipolar disorder. The study found that probiotics plus dietary fiber significantly reduced weight and prevented further deterioration of metabolic disturbances, and probiotics or dietary fiber alone could prevent further weight gain. Huang et al. [34] conducted two sequential, randomized clinical trials and reported that probiotics plus dietary fiber could reduce weight gain in drug-naïve, first-episode schizophrenia patients receiving antipsychotic drugs. The first study showed insignificant differences in weight gain between the olanzapine plus probiotics group and the olanzapine alone group at week 12. In the second study, the probiotics plus dietary fiber group gained less weight than the olanzapine alone group at week 12. The authors also found that probiotic supplementation significantly improved cognitive function and lowered FBS levels in these patients.
A recent study in rats indicated that co-administration of antibiotics ameliorated impairment in the microbiota and metabolic disorders due to olanzapine, including weight gain, visceral fat deposition, increased plasma-free fatty acids, and macrophage infiltration into adipose tissue [35]. Zhai et al. [36] indicated an early-onset nature of HDL-C abnormalities in drug-naïve first-episode schizophrenia patients. After an average of 22.7 days of antipsychotic exposure, lipid abnormalities, and insulin resistance markers were significantly elevated. Moreover, results of the recovery after an initial schizophrenia episode (RAISE) study [37] showed that after an average of 47.3 days of antipsychotic treatment, 48.3% of subjects were obese or overweight, 56.5% had dyslipidemia, 10.0% had hypertension, and 13.2% had metabolic syndrome. Therefore, improving metabolic indices in the probiotic group could be important, and the microbiota may be a novel approach for treating metabolic comorbidity in patients with schizophrenia.
Conclusions
We found no significant group effect on clinical psychiatric symptoms (BPRS and PANSS) in patients in the first episode of psychosis. Exploratory analyses have shown significant positive effects of probiotics on triglyceride, cholesterol, and FBS levels. Probiotics may have potential benefits in preventing or reducing antipsychotic side effects, particularly metabolic dysregulation. Therefore, an add-on probiotic strategy may be considered in patients with schizophrenia.
Study limitations
This study had some limitations. First, we did not control for non-pharmacological interventions prescribed to reduce antipsychotic-induced weight gain. Dietary counselling, exercise interventions, cognitive and behavioural strategies could have significant positive effects on weight loss, waist circumference, triglycerides, fasting blood sugar and insulin [38]. Secondly, as this was a 12-week trial, the beneficial effects of probiotic supplements on clinical signs of schizophrenia might be observed in studies with longer durations. The short study period may not have allowed sufficient time for intervention to impact outcomes. It is necessary for a long-term, confirmatory study to reinforce the current analyses. Thirdly, CGI-I has been considered a secondary objective in this study, thus our finding about this variable is exploratory and should be confirmed by more studies. Fourthly, the beneficial effects of probiotic supplements on cardiovascular risk factors are exploratory findings and our study was not designed to determine these effects. This must be tested in RCTs with acceptable power. Lastly, risperidone or olanzapine have different receptor profiles and consequently exhibit distinct adverse metabolic and endocrine effects, although the number of patients taking each medications did not show a significant difference.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were done in compliance with the ethical guidelines of the Declaration of Helsinki 2013. The trial was registered at the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20120603009934N2). The protocol was approved by the Ethics Committee of Guilan University of Medical Sciences (GUMS), Iran (Code: IR.GUMS.REC.1397.456). All subjects or their legal guardians signed informed consent before the start of the study.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization and methodology, writing, review, and editing: All authors; Investigation and writing the original draft: Seyede Melika Jalali; Resources and supervision: Robabeh Soleimani and Mir Mohammad Jalali.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank all the individuals who participated in the study.
References


Schizophrenia (SZ) is a chronic and serious psychiatric disorder. Its prevalence in the world population is about 1% [1]. The disease is characterized by positive symptoms, negative symptoms, and cognitive dysfunction, the latter two being particularly resistant to antipsychotics [2]. Cognitive impairment and negative symptoms present challenges in managing SZ [3, 4, 5, 6]. Therefore, it is necessary to regularly evaluate new combinations of nutritional and pharmacological therapy for these symptoms. Then again, antipsychotic medications are associated with cardiometabolic adverse effects, including weight gain, dyslipidemia, insulin resistance or frank type 2 diabetes mellitus, and hypertension [7].
Research into the pathogenesis of schizophrenia has pointed to a potential role for the human microbiome and the gut-brain axis, which involves bidirectional communication between the central and enteric nervous systems [8]. Disturbed gut microbiota is linked to increased systemic inflammation [9], and consequent neural inflammation may be directly related to schizophrenia [10].
Preclinical studies have consistently demonstrated that fecal microbiota transplantation from patients with different psychiatric disorders leads to the development of the behavioral and physiological profiles of the disease in germ-free mice [11-15]. In this regard, 4 studies [16-19] have examined the relationship between schizophrenia and probiotics and found that probiotic supplementation reduced the positive and negative syndrome scale (PANSS) scores but not significantly. On the other hand, evidence suggests that probiotics can reduce constipation [16, 20], weight gain, and metabolic side effects [21] commonly associated with antipsychotic use. Probiotics may improve metabolic parameters through anti-inflammatory and insulin-resistance properties [22]. Dickerson et al. [16] showed that probiotic supplementation for 14 weeks can prevent common physical symptoms in patients with SZ but have no effects on the clinical syndrome scale.
Despite the high prevalence of dyslipidemia in schizophrenia and the increased risk of metabolic complications and cardiovascular disease, the focus in this population is primarily on managing psychotic symptoms, and their physical health is generally overlooked.
To date, few studies have examined the lessening of metabolic impairments in patients with SZ, and the evidence regarding the effects of probiotics on metabolic status is insufficient. Research on schizophrenia needs more randomized clinical trials (RCTs) to provide sufficient proof, draw reasonable conclusions, and consequently determine the effect of probiotic supplements on the clinical symptoms and metabolic status of patients with SZ [23]. This study aimed to assess the impact of probiotic supplementation on improving clinical signs and adverse metabolic effects commonly observed in patients with first-episode schizophrenia receiving antipsychotics.
Materials and Methods
Study patients
From March 2019 to June 2021, a randomized, double-blind, placebo-controlled trial was conducted at Shafa Hospital, Rasht City, Iran. This study included inpatients from the Psychiatric Department who met the eligibility criteria: 1) Aged between 18 and 60 years, 2) First-episode schizophrenia diagnosis according to the DSM-5, and 3) Clinically stable for 4 weeks or more. Clinical stability was defined as 1) No change in oral antipsychotics (olanzapine or risperidone) dosage, 2) Clinical global impressions-improvement of illness (CGI-S) score ≤4, and 3) Positive symptom score ≤4 in brief psychiatric rating scale (BPRS). The exclusion criteria were as follows: 1) Duration of positive symptoms exceeding two years, 2) Diagnosis of serious neurologic or cardiovascular disease, 3) Using any drug for weight loss, 4) A history of substance use or alcohol in the last three months (except nicotine or caffeine), 5) Receiving antibiotics for any reason in the last two weeks, 6) Mental retardation (intelligent quotient of <70), and 7) Pregnancy.
Study design
After enrollment in the clinical study, the patients were treated on an outpatient basis. All patients received probiotic supplements (FamiLact capsule, Zist Takhmir Co., Tehran, Iran) or a placebo once daily. FamiLact capsules (500 mg) contain gram-positive organisms and 38.5 mg Fructooligosaccharides. The microorganisms are 9×109 colony-forming units (CFU)/g of viable, lyophilized Lactobacilli (Lactobacillus Acidophilus, Lactobacillus Casei, Lactobacillus Delbrueckii Subsp. Lactobacillus Bulgaricus, and Lactobacillus Rhamnosus), 1.25×1010 of Bifidobacteria (Bifidobacterium Longum and Bifidobacterium Breve), and 1.5×1010 of Streptococcus Salivarius Subsp. Thermophilus.
Randomization and blinding
Patients who met the inclusion criteria were randomly assigned to probiotic and placebo groups (equal numbers) for 12 weeks. Randomization and blinding were performed by an independent pharmacist using a sealed coded envelope. Block randomization (size 4) was performed using a computer-generated random allocation sequence. The probiotic supplement and placebo were indistinguishable in appearance and content.
Outcome measures
The primary endpoints were assessing clinical symptoms using BPRS and PANSS. Secondary endpoints included the severity of psychiatric symptoms using CGI-S, clinical response, and biometric and biochemical measures. Clinical response was defined as a ≥25% reduction in BPRS score after 3 months. Biometric and biochemical variables included blood pressure (BP), body mass index (BMI), triglycerides (TG), total cholesterol, and fasting blood sugar (FBS). Lipid profiles and FBS were measured using an enzymatic kit (Pars Azmun, Tehran, Iran) with intra-assay CVs of less than 5%. Primary objectives were measured at baseline and then 4, 8, and 12 weeks after the study started. All secondary objectives were assessed at baseline and 12 weeks later.
Sample size
The required sample size was computed using the Stata statistical software, version 14 (StataCorp LP, College Station, TX, USA). Leucht et al. [24] demonstrated a 25% BPRS score reduction as “minimally improved.” According to the included trials in this study, the standard deviation of the BPRS total score at baseline was 12.2. In addition, Leddy-Stacy and Rosenheck [25] suggested the minimum clinically important difference for the PANSS to be between 4.25 and 8.30 total points. From a previous study [18], we estimated the Mean±SD difference between active intervention and placebo group to be 70.9±11.4. Furthermore, we supposed that a 5-point difference in PANSS between active intervention and placebo would be clinically significant. Using analysis of covariance (ANCOVA) with a correlation coefficient of 0.7, 80% power, and 95% confidence at three measurement points, a total of 52 participants would be required. Accounting for a 20% loss, 62 participants (31 per arm) would be sufficient to estimate effect sizes for each of the two primary objectives.
Statistical analyses
We presented quantitative variables with Mean±SD and categorical variables with absolute frequencies and percentages. Data normality was assessed with the Shapiro-Wilk test. Repeated measures of analysis of variance (ANOVA) were used to analyze the effect of probiotics on primary objectives (BPRS and PANSS). Partial Eta squared (η2) was used to calculate effect sizes for significant main effects, with the following standards to determine small (0.01-0.059), medium (0.06-0.139), and large (>0.14) effect sizes.
Secondary analyses were exploratory without formal sample size estimation. The clinical response variable was considered a categorical variable. A chi-squared test was performed to assess the effect of probiotics on the clinical response. The chi-squared test compared the proportion of patients with a CGI-S score of 1 or 2 (very much or much improved). Other secondary objectives were analyzed using analysis of variance/covariance (ANOVA/ANCOVA) after adjusting for the baseline levels of variables. We presented effect sizes as standardized mean differences (Cohen’s d) or risk ratios (RR) with 95% confidence intervals (95% CI).
Data were analyzed according to the intention-to-treat principle using the Stata statistical software. To handle missing data, we used simple mean imputation [26]. Also, we used subgroup analysis, comparing the baseline characteristics between completers and dropouts. Statistical tests were two-tailed. P≤0.05 were considered to indicate statistical significance.
Results
Of 62 enrolled patients, 55(88.7%) completed the study. There were no significant differences in baseline characteristics between 7 patients who dropped out at week 8 or 12 and completers (Supplementary Table 1).

Figure 1 depicts the flow of patients’ enrolment through the study according to the consolidated reporting trial standards (CONSORT) statement. Table 1 presents the patients’ characteristics at baseline.

