Fri, Sep 20, 2024
Volume 7, Issue 1 (Winter 2021)
Caspian J Neurol Sci 2021, 7(1): 10-16 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shaygannejad V, Barzegar M, Manouchehri N, Esmaeil N, Nehzat N, Badihian S, et al . Anti-myelin Oligodendrocyte Glycoprotein in Aquaporin-4 Negative Neuromyelitis Optica Spectrum Disorder. Caspian J Neurol Sci 2021; 7 (1) :10-16
URL: http://cjns.gums.ac.ir/article-1-383-en.html
URL: http://cjns.gums.ac.ir/article-1-383-en.html
Vahid Shaygannejad * 

 1, Mahdi Barzegar2
1, Mahdi Barzegar2 

 , Navid Manouchehri2
, Navid Manouchehri2 

 , Nafiseh Esmaeil2
, Nafiseh Esmaeil2 

 , Nasim Nehzat2
, Nasim Nehzat2 

 , Shervin Badihian2
, Shervin Badihian2 

 , Fereshteh Ashtari2
, Fereshteh Ashtari2 

 , Omid Mirmosayyeb2
, Omid Mirmosayyeb2 




 1, Mahdi Barzegar2
1, Mahdi Barzegar2 

 , Navid Manouchehri2
, Navid Manouchehri2 

 , Nafiseh Esmaeil2
, Nafiseh Esmaeil2 

 , Nasim Nehzat2
, Nasim Nehzat2 

 , Shervin Badihian2
, Shervin Badihian2 

 , Fereshteh Ashtari2
, Fereshteh Ashtari2 

 , Omid Mirmosayyeb2
, Omid Mirmosayyeb2 


1- Department of Neurology, Isfahan Neurosciences Research Center, Alzahra Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran. , v.shaygannejad@gmail.com
2- Department of Neurology, Isfahan Neurosciences Research Center, Alzahra Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Department of Neurology, Isfahan Neurosciences Research Center, Alzahra Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran.
Full-Text [PDF 1219 kb]
(717 Downloads)
| Abstract (HTML) (1624 Views)
Full-Text: (723 Views)
Introduction
euromyelitis Optica Spectrum Disorder (NMOSD) is a demyelinating autoimmune disorder associated with optic neuritis and acute myelitis involving multiple spinal levels. Although the (AQP4-Ab also known as NMO-IgG) is positive in most NMOSD patients, there are patients with negative serology for NMO-IgG. The absence of NMO-IgG in 10% to 40% of the NMOSD patients prompts the search for other serologic markers in this disease [1].
A candidate target for such investigation is the Myelin Oligodendrocyte Glycoprotein (MOG) [2]. MOG is a protein component of the myelin sheets encapsulating the neural fibers. MOG is found exclusively in the central nervous system, highlighting its possible role in triggering the autoimmune responses in the brain and spinal cord while sparing the peripheral neural structures. Anti-MOG antibodies are found in a fraction of the patients diagnosed with NMOSD, most frequently in AQP-Ab seronegative patients [3]. Autoimmune diseases are best treated when the correct diagnosis is achieved early during the illness, and proper medications are administered. Therefore, finding serologic markers that guide the diagnosis process is of interest. Accordingly, in the present study, we assessed the presence of anti-MOG antibodies in a group of NMOSD patients with negative results for NMO-IgG and compared the measured levels with a healthy control group.
Materials and Methods
Study design
In this study, NMOSD patients with established negative results for NMO-IgG referred to the Neurology Clinic of Kashani University Hospital in Isfahan City, Iran, were consecutively enrolled in the study from March 2015 to March 2016. All patients were previously diagnosed as NMOSD cases according to international consensus diagnostic criteria in 2015 [4]. All patients were assessed with two neurologists with a specialist in Central Nervous System (CNS) Acute Disseminated Encephalomyelitis (ADEM) Vahid Shaygannejad and Fereshteh Ashtari in each patient, the NMOSD patients were enrolled in the study. Healthy controls, matched for age and sex, were also randomly enrolled in the study. Healthy controls were selected from the general population. They were caregivers of other patients with no history of neurological or autoimmune disorders. Any ambiguity regarding the confirmation of patients’ diagnosis, presence of other neurologic or muscular disorders, any history of head or spine injuries, and recent immunosuppressant therapy were defined as the exclusion criteria. To calculate the sample size, we used the equation to compare two means with a type I error of 1.96 and power of 0.84. We assess the previous study to determine the assumptions in two groups [5]. The sample size was calculated as at least 30 participants in each group. Informed consent was taken from all patients at the time of enrollment.
Data collection
Demographic and baseline data, including age, duration of the disease, initial symptoms of the disease, and MRI findings, were collected from all patients. Brain sequences included axial Posterior Duplication (PD), T2W, T2-FLAIR, contrast-enhanced-T1W, and PD. The following spine sequences were acquired: sagittal T2W, STIR, contrast-enhanced T1W, axial T2W, and contrast-enhanced T1W. Disease severity level and clinical disability were assessed in patients using the Extended Disability Status Scale (EDSS). The negative NMO-IgG status of the patients was also double-checked for all of the enrolled patients.
All patients and controls were then subjected to a session of venous blood sampling. The samples were tested for anti-MOG levels using the SensoLyte® Anti-Human MOG (1-125) Human quantitative ELISA Kit (AnaSpec, Fremont, CA, USA).
Data analysis
The obtained data were analyzed using the SPSS (version 18.0, Chicago, IL, USA). The categorical data were reported by using frequency reporting measures. The Student t-test was used to compare the quantitative data. When our sample data were not normally distributed, a non-parametric (Mann-Whitney U) test was used instead. The Chi-square test was used to assess the significance of differences between the categorical data. The correlations between data sets were investigated using the Pearson correlation coefficient. We defined r <0.5 as mild to moderate correlation and r >0.5 as strong correlation. The obtained data are represented as mean±SD or SEM, and statistical significance was defined as a two-tailed P-value of less than 0.05.
Results
A total of 30 seronegative NMOSD patients for NMO-Ab and 26 healthy controls were consecutively enrolled in the study. The data of 4 healthy controls (from 30 subjects) were missed and considered a dropout of the study. The mean±SD ages of the cases and controls were 32.9±7.7 and 33.9±8.7 years, respectively (P=0.356). Among cases and controls, 71.9% and 73.3% of the patients were female, respectively (P=0.931). The mean±SD of EDSS scores for the patients was 1.2±0.46, and the disease duration was 4.8±4.0.4 years. Among the patients, variables in the disease profile were not significantly different.
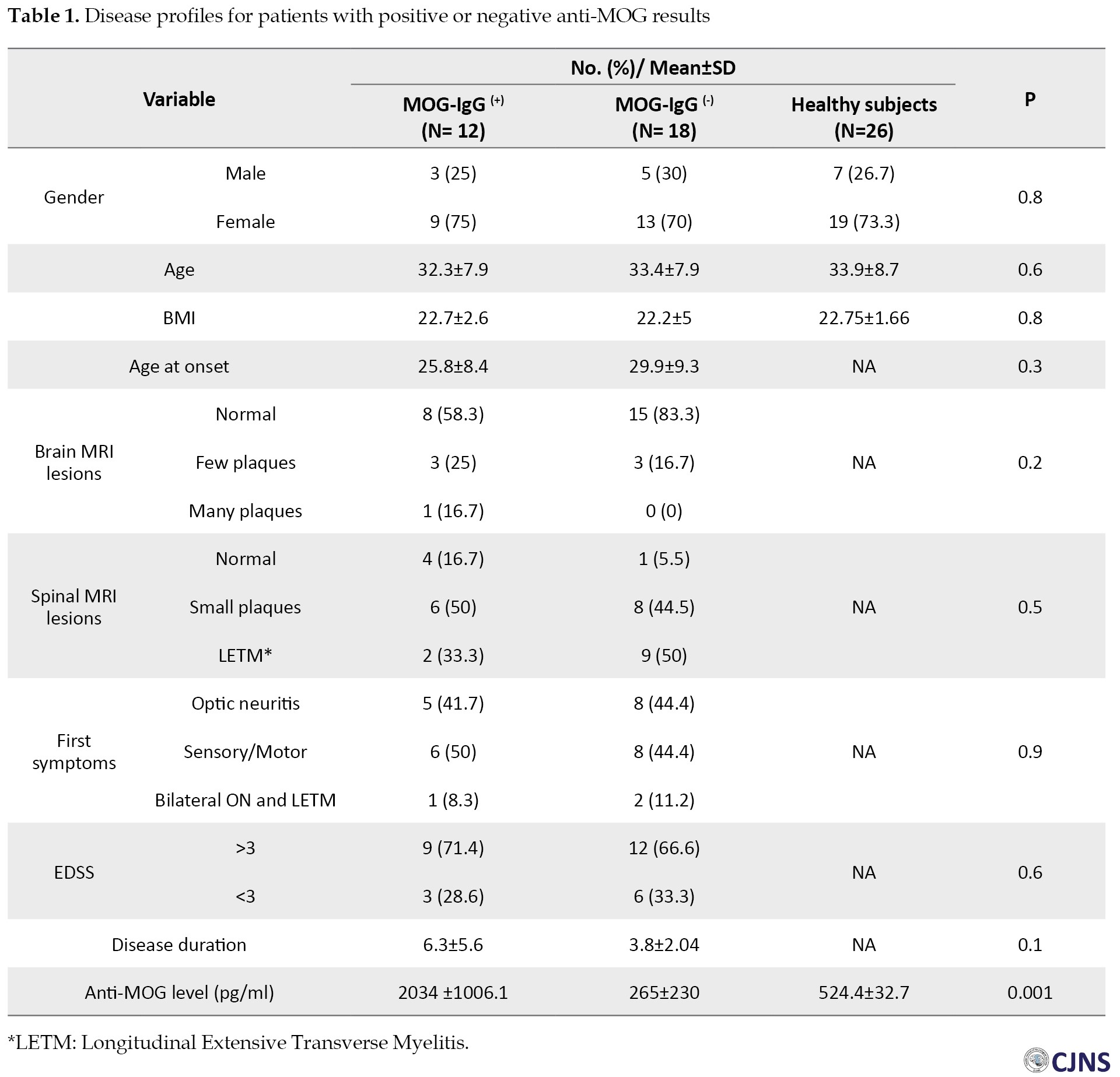
The anti-MOG test results were positive in 37.5% (n=12) and 0% (n= 0) of the patients and controls, respectively (P<0.0001). The mean±SEM values of the anti-MOG level of patients and controls were 966.5±201 and 524.4±32.7 pg/ml, respectively; as shown in Figure 1, the difference was statistically significant between the two groups (P=0.047). The mean±SEM value of anti-MOG level among patients with positive anti-MOG results was 2034±1006.1 pg/ml, and the difference of anti-MOG levels was statistically significant (P<0.0001) compared to the control group (Figure 2). Furthermore, the mean±SEM value of anti-MOG level among patients with a negative anti-MOG result was 265±230 pg/ml; patients with negative anti-MOG results had significantly lower anti-MOG levels compared to the control group (Figure 2) (P<0.001). Among the patients, variables in the disease profile were not significantly different between the anti-MOG positive and anti-MOG negative groups; however, anti-MOG levels were significantly (P<0.0001) different between the two patient groups (Figure 2). Table 1 presents the disease profiles in patients with positive and negative anti-MOG results.
There was no significant correlation between the level of anti-MOG and EDSS scores among patients (r=0.015, P=0.9). Also, no significant correlation was found between the anti-MOG levels and disease duration among patients (r=0.3, P=0.07).
Discussion
NMOSD correlation with NMO-IgG has been well established in the literature; however, some patients have clinical and radiologic findings of NMOSD without a positive serology for NMO-IgG. It has also been reported that in NMOSD patients, several autoantibodies are detectable, including anti-PLP, anti-MBP, anti-S100 beta, and anti-MOG [1, 2, 6]. However, anti-MOG stands out compared to the rest [6]. In this study, we measured the level of anti-MOG antibodies in a group of NMOSD patients with confirmed negative serology for NMO-IgG. The MOG-Ab was positive in a fraction (37.5%) of these cases, and its level was significantly higher compared to both the patients with negative MOG-Ab serology and healthy controls.
In NMOSD patients, anti-MOG could be proven as a secondary disease marker. However, MOG-Ab is also associated with other demyelinating diseases such as Acute Disseminated Encephalomyelitis (ADEM) and pediatric multiple sclerosis [7]. Unlike the NMO-IgG that was reported to be higher only in NMOSD patients than MS patients, elevated anti-MOG levels that were checked by the ELISA method have been reported in both MS and NMOSD patients without a significant difference by Chen et al. [8]. However, Correale et al. measured MOG-Ab using ELISPOT assays and reported a high frequency of MOG-Ab among NMOSD patients [9].
The role of these antibodies in the pathogenesis of NMOSD and other demyelinating autoimmune diseases has been studied. Autoimmune diseases of the central nervous system display a variety of symptoms, each different from the other. An NMO-IgG induced disease involves females, while the anti-MOG-induced disease has a higher male-to-female ratio. However, we did not find a significant difference between males and females in anti-MOG levels. MS patients are more often younger than patients with other diseases such as NMO-IgG positive NMOSD or MOG-ab positive patients. However, worse destructive events are observed among the anti-MOG induced cases.
In contrast, the patients with an NMO-IgG induced disease tend to have a worse prognosis regarding visual acuity [10]. This could be due to longer axial lesions during the acute phase of optic neuritis among NMO-IgG positive patients, which serves as a predictive factor for a worse prognosis [11]. The differences involve the MRI findings as well. When brain MRI of children with CNS autoimmune diseases was compared based on their anti-MOG status measured using live cell-based assays, it was revealed that anti-MOG seropositive cases had fewer periventricular demyelinating plaques and did not involve corpus callosum [12, 13]. NMO-IgG negative, anti-MOG positive NMOSD patients are younger and more men. The disease is often monophasic and included simultaneous optic neuritis and myelitis compared to NMO-IgG seropositive cases [14], while the relapse was more probable among the latter [15]. The anti-MOG-induced disease is also reported to have a more benign pattern [14]. MRI of the spinal cord lesions is less extended longitudinally in comparison to the NMO-IgG positive patients.
On the other hand, Ramanathan et al. assessed patients with positive cell-based assay anti-MOG and reported anti-MOG induced bilateral optic neuritis is more likely to be relapsing and responds well to long-term steroid treatment [16]. The MRI findings between anti-MOG positive and negative groups in our study were not significantly different. Hamid et al. tested MOG-Ab by cell-based assay and have reported more cortical lesions in MOG-Ab positive patients compared to AQP4-Ab NMOSD patients [17]. Additionally, in a nationwide study, Papp et al. tested the presence of anti-MOG in different methods, including tissue-based immunofluorescence assay, cell-based assay, ELISA kit, and in vitro translation/immunoprecipitation assay. They have assessed the presence of anti-MOG in two groups of MS and NMOSD patients, and consistent with our finding, they suggested that anti-MOG is associated with a broad range of clinical manifestations and should be considered in patients with atypical features [18]. The EDSS was also slightly lower in the MOG-Ab positive group and consistent with this finding, a good prognosis in anti-MOG positive subjects has been indicated. However, permanent disability, relapsing, and recurrent disease course have been reported in other studies that assessed anti-MOG status by cell-based assay [19, 20, 21, 22]. The reason may be the smaller sample size of anti-MOG positive cases, the effect of immunosuppressive drugs, or the method of anti-MOG measurement.
In our study, 62.5% of the patients were double negative for the NMO-IgG and MOG-Ab. This condition agrees with previous studies such as Cobocalvo et al. who tested ant-MOG status by cell-based assay, where the frequency of MOG-Ab positive patients in an NMO-IgG negative sample was reported as high as 23% [3].
In vivo experiments using NMO-IgG/anti-MOG double seronegative samples failed to reproduce lesion patterns seen regularly in NMOSD NMO-IgG negative patients, while the use of MOG-Ab positive samples created similar lesions. This finding has led to the hypothesis that MOG antigens are targeted during the pathogenesis of NMO-IgG negative NMOSD [23]. However, the existence of NMOSD patients with negative serology for both NMO-IgG and MOG-Ab patients promotes the possibility of other autoantibodies involved in the pathogenicity of seronegative NMOSD patients and that NMO-IgG negative NMOSD cases are a heterogeneous population which MOG-Ab positive cases being one of the subgroups [1, 2, 6]. Probstel et al. have suggested the use of cell-based-assay anti-MOG measurements as a means for better diagnosis of patients [24]. Previous studies have defined the specificity and sensitivity of cell-based-assay MOG-Ab tests in detecting the disease as 96%-99% and 46%-69%, respectively [24]. Reindle et al. have already highlighted that a unified method of measurement and a consensus for the cutoff point of positive results is essential for better cross-examination of studies [7]. The distinction of clinical presentation in MOG-Ab positive patients from NMO-IgG positive patients has supported the idea that MOG-Ab positive serology is associated with a spectrum of demyelinating diseases and not just a subset of another illness. The autoimmune response is different between NMO-IgG seropositive and anti-MOG seropositive cases. Anti-MOG disrupts the myelin formation and expression of action potential proteins with minimal astrocyte pathology and neuronal or axonal losses.
On the other hand, a response to NMO-IgG includes a complement-related inflammation that leads to both axonal and neuronal loss accompanied by extensive astrocyte pathology [26]. Other observations, including the role of MOG-Ab in pediatric disseminated encephalomyelitis and appearance of encephalomyelitis in adult populations with clinical and radiological outcomes, overlap both in the MS and NMOSD [27, 28]. Piccolo et al. suggested in their study that based on the clinical presentation in cell-based-assay MOG-Ab positive patients, an NMO-IgG negative non-MS optic neuritis is a possibility [29]. Based on a study by Zamvi et al., in the absence of astrocytopathy among NMO-IgG negative, we are not warranted to recognize the opticospinal demyelination phenotype in MOG-Ab positive NMOSD patients [30].
It is to be noted that we have limited access to a base-cell method in some centers, especially in Iran and other developing countries, and the only access is to the ELISA method. So, our findings may be useful in making a diagnosis in these centers. Our study highlights the lack of precision of ELISA MOG-Ab kits since a healthy sample might register higher MOG-Ab levels than a patient group. This condition emphasizes the importance of using cell-based MOG-Ab assays regarding the analysis of NMO-IgG negative NMOSD patients. Our study also highlighted heterogeneity in the anti-MOG response and characteristics of these patients concerning the assay’s methodology.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Research Ethics Committee of Isfahan University of Medical Sciences (Ethical code: 293397).
Funding
This research was supported by Isfahan University of Medical Sciences (Approval code: 293397)
Authors contributions
Conceptualization: Vahid Shaygannejad Mahdi Barzegar, Navid Manouchehri and Fereshteh Ashtari; Methodology: Vahid Shaygannejad Mahdi Barzegar, Shervin Badihian, Nafiseh Esmaeil, and Omid Mirmosayyeb; Writing the original draft: Mahdi Barzegar, Navid Manouchehr, and Shervin Badihian; Writing, review, and editing: Vahid Shaygannejad, Fereshteh Ashtari, and Nafiseh Esmaeil; Funding acquisition: Vahid Shaygannejad and Fereshteh Ashtari; Resources: Vahid Shaygannejad, Fereshteh Ashtari, Nafiseh Esmaeil, and Nasim Nehzat; Supervision: Vahid Shaygannejad, Fereshteh Ashtari, and Nafiseh Esmaeil; Investigation: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the patients for their collaboration. There was no conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
euromyelitis Optica Spectrum Disorder (NMOSD) is a demyelinating autoimmune disorder associated with optic neuritis and acute myelitis involving multiple spinal levels. Although the (AQP4-Ab also known as NMO-IgG) is positive in most NMOSD patients, there are patients with negative serology for NMO-IgG. The absence of NMO-IgG in 10% to 40% of the NMOSD patients prompts the search for other serologic markers in this disease [1].
A candidate target for such investigation is the Myelin Oligodendrocyte Glycoprotein (MOG) [2]. MOG is a protein component of the myelin sheets encapsulating the neural fibers. MOG is found exclusively in the central nervous system, highlighting its possible role in triggering the autoimmune responses in the brain and spinal cord while sparing the peripheral neural structures. Anti-MOG antibodies are found in a fraction of the patients diagnosed with NMOSD, most frequently in AQP-Ab seronegative patients [3]. Autoimmune diseases are best treated when the correct diagnosis is achieved early during the illness, and proper medications are administered. Therefore, finding serologic markers that guide the diagnosis process is of interest. Accordingly, in the present study, we assessed the presence of anti-MOG antibodies in a group of NMOSD patients with negative results for NMO-IgG and compared the measured levels with a healthy control group.
Materials and Methods
Study design
In this study, NMOSD patients with established negative results for NMO-IgG referred to the Neurology Clinic of Kashani University Hospital in Isfahan City, Iran, were consecutively enrolled in the study from March 2015 to March 2016. All patients were previously diagnosed as NMOSD cases according to international consensus diagnostic criteria in 2015 [4]. All patients were assessed with two neurologists with a specialist in Central Nervous System (CNS) Acute Disseminated Encephalomyelitis (ADEM) Vahid Shaygannejad and Fereshteh Ashtari in each patient, the NMOSD patients were enrolled in the study. Healthy controls, matched for age and sex, were also randomly enrolled in the study. Healthy controls were selected from the general population. They were caregivers of other patients with no history of neurological or autoimmune disorders. Any ambiguity regarding the confirmation of patients’ diagnosis, presence of other neurologic or muscular disorders, any history of head or spine injuries, and recent immunosuppressant therapy were defined as the exclusion criteria. To calculate the sample size, we used the equation to compare two means with a type I error of 1.96 and power of 0.84. We assess the previous study to determine the assumptions in two groups [5]. The sample size was calculated as at least 30 participants in each group. Informed consent was taken from all patients at the time of enrollment.
Data collection
Demographic and baseline data, including age, duration of the disease, initial symptoms of the disease, and MRI findings, were collected from all patients. Brain sequences included axial Posterior Duplication (PD), T2W, T2-FLAIR, contrast-enhanced-T1W, and PD. The following spine sequences were acquired: sagittal T2W, STIR, contrast-enhanced T1W, axial T2W, and contrast-enhanced T1W. Disease severity level and clinical disability were assessed in patients using the Extended Disability Status Scale (EDSS). The negative NMO-IgG status of the patients was also double-checked for all of the enrolled patients.
All patients and controls were then subjected to a session of venous blood sampling. The samples were tested for anti-MOG levels using the SensoLyte® Anti-Human MOG (1-125) Human quantitative ELISA Kit (AnaSpec, Fremont, CA, USA).
Data analysis
The obtained data were analyzed using the SPSS (version 18.0, Chicago, IL, USA). The categorical data were reported by using frequency reporting measures. The Student t-test was used to compare the quantitative data. When our sample data were not normally distributed, a non-parametric (Mann-Whitney U) test was used instead. The Chi-square test was used to assess the significance of differences between the categorical data. The correlations between data sets were investigated using the Pearson correlation coefficient. We defined r <0.5 as mild to moderate correlation and r >0.5 as strong correlation. The obtained data are represented as mean±SD or SEM, and statistical significance was defined as a two-tailed P-value of less than 0.05.
Results
A total of 30 seronegative NMOSD patients for NMO-Ab and 26 healthy controls were consecutively enrolled in the study. The data of 4 healthy controls (from 30 subjects) were missed and considered a dropout of the study. The mean±SD ages of the cases and controls were 32.9±7.7 and 33.9±8.7 years, respectively (P=0.356). Among cases and controls, 71.9% and 73.3% of the patients were female, respectively (P=0.931). The mean±SD of EDSS scores for the patients was 1.2±0.46, and the disease duration was 4.8±4.0.4 years. Among the patients, variables in the disease profile were not significantly different.
The anti-MOG test results were positive in 37.5% (n=12) and 0% (n= 0) of the patients and controls, respectively (P<0.0001). The mean±SEM values of the anti-MOG level of patients and controls were 966.5±201 and 524.4±32.7 pg/ml, respectively; as shown in Figure 1, the difference was statistically significant between the two groups (P=0.047). The mean±SEM value of anti-MOG level among patients with positive anti-MOG results was 2034±1006.1 pg/ml, and the difference of anti-MOG levels was statistically significant (P<0.0001) compared to the control group (Figure 2). Furthermore, the mean±SEM value of anti-MOG level among patients with a negative anti-MOG result was 265±230 pg/ml; patients with negative anti-MOG results had significantly lower anti-MOG levels compared to the control group (Figure 2) (P<0.001). Among the patients, variables in the disease profile were not significantly different between the anti-MOG positive and anti-MOG negative groups; however, anti-MOG levels were significantly (P<0.0001) different between the two patient groups (Figure 2). Table 1 presents the disease profiles in patients with positive and negative anti-MOG results.

There was no significant correlation between the level of anti-MOG and EDSS scores among patients (r=0.015, P=0.9). Also, no significant correlation was found between the anti-MOG levels and disease duration among patients (r=0.3, P=0.07).
Discussion
NMOSD correlation with NMO-IgG has been well established in the literature; however, some patients have clinical and radiologic findings of NMOSD without a positive serology for NMO-IgG. It has also been reported that in NMOSD patients, several autoantibodies are detectable, including anti-PLP, anti-MBP, anti-S100 beta, and anti-MOG [1, 2, 6]. However, anti-MOG stands out compared to the rest [6]. In this study, we measured the level of anti-MOG antibodies in a group of NMOSD patients with confirmed negative serology for NMO-IgG. The MOG-Ab was positive in a fraction (37.5%) of these cases, and its level was significantly higher compared to both the patients with negative MOG-Ab serology and healthy controls.
In NMOSD patients, anti-MOG could be proven as a secondary disease marker. However, MOG-Ab is also associated with other demyelinating diseases such as Acute Disseminated Encephalomyelitis (ADEM) and pediatric multiple sclerosis [7]. Unlike the NMO-IgG that was reported to be higher only in NMOSD patients than MS patients, elevated anti-MOG levels that were checked by the ELISA method have been reported in both MS and NMOSD patients without a significant difference by Chen et al. [8]. However, Correale et al. measured MOG-Ab using ELISPOT assays and reported a high frequency of MOG-Ab among NMOSD patients [9].
The role of these antibodies in the pathogenesis of NMOSD and other demyelinating autoimmune diseases has been studied. Autoimmune diseases of the central nervous system display a variety of symptoms, each different from the other. An NMO-IgG induced disease involves females, while the anti-MOG-induced disease has a higher male-to-female ratio. However, we did not find a significant difference between males and females in anti-MOG levels. MS patients are more often younger than patients with other diseases such as NMO-IgG positive NMOSD or MOG-ab positive patients. However, worse destructive events are observed among the anti-MOG induced cases.
In contrast, the patients with an NMO-IgG induced disease tend to have a worse prognosis regarding visual acuity [10]. This could be due to longer axial lesions during the acute phase of optic neuritis among NMO-IgG positive patients, which serves as a predictive factor for a worse prognosis [11]. The differences involve the MRI findings as well. When brain MRI of children with CNS autoimmune diseases was compared based on their anti-MOG status measured using live cell-based assays, it was revealed that anti-MOG seropositive cases had fewer periventricular demyelinating plaques and did not involve corpus callosum [12, 13]. NMO-IgG negative, anti-MOG positive NMOSD patients are younger and more men. The disease is often monophasic and included simultaneous optic neuritis and myelitis compared to NMO-IgG seropositive cases [14], while the relapse was more probable among the latter [15]. The anti-MOG-induced disease is also reported to have a more benign pattern [14]. MRI of the spinal cord lesions is less extended longitudinally in comparison to the NMO-IgG positive patients.
On the other hand, Ramanathan et al. assessed patients with positive cell-based assay anti-MOG and reported anti-MOG induced bilateral optic neuritis is more likely to be relapsing and responds well to long-term steroid treatment [16]. The MRI findings between anti-MOG positive and negative groups in our study were not significantly different. Hamid et al. tested MOG-Ab by cell-based assay and have reported more cortical lesions in MOG-Ab positive patients compared to AQP4-Ab NMOSD patients [17]. Additionally, in a nationwide study, Papp et al. tested the presence of anti-MOG in different methods, including tissue-based immunofluorescence assay, cell-based assay, ELISA kit, and in vitro translation/immunoprecipitation assay. They have assessed the presence of anti-MOG in two groups of MS and NMOSD patients, and consistent with our finding, they suggested that anti-MOG is associated with a broad range of clinical manifestations and should be considered in patients with atypical features [18]. The EDSS was also slightly lower in the MOG-Ab positive group and consistent with this finding, a good prognosis in anti-MOG positive subjects has been indicated. However, permanent disability, relapsing, and recurrent disease course have been reported in other studies that assessed anti-MOG status by cell-based assay [19, 20, 21, 22]. The reason may be the smaller sample size of anti-MOG positive cases, the effect of immunosuppressive drugs, or the method of anti-MOG measurement.
In our study, 62.5% of the patients were double negative for the NMO-IgG and MOG-Ab. This condition agrees with previous studies such as Cobocalvo et al. who tested ant-MOG status by cell-based assay, where the frequency of MOG-Ab positive patients in an NMO-IgG negative sample was reported as high as 23% [3].
In vivo experiments using NMO-IgG/anti-MOG double seronegative samples failed to reproduce lesion patterns seen regularly in NMOSD NMO-IgG negative patients, while the use of MOG-Ab positive samples created similar lesions. This finding has led to the hypothesis that MOG antigens are targeted during the pathogenesis of NMO-IgG negative NMOSD [23]. However, the existence of NMOSD patients with negative serology for both NMO-IgG and MOG-Ab patients promotes the possibility of other autoantibodies involved in the pathogenicity of seronegative NMOSD patients and that NMO-IgG negative NMOSD cases are a heterogeneous population which MOG-Ab positive cases being one of the subgroups [1, 2, 6]. Probstel et al. have suggested the use of cell-based-assay anti-MOG measurements as a means for better diagnosis of patients [24]. Previous studies have defined the specificity and sensitivity of cell-based-assay MOG-Ab tests in detecting the disease as 96%-99% and 46%-69%, respectively [24]. Reindle et al. have already highlighted that a unified method of measurement and a consensus for the cutoff point of positive results is essential for better cross-examination of studies [7]. The distinction of clinical presentation in MOG-Ab positive patients from NMO-IgG positive patients has supported the idea that MOG-Ab positive serology is associated with a spectrum of demyelinating diseases and not just a subset of another illness. The autoimmune response is different between NMO-IgG seropositive and anti-MOG seropositive cases. Anti-MOG disrupts the myelin formation and expression of action potential proteins with minimal astrocyte pathology and neuronal or axonal losses.
On the other hand, a response to NMO-IgG includes a complement-related inflammation that leads to both axonal and neuronal loss accompanied by extensive astrocyte pathology [26]. Other observations, including the role of MOG-Ab in pediatric disseminated encephalomyelitis and appearance of encephalomyelitis in adult populations with clinical and radiological outcomes, overlap both in the MS and NMOSD [27, 28]. Piccolo et al. suggested in their study that based on the clinical presentation in cell-based-assay MOG-Ab positive patients, an NMO-IgG negative non-MS optic neuritis is a possibility [29]. Based on a study by Zamvi et al., in the absence of astrocytopathy among NMO-IgG negative, we are not warranted to recognize the opticospinal demyelination phenotype in MOG-Ab positive NMOSD patients [30].
It is to be noted that we have limited access to a base-cell method in some centers, especially in Iran and other developing countries, and the only access is to the ELISA method. So, our findings may be useful in making a diagnosis in these centers. Our study highlights the lack of precision of ELISA MOG-Ab kits since a healthy sample might register higher MOG-Ab levels than a patient group. This condition emphasizes the importance of using cell-based MOG-Ab assays regarding the analysis of NMO-IgG negative NMOSD patients. Our study also highlighted heterogeneity in the anti-MOG response and characteristics of these patients concerning the assay’s methodology.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Research Ethics Committee of Isfahan University of Medical Sciences (Ethical code: 293397).
Funding
This research was supported by Isfahan University of Medical Sciences (Approval code: 293397)
Authors contributions
Conceptualization: Vahid Shaygannejad Mahdi Barzegar, Navid Manouchehri and Fereshteh Ashtari; Methodology: Vahid Shaygannejad Mahdi Barzegar, Shervin Badihian, Nafiseh Esmaeil, and Omid Mirmosayyeb; Writing the original draft: Mahdi Barzegar, Navid Manouchehr, and Shervin Badihian; Writing, review, and editing: Vahid Shaygannejad, Fereshteh Ashtari, and Nafiseh Esmaeil; Funding acquisition: Vahid Shaygannejad and Fereshteh Ashtari; Resources: Vahid Shaygannejad, Fereshteh Ashtari, Nafiseh Esmaeil, and Nasim Nehzat; Supervision: Vahid Shaygannejad, Fereshteh Ashtari, and Nafiseh Esmaeil; Investigation: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the patients for their collaboration. There was no conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Lana-Peixoto MA, Talim N. Neuromyelitis optica spectrum disorder and anti-MOG syndromes. Biomedicines. 2019; 7(2):42. [DOI:10.3390/biomedicines7020042] [PMID] [PMCID]
- Alves-Leon SV, Pimentel ML, Sant’Anna G, Malfetano FR, Estrada CD, Quirico-Santos T. Immune system markers of neuroinflammation in patients with clinical diagnose of neuromyelitis optica. Arquivos De Neuro-Psiquiatria. 2008; 66(3B):678-84. [DOI:10.1590/S0004-282X2008000500013] [PMID]
- Weber MS, Derfuss T, Metz I, Brück W. Defining distinct features of anti-MOG antibody associated central nervous system demyelination. Ther Adv Neurol Disord. 2018; 11:1756286418762083. [DOI:10.1177/1756286418762083] [PMID] [PMCID]
- Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015; 85(2):177-89. [DOI:https://doi.org/10.1212/WNL.0000000000001729]
- Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012; 79(12):1273-7. [DOI:10.1212/WNL.0b013e31826aac4e] [PMID]
- Rocca MA, Cacciaguerra L, Filippi M. Moving beyond anti-aquaporin-4 antibodies: emerging biomarkers in the spectrum of neuromyelitis optica. Expert Rev Neurother. 2020; 20(6):601-18. [DOI:10.1080/14737175.2020.1764352] [PMID]
- Hennes EM, Baumann M, Lechner C, Rostásy K. MOG spectrum disorders and role of MOG-antibodies in clinical practice. Neuropediatrics. 2018; 49(01):003-11. [DOI:10.1055/s-0037-1604404] [PMID]
- Chen H, Liu SM, Zhang XX, Liu YO, Li SZ, Liu Z, et al. Clinical Features of Patients with Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders. Chin Med J. 2016; 129(17):2079-84. [DOI:10.4103/0366-6999.189046] [PMID] [PMCID]
- Correale J, Fiol M. Activation of humoral immunity and eosinophils in neuromyelitis optica. Neurology. 2004; 63(12):2363-70. [DOI:10.1212/01.WNL.0000148481.80152.BF] [PMID]
- Vicini R, Brügger D, Abegg M, Salmen A, Grabe HM. Differences in morphology and visual function of myelin oligodendrocyte glycoprotein antibody and multiple sclerosis associated optic neuritis. J Neurol. 2020:1-9. [DOI:10.1007/s00415-020-10097-x] [PMID] [PMCID]
- Akaishi T, Nakashima I, Takeshita T, Mugikura S, Sato DK, Takahashi T, et al. Lesion length of optic neuritis impacts visual prognosis in neuromyelitis optica. J Neuroimmunol. 2016; 293:28-33. [DOI:10.1016/j.jneuroim.2016.02.004] [PMID]
- Fernandez-Carbonell C, Vargas-Lowy D, Musallam A, Healy B, McLaughlin K, Wucherpfennig KW, et al. Clinical and MRI phenotype of children with MOG antibodies. Mult Scler J. 2016; 22(2):174-84. [DOI:10.1177/1352458515587751] [PMID] [PMCID]
- Lechner C, Baumann M, Hennes EM, Schanda K, Marquard K, Karenfort M, et al. Antibodies to MOG and AQP4 in children with neuromyelitis optica and limited forms of the disease. J Neurol Neurosurg Psychiatry. 2016; 87(8):897-905. [DOI:10.1136/jnnp-2015-311743] [PMID]
- Tanaka S, Hashimoto B, Izaki S, Oji S, Fukaura H, Nomura K. Clinical and immunological differences between MOG associated disease and anti AQP4 antibody-positive neuromyelitis optica spectrum disorders: Blood-brain barrier breakdown and peripheral plasmablasts. Mult Scler Relat Disord. 2020; 41:102005. [DOI:10.1016/j.msard.2020.102005] [PMID]
- Kitley J, Waters P, Woodhall M, Leite MI, Murchison A, George J, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurology. 2014; 71(3):276-83. [DOI:10.1001/jamaneurol.2013.5857] [PMID]
- Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson AP, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016; 22(4):470-82. [DOI:10.1177/1352458515593406] [PMID]
- Hamid SH, Whittam D, Mutch K, Linaker S, Solomon T, Das K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017; 264(10):2088-94. [DOI:10.1007/s00415-017-8596-7] [PMID] [PMCID]
- Papp V, Illes Z, Magyari M, Koch-Henriksen N, Kant M, Pfleger CC, et al. Nationwide prevalence and incidence study of neuromyelitis optica spectrum disorder in Denmark. Neurology. 2018; 91(24):e2265-75.[DOI:10.1212/WNL.0000000000006645] [PMID] [PMCID]
- Fragoso YD, Sousa NA, Alves-Leon SV, Dias RM, Pimentel ML, Gomes S, et al. Clinical characteristics of 153 Brazilian patients with neuromyelitis optica spectrum disorder (NMOSD). Mult Scler Relat Disord. 2019; 27:392-6. [DOI:10.1016/j.msard.2018.11.031] [PMID]
- Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nature Rev Neurol. 2019; 15(2):89-102. [DOI:10.1038/s41582-018-0112-x] [PMID]
- Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflamm. 2016; 13(1):1-45. [DOI:10.1186/s12974-016-0718-0] [PMID] [PMCID]
- Nakajima H, Motomura M, Tanaka K, Fujikawa A, Nakata R, Maeda Y, et al. Antibodies to myelin oligodendrocyte glycoprotein in idiopathic optic neuritis. BMJ open. 2015; 5(4):e007766 [DOI:10.1136/bmjopen-2015-007766] [PMID] [PMCID]
- Wu X, Zhou M, Ding H, Xu S, Wang C, Chan P. Myelin oligodendrocyte glycoprotein induces aquaporin-4 autoantibodies in mouse experimental autoimmune encephalomyelitis. J Neuroinflamm. 2013; 261(1-2):1-6. [DOI:10.1016/j.jneuroim.2013.03.008] [PMID]
- Probstel AK, Rudolf G, Dornmair K, Collongues N, Chanson JB, Sanderson NS, et al. Anti-MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype. J Neuroinflamm. 2015; 12:46. [DOI:10.1186/s12974-015-0256-1] [PMID] [PMCID]
- Ramanathan S, Reddel SW, Henderson A, Parratt JD, Barnett M, Gatt PN, et al. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol(R) Neuroimmunol Neuroinflammation. 2014; 1(4):e40. [DOI:10.1212/NXI.0000000000000040] [PMID] [PMCID]
- Saadoun S, Waters P, Owens GP, Bennett JL, Vincent A, Papadopoulos MC. Neuromyelitis optica MOG-IgG causes reversible lesions in mouse brain. Acta Neuropathol Commun. 2014; 2:35. [DOI:10.1186/2051-5960-2-35] [PMID] [PMCID]
- Baumann M, Hennes EM, Schanda K, Karenfort M, Kornek B, Seidl R, et al. Children with multiphasic disseminated encephalomyelitis and antibodies to the myelin Oligodendrocyte Glycoprotein (MOG): extending the spectrum of MOG Antibody Positive Diseases. Mult Scler J. 2016; 22(14):1821-9. [DOI:10.1177/1352458516631038] [PMID]
- Sepulveda M, Armangue T, Martinez-Hernandez E, Arrambide G, Sola-Valls N, Sabater L, et al. Clinical spectrum associated with MOG autoimmunity in adults: significance of sharing rodent MOG epitopes. J Neurol. 2016; 263(7):1349-60. [DOI:10.1007/s00415-016-8147-7] [PMID] [PMCID]
- Piccolo L, Woodhall M, Tackley G, Jurynczyk M, Kong Y, Domingos J, et al. Isolated new onset ‘atypical’ optic neuritis in the NMO clinic: serum antibodies, prognoses and diagnoses at follow-up. J Neurol. 2016; 263(2):370-9. [DOI:10.1007/s00415-015-7983-1] [PMID]
- Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflammation. 2015; 2(1):e62. [DOI:10.1212/NXI.0000000000000062] [PMID] [PMCID]
Type of Study: Research |
Subject:
Special
Received: 2021/03/14 | Accepted: 2021/01/21 | Published: 2021/01/21
Received: 2021/03/14 | Accepted: 2021/01/21 | Published: 2021/01/21
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



.jpg)
.jpg)
