Sat, May 18, 2024
Volume 9, Issue 4 (Autumn 2023)
Caspian J Neurol Sci 2023, 9(4): 220-228 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rezaei M, Fakhri N, Afshari D, Khazaei M. Comparing the Effectiveness and Complications of Sodae and Sodium Valproate on Migraine. Caspian J Neurol Sci 2023; 9 (4) :220-228
URL: http://cjns.gums.ac.ir/article-1-664-en.html
URL: http://cjns.gums.ac.ir/article-1-664-en.html
1- Department of Biostatistics, Social Development and Health Promotion Research Center, School of Health, Research Institute for Health, Kermanshah University of Medical Sciences, Kermanshah, Iran
2- Student’s Research Committee, School of Health, Kermanshah University of Medical Sciences, Kermanshah, Iran
3- Department of Neurology, Imam Reza Hospital, School of Medicine, Kermanshah University of Medical Science, Kermanshah, Iran
4- Department of Neurology, Sina (Farshchian) Educational and Medical Center, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
2- Student’s Research Committee, School of Health, Kermanshah University of Medical Sciences, Kermanshah, Iran
3- Department of Neurology, Imam Reza Hospital, School of Medicine, Kermanshah University of Medical Science, Kermanshah, Iran
4- Department of Neurology, Sina (Farshchian) Educational and Medical Center, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
Full-Text [PDF 1338 kb]
(161 Downloads)
| Abstract (HTML) (369 Views)
Full-Text: (110 Views)
Introduction
As a primary headache disorder with significant pain, migraine is listed by the World Health Organization (WHO) among the 20 debilitating diseases [1, 2]. A migraine headache is characterized by moderate to severe throbbing pain, usually occurring on one side of the head, which is often accompanied by nausea, vomiting, photophobia, and phonophobia, all of which are usually aggravated by physical activity, according to the International Headache Society [3]. In Iran, migraine prevalence is estimated to be 14%, which is similar to or even higher than all over the world [4]. Migraine prevalence rates in Iran are 12.5% for women and 8.4% for men [5, 6]. The antiepileptic drug sodium valproate has been recommended as the first line of treatment for preventing migraine attacks since 1970 [7]. Sodium valproate is an effective migraine prevention treatment in many studies [8-12]. The effectiveness of sodium valproate in reducing migraine attack frequency, intensity, and maximum pain levels has been proven in numerous studies [9, 12]. Still, side effects such as fatigue, dizziness, nausea, tremors, and weight gain have led to poor compliance with the treatment [13, 14]. There have been reports of drowsiness, increased appetite, weight gain, insomnia, and dizziness associated with sodium valproate use [15-18]. Various studies investigated the use of herbal drugs in migraine prevention in addition to chemical medications [19-22]. In recent years, herbal medicines have been proposed as a promising strategy for the prevention and treatment of migraine with analgesic activity and minor side effects [23]. Plants such as feverfew (Tanacetum parthenium), butterbur (Petasites hybridus), marijuana (Cannabis spp.), Saint John’s Wort (Hypericum perforatum), and the Damask rose (Rosa+Damascena) have acceptable mechanisms of action and evidence for the treating migraine. Because of the widespread use of and the high tendency to use traditional medicinal plants, more extensive research should be designed in several areas of pharmacy and pharmacology of medicinal plants to gain proper information for pharmaceutical industries [24].
One of the herbal medicines in the treatment of migraine is the sodae, made by the Booali Daroo pharmaceutical company. The main components of this product with their scientific names are Turpethum, Commiphora Mukul, Rheum Officinale, and Terminalia Chebul, and the common names of these components are Indian jalap, Bdellium, Rhubarb, and Chebulic myrobalan. The sodae capsule has laxative effects, which cleanse the body and digestive tract of additives and toxins and create a lighter feeling in the head.
There was a clinical trial in Kermanshah City, Iran, in 2020 comparing sodae herbal capsule and placebo, and the results indicated a 65% recovery rate in the herbal medicine group and a 44% recovery rate in the placebo group, and the headache herbal medicine was recommended as a migraine prevention medicine [25]. Although no serious side effects were reported in this study, diarrhea and stomachache were the most common side effects. It seems necessary to examine this drug more closely regarding side effects and compare it with other migraine prevention drugs. Since no other study has been conducted, we embarked on comparing sodium valproate and sodae herbal headache medicine for their effectiveness and side effects.
Materials and Methods
Study design and research community
A randomized, double-blind, two-center clinical trial was conducted in Kermanshah and Hamadan cities, Iran, between December 2021 and July 2022. A co-investigator explained the study’s purpose to patients with episodic migraines who met the inclusion and exclusion criteria. A total of 96 migraine patients without aura participated in the study. The inclusion criteria for participants included those aged 18 to 65, more than 3 attacks per month in the past three months, migraine onset one year before the study, and migraine onset before 50. Non-entry criteria included not taking sodium valproate, risk factors for heart diseases (endothelial dysfunction), cardiac conduction disorders, headache between both migraine attacks that cannot be distinguished from the attacks, chronic tension headaches, or other headaches that occur more than 15 days a month, pregnancy and breastfeeding, history of asthma, a major psychiatric illness, alcoholism, and other illicit drug dependence. The exclusion criteria included not answering the phone or not accurately adhering to the treatment method.
Patients were divided into two groups of sodae and valproate using the quadruple random block method. After randomly numbering the headache and valproate packages, each individual was coded with the drug package number. The allocation of people to groups was blind to patients and doctors. There was a 3-month treatment period. A total of 96 patients were treated with medicine in this study, but 20 were excluded due to not answering the phone or not accurately adhering to the treatment method. A total of 76 people were enrolled in the study. Six patients had been in the study for less than 3 months and had not completed the headache impact test (HIT-6) questionnaire, so they were excluded too.
Study intervention
Booali Daroo Pharmaceutical Company developed the sodae capsule based on Iranian traditional medicine methods to treat migraine headaches. Sodium valproate 200 mg capsules were purchased from the manufacturer’s pharmaceutical company and packed in the same packages as sodae. In one group, routine medication was administered with sodae capsules; routine medicine with 200 mg of sodium valproate capsules in the other. The instructions were as follows: Take one sodae capsule or valproate each night before bedtime with warm water.
Data collection tools and methods
Researchers used a questionnaire to collect data on demographic characteristics, medical history, migraine attack status, and the HIT-6 questionnaire to assess headaches’ impact on patient’s lives. During the study, a co-researcher called patients to monitor drug use and record side effects and effectiveness. The HIT-6 questionnaire was completed every month. This questionnaire contains 6 questions with 5-option items: “Never” (6 points), “rarely” (8 points), “sometimes” (10 points), “often” (11 points), and “always” (13 points). The HIT-6 score is the sum of the scores of the options selected by the patients.
A numerical rating scale (NRS) (pain scale) was used to measure residual pain at the end of each month of treatment. The person rates their pain using a scale of 0 to 10. Zero means no pain, and 10 means the worst pain. To check the side effects, in addition to the side effects reported by the patients during the treatment, once at the beginning of the study and once after three months, liver function tests and complete blood cell count tests were performed for the patients.
Written informed consent was obtained from the patients to enter the study. Also, the use of routine drugs was not prohibited for patients.
Data analysis
The normality of the study variables was checked using the Kolmogorov-Smirnov test. In two groups of sodae and valproate, qualitative variables were compared using the chi-square test. In non-normal data, for quantitative variables, the Wilcoxon test was used to compare before and after in each group, and the Mann-Whitney test was used to compare two groups. In normal data, for quantitative variables, a paired t-test was used to compare before and after in each group, and an independent t-test was used to compare the two groups. The trend of HIT-6 score changes was investigated by repeated measures analysis for non-normal variables and the Friedman test. Data analysis was done using SPSS software, version 25.
Results
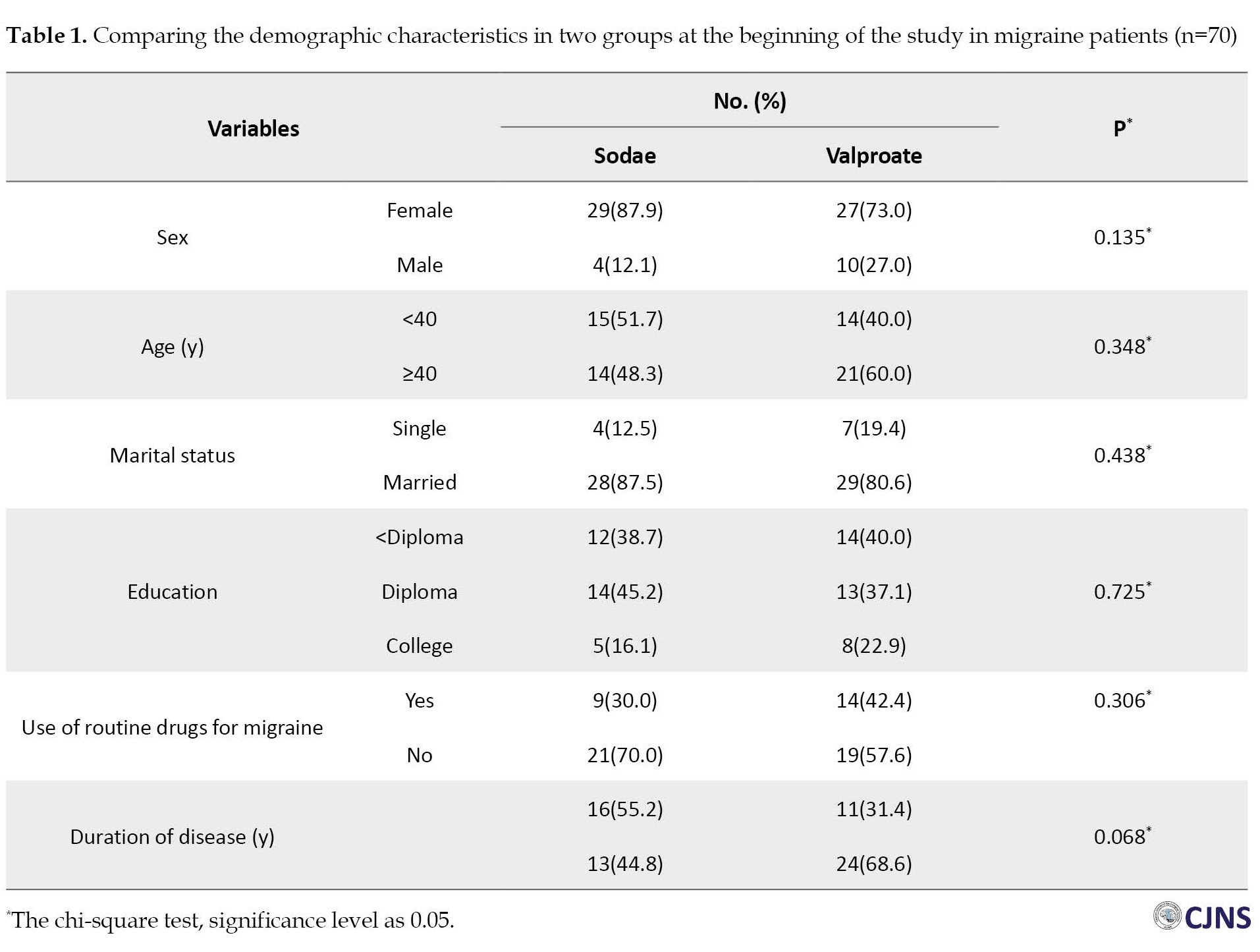
In this study, 70 migraine patients were evaluated, including 56 women (80%) and 14 men (20%). The sodae group consisted of 33 participants (47%), while the valproate group consisted of 37 participants (53%). For the sodae group, the Mean±SD age and duration of the disease were 40.77(11.2) and 7.27(8.9) years, and for the valproate group, it was 40.48(12.2) and 9.62(8.7) years, respectively. Demographic characteristics did not differ significantly between the sodae and valproate (Table 1).
The number, duration, and intensity of migraine attacks decreased significantly in both groups. At the end of the study, 25 people (75.8%) in the sodae group and 27(73%) in the valproate group reported an improvement of more than 70% in headaches and reduced the use of painkillers.
In both the sodae and valproate groups, the NRS scale showed that the pain level decreased consistently so that the NRS over months 1, 2, and 3 of treatment in the valproate group were 3.55, 2.57, and 2.14, respectively, and in the sodae group were 3.08, 2.53, and 2.14, respectively. There was no significant difference between the sodae and valproate groups (P=0.303). In both groups, the score of HIT-6 on patients’ lives was continuously decreasing significantly (P<0.001), and it had a similar trend in the two groups (Figure 1). According to the Friedman test, the average score of HIT-6 decreased significantly in both groups (P <0.001) (Table 2).
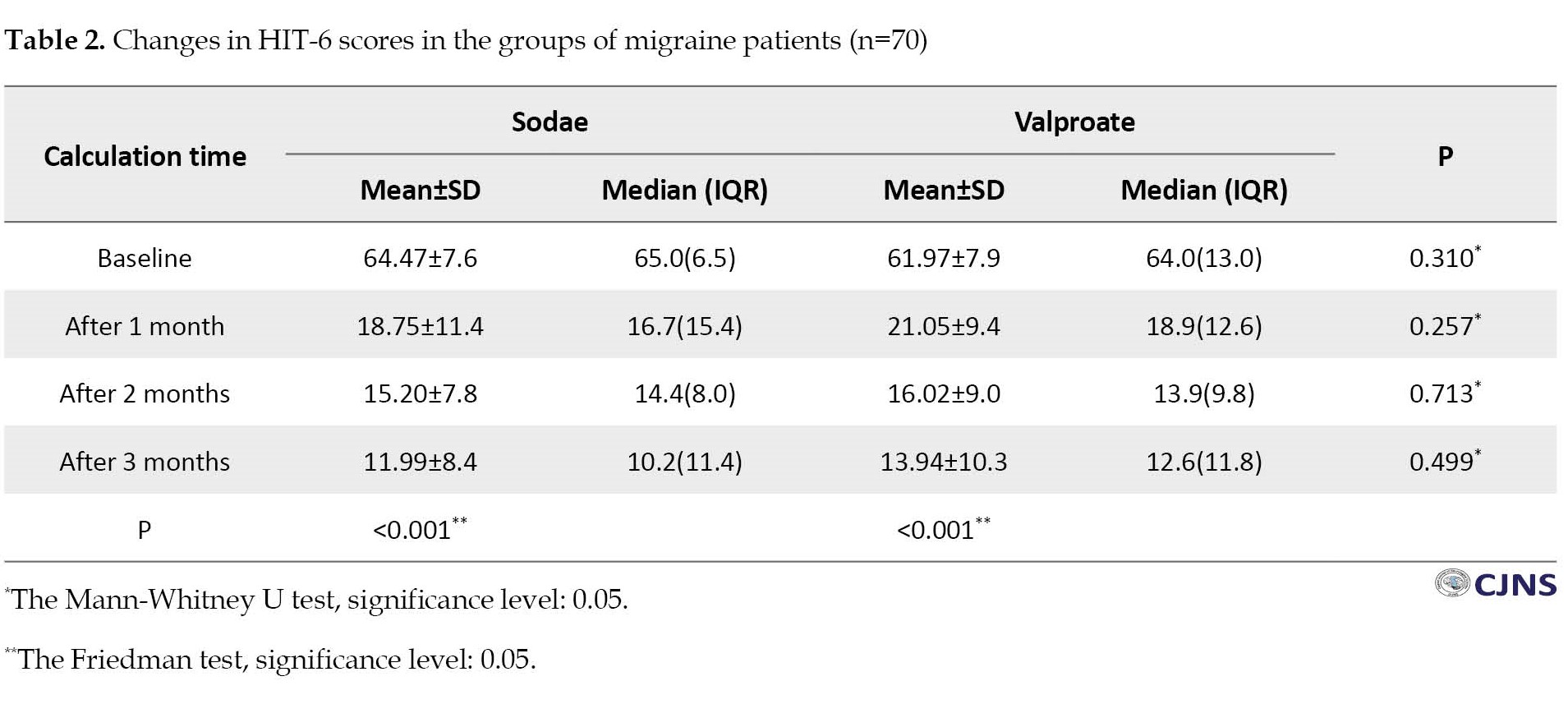
The Mann-Whitney test showed no significant difference in the HIT-6 score reduction in the two groups. (P for the first, second, and third months were 0.257, 0.713, 0.499, respectively)
Twenty people (54.05%) reported at least one side effect in the valproate group and 10 (30.30%) in the sodae group. Valproate patients reported significantly more side effects than the sodae patients (P=0.043).
In the valproate group, the most common side effects were drowsiness and boredom in 5 (13.5%) and 4 (10.8%) people, respectively, while in the sodae group, diarrhea and stomach pain were the most common side effects in 5 (15.1%) and 3 (9.1%) people (Table 3).

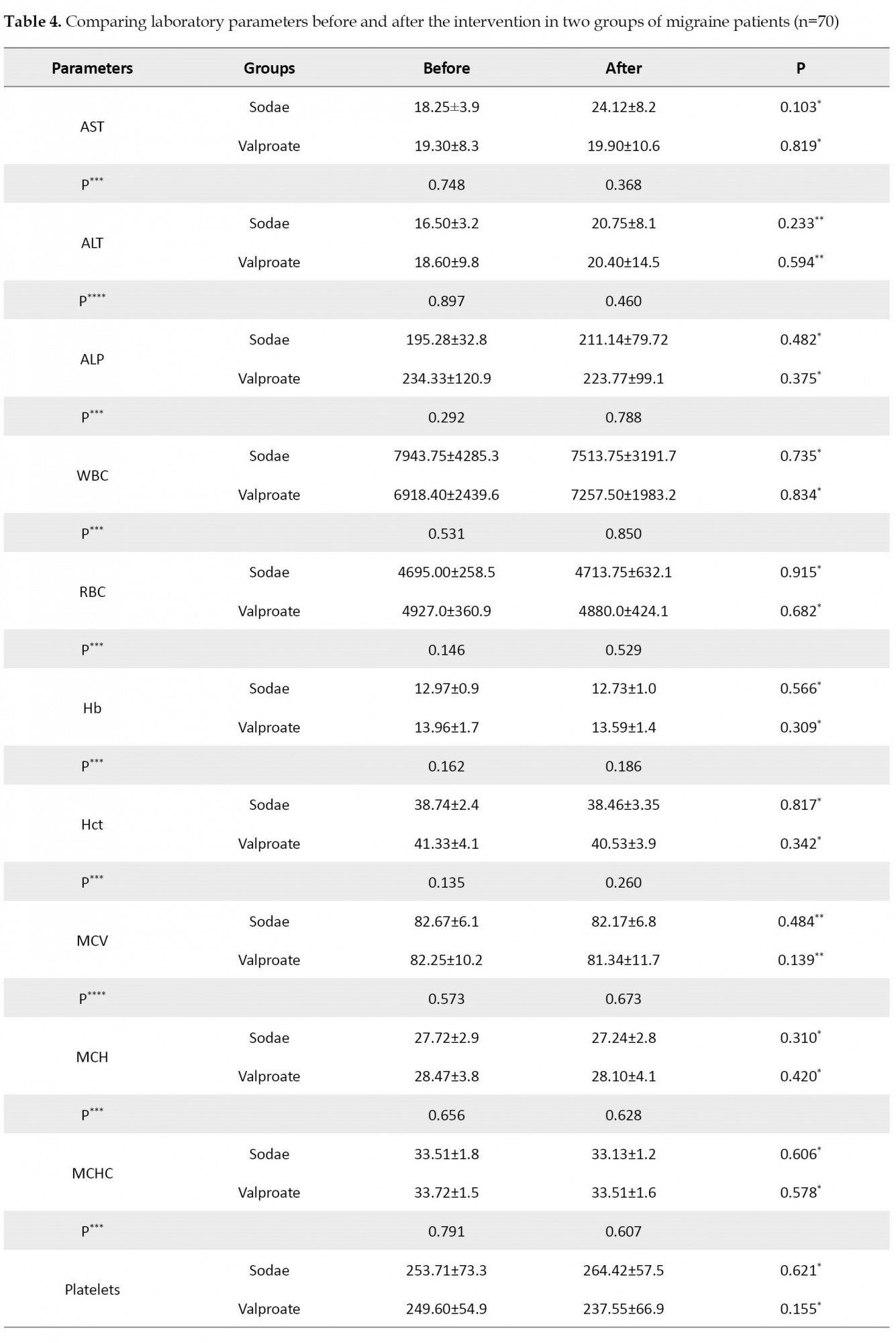
According to the paired t-test and Wilcoxon test, neither the sodae nor valproate groups’ average laboratory parameters changed significantly (P>0.05) after the study compared to before the study. Neither at the beginning of the research nor at the end, the independent t-test or Mann-Whitney test revealed significant (P>0.05) differences between the sodae and valproate groups in terms of mean laboratory parameters (Table 4).

Discussion
The efficacy and side effects of sodium valproate and sodae herbal capsules were compared in this study. In this 3-month study, 25 people (75.8%) in the sodae group and 27(73%) in the valproate group reported an overall improvement in headaches of more than 70% and a reduction in painkiller use. Sodae and valproate groups did not differ significantly in terms of headache improvement. HIT-6 scores decreased over time in both groups and had a similar trend. The side effects reported by patients in the valproate group (54.05%) were significantly higher than in the sodae group (30.30%). Drowsiness was the most common complication in the valproate group (13.5%), and diarrhea was the most common complication in the sodae group (15.1%). In both sodae and valproate groups, liver enzymes and blood count did not significantly change after the study compared to before.
Various studies have demonstrated sodium valproate’s effectiveness in improving migraine attacks, consistent with the current study’s findings [26-28]. Also, multiple studies have proven the effectiveness of different medicinal plants in treating headaches [21]. According to Dakhale et al.’s [10] study, sodium valproate significantly reduces migraine headache frequency, intensity, and duration. According to Mohammadtaheri et al.’s study, peppermint plant extract (menthol) combined with preventive drugs significantly reduces migraine attacks’ intensity, duration, and frequency [19]. Using traditional Japanese medicines (goshuyuto and chotosan), Hibino et al. reported that goshuyuto can significantly reduce migraines with aura by preventing excess platelet accumulation [20]. Goshuyuto is a mixture of four medicinal herbs: Zizyphi fructus, evodiae fructus, ginseng radix, and zingiberis rhizoma. Similarly, chotosan is manufactured from a mix of 11 medicinal herbs: Gypsum fibrosum, uncariae uncis cum ramulus, aurantii nobilis pericarpium, pinelliae tuber, ophiopogonis tuber, hoelen, ginseng radix, saposhnikoviae radix, chrysanthemi flos, glycyrrhizae radix, and zingiberis rhizoma. In this study, the most common complications reported in the sodae group were diarrhea and stomachache, reported by 5(15.1%) and 3(9.1%) people, respectively. According to Rezaei et al., who compared the effectiveness of sodae and placebo on migraine headaches, no significant difference (P=0.486) was found in side effects between the two groups. However, more people in the sodae group had diarrhea or stomach pain than in the placebo group (14% vs. 10%) [25]. In some people, diarrhea can occur because the sodae capsule cleanses the body and digestive system of additives and toxins. The patients in the valproate group reported significantly more side effects than those in the sodae group in the present study. Other studies have reported various side effects of sodium valproate in migraine patients, including drowsiness, appetite increase, weight gain, insomnia, and dizziness [15-18].
According to Dakhale et al. [10], 10 migraine patients (33.33%) reported one or more side effects receiving sodium valproate. According to the current study, 20 migraine patients (54.05%) treated with sodium valproate reported one or more side effects. In the present study, more participants experienced at least one side effect than in the study mentioned above. This difference may be caused because one of the co-researchers talked to the patients on the phone regularly to monitor the drug use and record its effectiveness and side effects. Still, in the Dakhale study, patients were evaluated at weeks 0 and 4, 8, and 12, and some side effects might be forgotten and not recorded until the re-evaluation of the patient 1 month later.
In both the sodae and valproate groups, liver enzymes and blood count did not significantly change after the study compared to before. Sodium valproate has been shown to influence laboratory parameters in studies [29, 30]
Conclusion
After 3 months of the study, more than 75% of the patients in the sodae and valproate groups reported more than 70% improvement in their headache and decreased painkiller use. The effectiveness of sodae and valproate was not significantly different. HIT-6 scores in both groups significantly reduced over time, and the decreases in HIT-6 scores were not significantly different between sodae and valproate. The side effects reported by patients in the valproate group were significantly higher than those reported by those in the sodae group. The most common side effect of valproate was drowsiness, while diarrhea was the most common side effect in the sodae group. Also, neither the sodae nor the valproate group showed significantly different laboratory parameters after the study. Liver enzyme tests and blood counts did not differ significantly between the valproate and sodae groups. With these descriptions, sodae is a suitable herbal medicine for preventing and controlling migraine attacks and can be used in migraine prophylaxis.
Ethical Considerations
Compliance with ethical guidelines
The study process was in compliance with the ethical guidelines of the Declaration of Helsinki 2013. This research was approved by the Ethics Committee of Kermanshah University of Medical Sciences (Code: IR.KUMS.REC.1398.1190) and registered on the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20200126046272N2).
Funding
This research was supported financially by Booali Daroo Pharmaceutical Company, which paid the project implementation cost to Kermanshah University of Medical Sciences.
Authors contributions
Project administration: Mansour Rezaei and Daryoush Afshari; Conceptualization and visiting patients: Daryoush Afshari and Mojtba Khazaei; Methodology and Formal analysis: Mansour Rezaei; Writing and review: Mansour Rezaei and Mojtba Khazaei; Data collection and software: Negin Fakhri; Editing: Negin Fakhri and Daryoush Afshari.
Conflict of interest
The authors declared no Conflict of interest.
Acknowledgements
The authors thank Booali Daroo Company for preparing the medications and the Research and Technology Deputy of Kermanshah University of Medical Sciences for approving this project and cooperating in its implementation. We thank Hamadan University of Medical Sciences for cooperating in implementing this project.
References
As a primary headache disorder with significant pain, migraine is listed by the World Health Organization (WHO) among the 20 debilitating diseases [1, 2]. A migraine headache is characterized by moderate to severe throbbing pain, usually occurring on one side of the head, which is often accompanied by nausea, vomiting, photophobia, and phonophobia, all of which are usually aggravated by physical activity, according to the International Headache Society [3]. In Iran, migraine prevalence is estimated to be 14%, which is similar to or even higher than all over the world [4]. Migraine prevalence rates in Iran are 12.5% for women and 8.4% for men [5, 6]. The antiepileptic drug sodium valproate has been recommended as the first line of treatment for preventing migraine attacks since 1970 [7]. Sodium valproate is an effective migraine prevention treatment in many studies [8-12]. The effectiveness of sodium valproate in reducing migraine attack frequency, intensity, and maximum pain levels has been proven in numerous studies [9, 12]. Still, side effects such as fatigue, dizziness, nausea, tremors, and weight gain have led to poor compliance with the treatment [13, 14]. There have been reports of drowsiness, increased appetite, weight gain, insomnia, and dizziness associated with sodium valproate use [15-18]. Various studies investigated the use of herbal drugs in migraine prevention in addition to chemical medications [19-22]. In recent years, herbal medicines have been proposed as a promising strategy for the prevention and treatment of migraine with analgesic activity and minor side effects [23]. Plants such as feverfew (Tanacetum parthenium), butterbur (Petasites hybridus), marijuana (Cannabis spp.), Saint John’s Wort (Hypericum perforatum), and the Damask rose (Rosa+Damascena) have acceptable mechanisms of action and evidence for the treating migraine. Because of the widespread use of and the high tendency to use traditional medicinal plants, more extensive research should be designed in several areas of pharmacy and pharmacology of medicinal plants to gain proper information for pharmaceutical industries [24].
One of the herbal medicines in the treatment of migraine is the sodae, made by the Booali Daroo pharmaceutical company. The main components of this product with their scientific names are Turpethum, Commiphora Mukul, Rheum Officinale, and Terminalia Chebul, and the common names of these components are Indian jalap, Bdellium, Rhubarb, and Chebulic myrobalan. The sodae capsule has laxative effects, which cleanse the body and digestive tract of additives and toxins and create a lighter feeling in the head.
There was a clinical trial in Kermanshah City, Iran, in 2020 comparing sodae herbal capsule and placebo, and the results indicated a 65% recovery rate in the herbal medicine group and a 44% recovery rate in the placebo group, and the headache herbal medicine was recommended as a migraine prevention medicine [25]. Although no serious side effects were reported in this study, diarrhea and stomachache were the most common side effects. It seems necessary to examine this drug more closely regarding side effects and compare it with other migraine prevention drugs. Since no other study has been conducted, we embarked on comparing sodium valproate and sodae herbal headache medicine for their effectiveness and side effects.
Materials and Methods
Study design and research community
A randomized, double-blind, two-center clinical trial was conducted in Kermanshah and Hamadan cities, Iran, between December 2021 and July 2022. A co-investigator explained the study’s purpose to patients with episodic migraines who met the inclusion and exclusion criteria. A total of 96 migraine patients without aura participated in the study. The inclusion criteria for participants included those aged 18 to 65, more than 3 attacks per month in the past three months, migraine onset one year before the study, and migraine onset before 50. Non-entry criteria included not taking sodium valproate, risk factors for heart diseases (endothelial dysfunction), cardiac conduction disorders, headache between both migraine attacks that cannot be distinguished from the attacks, chronic tension headaches, or other headaches that occur more than 15 days a month, pregnancy and breastfeeding, history of asthma, a major psychiatric illness, alcoholism, and other illicit drug dependence. The exclusion criteria included not answering the phone or not accurately adhering to the treatment method.
Patients were divided into two groups of sodae and valproate using the quadruple random block method. After randomly numbering the headache and valproate packages, each individual was coded with the drug package number. The allocation of people to groups was blind to patients and doctors. There was a 3-month treatment period. A total of 96 patients were treated with medicine in this study, but 20 were excluded due to not answering the phone or not accurately adhering to the treatment method. A total of 76 people were enrolled in the study. Six patients had been in the study for less than 3 months and had not completed the headache impact test (HIT-6) questionnaire, so they were excluded too.
Study intervention
Booali Daroo Pharmaceutical Company developed the sodae capsule based on Iranian traditional medicine methods to treat migraine headaches. Sodium valproate 200 mg capsules were purchased from the manufacturer’s pharmaceutical company and packed in the same packages as sodae. In one group, routine medication was administered with sodae capsules; routine medicine with 200 mg of sodium valproate capsules in the other. The instructions were as follows: Take one sodae capsule or valproate each night before bedtime with warm water.
Data collection tools and methods
Researchers used a questionnaire to collect data on demographic characteristics, medical history, migraine attack status, and the HIT-6 questionnaire to assess headaches’ impact on patient’s lives. During the study, a co-researcher called patients to monitor drug use and record side effects and effectiveness. The HIT-6 questionnaire was completed every month. This questionnaire contains 6 questions with 5-option items: “Never” (6 points), “rarely” (8 points), “sometimes” (10 points), “often” (11 points), and “always” (13 points). The HIT-6 score is the sum of the scores of the options selected by the patients.
A numerical rating scale (NRS) (pain scale) was used to measure residual pain at the end of each month of treatment. The person rates their pain using a scale of 0 to 10. Zero means no pain, and 10 means the worst pain. To check the side effects, in addition to the side effects reported by the patients during the treatment, once at the beginning of the study and once after three months, liver function tests and complete blood cell count tests were performed for the patients.
Written informed consent was obtained from the patients to enter the study. Also, the use of routine drugs was not prohibited for patients.
Data analysis
The normality of the study variables was checked using the Kolmogorov-Smirnov test. In two groups of sodae and valproate, qualitative variables were compared using the chi-square test. In non-normal data, for quantitative variables, the Wilcoxon test was used to compare before and after in each group, and the Mann-Whitney test was used to compare two groups. In normal data, for quantitative variables, a paired t-test was used to compare before and after in each group, and an independent t-test was used to compare the two groups. The trend of HIT-6 score changes was investigated by repeated measures analysis for non-normal variables and the Friedman test. Data analysis was done using SPSS software, version 25.
Results
In this study, 70 migraine patients were evaluated, including 56 women (80%) and 14 men (20%). The sodae group consisted of 33 participants (47%), while the valproate group consisted of 37 participants (53%). For the sodae group, the Mean±SD age and duration of the disease were 40.77(11.2) and 7.27(8.9) years, and for the valproate group, it was 40.48(12.2) and 9.62(8.7) years, respectively. Demographic characteristics did not differ significantly between the sodae and valproate (Table 1).

The number, duration, and intensity of migraine attacks decreased significantly in both groups. At the end of the study, 25 people (75.8%) in the sodae group and 27(73%) in the valproate group reported an improvement of more than 70% in headaches and reduced the use of painkillers.
In both the sodae and valproate groups, the NRS scale showed that the pain level decreased consistently so that the NRS over months 1, 2, and 3 of treatment in the valproate group were 3.55, 2.57, and 2.14, respectively, and in the sodae group were 3.08, 2.53, and 2.14, respectively. There was no significant difference between the sodae and valproate groups (P=0.303). In both groups, the score of HIT-6 on patients’ lives was continuously decreasing significantly (P<0.001), and it had a similar trend in the two groups (Figure 1). According to the Friedman test, the average score of HIT-6 decreased significantly in both groups (P <0.001) (Table 2).

The Mann-Whitney test showed no significant difference in the HIT-6 score reduction in the two groups. (P for the first, second, and third months were 0.257, 0.713, 0.499, respectively)
Twenty people (54.05%) reported at least one side effect in the valproate group and 10 (30.30%) in the sodae group. Valproate patients reported significantly more side effects than the sodae patients (P=0.043).
In the valproate group, the most common side effects were drowsiness and boredom in 5 (13.5%) and 4 (10.8%) people, respectively, while in the sodae group, diarrhea and stomach pain were the most common side effects in 5 (15.1%) and 3 (9.1%) people (Table 3).

According to the paired t-test and Wilcoxon test, neither the sodae nor valproate groups’ average laboratory parameters changed significantly (P>0.05) after the study compared to before the study. Neither at the beginning of the research nor at the end, the independent t-test or Mann-Whitney test revealed significant (P>0.05) differences between the sodae and valproate groups in terms of mean laboratory parameters (Table 4).


Discussion
The efficacy and side effects of sodium valproate and sodae herbal capsules were compared in this study. In this 3-month study, 25 people (75.8%) in the sodae group and 27(73%) in the valproate group reported an overall improvement in headaches of more than 70% and a reduction in painkiller use. Sodae and valproate groups did not differ significantly in terms of headache improvement. HIT-6 scores decreased over time in both groups and had a similar trend. The side effects reported by patients in the valproate group (54.05%) were significantly higher than in the sodae group (30.30%). Drowsiness was the most common complication in the valproate group (13.5%), and diarrhea was the most common complication in the sodae group (15.1%). In both sodae and valproate groups, liver enzymes and blood count did not significantly change after the study compared to before.
Various studies have demonstrated sodium valproate’s effectiveness in improving migraine attacks, consistent with the current study’s findings [26-28]. Also, multiple studies have proven the effectiveness of different medicinal plants in treating headaches [21]. According to Dakhale et al.’s [10] study, sodium valproate significantly reduces migraine headache frequency, intensity, and duration. According to Mohammadtaheri et al.’s study, peppermint plant extract (menthol) combined with preventive drugs significantly reduces migraine attacks’ intensity, duration, and frequency [19]. Using traditional Japanese medicines (goshuyuto and chotosan), Hibino et al. reported that goshuyuto can significantly reduce migraines with aura by preventing excess platelet accumulation [20]. Goshuyuto is a mixture of four medicinal herbs: Zizyphi fructus, evodiae fructus, ginseng radix, and zingiberis rhizoma. Similarly, chotosan is manufactured from a mix of 11 medicinal herbs: Gypsum fibrosum, uncariae uncis cum ramulus, aurantii nobilis pericarpium, pinelliae tuber, ophiopogonis tuber, hoelen, ginseng radix, saposhnikoviae radix, chrysanthemi flos, glycyrrhizae radix, and zingiberis rhizoma. In this study, the most common complications reported in the sodae group were diarrhea and stomachache, reported by 5(15.1%) and 3(9.1%) people, respectively. According to Rezaei et al., who compared the effectiveness of sodae and placebo on migraine headaches, no significant difference (P=0.486) was found in side effects between the two groups. However, more people in the sodae group had diarrhea or stomach pain than in the placebo group (14% vs. 10%) [25]. In some people, diarrhea can occur because the sodae capsule cleanses the body and digestive system of additives and toxins. The patients in the valproate group reported significantly more side effects than those in the sodae group in the present study. Other studies have reported various side effects of sodium valproate in migraine patients, including drowsiness, appetite increase, weight gain, insomnia, and dizziness [15-18].
According to Dakhale et al. [10], 10 migraine patients (33.33%) reported one or more side effects receiving sodium valproate. According to the current study, 20 migraine patients (54.05%) treated with sodium valproate reported one or more side effects. In the present study, more participants experienced at least one side effect than in the study mentioned above. This difference may be caused because one of the co-researchers talked to the patients on the phone regularly to monitor the drug use and record its effectiveness and side effects. Still, in the Dakhale study, patients were evaluated at weeks 0 and 4, 8, and 12, and some side effects might be forgotten and not recorded until the re-evaluation of the patient 1 month later.
In both the sodae and valproate groups, liver enzymes and blood count did not significantly change after the study compared to before. Sodium valproate has been shown to influence laboratory parameters in studies [29, 30]
Conclusion
After 3 months of the study, more than 75% of the patients in the sodae and valproate groups reported more than 70% improvement in their headache and decreased painkiller use. The effectiveness of sodae and valproate was not significantly different. HIT-6 scores in both groups significantly reduced over time, and the decreases in HIT-6 scores were not significantly different between sodae and valproate. The side effects reported by patients in the valproate group were significantly higher than those reported by those in the sodae group. The most common side effect of valproate was drowsiness, while diarrhea was the most common side effect in the sodae group. Also, neither the sodae nor the valproate group showed significantly different laboratory parameters after the study. Liver enzyme tests and blood counts did not differ significantly between the valproate and sodae groups. With these descriptions, sodae is a suitable herbal medicine for preventing and controlling migraine attacks and can be used in migraine prophylaxis.
Ethical Considerations
Compliance with ethical guidelines
The study process was in compliance with the ethical guidelines of the Declaration of Helsinki 2013. This research was approved by the Ethics Committee of Kermanshah University of Medical Sciences (Code: IR.KUMS.REC.1398.1190) and registered on the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20200126046272N2).
Funding
This research was supported financially by Booali Daroo Pharmaceutical Company, which paid the project implementation cost to Kermanshah University of Medical Sciences.
Authors contributions
Project administration: Mansour Rezaei and Daryoush Afshari; Conceptualization and visiting patients: Daryoush Afshari and Mojtba Khazaei; Methodology and Formal analysis: Mansour Rezaei; Writing and review: Mansour Rezaei and Mojtba Khazaei; Data collection and software: Negin Fakhri; Editing: Negin Fakhri and Daryoush Afshari.
Conflict of interest
The authors declared no Conflict of interest.
Acknowledgements
The authors thank Booali Daroo Company for preparing the medications and the Research and Technology Deputy of Kermanshah University of Medical Sciences for approving this project and cooperating in its implementation. We thank Hamadan University of Medical Sciences for cooperating in implementing this project.
References
- Steiner TJ, Stovner LJ, Vos T, Jensen R, Katsarava Z. Migraine is first cause of disability in under 50s: Will health politicians now take notice? J Headache Pain. 2018; 19(1):17. [DOI:10.1186/s10194-018-0846-2] [PMID] [PMCID]
- Leonardi M, Steiner TJ, Scher AT, Lipton RB. The global burden of migraine: Measuring disability in headache disorders with WHO’s Classification of Functioning, Disability and Health (ICF). J Headache Pain. 2005; 6(6):429-40. [DOI:10.1007/s10194-005-0252-4] [PMID] [PMCID]
- Olesen J, Steiner TJ. The International classification of headache disorders, 2nd edn (ICDH-II). J Neurol Neurosurg Psychiatry. 2004; 75(6):808-11. [DOI:10.1136/jnnp.2003.031286] [PMID] [PMCID]
- Farhadi Z, Alidoost S, Behzadifar M, Mohammadibakhsh R, Khodadadi N, Sepehrian R, et al. The prevalence of migraine in Iran: A systematic review and meta-analysis. Iran Red Crescent Med J. 2016; 18(10):e40061. [DOI:10.5812/ircmj.40061]
- Ayatollahi S, Khosravi A. Prevalence of migraine and tension-type headache in primary-school children in Shiraz. East Mediterr Health J. 2006; 12(6):809-17. 2006. [Link]
- Ayatollahi SM, Moradi F, Ayatollahi SA. Prevalences of migraine and tension-type headache in adolescent girls of Shiraz (southern Iran). Headache. 2002; 42(4):287-90. [DOI:10.1046/j.1526-4610.2002.02082.x] [PMID]
- Linde M, Mulleners WM, Chronicle EP, McCrory DC. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013; 2013(6):CD010610. [DOI:10.1002/14651858.CD010610] [PMID] [PMCID]
- Silberstein SD. Divalproex sodium in headache: Literature review and clinical guidelines. Headache. 1996; 36(9):547-55. [DOI:10.1046/j.1526-4610.1996.3609547.x] [PMID]
- Holland S, Silberstein SD, Freitag F, Dodick DW, Argoff C, Ashman E, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: Report of the quality standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012; 78(17):1346-53. [DOI:10.1212/WNL.0b013e3182535d0c] [PMID] [PMCID]
- Dakhale GN, Sharma VS, Thakre MN, Kalikar M. Low-dose sodium valproate versus low-dose propranolol in prophylaxis of common migraine headache: A randomized, prospective, parallel, open-label study. Indian J Pharmacol. 2019; 51(4):255-62. [DOI:10.4103/ijp.IJP_457_18] [PMID] [PMCID]
- Khani S, Hejazi SA, Yaghoubi M, Sharifipour E. Comparative study of magnesium, sodium valproate, and concurrent magnesium-sodium valproate therapy in the prevention of migraine headaches: A randomized controlled double-blind trial. J Headache Pain. 2021; 22(1):21. [DOI:10.1186/s10194-021-01234-6] [PMID] [PMCID]
- Yurekli VA, Akhan G, Kutluhan S, Uzar E, Koyuncuoglu HR, Gultekin F. The effect of sodium valproate on chronic daily headache and its subgroups. J Headache Pain. 2008; 9(1):37-41. [DOI:10.1007/s10194-008-0002-5] [PMID] [PMCID]
- Assarzadegan F, Tabesh H, Hosseini-Zijoud SM, Beale AD, Shoghli A, Ghafoori Yazdi M, et al. Comparing zonisamide with sodium valproate in the management of migraine headaches: Double-blind randomized clinical trial of efficacy and safety. Iran Red Crescent Med J. 2016; 18(9):e23768. [DOI:10.5812/ircmj.23768] [PMID] [PMCID]
- Romoli M, Costa C, Siliquini S, Corbelli I, Eusebi P, Bedetti C, et al. Antiepileptic drugs in migraine and epilepsy: Who is at increased risk of adverse events? Cephalalgia. 2018; 38(2):274-82. [DOI:10.1177/0333102416683925] [PMID]
- Jensen R, Brinck T, Olesen J. Sodium valproate has a prophylactic effect in migraine without aura: A triple-blind, placebo-controlled crossover study. Neurology. 1994; 44(4):647-51. [DOI:10.1212/WNL.44.4.647] [PMID]
- Afshari D, Rafizadeh S, Rezaei M. A comparative study of the effects of low-dose topiramate versus sodium valproate in migraine prophylaxis. Int J Neurosci. 2012; 122(2):60-8. [DOI:10.3109/00207454.2011.626908] [PMID]
- Bostani A, Rajabi A, Moradian N, Razazian N, Rezaei M. The effects of cinnarizine versus sodium valproate in migraine prophylaxis. Int J Neurosci. 2013; 123(7):487-93. [DOI:10.3109/00207454.2013.765419] [PMID]
- Takeshima T, Suzuki N, Matsumori Y, Shimmoto N, Kurihara Y, Gunji R, et al. Effectiveness and safety of an extended-release tablet of sodium valproate for the prophylactic treatment of migraine: Postmarketing surveillance in Japan. Neurol Clin Neurosci. 2016; 4(4):134-41. [DOI:10.1111/ncn3.12053] [PMID] [PMCID]
- Mohammadtaheri F, Tavakol K, Gheysari R, Akhlaghdoost M. [The effectiveness of oral peppermint extract on migraine (Persian)]. Anesthesiol Pain. 2016; 7(1):1-12. [Link]
- Hibino T, Yuzurihara M, Terawaki K, Kanno H, Kase Y, Takeda A. Goshuyuto, a traditional Japanese medicine for migraine, inhibits platelet aggregation in guinea-pig whole blood. J Pharmacol Sci. 2008; 108(1):89-94. [DOI:10.1254/jphs.08058FP] [PMID]
- Li JC, Shen XF, Meng XL, Zhang Y, Lai XR. Analgesic effect and mechanism of the three TCM-herbal drug-combination Tou Feng Yu pill on treatment of migraine. Phytomedicine. 2011; 18(8-9):788-94. [DOI:10.1016/j.phymed.2011.01.008] [PMID]
- Patni S. Comprehensive review of medicinal plants used in treatment of migraine. Asian J Res Pharm Sci. 2020; 10(3):189-94. [DOI:10.5958/2231-5659.2020.00036.3]
- Chen Y, Wang S, Wang Y. Role of herbal medicine for prevention and treatment of migraine. Phytother Res. 2022; 36(2):730-60. [DOI:10.1002/ptr.7339] [PMID]
- Rajapakse T, Davenport WJ. Phytomedicines in the treatment of migraine. CNS Drugs. 2019; 33(5):399-415. [DOI:10.1007/s40263-018-0597-2] [PMID]
- Rezaei M, Afshari D, Fakhri N. The effect of sodae herbal capsule on migraine headaches. J Med Plants. 2020; 19(73):143-51. [DOI:10.29252/jmp.1.73.143]
- Shahien R, Saleh SA, Bowirrat A. Intravenous sodium valproate aborts migraine headaches rapidly. Acta Neurol Scand. 2011; 123(4):257-65. [DOI:10.1111/j.1600-0404.2010.01394.x] [PMID]
- Linde M, Mulleners WM, Chronicle EP, McCrory DC. Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013; 2013(6):CD010611. [DOI:10.1002/14651858.CD010611] [PMID] [PMCID]
- Sarchielli P, Messina P, Cupini LM, Tedeschi G, Di Piero V, Livrea P, et al. Sodium valproate in migraine without aura and medication overuse headache: A randomized controlled trial. Eur Neuropsychopharmacol. 2014; 24(8):1289-97. [DOI:10.1016/j.euroneuro.2014.03.010] [PMID]
- Vasudev K, Keown P, Gibb I, McAllister-Williams RH. Hematological effects of valproate in psychiatric patients: What are the risk factors?J Clin Psychopharmacol. 2010; 30(3):282-5. [DOI:10.1097/JCP.0b013e3181db2684] [PMID]
- Hussein RR, Soliman RH, Ali AMA, Tawfeik MH, Abdelrahim ME. Effect of antiepileptic drugs on liver enzymes. Beni-Suef Univ J Basic Appl Sci. 2013; 2(1):14-9. [DOI:10.1016/j.bjbas.2013.09.002]
Type of Study: Research |
Subject:
Special
Received: 2023/10/11 | Accepted: 2023/10/17 | Published: 2023/10/17
Received: 2023/10/11 | Accepted: 2023/10/17 | Published: 2023/10/17
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