None of the variables with a P<0.05 were strong confounders for primary and secondary objectives [27, 28].
Primary outcomes
Clinical psychiatric symptoms (BPRS and PANSS) improved in both groups at 12 weeks (Table 2), and repeated measure ANOVA revealed a significant effect of time on these outcomes with medium effect sizes (Figure 2, Figure 3).
 The mean BPRS score was reduced from 44.4 to 29.4 in the probiotic group and 45.4 to 32.6 in the placebo group. However, the results indicated no significant group effect on clinical symptoms (P=0.296).
The mean BPRS score was reduced from 44.4 to 29.4 in the probiotic group and 45.4 to 32.6 in the placebo group. However, the results indicated no significant group effect on clinical symptoms (P=0.296). The mean total score of PANSS was reduced by 26.3, from 64.7 at the baseline to 38.4, after receiving antipsychotic drugs and probiotics for 12 weeks and by 21.2, from 61.45 at the baseline to 40.3, in the placebo group. Also, we observed no significant difference in PANSS score improvement between the two groups (P=0.995). Similar trends were also observed for all subscales of the PANSS (Supplementary Table 1). Mean differences of primary objectives at varying times and results of repeated measure ANOVA could be observed in Table 3.

In addition, we calculated effect sizes using Cohen’s d formula to examine the extent of treatment effects within the group. Effect sizes were classified as small (0.20–0.49), medium (0.50–0.79), and large (0.80 and more). Large effect sizes were observed in the BPRS and the PANSS in the probiotic group at 4 weeks (0.87 and 1.52), at 8 weeks (1.47 and 2.30), and at 12 weeks (2.02 and 2.73).
Secondary outcomes
Clinical responses in the probiotic and placebo groups were observed in 11 patients (39.3%) and 15 patients (55.6%), respectively (RR: 0.72; 95% CI, 0.42%-1.24%; X2=1.46; P=0.23). In terms of CGI-S, a score of 1 or 2 was observed in 19 patients (54.3%) in the probiotic group and 16 patients (45.7%) in the placebo group (RR: 1.20; 95% CI, 0.70%-2.06%; X2=0.44; P=0.51). Adjusting for baseline values, the systolic blood pressure and BMI were similar in the two groups. The mean diastolic blood pressure was 82.1(5.2) mm Hg with probiotics and 75.6(4.6) mm Hg with placebo (Cohen’s d: 4.79; 95% CI, 2.78%-6.80%; P<0.001). Interestingly, statistical analyses showed lower levels of all three biochemical variables (FBS, triglyceride, and cholesterol) in the probiotic group compared to the placebo group (P<0.05). The Cohen’s d for these biochemical variables were 0.70, 1.57, and 0.68, respectively. The magnitude of these values was interpreted as a medium-large effect size. Table 4 shows the results of ANOVA/ANCOVA analyses in detail.

Post-hoc analysis
The Mean±SD weight of patients in the probiotics and placebo groups was 81.9(9.1) kg and 85.1(11.0) kg, respectively. Patients in the probiotic group gained 7.9 kg, while those in the placebo group gained 10.6 kg. The difference in weight between the two groups was not significant (P=0.08). We identified individuals who experienced a weight gain of 7% or more [29]. In the first three months, 62.5% of individuals in the olanzapine group and 70.0% in the risperidone group gained weight by 7% or more from baseline in the probiotic group. However, the corresponding values in the placebo group were 100.0% and 66.7%, respectively.
Discussion
Antipsychotic medication is associated with side effects, including metabolic dysregulation, constipation, and cognitive impairment. Probiotics have been investigated as a potential intervention to prevent or reduce these side effects with several studies reporting promising results. A recent meta-analysis showed the effects of probiotic supplementation in several psychiatric disorders [30]. However, it is uncertain whether probiotics improve clinical symptoms in SZ patients. We could not observe any significant effects of the intervention on BPRS and PANSS (effect size of 0.02 for BPRS and <0.001 for PANSS). This result was consistent with previous studies [16, 31]. Dickerson treated SZ patients with probiotic or placebo supplementation in a randomized controlled trial. The researchers did not observe a significant difference in psychiatric symptom scores between probiotic and placebo-supplemented groups [16]. Similarly, two subsequent studies reported no significant difference in PANSS scores in probiotic supplementation [18, 19].
Contrary to these studies, Ghaderi et al. [17] observed an improvement in general and total PANSS scores in patients with chronic schizophrenia after taking probiotics and vitamin D for 12 weeks. However, it had no effects on negative and positive subscales and BPRS scores. The current disagreements could be explained through different doses of probiotics, various study designs, and participants’ diverse characteristics. We used an add-on design where all patients received standard therapy with either probiotics or placebo. Although this design is common in therapy trials for different diseases, such studies are not directly informative about a drug as monotherapy. Therefore, a larger research may need to be conducted to find a significant difference.
The present trial showed the positive effects of probiotic supplementation on weight, FBS, and lipid profile (metabolic indices). In line with the present study, Ghaderi et al. [17] reported the beneficial effects of vitamin D and probiotics on lipid profiles and glycemic control in chronic schizophrenia. Kang et al. [32] conducted an RCT to assess the efficacy and safety of probiotic supplements in mitigating antipsychotic-induced metabolic disturbance and increased body weight. Drug-naïve first-episode schizophrenia patients were randomly assigned to receive olanzapine plus probiotics or olanzapine alone. After 12 weeks of treatment with the addition of probiotics, the researchers found a nominal level of significant differences in BMI and body weight between treatments. Still, these changes became non-significant after adjusting for increased appetite. Lipid profiles (triglycerides, total cholesterol, high-density lipoprotein, and low-density lipoprotein) significantly increased in both groups (P<0.0001). However, only total cholesterol shows a significant difference between the two treatment groups (P=0.028). Another study [31] compared the effects of probiotics and dietary fiber on weight gain and metabolic disturbances in drug-naïve, first-episode schizophrenia patients receiving olanzapine. After 12 weeks, FBS and lipid profiles (except for HDL-C) showed no significant difference between the two groups. Huang et al. [33] assessed the effects of probiotics and dietary fiber alone or in combination on weight gain due to atypical antipsychotics in patients with schizophrenia or bipolar disorder. The study found that probiotics plus dietary fiber significantly reduced weight and prevented further deterioration of metabolic disturbances, and probiotics or dietary fiber alone could prevent further weight gain. Huang et al. [34] conducted two sequential, randomized clinical trials and reported that probiotics plus dietary fiber could reduce weight gain in drug-naïve, first-episode schizophrenia patients receiving antipsychotic drugs. The first study showed insignificant differences in weight gain between the olanzapine plus probiotics group and the olanzapine alone group at week 12. In the second study, the probiotics plus dietary fiber group gained less weight than the olanzapine alone group at week 12. The authors also found that probiotic supplementation significantly improved cognitive function and lowered FBS levels in these patients.
A recent study in rats indicated that co-administration of antibiotics ameliorated impairment in the microbiota and metabolic disorders due to olanzapine, including weight gain, visceral fat deposition, increased plasma-free fatty acids, and macrophage infiltration into adipose tissue [35]. Zhai et al. [36] indicated an early-onset nature of HDL-C abnormalities in drug-naïve first-episode schizophrenia patients. After an average of 22.7 days of antipsychotic exposure, lipid abnormalities, and insulin resistance markers were significantly elevated. Moreover, results of the recovery after an initial schizophrenia episode (RAISE) study [37] showed that after an average of 47.3 days of antipsychotic treatment, 48.3% of subjects were obese or overweight, 56.5% had dyslipidemia, 10.0% had hypertension, and 13.2% had metabolic syndrome. Therefore, improving metabolic indices in the probiotic group could be important, and the microbiota may be a novel approach for treating metabolic comorbidity in patients with schizophrenia.
Conclusions
We found no significant group effect on clinical psychiatric symptoms (BPRS and PANSS) in patients in the first episode of psychosis. Exploratory analyses have shown significant positive effects of probiotics on triglyceride, cholesterol, and FBS levels. Probiotics may have potential benefits in preventing or reducing antipsychotic side effects, particularly metabolic dysregulation. Therefore, an add-on probiotic strategy may be considered in patients with schizophrenia.
Study limitations
This study had some limitations. First, we did not control for non-pharmacological interventions prescribed to reduce antipsychotic-induced weight gain. Dietary counselling, exercise interventions, cognitive and behavioural strategies could have significant positive effects on weight loss, waist circumference, triglycerides, fasting blood sugar and insulin [38]. Secondly, as this was a 12-week trial, the beneficial effects of probiotic supplements on clinical signs of schizophrenia might be observed in studies with longer durations. The short study period may not have allowed sufficient time for intervention to impact outcomes. It is necessary for a long-term, confirmatory study to reinforce the current analyses. Thirdly, CGI-I has been considered a secondary objective in this study, thus our finding about this variable is exploratory and should be confirmed by more studies. Fourthly, the beneficial effects of probiotic supplements on cardiovascular risk factors are exploratory findings and our study was not designed to determine these effects. This must be tested in RCTs with acceptable power. Lastly, risperidone or olanzapine have different receptor profiles and consequently exhibit distinct adverse metabolic and endocrine effects, although the number of patients taking each medications did not show a significant difference.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were done in compliance with the ethical guidelines of the Declaration of Helsinki 2013. The trial was registered at the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20120603009934N2). The protocol was approved by the Ethics Committee of Guilan University of Medical Sciences (GUMS), Iran (Code: IR.GUMS.REC.1397.456). All subjects or their legal guardians signed informed consent before the start of the study.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization and methodology, writing, review, and editing: All authors; Investigation and writing the original draft: Seyede Melika Jalali; Resources and supervision: Robabeh Soleimani and Mir Mohammad Jalali.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank all the individuals who participated in the study.
References
- Seeman MV. Symptoms of schizophrenia: Normal adaptations to inability. Med Hypotheses. 2007; 69(2):253-7. [DOI:10.1016/j.mehy.2006.12.028] [PMID]
- Rabinowitz J, Levine SZ, Garibaldi G, Bugarski-Kirola D, Berardo CG, Kapur S. Negative symptoms have a greater impact on functioning than positive symptoms in schizophrenia: Analysis of CATIE data.Schizophr Res. 2012; 137(1-3):147-50. [DOI:10.1016/j.schres.2012.01.015] [PMID]
- Kaneko K. Negative symptoms and cognitive impairments in schizophrenia: Two key symptoms negatively influencing social functioning. Yonago Acta Med. 2018; 61(2):091-102. [DOI:10.33160/yam.2018.06.001] [PMID] [PMCID]
- MacKenzie NE, Kowalchuk C, Agarwal SM, Costa-Dookhan KA, Caravaggio F, Gerretsen P, et al. Antipsychotics, metabolic adverse effects, and cognitive function in schizophrenia.Front Psychiatry. 2018; 9:622. [DOI:10.3389/fpsyt.2018.00622] [PMID] [PMCID]
- Hamer S, Haddad PM. Adverse effects of antipsychotics as outcome measures. Br J Psychiatry Suppl. 2007; 50:s64-70. [DOI:10.1192/bjp.191.50.s64] [PMID]
- Lewis R. Should cognitive deficit be a diagnostic criterion for schizophrenia? J Psychiatry Neurosci. 2004; 29(2):102-13. [PMID]
- Abosi O, Lopes S, Schmitz S, Fiedorowicz JG. Cardiometabolic effects of psychotropic medications. Horm Mol Biol Clin Investig. 2018; 36(1):/j/hmbci.2018.36.issue-1/hmbci-2017-0065/hmbci-2017-0065.xml. [DOI:10.1515/hmbci-2017-0065] [PMID] [PMCID]
- Nemani K, Hosseini Ghomi R, McCormick B, Fan X. Schizophrenia and the gut-brain axis. Prog Neuropsychopharmacol Biol Psychiatry. 2015; 56:155-60. [DOI:10.1016/j.pnpbp.2014.08.018] [PMID]
- Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways.Mol Psychiatry. 2016; 21(6):738-48. [DOI:10.1038/mp.2016.50] [PMID] [PMCID]
- Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014; 48:277-86. [DOI:10.1016/j.pnpbp.2012.10.022] [PMID]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016; 21(6):786-96. [DOI:10.1038/mp.2016.44] [PMID]
- Kelly JR, Borre Y, O’Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016; 82:109-18. [DOI:10.1016/j.jpsychires.2016.07.019] [PMID]
- Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry. 2020; 25(11):2905-18. [DOI:10.1038/s41380-019-0475-4] [PMID]
- Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y, et al. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress. 2019; 22(5):592-602. [DOI:10.1080/10253890.2019.1617267] [PMID]
- Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019; 177(6):1600-18. e17. [DOI:10.1016/j.cell.2019.05.004] [PMID] [PMCID]
- Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CL, Schweinfurth LA, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: A randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014; 16(1):PCC.13m01579. [DOI:10.4088/PCC.13m01579] [PMID] [PMCID]
- Ghaderi A, Banafshe HR, Mirhosseini N, Moradi M, Karimi MA, Mehrzad F, et al. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry. 2019; 19(1):77. [DOI:10.1186/s12888-019-2059-x] [PMID] [PMCID]
- Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, et al. Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain Behav Immun. 2017; 62:41-5. [DOI:10.1016/j.bbi.2016.11.019] [PMID] [PMCID]
- Tomasik J, Yolken RH, Bahn S, Dickerson FB. Immunomodulatory effects of probiotic supplementation in schizophrenia patients: A randomized, placebo-controlled trial. Biomark Insights. 2015; 10:47-54. [DOI:10.4137/BMI.S22007] [PMID] [PMCID]
- Soh AYS, Kang JY, Siah KTH, Scarpignato C, Gwee KA. Searching for a definition for pharmacologically refractory constipation: A systematic review. J Gastroenterol Hepatol. 2018; 33(3):564-75. [DOI:10.1111/jgh.13998] [PMID]
- Sayon-Orea C, Martínez-González MA, Ruiz-Canela M, Bes-Rastrollo M. Associations between yogurt consumption and weight gain and risk of obesity and metabolic syndrome: A systematic review. Adv Nutr. 2017; 8(1):146S-54S. [DOI:10.3945/an.115.011536] [PMID] [PMCID]
- Ghoneim MA, Moselhy SS. Antioxidant status and hormonal profile reflected by experimental feeding of probiotics. Toxicol Ind Health. 2016; 32(4):741-50. [DOI:10.1177/0748233713506768] [PMID]
- Zagórska A, Marcinkowska M, Jamrozik M, Wiśniowska B, Paśko P. From probiotics to psychobiotics-the gut-brain axis in psychiatric disorders. Benef Microbes. 2020; 11(8):717-32. [DOI:10.3920/BM2020.0063] [PMID]
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. Br J Psychiatry. 2005; 187:366-71. [DOI:10.1192/bjp.187.4.366] [PMID]
- Leddy-Stacy MA, Rosenheck R. Obtaining employment as an anchor for estimating the minimum clinically important difference on the Positive and Negative Syndrome Scale (PANSS) in schizophrenia. Psychiatry Res. 2016; 238:304-9. [DOI:10.1016/j.psychres.2016.02.018] [PMID]
- Dziura JD, Post LA, Zhao Q, Fu Z, Peduzzi P. Strategies for dealing with missing data in clinical trials: From design to analysis. Yale J Biol Med. 2013; 86(3):343-58. [PMID]
- Basson BR, Kinon BJ, Taylor CC, Szymanski KA, Gilmore JA, Tollefson GD. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. J Clin Psychiatry. 2001; 62(4):62(4):231-8. [DOI:10.4088/JCP.v62n0404] [PMID]
- Chen YL, Chen KP, Chiu CC, Tai MH, Lung FW. Early predictors of poor treatment response in patients with schizophrenia treated with atypical antipsychotics. BMC Psychiatry. 2018; 18(1):376. [DOI:10.1186/s12888-018-1950-1] [PMID] [PMCID]
- De Hert M, Yu W, Detraux J, Sweers K, van Winkel R, Correll CU. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: A systematic review and exploratory meta-analysis. CNS Drugs. 2012; 26(9):733-59. [DOI:10.2165/11634500-000000000-00000] [PMID]
- Ng QX, Soh AYS, Venkatanarayanan N, Ho CYX, Lim DY, Yeo WS. A systematic review of the effect of probiotic supplementation on schizophrenia symptoms. Neuropsychobiology. 2019; 78(1):1-6. [DOI:10.1159/000498862] [PMID]
- Huang J, Kang D, Zhang F, Yang Y, Liu C, Xiao J, et al. Probiotics plus dietary fiber supplements attenuate olanzapine-induced weight gain in drug-naïve first-episode schizophrenia patients: Two randomized clinical trials. Schizophr Bull. 2022; 48(4):850-9. [DOI:10.1093/schbul/sbac044] [PMID] [PMCID]
- Kang D, Zhang F, Yang Y, Liu C, Xiao J, Long Y, et al. Probiotic supplements reduce antipsychotic-induced metabolic disturbances in drug-naive first-episode schizophrenia. medRxiv. 2021. [Unpublished]. [DOI:10.1101/2021.02.16.21251872]
- Huang J, Liu C, Yang Y, Kang D, Xiao J, Long Y, et al. The effects of probiotics plus dietary fiber on antipsychotic-induced weight gain: A randomized clinical trial. Transl Psychiatry. 2022; 12(1):185. [DOI:10.1038/s41398-022-01958-2] [PMID] [PMCID]
- Huang J, Kang D, Zhang F, Yang Y, Liu C, Xiao J, et al. Probiotics plus dietary fiber supplements attenuate olanzapine-induced weight gain in drug-naïve first-episode schizophrenia patients: Two randomized clinical trials. Schizophr Bull. 2022; 48(4):850-9. [DOI:10.1093/schbul/sbac044] [PMID] [PMCID]
- Davey KJ, Cotter PD, O’Sullivan O, Crispie F, Dinan TG, Cryan JF, et al. Antipsychotics and the gut microbiome: Olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry. 2013; 3(10):e309. [DOI:10.1038/tp.2013.83] [PMID] [PMCID]
- Zhai D, Cui T, Xu Y, Feng Y, Wang X, Yang Y, et al. Cardiometabolic risk in first-episode schizophrenia (FES) patients with the earliest stages of both illness and antipsychotic treatment. Schizophr Res. 2017; 179:41-9. [DOI:10.1016/j.schres.2016.09.001] [PMID]
- Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: Baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014; 71(12):1350-63. [DOI:10.1001/jamapsychiatry.2014.1314] [PMID]
- Bruins J, Jörg F, Bruggeman R, Slooff C, Corpeleijn E, Pijnenborg M. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: A meta-analysis. PloS One. 2014; 9(12):e112276. [DOI:10.1371/journal.pone.0112276] [PMID] [PMCID]



Type of Study: Research |
Subject:
Special
Received: 2023/10/11 | Accepted: 2023/10/17 | Published: 2023/10/17
Received: 2023/10/11 | Accepted: 2023/10/17 | Published: 2023/10/17
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |