Sat, Apr 27, 2024
Volume 3, Issue 4 (Autumn 2017)
Caspian J Neurol Sci 2017, 3(4): 185-195 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Taherian R, Arabahmadi M, Taherian M. Investigation of the Effect of Cycloserine on Motor Function in a Rat Model of Parkinson’s disease. Caspian J Neurol Sci 2017; 3 (4) :185-195
URL: http://cjns.gums.ac.ir/article-1-194-en.html
URL: http://cjns.gums.ac.ir/article-1-194-en.html
1- Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran; taherian.reza72@gmail.com
2- Brain Mapping Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3- School of Medicine, Semnan University of Medical Sciences, Tehran, Iran
2- Brain Mapping Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3- School of Medicine, Semnan University of Medical Sciences, Tehran, Iran
Full-Text [PDF 1187 kb]
(946 Downloads)
| Abstract (HTML) (3423 Views)
Materials and Methods
Fifty-six healthy adult male wistar rats with the weight of 200-300 gr prior to the study were examined. Rats have been used to model PD using MPTP toxin in previous studies (15-17). Although studies using primate models of PD have a higher evidence level compared to those using rat models, to our knowledge, no study has shown that results of studies in rat models cannot be extended to human.
Animals were housed under conditions of constant temperature (23±1°C) and humidity (55±5%) on a 12-h light–dark cycle. All rats were fed and given water ad libitum and were divided into seven groups:
A-A short period, i.e. 8 days, exposure of saline group which included eight rats receiving 1.5 ml/kg saline intraperitoneally 30 minutes before the first time of injection of MPTP and during eight days after that, twice a day.
B- A short-period, i.e. 8 days, exposure of cycloserine group which included eight rats receiving 100 mg/kg cycloserine intraperitoneally 30 minutes before the first time of injection of MPTP and during eight days after that, twice a day, at the given dose.
C- A short-period exposure, i.e. 8 days, of cycloserine group which included eight rats
receiving 200 mg/kg cycloserine intraperitoneally 30 minutes before the first time of injection of MPTP and during eight days after that, twice a day, at the given dose.
D- A long-period, i.e. 16 days, exposure of saline group which included eight rats and received 1.5 ml/kg saline intraperitoneally 30 minutes before the first time of injection of MPTP and during 16 days after that, twice a day.
E- A long-period, i.e. 16 days, exposure of cycloserine group which included eight rats and received 100 mg/kg cycloserine intraperitoneally 30 minutes before the first time of injection of MPTP and during16 days after that, twice a day, at the given dose.
F- A long-period, i.e. 16 days, exposure of cycloserine group which included eight rats and received 200 mg/kg cycloserine intraperitoneally 30 minutes before the first time of injection of MPTP and during 16 days after that, twice a day, at the given dose.
G- A healthy group which included eight rats not receiving MPTP or any other drug.
Although injection of MPTP into brain using stereotaxic surgery seems a better procedure than intraperitoneal injection of it to induce PD, intraperitoneal injection of MPTP has been performed in several previous studied (18) and was used in our study to prevent technical difficulties.
MPTP-treated model mice were prepared as described (19). Briefly, rats were treated with MPTP (25 mg/kg) once a day for five consecutive days. Apomorphine-induced rotational test (AIRT) (20), elevated body swing test (EBST) (21) and rotarod performance test (RPT) (22) were done to evaluate the behavioral performance of rats. In the long-period exposure groups, AIRT and EBST were done three, five and eight weeks after the last administration of MPTP
and RPT was done seven weeks after that. In the short period groups, AIRT and EBST were done four, six and eight weeks after the last administration of MPTP and RPT was done seven weeks after that.
Behavioral performance of rats was evaluated as follows:
1- To perform AIRT, animals received apomorphine hydrochloride (0.5 mg/kg, intraperitoneally). After the injection was done, number of rotations of rats in a cylindrical container was counted for 1 h at 10-min intervals. Rotations toward the lesion side were considered as positive scores while rotations far away the lesion side was considered as negative scores. Sum of negative and positive scores was considered as the net number of rotations.
2- To perform RPT, a rotarod apparatus with a 3-cm diameter rod set at a height of 63 cm was used. The apparatus was set at a rotation rate of 5 RPM initially which increased to 40 RPM during 180 sec. Then, the apparatus continued to rotate at 40 RPM for 60 sec. The latency of time to fall over this 4 min period was recorded. The test was conducted for three consecutive days, twice a day.
3- To perform EBST, the animal was placed in a cylindrical container and was allowed to habituate for 10 min. Then it was held approximately 2 cm from the base of its tail and elevated 2 cm vertically. During a period of 1 min, swing of animal’s head out of the vertical axis to left or right was recorded. Biased swing behavior was calculated using following equations:
L/(L+R)% for left-biased swings and R/(L+R)% for right-biased swing. Among the mentioned swings, the greater number was considered as the net biased swing.
The differences between results of behavioral tests before and after the administration of MPTP were analyzed using the student t-test and ANOVA. SPSS software ver.20 was used to perform statistical analyses and p-value<0.05 was considered as the level of significance.
All experimental procedures were performed according to the NIH Guide for the Care and Use of Laboratory Animals (2011) and were approved by the ethical committee of research and technology chancellor of Shahid Beheshti University of Medical Sciences. The study was conducted in brain mapping research center, Shahid Beheshti University of Medical Sciences in 2016.
Results
Results of the rotarod test showed that healthy rats learn to continue to walk on the rotarod in the third trial (figure 3). Although the rotarod performance times were higher in trials 5 and 6 compared to initial trials, they were not significantly higher than the fourth and third trials. In the control group, the performance of rats did not change during the trials (p>0.05). Treatment with low dose of cycloserine in short period group could not increase the performance time (p>0.05); however, when it was administrated in long period, it could increase the performance time in the 4th, 5th and 6th trials compared to initial trials (150±5, 164±11, 174±8 vs 105±14 for 4th, 5th and 6th trials and control group respectively, p=0.01). Treatment with high
dose of cycloserine increased the performance time in 5th and 6th trials in both short and long period groups (p<0.05). However, the performance time did not reach the control group in any of the rats treated with cycloserine. Moreover, the effect of high dose and low dose of cycloserine on increasing the performance time was clearly different when they were administrated in a short period; however, there was no significant difference between them in the 5th and 6th trials when they were administrated in a long period (164±11 vs 179±6 for low and high dose treatment with cycloserine in 5th trial respectively) (174±8 vs 186±7 for low and high dose treatment with cycloserine in 6th trial respectively).
Discussion
In the present study, we used three behavioral tests to investigate the effect of cycloserine on MPTP-induced motor disturbance in an animal model of PD. We showed that cycloserine can effectively, although not completely, block the neurodegeneration induced by MPTP. This effect had a time dependent, but not dose dependent manner.
Rats underwent three behavioral tests to assess behavioral performance after treatment of rats with cycloserine. These tests included AIRT, EBST and rotarod test which showed similar results; however, some differences were revealed. Each of these tests has different trials which help a better evaluation of motor function in PD-induce models. In most of the trials of these tests, long period administration of cycloserine in every dose was more effective than short period administration of it in reducing behavioral disturbances. This result emphasizes that the effect of cycloserine on motor function in PD is time dependent. Long period administration of high dose of cycloserine for PD treatment was not more beneficial than low dose of it in reducing behavioral in last trials of all tests except AIRT. However, Short period administration of high dose of cycloserine was more effective than low dose of it.
These results showed that in contrast with a short period administration, the beneficial effect of cycloserine on motor function in PD is not dose dependent in long period administration. All these results consequently agreed on this concept that long period administration of low dose cycloserine is the best option for treatment of Parkinsonian like behaviors and subsidence the adverse effect of high dose administration of this drug for long period.
The glutamatergic system heavily innervates the hippocampus and prefrontal cortex and participates in planning, attention, execution, and recognition. Glutamatergic activity and NMDA receptor density in the basal ganglia increase in patients with PD (23). Cycloserine is able to pass blood-brain barrier and enter the brain tissue where it acts as a partial agonist by binding to the binding site of the NMDA receptor (9). The effects of cycloserine on MPTP-induce motor disturbance may be related to increased neurotransmitter release and function in several inter-related cortical and subcortical systems. NMDA receptors release dopamine and also enhance dopamine neuronal firing; hence, part of the effect of cycloserine on PD may be due to its effect on enhancement of cortical and striatal dopamine activity especially in prefrontal cortex (24). Moreover, NMDA activation induces release of acetylcholine in striatum (24) and medial septum (25) which leads to development of cholinergic–glutamatergic interactions which modulate cognitive function (26). Therefore, effects of cycloserine on motor function may be related to the increased neurotransmitter release and function in several inter-related cortical and subcortical systems as well as its effects on enhancing glutamatergic neurotransmission directly.
Some previous studies have investigated the beneficial effects of cycloserine in PD. Schneider et al. (8) have reported that administration of cycloserine significantly improves variable delayed-response task (VDR) in animal model of PD; however, they have reported that only low doses of cycloserine has cognition-enhancing properties. This result is in contrast with the results of the current study and that of Ying-Jui et al. (27) which showed that both low
and high dose of cycloserine are beneficial in PD. This inconsistency may be due to consideration of different doses of cycloserine as high or low dose in these studies and also methodological differences such as the injection method in these studies. As Ying-Jui et al. reported both administration of cycloserine in low or high dose increase correct responses of MPTP-lesioned rats in T-maze test which shows the beneficial effects of both of the doses on MPTP-induced behavioral disturbances. Moreover, Ying-Jui et al. also showed that the effect of high and low dose of cycloserine on increasing correct responses in T-maze test is not different which further confirms the results of the current study.
Conclusion
In conclusion, our findings strongly suggest that administration of cycloserine significantly counteracts the motor disturbance in MPTP-induced PD. There is no difference between high dose and low dose administration of cycloserine in long period and it could be a novel finding to subside adverse effects of drug. Long period administration of cycloserine with low dose instead of short period administration of it with high dose can be a better option in the treatment of the PD. However, our results should be interpreted in the light of some limitations. The time of first and second trials of AIRT and EBST differed between short and long period groups about one week. The most important Trial in AIRT and EBST is the third trial which is done 8 weeks after the last administration of MPTP. Moreover, RPT was performed in both groups 7 weeks after the last administration of MPTP. Hence, we do not think that this limitation can significantly impact our conclusions.
Furthermore, some previous studies have used stereotaxic surgery to directly infuse MPTP into substantia nigra of rats (27) which may be a more precise model to induce parkinsonism compared to intraperitoneal injection of MPTP. Further investigations are needed to clarify exact and possible mechanism of drug action for better and additive therapeutic approaches
Acknowledgement
We would like to express our appreciation to the "Student Research Committee" and "Research and Technology chancellor" in Shahid Beheshti University of Medical Sciences for their financial support of this study. The funder was not involved in preparation of the manuscript or decision to publish.
Conflict of Interest
The authors have no conflict of interest.
References
1. Schrag A, Jahanshahi M, Quinn N. What
Contributes to Quality of Life in Patients with
Parkinson's Disease? J Neurol Neurosurg
Psychiatry 2000;69(3):308-12.
2. Levy OA, Malagelada C, Greene LA. Cell
Death Pathways in Parkinson’s Disease:
Proximal Triggers, Distal Effectors, and Final
Steps. Apoptosis 2009;14(4):478-500.
3. Ko HS, Lee Y, Shin J-H, Karuppagounder SS,
Gadad BS, Koleske AJ, et al. Phosphorylation
by the c-Abl Protein Tyrosine Kinase Inhibits
Parkin's Ubiquitination and Protective
Function. Proc Natl Acad Sci U S A
2010;107(38):16691-6.
4. Seet RC, Lee C-YJ, Lim EC, Tan JJ, Quek
AM, Chong W-L, et al. Oxidative Damage in
Parkinson Disease: Measurement Using
Accurate Biomarkers. Free Rad Biol
Medi2010;48(4):560-6.
5.Bronstein JM, Tagliati M, Alterman RL,
Lozano AM, Volkmann J, Stefani A, et al.
Deep Brain Stimulation for Parkinson
Disease: An Expert Consensus and Review of
Key Issues. Arch Neurol 2011;68(2):165-.
6. Duker AP, Espay AJ. Surgical treatment of
Parkinson disease: past, present, and future.
Neurologic clinics. 2013;31(3):799-808.
7. Aquino CC, Fox SH. Clinical Spectrum of
Levodopa‐induced Complications. Mov
Disord2015;30(1):80-9.
8. Schneider J, Tinker JP, Van Velson M,
Giardiniere M. Effects of the Partial Glycine
Agonist D-cycloserine on Cognitive
Functioning in Chronic Low Dose MPTPtreated
Monkeys. Brain Res2000;860(1):190-
4.
9. Billard JM, Rouaud E. Deficit of NMDA
Receptor Activation in CA1 Hippocampal
Area of Aged Rats Is Rescued by
D‐cycloserine. Eur J Neurosci
2007;25(8):2260-8.
10. Riekkinen M, Riekkinen Jr P. Nicotine and
D‐cycloserine Enhance Acquisition of Water
Maze Spatial Navigation in Aged Rats.
Neuroreport 1997;8(3):699-703.
11. Ho Y-J, Hsu L-S, Wang C-F, Hsu W-Y, Lai
T-J, Hsu C-C, et al. Behavioral Effects of Dcycloserine
in Rats: the Role of Anxiety
Level. Brain Res 2005;1043(1):179-85.
12. Wu S-L, Hsu L-S, Tu W-T, Wang W-F,
Huang Y-T, Pawlak CR, et al. Effects of Dcycloserine
on the Behavior and ERK
Activity in the Amygdala: Role of Individual
Anxiety Levels. Behav Brain Res
2008;187(2):246-53.
13. Wang A-L, Liou Y-M, Pawlak CR, Ho Y-J.
Involvement of NMDA Receptors in Both
MPTP-induced Neuroinflammation and
Deficits in Episodic-like Memory in Wistar
Rats. Behav Brain Res 2010;208(1):38-46.
14. Bhushan B, Chander R, Kajal N, Ranga V,
Gupta A, Bharti H. Profile of Adverse Drug
Reactions in Drug Resistant Tuberculosis
from Punjab. Indian JTuberc 2014;61(4):318-
24.
15. Kalinina TS, Nerobkova LN, Voronina TA,
Stovbun SV, Litvin AA, Sergienko VI. Study
of Antiparkinsonic Activity of Panavir on a
Model of Parkinson Syndrome Induced by
Systemic Administration of MPTP to Outbred
Rats and C57Bl/6 Mice. Bull Exp Biol Med
2005;140(1):55-7.
16. Castro AA, Wiemes BP, Matheus FC, Lapa
FR, Viola GG, Santos AR, et al. Atorvastatin
Improves Cognitive, Emotional and Motor
Impairments Induced by Intranasal 1-methyl-
4-phenyl-1, 2, 3, 6-Tetrahydropyridine
(MPTP) Administration in Rats, an
Experimental Model of Parkinson's Disease.
Brain Res 2013;1513:103-16.
17. Bisht R, Kaur B, Gupta H, Prakash A.
Ceftriaxone Mediated Rescue of Nigral
Oxidative Damage and Motor Deficits in
MPTP Model of Parkinson's Disease in Rats.
Neurotoxicology 2014;44:71-9.
18. Lin JG, Chen CJ, Yang HB, Chen YH, Hung
SY. Electroacupuncture Promotes Recovery
of Behaviorial Disturbance and Reduces
Dopaminergic Neuron Degeneration in
Rodent Models of Parkinson’s Disease. IntJ
Mol Sci 2017;18(9):1846.
19. Moriguchi S, Yabuki Y, Fukunaga K.
Reduced Calcium/Calmodulin‐Dependent
Protein Kinase II Activity in the
Hippocampus Is Associated with Impaired
Cognitive Function in MPTP‐treated Mice. J
Neurochem 2012;120(4):541-51.
20. Fujita M, Nishino H, Kumazaki M, Shimada
S, Tohyama M, Nishimura T. Expression of
Dopamine Transporter mRNA and Its Binding
Site in Fetal Nigral Cells Transplanted Into
the Striatum of 6-OHDA Lesioned Rat. Mol
Brain Res 1996;39(1):127-36.
21. Borlongan CV, Randall TS, Cahill DW,
Sanberg PR. Asymmetrical Motor Behavior in
Rats with Unilateral Striatal Excitotoxic
Lesions as Revealed by the Elevated Body
Swing Test. Brain Res 1995;676(1):231-4.
22. Lundblad M, Vaudano E, Cenci M. Cellular
and Behavioural Effects of the Adenosine
A2a Receptor Antagonist KW‐6002 in a Rat
Model of l‐DOPA‐induced Dyskinesia. J
Neurochem 2003;84(6):1398-410.
23. Ulas J, Weihmuller F, Brunner L, Joyce J,
Marshall J, Cotman C. Selective Increase of
NMDA-sensitive Glutamate Binding in the
Striatum of Parkinson's Disease, Alzheimer's
Disease, and Mixed Parkinson's
Disease/Alzheimer's Disease Patients: an
Autoradiographic Study. J Neurosci
1994;14(11):6317-24.
24. Ransom RW, Deschenes NL. Glycine
Modulation of NMDA-evoked Release of [3
H] Acetylcholine and [3 H] Dopamine from
Rat Striatal Slices. Neurosci Lett
1989;96(3):323-8.
25. Nishimura LM, Boegman RJ. N-methyl-Daspartate-
evoked Release of Acetylcholine
from the Medial Septum/Diagonal Band of
Rat Brain. Neurosci Lett 1990;115(2):259-64.
26. Matsuoka N, Aigner TG. The Glycine/NMDA
Receptor Antagonist HA-966 Impairs Visual
Recognition Memory in Rhesus Monkeys.
Brain Res 1996;731(1):72-8.
27. Ho Y-J, Ho S-C, Pawlak CR, Yeh K-Y.
Effects of D-cycloserine on MPTP-induced
Behavioral and Neurological Changes:
Potential for Treatment of Parkinson's Disease
Dementia. Behav Brain Res2011;219(2):280-
90.
Full-Text: (840 Views)
Introduction
parkinson’s disease (PD) is recognized as the second most prevalent neurodegenerative disorder globally. This disease is characterized by resting tremor, rigidity, bradykinesia, and postural instability (1). Lots of researches have been done to clarify the underlying pathophysiology of this disorder. Degeneration of dopaminergic neurons in midbrain is the most accepted theory in this relation. The degeneration may be the result of oxidative stress with or without glutamate excitotoxicity, which is induced through the inhibition of complex I of the electron transport chain of the mitochondria of the dopaminergic neurons by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (2-4). Although the pathophysiology of this disorder is now extensively revealed, there is less agreement onits treatment. Current treatments include L-DOPA, deep brain stimulation and surgical destruction of the globus pallidus. However, none of them could be accounted as a satisfactory treatment for this disorder (5,6). L-DOPA and dopamine agonists, improve the early symptoms of PD; however, they eventually become ineffective and also produce complications such as involuntary writing movements (7). Hence, investigating new treatments for PD seems crucial.
Cycloserine, an anti-tuberculosis antibiotic, is a partial agonist of the glycine binding site of the N-methyl-D-aspartate (NMDA) receptor which improves object recognition in MPTP-lesioned monkeys (8), spatial navigation and learning deficits in aged rats (9,10) and anxiety-like behavior in rats (11,12). Cycloserine also restores impairments in neurodegeneration and episodic-like memory in MPTP-induced rat model of PD (13). However, the effect of
cycloserine on motor function in PD is not well recognized. On the other hand, using cycloserine as a second line treatment of tuberculosis in high dose can induce adverse effects such as psychosis or hepatitis (14). Therefore the present study aimed to investigate the modulatory effect of different doses of cycloserine against MPTP-induced motor function in rat model of PD in long-period and short-period treatments with cycloserine.
Cycloserine, an anti-tuberculosis antibiotic, is a partial agonist of the glycine binding site of the N-methyl-D-aspartate (NMDA) receptor which improves object recognition in MPTP-lesioned monkeys (8), spatial navigation and learning deficits in aged rats (9,10) and anxiety-like behavior in rats (11,12). Cycloserine also restores impairments in neurodegeneration and episodic-like memory in MPTP-induced rat model of PD (13). However, the effect of
cycloserine on motor function in PD is not well recognized. On the other hand, using cycloserine as a second line treatment of tuberculosis in high dose can induce adverse effects such as psychosis or hepatitis (14). Therefore the present study aimed to investigate the modulatory effect of different doses of cycloserine against MPTP-induced motor function in rat model of PD in long-period and short-period treatments with cycloserine.
Materials and Methods
Fifty-six healthy adult male wistar rats with the weight of 200-300 gr prior to the study were examined. Rats have been used to model PD using MPTP toxin in previous studies (15-17). Although studies using primate models of PD have a higher evidence level compared to those using rat models, to our knowledge, no study has shown that results of studies in rat models cannot be extended to human.
Animals were housed under conditions of constant temperature (23±1°C) and humidity (55±5%) on a 12-h light–dark cycle. All rats were fed and given water ad libitum and were divided into seven groups:
A-A short period, i.e. 8 days, exposure of saline group which included eight rats receiving 1.5 ml/kg saline intraperitoneally 30 minutes before the first time of injection of MPTP and during eight days after that, twice a day.
B- A short-period, i.e. 8 days, exposure of cycloserine group which included eight rats receiving 100 mg/kg cycloserine intraperitoneally 30 minutes before the first time of injection of MPTP and during eight days after that, twice a day, at the given dose.
C- A short-period exposure, i.e. 8 days, of cycloserine group which included eight rats
receiving 200 mg/kg cycloserine intraperitoneally 30 minutes before the first time of injection of MPTP and during eight days after that, twice a day, at the given dose.
D- A long-period, i.e. 16 days, exposure of saline group which included eight rats and received 1.5 ml/kg saline intraperitoneally 30 minutes before the first time of injection of MPTP and during 16 days after that, twice a day.
E- A long-period, i.e. 16 days, exposure of cycloserine group which included eight rats and received 100 mg/kg cycloserine intraperitoneally 30 minutes before the first time of injection of MPTP and during16 days after that, twice a day, at the given dose.
F- A long-period, i.e. 16 days, exposure of cycloserine group which included eight rats and received 200 mg/kg cycloserine intraperitoneally 30 minutes before the first time of injection of MPTP and during 16 days after that, twice a day, at the given dose.
G- A healthy group which included eight rats not receiving MPTP or any other drug.
Although injection of MPTP into brain using stereotaxic surgery seems a better procedure than intraperitoneal injection of it to induce PD, intraperitoneal injection of MPTP has been performed in several previous studied (18) and was used in our study to prevent technical difficulties.
MPTP-treated model mice were prepared as described (19). Briefly, rats were treated with MPTP (25 mg/kg) once a day for five consecutive days. Apomorphine-induced rotational test (AIRT) (20), elevated body swing test (EBST) (21) and rotarod performance test (RPT) (22) were done to evaluate the behavioral performance of rats. In the long-period exposure groups, AIRT and EBST were done three, five and eight weeks after the last administration of MPTP
and RPT was done seven weeks after that. In the short period groups, AIRT and EBST were done four, six and eight weeks after the last administration of MPTP and RPT was done seven weeks after that.
Behavioral performance of rats was evaluated as follows:
1- To perform AIRT, animals received apomorphine hydrochloride (0.5 mg/kg, intraperitoneally). After the injection was done, number of rotations of rats in a cylindrical container was counted for 1 h at 10-min intervals. Rotations toward the lesion side were considered as positive scores while rotations far away the lesion side was considered as negative scores. Sum of negative and positive scores was considered as the net number of rotations.
2- To perform RPT, a rotarod apparatus with a 3-cm diameter rod set at a height of 63 cm was used. The apparatus was set at a rotation rate of 5 RPM initially which increased to 40 RPM during 180 sec. Then, the apparatus continued to rotate at 40 RPM for 60 sec. The latency of time to fall over this 4 min period was recorded. The test was conducted for three consecutive days, twice a day.
3- To perform EBST, the animal was placed in a cylindrical container and was allowed to habituate for 10 min. Then it was held approximately 2 cm from the base of its tail and elevated 2 cm vertically. During a period of 1 min, swing of animal’s head out of the vertical axis to left or right was recorded. Biased swing behavior was calculated using following equations:
L/(L+R)% for left-biased swings and R/(L+R)% for right-biased swing. Among the mentioned swings, the greater number was considered as the net biased swing.
The differences between results of behavioral tests before and after the administration of MPTP were analyzed using the student t-test and ANOVA. SPSS software ver.20 was used to perform statistical analyses and p-value<0.05 was considered as the level of significance.
All experimental procedures were performed according to the NIH Guide for the Care and Use of Laboratory Animals (2011) and were approved by the ethical committee of research and technology chancellor of Shahid Beheshti University of Medical Sciences. The study was conducted in brain mapping research center, Shahid Beheshti University of Medical Sciences in 2016.
Results
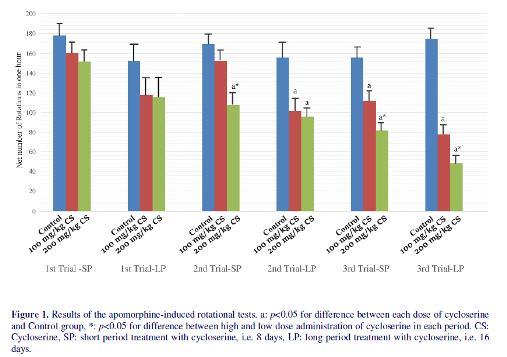
Fifty-six healthy adult male wistar rats with a weight of 200-300 in each group prior to the study were examined. All groups showed some degrees of rotations in AIRT (figure 1). Hence, treatment with cycloserine could not completely block the neurodegeneration induced by MPTP. In the first trial of AIRT, although the net number of rotations were significantly lower in long period treatment groups compared to short period treatment groups (p<0.01), there were no significant difference between treatment groups and control groups (118±18 and 116±21 vs 153±19 for low dose and high dose treatment group vs control group, respectively, p=0.12). In the second trial of AIRT, low dose of cycloserine could decrease number of rotations compared to the control group only when it was administrated in long period (102±12 vs 156±16 respectively, p<0.01). However, high dose of cycloserine reduced number of rotations both in short and long-period administrations and no significant difference was observed between them
(108±12 vs 96±9 respectively, p=0.22). Moreover, administration low dose of cycloserine for a long period treatment had a similar effect to its high dose in reducing number of rotations (102±12 vs 96±8 respectively, p=0.18). Similar to the second trial, in the third trial of AIRT, both short and long period administration of high dose of cycloserine (200 mg/kg) reduced the number of rotations (p=0.02 and p<0.01, respectively). The effect of long period administration of high dose of cycloserine was significantly higher than short period administration of it in the third trial of AIRT (48±8 vs 82±7 respectively, p=0.04). Regarding administration of high dose cycloserine, both short and long period treatment with the low dose of it (100 mg/kg), reduced number of rotations compared to control group (117±5 vs 156±8 for short period cycloserine and saline exposure, respectively) (75±11 vs 174±7 for long period cycloserine and saline exposure, respectively). The effect of long period treatment with low dose of cycloserine was significantly higher than short period treatment with it (78±10 vs 112±9 respectively, p=0.03); however, compared to long period treatment with a high dose of cycloserine, it was less effective.
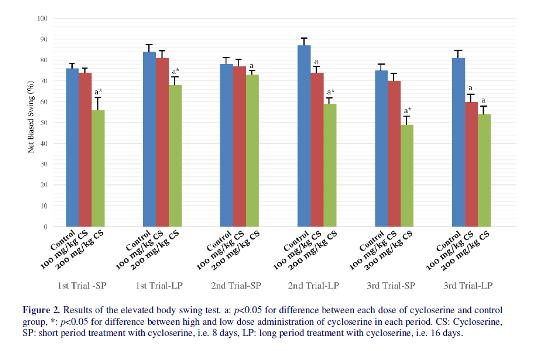
The results of the EBST were Similar to AIRT; however, some differences were revealed (figure 2). In the first trial, while low dose of cycloserine could not reduce biased swing even when it was administrated for long period (p=0.21), high dose of cycloserine reduced biased swing in both short and long period administration (56±11 vs 76±5 for short period administration of 200 mg/kg cycloserine and control group, respectively, p<0.01) (68±8 vs 84±6 for long-
period administration of 200 mg/kg cycloserine and control group, respectively, p<0.01). In the second trial, low dose of cycloserine reduced biased swing compared to the control group only when it was administrated for a long period (74±5 vs 87±6 respectively, p=0.02). Treatment with high dose of cycloserine reduced biased swing both in short period and long period administrations (p<0.05). In long-period administration, the effect of high dose of cycloserine on reducing biased swing was more than low dose of it (59±5 vs 74±6 respectively, p=0.04). In the third trial,
treatment with low dose of cycloserine for a short period could not alter biased swing (p>0.05). Both low dose and high dose of cycloserine reduced the biased swing compared to control group when they were administrated in long period (60±7 and 54±8 vs 81±8 for low and high dose treatment with cycloserine and control group respectively, p=0.02). In contrast to third trial of AIRT, no difference was observed between the effects of high and low dose of cycloserine in reducing biased swing when they were administrated for a long period (p>0.05).
(108±12 vs 96±9 respectively, p=0.22). Moreover, administration low dose of cycloserine for a long period treatment had a similar effect to its high dose in reducing number of rotations (102±12 vs 96±8 respectively, p=0.18). Similar to the second trial, in the third trial of AIRT, both short and long period administration of high dose of cycloserine (200 mg/kg) reduced the number of rotations (p=0.02 and p<0.01, respectively). The effect of long period administration of high dose of cycloserine was significantly higher than short period administration of it in the third trial of AIRT (48±8 vs 82±7 respectively, p=0.04). Regarding administration of high dose cycloserine, both short and long period treatment with the low dose of it (100 mg/kg), reduced number of rotations compared to control group (117±5 vs 156±8 for short period cycloserine and saline exposure, respectively) (75±11 vs 174±7 for long period cycloserine and saline exposure, respectively). The effect of long period treatment with low dose of cycloserine was significantly higher than short period treatment with it (78±10 vs 112±9 respectively, p=0.03); however, compared to long period treatment with a high dose of cycloserine, it was less effective.
The results of the EBST were Similar to AIRT; however, some differences were revealed (figure 2). In the first trial, while low dose of cycloserine could not reduce biased swing even when it was administrated for long period (p=0.21), high dose of cycloserine reduced biased swing in both short and long period administration (56±11 vs 76±5 for short period administration of 200 mg/kg cycloserine and control group, respectively, p<0.01) (68±8 vs 84±6 for long-
period administration of 200 mg/kg cycloserine and control group, respectively, p<0.01). In the second trial, low dose of cycloserine reduced biased swing compared to the control group only when it was administrated for a long period (74±5 vs 87±6 respectively, p=0.02). Treatment with high dose of cycloserine reduced biased swing both in short period and long period administrations (p<0.05). In long-period administration, the effect of high dose of cycloserine on reducing biased swing was more than low dose of it (59±5 vs 74±6 respectively, p=0.04). In the third trial,
treatment with low dose of cycloserine for a short period could not alter biased swing (p>0.05). Both low dose and high dose of cycloserine reduced the biased swing compared to control group when they were administrated in long period (60±7 and 54±8 vs 81±8 for low and high dose treatment with cycloserine and control group respectively, p=0.02). In contrast to third trial of AIRT, no difference was observed between the effects of high and low dose of cycloserine in reducing biased swing when they were administrated for a long period (p>0.05).
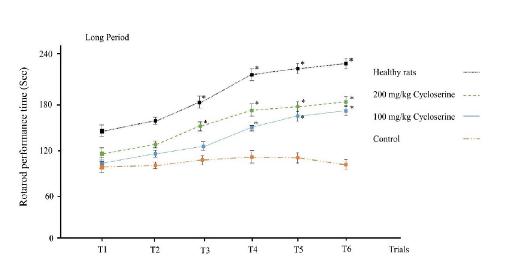
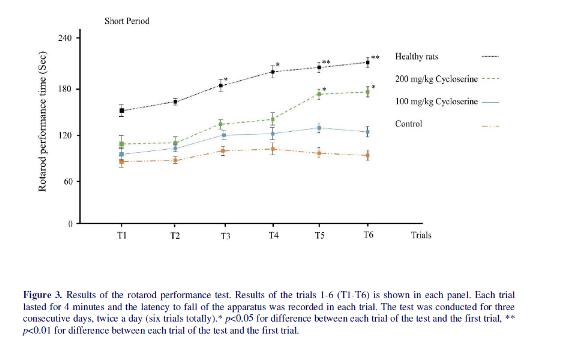
Results of the rotarod test showed that healthy rats learn to continue to walk on the rotarod in the third trial (figure 3). Although the rotarod performance times were higher in trials 5 and 6 compared to initial trials, they were not significantly higher than the fourth and third trials. In the control group, the performance of rats did not change during the trials (p>0.05). Treatment with low dose of cycloserine in short period group could not increase the performance time (p>0.05); however, when it was administrated in long period, it could increase the performance time in the 4th, 5th and 6th trials compared to initial trials (150±5, 164±11, 174±8 vs 105±14 for 4th, 5th and 6th trials and control group respectively, p=0.01). Treatment with high
dose of cycloserine increased the performance time in 5th and 6th trials in both short and long period groups (p<0.05). However, the performance time did not reach the control group in any of the rats treated with cycloserine. Moreover, the effect of high dose and low dose of cycloserine on increasing the performance time was clearly different when they were administrated in a short period; however, there was no significant difference between them in the 5th and 6th trials when they were administrated in a long period (164±11 vs 179±6 for low and high dose treatment with cycloserine in 5th trial respectively) (174±8 vs 186±7 for low and high dose treatment with cycloserine in 6th trial respectively).
Discussion
In the present study, we used three behavioral tests to investigate the effect of cycloserine on MPTP-induced motor disturbance in an animal model of PD. We showed that cycloserine can effectively, although not completely, block the neurodegeneration induced by MPTP. This effect had a time dependent, but not dose dependent manner.
Rats underwent three behavioral tests to assess behavioral performance after treatment of rats with cycloserine. These tests included AIRT, EBST and rotarod test which showed similar results; however, some differences were revealed. Each of these tests has different trials which help a better evaluation of motor function in PD-induce models. In most of the trials of these tests, long period administration of cycloserine in every dose was more effective than short period administration of it in reducing behavioral disturbances. This result emphasizes that the effect of cycloserine on motor function in PD is time dependent. Long period administration of high dose of cycloserine for PD treatment was not more beneficial than low dose of it in reducing behavioral in last trials of all tests except AIRT. However, Short period administration of high dose of cycloserine was more effective than low dose of it.
These results showed that in contrast with a short period administration, the beneficial effect of cycloserine on motor function in PD is not dose dependent in long period administration. All these results consequently agreed on this concept that long period administration of low dose cycloserine is the best option for treatment of Parkinsonian like behaviors and subsidence the adverse effect of high dose administration of this drug for long period.
The glutamatergic system heavily innervates the hippocampus and prefrontal cortex and participates in planning, attention, execution, and recognition. Glutamatergic activity and NMDA receptor density in the basal ganglia increase in patients with PD (23). Cycloserine is able to pass blood-brain barrier and enter the brain tissue where it acts as a partial agonist by binding to the binding site of the NMDA receptor (9). The effects of cycloserine on MPTP-induce motor disturbance may be related to increased neurotransmitter release and function in several inter-related cortical and subcortical systems. NMDA receptors release dopamine and also enhance dopamine neuronal firing; hence, part of the effect of cycloserine on PD may be due to its effect on enhancement of cortical and striatal dopamine activity especially in prefrontal cortex (24). Moreover, NMDA activation induces release of acetylcholine in striatum (24) and medial septum (25) which leads to development of cholinergic–glutamatergic interactions which modulate cognitive function (26). Therefore, effects of cycloserine on motor function may be related to the increased neurotransmitter release and function in several inter-related cortical and subcortical systems as well as its effects on enhancing glutamatergic neurotransmission directly.
Some previous studies have investigated the beneficial effects of cycloserine in PD. Schneider et al. (8) have reported that administration of cycloserine significantly improves variable delayed-response task (VDR) in animal model of PD; however, they have reported that only low doses of cycloserine has cognition-enhancing properties. This result is in contrast with the results of the current study and that of Ying-Jui et al. (27) which showed that both low
and high dose of cycloserine are beneficial in PD. This inconsistency may be due to consideration of different doses of cycloserine as high or low dose in these studies and also methodological differences such as the injection method in these studies. As Ying-Jui et al. reported both administration of cycloserine in low or high dose increase correct responses of MPTP-lesioned rats in T-maze test which shows the beneficial effects of both of the doses on MPTP-induced behavioral disturbances. Moreover, Ying-Jui et al. also showed that the effect of high and low dose of cycloserine on increasing correct responses in T-maze test is not different which further confirms the results of the current study.
Conclusion
In conclusion, our findings strongly suggest that administration of cycloserine significantly counteracts the motor disturbance in MPTP-induced PD. There is no difference between high dose and low dose administration of cycloserine in long period and it could be a novel finding to subside adverse effects of drug. Long period administration of cycloserine with low dose instead of short period administration of it with high dose can be a better option in the treatment of the PD. However, our results should be interpreted in the light of some limitations. The time of first and second trials of AIRT and EBST differed between short and long period groups about one week. The most important Trial in AIRT and EBST is the third trial which is done 8 weeks after the last administration of MPTP. Moreover, RPT was performed in both groups 7 weeks after the last administration of MPTP. Hence, we do not think that this limitation can significantly impact our conclusions.
Furthermore, some previous studies have used stereotaxic surgery to directly infuse MPTP into substantia nigra of rats (27) which may be a more precise model to induce parkinsonism compared to intraperitoneal injection of MPTP. Further investigations are needed to clarify exact and possible mechanism of drug action for better and additive therapeutic approaches
Acknowledgement
We would like to express our appreciation to the "Student Research Committee" and "Research and Technology chancellor" in Shahid Beheshti University of Medical Sciences for their financial support of this study. The funder was not involved in preparation of the manuscript or decision to publish.
Conflict of Interest
The authors have no conflict of interest.
References
1. Schrag A, Jahanshahi M, Quinn N. What
Contributes to Quality of Life in Patients with
Parkinson's Disease? J Neurol Neurosurg
Psychiatry 2000;69(3):308-12.
2. Levy OA, Malagelada C, Greene LA. Cell
Death Pathways in Parkinson’s Disease:
Proximal Triggers, Distal Effectors, and Final
Steps. Apoptosis 2009;14(4):478-500.
3. Ko HS, Lee Y, Shin J-H, Karuppagounder SS,
Gadad BS, Koleske AJ, et al. Phosphorylation
by the c-Abl Protein Tyrosine Kinase Inhibits
Parkin's Ubiquitination and Protective
Function. Proc Natl Acad Sci U S A
2010;107(38):16691-6.
4. Seet RC, Lee C-YJ, Lim EC, Tan JJ, Quek
AM, Chong W-L, et al. Oxidative Damage in
Parkinson Disease: Measurement Using
Accurate Biomarkers. Free Rad Biol
Medi2010;48(4):560-6.
5.Bronstein JM, Tagliati M, Alterman RL,
Lozano AM, Volkmann J, Stefani A, et al.
Deep Brain Stimulation for Parkinson
Disease: An Expert Consensus and Review of
Key Issues. Arch Neurol 2011;68(2):165-.
6. Duker AP, Espay AJ. Surgical treatment of
Parkinson disease: past, present, and future.
Neurologic clinics. 2013;31(3):799-808.
7. Aquino CC, Fox SH. Clinical Spectrum of
Levodopa‐induced Complications. Mov
Disord2015;30(1):80-9.
8. Schneider J, Tinker JP, Van Velson M,
Giardiniere M. Effects of the Partial Glycine
Agonist D-cycloserine on Cognitive
Functioning in Chronic Low Dose MPTPtreated
Monkeys. Brain Res2000;860(1):190-
4.
9. Billard JM, Rouaud E. Deficit of NMDA
Receptor Activation in CA1 Hippocampal
Area of Aged Rats Is Rescued by
D‐cycloserine. Eur J Neurosci
2007;25(8):2260-8.
10. Riekkinen M, Riekkinen Jr P. Nicotine and
D‐cycloserine Enhance Acquisition of Water
Maze Spatial Navigation in Aged Rats.
Neuroreport 1997;8(3):699-703.
11. Ho Y-J, Hsu L-S, Wang C-F, Hsu W-Y, Lai
T-J, Hsu C-C, et al. Behavioral Effects of Dcycloserine
in Rats: the Role of Anxiety
Level. Brain Res 2005;1043(1):179-85.
12. Wu S-L, Hsu L-S, Tu W-T, Wang W-F,
Huang Y-T, Pawlak CR, et al. Effects of Dcycloserine
on the Behavior and ERK
Activity in the Amygdala: Role of Individual
Anxiety Levels. Behav Brain Res
2008;187(2):246-53.
13. Wang A-L, Liou Y-M, Pawlak CR, Ho Y-J.
Involvement of NMDA Receptors in Both
MPTP-induced Neuroinflammation and
Deficits in Episodic-like Memory in Wistar
Rats. Behav Brain Res 2010;208(1):38-46.
14. Bhushan B, Chander R, Kajal N, Ranga V,
Gupta A, Bharti H. Profile of Adverse Drug
Reactions in Drug Resistant Tuberculosis
from Punjab. Indian JTuberc 2014;61(4):318-
24.
15. Kalinina TS, Nerobkova LN, Voronina TA,
Stovbun SV, Litvin AA, Sergienko VI. Study
of Antiparkinsonic Activity of Panavir on a
Model of Parkinson Syndrome Induced by
Systemic Administration of MPTP to Outbred
Rats and C57Bl/6 Mice. Bull Exp Biol Med
2005;140(1):55-7.
16. Castro AA, Wiemes BP, Matheus FC, Lapa
FR, Viola GG, Santos AR, et al. Atorvastatin
Improves Cognitive, Emotional and Motor
Impairments Induced by Intranasal 1-methyl-
4-phenyl-1, 2, 3, 6-Tetrahydropyridine
(MPTP) Administration in Rats, an
Experimental Model of Parkinson's Disease.
Brain Res 2013;1513:103-16.
17. Bisht R, Kaur B, Gupta H, Prakash A.
Ceftriaxone Mediated Rescue of Nigral
Oxidative Damage and Motor Deficits in
MPTP Model of Parkinson's Disease in Rats.
Neurotoxicology 2014;44:71-9.
18. Lin JG, Chen CJ, Yang HB, Chen YH, Hung
SY. Electroacupuncture Promotes Recovery
of Behaviorial Disturbance and Reduces
Dopaminergic Neuron Degeneration in
Rodent Models of Parkinson’s Disease. IntJ
Mol Sci 2017;18(9):1846.
19. Moriguchi S, Yabuki Y, Fukunaga K.
Reduced Calcium/Calmodulin‐Dependent
Protein Kinase II Activity in the
Hippocampus Is Associated with Impaired
Cognitive Function in MPTP‐treated Mice. J
Neurochem 2012;120(4):541-51.
20. Fujita M, Nishino H, Kumazaki M, Shimada
S, Tohyama M, Nishimura T. Expression of
Dopamine Transporter mRNA and Its Binding
Site in Fetal Nigral Cells Transplanted Into
the Striatum of 6-OHDA Lesioned Rat. Mol
Brain Res 1996;39(1):127-36.
21. Borlongan CV, Randall TS, Cahill DW,
Sanberg PR. Asymmetrical Motor Behavior in
Rats with Unilateral Striatal Excitotoxic
Lesions as Revealed by the Elevated Body
Swing Test. Brain Res 1995;676(1):231-4.
22. Lundblad M, Vaudano E, Cenci M. Cellular
and Behavioural Effects of the Adenosine
A2a Receptor Antagonist KW‐6002 in a Rat
Model of l‐DOPA‐induced Dyskinesia. J
Neurochem 2003;84(6):1398-410.
23. Ulas J, Weihmuller F, Brunner L, Joyce J,
Marshall J, Cotman C. Selective Increase of
NMDA-sensitive Glutamate Binding in the
Striatum of Parkinson's Disease, Alzheimer's
Disease, and Mixed Parkinson's
Disease/Alzheimer's Disease Patients: an
Autoradiographic Study. J Neurosci
1994;14(11):6317-24.
24. Ransom RW, Deschenes NL. Glycine
Modulation of NMDA-evoked Release of [3
H] Acetylcholine and [3 H] Dopamine from
Rat Striatal Slices. Neurosci Lett
1989;96(3):323-8.
25. Nishimura LM, Boegman RJ. N-methyl-Daspartate-
evoked Release of Acetylcholine
from the Medial Septum/Diagonal Band of
Rat Brain. Neurosci Lett 1990;115(2):259-64.
26. Matsuoka N, Aigner TG. The Glycine/NMDA
Receptor Antagonist HA-966 Impairs Visual
Recognition Memory in Rhesus Monkeys.
Brain Res 1996;731(1):72-8.
27. Ho Y-J, Ho S-C, Pawlak CR, Yeh K-Y.
Effects of D-cycloserine on MPTP-induced
Behavioral and Neurological Changes:
Potential for Treatment of Parkinson's Disease
Dementia. Behav Brain Res2011;219(2):280-
90.
Type of Study: Research |
Subject:
Special
Received: 2017/10/18 | Accepted: 2017/10/18 | Published: 2017/10/18
Received: 2017/10/18 | Accepted: 2017/10/18 | Published: 2017/10/18
References
1. Schrag A, Jahanshahi M, Quinn N. What Contributes to Quality of Life in Patients with Parkinson's Disease? J Neurol Neurosurg Psychiatry 2000;69(3):308-12. [DOI:10.1136/jnnp.69.3.308] [PMID] [PMCID]
2. Levy OA, Malagelada C, Greene LA. Cell Death Pathways in Parkinson's Disease: Proximal Triggers, Distal Effectors, and Final Steps. Apoptosis 2009;14(4):478-500. [DOI:10.1007/s10495-008-0309-3] [PMID] [PMCID]
3. Ko HS, Lee Y, Shin J-H, Karuppagounder SS, Gadad BS, Koleske AJ, et al. Phosphorylation by the c-Abl Protein Tyrosine Kinase Inhibits Parkin's Ubiquitination and Protective Function. Proc Natl Acad Sci U S A 2010;107(38):16691-6. [DOI:10.1073/pnas.1006083107] [PMID] [PMCID]
4. Seet RC, Lee C-YJ, Lim EC, Tan JJ, Quek AM, Chong W-L, et al. Oxidative Damage in Parkinson Disease: Measurement Using Accurate Biomarkers. Free Rad Biol Medi2010;48(4):560-6. [DOI:10.1016/j.freeradbiomed.2009.11.026] [PMID]
5. Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. Deep Brain Stimulation for Parkinson Disease: An Expert Consensus and Review of Key Issues. Arch Neurol 2011;68(2):165-. [DOI:10.1001/archneurol.2010.260] [PMID] [PMCID]
6. Duker AP, Espay AJ. Surgical treatment of Parkinson disease: past, present, and future. Neurologic clinics. 2013;31(3):799-808. [DOI:10.1016/j.ncl.2013.03.007] [PMID] [PMCID]
7. Aquino CC, Fox SH. Clinical Spectrum of Levodopa‐induced Complications. Mov Disord2015;30(1):80-9. [DOI:10.1002/mds.26125] [PMID]
8. Schneider J, Tinker JP, Van Velson M, Giardiniere M. Effects of the Partial Glycine Agonist D-cycloserine on Cognitive Functioning in Chronic Low Dose MPTP-treated Monkeys. Brain Res2000;860(1):190-4. [DOI:10.1016/S0006-8993(00)02036-9]
9. Billard JM, Rouaud E. Deficit of NMDA Receptor Activation in CA1 Hippocampal Area of Aged Rats Is Rescued by D‐cycloserine. Eur J Neurosci 2007;25(8):2260-8. [DOI:10.1111/j.1460-9568.2007.05488.x] [PMID]
10. Riekkinen M, Riekkinen Jr P. Nicotine and D‐cycloserine Enhance Acquisition of Water Maze Spatial Navigation in Aged Rats. Neuroreport 1997;8(3):699-703. [DOI:10.1097/00001756-199702100-00024] [PMID]
11. Ho Y-J, Hsu L-S, Wang C-F, Hsu W-Y, Lai T-J, Hsu C-C, et al. Behavioral Effects of D-cycloserine in Rats: the Role of Anxiety Level. Brain Res 2005;1043(1):179-85. [DOI:10.1016/j.brainres.2005.02.057] [PMID]
12. Wu S-L, Hsu L-S, Tu W-T, Wang W-F, Huang Y-T, Pawlak CR, et al. Effects of D-cycloserine on the Behavior and ERK Activity in the Amygdala: Role of Individual Anxiety Levels. Behav Brain Res 2008;187(2):246-53. [DOI:10.1016/j.bbr.2007.09.013] [PMID]
13. Wang A-L, Liou Y-M, Pawlak CR, Ho Y-J. Involvement of NMDA Receptors in Both MPTP-induced Neuroinflammation and Deficits in Episodic-like Memory in Wistar Rats. Behav Brain Res 2010;208(1):38-46. [DOI:10.1016/j.bbr.2009.11.006] [PMID]
14. Bhushan B, Chander R, Kajal N, Ranga V, Gupta A, Bharti H. Profile of Adverse Drug Reactions in Drug Resistant Tuberculosis from Punjab. Indian JTuberc 2014;61(4):318-24. [PMID]
15. Kalinina TS, Nerobkova LN, Voronina TA, Stovbun SV, Litvin AA, Sergienko VI. Study of Antiparkinsonic Activity of Panavir on a Model of Parkinson Syndrome Induced by Systemic Administration of MPTP to Outbred Rats and C57Bl/6 Mice. Bull Exp Biol Med 2005;140(1):55-7. [DOI:10.1007/s10517-005-0410-3] [PMID]
16. Castro AA, Wiemes BP, Matheus FC, Lapa FR, Viola GG, Santos AR, et al. Atorvastatin Improves Cognitive, Emotional and Motor Impairments Induced by Intranasal 1-methyl-4-phenyl-1, 2, 3, 6-Tetrahydropyridine (MPTP) Administration in Rats, an Experimental Model of Parkinson's Disease. Brain Res 2013;1513:103-16. [DOI:10.1016/j.brainres.2013.03.029] [PMID]
17. Bisht R, Kaur B, Gupta H, Prakash A. Ceftriaxone Mediated Rescue of Nigral Oxidative Damage and Motor Deficits in MPTP Model of Parkinson's Disease in Rats. Neurotoxicology 2014;44:71-9. [DOI:10.1016/j.neuro.2014.05.009] [PMID]
18. Lin JG, Chen CJ, Yang HB, Chen YH, Hung SY. Electroacupuncture Promotes Recovery of Behaviorial Disturbance and Reduces Dopaminergic Neuron Degeneration in Rodent Models of Parkinson's Disease. IntJ Mol Sci 2017;18(9):1846. [DOI:10.3390/ijms18091846] [PMID] [PMCID]
19. Moriguchi S, Yabuki Y, Fukunaga K. Reduced Calcium/Calmodulin‐Dependent Protein Kinase II Activity in the Hippocampus Is Associated with Impaired Cognitive Function in MPTP‐treated Mice. J Neurochem 2012;120(4):541-51. [DOI:10.1111/j.1471-4159.2011.07608.x] [PMID]
20. Fujita M, Nishino H, Kumazaki M, Shimada S, Tohyama M, Nishimura T. Expression of Dopamine Transporter mRNA and Its Binding Site in Fetal Nigral Cells Transplanted Into the Striatum of 6-OHDA Lesioned Rat. Mol Brain Res 1996;39(1):127-36. [DOI:10.1016/0169-328X(96)00018-6]
21. Borlongan CV, Randall TS, Cahill DW, Sanberg PR. Asymmetrical Motor Behavior in Rats with Unilateral Striatal Excitotoxic Lesions as Revealed by the Elevated Body Swing Test. Brain Res 1995;676(1):231-4. [DOI:10.1016/0006-8993(95)00150-O]
22. Lundblad M, Vaudano E, Cenci M. Cellular and Behavioural Effects of the Adenosine A2a Receptor Antagonist KW‐6002 in a Rat Model of l‐DOPA‐induced Dyskinesia. J Neurochem 2003;84(6):1398-410. [DOI:10.1046/j.1471-4159.2003.01632.x] [PMID]
23. Ulas J, Weihmuller F, Brunner L, Joyce J, Marshall J, Cotman C. Selective Increase of NMDA-sensitive Glutamate Binding in the Striatum of Parkinson's Disease, Alzheimer's Disease, and Mixed Parkinson's Disease/Alzheimer's Disease Patients: an Autoradiographic Study. J Neurosci 1994;14(11):6317-24. [PMID]
24. Ransom RW, Deschenes NL. Glycine Modulation of NMDA-evoked Release of [3 H] Acetylcholine and [3 H] Dopamine from Rat Striatal Slices. Neurosci Lett 1989;96(3):323-8. [DOI:10.1016/0304-3940(89)90399-6]
25. Nishimura LM, Boegman RJ. N-methyl-D-aspartate-evoked Release of Acetylcholine from the Medial Septum/Diagonal Band of Rat Brain. Neurosci Lett 1990;115(2):259-64. [DOI:10.1016/0304-3940(90)90465-L]
26. Matsuoka N, Aigner TG. The Glycine/NMDA Receptor Antagonist HA-966 Impairs Visual Recognition Memory in Rhesus Monkeys. Brain Res 1996;731(1):72-8. [DOI:10.1016/0006-8993(96)00463-5]
27. Ho Y-J, Ho S-C, Pawlak CR, Yeh K-Y. Effects of D-cycloserine on MPTP-induced Behavioral and Neurological Changes: Potential for Treatment of Parkinson's Disease Dementia. Behav Brain Res2011;219(2):280-90. [DOI:10.1016/j.bbr.2011.01.028] [PMID]
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |