Sat, May 18, 2024
Volume 9, Issue 4 (Autumn 2023)
Caspian J Neurol Sci 2023, 9(4): 201-209 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Keymoradzadeh A, Saberi A, Soleymanpour A, Roshan A, Mohammadi P, Bakhshi A. Systemic Inflammation Biomarkers Ratio as Predictors of Clinical Outcomes in Ischemic Stroke. Caspian J Neurol Sci 2023; 9 (4) :201-209
URL: http://cjns.gums.ac.ir/article-1-662-en.html
URL: http://cjns.gums.ac.ir/article-1-662-en.html
Arman Keymoradzadeh * 

 1, Alia Saberi2
1, Alia Saberi2 

 , Armin Soleymanpour3
, Armin Soleymanpour3 

 , Amirhossein Roshan3
, Amirhossein Roshan3 

 , Parastoo Mohammadi3
, Parastoo Mohammadi3 

 , Arash Bakhshi3
, Arash Bakhshi3 




 1, Alia Saberi2
1, Alia Saberi2 

 , Armin Soleymanpour3
, Armin Soleymanpour3 

 , Amirhossein Roshan3
, Amirhossein Roshan3 

 , Parastoo Mohammadi3
, Parastoo Mohammadi3 

 , Arash Bakhshi3
, Arash Bakhshi3 


1- Department of Neurosurgery, School of Medicine, Imam Hossein Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2- Department of Neurology, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran
3- Student Research Committee, School of Medicine, Guilan University of Medical Sciences, Iran
2- Department of Neurology, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran
3- Student Research Committee, School of Medicine, Guilan University of Medical Sciences, Iran
Keywords: Ischemic stroke, Patient outcomes assessment, Leukocyte counts, C-reactive protein, Blood sedimentation
Full-Text [PDF 1357 kb]
(195 Downloads)
| Abstract (HTML) (409 Views)
Full-Text: (136 Views)
Introduction
Strokes are the world’s second most common mortality cause and the third most common disability-causing condition [1]. Early prediction of stroke outcomes could benefit patients and can assist clinicians in ensuring effective stroke treatment and functional recovery when individuals with stroke receive hospitalization. In this regard, researchers have studied biomarkers that can predict stroke treatment response and outcome. They can vary significantly from patient to patient. Several factors affect the prognosis of stroke, including stroke type, patient age, and stroke severity [2, 3, 4]. Some studies suggest laboratory findings are prognostic factors in acute ischemic stroke (AIS) [5, 6].
An important pathophysiologic feature of AIS is inflammation [7]. A wealth of evidence suggests that immune system components play a crucial role by initiating and propagating ischemic neurological damage to the brain. The development of the immunosuppressive response to brain ischemia could lead to concurrent infectious diseases [8]. Lymphocyte-monocyte ratio (LMR) and neutrophil-lymphocyte ratio (NLR) have been reported as potential biomarkers of baseline inflammation and AIS morbidity and mortality [9, 10, 11]. However, the platelet-lymphocyte ratio (PLR) offers significantly better prognostic value than single platelet counts in stroke. It is an inexpensive, readily accessible, and comprehensive marker of inflammatory processes. PLR offers primarily two main potential benefits: 1) An integrated measure that provides supplementary data in addition to current measures and 2) More consistency compared to individual blood parameters, which are susceptible to variation due to dehydration, excessive hydration, and the condition of the blood samples [12]. The C-reactive protein (CRP), an acute-phase protein, is the most widely used marker of inflammation in peripheral blood [13]. A higher level of CRP in the blood is also independently associated with a greater risk of future vascular events or mortality [14]. We found that few studies examined the association of AIS prognosis and mortality with NLR, PLR, LMR, erythrocyte sedimentation rate (ESR), CRP, and ESR-CRP ratio (ECR) variables. Accordingly, this investigation aimed to determine the potential associations of NLR, PLR, LMR, and ECR levels with the AIS prognosis (defined as mRS score) and mortality within 3 months after stroke.
Materials and Methods
We conducted the present cross-sectional investigation at an academic hospital in northern Iran. This study included 614 AIS patients admitted between September 2019 and June 2021 for analysis. This study was conducted using subjects who met with all (2) these conditions: 1) Adults (over the age of 18), 2) Documented clinical diagnosis of AIS. The exclusion criteria constituted 1) Those with terminal cancers, hematological disorders, recently undergoing major injuries or surgeries, and severe liver, other neurological, or renal diseases based on clinical history and laboratory findings; 2) Those taking immunosuppressive medications; 3) History of active infectious diseases within two weeks before admission, myocardial infarction within 4 weeks before admission; 4) Usage of steroid, anti-platelet or anti-coagulant; 5) Patients suspected of having COVID-19 based on the findings of blood sample tests (leukocytosis, lymphopenia and elevated CRP or chest CT scans; 6) Individuals who reported symptoms of COVID-19 subjectively at any stage of the study.
Information obtained from clinical documentation included age, gender, predisposing diseases, duration of hospitalization, smoking, and rehabilitation after stroke (10 sessions or more). After applying the inclusion-exclusion criteria, 241 individuals were not eligible for inclusion. Moreover, 32 participants did not follow up with the research team 3 months after their acute ischemic stroke.
Demographic characteristics such as age, sex, duration of hospitalization, and baseline vascular risk factors (smoking, diabetes mellitus, dyslipidemia, hypertension, previous strokes, atrial fibrillation, and coronary artery diseases) were obtained using the institution’s databases. Laboratory testing of the blood samples was performed no later than 24 hours from the onset of symptoms. Laboratory and imaging data were collected using an automated testing method. Laboratory findings included a total blood count with white blood cell differentials, urea and electrolytes, hepatic function assessments, and ESR and CRP checked on admission.
A neurology specialist and 3 medical students assessed the outcomes with the modified rankin scale (mRS) [15] following 90 days after the initial assessment.
Statistical analysis
Data analysis was conducted using descriptive statistics such as percentage, frequency, and Mean±SD. We performed the Kolmogorov-Smirnov test to assess the normality of the results and applied Levene’s test for variance homogeneity. We utilized the Spearman’s rank correlation coefficient for determining the associations of NLR, LMR, PLR, ESR, CRP, and ECR with 3-month clinical outcomes. The Mann-Whitney U test was used to compare means between alive and dead patients. Linear or ordinal regression analyses were conducted to examine the interplay among multiple independent factors on outcomes. We ran the analysis using statistics of IBM SPSS software, version 26 at a significance level of P<0.05.
Results
Three hundred and forty-one subjects were included in the study, and their information was analyzed. As shown in Table 1, the sample population consists of the following general characteristics and laboratory results.
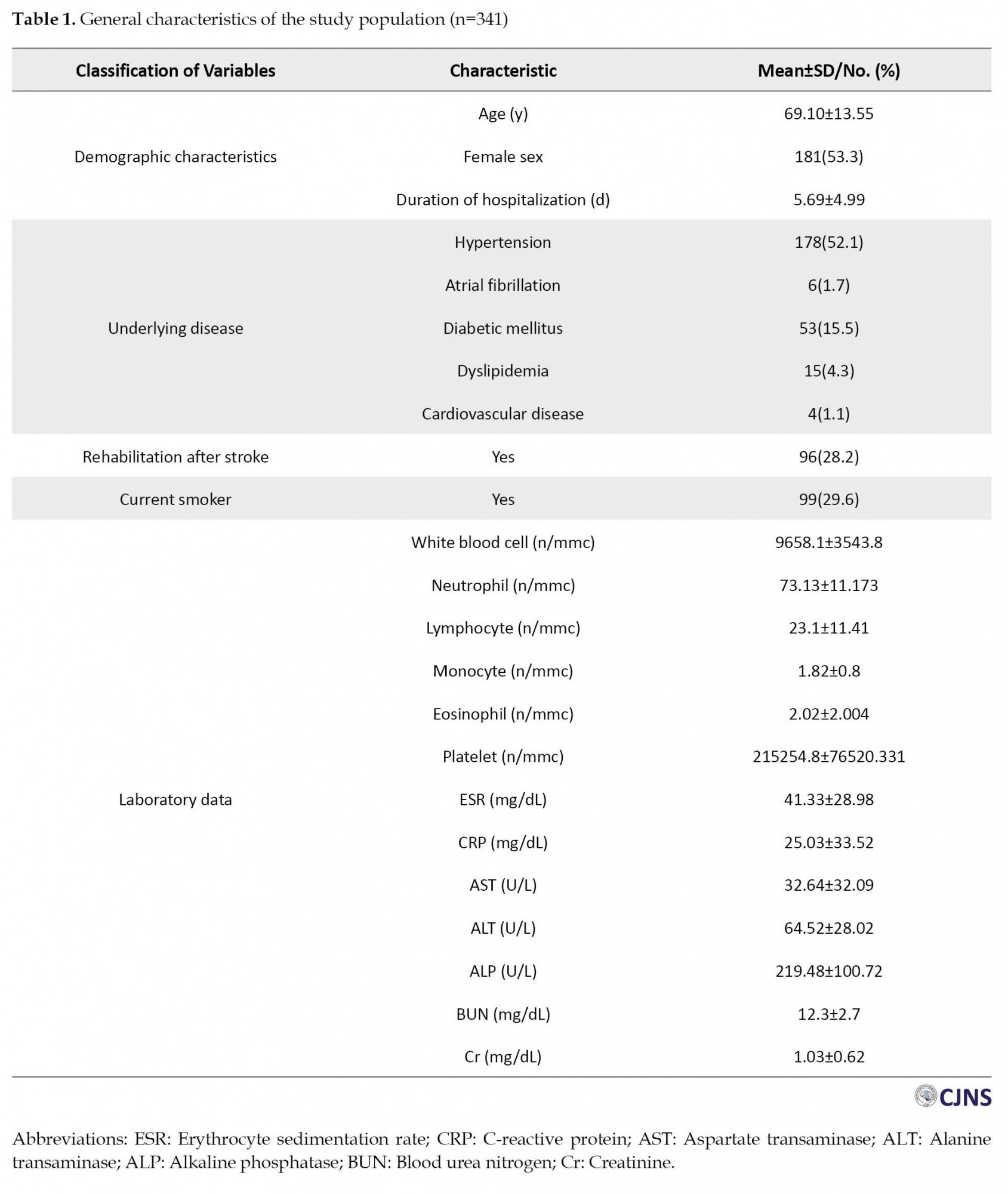
The correlation of NLR, LMR, PLR, ESR, CRP, and ECR levels with 3-month mRS
The univariate analysis shows that higher NLR, RLR, and CRP and lower LMR and ECR indicate poorer outcomes based on the 3-month mRS (Table 2).
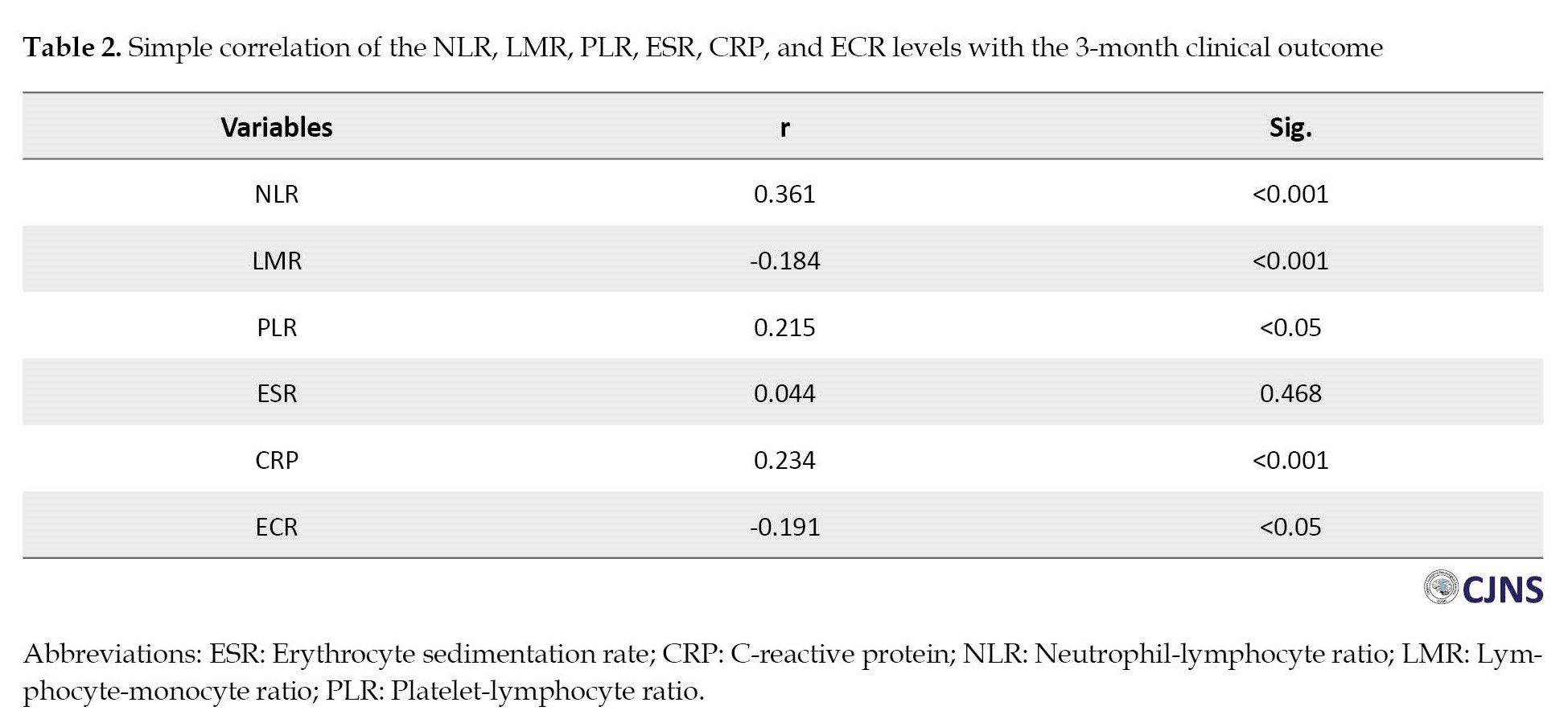
Although a significant correlation exists between NLR, RLR, CRP, LMR, and ECR with 3-month mRS, the fact that r values are less than 0.4 suggests the study variables lack a strong correlation. After adjusting for age, gender, mRS upon hospital discharge, smoking, duration of hospitalization, stroke rehabilitation, and underlying diseases, no significant relationship was found between NLR, LMR PLR, CPR, ECR, and 3-month mRS (Table 3).
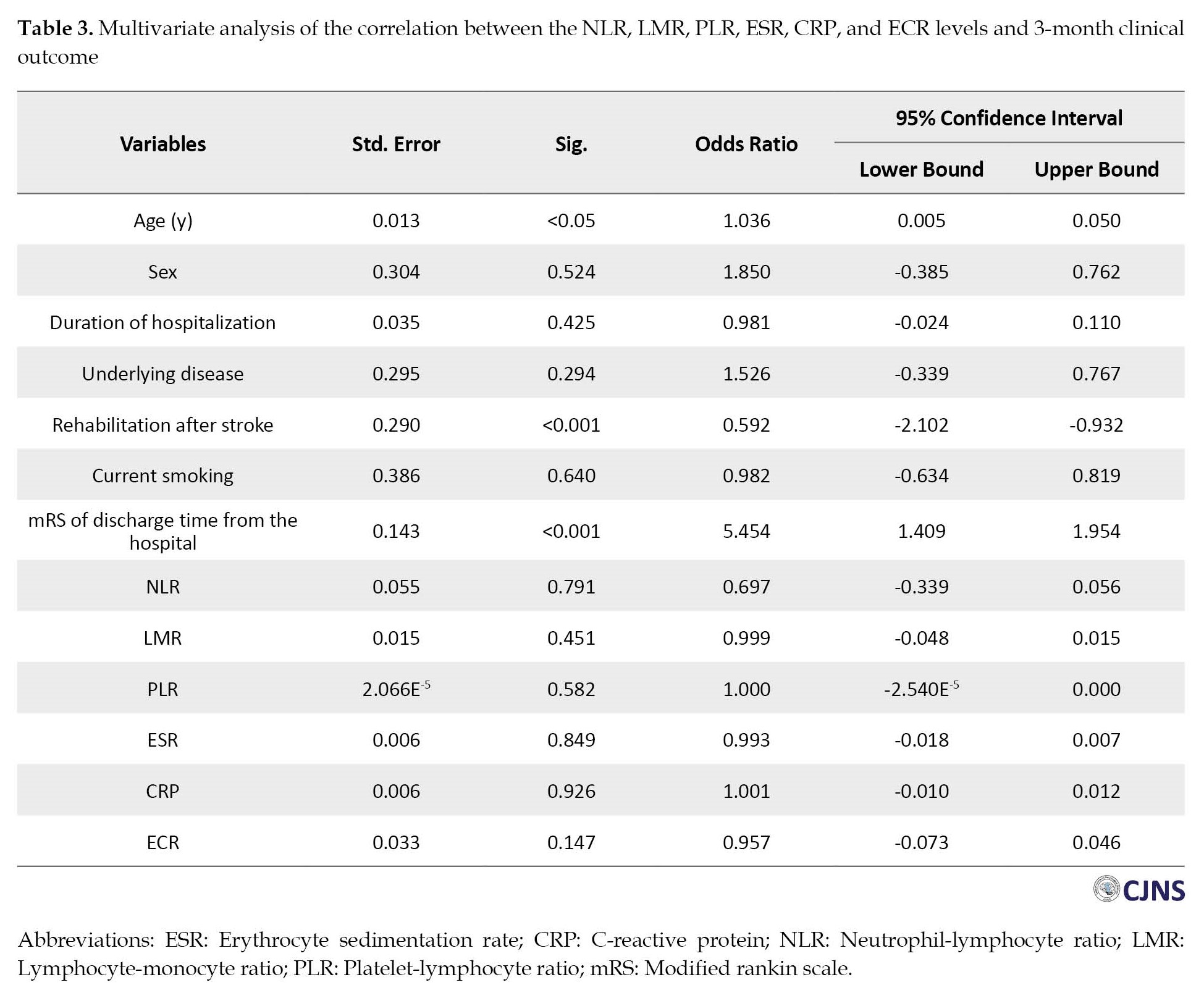
The correlations of NLR, LMR, PLR, ESR, CRP, and ECR levels with 3 months mortality in AIS patients
The three-month follow-up showed that 128 stroke patients (46.2%) had died, while 149 patients (53.8%) survived (Table 4).

Patients who died had a higher age compared to those who survived. There were no significant differences in other variables among participants. The univariate analysis showed that NLR, PLR, and CRP were higher among those who lost their lives within three months than the survivors. However, LMR and ECR were lower in those who died. In multivariate analysis, ECR remained independently associated with 3 months mortality (P<0.05), but NLR, LMR, PLR, ESR, and CRP were not associated with 3 months mortality (Table 5).

Discussion
Our investigation evaluated the clinical significance of LMR, PLR, NLR, ESR, CRP, and ECR in predicting 3-month functional outcomes and mortality after acute ischemic stroke. Based on the univariate analysis, NLR, PLR, and CRP were higher among those who lost their lives within 3 months than the survivors, but LMR and ECR were lower in those who died. In multivariate analysis, ECR remained independently associated with the 3-month mortality rate, but the relationship between other variables and mortality was not significant. The present research findings show a weak relationship exists between NLR, PLR, CRP, LMR, and ECR with the 3-month mRS. Monocytes, lymphocytes, platelets, and neutrophils may have distinct roles in inflammatory processes and the development of different diseases. When AIS occurs, platelets do not function normally [16], leading to overactivation and accumulation of platelets and causing clots and blockage of blood vessels [17]. It has been shown that high neutrophil counts confer an adverse prognosis on cardiovascular patients, whereas high lymphocyte counts confer a protective effect [18, 19]. In acute ischemic events, stress activates the hypothalamic-pituitary-adrenal system.
Consequently, a greater level of cortisol release results in a decreased level of lymphocytes [20]. Considering these factors separately may miss their interactions and associations with different medical conditions, yet exploring them together may not shed any light on the opposing roles they seem to have. Therefore, dynamic measurements of NLR, MLR, and PLR may serve as a more accurate outcome measurement than single measurements.
Firstly, our data indicated high NLR was associated with unfavorable 3-month functional outcomes and death. In previous studies, higher NLR was associated with poor functional outcomes at discharge from the hospital during the first 3 days of the stroke occurrence [21]. Also, studies have shown that increased NLR among those with acute myocardial infarction reliably predicted death and morbidity during hospitalization [22], along with inefficient heart perfusion following percutaneous coronary angioplasty [23]. In another study, a high admission NLR is associated with independent prediction of function, recanalization, and treatment with IV rtPA (recombinant tissue plasminogen activator) after AIS [11].
In addition, our data indicated that elevated levels of PLR significantly predicted poor outcomes in terms of function and mortality within 3 months. PLR might play a role in determining the outcome of acute ischemic strokes. It has been shown that platelets and lymphocytes determine the outcome of ischemic vascular diseases, such as myocardial infarction and cerebral infarction [24–27]. PLR data may offer useful information about ischemic events. There have been several studies examining this perspective. In individuals with acute myocardial infarction, PLR was an independent predictor of mortality and incidence of major adverse cardiovascular events in [28] hospital and long-term [29, 30]. A high PLR level was observed in unstable angina pectoris sufferers having impaired coronary collateral circulation [31]. Gary et al. [32] also identified a significant association between elevated PLR and increased chances of severe limb ischemia in critically ill patients. This association may be useful in identifying patients with an increased risk of vascular complications. Studies of cerebrovascular diseases have shown that a higher PLR is associated with stroke [33]. Another study used A high PLR value as an indirect measure of stroke patients’ infarcted area and a relatively low recanalization rate following thrombectomy treatment [34].
Our data indicated low LMR levels were associated with unfavorable 3-month functional outcomes and death. According to the reports, LMR is linked to poor outcomes in various cancers [35, 36] and coronary artery disease [37, 38]. Similarly, in a study, lymphocyte and monocyte counts were measured before and 24 hours following mechanical thrombectomy in AIS patients. In that study, a lower LMR 24 hours after mechanical thrombectomy significantly predicted impaired functional prognosis. However, admission LMR was not significant as a predictor of 3-month mRS [8].
Two possible explanations support the association between plasma CRP after an ischemic stroke and clinical outcomes. The first scenario involves worsening clinical outcomes after cerebral infarction caused by CRP. However, if CRP serves a beneficial rather than a harmful function by eliminating necrotic and apoptotic cells [39], plasma CRP may increase to counteract an exacerbating factor. For example, a recent study showed that the administration of pure human CRP to healthy adult volunteers showed no proinflammatory effects [40]. Further, CRP-deficient mice do not offer a reduced risk of atherosclerosis, debunking the concept that CRP might contribute to atherosclerosis [41]. There is a need for further studies to investigate plasma CRP’s pathophysiological role in acute ischemic stroke. As a result of this investigation, those with acute ischemic stroke who died or had an unfavorable outcome had higher CRP levels and lower ECR. A published prospective case-control investigation demonstrated that increased CRP upon hospitalization independently predicted functional prognosis one month after an acute ischemic stroke [42]. Similarly, in a study, higher serum CRP levels were significantly associated with unfavorable outcomes after AIS [14].
Conclusion
In this study, ECR within 24 hours of symptoms onset was related to functional outcomes and mortality at 3-month follow-up, suggesting ECR as a cost-effective and useful prognostic indicator.
Study limitations
The current study’s main limitation was the COVID-19 outbreak, which eliminated many patients suspected of being infected. At the same time, it was impossible to state whether they were infected. Also, in our study, one limitation was that we did not know exactly when the blood samples were collected, even if they were collected within 24 hours. Thus, the time elapsed from stroke onset could not be adjusted. It is also necessary to further investigate the effect of other inflammatory cytokines, such as interleukin (IL)-8, IL-6, IL-4, and IL-1 on long-term outcomes in AIS.
Stroke severity is one of the main determinants of mortality and poor prognosis, which should have been included as a confounding variable, or the study was conducted in patients with a more limited range of stroke severity. However, it was not possible because of the limited number of samples.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki 2013. The study protocol was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1398.506).
Funding
This work was supported by the Student Research Committee (SRC) of Guilan University of Medical Sciences.
Authors contributions
Conceptualization and study design: Arman Keymoradzadeh and Alia Saberi; Data acquisition: Parastoo Mohammadi, Amirhossein Roshan, and Alia Saberi; Statistical analysis: Arman Keymoradzadeh; Data interpretation: Alia Saberi, Arman Keymoradzadeh, Amirhossein Roshan, and Parastoo Mohammadi; Writing–original draft: Alia Saberi, Arash Bakhshi, and Arman Keymoradzadeh; Data analysis, writing the original draft, review, editing, and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank all the members of the Student Research Committee of Guilan University of Medical Sciences for their participation in this study.
References
Strokes are the world’s second most common mortality cause and the third most common disability-causing condition [1]. Early prediction of stroke outcomes could benefit patients and can assist clinicians in ensuring effective stroke treatment and functional recovery when individuals with stroke receive hospitalization. In this regard, researchers have studied biomarkers that can predict stroke treatment response and outcome. They can vary significantly from patient to patient. Several factors affect the prognosis of stroke, including stroke type, patient age, and stroke severity [2, 3, 4]. Some studies suggest laboratory findings are prognostic factors in acute ischemic stroke (AIS) [5, 6].
An important pathophysiologic feature of AIS is inflammation [7]. A wealth of evidence suggests that immune system components play a crucial role by initiating and propagating ischemic neurological damage to the brain. The development of the immunosuppressive response to brain ischemia could lead to concurrent infectious diseases [8]. Lymphocyte-monocyte ratio (LMR) and neutrophil-lymphocyte ratio (NLR) have been reported as potential biomarkers of baseline inflammation and AIS morbidity and mortality [9, 10, 11]. However, the platelet-lymphocyte ratio (PLR) offers significantly better prognostic value than single platelet counts in stroke. It is an inexpensive, readily accessible, and comprehensive marker of inflammatory processes. PLR offers primarily two main potential benefits: 1) An integrated measure that provides supplementary data in addition to current measures and 2) More consistency compared to individual blood parameters, which are susceptible to variation due to dehydration, excessive hydration, and the condition of the blood samples [12]. The C-reactive protein (CRP), an acute-phase protein, is the most widely used marker of inflammation in peripheral blood [13]. A higher level of CRP in the blood is also independently associated with a greater risk of future vascular events or mortality [14]. We found that few studies examined the association of AIS prognosis and mortality with NLR, PLR, LMR, erythrocyte sedimentation rate (ESR), CRP, and ESR-CRP ratio (ECR) variables. Accordingly, this investigation aimed to determine the potential associations of NLR, PLR, LMR, and ECR levels with the AIS prognosis (defined as mRS score) and mortality within 3 months after stroke.
Materials and Methods
We conducted the present cross-sectional investigation at an academic hospital in northern Iran. This study included 614 AIS patients admitted between September 2019 and June 2021 for analysis. This study was conducted using subjects who met with all (2) these conditions: 1) Adults (over the age of 18), 2) Documented clinical diagnosis of AIS. The exclusion criteria constituted 1) Those with terminal cancers, hematological disorders, recently undergoing major injuries or surgeries, and severe liver, other neurological, or renal diseases based on clinical history and laboratory findings; 2) Those taking immunosuppressive medications; 3) History of active infectious diseases within two weeks before admission, myocardial infarction within 4 weeks before admission; 4) Usage of steroid, anti-platelet or anti-coagulant; 5) Patients suspected of having COVID-19 based on the findings of blood sample tests (leukocytosis, lymphopenia and elevated CRP or chest CT scans; 6) Individuals who reported symptoms of COVID-19 subjectively at any stage of the study.
Information obtained from clinical documentation included age, gender, predisposing diseases, duration of hospitalization, smoking, and rehabilitation after stroke (10 sessions or more). After applying the inclusion-exclusion criteria, 241 individuals were not eligible for inclusion. Moreover, 32 participants did not follow up with the research team 3 months after their acute ischemic stroke.
Demographic characteristics such as age, sex, duration of hospitalization, and baseline vascular risk factors (smoking, diabetes mellitus, dyslipidemia, hypertension, previous strokes, atrial fibrillation, and coronary artery diseases) were obtained using the institution’s databases. Laboratory testing of the blood samples was performed no later than 24 hours from the onset of symptoms. Laboratory and imaging data were collected using an automated testing method. Laboratory findings included a total blood count with white blood cell differentials, urea and electrolytes, hepatic function assessments, and ESR and CRP checked on admission.
A neurology specialist and 3 medical students assessed the outcomes with the modified rankin scale (mRS) [15] following 90 days after the initial assessment.
Statistical analysis
Data analysis was conducted using descriptive statistics such as percentage, frequency, and Mean±SD. We performed the Kolmogorov-Smirnov test to assess the normality of the results and applied Levene’s test for variance homogeneity. We utilized the Spearman’s rank correlation coefficient for determining the associations of NLR, LMR, PLR, ESR, CRP, and ECR with 3-month clinical outcomes. The Mann-Whitney U test was used to compare means between alive and dead patients. Linear or ordinal regression analyses were conducted to examine the interplay among multiple independent factors on outcomes. We ran the analysis using statistics of IBM SPSS software, version 26 at a significance level of P<0.05.
Results
Three hundred and forty-one subjects were included in the study, and their information was analyzed. As shown in Table 1, the sample population consists of the following general characteristics and laboratory results.

The correlation of NLR, LMR, PLR, ESR, CRP, and ECR levels with 3-month mRS
The univariate analysis shows that higher NLR, RLR, and CRP and lower LMR and ECR indicate poorer outcomes based on the 3-month mRS (Table 2).

Although a significant correlation exists between NLR, RLR, CRP, LMR, and ECR with 3-month mRS, the fact that r values are less than 0.4 suggests the study variables lack a strong correlation. After adjusting for age, gender, mRS upon hospital discharge, smoking, duration of hospitalization, stroke rehabilitation, and underlying diseases, no significant relationship was found between NLR, LMR PLR, CPR, ECR, and 3-month mRS (Table 3).

The correlations of NLR, LMR, PLR, ESR, CRP, and ECR levels with 3 months mortality in AIS patients
The three-month follow-up showed that 128 stroke patients (46.2%) had died, while 149 patients (53.8%) survived (Table 4).

Patients who died had a higher age compared to those who survived. There were no significant differences in other variables among participants. The univariate analysis showed that NLR, PLR, and CRP were higher among those who lost their lives within three months than the survivors. However, LMR and ECR were lower in those who died. In multivariate analysis, ECR remained independently associated with 3 months mortality (P<0.05), but NLR, LMR, PLR, ESR, and CRP were not associated with 3 months mortality (Table 5).

Discussion
Our investigation evaluated the clinical significance of LMR, PLR, NLR, ESR, CRP, and ECR in predicting 3-month functional outcomes and mortality after acute ischemic stroke. Based on the univariate analysis, NLR, PLR, and CRP were higher among those who lost their lives within 3 months than the survivors, but LMR and ECR were lower in those who died. In multivariate analysis, ECR remained independently associated with the 3-month mortality rate, but the relationship between other variables and mortality was not significant. The present research findings show a weak relationship exists between NLR, PLR, CRP, LMR, and ECR with the 3-month mRS. Monocytes, lymphocytes, platelets, and neutrophils may have distinct roles in inflammatory processes and the development of different diseases. When AIS occurs, platelets do not function normally [16], leading to overactivation and accumulation of platelets and causing clots and blockage of blood vessels [17]. It has been shown that high neutrophil counts confer an adverse prognosis on cardiovascular patients, whereas high lymphocyte counts confer a protective effect [18, 19]. In acute ischemic events, stress activates the hypothalamic-pituitary-adrenal system.
Consequently, a greater level of cortisol release results in a decreased level of lymphocytes [20]. Considering these factors separately may miss their interactions and associations with different medical conditions, yet exploring them together may not shed any light on the opposing roles they seem to have. Therefore, dynamic measurements of NLR, MLR, and PLR may serve as a more accurate outcome measurement than single measurements.
Firstly, our data indicated high NLR was associated with unfavorable 3-month functional outcomes and death. In previous studies, higher NLR was associated with poor functional outcomes at discharge from the hospital during the first 3 days of the stroke occurrence [21]. Also, studies have shown that increased NLR among those with acute myocardial infarction reliably predicted death and morbidity during hospitalization [22], along with inefficient heart perfusion following percutaneous coronary angioplasty [23]. In another study, a high admission NLR is associated with independent prediction of function, recanalization, and treatment with IV rtPA (recombinant tissue plasminogen activator) after AIS [11].
In addition, our data indicated that elevated levels of PLR significantly predicted poor outcomes in terms of function and mortality within 3 months. PLR might play a role in determining the outcome of acute ischemic strokes. It has been shown that platelets and lymphocytes determine the outcome of ischemic vascular diseases, such as myocardial infarction and cerebral infarction [24–27]. PLR data may offer useful information about ischemic events. There have been several studies examining this perspective. In individuals with acute myocardial infarction, PLR was an independent predictor of mortality and incidence of major adverse cardiovascular events in [28] hospital and long-term [29, 30]. A high PLR level was observed in unstable angina pectoris sufferers having impaired coronary collateral circulation [31]. Gary et al. [32] also identified a significant association between elevated PLR and increased chances of severe limb ischemia in critically ill patients. This association may be useful in identifying patients with an increased risk of vascular complications. Studies of cerebrovascular diseases have shown that a higher PLR is associated with stroke [33]. Another study used A high PLR value as an indirect measure of stroke patients’ infarcted area and a relatively low recanalization rate following thrombectomy treatment [34].
Our data indicated low LMR levels were associated with unfavorable 3-month functional outcomes and death. According to the reports, LMR is linked to poor outcomes in various cancers [35, 36] and coronary artery disease [37, 38]. Similarly, in a study, lymphocyte and monocyte counts were measured before and 24 hours following mechanical thrombectomy in AIS patients. In that study, a lower LMR 24 hours after mechanical thrombectomy significantly predicted impaired functional prognosis. However, admission LMR was not significant as a predictor of 3-month mRS [8].
Two possible explanations support the association between plasma CRP after an ischemic stroke and clinical outcomes. The first scenario involves worsening clinical outcomes after cerebral infarction caused by CRP. However, if CRP serves a beneficial rather than a harmful function by eliminating necrotic and apoptotic cells [39], plasma CRP may increase to counteract an exacerbating factor. For example, a recent study showed that the administration of pure human CRP to healthy adult volunteers showed no proinflammatory effects [40]. Further, CRP-deficient mice do not offer a reduced risk of atherosclerosis, debunking the concept that CRP might contribute to atherosclerosis [41]. There is a need for further studies to investigate plasma CRP’s pathophysiological role in acute ischemic stroke. As a result of this investigation, those with acute ischemic stroke who died or had an unfavorable outcome had higher CRP levels and lower ECR. A published prospective case-control investigation demonstrated that increased CRP upon hospitalization independently predicted functional prognosis one month after an acute ischemic stroke [42]. Similarly, in a study, higher serum CRP levels were significantly associated with unfavorable outcomes after AIS [14].
Conclusion
In this study, ECR within 24 hours of symptoms onset was related to functional outcomes and mortality at 3-month follow-up, suggesting ECR as a cost-effective and useful prognostic indicator.
Study limitations
The current study’s main limitation was the COVID-19 outbreak, which eliminated many patients suspected of being infected. At the same time, it was impossible to state whether they were infected. Also, in our study, one limitation was that we did not know exactly when the blood samples were collected, even if they were collected within 24 hours. Thus, the time elapsed from stroke onset could not be adjusted. It is also necessary to further investigate the effect of other inflammatory cytokines, such as interleukin (IL)-8, IL-6, IL-4, and IL-1 on long-term outcomes in AIS.
Stroke severity is one of the main determinants of mortality and poor prognosis, which should have been included as a confounding variable, or the study was conducted in patients with a more limited range of stroke severity. However, it was not possible because of the limited number of samples.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki 2013. The study protocol was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1398.506).
Funding
This work was supported by the Student Research Committee (SRC) of Guilan University of Medical Sciences.
Authors contributions
Conceptualization and study design: Arman Keymoradzadeh and Alia Saberi; Data acquisition: Parastoo Mohammadi, Amirhossein Roshan, and Alia Saberi; Statistical analysis: Arman Keymoradzadeh; Data interpretation: Alia Saberi, Arman Keymoradzadeh, Amirhossein Roshan, and Parastoo Mohammadi; Writing–original draft: Alia Saberi, Arash Bakhshi, and Arman Keymoradzadeh; Data analysis, writing the original draft, review, editing, and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank all the members of the Student Research Committee of Guilan University of Medical Sciences for their participation in this study.
References
- Nutakki A, Chomba M, Chishimba L, Zimba S, Gottesman RF, Bahouth MN, et al. Risk factors and outcomes of hospitalized stroke patients in Lusaka, Zambia. J Neurol Sci. 2021; 424:117404. [DOI:10.1016/j.jns.2021.117404] [PMID] [PMCID]
- Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC; German Stroke Study Collaboration. Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: Development and external validation of prognostic models. Stroke. 2004; 35(1):158-62. [DOI:10.1161/01.STR.0000106761.94985.8B] [PMID]
- Saposnik G, Kapral MK, Liu Y, Hall R, O’Donnell M, Raptis S, et al. IScore: A risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation. 2011; 123(7):739-49. [DOI:10.1161/CIRCULATIONAHA.110.983353] [PMID]
- Koennecke HC, Belz W, Berfelde D, Endres M, Fitzek S, Hamilton F, et al. Factors influencing in-hospital mortality and morbidity in patients treated on a stroke unit. Neurology. 2011; 77(10):965-72. [DOI:10.1212/WNL.0b013e31822dc795] [PMID]
- Ntaios G, Gurer O, Faouzi M, Aubert C, Michel P. Red cell distribution width does not predict stroke severity or functional outcome. Int J Stroke. 2012; 7(1):2-6. [DOI:10.1111/j.1747-4949.2011.00609.x] [PMID]
- Hatamian H, Saberi A, Pourghasem M. The relationship between stroke mortality and red blood cell parameters. Iran J Neurol. 2014; 13(4):237-40. [PMID]
- Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nat Med. 2011; 17(7):796-808. [DOI:10.1038/nm.2399] [PMID] [PMCID]
- Lux D, Alakbarzade V, Bridge L, Clark CN, Clarke B, Zhang L, et al. The association of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio with 3-month clinical outcome after mechanical thrombectomy following stroke. J Neuroinflammation. 2020; 17(1):60. [DOI:10.1186/s12974-020-01739-y] [PMID] [PMCID]
- Tokgoz S, Kayrak M, Akpinar Z, Seyithanoğlu A, Güney F, Yürüten B. Neutrophil lymphocyte ratio as a predictor of stroke. J Stroke Cerebrovasc Dis. 2013; 22(7):1169-74. [DOI:10.1016/j.jstrokecerebrovasdis.2013.01.011] [PMID]
- Zhu B, Pan Y, Jing J, Meng X, Zhao X, Liu L, et al. Neutrophil counts, neutrophil ratio, and new stroke in minor ischemic stroke or TIA. Neurology. 2018; 90(21):e1870-8. [DOI:10.1212/WNL.0000000000005554] [PMID]
- Brooks SD, Spears C, Cummings C, VanGilder RL, Stinehart KR, Gutmann L, et al. Admission neutrophil-lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J Neurointerv Surg. 2014; 6(8):578-83. [DOI:10.1136/neurintsurg-2013-010780] [PMID] [PMCID]
- Balta S, Ozturk C. The platelet-lymphocyte ratio: A simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets. 2015; 26(7):680-1. [DOI:10.3109/09537104.2014.979340] [PMID]
- Torzewski J, Fan J, Schunkert H, Szalai A, Torzewski M. C-reactive protein and arteriosclerosis. Mediators Inflamm. 2014; 2014:646817. [DOI:10.1155/2014/646817] [PMID] [PMCID]
- Matsuo R, Ago T, Hata J, Wakisaka Y, Kuroda J, Kuwashiro T, et al. Plasma C-reactive protein and clinical outcomes after acute ischemic stroke: A prospective observational study. PLoS One. 2016; 11(6):e0156790. [DOI:10.1371/journal.pone.0156790] [PMID] [PMCID]
- Xu JH, He XW, Li Q, Liu JR, Zhuang MT, Huang FF, et al. Higher platelet-to-lymphocyte ratio is associated with worse outcomes after intravenous thrombolysis in acute ischaemic stroke. Front Neurol. 2019; 10:1192. [DOI:10.3389/fneur.2019.01192] [PMID] [PMCID]
- Xu XR, Zhang D, Oswald BE, Carrim N, Wang X, Hou Y, et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci. 2016; 53(6):409-30. [DOI:10.1080/10408363.2016.1200008] [PMID]
- Franks ZG, Campbell RA, Weyrich AS, Rondina MT. Platelet-leukocyte interactions link inflammatory and thromboembolic events in ischemic stroke. Ann N Y Acad Sci. 2010; 1207:11-7. [DOI:10.1111/j.1749-6632.2010.05733.x] [PMID] [PMCID]
- Esquinas-Requena JL, Lozoya-Moreno S, García-Nogueras I, Atienzar-Núñez P, Sánchez-Jurado PM, Abizanda P. [Anemia increases mortality risk associated with frailty or disability in older adults. The FRADEA study (Spanish)]. Aten Primaria. 2020; 52(7):452-61. [DOI:10.1016/j.aprim.2019.07.001] [PMID] [PMCID]
- Sathvik M, Vuppuluri K, Dulipala P. The association of the neutrophil-lymphocyte ratio with the outcome of diabetic foot ulcer. Cureus. 2023; 15(1):e33891. [DOI:10.7759/cureus.33891]
- Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM, et al. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol. 2016; 68(3):265-71. [DOI:10.1016/j.jacc.2016.04.053] [PMID] [PMCID]
- Yu S, Arima H, Bertmar C, Clarke S, Herkes G, Krause M. Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. J Neurol Sci. 2018; 387:115-8. [DOI:10.1016/j.jns.2018.02.002] [PMID]
- Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008; 102(6):653-7. [DOI:10.1016/j.amjcard.2008.05.006] [PMID]
- Akpek M, Kaya MG, Lam YY, Sahin O, Elcik D, Celik T, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012; 110(5):621-7. [DOI:10.1016/j.amjcard.2012.04.041] [PMID]
- Mueller C, Neumann FJ, Hochholzer W, Trenk D, Zeller T, Perruchoud AP, et al. The impact of platelet count on mortality in unstable angina/non-ST-segment elevation myocardial infarction. Am Heart J. 2006; 151(6):1214-e1. [DOI:10.1016/j.ahj.2006.03.011] [PMID]
- Yang M, Pan Y, Li Z, Yan H, Zhao X, Liu L, et al. Platelet count predicts adverse clinical outcomes after ischemic stroke or TIA: Subgroup analysis of CNSR II. Front Neurol. 2019; 10:370. [DOI:10.3389/fneur.2019.00370] [PMID] [PMCID]
- Forteza MJ, Trapero I, Hervas A, de Dios E, Ruiz-Sauri A, Minana G, et al. Apoptosis and mobilization of lymphocytes to cardiac tissue is associated with myocardial infarction in a reperfused porcine model and infarct size in post-PCI patients. Oxid Med Cell Longev. 2018; 2018:1975167. [DOI:10.1155/2018/1975167] [PMID] [PMCID]
- Zierath D, Tanzi P, Shibata D, Becker KJ. Cortisol is more important than metanephrines in driving changes in leukocyte counts after stroke. J Stroke Cerebrovasc Dis. 2018; 27(3):555-62. [DOI:10.1016/j.jstrokecerebrovasdis.2017.09.048] [PMID] [PMCID]
- GBD 2016 epilepsy collaborators. Global, regional, and national burden of epilepsy, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019; 18(4):357-75. [DOI:10.1016/S1474-4422(18)30454-X] [PMID]
- Ozcan Cetin EH, Cetin MS, Aras D, Topaloglu S, Temizhan A, Kisacik HL, et al. Platelet to lymphocyte ratio as a prognostic marker of in-hospital and long-term major adverse cardiovascular events in ST-segment elevation myocardial infarction. Angiology. 2016; 67(4):336-45. [DOI:10.1177/0003319715591751] [PMID]
- Temiz A, Gazi E, Güngör Ö, Barutçu A, Altun B, Bekler A, et al. Platelet/lymphocyte ratio and risk of in-hospital mortality in patients with ST-elevated myocardial infarction. Med Sci Monit. 2014; 20:660-5. [DOI:10.12659/MSM.890152] [PMID] [PMCID]
- Açar G, Kalkan ME, Avci A, Alizade E, Tabakci MM, Toprak C, et al. The relation of platelet-lymphocyte ratio and coronary collateral circulation in patients with stable angina pectoris and chronic total occlusion. Clin Appl Thromb Hemost. 2015; 21(5):462-8. [DOI:10.1177/1076029613508599] [PMID]
- Gary T, Pichler M, Belaj K, Hafner F, Gerger A, Froehlich H, et al. Platelet-to-lymphocyte ratio: A novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS One. 2013; 8(7):e67688. [DOI:10.1371/journal.pone.0067688] [PMID] [PMCID]
- Altintas O, Tasal A, Niftaliyev E, Kucukdagli OT, Asil T. Association of platelet-to-lymphocyte ratio with silent brain infarcts in patients with paroxysmal atrial fibrillation. Neurol Res. 2016; 38(9):753-8. [DOI:10.1080/01616412.2016.1210357] [PMID]
- Altintas O, Altintas MO, Tasal A, Kucukdagli OT, Asil T. The relationship of platelet-to-lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone endovascular therapy. Neurol Res. 2016; 38(9):759-65. [DOI:10.1080/01616412.2016.1215030] [PMID]
- Zhu JY, Liu CC, Wang L, Zhong M, Tang HL, Wang H. Peripheral blood lymphocyte-to-monocyte ratio as a prognostic factor in advanced epithelial ovarian cancer: A multicenter retrospective study. J Cancer. 2017; 8(5):737-43. [DOI:10.7150/jca.17668] [PMID] [PMCID]
- Song W, Tian C, Wang K, Zhang R, Zou SB. The pretreatment lymphocyte to monocyte ratio predicts clinical outcome for patients with hepatocellular carcinoma: A meta-analysis. Sci Rep. 2017; 7:46601. [DOI:10.1038/srep46601] [PMID] [PMCID]
- Ji H, Li Y, Fan Z, Zuo B, Jian X, Li L, et al. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: A syntax score assessment. BMC Cardiovasc Disord. 2017; 17(1):90. [DOI:10.1186/s12872-017-0507-4] [PMID] [PMCID]
- Kiris T, Çelik A, Variş E, Akan E, Akyildiz ZI, Karaca M, et al. Association of lymphocyte-to-monocyte ratio with the mortality in patients with ST-elevation myocardial infarction who underwent primary percutaneous coronary intervention. Angiology. 2017; 68(8):707-15. [DOI:10.1177/0003319716685480] [PMID]
- Di Napoli M, Schwaninger M, Cappelli R, Ceccarelli E, Di Gianfilippo G, Donati C, et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: A statement for health care professionals from the CRP Pooling Project members. Stroke. 2005; 36(6):1316-29. [DOI:10.1161/01.STR.0000165929.78756.ed] [PMID]
- Lane T, Wassef N, Poole S, Mistry Y, Lachmann HJ, Gillmore JD, et al. Infusion of pharmaceutical-grade natural human C-reactive protein is not proinflammatory in healthy adult human volunteers. Circ Res. 2014; 114(4):672-6. [DOI:10.1161/CIRCRESAHA.114.302770] [PMID]
- Teupser D, Weber O, Rao TN, Sass K, Thiery J, Fehling HJ. No reduction of atherosclerosis in C-reactive protein (CRP)-deficient mice. J Biol Chem. 2011; 286(8):6272-9. [DOI:10.1074/jbc.M110.161414] [PMID] [PMCID]
- Abubakar SA, Okubadejo NU, Ojo OO, Oladipo O, Ojini FI, Danesi MA. Relationship between admission serum C-reactive protein and short term outcome following acute ischaemic stroke at a tertiary health institution in Nigeria. Niger J Clin Pract. 2013; 16(3):320-4. [DOI:10.4103/1119-3077.113454] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2023/10/11 | Accepted: 2023/10/17 | Published: 2023/10/17
Received: 2023/10/11 | Accepted: 2023/10/17 | Published: 2023/10/17
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



