Sun, Feb 22, 2026
Volume 11, Issue 4 (Autumn 2025)
Caspian J Neurol Sci 2025, 11(4): 306-316 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
R Prabhu R. Induction of αCaMKII Expression by KCl in Cultured Primary Cerebellar Granule Neurons. Caspian J Neurol Sci 2025; 11 (4) :306-316
URL: http://cjns.gums.ac.ir/article-1-787-en.html
URL: http://cjns.gums.ac.ir/article-1-787-en.html
Department of Biotechnology, Government Arts College, Thiruvananthapuram, India. & Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, India. , ramyarprabhu@gmail.com
Keywords: Cerebellum, Cultured cells, CaMKinase II alpha (αCaMKII), Depolarisation, Potassium chloride
Full-Text [PDF 2838 kb]
(353 Downloads)
| Abstract (HTML) (455 Views)
Full-Text: (152 Views)
Introduction
The cerebellum is the seat of motor learning and memory in the brain [1]. It mainly consists of two major cell types: Cerebellar granule (CG) cells and Purkinje cells, both of which are excitatory in nature. The CG cells constitute the most abundant cell type in the mammalian brain. These cells receive innervation from deeper parts of the cerebellum through mossy fibres, and their output is modulated through parallel-fibre Purkinje neurons [2]. The projections from Purkinje cells are the sole output from the cerebellum.
Calcium/calmodulin-dependent protein kinase type II (CaMKII) is important for inducing and maintaining synaptic plasticity in the hippocampus and is known as the ‘memory molecule’ [3]. Calcium entry through the N-methyl-D-aspartate receptors (NMDARs) activates CaMKII [4]. CaMKII comprises a family of isoforms derived from four closely related yet distinct genes designated as α, β, γ, and δ. The predominant isoforms of CaMKII are α and β, which are found in the ratio of 3:1 in the forebrain and 1:4 in the cerebellum, respectively [5]. In the cerebellum, the promoter for the α-isoform of CaMKII uses an alternate transcription initiation site that is weaker compared to the site that is functional in the hippocampus [6]. However, the specific cell type in the cerebellum in which the alternate transcriptional control operates has not been demonstrated. The expression of αCaMKII is confined solely to Purkinje cells in the cerebellum, as observed by immunohistochemical analysis using an antibody specific to αCaMKII [7-9]. The mRNA encoding αCaMKII is also enriched in the Purkinje layer, as seen by in situ hybridisation [10, 11]. αCaMKII plays a role in long-term depression in Purkinje neurons [12]. The CG cell layer is found to be completely devoid of αCaMKII [9]. Instead, the CG cells express βCaMKII, which differs in its biochemical properties compared to αCaMKII, such as increased sensitivity to calcium [13, 14] and the ability to bind to the actin cytoskeleton [15]. The absence of αCaMKII in CG cells suggests that the calcium signalling pathways in these cells may be unique.
To investigate the mechanisms of calcium signaling in CG cells, we utilized primary cultures of CG cells. Primary CG cells have been employed as model systems for studying neural activity and Ca²-dependent gene expression [16]. CG cells can be significantly enriched in culture and possess characteristics that resemble those of granule cells in the cerebellum [17]. CG cells are grown in medium containing high KCl to induce depolarization, which mimics stimulation in vivo by the mossy fibers that innervate them. This treatment enhances long-term survival of CG cells in vitro [17-19]. Depolarising conditions facilitate Ca2+ entry into these cells via voltage-gated calcium channels [17]. However, some studies have shown that CGs grown in high concentrations of KCl exhibit an aberrant phenotype that differs from that observed in vivo [20].
Our study aimed to develop a primary culture of CG cells for use as a model to study the interaction of transfected αCaMKII with neuronal receptors upon calcium signaling. However, we observed an aberrant expression of αCaMKII in the primary CG culture, despite it being known to be absent in vivo. Previous studies had not reported this anomaly, which intrigued us to investigate the factors behind the induction of αCaMKII expression.
Materials and Methods
Materials
Normal goat serum was from Santacruz, USA. Paraformaldehyde, Triton-X-100, and Tween-20 were from SRL chemicals, India. DAPI, cytosine arabinoside (Ara C), DNase 1, Poly-D-lysine, Laminin, dNTPs, Taq polymerase, RIPA buffer, and monoclonal antibody against β-actin (A-5441) were from Sigma Chemicals, USA. Fluoromount G and optimal cutting temperature (OCT) compound were from Electron Microscopic Sciences. NBM, Trypsin, Glutamax, and Antibiotic/antimycotic solution were from Invitrogen/GIBCO. Monoclonal antibodies against α (MA1-048) and β subunits (PA1-41150) of CaMKII were from Thermo Scientific, USA. Monoclonal antibody against GluN2B subunit of NMDA receptor (GluN2B) (ab93610) was from Abcam, UK. Polyclonal antibody for GABA-A receptor α6 subunit (α6GABA) (AB-5453) was from Millipore, USA. Secondary antibodies with alkaline phosphatase (AP) conjugates, as well as horse radish peroxidase (HRP) conjugates, were from Sigma Chemicals, USA. The secondary antibodies with fluorescence conjugates were from either Sigma or Jackson Immunoresearch, USA. The kits used included the RNaseasy kit from Qiagen, USA (74104), the DNase digestion kit from Ambion, USA (18068015), and the cDNA synthesis kit, Superscript III from Invitrogen (11304011).
Methods
Immunohistochemistry
All animal experiments were approved by the Institutional Animal Ethics Committee. Rat pups of the required age, postnatal day 7 (P7), were provided by the Animal Research Facility of the Rajiv Gandhi Centre for Biotechnology (RGCB), in accordance with the requirements of the approved experiments. All procedures performed in studies involving animals adhered to the ethical standards of the institution or practice at which the studies were conducted. The cerebella were dissected from P7 pups and immediately immersed in the fixative (4% PFA in 1X PBS, pH 7.4) overnight at 4 °C. They were then cryoprotected in 30% sucrose in 1X PBS overnight at 4 °C for two days. The brains were subsequently embedded in OCT compound. Blocks were created by slowly immersing the brain in liquid nitrogen, and the frozen blocks were stored at -70 °C. Brain samples were sectioned sagitally at a thickness of 14 µM using a cryostat (Leica 3050S). Sections were processed for immunohistochemical to assess the expression of αCaMKII.
Antigen retrieval using citrate buffer was performed to expose any antigen-binding sites that might be inaccessible to the antibody. The sections were subjected to high temperatures of about 90-100 °C in citrate buffer (10 mM citric acid, 0.05% Tween-20, pH 6.0) for about 10-15 minutes. The sections were then washed in 1X PBS three times for about 5 minutes each. The sections were then permeabilized and blocked with 5% normal goat serum (Chemicon) containing 0.2% Triton-X-100 in PBS for 1 h at room temperature, followed by incubation with the primary antibodies in the same solution at 4 °C overnight. The sections were again subjected to washes with 1X PBS three times for 5 minutes each and were incubated with the corresponding secondary antibody conjugated to a fluorophore at room temperature for about 90 minutes. The sections were again washed in 1X PBS three times for 5 minutes each. Between the first and second washes, the sections were stained with the nuclear stain DAPI (1:50,000) by incubating at room temperature. Finally, the sections were mounted with Fluromount G.
Preparation of primary CG neurons
The primary CG neuronal culture was prepared from P7 Sprague Dawley rat pups. The pups were wiped with 70% alcohol and decapitated. The cerebellum was carefully dissected out. After removing the meninges, the cerebellum was minced into pieces. All dissections were done in sterile ice-cold PBS. The cerebellum was then trypsinized in 0.05% trypsin at 37 oC for about 12 minutes. The tissue was centrifuged at 1000 rpm for about one minute to settle. The trypsin was aspirated out gently, and the tissue was washed with PBS. About 20 µL of DNase (4000 U) was added to about 2 mL of plain neurobasal medium (NBM) and mixed with the tissue. To disintegrate the tissue and obtain isolated cells, it was subjected to mechanical stress by pipetting approximately 20-50 times. This supernatant was gently harvested, PBS was added, and it was again centrifuged at about 1000 rpm for 5 minutes. The resulting pellet contained the cells. Reconstituted NBM [containing 0.5% B27 supplement, 20 mM added KCl (5 or 10 mM KCl in certain experiments), 1X Glutamax, 33 mM glucose, and 1X Antibiotic/Antimycotic] was added to the cells. Since NBM already contains 5 mM KCl, the total concentration of KCl becomes 25 mM when 20 mM KCl is added. The cells were seeded onto plates pre-coated with poly-D-lysine and laminin. After 24 hours, the medium was removed, and fresh reconstituted NBM was added. In some cases, after 48 hours, the cells were treated with 10 µM Ara C to prevent the proliferation of glial cells. The cells were maintained in vitro for up to 24 days. The medium was regularly changed, and Ara C treatment was administered intermittently to prevent glial proliferation.
The cells were harvested using 1X PBS on fixed days, specifically DIV 0, 2, 4, 6, 8, 10, 12, 15, 19, and 24. The cells were either lysed using RIPA buffer for Western blot analysis or total RNA was isolated from the cells using RNAeasy kit, in preparation for cDNA synthesis. Various genes were amplified using suitable primers through reverse transcriptase polymerase chain reaction (RT-PCR). The cells were also subjected to immunocytochemical analysis to assess the expression of proteins of interest.
Transcriptomic analysis
Total RNA was isolated from primary CG cells using the Qiagen RNAeasy kit according to the manufacturer’s protocol. DNA contamination in RNA preparation mentioned above was removed by DNase digestion using the DNase digestion kit. Reverse transcription was carried out on the RNA preparation to obtain cDNA. The cDNA was subjected to amplification for the gene of interest using the corresponding primers and Taq polymerase.
Western blot analysis
The cells were harvested on different days and were lysed in RIPA buffer. Protein estimation was done using the bicinchonic acid (BCA) method [21]. The lysate was subjected to SDS-PAGE followed by Western blotting to analyze the expression of various proteins of interest. The Western blots were developed using either alkaline phosphatase-conjugated secondary antibodies or by chemiluminescence with HRP-conjugated secondary antibodies.
Quantitation of the Western blots was carried out by densitometric analysis of the images using Quantity One software, version 4.6.3 from Bio-Rad.
Immunocytochemical analysis
Cells seeded on 12 mm diameter coverslips in 24-well plates were fixed with 4% paraformaldehyde after the respective treatments. The cells were washed three times with PBS for 10 min each. Permeabilization and blocking of non-specific binding were carried out by treating the cells with 3% BSA and 0.2% Triton X-100 in PBS. The cells were then incubated in the primary antibody diluted in the same buffer overnight at 4 °C. Subsequently, the cells were washed three times with PBS for 10 min each. The secondary antibody conjugate was then added in the same buffer and incubated for 1 h at room temperature. The cells were washed 3 times with PBS, for 10 min each. Coverslips were mounted using Fluromount G onto glass slides. DPX mountant was used to seal the coverslips. Images were captured using either an epifluorescence microscope (Leica DMI 4000B inverted microscope with Leica DFC 350 FX model CCD camera and Leica Application Suite software, version 2.8.1) or a confocal fluorescence microscope (Leica Confocal Microscope, TCS SP2 AOBS, with LCS lite imaging software or Nikon Eclipse Ti A1R microscope with NIS Elements software, version 4.0).
Effect of KCl on primary CG cells
The CG cells were grown in reconstituted NBM containing different concentrations of KCl, specifically 10 mM, 15 mM, and 25 mM (with 5 mM KCl present in NBM and an additional 5, 10, and 20 mM of KCl, respectively), starting from DIV 0. These cells were monitored regularly to ensure they remained viable and healthy at the different KCl concentrations. The cells were either harvested for Western blot analysis or fixed with 4% paraformaldehyde for immunocytochemistry on different days to study the expression of αCaMKII.
Results
Immunohistochemical analysis of the rat cerebellum to study the expression of αCaMKII
The cerebellum obtained from P7 rat pups was analyzed for the presence of αCaMKII in vivo through immunohistochemical analysis. The expression of αCaMKII was confined only to the Purkinje layer and was not present in either the external granular layer or the internal granular layer (Figure 1).
confirming the previous findings [9] that αCaMKII is not expressed in the cerebellar granular layer and is restricted to the Purkinje layer.
Characterization of primary CG neuron preparation
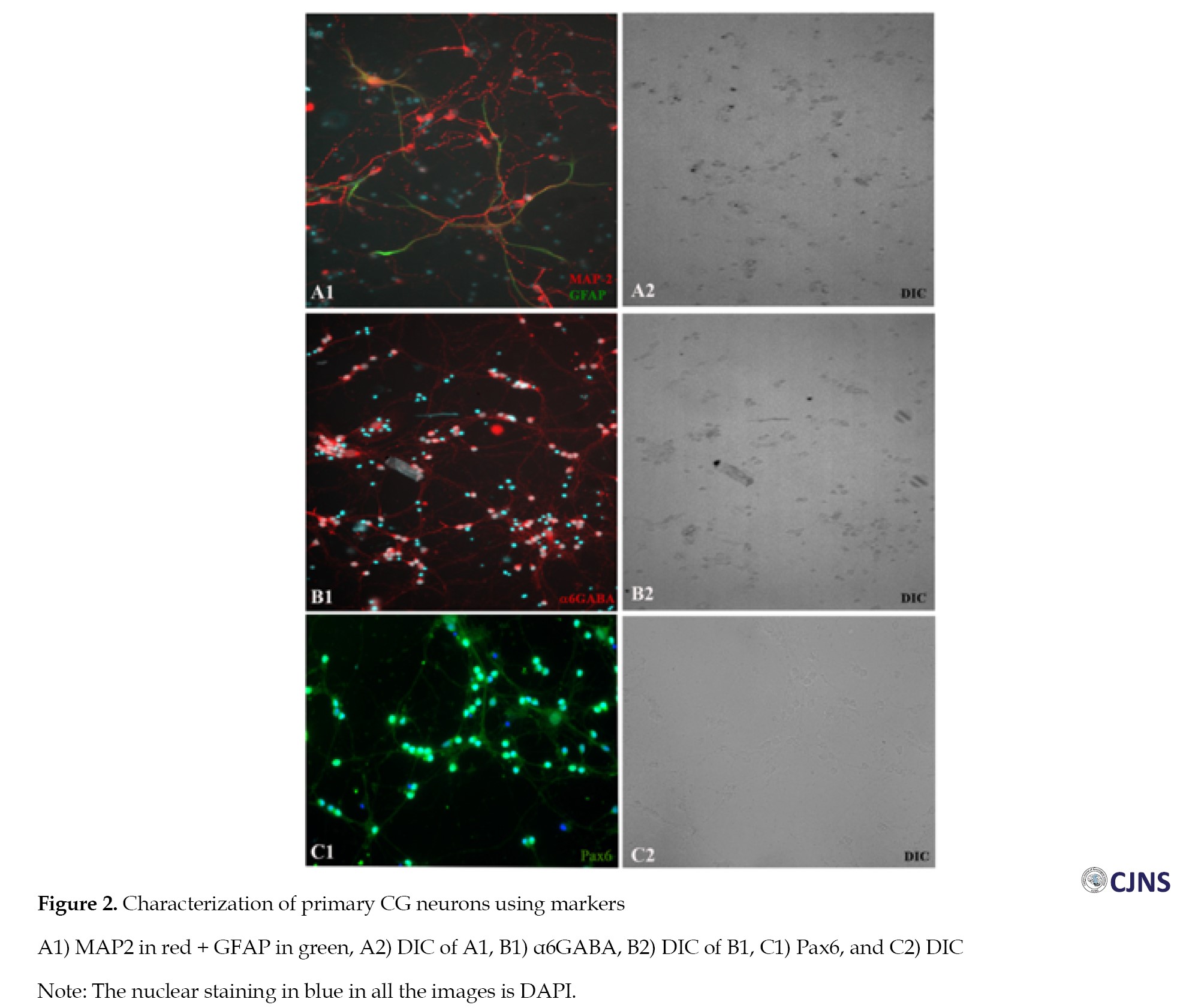
The primary CG neurons prepared from P7 pups were characterised by immunostaining for the presence of various markers. Most of the cells were positive for the neuronal marker microtubule-associated protein (MAP-2), whereas a few cells were stained for glial fibrillary acidic protein (GFAP), showing the presence of astrocytes. The cells were positive for α6GABA, the marker for CG cells [22], confirming that most of the cells in the preparation consisted solely of CG cells (Figure 2).
The cells were also immunostained for another well-known marker for CG cells, namely Pax6 [23].
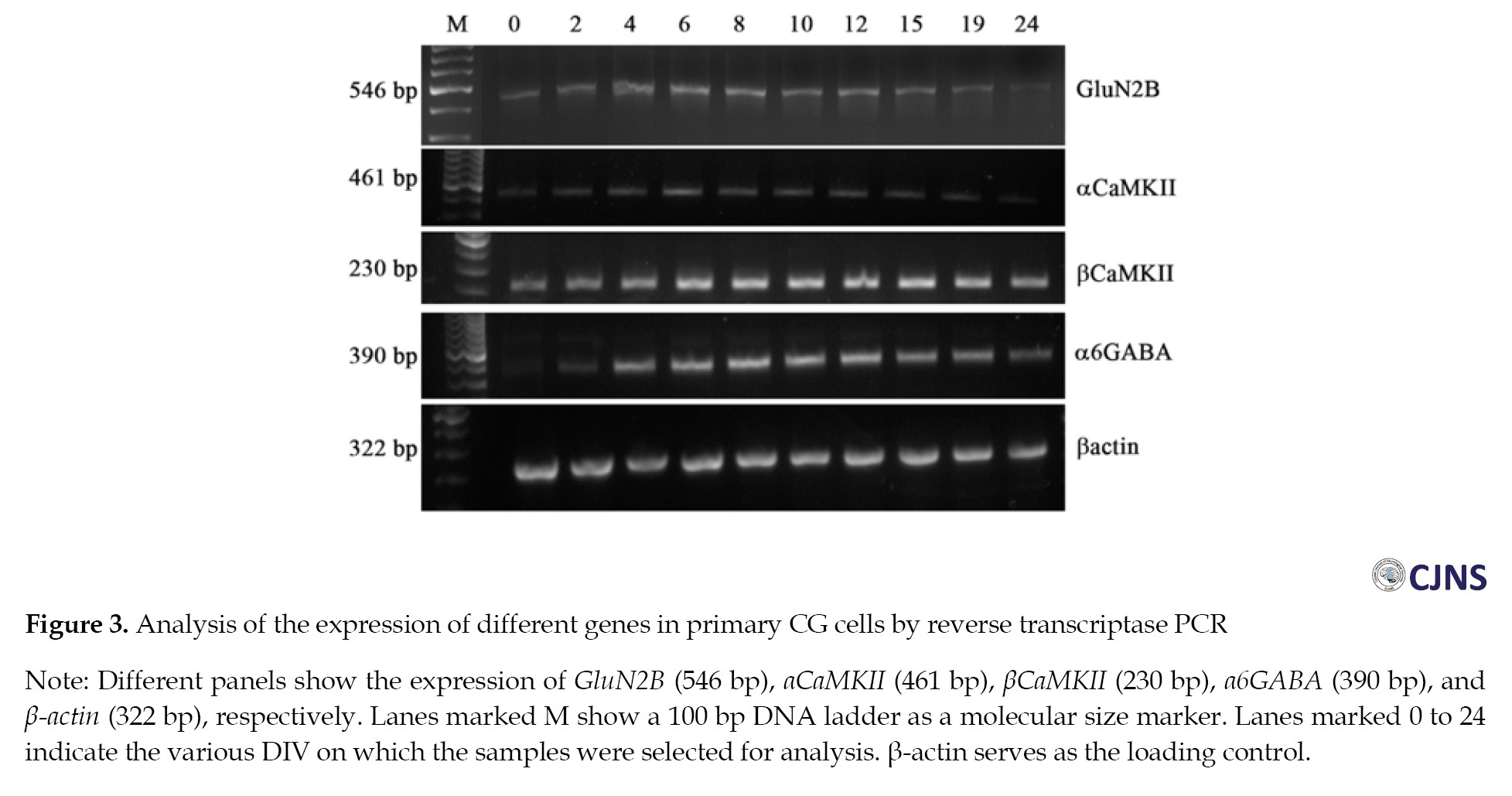
RT-PCR analyses for the transcripts of GluN2B, αCaMKII, βCaMKII, α6GABA, and β-actin were conducted in primary CG cells that were harvested on DIV 2, 4, 6, 8, 10, 12, 15, 19, and 24. Expression of these genes was detected on almost all the days investigated. The marker gene α6GABA showed a reduced expression on DIV 0 and 2 but gradually increased till DIV 10 to 12 as the culture matured. βCaMKII was present on all the days, showing a more or less uniform expression pattern. The receptor GluN2B was detected on all the days studied but exhibited reduced expression at early and much later time points, specifically DIV 0, 2, and DIV 19, 24, respectively. This is consistent with earlier studies indicating that in CG cells, the GluN2B subunit is completely downregulated and replaced by GluN2A and GluN2C by DIV 21 [24, 25]. Surprisingly, αCaMKII was also found to be present at low levels on all the days, with the expression further decreasing at later time points, similar to the pattern observed for GluN2B (Figure 3).
The primary CG cells were subjected to cell lysis, followed by Western blotting on DIV 2, 4, 6, 8, 10, 12, 15, 19, and 24 (Figure 4A).
βCaMKII showed a gradual increase in expression starting from DIV 2 to DIV 24. GluN2B was present on all the days analyzed, but its expression decreased towards DIV 19 and DIV 24. Consistent with the transcriptomic data, the presence of αCaMKII was also detected on all the days studied, except for DIV 0, where there was no detectable expression (Figure 4B).
KCl induces the expression of αCaMKII in CG cells
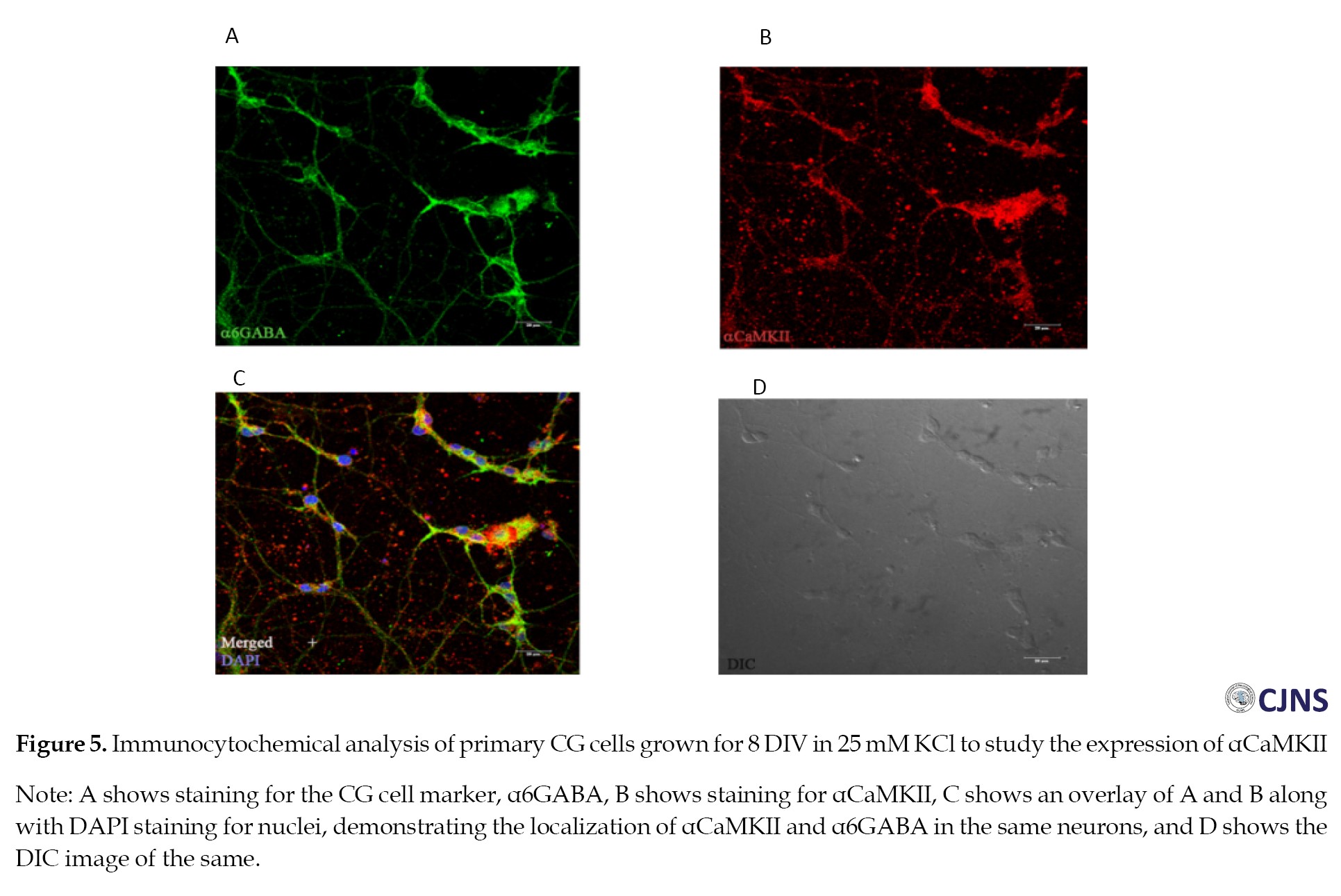
The expression of αCaMKII in primary CG cells was contrary to expectations. The colocalization of αCaMKII with α6GABA shows that the expression of αCaMKII is indeed present in CG cells (Figure 5).
We investigated the factor that could be responsible for inducing αCaMKII expression in primary CG cells. One of the prominent components added to the culture medium is KCl; hence, we tested whether KCl is responsible for inducing αCaMKII expression. The primary CG cells were grown in different concentrations of KCl to study its effect on the expression of αCaMKII. It was found that the cells grown in 25 mM KCl exhibited αCaMKII expression, as detected by Western blotting (Figure 6) and immunocytochemistry (Figure 7). There was no detectable expression or very faint expression of αCaMKII in cells grown in 10 mM KCl and 15 mM KCl on DIV 4 (Figures 6 and 7), and on DIV 8 (Figures 6 and 7). However, the effect was pronounced when cells were treated with 25 mM KCl on both DIV 4 and 8, with increased expression observed on DIV 8. This indicates that KCl is responsible for inducing the expression of αCaMKII in cultured CG cells.
We also tested the effect of KCl by altering its concentration in the medium. When cells grown in 10 mM KCl for 2 days were switched to a medium containing 25 mM KCl, there was a clear increase in αCaMKII expression compared to cells maintained in 10 mM KCl, further demonstrating that KCl induces αCaMKII expression in a concentration-dependent manner.
Discussion
αCaMKII is an essential molecule involved in calcium signal transduction in excitatory synapses involved in learning and memory. However, CG cells, which are primarily excitatory, are found to be devoid of αCaMKII in vivo [9]. The promoter for αCaMKII in the cerebellum is weak, resulting in negligible transcription [6]. Although some of the functions supported by αCaMKII could be carried out by βCaMKII, which is expressed in CG cells, it is still intriguing how CG cells compensate for the absence of αCaMKII, given that there are key differences in the biochemical properties between βCaMKII and αCaMKII, particularly regarding affinity for calmodulin, actin binding, etc. [13]. To understand the molecular mechanisms associated with calcium signaling in CG cells, we established a primary culture of CG cells using standard procedures adapted from previous reports [26, 27]. The preparation mostly consisted of CG cells, as they were immunostained positively for α6GABA and Pax6 [22]. In the cultured CG cells, the expression of the GluN2B subunit (the receptor to which CaMKII is known to bind) was consistent with in vivo studies [24, 25].
Surprisingly, we found that the primary culture of CG cells showed the presence of αCaMKII, as demonstrated by mRNA expression analysis, Western blotting, and immunocytochemical analysis. Although mRNA of αCaMKII was present in CG cells at very low levels on DIV 0 (Figure 3), Western blot analysis showed that αCaMKII protein level could not always be detected (Figures 4B and 6), which is consistent with data from immunohistochemical analysis of rat cerebellum (Figure 1). This suggests that the expression is induced by some factor, which could possibly be a component of the medium used to grow and maintain the cells. Previous research shows that KCl, a major component of the medium used for the maintenance of CG cells, can induce the expression of certain genes in CG cells [20]. According to this study, various genes are upregulated and downregulated due to the chronic depolarization occurring in CGs maintained in 25 mM KCl compared to those in 5 mM KCl, as shown by gene expression analysis. Some of the upregulated mRNAs include those for channels, like Itpr1, neurotensin receptor 2, voltage-gated K+ channels, ligands, like BDNF, and enzymes, like Cyt c oxidase. It is also known that different durations of KCl-induced depolarization lead to the activation of different signaling pathways based on the concentration of Ca2+ entry, resulting in differential transcriptional activity and gene expression [28]. Our study also showed a similar effect, namely the induction and expression of αCaMKII, which is not typically expressed in vivo.
In our experiments, αCaMKII is expressed when the cells are maintained in elevated concentrations of KCl, specifically 25 mM. Lower concentrations of KCl, such as 10 mM and 15 mM, did not induce significant expression of αCaMKII. However, these conditions do not support long-term survival of CG cells [17-19]. Depolarization induces the opening of VGCCs, facilitating the entry of calcium, which promotes cell survival [17]. Although depolarization induced by KCl and subsequent Ca2+ influx is mediated through different channels, such as NMDAR and AMPAR, certain studies have clearly shown that the major mediators of calcium influx are L-type VGCCs [29, 30]. This influx also induces specific activity-dependent changes in neurons in response to downstream signaling of Ca2+ influx. It is also possible that in our study, KCl could mediate membrane depolarization, which subsequently activated VGCCs, resulting in the induction of αCaMKII.
The study suggests that the mechanisms by which αCaMKII expression is prevented in CG cells in vivo are likely reversed when CG cells are in culture due to the depolarization caused by KCl. This observation is supported by studies showing that retinoic acid stimulates αCaMKII gene expression in PC12 cells, which have a distinct transcription initiation site and are similar to that present in cerebellar tissue. This is different from the αCaMKII transcription initiation site in the hippocampus and accounts for the difference in the level of expression of αCaMKII between these tissues [6]. It is possible that the KCl-induced expression of αCaMKII in CG cells depends on this difference in the transcription apparatus. Understanding the mechanisms of αCaMKII induction in the CG primary culture system may help elucidate the mechanisms by which expression is suppressed in vivo. Recent studies have investigated the action of scorpion toxin Bmk NT1, which induces neurotoxicity via PKC/CaMKII-dependent ERK1/2 signaling in primary CG cells [31]. Although these studies have not addressed whether the pathway is mediated specifically by αCaMKII or βCaMKII signaling, it would be interesting to determine which of the kinases are involved.
Conclusion
The study shows the expression of αCaMKII in the CG primary culture cells, although the same is not present in vivo in the animal. The induction and expression of αCaMKII are attributed to the higher concentrations of KCl used to maintain the cells in vitro. Previous studies have shown that KCl can lead to aberrant expression of genes in primary cells cultured in vitro. This study reports for the first time the concentration-dependent induction of αCaMKII expression in CG primary cells, despite its absence in vivo. Although primary cultures of CG cells have been used as an in vitro model, culture conditions that induce alterations in the expression of key proteins, such as αCaMKII, could lead to significant changes in the signaling mechanisms within these cells. Therefore, primary cultures of CG cells could serve as a model to delineate the mechanisms involved in inducing the expression of αCaMKII, which would help in understanding the tight regulation that suppresses the expression of this enzyme in vivo. While this emphasizes the need for caution in extrapolating data from CG cell cultures to in vivo conditions, such alterations may also reveal mechanisms that are otherwise difficult to detect using in vivo systems.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were done in compliance with the ethical guidelines of the Declaration of Helsinki 2013. This study was approved by the Rajiv Gandhi Centre for Biotechnology (RGCB), Thiruvananthapuram, India, (Code: IAEC/100/OMK/2010).
Funding
This study was supported by Research grants and fellowships from the Rajiv Gandhi Centre for Biotechnology (RGCB), Department of Science and Technology, and Council of Scientific and Industrial Research of the Government of India (Grant No.: 10-2(5)/2007(ii)-E.U.II; Dated 15-05-2008).
Conflict of interest
The author declared no conflict of interest.
Acknowledgements
The author thanks the PhD supervisor, the Director, faculty, and students of Rajiv Gandhi Centre for Biotechnology (RGCB), as well as the Principal, faculty, and students at Government Arts College, for their support in designing and carrying out the work. The support extended by Ani V Das in establishing primary CG cultures and the facilities provided by RGCB for conducting the research are deeply acknowledged.
References
The cerebellum is the seat of motor learning and memory in the brain [1]. It mainly consists of two major cell types: Cerebellar granule (CG) cells and Purkinje cells, both of which are excitatory in nature. The CG cells constitute the most abundant cell type in the mammalian brain. These cells receive innervation from deeper parts of the cerebellum through mossy fibres, and their output is modulated through parallel-fibre Purkinje neurons [2]. The projections from Purkinje cells are the sole output from the cerebellum.
Calcium/calmodulin-dependent protein kinase type II (CaMKII) is important for inducing and maintaining synaptic plasticity in the hippocampus and is known as the ‘memory molecule’ [3]. Calcium entry through the N-methyl-D-aspartate receptors (NMDARs) activates CaMKII [4]. CaMKII comprises a family of isoforms derived from four closely related yet distinct genes designated as α, β, γ, and δ. The predominant isoforms of CaMKII are α and β, which are found in the ratio of 3:1 in the forebrain and 1:4 in the cerebellum, respectively [5]. In the cerebellum, the promoter for the α-isoform of CaMKII uses an alternate transcription initiation site that is weaker compared to the site that is functional in the hippocampus [6]. However, the specific cell type in the cerebellum in which the alternate transcriptional control operates has not been demonstrated. The expression of αCaMKII is confined solely to Purkinje cells in the cerebellum, as observed by immunohistochemical analysis using an antibody specific to αCaMKII [7-9]. The mRNA encoding αCaMKII is also enriched in the Purkinje layer, as seen by in situ hybridisation [10, 11]. αCaMKII plays a role in long-term depression in Purkinje neurons [12]. The CG cell layer is found to be completely devoid of αCaMKII [9]. Instead, the CG cells express βCaMKII, which differs in its biochemical properties compared to αCaMKII, such as increased sensitivity to calcium [13, 14] and the ability to bind to the actin cytoskeleton [15]. The absence of αCaMKII in CG cells suggests that the calcium signalling pathways in these cells may be unique.
To investigate the mechanisms of calcium signaling in CG cells, we utilized primary cultures of CG cells. Primary CG cells have been employed as model systems for studying neural activity and Ca²-dependent gene expression [16]. CG cells can be significantly enriched in culture and possess characteristics that resemble those of granule cells in the cerebellum [17]. CG cells are grown in medium containing high KCl to induce depolarization, which mimics stimulation in vivo by the mossy fibers that innervate them. This treatment enhances long-term survival of CG cells in vitro [17-19]. Depolarising conditions facilitate Ca2+ entry into these cells via voltage-gated calcium channels [17]. However, some studies have shown that CGs grown in high concentrations of KCl exhibit an aberrant phenotype that differs from that observed in vivo [20].
Our study aimed to develop a primary culture of CG cells for use as a model to study the interaction of transfected αCaMKII with neuronal receptors upon calcium signaling. However, we observed an aberrant expression of αCaMKII in the primary CG culture, despite it being known to be absent in vivo. Previous studies had not reported this anomaly, which intrigued us to investigate the factors behind the induction of αCaMKII expression.
Materials and Methods
Materials
Normal goat serum was from Santacruz, USA. Paraformaldehyde, Triton-X-100, and Tween-20 were from SRL chemicals, India. DAPI, cytosine arabinoside (Ara C), DNase 1, Poly-D-lysine, Laminin, dNTPs, Taq polymerase, RIPA buffer, and monoclonal antibody against β-actin (A-5441) were from Sigma Chemicals, USA. Fluoromount G and optimal cutting temperature (OCT) compound were from Electron Microscopic Sciences. NBM, Trypsin, Glutamax, and Antibiotic/antimycotic solution were from Invitrogen/GIBCO. Monoclonal antibodies against α (MA1-048) and β subunits (PA1-41150) of CaMKII were from Thermo Scientific, USA. Monoclonal antibody against GluN2B subunit of NMDA receptor (GluN2B) (ab93610) was from Abcam, UK. Polyclonal antibody for GABA-A receptor α6 subunit (α6GABA) (AB-5453) was from Millipore, USA. Secondary antibodies with alkaline phosphatase (AP) conjugates, as well as horse radish peroxidase (HRP) conjugates, were from Sigma Chemicals, USA. The secondary antibodies with fluorescence conjugates were from either Sigma or Jackson Immunoresearch, USA. The kits used included the RNaseasy kit from Qiagen, USA (74104), the DNase digestion kit from Ambion, USA (18068015), and the cDNA synthesis kit, Superscript III from Invitrogen (11304011).
Methods
Immunohistochemistry
All animal experiments were approved by the Institutional Animal Ethics Committee. Rat pups of the required age, postnatal day 7 (P7), were provided by the Animal Research Facility of the Rajiv Gandhi Centre for Biotechnology (RGCB), in accordance with the requirements of the approved experiments. All procedures performed in studies involving animals adhered to the ethical standards of the institution or practice at which the studies were conducted. The cerebella were dissected from P7 pups and immediately immersed in the fixative (4% PFA in 1X PBS, pH 7.4) overnight at 4 °C. They were then cryoprotected in 30% sucrose in 1X PBS overnight at 4 °C for two days. The brains were subsequently embedded in OCT compound. Blocks were created by slowly immersing the brain in liquid nitrogen, and the frozen blocks were stored at -70 °C. Brain samples were sectioned sagitally at a thickness of 14 µM using a cryostat (Leica 3050S). Sections were processed for immunohistochemical to assess the expression of αCaMKII.
Antigen retrieval using citrate buffer was performed to expose any antigen-binding sites that might be inaccessible to the antibody. The sections were subjected to high temperatures of about 90-100 °C in citrate buffer (10 mM citric acid, 0.05% Tween-20, pH 6.0) for about 10-15 minutes. The sections were then washed in 1X PBS three times for about 5 minutes each. The sections were then permeabilized and blocked with 5% normal goat serum (Chemicon) containing 0.2% Triton-X-100 in PBS for 1 h at room temperature, followed by incubation with the primary antibodies in the same solution at 4 °C overnight. The sections were again subjected to washes with 1X PBS three times for 5 minutes each and were incubated with the corresponding secondary antibody conjugated to a fluorophore at room temperature for about 90 minutes. The sections were again washed in 1X PBS three times for 5 minutes each. Between the first and second washes, the sections were stained with the nuclear stain DAPI (1:50,000) by incubating at room temperature. Finally, the sections were mounted with Fluromount G.
Preparation of primary CG neurons
The primary CG neuronal culture was prepared from P7 Sprague Dawley rat pups. The pups were wiped with 70% alcohol and decapitated. The cerebellum was carefully dissected out. After removing the meninges, the cerebellum was minced into pieces. All dissections were done in sterile ice-cold PBS. The cerebellum was then trypsinized in 0.05% trypsin at 37 oC for about 12 minutes. The tissue was centrifuged at 1000 rpm for about one minute to settle. The trypsin was aspirated out gently, and the tissue was washed with PBS. About 20 µL of DNase (4000 U) was added to about 2 mL of plain neurobasal medium (NBM) and mixed with the tissue. To disintegrate the tissue and obtain isolated cells, it was subjected to mechanical stress by pipetting approximately 20-50 times. This supernatant was gently harvested, PBS was added, and it was again centrifuged at about 1000 rpm for 5 minutes. The resulting pellet contained the cells. Reconstituted NBM [containing 0.5% B27 supplement, 20 mM added KCl (5 or 10 mM KCl in certain experiments), 1X Glutamax, 33 mM glucose, and 1X Antibiotic/Antimycotic] was added to the cells. Since NBM already contains 5 mM KCl, the total concentration of KCl becomes 25 mM when 20 mM KCl is added. The cells were seeded onto plates pre-coated with poly-D-lysine and laminin. After 24 hours, the medium was removed, and fresh reconstituted NBM was added. In some cases, after 48 hours, the cells were treated with 10 µM Ara C to prevent the proliferation of glial cells. The cells were maintained in vitro for up to 24 days. The medium was regularly changed, and Ara C treatment was administered intermittently to prevent glial proliferation.
The cells were harvested using 1X PBS on fixed days, specifically DIV 0, 2, 4, 6, 8, 10, 12, 15, 19, and 24. The cells were either lysed using RIPA buffer for Western blot analysis or total RNA was isolated from the cells using RNAeasy kit, in preparation for cDNA synthesis. Various genes were amplified using suitable primers through reverse transcriptase polymerase chain reaction (RT-PCR). The cells were also subjected to immunocytochemical analysis to assess the expression of proteins of interest.
Transcriptomic analysis
Total RNA was isolated from primary CG cells using the Qiagen RNAeasy kit according to the manufacturer’s protocol. DNA contamination in RNA preparation mentioned above was removed by DNase digestion using the DNase digestion kit. Reverse transcription was carried out on the RNA preparation to obtain cDNA. The cDNA was subjected to amplification for the gene of interest using the corresponding primers and Taq polymerase.
Western blot analysis
The cells were harvested on different days and were lysed in RIPA buffer. Protein estimation was done using the bicinchonic acid (BCA) method [21]. The lysate was subjected to SDS-PAGE followed by Western blotting to analyze the expression of various proteins of interest. The Western blots were developed using either alkaline phosphatase-conjugated secondary antibodies or by chemiluminescence with HRP-conjugated secondary antibodies.
Quantitation of the Western blots was carried out by densitometric analysis of the images using Quantity One software, version 4.6.3 from Bio-Rad.
Immunocytochemical analysis
Cells seeded on 12 mm diameter coverslips in 24-well plates were fixed with 4% paraformaldehyde after the respective treatments. The cells were washed three times with PBS for 10 min each. Permeabilization and blocking of non-specific binding were carried out by treating the cells with 3% BSA and 0.2% Triton X-100 in PBS. The cells were then incubated in the primary antibody diluted in the same buffer overnight at 4 °C. Subsequently, the cells were washed three times with PBS for 10 min each. The secondary antibody conjugate was then added in the same buffer and incubated for 1 h at room temperature. The cells were washed 3 times with PBS, for 10 min each. Coverslips were mounted using Fluromount G onto glass slides. DPX mountant was used to seal the coverslips. Images were captured using either an epifluorescence microscope (Leica DMI 4000B inverted microscope with Leica DFC 350 FX model CCD camera and Leica Application Suite software, version 2.8.1) or a confocal fluorescence microscope (Leica Confocal Microscope, TCS SP2 AOBS, with LCS lite imaging software or Nikon Eclipse Ti A1R microscope with NIS Elements software, version 4.0).
Effect of KCl on primary CG cells
The CG cells were grown in reconstituted NBM containing different concentrations of KCl, specifically 10 mM, 15 mM, and 25 mM (with 5 mM KCl present in NBM and an additional 5, 10, and 20 mM of KCl, respectively), starting from DIV 0. These cells were monitored regularly to ensure they remained viable and healthy at the different KCl concentrations. The cells were either harvested for Western blot analysis or fixed with 4% paraformaldehyde for immunocytochemistry on different days to study the expression of αCaMKII.
Results
Immunohistochemical analysis of the rat cerebellum to study the expression of αCaMKII
The cerebellum obtained from P7 rat pups was analyzed for the presence of αCaMKII in vivo through immunohistochemical analysis. The expression of αCaMKII was confined only to the Purkinje layer and was not present in either the external granular layer or the internal granular layer (Figure 1).
confirming the previous findings [9] that αCaMKII is not expressed in the cerebellar granular layer and is restricted to the Purkinje layer.
Characterization of primary CG neuron preparation
The primary CG neurons prepared from P7 pups were characterised by immunostaining for the presence of various markers. Most of the cells were positive for the neuronal marker microtubule-associated protein (MAP-2), whereas a few cells were stained for glial fibrillary acidic protein (GFAP), showing the presence of astrocytes. The cells were positive for α6GABA, the marker for CG cells [22], confirming that most of the cells in the preparation consisted solely of CG cells (Figure 2).
The cells were also immunostained for another well-known marker for CG cells, namely Pax6 [23].
RT-PCR analyses for the transcripts of GluN2B, αCaMKII, βCaMKII, α6GABA, and β-actin were conducted in primary CG cells that were harvested on DIV 2, 4, 6, 8, 10, 12, 15, 19, and 24. Expression of these genes was detected on almost all the days investigated. The marker gene α6GABA showed a reduced expression on DIV 0 and 2 but gradually increased till DIV 10 to 12 as the culture matured. βCaMKII was present on all the days, showing a more or less uniform expression pattern. The receptor GluN2B was detected on all the days studied but exhibited reduced expression at early and much later time points, specifically DIV 0, 2, and DIV 19, 24, respectively. This is consistent with earlier studies indicating that in CG cells, the GluN2B subunit is completely downregulated and replaced by GluN2A and GluN2C by DIV 21 [24, 25]. Surprisingly, αCaMKII was also found to be present at low levels on all the days, with the expression further decreasing at later time points, similar to the pattern observed for GluN2B (Figure 3).
The primary CG cells were subjected to cell lysis, followed by Western blotting on DIV 2, 4, 6, 8, 10, 12, 15, 19, and 24 (Figure 4A).
βCaMKII showed a gradual increase in expression starting from DIV 2 to DIV 24. GluN2B was present on all the days analyzed, but its expression decreased towards DIV 19 and DIV 24. Consistent with the transcriptomic data, the presence of αCaMKII was also detected on all the days studied, except for DIV 0, where there was no detectable expression (Figure 4B).
KCl induces the expression of αCaMKII in CG cells
The expression of αCaMKII in primary CG cells was contrary to expectations. The colocalization of αCaMKII with α6GABA shows that the expression of αCaMKII is indeed present in CG cells (Figure 5).
We investigated the factor that could be responsible for inducing αCaMKII expression in primary CG cells. One of the prominent components added to the culture medium is KCl; hence, we tested whether KCl is responsible for inducing αCaMKII expression. The primary CG cells were grown in different concentrations of KCl to study its effect on the expression of αCaMKII. It was found that the cells grown in 25 mM KCl exhibited αCaMKII expression, as detected by Western blotting (Figure 6) and immunocytochemistry (Figure 7). There was no detectable expression or very faint expression of αCaMKII in cells grown in 10 mM KCl and 15 mM KCl on DIV 4 (Figures 6 and 7), and on DIV 8 (Figures 6 and 7). However, the effect was pronounced when cells were treated with 25 mM KCl on both DIV 4 and 8, with increased expression observed on DIV 8. This indicates that KCl is responsible for inducing the expression of αCaMKII in cultured CG cells.
We also tested the effect of KCl by altering its concentration in the medium. When cells grown in 10 mM KCl for 2 days were switched to a medium containing 25 mM KCl, there was a clear increase in αCaMKII expression compared to cells maintained in 10 mM KCl, further demonstrating that KCl induces αCaMKII expression in a concentration-dependent manner.
Discussion
αCaMKII is an essential molecule involved in calcium signal transduction in excitatory synapses involved in learning and memory. However, CG cells, which are primarily excitatory, are found to be devoid of αCaMKII in vivo [9]. The promoter for αCaMKII in the cerebellum is weak, resulting in negligible transcription [6]. Although some of the functions supported by αCaMKII could be carried out by βCaMKII, which is expressed in CG cells, it is still intriguing how CG cells compensate for the absence of αCaMKII, given that there are key differences in the biochemical properties between βCaMKII and αCaMKII, particularly regarding affinity for calmodulin, actin binding, etc. [13]. To understand the molecular mechanisms associated with calcium signaling in CG cells, we established a primary culture of CG cells using standard procedures adapted from previous reports [26, 27]. The preparation mostly consisted of CG cells, as they were immunostained positively for α6GABA and Pax6 [22]. In the cultured CG cells, the expression of the GluN2B subunit (the receptor to which CaMKII is known to bind) was consistent with in vivo studies [24, 25].
Surprisingly, we found that the primary culture of CG cells showed the presence of αCaMKII, as demonstrated by mRNA expression analysis, Western blotting, and immunocytochemical analysis. Although mRNA of αCaMKII was present in CG cells at very low levels on DIV 0 (Figure 3), Western blot analysis showed that αCaMKII protein level could not always be detected (Figures 4B and 6), which is consistent with data from immunohistochemical analysis of rat cerebellum (Figure 1). This suggests that the expression is induced by some factor, which could possibly be a component of the medium used to grow and maintain the cells. Previous research shows that KCl, a major component of the medium used for the maintenance of CG cells, can induce the expression of certain genes in CG cells [20]. According to this study, various genes are upregulated and downregulated due to the chronic depolarization occurring in CGs maintained in 25 mM KCl compared to those in 5 mM KCl, as shown by gene expression analysis. Some of the upregulated mRNAs include those for channels, like Itpr1, neurotensin receptor 2, voltage-gated K+ channels, ligands, like BDNF, and enzymes, like Cyt c oxidase. It is also known that different durations of KCl-induced depolarization lead to the activation of different signaling pathways based on the concentration of Ca2+ entry, resulting in differential transcriptional activity and gene expression [28]. Our study also showed a similar effect, namely the induction and expression of αCaMKII, which is not typically expressed in vivo.
In our experiments, αCaMKII is expressed when the cells are maintained in elevated concentrations of KCl, specifically 25 mM. Lower concentrations of KCl, such as 10 mM and 15 mM, did not induce significant expression of αCaMKII. However, these conditions do not support long-term survival of CG cells [17-19]. Depolarization induces the opening of VGCCs, facilitating the entry of calcium, which promotes cell survival [17]. Although depolarization induced by KCl and subsequent Ca2+ influx is mediated through different channels, such as NMDAR and AMPAR, certain studies have clearly shown that the major mediators of calcium influx are L-type VGCCs [29, 30]. This influx also induces specific activity-dependent changes in neurons in response to downstream signaling of Ca2+ influx. It is also possible that in our study, KCl could mediate membrane depolarization, which subsequently activated VGCCs, resulting in the induction of αCaMKII.
The study suggests that the mechanisms by which αCaMKII expression is prevented in CG cells in vivo are likely reversed when CG cells are in culture due to the depolarization caused by KCl. This observation is supported by studies showing that retinoic acid stimulates αCaMKII gene expression in PC12 cells, which have a distinct transcription initiation site and are similar to that present in cerebellar tissue. This is different from the αCaMKII transcription initiation site in the hippocampus and accounts for the difference in the level of expression of αCaMKII between these tissues [6]. It is possible that the KCl-induced expression of αCaMKII in CG cells depends on this difference in the transcription apparatus. Understanding the mechanisms of αCaMKII induction in the CG primary culture system may help elucidate the mechanisms by which expression is suppressed in vivo. Recent studies have investigated the action of scorpion toxin Bmk NT1, which induces neurotoxicity via PKC/CaMKII-dependent ERK1/2 signaling in primary CG cells [31]. Although these studies have not addressed whether the pathway is mediated specifically by αCaMKII or βCaMKII signaling, it would be interesting to determine which of the kinases are involved.
Conclusion
The study shows the expression of αCaMKII in the CG primary culture cells, although the same is not present in vivo in the animal. The induction and expression of αCaMKII are attributed to the higher concentrations of KCl used to maintain the cells in vitro. Previous studies have shown that KCl can lead to aberrant expression of genes in primary cells cultured in vitro. This study reports for the first time the concentration-dependent induction of αCaMKII expression in CG primary cells, despite its absence in vivo. Although primary cultures of CG cells have been used as an in vitro model, culture conditions that induce alterations in the expression of key proteins, such as αCaMKII, could lead to significant changes in the signaling mechanisms within these cells. Therefore, primary cultures of CG cells could serve as a model to delineate the mechanisms involved in inducing the expression of αCaMKII, which would help in understanding the tight regulation that suppresses the expression of this enzyme in vivo. While this emphasizes the need for caution in extrapolating data from CG cell cultures to in vivo conditions, such alterations may also reveal mechanisms that are otherwise difficult to detect using in vivo systems.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were done in compliance with the ethical guidelines of the Declaration of Helsinki 2013. This study was approved by the Rajiv Gandhi Centre for Biotechnology (RGCB), Thiruvananthapuram, India, (Code: IAEC/100/OMK/2010).
Funding
This study was supported by Research grants and fellowships from the Rajiv Gandhi Centre for Biotechnology (RGCB), Department of Science and Technology, and Council of Scientific and Industrial Research of the Government of India (Grant No.: 10-2(5)/2007(ii)-E.U.II; Dated 15-05-2008).
Conflict of interest
The author declared no conflict of interest.
Acknowledgements
The author thanks the PhD supervisor, the Director, faculty, and students of Rajiv Gandhi Centre for Biotechnology (RGCB), as well as the Principal, faculty, and students at Government Arts College, for their support in designing and carrying out the work. The support extended by Ani V Das in establishing primary CG cultures and the facilities provided by RGCB for conducting the research are deeply acknowledged.
References
- De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Curr Opin Neurobiol. 2005; 15(6):667-74. [DOI:10.1016/j.conb.2005.10.008] [PMID]
- Ito M. Cerebellar long-term depression: Characterization, signal transduction, and functional roles. Physiol Rev. 2001; 81(3):1143-95. [DOI:10.1152/physrev.2001.81.3.1143] [PMID]
- Sanhueza M, Lisman J. The CaMKII/NMDAR complex as a molecular memory. Mol Brain. 2013; 6:10. [DOI:10.1186/1756-6606-6-10] [PMID]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001; 411(6839):801-5. [DOI:10.1038/35081080] [PMID]
- Miller SG, Kennedy MB. Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J Biol Chem. 1985; 260(15):9039-46. [DOI:10.1016/S0021-9258(17)39454-1]
- Chen J, Kelly PT. Retinoic acid stimulates alpha-CAMKII gene expression in PC12 cells at a distinct transcription initiation site. J Neurosci. 1996;16(18):5704-14. [DOI:10.1523/JNEUROSCI.16-18-05704.1996] [PMID]
- Sola C, Tusell JM, Serratosa J. Comparative study of the distribution of calmodulin kinase II and calcineurin in the mouse brain. J Neurosci Res. 1999; 57(5):651-62. [DOI:10.1002/(SICI)1097-4547(19990901)57:5<651::AID-JNR7>3.0.CO;2-G]
- Ichikawa T, Sekihara S, Ohsako S, Hirata Y, Yamauchi T. Ca2+/calmodulin-dependent protein kinase II in the rat cerebellum: An immunohistochemical study with monoclonal antibodies specific to either alpha or beta subunit. J Chem Neuroanat. 1992; 5(5):383-90. [DOI:10.1016/0891-0618(92)90054-T] [PMID]
- Walaas SI, Lai Y, Gorelick FS, DeCamilli P, Moretti M, Greengard P. Cell-specific localization of the alpha-subunit of calcium/calmodulin-dependent protein kinase II in Purkinje cells in rodent cerebellum. Brain Res. 1988; 464(3):233-42. [DOI:10.1016/0169-328X(88)90029-0] [PMID]
- Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990; 10(6):1788-98. [DOI:10.1523/JNEUROSCI.10-06-01788.1990] [PMID]
- Lin CR, Kapiloff MS, Durgerian S, Tatemoto K, Russo AF, Hanson P, et al. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci USA. 1987; 84(16):5962-6. [DOI:10.1073/pnas.84.16.5962] [PMID]
- Hansel C, de Jeu M, Belmeguenai A, Houtman SH, Buitendijk GH, Andreev D, et al. alphaCaMKII Is essential for cerebellar LTD and motor learning. Neuron. 2006; 51(6):835-43. [DOI:10.1016/j.neuron.2006.08.013] [PMID]
- Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002; 364(Pt 3):593-611. [DOI:10.1042/bj20020228] [PMID]
- De Koninck P, Schulman H. Sensitivity of Ca2+/calmodulin-dependent protein kinase II to the frequency of Ca2+ oscillations. Science. 1998; 279(5348):227-30. [DOI:10.1126/science.279.5348.227] [PMID]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999; 284(5411):162-6. [PMID]
- Vaudry D, Falluel-Morel A, Leuillet S, Vaudry H, Gonzalez BJ. Regulators of cerebellar granule cell development act through specific signaling pathways. Science. 2003; 300(5625):1532-4. [DOI:10.1126/science.1085260] [PMID]
- Gallo V, Kingsbury A, Balazs R, Jorgensen OS. The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. J Neurosci. 1987; 7(7):2203-13.[DOI:10.1523/JNEUROSCI.07-07-02203.1987] [PMID]
- D’Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci USA. 1993; 90(23):10989-93. [DOI:10.1073/pnas.90.23.10989] [PMID]
- Isaev NK, Stelmashook EV, Halle A, Harms C, Lautenschlager M, Weih M, et al. Inhibition of Na(+),K(+)-ATPase activity in cultured rat cerebellar granule cells prevents the onset of apoptosis induced by low potassium. Neurosci Lett. 2000; 283(1):41-4. [DOI:10.1016/S0304-3940(00)00903-4] [PMID]
- Bui CJ, McGann AC, Middleton FA, Beaman-Hall CM, Vallano ML. Transcriptional profiling of depolarization-dependent phenotypic alterations in primary cultures of developing granule neurons. Brain Res. 2006; 1119(1):13-25. [DOI:10.1016/j.brainres.2006.08.043] [PMID]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985; 150(1):76-87. [DOI:10.1016/0003-2697(85)90442-7] [PMID]
- Stykova A, Gruss P. Roles of Pax-genes expression patterns in developing and adult brain as suggested by expression patterns. J Neurosci. 1994; 14(3 Pt 2):1395-412. [DOI:10.1523/JNEUROSCI.14-03-01395.1994] [PMID]
- Jones A, Bahn S, Grant AL, Kohler M, Wisden W. Characterization of a cerebellar granule cell-specific gene encoding the gamma-aminobutyric acid type A receptor alpha 6 subunit. J Neurochem. 1996; 67(3):907-16. [DOI:10.1046/j.1471-4159.1996.67030907.x] [PMID]
- Farrant M, Feldmeyer D, Takahashi T, Cull-Candy SG. NMDA-receptor channel diversity in the developing cerebellum. Nature. 1994; 368(6469):335-9. [DOI:10.1038/368335a0] [PMID]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994; 12(3):529-40. [DOI:10.1016/0896-6273(94)90210-0] [PMID]
- Houston CM, Hosie AM, Smart TG. Distinct regulation of beta2 and beta3 subunit-containing cerebellar synaptic GABAA receptors by calcium/calmodulin-dependent protein kinase II. J Neurosci. 2008; 28(30):7574-84. [DOI:10.1523/JNEUROSCI.5531-07.2008] [PMID]
- Facci L, Skaper SD. Culture of rat cerebellar granule neurons and application to identify neuroprotective agents. Methods Mol Biol. 2012; 846:23-37. [DOI:10.1007/978-1-61779-536-7_3] [PMID]
- Tyssowski KM, DeStefino NR, Cho JH, Dunn CJ, Poston RG, Carty CE, et al. Different neuronal activity patterns induce different gene expression programs. Neuron. 2018; 98(3):530-46. [DOI:10.1016/j.neuron.2018.04.001] [PMID]
- Rienecker KD, Poston RG, Saha RN. Merits and limitations of studying neuronal depolarization-dependent processes using elevated external potassium. ASN Neuro. 2020; 12:1759091420974807. [DOI:10.1177/1759091420974807] [PMID]
- Lyons MR, West AE. Mechanisms of specificity in neuronal activity-regulated gene transcription. Prog Neurobiol. 2011; 94(3):259-95. [DOI:10.1016/j.pneurobio.2011.05.003] [PMID]
- Shen L, Yang Q, He Y, Zou X, Cao Z. BmK NT1-induced neurotoxicity is mediated by PKC/CaMKII-dependent ERK1/2 and p38 activation in primary cultured cerebellar granule cells. Toxicology. 2019; 421:22-9. [DOI:10.1016/j.tox.2019.03.012] [PMID]
Type of Study: Research |
Subject:
General
Received: 2025/04/12 | Accepted: 2025/09/22 | Published: 2025/10/26
Received: 2025/04/12 | Accepted: 2025/09/22 | Published: 2025/10/26
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |