Fri, Apr 26, 2024
Volume 3, Issue 4 (Autumn 2017)
Caspian J Neurol Sci 2017, 3(4): 231-240 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Etemadifar M, Fazli A. Myelin-Oligodendrocyte Glycoprotein Antibodies Spectrum Disorders. Caspian J Neurol Sci 2017; 3 (4) :231-240
URL: http://cjns.gums.ac.ir/article-1-206-en.html
URL: http://cjns.gums.ac.ir/article-1-206-en.html
1- Professor of Neurology, Department of Neurology, Isfahan University of Medical Science, Isfahan, Iran
2- Department of Neurology, Isfahan University of Medical Science, Isfahan, Iran; ali96fazli@yahoo.com
2- Department of Neurology, Isfahan University of Medical Science, Isfahan, Iran; ali96fazli@yahoo.com
Keywords: Myelin-Oligodendrocyte Glycoprotein, Immunoglobulins, Myelitis, Transverse, Optic Neuritis
Full-Text [PDF 622 kb]
(1568 Downloads)
| Abstract (HTML) (3312 Views)
Full-Text: (1191 Views)
Introduction
Myelin-Oligodendrocyte Glycoprotein (MOG) biochemical review
Myelin-Oligodendrocyte Glycoprotein (MOG), which is encoded by MOG gene in human oligodendrocytes, plays an important role in central nervous system nerve myelination. Although the primary molecular function is not well known yet MOG is considered to serve as an adhesive molecule and be responsible for myelinsheath structural integrity and maintenance. The MOG gene is located on chromosome 6p21.3-p22. The
gene expresses a transmembrane protein (minor type 1 transmembrane protein) on the surface of oligodendrocytes. This protein is perched on the outermost layer of sheaths of myelin. While MOG is located on the surface of myelin sheath, a single immunoglobulin domain is exposed that allows antibodies to have easy access (1-3).
There are proved to be 9 isoforms of myelin oligodendrocyte glycoprotein because human mRNA of MOG can participate in alternative splicing, the mechanism which can increase the variety. X-ray diffraction at 1.45 angstrom resolution observing Norway rat MOG protein crystal structure determined that MOG is a member of immunoglobulin superfamily and 139 residues long. Secondary structure of MOG consists of 6% helical and 43% beta sheath. It has 10 beta strands and so this complex forms an immunoglobulin-like beta-sandwich fold. Experiments suggested that MOG is dimerized in solution and its shape complementarity index was observed to be high at the dimer interface resulting in hypothesis of the biologically relevant MOG dimer (4-6).
Patients with spectrum of inflammatory demyelinating disease particularly in CNS are reported to have antibodies against MOG protein (7). Although anti-MOG antibodies do not seem to have primary pathogenicity, discovering their presence can be useful for diagnosing diseases such as multiple sclerosis, neuromyelitis optica, acute disseminated encephalomyelitis, transverse myelitis, and optic neuritis.
Anti-MOG antibodies in Multiple sclerosis
Multiple sclerosis, an inflammatory demyelinating disease in which blurred vision, limbs paresthesia, ataxia etc. may
occur, has several types such as relapsing or progressive forms in which attacks are built up over time. Symptoms may disappear completely between attacks but permanent complications often remain, especially when the disease is advanced (7). Due to an unclear cause, the mechanism of this disease is thought to be myelin production failure or destruction by immunoglobulin responses. As explained above, in cases which anti-MOG antibodies are present, the immunological responses against myelin sheath can be observed and so the symptoms are shown (8).
A recent study observing IgM, IgA and IgG against MOG protein in 261 MS patients reported that 72% of cases were anti-MOG antibody positive. The dominant immunoglobulin was anti-MOG IgM. There was found a significant correlation between anti-MOG IgM serum titer and disease progression. A comparison between patients suffering from relapses and the ones in remission showed that IgG3 was clearly more often observed in the first group. In patients treated by intravenous immunoglobulin or interferon β, anti-MOG antibodies were reported to be reduced (9). Studying 30 MS patients reported anti-MOG antibodies present in cerebrospinal fluid but no immunoglobulin was demonstrated in plasma. Measuring serum samples for anti-MOG antibodies resulted in finding immunoglobulin from the family of complement activating IgG subclass (IgG1) against myelin oligodendrocyte glycoprotein (10-12).
Clinically isolated syndrome is considered as a clinical stage of multiple sclerosis; however recent studies rarely detected high serum titer of anti-MOG antibodies in it (6,13).
Anti-MOG antibodies in neuromyelitis optica
Neuromyelitis optica also known as Devic syndrome, a rare autoimmune disorder, consists of simultaneous demyelination and inflammation of optic nerve and spinal cord causing optic neuritis and myelitis. NMO hallmarks are specific antibodies against Aquaporin molecule, from subclass of IgG. If antibodies for Aquaporin are present (AQP4+ patient) the disease is called Astrocytopathy because it is the result of autoimmune interactions against astrocytes in the spinal cord and around optic nerve. Cause of AQP4 negative variant is unknown but it is currently observed that astrocytes are spared in this case too. Although inflammation can affect the brain, lesions aren’t similar to those observed in multiple sclerosis, and this can be a method for diagnosing (14-16).
A recent study on 48 AQP4 seronegative and seropositive NMO patients showed that in 4/17 seronegative cases anti-MOG antibodies were found but this immunoglobulin was demonstrated in none of seropositive patients. Anti-MOG antibodies can be used in both diagnostic and prognostic pathways in AQP4 seronegative NMO patients (17). Observing 215 NMOSD cases proved that 16/215 were anti-MOG antibody positive and 139/215 were AQP4 antibody positive but none of the cases were reported to be positive for both. In a comparison between AQP4 antibody positive patients and anti-MOG antibody positive ones, the latter was more frequently males and their optic nerve symptoms were more obvious than spinal cord. Single attacks occurred more often in anti-MOG antibody positive patients, however after attacks, their functional recovery was observed to be better (18). Live cell-based assays testing serum samples of 45 children with NMO (n=12), recurrent optic
neuritis (n=12), bilateral optic neuritis (n=6), longitudinal extensive transverse myelitis (n=14) and brain stem syndrome (n=1) for antibodies resulted in following: overall 25/45 (56%) cases were anti-MOG antibody seropositive (7 NMO, 8 ON, 4 bilateral ON, 6 LETM). It is suggested that when this immunoglobulin is present in children it can cause them to have a monophasic course in a year, simultaneous longitudinal extensive transverse myelitis and optic neuritis and to be less likely for receiving immunosuppressive therapies. MRI in these cases shows less frequently periependymal lesions rather than seronegative patients (19)
Anti-MOG antibodies in Acute disseminated encephalomyelitis
Acute disseminated encephalomyelitis (ADEM) or acute demyelinating encephalomyelitis, an uncommon autoimmune disorder, is illustrated by sudden and widespread attacks of inflammation in central nervous system therefore the myelin insulation damages occur, leading to white matter destruction. ADEM symptoms are similar to the ones in multiple sclerosis, so it is classified as a multiple sclerosis spectrum disease. Unlike MS, ADEM usually occurs in childhood and presents with fever, encephalopathy, and loss of consciousness, coma and death, which are rare in MS, except in severe cases (20, 21).
When a patient suffers from more than one; demyelinating episode of acute disseminated encephalomyelitis, then it is called recurrent disseminated encephalomyelitis or multiphasic disseminated encephalomyelitis (MDEM). It is proved that anti-MOG antibodies are playing a critical role in this type of disease (22). Another variant of ADEM in adults is
related to this immunoglobulin, it is clinically ADEM, but showing MS-like lesions on autopsy. The last condition is called fulminant disseminated encephalomyelitis (23). Reviewing the literature, we found that while anti-MOG antibodies participate in development of ADEM, years later it can lead to Optic Neuritis. A 37 years old woman diagnosed with ADEM when she was four years old demonstrated optic neuritis 33 years after ADEM onset, while her serum analysis showed anti-MOG antibody positivity (24). A five year old girl with ADEM and serum anti-MOG antibody positivity developed optic neuritis 71 days after ADEM onset (25). These cases may lead us to the conclusion that anti-MOG antibodies are responsible for ADEM and the optic neuritis followed by that.
Anti-MOG antibodies in idiopathic recurrent Transverse myelitis
Transverse myelitis, a neurological condition in which the spinal cord is inflamed and the nerve fibers are damaged, resulting in losing their myelin coating, can lead to decreased electrical conductivity in CNS. The term transverse implies that inflammation can extend across entire width of spinal cord. Malfunction in motor and sensory nerves and disturbances in autonomic nervous system occur as symptoms. The signs depend on the area of lesion in spinal cord. The lesions are sorted in groups of cervical, thoracic and lumbar. In some cases of transverse myelitis if spinal cord lesions extend over 3 vertebral segments, the condition is called longitudinally extensive myelitis (LETM) (26, 27).
A case study reported the correlation of anti-MOG antibodies and longitudinally extensive transverse myelitis. The case was a
32 year old male, developing transverse myelitis after an influenza type A infection. MRI revealed an extensive spinal cord lesion, however no sign of inflammation was found in brain or optic nerves. Cell based immunoassay proved his serum anti-MOG antibody positivity with high titer (28). It can be concluded that anti-MOG antibodies are certainly participating in transverse myelitis but further studies are required to clarify their exact role.
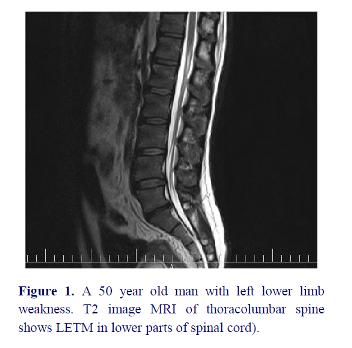
MRI findings in these patients demonstrated spinal lesion in lower parts of spinal cord (figure 1).
Myelin-Oligodendrocyte Glycoprotein (MOG) biochemical review
Myelin-Oligodendrocyte Glycoprotein (MOG), which is encoded by MOG gene in human oligodendrocytes, plays an important role in central nervous system nerve myelination. Although the primary molecular function is not well known yet MOG is considered to serve as an adhesive molecule and be responsible for myelinsheath structural integrity and maintenance. The MOG gene is located on chromosome 6p21.3-p22. The
gene expresses a transmembrane protein (minor type 1 transmembrane protein) on the surface of oligodendrocytes. This protein is perched on the outermost layer of sheaths of myelin. While MOG is located on the surface of myelin sheath, a single immunoglobulin domain is exposed that allows antibodies to have easy access (1-3).
There are proved to be 9 isoforms of myelin oligodendrocyte glycoprotein because human mRNA of MOG can participate in alternative splicing, the mechanism which can increase the variety. X-ray diffraction at 1.45 angstrom resolution observing Norway rat MOG protein crystal structure determined that MOG is a member of immunoglobulin superfamily and 139 residues long. Secondary structure of MOG consists of 6% helical and 43% beta sheath. It has 10 beta strands and so this complex forms an immunoglobulin-like beta-sandwich fold. Experiments suggested that MOG is dimerized in solution and its shape complementarity index was observed to be high at the dimer interface resulting in hypothesis of the biologically relevant MOG dimer (4-6).
Patients with spectrum of inflammatory demyelinating disease particularly in CNS are reported to have antibodies against MOG protein (7). Although anti-MOG antibodies do not seem to have primary pathogenicity, discovering their presence can be useful for diagnosing diseases such as multiple sclerosis, neuromyelitis optica, acute disseminated encephalomyelitis, transverse myelitis, and optic neuritis.
Anti-MOG antibodies in Multiple sclerosis
Multiple sclerosis, an inflammatory demyelinating disease in which blurred vision, limbs paresthesia, ataxia etc. may
occur, has several types such as relapsing or progressive forms in which attacks are built up over time. Symptoms may disappear completely between attacks but permanent complications often remain, especially when the disease is advanced (7). Due to an unclear cause, the mechanism of this disease is thought to be myelin production failure or destruction by immunoglobulin responses. As explained above, in cases which anti-MOG antibodies are present, the immunological responses against myelin sheath can be observed and so the symptoms are shown (8).
A recent study observing IgM, IgA and IgG against MOG protein in 261 MS patients reported that 72% of cases were anti-MOG antibody positive. The dominant immunoglobulin was anti-MOG IgM. There was found a significant correlation between anti-MOG IgM serum titer and disease progression. A comparison between patients suffering from relapses and the ones in remission showed that IgG3 was clearly more often observed in the first group. In patients treated by intravenous immunoglobulin or interferon β, anti-MOG antibodies were reported to be reduced (9). Studying 30 MS patients reported anti-MOG antibodies present in cerebrospinal fluid but no immunoglobulin was demonstrated in plasma. Measuring serum samples for anti-MOG antibodies resulted in finding immunoglobulin from the family of complement activating IgG subclass (IgG1) against myelin oligodendrocyte glycoprotein (10-12).
Clinically isolated syndrome is considered as a clinical stage of multiple sclerosis; however recent studies rarely detected high serum titer of anti-MOG antibodies in it (6,13).
Anti-MOG antibodies in neuromyelitis optica
Neuromyelitis optica also known as Devic syndrome, a rare autoimmune disorder, consists of simultaneous demyelination and inflammation of optic nerve and spinal cord causing optic neuritis and myelitis. NMO hallmarks are specific antibodies against Aquaporin molecule, from subclass of IgG. If antibodies for Aquaporin are present (AQP4+ patient) the disease is called Astrocytopathy because it is the result of autoimmune interactions against astrocytes in the spinal cord and around optic nerve. Cause of AQP4 negative variant is unknown but it is currently observed that astrocytes are spared in this case too. Although inflammation can affect the brain, lesions aren’t similar to those observed in multiple sclerosis, and this can be a method for diagnosing (14-16).
A recent study on 48 AQP4 seronegative and seropositive NMO patients showed that in 4/17 seronegative cases anti-MOG antibodies were found but this immunoglobulin was demonstrated in none of seropositive patients. Anti-MOG antibodies can be used in both diagnostic and prognostic pathways in AQP4 seronegative NMO patients (17). Observing 215 NMOSD cases proved that 16/215 were anti-MOG antibody positive and 139/215 were AQP4 antibody positive but none of the cases were reported to be positive for both. In a comparison between AQP4 antibody positive patients and anti-MOG antibody positive ones, the latter was more frequently males and their optic nerve symptoms were more obvious than spinal cord. Single attacks occurred more often in anti-MOG antibody positive patients, however after attacks, their functional recovery was observed to be better (18). Live cell-based assays testing serum samples of 45 children with NMO (n=12), recurrent optic
neuritis (n=12), bilateral optic neuritis (n=6), longitudinal extensive transverse myelitis (n=14) and brain stem syndrome (n=1) for antibodies resulted in following: overall 25/45 (56%) cases were anti-MOG antibody seropositive (7 NMO, 8 ON, 4 bilateral ON, 6 LETM). It is suggested that when this immunoglobulin is present in children it can cause them to have a monophasic course in a year, simultaneous longitudinal extensive transverse myelitis and optic neuritis and to be less likely for receiving immunosuppressive therapies. MRI in these cases shows less frequently periependymal lesions rather than seronegative patients (19)
Anti-MOG antibodies in Acute disseminated encephalomyelitis
Acute disseminated encephalomyelitis (ADEM) or acute demyelinating encephalomyelitis, an uncommon autoimmune disorder, is illustrated by sudden and widespread attacks of inflammation in central nervous system therefore the myelin insulation damages occur, leading to white matter destruction. ADEM symptoms are similar to the ones in multiple sclerosis, so it is classified as a multiple sclerosis spectrum disease. Unlike MS, ADEM usually occurs in childhood and presents with fever, encephalopathy, and loss of consciousness, coma and death, which are rare in MS, except in severe cases (20, 21).
When a patient suffers from more than one; demyelinating episode of acute disseminated encephalomyelitis, then it is called recurrent disseminated encephalomyelitis or multiphasic disseminated encephalomyelitis (MDEM). It is proved that anti-MOG antibodies are playing a critical role in this type of disease (22). Another variant of ADEM in adults is
related to this immunoglobulin, it is clinically ADEM, but showing MS-like lesions on autopsy. The last condition is called fulminant disseminated encephalomyelitis (23). Reviewing the literature, we found that while anti-MOG antibodies participate in development of ADEM, years later it can lead to Optic Neuritis. A 37 years old woman diagnosed with ADEM when she was four years old demonstrated optic neuritis 33 years after ADEM onset, while her serum analysis showed anti-MOG antibody positivity (24). A five year old girl with ADEM and serum anti-MOG antibody positivity developed optic neuritis 71 days after ADEM onset (25). These cases may lead us to the conclusion that anti-MOG antibodies are responsible for ADEM and the optic neuritis followed by that.
Anti-MOG antibodies in idiopathic recurrent Transverse myelitis
Transverse myelitis, a neurological condition in which the spinal cord is inflamed and the nerve fibers are damaged, resulting in losing their myelin coating, can lead to decreased electrical conductivity in CNS. The term transverse implies that inflammation can extend across entire width of spinal cord. Malfunction in motor and sensory nerves and disturbances in autonomic nervous system occur as symptoms. The signs depend on the area of lesion in spinal cord. The lesions are sorted in groups of cervical, thoracic and lumbar. In some cases of transverse myelitis if spinal cord lesions extend over 3 vertebral segments, the condition is called longitudinally extensive myelitis (LETM) (26, 27).
A case study reported the correlation of anti-MOG antibodies and longitudinally extensive transverse myelitis. The case was a
32 year old male, developing transverse myelitis after an influenza type A infection. MRI revealed an extensive spinal cord lesion, however no sign of inflammation was found in brain or optic nerves. Cell based immunoassay proved his serum anti-MOG antibody positivity with high titer (28). It can be concluded that anti-MOG antibodies are certainly participating in transverse myelitis but further studies are required to clarify their exact role.
MRI findings in these patients demonstrated spinal lesion in lower parts of spinal cord (figure 1).
Anti-MOG antibodies in Optic neuritis
Optic neuritis is most often a consequence of multiple sclerosis and can lead to partial or complete loss of vision in one or both eyes. Autoimmune mechanisms like producing antibodies against myelin sheath are the causes of this disorder. When idiopathic optic neuritis occurs repeatedly it is considered to be a distinct clinical condition and if accompanied by demyelination it’s believed to be associated with anti-MOG antibodies (29).
While observing thirty three eyes of 23 patients with optic neuritis and measuring their serum anti-MOG antibodies concentration, 34% of the cases were seropositive (and 26% of cases were seropositive for both MOG and NMO antibodies).This immunoglobulin is considered to be a biomarker to investigate visual prognosis in optic neuritis patients (30). A study on 37 cases, 18 or under 18 years old with recurrent or single episodes of optic neuritis, suggested that generally these patients had high titer serum anti-MOG antibodies from the subclass of IgG, especially in patients suffering from recurrent episodes of optic neuritis (31). In a group of pediatrics suffering from recurrent or monophasic ADEM, optic neuritis was diagnosed. Their serum immunoglobulin against MOG protein was measured high titer (32). It is concluded that anti-MOG antibodies can exert a direct role in optic neuritis pathogenesis. This antibody is significantly associated with bilateral optic neuritis and can play the role of clinical biomarker. Anti-MOG antibody-associated bilateral ON, which is a relapsing disorder, is frequently steroid dependent (33). And as Tsuburaya stated, recurrent ON patients are candidates for serum anti-MOG antibody analysis (34).
Anti-MOG antibodies in other disorders
A recent study on 10 arthritis rheumatoid cases resulted in anti-MOG antibody seropositivity in only one patient. It leads to the conclusion that although these antibodies exclusively serve in CNS but we can find them in chronic systemic inflammatory disorders in which CNS is not involved (35).
A general survey carried out by Mader and his team presented high MOG-IgG serum
concentration in 2/27 of systemic lupus erythematosus (SLE) cases (36). However exploring serum of 6 systemic lupus erythematosus cases indicated no sign of anti-MOG IgG/IgM (37).
Treatment
Treatment of MOG spectrum patients is divided in two major parts: treatment of attacks and prevention. The acute phase is generally treated by corticosteroid therapy.
There is no certain agreement about the treatment for MOG spectrum disorder patients however in acute attacks we can use methylprednisolone intravenous (1gr per day, for 3-7 days) for treating acute attacks in patients presented with optic neuritis and transverse myelitis and other symptoms of central nervous system involvement. If the patient showed no response to treatments we can repeat the mentioned dose and plasmapheresis is can be used after intravenous methylprednisolone. Oshiro et al. reported a 10 year old boy with an NMO spectrum disorder who underwent plasma exchange therapy. Only after anti-MOG antibody serum titer was decreased to the cut-out level did his symptoms obviously stop; however as they suggested further studies are required to establish this method as a therapeutic pathway (38).
No certain therapy is suggested for prevention yet, however due to recent studies these patients are advised to administrate azathioprine or methotrexate and low dose of prednisolone (for 6-12 months).
A 16 year old girl with optic neuritis accompanied by spinal and cerebral lesions demonstrated anti-MOG antibody seropositivity and fingolimod therapy brought about a relapsing condition. It is suggested
that fingolimod can’t be an effective choice in MOG spectrum disorders (39).
A general study on patients with ADEM, LETM and NMOSD, all were demonstrating high anti-MOG antibody serum titer, suggested that intravenous immunoglobulin followed by rituximab significantly reduces the relapse rate. While comparing the cases according to their age, it was proved that children with MOG antibody spectrum disorder recovered better after steroid and IVIG therapy (40).
Relapsing course can be observed in some anti-MOG seropositive patients. These cases fulfill MS diagnosis but they may have a poor response to MS drug treatments like interferon β (41). An eleven year old girl whose MRI showed transverse myelitis-like lesions, underwent systemic steroid treatment and after initial improvements she lost bowel and bladder control and the lower limb weakness worsened. Vast serum investigations revealed anti-MOG antibody seropositivity; however after frequent courses of plasmapheresis, this condition continued to deteriorate. Finally, extreme immunosuppressive therapy such as cyclophosphamide and rituximab for one year was able to omit further relapses but paraparesis was not eliminated (42).
Discussion
Myelin Oligodendrocyte Glycoprotein, a CNS exclusive glycoprotein located on the outermost layer of myelin sheath, is produced by oligodendrocytes. This adhesive protein plays an important role in myelin sheath structural integrity and maintenance. Thus any malfunction in MOG protein might result in myelin sheath malformation, which can be seen as signs of demyelinating disorders: For instance immune responses are capable of
endangering MOG molecule entirety. Antibodies against MOG not only bind to the extracellular domain of this protein, but induce natural killer cells to terminate the cells which express MOG (43).
As mentioned above in a reliable research Egg and his group concluded that Anti-MOG antibodies were present in most cases of Multiple sclerosis, though in contrast with that, with due attention to Jarius et al. large study’s observing anti-neural antibodies, MOG-IgG might play a noticeable role in a small percentage of cases diagnosed with pattern II MS. Also according to a research performed in 2016 on 104 preselected MS patients, antibodies to MOG were existent in almost 5% of cases. So it seems the elevation of MOG antibodies is not explicit in MS patients. On brain MRI of all seropositive cases lesions were similar to those of typical MS patients. Following them up for more than 9 years to have a longitudinal analysis revealed fluctuations in Anti-MOG antibody serum titer and activity (9, 12, 44).
While researches done in fields of acute disseminated encephalomyelitis, transverse myelitis, optic neuritis and clinically isolated syndrome were suggesting the important destructive role of anti-MOG antibodies in these disorders, the results were not strongly proved or number of cases were not large enough, therefore further investigations are required to gather a solid opinion on this issue. Nonetheless a research covering the Anti-MOG antibodies in CNS demyelinating diseases, a rare study on this specific subject, reported the following: age is a significant factor in ADEM disease; this means that predominant high titer antibody against MOG was demonstrated in pediatrics. An important finding suggested that recombinant human MOG ELISA couldn’t detect these
antibodies. Other than that these antibodies were rarely frequent in CIS cases’ serum (13). In contrast with the above study, Brilot et al. detected Anti-MOG antibodies in 40% of their CIS/ADEM patients by a cell-based bioassay. They also asserted that cases which were seropositive for antibodies were younger, as it was suggested in the previous reference, and that anti-MOG IgG titer was not different between the CIS cases and the ADEM ones (43).
A recent case report studying on a positive anti-MOG antibody Japanese boy supported the relationship of the former immunoglobulin with Optic Neuritis, although additional research is needed in this field (7). Anti-MOG antibodies are detected in different kinds of immune-mediated Optic Neuritis; however in NMO related Optic Neuritis they’re not found. By contrast the expression of the mentioned immunoglobulin was significantly higher in MS related Optic Neuritis rather than other NMO spectrum disorders’ group (45).
To conclude all above it can be stated that Anti-MOG antibodies can be considered as biomarkers for central nervous system autoimmune demyelinating disorders. Detecting these immunoglobulins in patients’ serums can be useful in diagnosis and prognosis pathways. It is possible that anti-MOG antibodies in patients presenting several symptoms such as myelitis, optic neuritis and so on can be sign of a new disease, so we choose MOG spectrum disorder for Anti-MOG antibody seropositive patients presenting mentioned neurological characteristics. Still MOG spectrum disorder is a new term in nervous system demyelinating diseases field.
Conclusion
To conclude all above we can state that for prophylactic treatment of MOG spectrum disorder azathioprine, methotrexate, rituximab and cyclophosphamide are suggested. On the other hand methyl prednisolone and plasmapheresis are suitable for acute phase. Intravenous immunoglobulin can be useful in relapsing forms but it needs more investigations to be proved. According to similar physiopathology of NMOSD and MOG spectrum disorder it is noticeable that fingolimod and interferons may deteriorate the conditions in both of them.
Conflict of Interest
The authors have no conflict of interest.
References
1. Roth M-P, Malfroy L, Offer C, Sevin J,
Enault G, Borot N, et al. The Human Myelin
Oligodendrocyte Glycoprotein (MOG) Gene:
Complete Nucleotide Sequence and Structural
Characterization. Genomics 1995;28(2):241-
50.
2. Pham-Dinh D, Allinquant B, Ruberg M, Della
Gaspera B, Nussbaum JL, Dautigny A.
Characterization and Expression of the cDNA
Coding for the Human
Myelin/Oligodendrocyte Glycoprotein. J
Neurochem 1994;63(6):2353-6.
3. Pham-Dinh D, Mattei M-G, Nussbaum J-L,
Roussel G, Pontarotti P, Roeckel N, et al.
Myelin/Oligodendrocyte Glycoprotein Is a
Member of a Subset of the Immunoglobulin
Superfamily Encoded within the Major
Histocompatibility Complex. Proc Natl Acad
Sci U S A 1993;90(17):7990-4.
4. Boyle LH, Traherne JA, Plotnek G, Ward R,
Trowsdale J. Splice Variation in the
Cytoplasmic Domains of Myelin
Oligodendrocyte Glycoprotein Affects Its
Cellular Localisation and Transport1. J
Neurochem 2007;102(6):1853-62.
5. Breithaupt C, Schubart A, Zander H, Skerra
A, Huber R, Linington C, et al. Structural
Insights into the Antigenicity of Myelin
Oligodendrocyte Glycoprotein. Proc Natl
Acad Sci U S A 2003;100(16):9446-51.
6. Clements CS, Reid HH, Beddoe T, Tynan FE,
Perugini MA, Johns TG, et al. The Crystal
Structure of Myelin Oligodendrocyte
Glycoprotein, a Key Autoantigen in Multiple
Sclerosis. Proc Natl Acad Sci U S A
2003;100(19):11059-64.
7. Tsuburaya RS, Miki N, Tanaka K, Kageyama
T, Irahara K, Mukaida S, et al. Anti-myelin
Oligodendrocyte Glycoprotein (MOG)
Antibodies in a Japanese Boy with Recurrent
Optic Neuritis. Brain Dev 2015;37(1):145-8.
8. Ascherio A, Munger KL. Environmental Risk
Factors for Multiple Sclerosis. Part I: the Role
of Infection. Ann Neurol 2007;61(4):288-99.
9. Egg R, Reindl M, Deisenhammer F, Linington
C, Berger T. Anti-MOG and Anti-MBP
Antibody Subclasses in Multiple Sclerosis.
Mult Scler 2001;7(5):285-9.
10. Xiao B-G, Linington C, Link Hd. Antibodies
to Myelin-Oligodendrocyte Glycoprotein in
Cerebrospinal Fluid from Patients with
Multiple Sclerosis and Controls. J
Neuroimmunol 1991;31(2):91-6.
11. Lampasona V, Franciotta D, Furlan R,
Zanaboni S, Fazio R, Bonifacio E, et al.
Similar Low Frequency of anti-MOG IgG and
IgM in MS Patients and Healthy Subjects.
Neurology 2004;62(11):2092-4.
12. Jarius S, Metz I, König FB, Ruprecht K,
Reindl M, Paul F, et al. Screening for MOGIgG
and 27 Other Anti-glial and Antineuronal
Autoantibodies in ‘Pattern II
Multiple Sclerosis’ and Brain Biopsy Findings
in a MOG-IgG-positive Case. Mult Scler
2016;1541-9.
13. Di Pauli F, Mader S, Rostasy K, Schanda K,
Bajer-Kornek B, Ehling R, et al. Temporal
Dynamics of anti-MOG Antibodies in CNS
Demyelinating Diseases. Clin Immunol
2011;138(3):247-54.
14. Bernard-Valnet R, Liblau RS, Vukusic S,
Marignier R. Neuromyelitis Optica: a Positive
Appraisal of Seronegative Cases. Eur J Neurol
2015;22(12):1511-8, e82-3.
15. Lucchinetti CF, Guo Y, Popescu BFG,
Fujihara K, Itoyama Y, Misu T. The
Pathology of an Autoimmune Astrocytopathy:
Lessons Learned from Neuromyelitis Optica.
Brain Pathol 2014;24(1):83-97.
16. Ikeda K, Kiyota N, Kuroda H, Sato DK,
Nishiyama S, Takahashi T, et al. Severe
Demyelination but no Astrocytopathy in
Clinically Definite Neuromyelitis Optica with
Anti-Myelin-Oligodendrocyte Glycoprotein
Antibody. Mult Scler 2015;21(5):656-9.
17. Pröbstel A-K, Rudolf G, Dornmair K,
Collongues N, Chanson J-B, Sanderson NS, et
al. Anti-MOG Antibodies Are Present in a
Subgroup of Patients with a Neuromyelitis
Optica Phenotype. J Neuroinflammation
2015;12(1):1.
18. Sato DK, Callegaro D, Lana-Peixoto MA,
Waters PJ, de Haidar Jorge FM, Takahashi T,
et al. Distinction between MOG Antibody-
Positive and AQP4 Antibody-Positive NMO
Spectrum Disorders. Neurology
2014;82(6):474-81.
19. Lechner C, Baumann M, Hennes EM,
Schanda K, Marquard K, Karenfort M, et al.
Antibodies to MOG and AQP4 in Children
with Neuromyelitis Optica and Limited Forms
of the Disease. J Neurol Neurosurg Psychiatry
2016; 87(8):897-905.
20. Dale RC. Acute Disseminated
Encephalomyelitis. Semin Pediatr Infect Dis
2003;14(2):90-5.
21. Huynh W, Cordato DJ, Kehdi E, Masters LT,
Dedousis C. Post-Vaccination
Encephalomyelitis: Literature Review and
Illustrative Case. J Clin Neurosci
2008;15(12):1315-22.
22. Baumann M, Hennes E, Schanda K, Karenfort
M, Bajer-Kornek B, Diepold K, et al. OP65–
3006: Clinical Characteristics and
Neuroradiological Findings in Children with
Multiphasic Demyelinating
Encephalomyelitis and MOG Antibodies. Eur
J Paediatr Neurol 2015;19:S21.
23. Di Pauli F, Höftberger R, Reindl M, Beer R,
Rhomberg P, Schanda K, et al. Fulminant
Demyelinating Encephalomyelitis Insights
from Antibody Studies and Neuropathology.
Neurol Neuroimmunol Neuroinflamm
2015;2(6):e175.
24. Numa S, Kasai T, Kondo T, Kushimura Y,
Kimura A, Takahashi H, et al. An Adult Case
of Anti-Myelin Oligodendrocyte Glycoprotein (MOG) Antibody-associated Multiphasic
Acute Disseminated Encephalomyelitis at 33-
year Intervals. Intern Med 2016;55(6):699-
702.
25. Miyauchi A, Monden Y, Watanabe M, Sugie
H, Morita M, Kezuka T, et al. Persistent
Presence of the Anti-Myelin Oligodendrocyte
Glycoprotein Autoantibody in a Pediatric
Case of Acute Disseminated
EncephalomyElitis Followed by Optic
Neuritis. Neuropediatrics 2014;45(3):196-9.
26. Pekcevik Y, Mitchell CH, Mealy MA, Orman
G, Lee IH, Newsome SD, et al.
Differentiating Neuromyelitis Optica from
Other Causes of Longitudinally Extensive
Transverse Myelitis on Spinal Magnetic
Resonance Imaging. Mult Scler
2016;22(3):302-11.
27. Cobo-Calvo A, Sepulveda M, Bernard-Valnet
R, Ruiz A, Brassat D, Martinez-Yelamos S, et
al. Antibodies to Myelin Oligodendrocyte
Glycoprotein in Aquaporin 4 Antibody
Seronegative Longitudinally Extensive
Transverse Myelitis: Clinical and Prognostic
Implications. Mult Scler 2016;22(3):312-9.
28. Amano H, Miyamoto N, Shimura H, Sato DK,
Fujihara K, Ueno S, et al. Influenzaassociated
MOG Antibody-Positive
Longitudinally Extensive Transverse Myelitis:
A Case Report. BMC Neurol 2014;14:224.
29. Chalmoukou K, Stathopoulos P, Alexopoulos
H, Akrivou S, Dalakas M. Recurrent Optic
Neuritis (rON) Is Characterised by Anti-MOG
Antibodies: A Follow-up Study (P5. 274).
Neurology 2015;84(14 Supplement):P5. 274.
30. Kezuka T, Usui Y, Yamakawa N, Matsunaga
Y, Matsuda R, Masuda M, et al. Relationship
between NMO-Antibody and Anti–MOG
Antibody in Optic Neuritis. J
Neuroophthalmol 2012;32(2):107-10.
31. Rostasy K, Mader S, Schanda K, Huppke P,
Gärtner J, Kraus V, et al. Anti–myelin
Oligodendrocyte Glycoprotein Antibodies in
Pediatric Patients with Optic Neuritis. Arch
Neurol 2012;69(6):752-6.
32. Huppke P, Rostasy K, Karenfort M, Huppke
B, Seidl R, Leiz S, et al. Acute Disseminated
Encephalomyelitis Followed by Recurrent or
Monophasic Optic Neuritis in Pediatric
Patients. Mult Scler 2013;19(7):941-6.
33. Ramanathan S, Reddel SW, Henderson A,
Parratt JD, Barnett M, Gatt PN, et al.
Antibodies to Myelin Oligodendrocyte
Glycoprotein in Bilateral and Recurrent Optic
Neuritis. Neurol Neuroimmunol
Neuroinflamm 2014;1(4):e40.
34. Tsuburaya RS, Miki N, Tanaka K, Kageyama
T, Irahara K, Mukaida S, et al. Anti-myelin
Oligodendrocyte Glycoprotein (MOG)
Antibodies in a Japanese Boy with Recurrent
Optic Neuritis. Brain Dev 2015;37(1):145-8.
35. Reindl M, Linington C, Brehm U, Egg R,
Dilitz E, Deisenhammer F, et al. Antibodies
Against the Myelin Oligodendrocyte
Glycoprotein and the Myelin Basic Protein in
Multiple Sclerosis and Other Neurological
Diseases: A Comparative Study. Brain
1999;122 ( Pt 11):2047-56.
36. Mader S, Gredler V, Schanda K, Rostasy K,
Dujmovic I, Pfaller K, et al. Complement
Activating Antibodies to Myelin
Oligodendrocyte Glycoprotein in
Neuromyelitis Optica and Related Disorders.
J Neuroinflammation 2011; 8:184.
37. Kovacs KT, Kalluri SR, Boza-Serrano A,
Deierborg T, Csepany T, Simo M, et al.
Change in Autoantibody and Cytokine
Responses During the Evolution of
Neuromyelitis Optica in Patients with
Systemic Lupus Erythematosus: A
Preliminary Study. Mult Scler
2016;22(9):1192-201.
38. Oshiro A, Nakamura S, Tamashiro K,
Fujihara K. Anti-MOG + Neuromyelitis
Optica Spectrum Disorders Treated with
Plasmapheresis. No To Hattatsu
2016;48(3):199-203. [Text in Japanese]
39. Miyazaki T, Nakajima H, Motomura M,
Tanaka K, Maeda Y, Shiraishi H, et al. A
Case of Recurrent Optic Neuritis Associated
with Cerebral and Spinal Cord Lesions and
Autoantibodies Against Myelin
Oligodendrocyte Glycoprotein Relapsed after
Fingolimod Therapy. Rinsho Shinkeigaku
2016;56(4):265-9.
40. Thulasirajah S, Pohl D, Davila-Acosta J,
Venkateswaran S. Myelin Oligodendrocyte
Glycoprotein-Associated Pediatric Central
Nervous System Demyelination: Clinical
Course, Neuroimaging Findings, and Response to Therapy. Neuropediatrics 2016;
47(4):245-52.
41. Hacohen Y, Absoud M, Gadian J, Clarke A,
Hemingway C, Palace J, et al. PP08.4 –
2929:Relapsing Central Nervous System
Demyelination in Children with Myelin
Oligodendrocyte Glycoprotein (MOG)
Antibodies. Eur J Paediatr Neurol
2015;19(supplement 1):S61.
42. Ram D, Hughes M. PP08. 6–2786: Relapsing
MOG Antibody Associated
Encephalomyelitis: A Case Report. Eur J
Paediatr Neurol 2015;19:S61.
43. Brilot F, Dale RC, Selter RC, Grummel V,
Reddy Kalluri S, Aslam M, et al. Antibodies
to Native Myelin Oligodendrocyte
Glycoprotein in Children with Inflammatory
Demyelinating Central Nervous System
Disease. Ann Neurol 2009;66(6):833-42.
44. Spadaro M, Gerdes LA, Krumbholz M, Ertl-
Wagner B, Thaler FS, Schuh E, et al.
Autoantibodies to MOG in a Distinct
Subgroup of Adult Multiple Sclerosis. Neurol
Neuroimmunol Neuroinflamm
2016;3(5):e257.
45. Kong XY, Peng JT, Liu LJ, Yan R, Zhang XJ.
Anti-MOG antibody in different types of
immune-mediated optic neuritis. Zhonghua
Yan Ke Za Zhi 2012;48(12):1069-72. [Text in
Chinese]
Downloaded
Optic neuritis is most often a consequence of multiple sclerosis and can lead to partial or complete loss of vision in one or both eyes. Autoimmune mechanisms like producing antibodies against myelin sheath are the causes of this disorder. When idiopathic optic neuritis occurs repeatedly it is considered to be a distinct clinical condition and if accompanied by demyelination it’s believed to be associated with anti-MOG antibodies (29).
While observing thirty three eyes of 23 patients with optic neuritis and measuring their serum anti-MOG antibodies concentration, 34% of the cases were seropositive (and 26% of cases were seropositive for both MOG and NMO antibodies).This immunoglobulin is considered to be a biomarker to investigate visual prognosis in optic neuritis patients (30). A study on 37 cases, 18 or under 18 years old with recurrent or single episodes of optic neuritis, suggested that generally these patients had high titer serum anti-MOG antibodies from the subclass of IgG, especially in patients suffering from recurrent episodes of optic neuritis (31). In a group of pediatrics suffering from recurrent or monophasic ADEM, optic neuritis was diagnosed. Their serum immunoglobulin against MOG protein was measured high titer (32). It is concluded that anti-MOG antibodies can exert a direct role in optic neuritis pathogenesis. This antibody is significantly associated with bilateral optic neuritis and can play the role of clinical biomarker. Anti-MOG antibody-associated bilateral ON, which is a relapsing disorder, is frequently steroid dependent (33). And as Tsuburaya stated, recurrent ON patients are candidates for serum anti-MOG antibody analysis (34).
Anti-MOG antibodies in other disorders
A recent study on 10 arthritis rheumatoid cases resulted in anti-MOG antibody seropositivity in only one patient. It leads to the conclusion that although these antibodies exclusively serve in CNS but we can find them in chronic systemic inflammatory disorders in which CNS is not involved (35).
A general survey carried out by Mader and his team presented high MOG-IgG serum
concentration in 2/27 of systemic lupus erythematosus (SLE) cases (36). However exploring serum of 6 systemic lupus erythematosus cases indicated no sign of anti-MOG IgG/IgM (37).
Treatment
Treatment of MOG spectrum patients is divided in two major parts: treatment of attacks and prevention. The acute phase is generally treated by corticosteroid therapy.
There is no certain agreement about the treatment for MOG spectrum disorder patients however in acute attacks we can use methylprednisolone intravenous (1gr per day, for 3-7 days) for treating acute attacks in patients presented with optic neuritis and transverse myelitis and other symptoms of central nervous system involvement. If the patient showed no response to treatments we can repeat the mentioned dose and plasmapheresis is can be used after intravenous methylprednisolone. Oshiro et al. reported a 10 year old boy with an NMO spectrum disorder who underwent plasma exchange therapy. Only after anti-MOG antibody serum titer was decreased to the cut-out level did his symptoms obviously stop; however as they suggested further studies are required to establish this method as a therapeutic pathway (38).
No certain therapy is suggested for prevention yet, however due to recent studies these patients are advised to administrate azathioprine or methotrexate and low dose of prednisolone (for 6-12 months).
A 16 year old girl with optic neuritis accompanied by spinal and cerebral lesions demonstrated anti-MOG antibody seropositivity and fingolimod therapy brought about a relapsing condition. It is suggested
that fingolimod can’t be an effective choice in MOG spectrum disorders (39).
A general study on patients with ADEM, LETM and NMOSD, all were demonstrating high anti-MOG antibody serum titer, suggested that intravenous immunoglobulin followed by rituximab significantly reduces the relapse rate. While comparing the cases according to their age, it was proved that children with MOG antibody spectrum disorder recovered better after steroid and IVIG therapy (40).
Relapsing course can be observed in some anti-MOG seropositive patients. These cases fulfill MS diagnosis but they may have a poor response to MS drug treatments like interferon β (41). An eleven year old girl whose MRI showed transverse myelitis-like lesions, underwent systemic steroid treatment and after initial improvements she lost bowel and bladder control and the lower limb weakness worsened. Vast serum investigations revealed anti-MOG antibody seropositivity; however after frequent courses of plasmapheresis, this condition continued to deteriorate. Finally, extreme immunosuppressive therapy such as cyclophosphamide and rituximab for one year was able to omit further relapses but paraparesis was not eliminated (42).
Discussion
Myelin Oligodendrocyte Glycoprotein, a CNS exclusive glycoprotein located on the outermost layer of myelin sheath, is produced by oligodendrocytes. This adhesive protein plays an important role in myelin sheath structural integrity and maintenance. Thus any malfunction in MOG protein might result in myelin sheath malformation, which can be seen as signs of demyelinating disorders: For instance immune responses are capable of
endangering MOG molecule entirety. Antibodies against MOG not only bind to the extracellular domain of this protein, but induce natural killer cells to terminate the cells which express MOG (43).
As mentioned above in a reliable research Egg and his group concluded that Anti-MOG antibodies were present in most cases of Multiple sclerosis, though in contrast with that, with due attention to Jarius et al. large study’s observing anti-neural antibodies, MOG-IgG might play a noticeable role in a small percentage of cases diagnosed with pattern II MS. Also according to a research performed in 2016 on 104 preselected MS patients, antibodies to MOG were existent in almost 5% of cases. So it seems the elevation of MOG antibodies is not explicit in MS patients. On brain MRI of all seropositive cases lesions were similar to those of typical MS patients. Following them up for more than 9 years to have a longitudinal analysis revealed fluctuations in Anti-MOG antibody serum titer and activity (9, 12, 44).
While researches done in fields of acute disseminated encephalomyelitis, transverse myelitis, optic neuritis and clinically isolated syndrome were suggesting the important destructive role of anti-MOG antibodies in these disorders, the results were not strongly proved or number of cases were not large enough, therefore further investigations are required to gather a solid opinion on this issue. Nonetheless a research covering the Anti-MOG antibodies in CNS demyelinating diseases, a rare study on this specific subject, reported the following: age is a significant factor in ADEM disease; this means that predominant high titer antibody against MOG was demonstrated in pediatrics. An important finding suggested that recombinant human MOG ELISA couldn’t detect these
antibodies. Other than that these antibodies were rarely frequent in CIS cases’ serum (13). In contrast with the above study, Brilot et al. detected Anti-MOG antibodies in 40% of their CIS/ADEM patients by a cell-based bioassay. They also asserted that cases which were seropositive for antibodies were younger, as it was suggested in the previous reference, and that anti-MOG IgG titer was not different between the CIS cases and the ADEM ones (43).
A recent case report studying on a positive anti-MOG antibody Japanese boy supported the relationship of the former immunoglobulin with Optic Neuritis, although additional research is needed in this field (7). Anti-MOG antibodies are detected in different kinds of immune-mediated Optic Neuritis; however in NMO related Optic Neuritis they’re not found. By contrast the expression of the mentioned immunoglobulin was significantly higher in MS related Optic Neuritis rather than other NMO spectrum disorders’ group (45).
To conclude all above it can be stated that Anti-MOG antibodies can be considered as biomarkers for central nervous system autoimmune demyelinating disorders. Detecting these immunoglobulins in patients’ serums can be useful in diagnosis and prognosis pathways. It is possible that anti-MOG antibodies in patients presenting several symptoms such as myelitis, optic neuritis and so on can be sign of a new disease, so we choose MOG spectrum disorder for Anti-MOG antibody seropositive patients presenting mentioned neurological characteristics. Still MOG spectrum disorder is a new term in nervous system demyelinating diseases field.
Conclusion
To conclude all above we can state that for prophylactic treatment of MOG spectrum disorder azathioprine, methotrexate, rituximab and cyclophosphamide are suggested. On the other hand methyl prednisolone and plasmapheresis are suitable for acute phase. Intravenous immunoglobulin can be useful in relapsing forms but it needs more investigations to be proved. According to similar physiopathology of NMOSD and MOG spectrum disorder it is noticeable that fingolimod and interferons may deteriorate the conditions in both of them.
Conflict of Interest
The authors have no conflict of interest.
References
1. Roth M-P, Malfroy L, Offer C, Sevin J,
Enault G, Borot N, et al. The Human Myelin
Oligodendrocyte Glycoprotein (MOG) Gene:
Complete Nucleotide Sequence and Structural
Characterization. Genomics 1995;28(2):241-
50.
2. Pham-Dinh D, Allinquant B, Ruberg M, Della
Gaspera B, Nussbaum JL, Dautigny A.
Characterization and Expression of the cDNA
Coding for the Human
Myelin/Oligodendrocyte Glycoprotein. J
Neurochem 1994;63(6):2353-6.
3. Pham-Dinh D, Mattei M-G, Nussbaum J-L,
Roussel G, Pontarotti P, Roeckel N, et al.
Myelin/Oligodendrocyte Glycoprotein Is a
Member of a Subset of the Immunoglobulin
Superfamily Encoded within the Major
Histocompatibility Complex. Proc Natl Acad
Sci U S A 1993;90(17):7990-4.
4. Boyle LH, Traherne JA, Plotnek G, Ward R,
Trowsdale J. Splice Variation in the
Cytoplasmic Domains of Myelin
Oligodendrocyte Glycoprotein Affects Its
Cellular Localisation and Transport1. J
Neurochem 2007;102(6):1853-62.
5. Breithaupt C, Schubart A, Zander H, Skerra
A, Huber R, Linington C, et al. Structural
Insights into the Antigenicity of Myelin
Oligodendrocyte Glycoprotein. Proc Natl
Acad Sci U S A 2003;100(16):9446-51.
6. Clements CS, Reid HH, Beddoe T, Tynan FE,
Perugini MA, Johns TG, et al. The Crystal
Structure of Myelin Oligodendrocyte
Glycoprotein, a Key Autoantigen in Multiple
Sclerosis. Proc Natl Acad Sci U S A
2003;100(19):11059-64.
7. Tsuburaya RS, Miki N, Tanaka K, Kageyama
T, Irahara K, Mukaida S, et al. Anti-myelin
Oligodendrocyte Glycoprotein (MOG)
Antibodies in a Japanese Boy with Recurrent
Optic Neuritis. Brain Dev 2015;37(1):145-8.
8. Ascherio A, Munger KL. Environmental Risk
Factors for Multiple Sclerosis. Part I: the Role
of Infection. Ann Neurol 2007;61(4):288-99.
9. Egg R, Reindl M, Deisenhammer F, Linington
C, Berger T. Anti-MOG and Anti-MBP
Antibody Subclasses in Multiple Sclerosis.
Mult Scler 2001;7(5):285-9.
10. Xiao B-G, Linington C, Link Hd. Antibodies
to Myelin-Oligodendrocyte Glycoprotein in
Cerebrospinal Fluid from Patients with
Multiple Sclerosis and Controls. J
Neuroimmunol 1991;31(2):91-6.
11. Lampasona V, Franciotta D, Furlan R,
Zanaboni S, Fazio R, Bonifacio E, et al.
Similar Low Frequency of anti-MOG IgG and
IgM in MS Patients and Healthy Subjects.
Neurology 2004;62(11):2092-4.
12. Jarius S, Metz I, König FB, Ruprecht K,
Reindl M, Paul F, et al. Screening for MOGIgG
and 27 Other Anti-glial and Antineuronal
Autoantibodies in ‘Pattern II
Multiple Sclerosis’ and Brain Biopsy Findings
in a MOG-IgG-positive Case. Mult Scler
2016;1541-9.
13. Di Pauli F, Mader S, Rostasy K, Schanda K,
Bajer-Kornek B, Ehling R, et al. Temporal
Dynamics of anti-MOG Antibodies in CNS
Demyelinating Diseases. Clin Immunol
2011;138(3):247-54.
14. Bernard-Valnet R, Liblau RS, Vukusic S,
Marignier R. Neuromyelitis Optica: a Positive
Appraisal of Seronegative Cases. Eur J Neurol
2015;22(12):1511-8, e82-3.
15. Lucchinetti CF, Guo Y, Popescu BFG,
Fujihara K, Itoyama Y, Misu T. The
Pathology of an Autoimmune Astrocytopathy:
Lessons Learned from Neuromyelitis Optica.
Brain Pathol 2014;24(1):83-97.
16. Ikeda K, Kiyota N, Kuroda H, Sato DK,
Nishiyama S, Takahashi T, et al. Severe
Demyelination but no Astrocytopathy in
Clinically Definite Neuromyelitis Optica with
Anti-Myelin-Oligodendrocyte Glycoprotein
Antibody. Mult Scler 2015;21(5):656-9.
17. Pröbstel A-K, Rudolf G, Dornmair K,
Collongues N, Chanson J-B, Sanderson NS, et
al. Anti-MOG Antibodies Are Present in a
Subgroup of Patients with a Neuromyelitis
Optica Phenotype. J Neuroinflammation
2015;12(1):1.
18. Sato DK, Callegaro D, Lana-Peixoto MA,
Waters PJ, de Haidar Jorge FM, Takahashi T,
et al. Distinction between MOG Antibody-
Positive and AQP4 Antibody-Positive NMO
Spectrum Disorders. Neurology
2014;82(6):474-81.
19. Lechner C, Baumann M, Hennes EM,
Schanda K, Marquard K, Karenfort M, et al.
Antibodies to MOG and AQP4 in Children
with Neuromyelitis Optica and Limited Forms
of the Disease. J Neurol Neurosurg Psychiatry
2016; 87(8):897-905.
20. Dale RC. Acute Disseminated
Encephalomyelitis. Semin Pediatr Infect Dis
2003;14(2):90-5.
21. Huynh W, Cordato DJ, Kehdi E, Masters LT,
Dedousis C. Post-Vaccination
Encephalomyelitis: Literature Review and
Illustrative Case. J Clin Neurosci
2008;15(12):1315-22.
22. Baumann M, Hennes E, Schanda K, Karenfort
M, Bajer-Kornek B, Diepold K, et al. OP65–
3006: Clinical Characteristics and
Neuroradiological Findings in Children with
Multiphasic Demyelinating
Encephalomyelitis and MOG Antibodies. Eur
J Paediatr Neurol 2015;19:S21.
23. Di Pauli F, Höftberger R, Reindl M, Beer R,
Rhomberg P, Schanda K, et al. Fulminant
Demyelinating Encephalomyelitis Insights
from Antibody Studies and Neuropathology.
Neurol Neuroimmunol Neuroinflamm
2015;2(6):e175.
24. Numa S, Kasai T, Kondo T, Kushimura Y,
Kimura A, Takahashi H, et al. An Adult Case
of Anti-Myelin Oligodendrocyte Glycoprotein (MOG) Antibody-associated Multiphasic
Acute Disseminated Encephalomyelitis at 33-
year Intervals. Intern Med 2016;55(6):699-
702.
25. Miyauchi A, Monden Y, Watanabe M, Sugie
H, Morita M, Kezuka T, et al. Persistent
Presence of the Anti-Myelin Oligodendrocyte
Glycoprotein Autoantibody in a Pediatric
Case of Acute Disseminated
EncephalomyElitis Followed by Optic
Neuritis. Neuropediatrics 2014;45(3):196-9.
26. Pekcevik Y, Mitchell CH, Mealy MA, Orman
G, Lee IH, Newsome SD, et al.
Differentiating Neuromyelitis Optica from
Other Causes of Longitudinally Extensive
Transverse Myelitis on Spinal Magnetic
Resonance Imaging. Mult Scler
2016;22(3):302-11.
27. Cobo-Calvo A, Sepulveda M, Bernard-Valnet
R, Ruiz A, Brassat D, Martinez-Yelamos S, et
al. Antibodies to Myelin Oligodendrocyte
Glycoprotein in Aquaporin 4 Antibody
Seronegative Longitudinally Extensive
Transverse Myelitis: Clinical and Prognostic
Implications. Mult Scler 2016;22(3):312-9.
28. Amano H, Miyamoto N, Shimura H, Sato DK,
Fujihara K, Ueno S, et al. Influenzaassociated
MOG Antibody-Positive
Longitudinally Extensive Transverse Myelitis:
A Case Report. BMC Neurol 2014;14:224.
29. Chalmoukou K, Stathopoulos P, Alexopoulos
H, Akrivou S, Dalakas M. Recurrent Optic
Neuritis (rON) Is Characterised by Anti-MOG
Antibodies: A Follow-up Study (P5. 274).
Neurology 2015;84(14 Supplement):P5. 274.
30. Kezuka T, Usui Y, Yamakawa N, Matsunaga
Y, Matsuda R, Masuda M, et al. Relationship
between NMO-Antibody and Anti–MOG
Antibody in Optic Neuritis. J
Neuroophthalmol 2012;32(2):107-10.
31. Rostasy K, Mader S, Schanda K, Huppke P,
Gärtner J, Kraus V, et al. Anti–myelin
Oligodendrocyte Glycoprotein Antibodies in
Pediatric Patients with Optic Neuritis. Arch
Neurol 2012;69(6):752-6.
32. Huppke P, Rostasy K, Karenfort M, Huppke
B, Seidl R, Leiz S, et al. Acute Disseminated
Encephalomyelitis Followed by Recurrent or
Monophasic Optic Neuritis in Pediatric
Patients. Mult Scler 2013;19(7):941-6.
33. Ramanathan S, Reddel SW, Henderson A,
Parratt JD, Barnett M, Gatt PN, et al.
Antibodies to Myelin Oligodendrocyte
Glycoprotein in Bilateral and Recurrent Optic
Neuritis. Neurol Neuroimmunol
Neuroinflamm 2014;1(4):e40.
34. Tsuburaya RS, Miki N, Tanaka K, Kageyama
T, Irahara K, Mukaida S, et al. Anti-myelin
Oligodendrocyte Glycoprotein (MOG)
Antibodies in a Japanese Boy with Recurrent
Optic Neuritis. Brain Dev 2015;37(1):145-8.
35. Reindl M, Linington C, Brehm U, Egg R,
Dilitz E, Deisenhammer F, et al. Antibodies
Against the Myelin Oligodendrocyte
Glycoprotein and the Myelin Basic Protein in
Multiple Sclerosis and Other Neurological
Diseases: A Comparative Study. Brain
1999;122 ( Pt 11):2047-56.
36. Mader S, Gredler V, Schanda K, Rostasy K,
Dujmovic I, Pfaller K, et al. Complement
Activating Antibodies to Myelin
Oligodendrocyte Glycoprotein in
Neuromyelitis Optica and Related Disorders.
J Neuroinflammation 2011; 8:184.
37. Kovacs KT, Kalluri SR, Boza-Serrano A,
Deierborg T, Csepany T, Simo M, et al.
Change in Autoantibody and Cytokine
Responses During the Evolution of
Neuromyelitis Optica in Patients with
Systemic Lupus Erythematosus: A
Preliminary Study. Mult Scler
2016;22(9):1192-201.
38. Oshiro A, Nakamura S, Tamashiro K,
Fujihara K. Anti-MOG + Neuromyelitis
Optica Spectrum Disorders Treated with
Plasmapheresis. No To Hattatsu
2016;48(3):199-203. [Text in Japanese]
39. Miyazaki T, Nakajima H, Motomura M,
Tanaka K, Maeda Y, Shiraishi H, et al. A
Case of Recurrent Optic Neuritis Associated
with Cerebral and Spinal Cord Lesions and
Autoantibodies Against Myelin
Oligodendrocyte Glycoprotein Relapsed after
Fingolimod Therapy. Rinsho Shinkeigaku
2016;56(4):265-9.
40. Thulasirajah S, Pohl D, Davila-Acosta J,
Venkateswaran S. Myelin Oligodendrocyte
Glycoprotein-Associated Pediatric Central
Nervous System Demyelination: Clinical
Course, Neuroimaging Findings, and Response to Therapy. Neuropediatrics 2016;
47(4):245-52.
41. Hacohen Y, Absoud M, Gadian J, Clarke A,
Hemingway C, Palace J, et al. PP08.4 –
2929:Relapsing Central Nervous System
Demyelination in Children with Myelin
Oligodendrocyte Glycoprotein (MOG)
Antibodies. Eur J Paediatr Neurol
2015;19(supplement 1):S61.
42. Ram D, Hughes M. PP08. 6–2786: Relapsing
MOG Antibody Associated
Encephalomyelitis: A Case Report. Eur J
Paediatr Neurol 2015;19:S61.
43. Brilot F, Dale RC, Selter RC, Grummel V,
Reddy Kalluri S, Aslam M, et al. Antibodies
to Native Myelin Oligodendrocyte
Glycoprotein in Children with Inflammatory
Demyelinating Central Nervous System
Disease. Ann Neurol 2009;66(6):833-42.
44. Spadaro M, Gerdes LA, Krumbholz M, Ertl-
Wagner B, Thaler FS, Schuh E, et al.
Autoantibodies to MOG in a Distinct
Subgroup of Adult Multiple Sclerosis. Neurol
Neuroimmunol Neuroinflamm
2016;3(5):e257.
45. Kong XY, Peng JT, Liu LJ, Yan R, Zhang XJ.
Anti-MOG antibody in different types of
immune-mediated optic neuritis. Zhonghua
Yan Ke Za Zhi 2012;48(12):1069-72. [Text in
Chinese]
Downloaded
Type of Study: Review |
Subject:
Special
Received: 2017/11/30 | Accepted: 2017/11/30 | Published: 2017/11/30
Received: 2017/11/30 | Accepted: 2017/11/30 | Published: 2017/11/30
References
1. Roth M-P, Malfroy L, Offer C, Sevin J, Enault G, Borot N, et al. The Human Myelin Oligodendrocyte Glycoprotein (MOG) Gene: Complete Nucleotide Sequence and Structural Characterization. Genomics 1995;28(2):241-50. [DOI:10.1006/geno.1995.1137] [PMID]
2. Pham-Dinh D, Allinquant B, Ruberg M, Della Gaspera B, Nussbaum JL, Dautigny A. Characterization and Expression of the cDNA Coding for the Human Myelin/Oligodendrocyte Glycoprotein. J Neurochem 1994;63(6):2353-6. [DOI:10.1046/j.1471-4159.1994.63062353.x] [PMID]
3. Pham-Dinh D, Mattei M-G, Nussbaum J-L, Roussel G, Pontarotti P, Roeckel N, et al. Myelin/Oligodendrocyte Glycoprotein Is a Member of a Subset of the Immunoglobulin Superfamily Encoded within the Major Histocompatibility Complex. Proc Natl Acad Sci U S A 1993;90(17):7990-4. [DOI:10.1073/pnas.90.17.7990] [PMID] [PMCID]
4. Boyle LH, Traherne JA, Plotnek G, Ward R, Trowsdale J. Splice Variation in the Cytoplasmic Domains of Myelin Oligodendrocyte Glycoprotein Affects Its Cellular Localisation and Transport1. J Neurochem 2007;102(6):1853-62. [DOI:10.1111/j.1471-4159.2007.04687.x] [PMID] [PMCID]
5. Breithaupt C, Schubart A, Zander H, Skerra A, Huber R, Linington C, et al. Structural Insights into the Antigenicity of Myelin Oligodendrocyte Glycoprotein. Proc Natl Acad Sci U S A 2003;100(16):9446-51. [DOI:10.1073/pnas.1133443100] [PMID] [PMCID]
6. Clements CS, Reid HH, Beddoe T, Tynan FE, Perugini MA, Johns TG, et al. The Crystal Structure of Myelin Oligodendrocyte Glycoprotein, a Key Autoantigen in Multiple Sclerosis. Proc Natl Acad Sci U S A 2003;100(19):11059-64. [DOI:10.1073/pnas.1833158100] [PMID] [PMCID]
7. Tsuburaya RS, Miki N, Tanaka K, Kageyama T, Irahara K, Mukaida S, et al. Anti-myelin Oligodendrocyte Glycoprotein (MOG) Antibodies in a Japanese Boy with Recurrent Optic Neuritis. Brain Dev 2015;37(1):145-8. [DOI:10.1016/j.braindev.2014.02.002] [PMID]
8. Ascherio A, Munger KL. Environmental Risk Factors for Multiple Sclerosis. Part I: the Role of Infection. Ann Neurol 2007;61(4):288-99. [DOI:10.1002/ana.21117] [PMID]
9. Egg R, Reindl M, Deisenhammer F, Linington C, Berger T. Anti-MOG and Anti-MBP Antibody Subclasses in Multiple Sclerosis. Mult Scler 2001;7(5):285-9.
https://doi.org/10.1177/135245850100700503 [DOI:10.1191/135245801681137979] [PMID]
10. Xiao B-G, Linington C, Link Hd. Antibodies to Myelin-Oligodendrocyte Glycoprotein in Cerebrospinal Fluid from Patients with Multiple Sclerosis and Controls. J Neuroimmunol 1991;31(2):91-6. [DOI:10.1016/0165-5728(91)90014-X]
11. Lampasona V, Franciotta D, Furlan R, Zanaboni S, Fazio R, Bonifacio E, et al. Similar Low Frequency of anti-MOG IgG and IgM in MS Patients and Healthy Subjects. Neurology 2004;62(11):2092-4. [DOI:10.1212/01.WNL.0000127615.15768.AE] [PMID]
12. Jarius S, Metz I, König FB, Ruprecht K, Reindl M, Paul F, et al. Screening for MOG-IgG and 27 Other Anti-glial and Anti-neuronal Autoantibodies in 'Pattern II Multiple Sclerosis' and Brain Biopsy Findings in a MOG-IgG-positive Case. Mult Scler 2016;1541-9. [DOI:10.1177/1352458515622986] [PMID]
13. Di Pauli F, Mader S, Rostasy K, Schanda K, Bajer-Kornek B, Ehling R, et al. Temporal Dynamics of anti-MOG Antibodies in CNS Demyelinating Diseases. Clin Immunol 2011;138(3):247-54. [DOI:10.1016/j.clim.2010.11.013] [PMID]
14. Bernard-Valnet R, Liblau RS, Vukusic S, Marignier R. Neuromyelitis Optica: a Positive Appraisal of Seronegative Cases. Eur J Neurol 2015;22(12):1511-8, e82-3.
15. Lucchinetti CF, Guo Y, Popescu BFG, Fujihara K, Itoyama Y, Misu T. The Pathology of an Autoimmune Astrocytopathy: Lessons Learned from Neuromyelitis Optica. Brain Pathol 2014;24(1):83-97. [DOI:10.1111/bpa.12099] [PMID] [PMCID]
16. Ikeda K, Kiyota N, Kuroda H, Sato DK, Nishiyama S, Takahashi T, et al. Severe Demyelination but no Astrocytopathy in Clinically Definite Neuromyelitis Optica with Anti-Myelin-Oligodendrocyte Glycoprotein Antibody. Mult Scler 2015;21(5):656-9. [DOI:10.1177/1352458514551455] [PMID]
17. Pröbstel A-K, Rudolf G, Dornmair K, Collongues N, Chanson J-B, Sanderson NS, et al. Anti-MOG Antibodies Are Present in a Subgroup of Patients with a Neuromyelitis Optica Phenotype. J Neuroinflammation 2015;12(1):1. [DOI:10.1186/s12974-015-0256-1]
18. Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, de Haidar Jorge FM, Takahashi T, et al. Distinction between MOG Antibody-Positive and AQP4 Antibody-Positive NMO Spectrum Disorders. Neurology 2014;82(6):474-81. [DOI:10.1212/WNL.0000000000000101] [PMID] [PMCID]
19. Lechner C, Baumann M, Hennes EM, Schanda K, Marquard K, Karenfort M, et al. Antibodies to MOG and AQP4 in Children with Neuromyelitis Optica and Limited Forms of the Disease. J Neurol Neurosurg Psychiatry 2016; 87(8):897-905. [DOI:10.1136/jnnp-2015-311743] [PMID]
20. Dale RC. Acute Disseminated Encephalomyelitis. Semin Pediatr Infect Dis 2003;14(2):90-5. [DOI:10.1053/spid.2003.127225] [PMID]
21. Huynh W, Cordato DJ, Kehdi E, Masters LT, Dedousis C. Post-Vaccination Encephalomyelitis: Literature Review and Illustrative Case. J Clin Neurosci 2008;15(12):1315-22. [DOI:10.1016/j.jocn.2008.05.002] [PMID]
22. Baumann M, Hennes E, Schanda K, Karenfort M, Bajer-Kornek B, Diepold K, et al. OP65–3006: Clinical Characteristics and Neuroradiological Findings in Children with Multiphasic Demyelinating Encephalomyelitis and MOG Antibodies. Eur J Paediatr Neurol 2015;19:S21. [DOI:10.1016/S1090-3798(15)30066-0]
23. Di Pauli F, Höftberger R, Reindl M, Beer R, Rhomberg P, Schanda K, et al. Fulminant Demyelinating Encephalomyelitis Insights from Antibody Studies and Neuropathology. Neurol Neuroimmunol Neuroinflamm 2015;2(6):e175. [DOI:10.1212/NXI.0000000000000175] [PMID] [PMCID]
24. Numa S, Kasai T, Kondo T, Kushimura Y, Kimura A, Takahashi H, et al. An Adult Case of Anti-Myelin Oligodendrocyte Glycoprotein (MOG) Antibody-associated Multiphasic Acute Disseminated Encephalomyelitis at 33-year Intervals. Intern Med 2016;55(6):699-702. [DOI:10.2169/internalmedicine.55.5727] [PMID]
25. Miyauchi A, Monden Y, Watanabe M, Sugie H, Morita M, Kezuka T, et al. Persistent Presence of the Anti-Myelin Oligodendrocyte Glycoprotein Autoantibody in a Pediatric Case of Acute Disseminated EncephalomyElitis Followed by Optic Neuritis. Neuropediatrics 2014;45(3):196-9.
https://doi.org/10.1055/s-0034-1371179 [DOI:10.1055/s-0034-1372693] [PMID]
26. Pekcevik Y, Mitchell CH, Mealy MA, Orman G, Lee IH, Newsome SD, et al. Differentiating Neuromyelitis Optica from Other Causes of Longitudinally Extensive Transverse Myelitis on Spinal Magnetic Resonance Imaging. Mult Scler 2016;22(3):302-11. [DOI:10.1177/1352458515591069] [PMID] [PMCID]
27. Cobo-Calvo A, Sepulveda M, Bernard-Valnet R, Ruiz A, Brassat D, Martinez-Yelamos S, et al. Antibodies to Myelin Oligodendrocyte Glycoprotein in Aquaporin 4 Antibody Seronegative Longitudinally Extensive Transverse Myelitis: Clinical and Prognostic Implications. Mult Scler 2016;22(3):312-9. [DOI:10.1177/1352458515591071] [PMID]
28. Amano H, Miyamoto N, Shimura H, Sato DK, Fujihara K, Ueno S, et al. Influenza-associated MOG Antibody-Positive Longitudinally Extensive Transverse Myelitis: A Case Report. BMC Neurol 2014;14:224. [DOI:10.1186/s12883-014-0224-x] [PMID] [PMCID]
29. Chalmoukou K, Stathopoulos P, Alexopoulos H, Akrivou S, Dalakas M. Recurrent Optic Neuritis (rON) Is Characterised by Anti-MOG Antibodies: A Follow-up Study (P5. 274). Neurology 2015;84(14 Supplement):P5. 274.
30. Kezuka T, Usui Y, Yamakawa N, Matsunaga Y, Matsuda R, Masuda M, et al. Relationship between NMO-Antibody and Anti–MOG Antibody in Optic Neuritis. J Neuroophthalmol 2012;32(2):107-10. [DOI:10.1097/WNO.0b013e31823c9b6c] [PMID]
31. Rostasy K, Mader S, Schanda K, Huppke P, Gärtner J, Kraus V, et al. Anti–myelin Oligodendrocyte Glycoprotein Antibodies in Pediatric Patients with Optic Neuritis. Arch Neurol 2012;69(6):752-6. [DOI:10.1001/archneurol.2011.2956] [PMID]
32. Huppke P, Rostasy K, Karenfort M, Huppke B, Seidl R, Leiz S, et al. Acute Disseminated Encephalomyelitis Followed by Recurrent or Monophasic Optic Neuritis in Pediatric Patients. Mult Scler 2013;19(7):941-6. [DOI:10.1177/1352458512466317] [PMID]
33. Ramanathan S, Reddel SW, Henderson A, Parratt JD, Barnett M, Gatt PN, et al. Antibodies to Myelin Oligodendrocyte Glycoprotein in Bilateral and Recurrent Optic Neuritis. Neurol Neuroimmunol Neuroinflamm 2014;1(4):e40. [DOI:10.1212/NXI.0000000000000040] [PMID] [PMCID]
34. Tsuburaya RS, Miki N, Tanaka K, Kageyama T, Irahara K, Mukaida S, et al. Anti-myelin Oligodendrocyte Glycoprotein (MOG) Antibodies in a Japanese Boy with Recurrent Optic Neuritis. Brain Dev 2015;37(1):145-8. [DOI:10.1016/j.braindev.2014.02.002] [PMID]
35. Reindl M, Linington C, Brehm U, Egg R, Dilitz E, Deisenhammer F, et al. Antibodies Against the Myelin Oligodendrocyte Glycoprotein and the Myelin Basic Protein in Multiple Sclerosis and Other Neurological Diseases: A Comparative Study. Brain 1999;122 (Pt 11):2047-56. [DOI:10.1093/brain/122.11.2047] [PMID]
36. Mader S, Gredler V, Schanda K, Rostasy K, Dujmovic I, Pfaller K, et al. Complement Activating Antibodies to Myelin Oligodendrocyte Glycoprotein in Neuromyelitis Optica and Related Disorders. J Neuroinflammation 2011; 8:184. [DOI:10.1186/1742-2094-8-184] [PMID] [PMCID]
37. Kovacs KT, Kalluri SR, Boza-Serrano A, Deierborg T, Csepany T, Simo M, et al. Change in Autoantibody and Cytokine Responses During the Evolution of Neuromyelitis Optica in Patients with Systemic Lupus Erythematosus: A Preliminary Study. Mult Scler 2016;22(9):1192-201. [DOI:10.1177/1352458515613165] [PMID]
38. Oshiro A, Nakamura S, Tamashiro K, Fujihara K. Anti-MOG + Neuromyelitis Optica Spectrum Disorders Treated with Plasmapheresis. No To Hattatsu 2016;48(3):199-203. [Text in Japanese] [PMID]
39. Miyazaki T, Nakajima H, Motomura M, Tanaka K, Maeda Y, Shiraishi H, et al. A Case of Recurrent Optic Neuritis Associated with Cerebral and Spinal Cord Lesions and Autoantibodies Against Myelin Oligodendrocyte Glycoprotein Relapsed after Fingolimod Therapy. Rinsho Shinkeigaku 2016;56(4):265-9. [DOI:10.5692/clinicalneurol.cn-000756] [PMID]
40. Thulasirajah S, Pohl D, Davila-Acosta J, Venkateswaran S. Myelin Oligodendrocyte Glycoprotein-Associated Pediatric Central Nervous System Demyelination: Clinical Course, Neuroimaging Findings, and Response to Therapy. Neuropediatrics 2016; 47(4):245-52. [DOI:10.1055/s-0036-1583184] [PMID]
41. Hacohen Y, Absoud M, Gadian J, Clarke A, Hemingway C, Palace J, et al. PP08.4 – 2929:Relapsing Central Nervous System Demyelination in Children with Myelin Oligodendrocyte Glycoprotein (MOG) Antibodies. Eur J Paediatr Neurol 2015;19(supplement 1):S61. [DOI:10.1016/S1090-3798(15)30200-2]
42. Ram D, Hughes M. PP08. 6–2786: Relapsing MOG Antibody Associated Encephalomyelitis: A Case Report. Eur J Paediatr Neurol 2015;19:S61. [DOI:10.1016/S1090-3798(15)30202-6]
43. Brilot F, Dale RC, Selter RC, Grummel V, Reddy Kalluri S, Aslam M, et al. Antibodies to Native Myelin Oligodendrocyte Glycoprotein in Children with Inflammatory Demyelinating Central Nervous System Disease. Ann Neurol 2009;66(6):833-42. [DOI:10.1002/ana.21916] [PMID]
44. Spadaro M, Gerdes LA, Krumbholz M, Ertl-Wagner B, Thaler FS, Schuh E, et al. Autoantibodies to MOG in a Distinct Subgroup of Adult Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm 2016;3(5):e257. [DOI:10.1212/NXI.0000000000000257] [PMID] [PMCID]
45. Kong XY, Peng JT, Liu LJ, Yan R, Zhang XJ. Anti-MOG antibody in different types of immune-mediated optic neuritis. Zhonghua Yan Ke Za Zhi 2012;48(12):1069-72. [Text in Chinese] [PMID]
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |