Sat, Jan 31, 2026
Volume 11, Issue 3 (Summer 2025)
Caspian J Neurol Sci 2025, 11(3): 185-197 |
Back to browse issues page
Ethics code: NA
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Narayanan M, Prabhu N. The Effect of Bioactive Compounds in Multiple Sclerosis as the Adjuvant Immunomodulatory: A Comprehensive Review. Caspian J Neurol Sci 2025; 11 (3) :185-197
URL: http://cjns.gums.ac.ir/article-1-788-en.html
URL: http://cjns.gums.ac.ir/article-1-788-en.html
1- Research and Innovations, Department of Biotechnology, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences, Chennai, India.
2- Research and Innovations, Department of Biotechnology, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences, Chennai, India. ,bioscitecsol@gmail.com
2- Research and Innovations, Department of Biotechnology, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences, Chennai, India. ,
Full-Text [PDF 2676 kb]
(743 Downloads)
| Abstract (HTML) (718 Views)
Full-Text: (847 Views)
Introduction
Multiple sclerosis (MS) is a chronic, immune-mediated, neuroinflammatory condition affecting the central nervous system (CNS), primarily affecting young adults aged 20-40 [1]. The immunological response results in inflammation, demyelination, and subsequent degeneration of the axons (Figure 1). CD4+ or T helper cells, particularly Th1 and Th17 subsets, are pivotal in immunopathogenesis, triggering localized inflammatory reactions. B lymphocytes also contribute by generating autoantibodies targeting myelin antigens and establishing ectopic lymphoid follicles within the CNS [2]. Current pharmacological interventions for MS predominantly consist of disease-modifying therapies (DMTs) designed to decelerate the disease’s progression and diminish relapse incidence. These therapies are classified as intravenous (glatiramer acetate) [3], oral (teriflunomide, fingolimod, and dimethyl fumarate) [4], and infused treatments (alemtuzumab, natalizumab, and ocrelizumab). They provide significant efficacy while presenting risks like progressive multifocal leukoencephalopathy (PML), infusion reactions, infections, and autoimmune complications [5]. This review seeks to critically assess and summarize existing research about the immunomodulatory effects of natural bioactive compounds—alkaloids, polyphenols, flavonoids, and essential fatty acids—concerning MS. This work’s originality resides in its thorough synthesis of preclinical and clinical findings, emphasizing the several pathways by which these chemicals influence immunological responses, including antioxidant action, cytokine regulation, and immune cell modulation. The review offers novel insights into incorporating bioactive substances as adjunctive agents in MS treatment strategies by examining their therapeutic potential, safety profiles, and limitations, including bioavailability and standardization, an area inadequately addressed in current literature.

Autoimmune Mechanisms in MS
The autoimmune characteristic of MS is mostly caused by the disruption of self-tolerance, resulting in an induction of autoreactive lymphocytic cells. Multiple mechanisms are associated with this process. Molecular imitation transpires when specific viral and bacterial antigens, including those from Epstein-Barr virus (EBV), resemble CNS self-antigens, including myelin core protein, thus eliciting cross-reactivity stimulation of T cells [6]. Epitope dissemination entails the liberation of novel CNS antigens as tissue damage advances, subsequently exposed to the immune response, thereby stimulating formerly non-autoreactive T and B cells. Impaired peripheral tolerance is crucial, as the inability of central and peripheral systems to eradicate or inhibit autoreactive cells facilitates the establishment of the illness [7]. Furthermore, the breakdown of the blood-brain barrier (BBB) is a defining characteristic of MS, wherein the BBB remains permeable, permitting the infiltration of periphery immune systems into the CNS. Upon entry, these immune cells sustain inflammation and intensify tissue damage, aggravating the disease progression.
Inflammation versus regulatory immune equilibrium
An essential aspect of MS pathogenesis includes the disparity between pro-inflammatory and regulatory immunity elements. Pro-inflammatory (axis) chemicals characterized through Th1 and Th17 reactions perpetuate prolonged inflammation and CNS injury via cytokines, including interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-17, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-6 [8]. Such cytokines compromise the BBB, activate resident microglia, and induce endothelial and astrocyte cells to enhance immune cell infiltration. The regulatory axis comprises Tregs, tolerogenic dendritic cells, IL-10, and transforming growth factor (TGF)-β. Such components typically inhibit immune activation and sustain peripheral tolerance [9]. In MS, their quantity as well as functionality are diminished, further exacerbating the predisposition to autoimmunity. A Defining characteristic of MS is the lack of regulatory oversight of autoreactive lymphocytes constitutes. Moreover, current research indicates that metabolic disorders in immune cells and changes in the intestinal microbiota could exacerbate this immune disequilibrium. Immune instability in MS is a multifaceted process characterized by the abnormal activation of innate and adaptive immunity. The equilibrium among pro-inflammatory and regulatory mechanisms is disturbed, resulting in persistent neurodegeneration and CNS inflammation [10]. Comprehending such immunological pathways is crucial for pinpointing therapeutic goals, such as bioactive substances that could aid in re-establishing immunological equilibrium in MS.
Bioactive Compounds and Their Immunomodulatory Functions
Definition and category
Bioactive chemicals are organic chemicals in plants, fungi, marine life, and microbes that produce biological effects on humans. Such substances can influence multiple biological functions and pathways, so they are frequently categorized according to their chemical structure, origin, or biological function [11] like support the management of MS (Figure 2). Phenolic chemicals constitute a primary category, encompassing flavonoids, polyphenols, along with tannins. These chemicals are recognized for their anti-inflammatory and antioxidant characteristics, with quercetin (present in onions and apples) and epigallocatechin gallate (EGCG) found in green tea serving as notable examples [12, 13]. The alkaloids are chemical molecules, frequently sourced from vegetation, that demonstrate diverse pharmacological effects, notably regulating the immunity system. Alkaloid examples comprise morphine derived from poppies as well as quinine extracted from Cinchona tree bark. Terpenoids, a varied class of organic chemicals, possess anti-inflammatory, antibacterial [14], and immunomodulatory properties, exemplified by turmerone from ginsenosides and turmeric from ginseng [15, 16]. Glycosides, predominantly sourced from plants, provide significant immune-enhancing properties, as shown by flavonoid glycosides from citrus and saponins from ginseng. Peptides and proteins, frequently sourced from plants, fungi, or marine life, might influence immunity and inflammation. Beta-glucans derived from mushrooms such as Ganoderma lucidum are recognized for augmenting immune function [17]. Finally, fatty acids, notably omega-3 and -6 from marine life like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) found in fish oil, exhibit substantial immunomodulatory effects, especially for mitigating chronic inflammation [17]. Such bioactive chemicals have diverse physiological consequences, encompassing antioxidant, anti-inflammatory, antibacterial, and immune-modulating capabilities, enhancing their potential therapeutic advantages in numerous health disorders.

Natural sources: Flora, fungi, and marine living things
Bioactive chemicals are prevalent, particularly in plants, fungi, and marine life, each presenting distinct therapeutic potential owing to their varied bioactive qualities [18, 19]. Numerous medicinal plant varieties are renowned for their health advantages, with bioactive components such as alkaloids, flavonoids, terpenoids, and phenolic acids significantly influencing immune function and inflammation. Curcumin derived from Curcuma longa exhibits anti-inflammatory activities, resveratrol from Vitis vinifera possesses antioxidant effects, and quercetin in numerous veggies and fruits also displays anti-inflammatory abilities [20, 21]. Fungi, particularly mushrooms, contain bioactive molecules such as polysaccharides (e.g. β-glucans), terpenoids, phenolics, and lectins, which are associated with various medicinal properties, including immunomodulatory, antimicrobial, antioxidant, and anticancer effects.
Immunomodulation mechanisms
The immune-modulating properties of bioactive chemicals are intricate and multifaceted since these substances engage with immune cells and molecular signaling systems to augment or inhibit immunological responses contingent upon their surroundings. A primary method is cytokine modulation, wherein numerous bioactive chemicals affect the synthesis of crucial cytokines, including ILs, TNF-α, and IFN-γ [22]. Curcumin has demonstrated the ability to diminish pro-inflammatory cytokines, including IL-6 and TNF-α, while elevating anti-inflammatory cytokines, such as IL-10. A vital process is T cell activation, wherein bioactive substances can influence the stimulation along with T cell differentiation [23]. Flavonoids, including quercetin, affect the development of CD4⁺ T cells towards Th1 and Th2 subsets, either augmenting immunity or fostering tolerance, contingent upon the context. Compounds such as β-glucans facilitate the activation of natural killer (NK) cells, enhancing innate immune defense. Macrophage stimulation is a significant mechanism, as bioactive substances can modulate macrophage activity, fostering either anti-inflammatory M2 macrophages or pro-inflammatory M1 macrophages [24]. EPA, a marine-sourced omega-3 fatty acid, can promote M2 macrophage polarization, augmenting anti-inflammatory activity. Numerous bioactive substances modulate the nuclear factor-kappa B (NF-κB) pathway, a crucial regulator of immune systems and inflammation. Chemicals like curcumin, EGCG, and resveratrol from green tea impede NF-κB activation, hence diminishing chronic pain and autoimmune [25]. Antioxidant function represents a notable characteristic, given that oxidative stress significantly contributes to immunological dysregulation. Bioactive substances such as catechins and flavonoids derived from green tea mitigate reactive oxygen species (ROS), inhibiting the stimulation of inflammatory mechanisms and diminishing the activity of immune cells along with tissue damage. Furthermore, specific bioactive chemicals modulate immune-mediated apoptosis, affecting the viability of immune cells apoptosis. Terpenoids and polyphenols can modulate the death of autoreactive T cells, thus averting tissue harm in prolonged inflammation [26]. Gut-microbiota immune regulation is an evolving field, with research indicating that bioactive substances affect immunological responses by altering the composition of intestinal microbiotas. Polyphenols found in veggies and fruits, including resveratrol, impact the microbiota, and this can modulate systemic defenses and diminish inflammation in autoimmune conditions, including MS [27]. Bioactive substances derived from plants, fungi, and marine organisms demonstrate various immunomodulatory actions, presenting the potential for use in autoimmune illnesses by re-establishing immunological equilibrium and diminishing persistent inflammation. This natural origin and varied biological activity render them interesting candidates for synergistic and complementary therapy in immune-driven disorders.
Case and exploratory studies
A multitude of case reports, as well as laboratory studies, underscore their capacity to modulate immunological responses [28]. A research investigation on Annona reticulata leaf extract revealed its immunomodulatory properties in cultured immune cell lines and mice, indicating that substances such as quercetin and β-sitosterol can elicit significant immunological reactions [29]. A polyherbal formulation of Tinospora cordifolia, Phyllanthus emblica, Withania somnifera, and Piper nigrum was observed to augment macrophage along with NK cell activity, hence enhancing the immunological response in rats immunosuppressed by cyclophosphamide [30]. Additional research has investigated substances such as curcumin from C. longa and quercetin from different vegetables and fruits, demonstrating encouraging anti-inflammatory properties [31]. Mushrooms like Ganoderma lucidum possess β-glucans that activate immune cells, whereas sea algae such as Spirulina and Chlorella are recognized for stimulating immunological responses via their bioactive peptides. Field investigations revealed that Euphorbia deccanensis, a plant native to South India, exhibited notable antioxidant and anti-inflammatory activities, reinforcing the medicinal potential of its bioactive components [32]. Moreover, Kaempferia parviflora could avert the depletion of splenic constituents in immunosuppressed rats, underscoring its immunomodulatory capabilities [33]. These results highlight the extensive biological actions of bioactive chemicals, potentially resulting in innovative therapy strategies for immune-related disorders. Nonetheless, additional clinical trials are required to validate their safety and effectiveness for human application.
Types of Bioactive Substances With MS Treating Potentials
Polyphenols
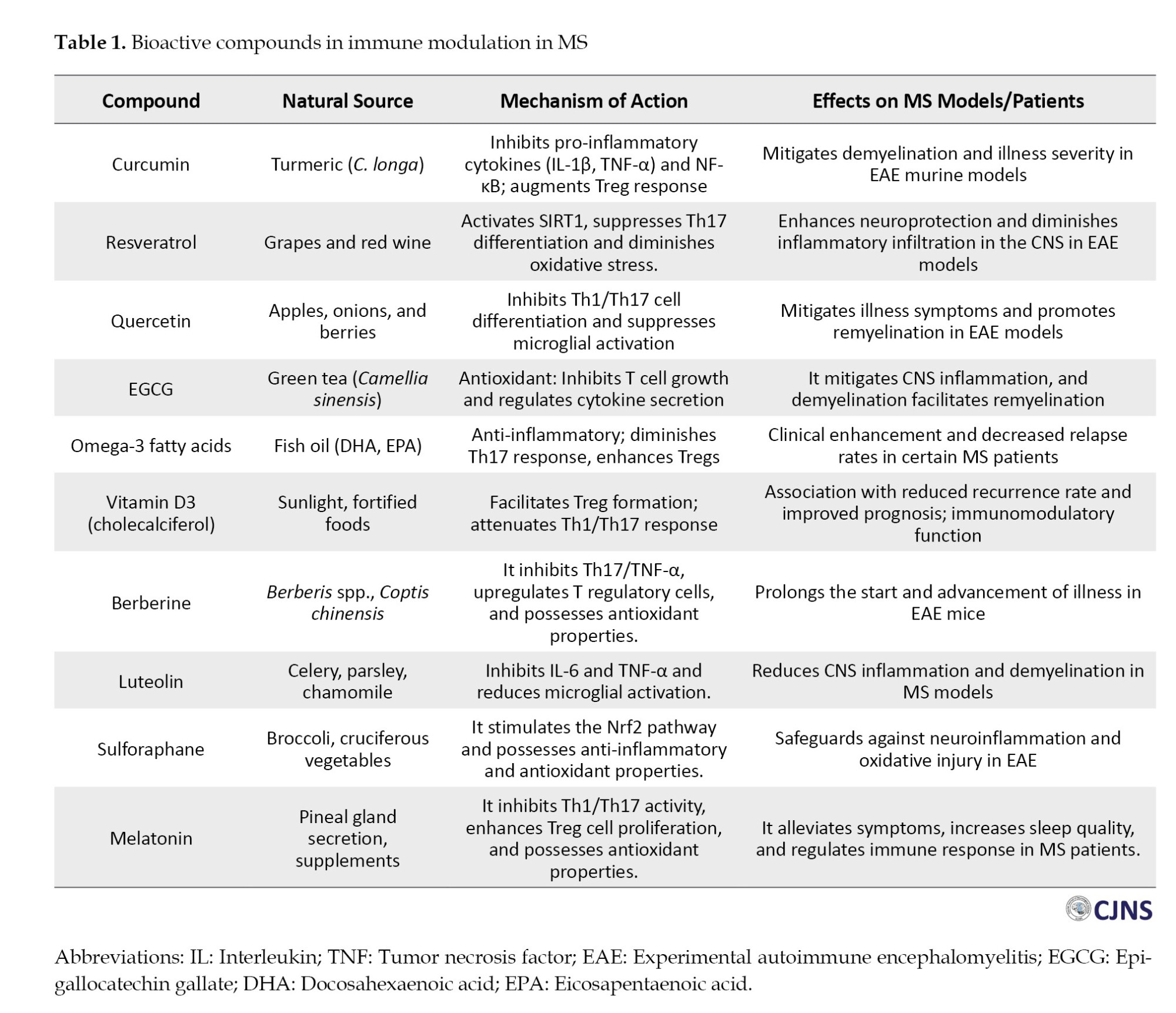
Polyphenols are organic chemicals extensively found in plants, with more than 8000 varieties documented. These chemicals are essential in mitigating inflammation and oxidative stress and regulating immunological responses [34]. Curcumin, obtained from C. longa, represents one of the more extensively researched polyphenolic substances [35]. Curcumin possesses significant antioxidant and anti-inflammatory characteristics, suppressing pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, which play a vital role in autoimmune disorders such as MS and rheumatoid arthritis (RA). It regulates the NF-κB pathway, which is crucial for inflammatory regulation, and research indicates that curcumin may serve as a complementary treatment to manage inflammation in autoimmune diseases [36]. Resveratrol, another notable polyphenol, is present within the V. vinifera skin. Resveratrol has demonstrated the ability to diminish oxidative stress, suppress inflammation, and modulate immunological function by activating sirtuin proteins, which govern immune cell activities [37]. It can inhibit the stimulation of pro-inflammatory T cells while decreasing the synthesis of pro-inflammatory cytokines such as IL-1 and IL-6, rendering it advantageous for conditions like lupus and arthritis. Ellagic acid, present in pomegranate, raspberries, and strawberries, is a polyphenolic molecule recognized for its anti-inflammatory and antioxidant characteristics [38]. According to research, it improves immunological responses by augmenting T-cell proliferation and elevating the synthesis of anti-inflammatory cytokines such as IL-10 [39]. Ellagic acid may additionally lower the likelihood of cancer along with cardiovascular illnesses. Table 1 states various bioactive compounds used in MS treatment from different sources, and each compound exhibits a specific mechanism.
Flavonoids
Flavonoids are bioactive compounds derived from plants with antioxidant, immune-modulatory, and anti-inflammatory characteristics. Quercetin, present in fruits and vegetables, possesses significant antioxidant and anti-inflammatory capabilities, modulating immune cell types such as T cells, dendritic cells, and macrophages [40]. It also mitigates allergic symptoms by inhibiting histamine release and immune cell activation. Luteolin, present in olive oil, celery, and peppers, can diminish pro-inflammatory cytokine activation and improve the efficacy of T cell regulators by inhibiting the NF-κB pathway [41]. Research on animals with inflammatory bowel illness and neuroinflammation has shown its effectiveness in diminishing pro-inflammatory cell proliferation in the gastrointestinal tract and CNS [42]. Kaempferol, present in kale, apples, and spinach, possesses antioxidant and anti-inflammatory characteristics, bolstering immunity by modulating macrophage function and reducing the production of IL-6 and TNF-α [43].
Alkaloids
Alkaloids, nitrogenous organic compounds in plants, fungi, and marine organisms, possess notable pharmacological features such as immunological modulation, anti-inflammatory effects, and antibacterial activity [44]. Morphine, an analgesic derived from Papaver somniferum, can block pro-inflammatory cytokines, potentially affecting adaptive and innate immunity [45]. Nonetheless, its utilization needs meticulous regulation owing to the risk of addiction. Quinine, an alkaloid derived from the bark of Cinchona trees, possesses antimalarial effects and has been utilized in treating malaria [46]. It modulates immune responses by enhancing macrophage phagocytic activity and promoting nitric oxide production, which possesses antibacterial effects. Quinine additionally affects the formation of immune cells in inflammatory situations [47]. Berberine, a botanical alkaloid, possesses antibacterial, anti-inflammatory, and immunomodulatory characteristics [48]. It regulates macrophage polarization, enhancing anti-inflammatory M2 phenotypes, and may offer possible treatments for diabetes and inflammatory bowel disease.
Terpenoids
Terpenoids, or isoprenoids, constitute a substantial category of chemicals naturally seen in plants, fungi, and marine life [49]. These chemicals are recognized for their diverse biological actions, encompassing anti-inflammatory, immunomodulatory, and antioxidant properties. An instance is turmerone, a terpenoid chemical in C. longa, in conjunction with curcumin [50]. Turmerone exhibits anti-inflammatory activities by regulating the release of cytokines and blocking the NF-κB cascade [51]. It can also augment the immune system’s reaction by elevating the activity of T cells and phagocytes, thereby providing therapeutic advantages in situations like autoimmune and asthma disorders. Another significant instance is ginsenosides, saponins present in Panax ginseng. Ginsenosides are recognized for their ability to augment the functions of immune systems, namely the stimulation of T cells, macrophages, and NK cells [52]. They also govern the synthesis of pro-inflammatory cytokines, including IL-6 and TNF-α, which play a role in inflammation and autoimmune disorders like lupus and RA.
Fatty acids
Fatty acids, especially omega-3 and omega-6 polyunsaturated fatty acids, are crucial for immunological regulation and maintaining equilibrium among pro-inflammatory and anti-inflammatory mechanisms [53]. Omega-3 fatty acids, including DHA and EPA, present in chia seeds, fish oil, and flaxseed, have been extensively researched for their capacity to mitigate chronic inflammation [54]. Such fatty acids reduce pro-inflammatory cytokines, including TNF-α and IL-1, while enhancing the synthesis of anti-inflammatory mediators like resolvins. Omega-3 fatty acids are particularly efficacious in autoimmune disorders like RA and inflammatory bowel illness. Conversely, omega-6 fatty acids, including in sunflower and safflower oils, are generally pro-inflammatory but essential for immune system functionality when harmonized with omega-3 fatty acids [55]. Such fatty acids are crucial for the effective functioning of immune systems, including macrophages, while preserving the mucosal barriers and skin integrity.
Preclinical and clinical evidence
Preclinical along with clinical evidence is critical for confirming the curative value of bioactive substances in autoimmune illnesses like MS. A thorough comprehension develops over multiple phases of research, encompassing in vitro investigations, animal models, as well as human clinical studies, which investigate mechanisms of action, efficacy in therapy, as well as risk concerns.
In vitro studies
In vitro investigations are essential for clarifying the molecular processes via which bioactive substances influence immune regulation. Curcumin, a polyphenolic substance derived from C. longa, has demonstrated the ability to suppress the stimulation of NF-κB process, a key mediator of inflammation [56]. In cultivated human immune cells, curcumin markedly reduces the synthesis of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, essential contributors to MS pathogenesis. Moreover, curcumin augments the functionality of Tregs, which are essential for sustaining immunological tolerance and averting autoimmune assaults. Quercetin, a flavonoid found in fruits such as onions and apples, reduces histamine release from the mast cells and regulates T-cell multiplication [18]. In vitro studies indicate that quercetin can diminish the expression of MHC class II compounds on antigen-presenting cells, thereby attenuating the stimulation of autoreactive T lymphocytes [57]. These results highlight the immunomodulatory capacity of bioactive substances in modulating the activation of immune cells as well as inflammation at the level of cells.
Animal models of MS
The application of curcumin in experimental autoimmune encephalomyelitis (EAE) animals has demonstrated a reduction in clinical manifestations of MS, notably paralysis, through the modulation of innate and adaptive immune responses [58]. Curcumin particularly diminishes the proliferation of pro-inflammatory Th17 cells and Th1, crucial in developing MS-like disease. It also facilitates the proliferation of Tregs, so fostering immunological tolerance. Moreover, curcumin has been shown to alleviate oxidative stress by enhancing the activity of antioxidant enzymes, including catalase and superoxide dismutase, thereby preventing neuronal damage [58]. Resveratrol, a polyphenolic molecule in grapes and red wine, demonstrates potential in EAE models by mitigating demyelination and CNS inflammation. It suppresses the stimulation of astrocytes and microglia, two glial cells implicated in neurological inflammation, while concurrently safeguarding oligodendrocytes, the cells accountable for myelination. Furthermore, resveratrol enhances the functioning of the BBB, inhibiting the influx of periphery immune systems into the CNS [59]. The results from these animal models indicate that bioactive substances such as curcumin and resveratrol may influence disease development and safeguard the CNS in MS.
Human clinical trials and observational research
A randomized, double-blind, placebo-controlled research showed that curcumin administration decreased pro-inflammatory cytokine amounts in MS patients, enhancing physical function and quality of life [60]. A separate study indicated that omega-3 fatty acids, specifically EPA and DHA, decreased the rate of relapse and inflammatory indicators among people with relapsing-remitting MS. Such fatty acids alter the body’s reaction to an anti-inflammatory characteristic by diminishing pro-inflammatory cytokines and promoting resolving production. Polyphenols in veggies, fruits, and green tea are associated with reduced inflammation and decreased oxidative damage in MS patients [25, 54]. Flavonoids such as luteolin and quercetin have demonstrated encouraging outcomes in enhancing immune cell functionality and regulating the intestinal microbiota in patients with MS [45]. These findings indicate that bioactive substances can markedly influence immune function and inflammation in MS, diminishing inflammation, enhancing neuroprotection, and enhancing overall disease outcomes. Nonetheless, additional extensive and rigorously planned clinical trials are required to validate the effectiveness, safety, and lasting benefits of such medicines for patients with MS.
Synergistic Effects and Combination Therapies
There is growing interest in combination therapies that integrate bioactive molecules to augment traditional treatments and improve their efficacy (Table 2) [61].

Integration with traditional MS therapies
Bioactive substances demonstrate the potential to enhance the efficacy of traditional MS therapies by targeting immunological pathways and mitigating negative effects. Curcumin, an antioxidant and anti-inflammatory compound, has been investigated for its possible synergistic effects when administered alongside IFN-β, a primary treatment for relapsing-remitting MS [62]. Curcumin obstructs the NF-κB pathway and diminishes pro-inflammatory cytokines, potentially enhancing therapy efficacy and lowering relapse rates in MS patients. Likewise, resveratrol and glatiramer acetate (GA), a frequently utilized disease-modifying therapy, have demonstrated encouraging outcomes in mitigating neuroinflammation and safeguarding against oxidative stress in animal studies of MS [63]. Bioactive substances such as flavonoids and omega-3 fatty acids may augment the immune-regulating effects of DMTs by transitioning their immune system from a pro-inflammatory condition to a more equilibrated, anti-inflammatory character [25]. These chemicals may also mitigate adverse effects linked to traditional therapies, including gastrointestinal pain and fatigue, enhancing patient adherence and quality of life.
Gut microbiota modulation
Combination therapy for MS entails the regulation of gut microbiota, which is essential for immune function. Dysbiosis of gut microbiota has been associated with autoimmune disorders, including MS [64]. Bioactive chemicals such as polyphenols and prebiotics can enhance the proliferation of beneficial microbes while suppressing detrimental strains, modulating systemic immune reactions, and perhaps diminishing autoimmune reactivity. Resveratrol improves gut barrier integrity and modulates immune cell activity in gut-associated lymphoid tissue (GALT), enhancing immunological tolerance [65]. Integrating bioactive substances with probiotics can re-establish immunological homeostasis and mitigate inflammation in MS. This method addresses immunological dysregulation, mitigates inflammation, and safeguards the CNS from more harm. The combinatorial impacts of several medicines, including gut microbiota manipulation, immune cell stimulation, and oxidative stress, underscore the possibility of personalized, integrative treatment techniques in MS [66]. Nonetheless, additional research and clinical studies are requisite to ascertain the appropriate dosages, combinations, and lasting impacts of these therapies in treating MS.
Limitations, Challenges, and Future Directions
Bioactive compounds possess considerable potential for treating MS, although they encounter obstacles, including insufficient bioavailability, variability in individual responses, and the necessity for more rigorous clinical trials. Researchers are investigating approaches to improve the distribution and bioavailability of bioactive compounds, including nano-formulations, biocompatible polymers, and encapsulating techniques. Inter-individual heterogeneity in response patterns is challenging, as enzyme genetic differences influence absorption and efficacy. The gut microbiota additionally affects metabolic and immune responses to bioactive compounds. Characterization of the microbiome and personalized dietary strategies may facilitate the customization of bioactive chemical therapy for enhanced efficacy in MS patients. Future research must prioritize comprehensive, randomized, placebo-controlled trials, sophisticated drug delivery technologies, biomarker development, personalized medicine approaches, combination therapies, and long-term safety and toxicology studies.
Conclusion
Bioactive substances derived from plants, fungi, marine life, and microbes potentially modify immune responses in MS. These compounds, comprising polyphenols, alkaloids, flavonoids, and so on, have immunoregulatory, anti-inflammatory, and antioxidant properties. Preclinical investigations indicate that they may mitigate disease development, diminish inflammatory cytokines, and re-establish immunological equilibrium. Nevertheless, low bioavailability and a scarcity of extensive trials impede their broad implementation. Novel delivery systems and personalized treatment strategies may assist in surmounting these obstacles. Future investigations should concentrate on stringent clinical studies, mechanistic elucidations, and evaluations of long-term safety.
Ethical Considerations
Compliance with ethical guidelines
This review manuscript includes no original research involving human subjects or animals. Consequently, ethical approval, along with informed consent, was unnecessary. All sources and studies referenced in this review have been acknowledged to uphold academic honesty and prevent plagiarism. The writers have diligently endeavored to present the reviewed material with objectivity, accuracy, and balance, honoring the contributions and discoveries of the original authors.
This article is a review and does not include any experiments involving human participants or animals conducted by writers. Consequently, ethical approval and informed consent were not relevant. All data and information presented in this review have been sourced from previously published materials, which have been appropriately attributed. The authors assert adherence to all pertinent ethical publication norms during the manuscript’s development.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors contributed equally to the main manuscript preparation and data analysis and reviewed the manuscript.
Conflict of interest
The authors declared no conflict interests.
Acknowledgements
All authors thank the Saveetha Institute of Medical and Technical Sciences (SIMATS), Kuthambakkam, India for their constant support.
References
Multiple sclerosis (MS) is a chronic, immune-mediated, neuroinflammatory condition affecting the central nervous system (CNS), primarily affecting young adults aged 20-40 [1]. The immunological response results in inflammation, demyelination, and subsequent degeneration of the axons (Figure 1). CD4+ or T helper cells, particularly Th1 and Th17 subsets, are pivotal in immunopathogenesis, triggering localized inflammatory reactions. B lymphocytes also contribute by generating autoantibodies targeting myelin antigens and establishing ectopic lymphoid follicles within the CNS [2]. Current pharmacological interventions for MS predominantly consist of disease-modifying therapies (DMTs) designed to decelerate the disease’s progression and diminish relapse incidence. These therapies are classified as intravenous (glatiramer acetate) [3], oral (teriflunomide, fingolimod, and dimethyl fumarate) [4], and infused treatments (alemtuzumab, natalizumab, and ocrelizumab). They provide significant efficacy while presenting risks like progressive multifocal leukoencephalopathy (PML), infusion reactions, infections, and autoimmune complications [5]. This review seeks to critically assess and summarize existing research about the immunomodulatory effects of natural bioactive compounds—alkaloids, polyphenols, flavonoids, and essential fatty acids—concerning MS. This work’s originality resides in its thorough synthesis of preclinical and clinical findings, emphasizing the several pathways by which these chemicals influence immunological responses, including antioxidant action, cytokine regulation, and immune cell modulation. The review offers novel insights into incorporating bioactive substances as adjunctive agents in MS treatment strategies by examining their therapeutic potential, safety profiles, and limitations, including bioavailability and standardization, an area inadequately addressed in current literature.

Autoimmune Mechanisms in MS
The autoimmune characteristic of MS is mostly caused by the disruption of self-tolerance, resulting in an induction of autoreactive lymphocytic cells. Multiple mechanisms are associated with this process. Molecular imitation transpires when specific viral and bacterial antigens, including those from Epstein-Barr virus (EBV), resemble CNS self-antigens, including myelin core protein, thus eliciting cross-reactivity stimulation of T cells [6]. Epitope dissemination entails the liberation of novel CNS antigens as tissue damage advances, subsequently exposed to the immune response, thereby stimulating formerly non-autoreactive T and B cells. Impaired peripheral tolerance is crucial, as the inability of central and peripheral systems to eradicate or inhibit autoreactive cells facilitates the establishment of the illness [7]. Furthermore, the breakdown of the blood-brain barrier (BBB) is a defining characteristic of MS, wherein the BBB remains permeable, permitting the infiltration of periphery immune systems into the CNS. Upon entry, these immune cells sustain inflammation and intensify tissue damage, aggravating the disease progression.
Inflammation versus regulatory immune equilibrium
An essential aspect of MS pathogenesis includes the disparity between pro-inflammatory and regulatory immunity elements. Pro-inflammatory (axis) chemicals characterized through Th1 and Th17 reactions perpetuate prolonged inflammation and CNS injury via cytokines, including interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-17, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-6 [8]. Such cytokines compromise the BBB, activate resident microglia, and induce endothelial and astrocyte cells to enhance immune cell infiltration. The regulatory axis comprises Tregs, tolerogenic dendritic cells, IL-10, and transforming growth factor (TGF)-β. Such components typically inhibit immune activation and sustain peripheral tolerance [9]. In MS, their quantity as well as functionality are diminished, further exacerbating the predisposition to autoimmunity. A Defining characteristic of MS is the lack of regulatory oversight of autoreactive lymphocytes constitutes. Moreover, current research indicates that metabolic disorders in immune cells and changes in the intestinal microbiota could exacerbate this immune disequilibrium. Immune instability in MS is a multifaceted process characterized by the abnormal activation of innate and adaptive immunity. The equilibrium among pro-inflammatory and regulatory mechanisms is disturbed, resulting in persistent neurodegeneration and CNS inflammation [10]. Comprehending such immunological pathways is crucial for pinpointing therapeutic goals, such as bioactive substances that could aid in re-establishing immunological equilibrium in MS.
Bioactive Compounds and Their Immunomodulatory Functions
Definition and category
Bioactive chemicals are organic chemicals in plants, fungi, marine life, and microbes that produce biological effects on humans. Such substances can influence multiple biological functions and pathways, so they are frequently categorized according to their chemical structure, origin, or biological function [11] like support the management of MS (Figure 2). Phenolic chemicals constitute a primary category, encompassing flavonoids, polyphenols, along with tannins. These chemicals are recognized for their anti-inflammatory and antioxidant characteristics, with quercetin (present in onions and apples) and epigallocatechin gallate (EGCG) found in green tea serving as notable examples [12, 13]. The alkaloids are chemical molecules, frequently sourced from vegetation, that demonstrate diverse pharmacological effects, notably regulating the immunity system. Alkaloid examples comprise morphine derived from poppies as well as quinine extracted from Cinchona tree bark. Terpenoids, a varied class of organic chemicals, possess anti-inflammatory, antibacterial [14], and immunomodulatory properties, exemplified by turmerone from ginsenosides and turmeric from ginseng [15, 16]. Glycosides, predominantly sourced from plants, provide significant immune-enhancing properties, as shown by flavonoid glycosides from citrus and saponins from ginseng. Peptides and proteins, frequently sourced from plants, fungi, or marine life, might influence immunity and inflammation. Beta-glucans derived from mushrooms such as Ganoderma lucidum are recognized for augmenting immune function [17]. Finally, fatty acids, notably omega-3 and -6 from marine life like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) found in fish oil, exhibit substantial immunomodulatory effects, especially for mitigating chronic inflammation [17]. Such bioactive chemicals have diverse physiological consequences, encompassing antioxidant, anti-inflammatory, antibacterial, and immune-modulating capabilities, enhancing their potential therapeutic advantages in numerous health disorders.

Natural sources: Flora, fungi, and marine living things
Bioactive chemicals are prevalent, particularly in plants, fungi, and marine life, each presenting distinct therapeutic potential owing to their varied bioactive qualities [18, 19]. Numerous medicinal plant varieties are renowned for their health advantages, with bioactive components such as alkaloids, flavonoids, terpenoids, and phenolic acids significantly influencing immune function and inflammation. Curcumin derived from Curcuma longa exhibits anti-inflammatory activities, resveratrol from Vitis vinifera possesses antioxidant effects, and quercetin in numerous veggies and fruits also displays anti-inflammatory abilities [20, 21]. Fungi, particularly mushrooms, contain bioactive molecules such as polysaccharides (e.g. β-glucans), terpenoids, phenolics, and lectins, which are associated with various medicinal properties, including immunomodulatory, antimicrobial, antioxidant, and anticancer effects.
Immunomodulation mechanisms
The immune-modulating properties of bioactive chemicals are intricate and multifaceted since these substances engage with immune cells and molecular signaling systems to augment or inhibit immunological responses contingent upon their surroundings. A primary method is cytokine modulation, wherein numerous bioactive chemicals affect the synthesis of crucial cytokines, including ILs, TNF-α, and IFN-γ [22]. Curcumin has demonstrated the ability to diminish pro-inflammatory cytokines, including IL-6 and TNF-α, while elevating anti-inflammatory cytokines, such as IL-10. A vital process is T cell activation, wherein bioactive substances can influence the stimulation along with T cell differentiation [23]. Flavonoids, including quercetin, affect the development of CD4⁺ T cells towards Th1 and Th2 subsets, either augmenting immunity or fostering tolerance, contingent upon the context. Compounds such as β-glucans facilitate the activation of natural killer (NK) cells, enhancing innate immune defense. Macrophage stimulation is a significant mechanism, as bioactive substances can modulate macrophage activity, fostering either anti-inflammatory M2 macrophages or pro-inflammatory M1 macrophages [24]. EPA, a marine-sourced omega-3 fatty acid, can promote M2 macrophage polarization, augmenting anti-inflammatory activity. Numerous bioactive substances modulate the nuclear factor-kappa B (NF-κB) pathway, a crucial regulator of immune systems and inflammation. Chemicals like curcumin, EGCG, and resveratrol from green tea impede NF-κB activation, hence diminishing chronic pain and autoimmune [25]. Antioxidant function represents a notable characteristic, given that oxidative stress significantly contributes to immunological dysregulation. Bioactive substances such as catechins and flavonoids derived from green tea mitigate reactive oxygen species (ROS), inhibiting the stimulation of inflammatory mechanisms and diminishing the activity of immune cells along with tissue damage. Furthermore, specific bioactive chemicals modulate immune-mediated apoptosis, affecting the viability of immune cells apoptosis. Terpenoids and polyphenols can modulate the death of autoreactive T cells, thus averting tissue harm in prolonged inflammation [26]. Gut-microbiota immune regulation is an evolving field, with research indicating that bioactive substances affect immunological responses by altering the composition of intestinal microbiotas. Polyphenols found in veggies and fruits, including resveratrol, impact the microbiota, and this can modulate systemic defenses and diminish inflammation in autoimmune conditions, including MS [27]. Bioactive substances derived from plants, fungi, and marine organisms demonstrate various immunomodulatory actions, presenting the potential for use in autoimmune illnesses by re-establishing immunological equilibrium and diminishing persistent inflammation. This natural origin and varied biological activity render them interesting candidates for synergistic and complementary therapy in immune-driven disorders.
Case and exploratory studies
A multitude of case reports, as well as laboratory studies, underscore their capacity to modulate immunological responses [28]. A research investigation on Annona reticulata leaf extract revealed its immunomodulatory properties in cultured immune cell lines and mice, indicating that substances such as quercetin and β-sitosterol can elicit significant immunological reactions [29]. A polyherbal formulation of Tinospora cordifolia, Phyllanthus emblica, Withania somnifera, and Piper nigrum was observed to augment macrophage along with NK cell activity, hence enhancing the immunological response in rats immunosuppressed by cyclophosphamide [30]. Additional research has investigated substances such as curcumin from C. longa and quercetin from different vegetables and fruits, demonstrating encouraging anti-inflammatory properties [31]. Mushrooms like Ganoderma lucidum possess β-glucans that activate immune cells, whereas sea algae such as Spirulina and Chlorella are recognized for stimulating immunological responses via their bioactive peptides. Field investigations revealed that Euphorbia deccanensis, a plant native to South India, exhibited notable antioxidant and anti-inflammatory activities, reinforcing the medicinal potential of its bioactive components [32]. Moreover, Kaempferia parviflora could avert the depletion of splenic constituents in immunosuppressed rats, underscoring its immunomodulatory capabilities [33]. These results highlight the extensive biological actions of bioactive chemicals, potentially resulting in innovative therapy strategies for immune-related disorders. Nonetheless, additional clinical trials are required to validate their safety and effectiveness for human application.
Types of Bioactive Substances With MS Treating Potentials
Polyphenols
Polyphenols are organic chemicals extensively found in plants, with more than 8000 varieties documented. These chemicals are essential in mitigating inflammation and oxidative stress and regulating immunological responses [34]. Curcumin, obtained from C. longa, represents one of the more extensively researched polyphenolic substances [35]. Curcumin possesses significant antioxidant and anti-inflammatory characteristics, suppressing pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, which play a vital role in autoimmune disorders such as MS and rheumatoid arthritis (RA). It regulates the NF-κB pathway, which is crucial for inflammatory regulation, and research indicates that curcumin may serve as a complementary treatment to manage inflammation in autoimmune diseases [36]. Resveratrol, another notable polyphenol, is present within the V. vinifera skin. Resveratrol has demonstrated the ability to diminish oxidative stress, suppress inflammation, and modulate immunological function by activating sirtuin proteins, which govern immune cell activities [37]. It can inhibit the stimulation of pro-inflammatory T cells while decreasing the synthesis of pro-inflammatory cytokines such as IL-1 and IL-6, rendering it advantageous for conditions like lupus and arthritis. Ellagic acid, present in pomegranate, raspberries, and strawberries, is a polyphenolic molecule recognized for its anti-inflammatory and antioxidant characteristics [38]. According to research, it improves immunological responses by augmenting T-cell proliferation and elevating the synthesis of anti-inflammatory cytokines such as IL-10 [39]. Ellagic acid may additionally lower the likelihood of cancer along with cardiovascular illnesses. Table 1 states various bioactive compounds used in MS treatment from different sources, and each compound exhibits a specific mechanism.

Flavonoids
Flavonoids are bioactive compounds derived from plants with antioxidant, immune-modulatory, and anti-inflammatory characteristics. Quercetin, present in fruits and vegetables, possesses significant antioxidant and anti-inflammatory capabilities, modulating immune cell types such as T cells, dendritic cells, and macrophages [40]. It also mitigates allergic symptoms by inhibiting histamine release and immune cell activation. Luteolin, present in olive oil, celery, and peppers, can diminish pro-inflammatory cytokine activation and improve the efficacy of T cell regulators by inhibiting the NF-κB pathway [41]. Research on animals with inflammatory bowel illness and neuroinflammation has shown its effectiveness in diminishing pro-inflammatory cell proliferation in the gastrointestinal tract and CNS [42]. Kaempferol, present in kale, apples, and spinach, possesses antioxidant and anti-inflammatory characteristics, bolstering immunity by modulating macrophage function and reducing the production of IL-6 and TNF-α [43].
Alkaloids
Alkaloids, nitrogenous organic compounds in plants, fungi, and marine organisms, possess notable pharmacological features such as immunological modulation, anti-inflammatory effects, and antibacterial activity [44]. Morphine, an analgesic derived from Papaver somniferum, can block pro-inflammatory cytokines, potentially affecting adaptive and innate immunity [45]. Nonetheless, its utilization needs meticulous regulation owing to the risk of addiction. Quinine, an alkaloid derived from the bark of Cinchona trees, possesses antimalarial effects and has been utilized in treating malaria [46]. It modulates immune responses by enhancing macrophage phagocytic activity and promoting nitric oxide production, which possesses antibacterial effects. Quinine additionally affects the formation of immune cells in inflammatory situations [47]. Berberine, a botanical alkaloid, possesses antibacterial, anti-inflammatory, and immunomodulatory characteristics [48]. It regulates macrophage polarization, enhancing anti-inflammatory M2 phenotypes, and may offer possible treatments for diabetes and inflammatory bowel disease.
Terpenoids
Terpenoids, or isoprenoids, constitute a substantial category of chemicals naturally seen in plants, fungi, and marine life [49]. These chemicals are recognized for their diverse biological actions, encompassing anti-inflammatory, immunomodulatory, and antioxidant properties. An instance is turmerone, a terpenoid chemical in C. longa, in conjunction with curcumin [50]. Turmerone exhibits anti-inflammatory activities by regulating the release of cytokines and blocking the NF-κB cascade [51]. It can also augment the immune system’s reaction by elevating the activity of T cells and phagocytes, thereby providing therapeutic advantages in situations like autoimmune and asthma disorders. Another significant instance is ginsenosides, saponins present in Panax ginseng. Ginsenosides are recognized for their ability to augment the functions of immune systems, namely the stimulation of T cells, macrophages, and NK cells [52]. They also govern the synthesis of pro-inflammatory cytokines, including IL-6 and TNF-α, which play a role in inflammation and autoimmune disorders like lupus and RA.
Fatty acids
Fatty acids, especially omega-3 and omega-6 polyunsaturated fatty acids, are crucial for immunological regulation and maintaining equilibrium among pro-inflammatory and anti-inflammatory mechanisms [53]. Omega-3 fatty acids, including DHA and EPA, present in chia seeds, fish oil, and flaxseed, have been extensively researched for their capacity to mitigate chronic inflammation [54]. Such fatty acids reduce pro-inflammatory cytokines, including TNF-α and IL-1, while enhancing the synthesis of anti-inflammatory mediators like resolvins. Omega-3 fatty acids are particularly efficacious in autoimmune disorders like RA and inflammatory bowel illness. Conversely, omega-6 fatty acids, including in sunflower and safflower oils, are generally pro-inflammatory but essential for immune system functionality when harmonized with omega-3 fatty acids [55]. Such fatty acids are crucial for the effective functioning of immune systems, including macrophages, while preserving the mucosal barriers and skin integrity.
Preclinical and clinical evidence
Preclinical along with clinical evidence is critical for confirming the curative value of bioactive substances in autoimmune illnesses like MS. A thorough comprehension develops over multiple phases of research, encompassing in vitro investigations, animal models, as well as human clinical studies, which investigate mechanisms of action, efficacy in therapy, as well as risk concerns.
In vitro studies
In vitro investigations are essential for clarifying the molecular processes via which bioactive substances influence immune regulation. Curcumin, a polyphenolic substance derived from C. longa, has demonstrated the ability to suppress the stimulation of NF-κB process, a key mediator of inflammation [56]. In cultivated human immune cells, curcumin markedly reduces the synthesis of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, essential contributors to MS pathogenesis. Moreover, curcumin augments the functionality of Tregs, which are essential for sustaining immunological tolerance and averting autoimmune assaults. Quercetin, a flavonoid found in fruits such as onions and apples, reduces histamine release from the mast cells and regulates T-cell multiplication [18]. In vitro studies indicate that quercetin can diminish the expression of MHC class II compounds on antigen-presenting cells, thereby attenuating the stimulation of autoreactive T lymphocytes [57]. These results highlight the immunomodulatory capacity of bioactive substances in modulating the activation of immune cells as well as inflammation at the level of cells.
Animal models of MS
The application of curcumin in experimental autoimmune encephalomyelitis (EAE) animals has demonstrated a reduction in clinical manifestations of MS, notably paralysis, through the modulation of innate and adaptive immune responses [58]. Curcumin particularly diminishes the proliferation of pro-inflammatory Th17 cells and Th1, crucial in developing MS-like disease. It also facilitates the proliferation of Tregs, so fostering immunological tolerance. Moreover, curcumin has been shown to alleviate oxidative stress by enhancing the activity of antioxidant enzymes, including catalase and superoxide dismutase, thereby preventing neuronal damage [58]. Resveratrol, a polyphenolic molecule in grapes and red wine, demonstrates potential in EAE models by mitigating demyelination and CNS inflammation. It suppresses the stimulation of astrocytes and microglia, two glial cells implicated in neurological inflammation, while concurrently safeguarding oligodendrocytes, the cells accountable for myelination. Furthermore, resveratrol enhances the functioning of the BBB, inhibiting the influx of periphery immune systems into the CNS [59]. The results from these animal models indicate that bioactive substances such as curcumin and resveratrol may influence disease development and safeguard the CNS in MS.
Human clinical trials and observational research
A randomized, double-blind, placebo-controlled research showed that curcumin administration decreased pro-inflammatory cytokine amounts in MS patients, enhancing physical function and quality of life [60]. A separate study indicated that omega-3 fatty acids, specifically EPA and DHA, decreased the rate of relapse and inflammatory indicators among people with relapsing-remitting MS. Such fatty acids alter the body’s reaction to an anti-inflammatory characteristic by diminishing pro-inflammatory cytokines and promoting resolving production. Polyphenols in veggies, fruits, and green tea are associated with reduced inflammation and decreased oxidative damage in MS patients [25, 54]. Flavonoids such as luteolin and quercetin have demonstrated encouraging outcomes in enhancing immune cell functionality and regulating the intestinal microbiota in patients with MS [45]. These findings indicate that bioactive substances can markedly influence immune function and inflammation in MS, diminishing inflammation, enhancing neuroprotection, and enhancing overall disease outcomes. Nonetheless, additional extensive and rigorously planned clinical trials are required to validate the effectiveness, safety, and lasting benefits of such medicines for patients with MS.
Synergistic Effects and Combination Therapies
There is growing interest in combination therapies that integrate bioactive molecules to augment traditional treatments and improve their efficacy (Table 2) [61].

Integration with traditional MS therapies
Bioactive substances demonstrate the potential to enhance the efficacy of traditional MS therapies by targeting immunological pathways and mitigating negative effects. Curcumin, an antioxidant and anti-inflammatory compound, has been investigated for its possible synergistic effects when administered alongside IFN-β, a primary treatment for relapsing-remitting MS [62]. Curcumin obstructs the NF-κB pathway and diminishes pro-inflammatory cytokines, potentially enhancing therapy efficacy and lowering relapse rates in MS patients. Likewise, resveratrol and glatiramer acetate (GA), a frequently utilized disease-modifying therapy, have demonstrated encouraging outcomes in mitigating neuroinflammation and safeguarding against oxidative stress in animal studies of MS [63]. Bioactive substances such as flavonoids and omega-3 fatty acids may augment the immune-regulating effects of DMTs by transitioning their immune system from a pro-inflammatory condition to a more equilibrated, anti-inflammatory character [25]. These chemicals may also mitigate adverse effects linked to traditional therapies, including gastrointestinal pain and fatigue, enhancing patient adherence and quality of life.
Gut microbiota modulation
Combination therapy for MS entails the regulation of gut microbiota, which is essential for immune function. Dysbiosis of gut microbiota has been associated with autoimmune disorders, including MS [64]. Bioactive chemicals such as polyphenols and prebiotics can enhance the proliferation of beneficial microbes while suppressing detrimental strains, modulating systemic immune reactions, and perhaps diminishing autoimmune reactivity. Resveratrol improves gut barrier integrity and modulates immune cell activity in gut-associated lymphoid tissue (GALT), enhancing immunological tolerance [65]. Integrating bioactive substances with probiotics can re-establish immunological homeostasis and mitigate inflammation in MS. This method addresses immunological dysregulation, mitigates inflammation, and safeguards the CNS from more harm. The combinatorial impacts of several medicines, including gut microbiota manipulation, immune cell stimulation, and oxidative stress, underscore the possibility of personalized, integrative treatment techniques in MS [66]. Nonetheless, additional research and clinical studies are requisite to ascertain the appropriate dosages, combinations, and lasting impacts of these therapies in treating MS.
Limitations, Challenges, and Future Directions
Bioactive compounds possess considerable potential for treating MS, although they encounter obstacles, including insufficient bioavailability, variability in individual responses, and the necessity for more rigorous clinical trials. Researchers are investigating approaches to improve the distribution and bioavailability of bioactive compounds, including nano-formulations, biocompatible polymers, and encapsulating techniques. Inter-individual heterogeneity in response patterns is challenging, as enzyme genetic differences influence absorption and efficacy. The gut microbiota additionally affects metabolic and immune responses to bioactive compounds. Characterization of the microbiome and personalized dietary strategies may facilitate the customization of bioactive chemical therapy for enhanced efficacy in MS patients. Future research must prioritize comprehensive, randomized, placebo-controlled trials, sophisticated drug delivery technologies, biomarker development, personalized medicine approaches, combination therapies, and long-term safety and toxicology studies.
Conclusion
Bioactive substances derived from plants, fungi, marine life, and microbes potentially modify immune responses in MS. These compounds, comprising polyphenols, alkaloids, flavonoids, and so on, have immunoregulatory, anti-inflammatory, and antioxidant properties. Preclinical investigations indicate that they may mitigate disease development, diminish inflammatory cytokines, and re-establish immunological equilibrium. Nevertheless, low bioavailability and a scarcity of extensive trials impede their broad implementation. Novel delivery systems and personalized treatment strategies may assist in surmounting these obstacles. Future investigations should concentrate on stringent clinical studies, mechanistic elucidations, and evaluations of long-term safety.
Ethical Considerations
Compliance with ethical guidelines
This review manuscript includes no original research involving human subjects or animals. Consequently, ethical approval, along with informed consent, was unnecessary. All sources and studies referenced in this review have been acknowledged to uphold academic honesty and prevent plagiarism. The writers have diligently endeavored to present the reviewed material with objectivity, accuracy, and balance, honoring the contributions and discoveries of the original authors.
This article is a review and does not include any experiments involving human participants or animals conducted by writers. Consequently, ethical approval and informed consent were not relevant. All data and information presented in this review have been sourced from previously published materials, which have been appropriately attributed. The authors assert adherence to all pertinent ethical publication norms during the manuscript’s development.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors contributed equally to the main manuscript preparation and data analysis and reviewed the manuscript.
Conflict of interest
The authors declared no conflict interests.
Acknowledgements
All authors thank the Saveetha Institute of Medical and Technical Sciences (SIMATS), Kuthambakkam, India for their constant support.
References
- Aliyu M, Zohora FT, Ceylan A, Hossain F, Yazdani R, Azizi G. Immunopathogenesis of multiple sclerosis: Molecular and cellular mechanisms and new immunotherapeutic approaches. Immunopharmacol Immunotoxicol. 2024; 46(3):355-77. [DOI:10.1080/08923973.2024.2330642] [PMID]
- Jain RW, Yong VW. B cells in central nervous system disease: Diversity, locations and pathophysiology. Nat Rev Immunol. 2022; 22(8):513-24. [DOI:10.1038/s41577-021-00652-6] [PMID]
- Jalkh G, Abi Nahed R, Macaron G, Rensel M. Safety of Newer Disease Modifying Therapies in Multiple Sclerosis. Vaccines (Basel). 2020; 9(1):12. [DOI:10.3390/vaccines9010012] [PMID]
- Kourakis S, Timpani CA, de Haan JB, Gueven N, Fischer D, Rybalka E. Dimethyl fumarate and its esters: A drug with broad clinical utility? Pharmaceuticals (Basel). 2020; 13(10):306. [DOI:10.3390/ph13100306] [PMID]
- Scavone C, Liguori V, Adungba OJ, Cesare DDG, Sullo MG, Andreone V, et al. Disease-modifying therapies and hematological disorders: A systematic review of case reports and case series. Front Neurol. 2024; 15:1386527. [DOI:10.3389/fneur.2024.1386527] [PMID]
- Suliman BA. Potential clinical implications of molecular mimicry-induced autoimmunity. Immun Inflamm Dis. 2024; 12(2):e1178. [DOI:10.1002/iid3.1178] [PMID]
- Schwartz M, Baruch K. Breaking peripheral immune tolerance to CNS antigens in neurodegenerative diseases: Boosting autoimmunity to fight-off chronic neuroinflammation. J Autoimmun. 2014; 54:8-14. [DOI:10.1016/j.jaut.2014.08.002] [PMID]
- Khan Z, Mehan S, Gupta GD, Narula AS. Immune system dysregulation in the progression of multiple sclerosis: Molecular insights and therapeutic implications. Neuroscience. 2024; 548:9-26. [DOI:10.1016/j.neuroscience.2024.04.004] [PMID]
- Palomares O, Martín-Fontecha M, Lauener R, Traidl-Hoffmann C, Cavkaytar O, Akdis M, et al. Regulatory T cells and immune regulation of allergic diseases: Roles of IL-10 and TGF-β. Genes Immun. 2014; 15(8):511-20. [DOI:10.1038/gene.2014.45] [PMID]
- Rauf A, Badoni H, Abu-Izneid T, Olatunde A, Rahman MM, Painuli S, et al. Neuroinflammatory Markers: Key indicators in the pathology of neurodegenerative diseases. Molecules. 2022; 27(10):3194. [DOI:10.3390/molecules27103194] [PMID]
- Haruna A, Yahaya SM. Recent advances in the chemistry of bioactive compounds from plants and soil microbes: A review. Chem Afr. 2021; 4(2):231-48. [Link]
- Batiha GE, Beshbishy AM, Ikram M, Mulla ZS, El-Hack MEA, Taha AE, et al. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods. 2020; 9(3):374. [DOI:10.3390/foods9030374] [PMID]
- Gupta A, Roy A, Roy A, Raja V, Sharma K, Verma R. Role of Medicinal Plants in the Management of Multiple Sclerosis. Curr Pharm Biotechnol. 2025; 26(5):665-79. [DOI:10.2174/0113892010324850240923181408] [PMID]
- Narayanan M. An evaluation of antibacterial, antioxidant, and biocompatibility (hemocompatibility) nature of green synthesized ZnONPs: An in-vitro approach. Dubai Med J. 2025; 1-1. [Link]
- Balasubramaniam M, Sapuan S, Hashim IF, Ismail NI, Yaakop AS, Kamaruzaman NA, et al. The properties and mechanism of action of plant immunomodulators in regulation of immune response - A narrative review focusing on Curcuma longa L., Panax ginseng C. A. Meyer and Moringa oleifera Lam. Heliyon. 2024; 10(7):e28261. [DOI:10.1016/j.heliyon.2024.e28261] [PMID]
- Sathishkumar K. From nature to medicine: Bioactive compounds in the battle against SARS-CoV-2. Nat Prod Res. 2024; 1-2. [DOI:10.1080/14786419.2024.2375754]
- Drzewiecka B, Wessely-Szponder J, Świeca M, Espinal P, Fusté E, Fernández-De La Cruz E. Bioactive peptides and other immunomodulators of mushroom origin. Biomedicines. 2024; 12(7):1483 [DOI:10.3390/biomedicines12071483] [PMID]
- Kiuru P, DʼAuria MV, Muller CD, Tammela P, Vuorela H, Yli-Kauhaluoma J. Exploring marine resources for bioactive compounds. Planta Med. 2014; 80(14):1234-46.[DOI:10.1055/s-0034-1383001] [PMID]
- Murugan R. Innovative polysaccharide-based hydrogels: A promising vehicle for bioactive compounds in oral cancer therapy. Nat Prod Res. 2024; 1-2. [DOI:10.1080/14786419.2024.2383269]
- Cecerska-Heryć E, Wiśniewska Z, Serwin N, Polikowska A, Goszka M, Engwert W, et al. Can compounds of natural origin be important in chemoprevention? Anticancer properties of quercetin, resveratrol, and curcumin-A comprehensive review. Int J Mol Sci. 2024; 25(8):4505. [DOI:10.3390/ijms25084505] [PMID]
- Sathishkumar K. Marine biotechnology in dermatology: Exploring bioactive compounds for skin health. Nat Prod Res. 2024; 1-2. [DOI:10.1080/14786419.2024.2383271]
- Bhol NK, Bhanjadeo MM, Singh AK, Dash UC, Ojha RR, Majhi S, et al. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed Pharmacother. 2024; 178:117177. [DOI:10.1016/j.biopha.2024.117177] [PMID]
- Makuch S, Więcek K, Woźniak M. The immunomodulatory and anti-inflammatory effect of curcumin on immune cell populations, cytokines, and in vivo models of rheumatoid arthritis. Pharmaceuticals (Basel). 2021; 14(4):309. [DOI:10.3390/ph14040309] [PMID]
- Wang Y, Smith W, Hao D, He B, Kong L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int Immunopharmacol. 2019; 70:459-66. [DOI:10.1016/j.intimp.2019.02.050] [PMID]
- Saleem U, Farrukh M, Saadullah M, Siddique R, Gul H, Ahmad A, et al. Role of polyphenolics in the management of rheumatoid arthritis through intracellular signaling pathways: A mechanistic review. Inflammopharmacology. 2025; 33(5):2263-75. [DOI:10.1007/s10787-025-01731-z] [PMID]
- Moody R, Wilson K, Jaworowski A, Plebanski M. Natural compounds with potential to modulate cancer therapies and self-reactive immune cells. Cancers. 2020; 12(3):673. [DOI:10.3390/cancers12030673] [PMID]
- La Rosa G, Lonardo MS, Cacciapuoti N, Muscariello E, Guida B, Faraonio R, et al. Dietary polyphenols, microbiome, and multiple sclerosis: From molecular anti-inflammatory and neuroprotective mechanisms to clinical evidence. Int J Mol Sci. 2023; 24(8):7247. [DOI:10.3390/ijms24087247] [PMID]
- Parveen B, Narayanan M, Rajinikanth V. Empowering patient self-care in plantar hyperkeratotic/palmoplantar keratodermas eczema: A case report. Dubai Med J. 2025; 8(1):42-7.[Link]
- Mazumdar S, Ghosh AK, Purohit S, Das AK, Bhattacharyya A, Karmakar P. Immunomodulatory activity of ethanol extract of Annona reticulata L. leaf in cultured immune cells and in Swiss albino mice. J Ayurveda Integr Med. 2022; 13(2):100554. [DOI:10.1016/j.jaim.2022.100554] [PMID]
- Hooda P, Malik R, Bhatia S, Al-Harrasi A, Najmi A, Zoghebi K, et al. Phytoimmunomodulators: A review of natural modulators for complex immune system. Heliyon. 2023; 10(1):e23790. [DOI:10.1016/j.heliyon.2023.e23790] [PMID]
- Razavi BM, Ghasemzadeh Rahbardar M, Hosseinzadeh H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytother Res. 2021; 35(12):6489-513. [DOI:10.1002/ptr.7224] [PMID]
- Sameena VP, Thoppil JE. Assessment of phytometabolite distribution, in vitro antioxidant and anti-inflammatory potential of novel plant, Euphorbia deccanensis—endemic to South India. Vegetos. 2024; 37(4):1513-25. [Link]
- Tiwari R, Latheef SK, Ahmed I, Iqbal HMN, Bule MH, Dhama K, et al. Herbal Immunomodulators - A remedial panacea for designing and developing effective drugs and medicines: Current scenario and future prospects. Curr Drug Metab. 2018; 19(3):264-301. [DOI:10.2174/1389200219666180129125436] [PMID]
- Rudrapal M, Khairnar SJ, Khan J, Dukhyil AB, Ansari MA, Alomary MN, et al. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front Pharmacol. 2022; 13:806470. [DOI:10.3389/fphar.2022.806470] [PMID]
- Jyotirmayee B, Mahalik G. A review on selected pharmacological activities of Curcuma longa L. Int J Food Prop. 2022; 25(1):1377-98. [Link]
- Moudgil KD, Venkatesha SH. The anti-inflammatory and immunomodulatory activities of natural products to control autoimmune inflammation. Int J Mol Sci. 2022; 24(1):95. [DOI:10.3390/ijms24010095] [PMID]
- Alesci A, Nicosia N, Fumia A, Giorgianni F, Santini A, Cicero N. Resveratrol and Immune Cells: A link to improve human health. Molecules. 2022; 27(2):424. [DOI:10.3390/molecules27020424] [PMID]
- Garcia G, Pais TF, Pinto P, Dobson G, McDougall GJ, Stewart D, et al. Bioaccessible raspberry extracts enriched in ellagitannins and ellagic acid derivatives have anti-neuroinflammatory properties. Antioxidants (Basel). 2020; 9(10):970. [DOI:10.3390/antiox9100970] [PMID]
- Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A. Immune modulation by curcumin: The role of interleukin-10. Crit Rev Food Sci Nutr. 2019; 59(1):89-101. [DOI:10.1080/10408398.2017.1358139] [PMID]
- Rakha A, Umar N, Rabail R, Butt MS, Kieliszek M, Hassoun A, et al. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed Pharmacother. 2022; 156:113945. [DOI:10.1016/j.biopha.2022.113945] [PMID]
- Huang L, Kim MY, Cho JY. Immunopharmacological Activities of Luteolin in Chronic Diseases. Int J Mol Sci. 2023; 24(3):2136. [DOI:10.3390/ijms24032136] [PMID]
- Populin L, Stebbing MJ, Furness JB. Neuronal regulation of the gut immune system and neuromodulation for treating inflammatory bowel disease. FASEB Bioadv. 2021; 3(11):953-66. [DOI:10.1096/fba.2021-00070] [PMID]
- Alrumaihi F, Almatroodi SA, Alharbi HOA, Alwanian WM, Alharbi FA, Almatroudi A, et al. Pharmacological potential of kaempferol, a flavonoid in the management of pathogenesis via modulation of inflammation and other biological activities. Molecules. 2024; 29(9):2007. [DOI:10.3390/molecules29092007] [PMID]
- Thawabteh A, Juma S, Bader M, Karaman D, Scrano L, Bufo SA, et al. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins (Basel). 2019; 11(11):656. [DOI:10.3390/toxins11110656] [PMID]
- Eisenstein TK. The role of opioid receptors in immune system function. Front Immunol. 2019; 10:2904. [DOI:10.3389/fimmu.2019.02904] [PMID]
- Uzor PF. Alkaloids from Plants with Antimalarial Activity: A review of recent studies. Evid Based Complement Alternat Med. 2020; 2020:8749083. [DOI:10.1155/2020/8749083] [PMID]
- Liles NW, Page EE, Liles AL, Vesely SK, Raskob GE, George JN. Diversity and severity of adverse reactions to quinine: A systematic review. Am J Hematol. 2016; 91(5):461-6. [DOI:10.1002/ajh.24314] [PMID]
- Qin Z, Tang R, Liang J, Jia X. Berberine, a natural alkaloid: Advances in its pharmacological effects and mechanisms in the treatment of autoimmune diseases. Int Immunopharmacol. 2024; 137:112422. [DOI:10.1016/j.intimp.2024.112422] [PMID]
- Gozari M, Alborz M, El-Seedi HR, Jassbi AR. Chemistry, biosynthesis and biological activity of terpenoids and meroterpenoids in bacteria and fungi isolated from different marine habitats. Eur J Med Chem. 2021; 210:112957. [DOI:10.1016/j.ejmech.2020.112957] [PMID]
- Ağören BK, Akkol EK. Secondary metabolites of turmeric extract and essential oils. In: Rai M, Feitosa CM, editors. Curcumin and neurodegenerative diseases. Singapore: Springer; 2023. [DOI:10.1007/978-981-99-7731-4_5]
- Yang S, Liu J, Jiao J, Jiao L. Ar-Turmerone Exerts Anti-proliferative and Anti-inflammatory Activities in HaCaT Keratinocytes by Inactivating Hedgehog Pathway. Inflammation. 2020; 43(2):478-86. [DOI:10.1007/s10753-019-01131-w] [PMID]
- Ratan ZA, Youn SH, Kwak YS, Han CK, Haidere MF, Kim JK, et al. Adaptogenic effects of Panax ginseng on modulation of immune functions. J Ginseng Res. 2021; 45(1):32-40. [DOI:10.1016/j.jgr.2020.09.004] [PMID]
- Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018; 132:41-48. [DOI:10.1016/j.plefa.2018.03.004] [PMID]
- Saini RK, Prasad P, Sreedhar RV, Akhilender Naidu K, Shang X, Keum YS. Omega-3 Polyunsaturated Fatty Acids (PUFAs): Emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits-A review. Antioxidants (Basel). 2021; 10(10):1627. [DOI:10.3390/antiox10101627] [PMID]
- Balić A, Vlašić D, Žužul K, Marinović B, Bukvić Mokos Z. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. 2020; 21(3):741. [DOI:10.3390/ijms21030741] [PMID]
- Memarzia A, Khazdair MR, Behrouz S, Gholamnezhad Z, Jafarnezhad M, Saadat S, et al. Experimental and clinical reports on anti-inflammatory, antioxidant, and immunomodulatory effects of Curcuma longa and curcumin, an updated and comprehensive review. Biofactors. 2021; 47(3):311-50. [DOI:10.1002/biof.1716] [PMID]
- Han L, Fu Q, Deng C, Luo L, Xiang T, Zhao H. Immunomodulatory potential of flavonoids for the treatment of autoimmune diseases and tumour. Scand J Immunol. 2022; 95(1):e13106. [DOI:10.1111/sji.13106]
- Bjelobaba I, Begovic-Kupresanin V, Pekovic S, Lavrnja I. Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. J Neurosci Res. 2018; 96(6):1021-42. [DOI:10.1002/jnr.24224] [PMID]
- Komorowska J, Wątroba M, Bednarzak M, Grabowska AD, Szukiewicz D. Anti-inflammatory action of resveratrol in the central nervous system in relation to glucose concentration-an in vitro study on a blood-brain barrier model. Int J Mol Sci. 2024; 25(6):3110. [DOI:10.3390/ijms25063110] [PMID]
- Petracca M, Quarantelli M, Moccia M, Vacca G, Satelliti B, D'Ambrosio G, et al. ProspeCtive study to evaluate efficacy, safety and tOlerability of dietary supplemeNT of Curcumin (BCM95) in subjects with Active relapsing MultIple Sclerosis treated with subcutaNeous Interferon beta 1a 44 mcg TIW (CONTAIN): A randomized, controlled trial. Mult Scler Relat Disord. 2021; 56:103274. [DOI:10.1016/j.msard.2021.103274] [PMID]
- Abe M, Abe H. Lifestyle medicine–an evidence based approach to nutrition, sleep, physical activity, and stress management on health and chronic illness. Per Med Universe. 2019; 8:3-9. [DOI:10.1016/j.pmu.2019.05.002]
- Miller ED, Dziedzic A, Saluk-Bijak J, Bijak M. A review of various antioxidant compounds and their potential utility as complementary therapy in multiple sclerosis. Nutrients. 2019; 11(7):1528. [DOI:10.3390/nu11071528] [PMID]
- Theodosis-Nobelos P, Rekka EA. The multiple sclerosis modulatory potential of natural multi-targeting antioxidants. Molecules. 2022; 27(23):8402. [DOI:10.3390/molecules27238402] [PMID]
- Correale J, Hohlfeld R, Baranzini SE. The role of the gut microbiota in multiple sclerosis. Nat Rev Neurol. 2022; 18(9):544-58. [DOI:10.1038/s41582-022-00697-8] [PMID]
- Drabińska N, Jarocka-Cyrta E. Crosstalk between resveratrol and gut barrier: A review. Int J Mol Sci. 2022 3; 23(23):15279. [DOI:10.3390/ijms232315279] [PMID]
- Calvo-Barreiro L, Eixarch H, Montalban X, Espejo C. Combined therapies to treat complex diseases: The role of the gut microbiota in multiple sclerosis. Autoimmun Rev. 2018; 17(2):165174. [DOI:10.1016/j.autrev.2017.11.019] [PMID]
- Tancreda G, Ravera S, Panfoli I. Exploring the therapeutic potential: Bioactive molecules and dietary interventions in multiple sclerosis management. Curr Issues Mol Biol. 2024; 46(6):5595-613. [DOI:10.3390/cimb46060335] [PMID]
Type of Study: Review |
Subject:
General
Received: 2025/03/18 | Accepted: 2025/06/2 | Published: 2025/07/1
Received: 2025/03/18 | Accepted: 2025/06/2 | Published: 2025/07/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






