Tue, Feb 3, 2026
Volume 11, Issue 4 (Autumn 2025)
Caspian J Neurol Sci 2025, 11(4): 263-275 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mathiyazhagan N, Parveen B. Plant Bioactive Chemicals With Antiepileptic Properties and Their Promising Mechanisms-A Systematic Review. Caspian J Neurol Sci 2025; 11 (4) :263-275
URL: http://cjns.gums.ac.ir/article-1-785-en.html
URL: http://cjns.gums.ac.ir/article-1-785-en.html
1- Department of Biotechnology, Center for Research and Innovations, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences (SIMATS), Thandalam, India.
2- Department of Biotechnology, Center for Research and Innovations, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences (SIMATS), Thandalam, India. ,hajaraparveen22@gmail.com
2- Department of Biotechnology, Center for Research and Innovations, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences (SIMATS), Thandalam, India. ,
Full-Text [PDF 2803 kb]
(556 Downloads)
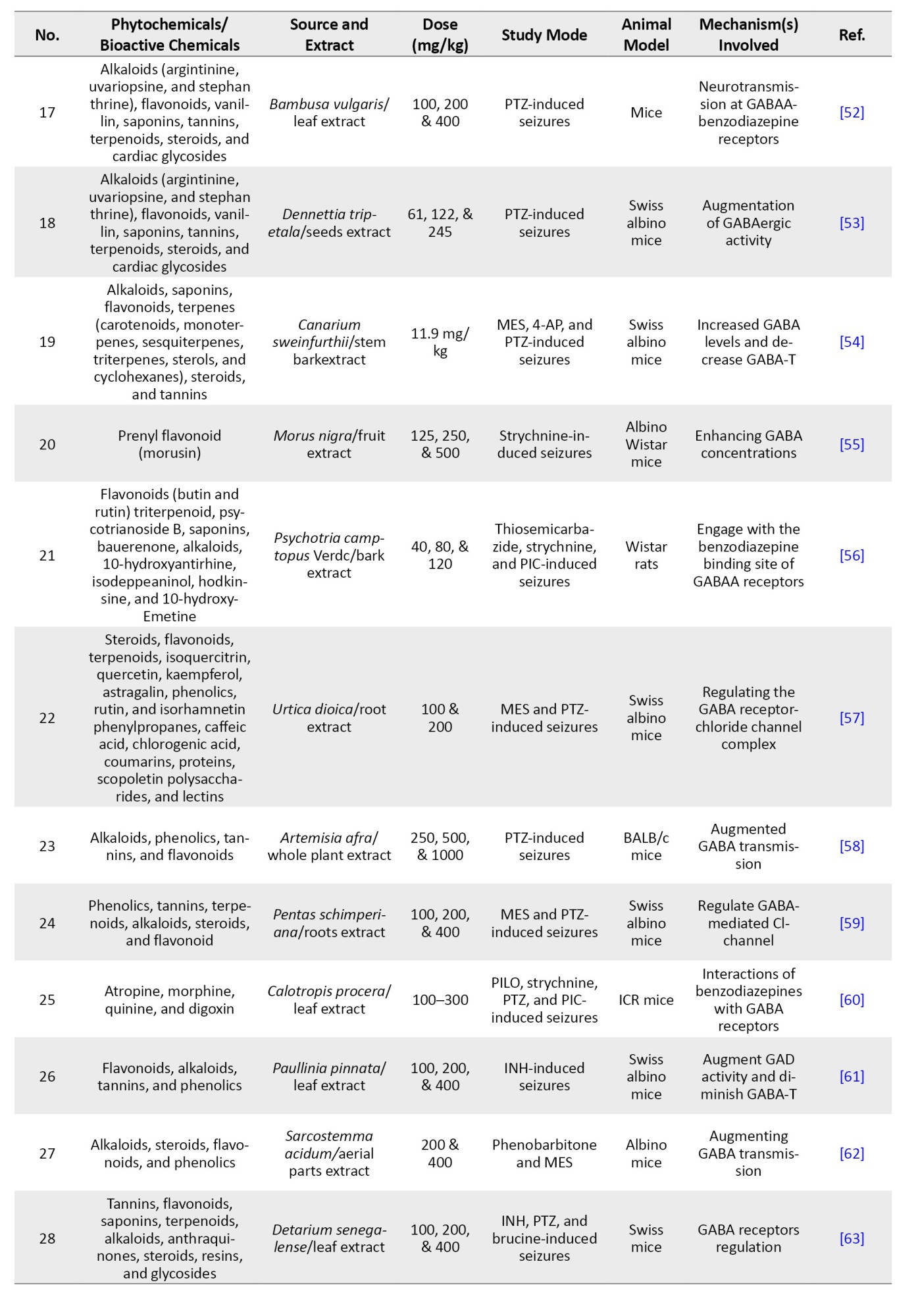
| Abstract (HTML) (457 Views)
References
Full-Text: (179 Views)
Introduction
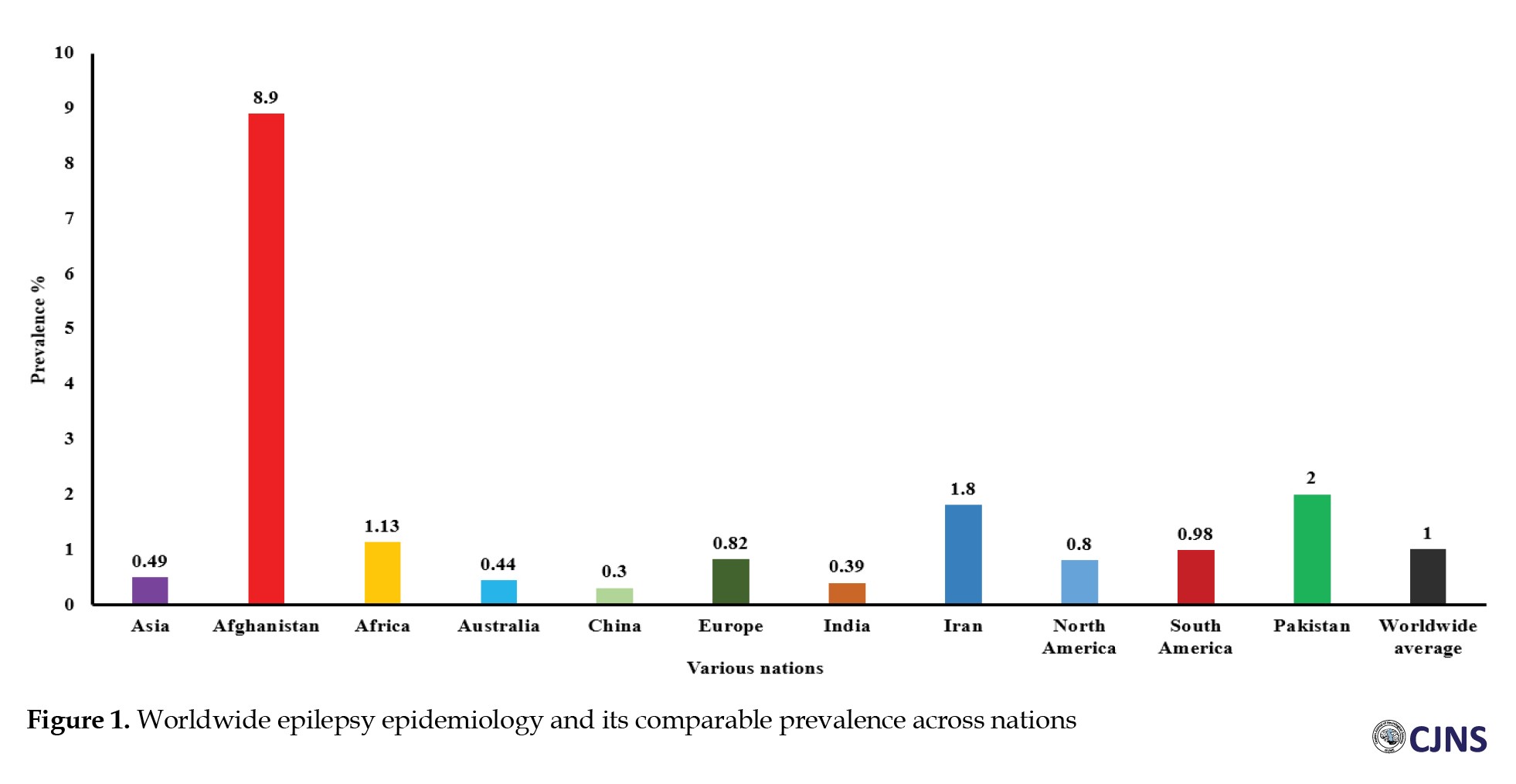
Epilepsy is among the most prevalent and severe neurological disorders worldwide (Figure 1).
Epilepsy is among the most prevalent and severe neurological disorders worldwide (Figure 1).
Approximately 1% of the general population is afflicted with epilepsy, with roughly one-third of these cases suffering from refractory epilepsy, characterized by the continuation of seizures despite sufficient trials of at least two suitably selected and tolerated antiepileptic medications [1]. About 75% of epilepsy manifests throughout infancy, indicating the growing brain’s vulnerability to seizures. Nonetheless, the prevalence in children has declined over the past 30 years in affluent nations, accompanied by another increase in the elderly [2]. A clinical condition often possesses multiple potential aetiologies that may result in diverse epileptic manifestations. An epilepsy syndrome denotes a collection of clinical features that coexist with similar seizure types, age of onset, electroencephalogram abnormalities, precipitating factors, genetic predispositions, natural history, prognosis, and responses to antiepileptic medications [3].
The prevalent forms of epilepsy are complex conditions influenced by specific genetic variations. A seizure can be described as “a condition resulting from an aberrant uncontrolled neuronal activation across the central nervous system” [4]. A convulsion is abrupt, involuntary muscle contractions and relaxations, frequently associated with atypical motions or postures, which may arise from excessive and synchronized neuronal discharges in the brain. The term “seizure of epilepsy” is used to distinguish a seizure resulting from abnormal neural activity from a non-epileptic event, such as psychogenic epilepsy [5, 6]. Some epilepsies are classified as electro-clinical disorders, and this can be achieved through modern computerized technologies [7-9]. Rare epilepsy disorders with monogenic inheritance are linked to abnormalities in genes encoding components of voltage-gated and ligand-gated ion channels. Mutations in voltage-gated Na+, Cl-, and K+ channels are linked to various kinds of generalized epilepsy along with juvenile seizure disorders [10]. Absence seizures are linked to the impairment of P/Q-type calcium channels with voltage regulation. Nicotinic ligand-gated ions channels, cholinergic receptors, and GABA receptor monomers are linked to prefrontal and generalized epilepsies [11].
The notable characteristics vary among epileptic phenotypes and are linked to the identified genetic mutations that cause all recognized monogenic disorders. Modifications in two non-ion channel encoding genes were discovered in idiopathic epileptic seizures. Current antiepileptic medications inhibit seizures despite addressing the root cause of seizure generation, demonstrating efficacy in 60–70% of patients [12]. Synthetic pharmaceuticals for neurological illnesses are costly and may exhibit severe and unavoidable adverse effects, resulting in low patient adherence. Consequently, medicinal and traditional therapies are favored over synthetic pharmaceuticals for neurological illnesses [13]. The accessibility, low occurrence of adverse effects, and cost efficiency of botanical medications provide significant advantages over synthetic drugs [14, 15]. Around 70% of individuals in impoverished nations continue to depend on alternative and complementary treatments despite advancements in traditional care [16]. This review aimed to highlight phytochemicals (alkaloids, coumarins, flavonoids, glycosides, terpenoids, etc.) that possess antiepileptic activities and their potential mechanisms, as supported by documented global research.
Materials and Methods
Search methodology
This study encompassed both human clinical trials and experimental animal research that examined the antiepileptic potential and mechanisms of bioactive substances derived from plants were retrieved from scientific databases (Scopus, PubMed, Web of Science (WoS), Medline, Frontiers, MDPI, and Google Scholar). Particular search terms, such as epilepsy, pathogenesis, approved drugs for epilepsy, antiepileptic, prevalence, epidemiology, phytochemicals (alkaloids, coumarins, flavonoids, glycosides, terpenoid, etc.), antiseizures, and antiepileptic mechanisms were used in searching. Each article was subsequently examined individually, and the phytochemicals identified as effective for epilepsy were compiled and organized into a table. Data regarding the local utilization and methods of using phytochemicals to treat epilepsy were gathered from practitioners of informal medicine, herbalists, and knowledgeable elderly individuals familiar with phytochemical remedies.
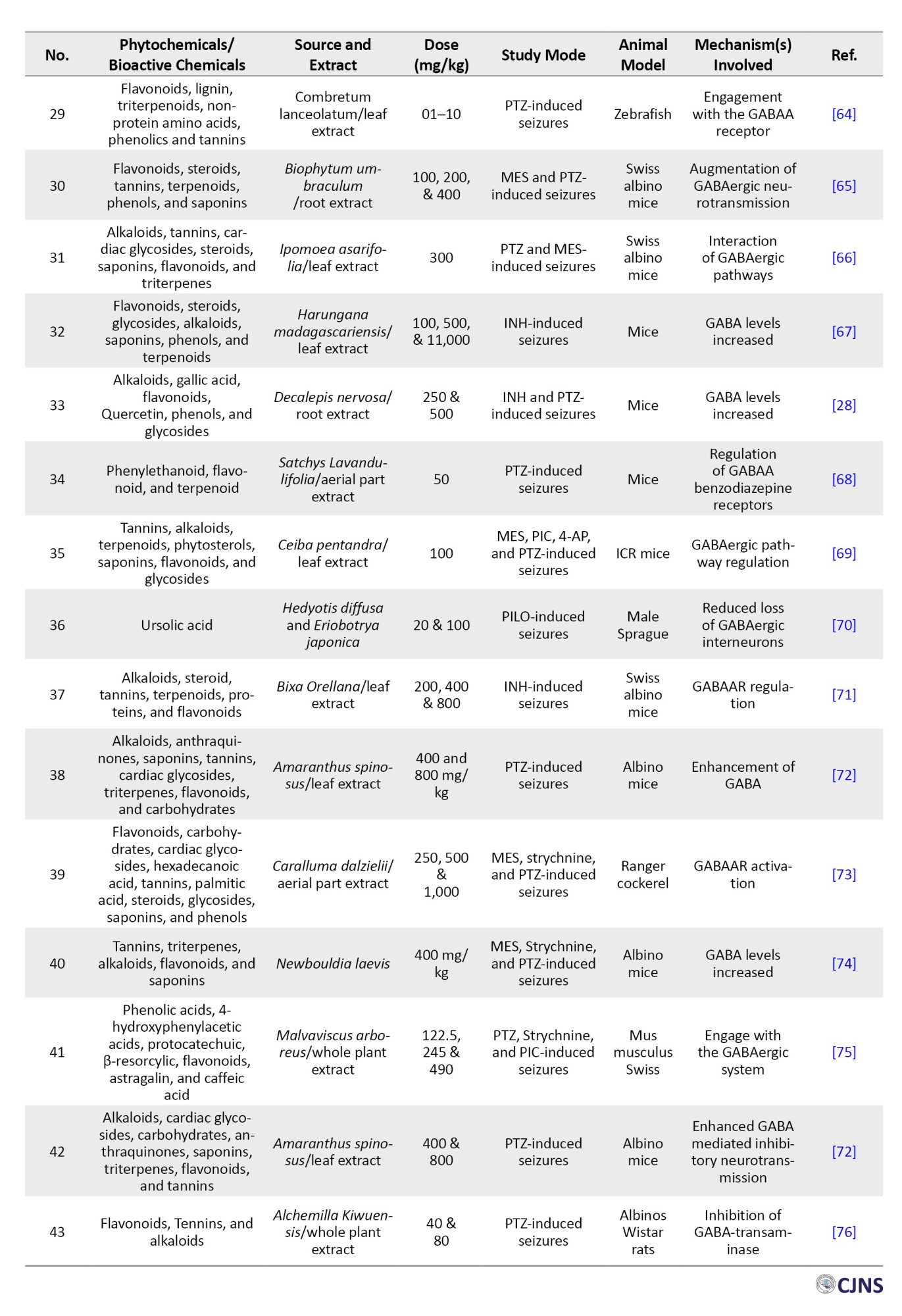
Data extraction
The authors individually evaluated all titles and abstracts identified during the search according to the inclusion criteria. All potentially eligible studies, including those with undefined eligibility, were meticulously evaluated. Following this phase, the complete publications were thoroughly reviewed, and any discrepancies in viewpoints were reconciled.
Inclusion and exclusion criteria
The review encompassed peer-reviewed papers and randomized controlled trials (RCTs) investigating plant bioactive substances with antiepileptic effects. The research examined plant-derived bioactive substances exhibiting significant antiepileptic or neuroprotective properties, utilizing experimental models of epilepsy, clinical trials on humans, or cultures of cells pertinent to epilepsy investigations. Qualified research evaluated seizure reduction, neuroprotective benefits, control of oxidative stress, neurotransmitter equilibrium, ion channel regulation, or other pathways linked to antiepileptic efficacy. Only full-text, peer-reviewed journal publications published in English over the past 10 years were included. The exclusion criteria were applied to research that examined synthetic medicines or non-bioactive extracts of plants lacking documented antiepileptic properties [17]. Studies that failed to evaluate antiepileptic efficacy or relevant mechanisms were also omitted. Narrative reviews, editorials, letters to the editor, and non-systematic reviews were excluded. Furthermore, publications in languages other than English were eliminated unless a high-quality translation was provided. Unpublished works, such as preprints, conference abstracts, and dissertations without peer review, were excluded from consideration. Additionally, this review excluded studies that analyzed mixed plant extracts without separating and assessing individual bioactive components.
Results
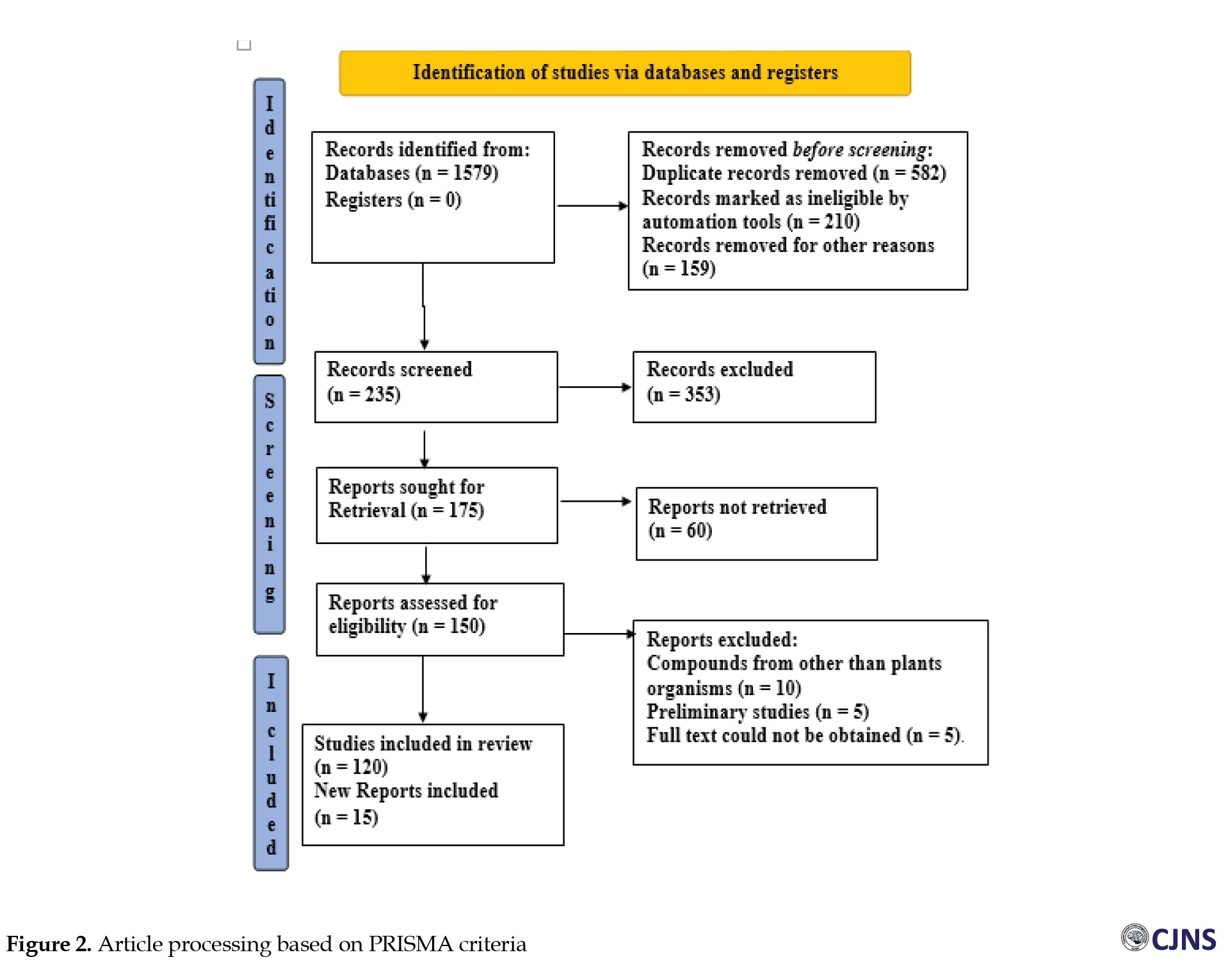
A total of 1579 documents (research, review, and case reports) were obtained through the searches, along with 135 papers that were individually evaluated and selected according to the inclusion and exclusion criteria of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) templates (Figure 2).
The prevalent forms of epilepsy are complex conditions influenced by specific genetic variations. A seizure can be described as “a condition resulting from an aberrant uncontrolled neuronal activation across the central nervous system” [4]. A convulsion is abrupt, involuntary muscle contractions and relaxations, frequently associated with atypical motions or postures, which may arise from excessive and synchronized neuronal discharges in the brain. The term “seizure of epilepsy” is used to distinguish a seizure resulting from abnormal neural activity from a non-epileptic event, such as psychogenic epilepsy [5, 6]. Some epilepsies are classified as electro-clinical disorders, and this can be achieved through modern computerized technologies [7-9]. Rare epilepsy disorders with monogenic inheritance are linked to abnormalities in genes encoding components of voltage-gated and ligand-gated ion channels. Mutations in voltage-gated Na+, Cl-, and K+ channels are linked to various kinds of generalized epilepsy along with juvenile seizure disorders [10]. Absence seizures are linked to the impairment of P/Q-type calcium channels with voltage regulation. Nicotinic ligand-gated ions channels, cholinergic receptors, and GABA receptor monomers are linked to prefrontal and generalized epilepsies [11].
The notable characteristics vary among epileptic phenotypes and are linked to the identified genetic mutations that cause all recognized monogenic disorders. Modifications in two non-ion channel encoding genes were discovered in idiopathic epileptic seizures. Current antiepileptic medications inhibit seizures despite addressing the root cause of seizure generation, demonstrating efficacy in 60–70% of patients [12]. Synthetic pharmaceuticals for neurological illnesses are costly and may exhibit severe and unavoidable adverse effects, resulting in low patient adherence. Consequently, medicinal and traditional therapies are favored over synthetic pharmaceuticals for neurological illnesses [13]. The accessibility, low occurrence of adverse effects, and cost efficiency of botanical medications provide significant advantages over synthetic drugs [14, 15]. Around 70% of individuals in impoverished nations continue to depend on alternative and complementary treatments despite advancements in traditional care [16]. This review aimed to highlight phytochemicals (alkaloids, coumarins, flavonoids, glycosides, terpenoids, etc.) that possess antiepileptic activities and their potential mechanisms, as supported by documented global research.
Materials and Methods
Search methodology
This study encompassed both human clinical trials and experimental animal research that examined the antiepileptic potential and mechanisms of bioactive substances derived from plants were retrieved from scientific databases (Scopus, PubMed, Web of Science (WoS), Medline, Frontiers, MDPI, and Google Scholar). Particular search terms, such as epilepsy, pathogenesis, approved drugs for epilepsy, antiepileptic, prevalence, epidemiology, phytochemicals (alkaloids, coumarins, flavonoids, glycosides, terpenoid, etc.), antiseizures, and antiepileptic mechanisms were used in searching. Each article was subsequently examined individually, and the phytochemicals identified as effective for epilepsy were compiled and organized into a table. Data regarding the local utilization and methods of using phytochemicals to treat epilepsy were gathered from practitioners of informal medicine, herbalists, and knowledgeable elderly individuals familiar with phytochemical remedies.
Data extraction
The authors individually evaluated all titles and abstracts identified during the search according to the inclusion criteria. All potentially eligible studies, including those with undefined eligibility, were meticulously evaluated. Following this phase, the complete publications were thoroughly reviewed, and any discrepancies in viewpoints were reconciled.
Inclusion and exclusion criteria
The review encompassed peer-reviewed papers and randomized controlled trials (RCTs) investigating plant bioactive substances with antiepileptic effects. The research examined plant-derived bioactive substances exhibiting significant antiepileptic or neuroprotective properties, utilizing experimental models of epilepsy, clinical trials on humans, or cultures of cells pertinent to epilepsy investigations. Qualified research evaluated seizure reduction, neuroprotective benefits, control of oxidative stress, neurotransmitter equilibrium, ion channel regulation, or other pathways linked to antiepileptic efficacy. Only full-text, peer-reviewed journal publications published in English over the past 10 years were included. The exclusion criteria were applied to research that examined synthetic medicines or non-bioactive extracts of plants lacking documented antiepileptic properties [17]. Studies that failed to evaluate antiepileptic efficacy or relevant mechanisms were also omitted. Narrative reviews, editorials, letters to the editor, and non-systematic reviews were excluded. Furthermore, publications in languages other than English were eliminated unless a high-quality translation was provided. Unpublished works, such as preprints, conference abstracts, and dissertations without peer review, were excluded from consideration. Additionally, this review excluded studies that analyzed mixed plant extracts without separating and assessing individual bioactive components.
Results
A total of 1579 documents (research, review, and case reports) were obtained through the searches, along with 135 papers that were individually evaluated and selected according to the inclusion and exclusion criteria of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) templates (Figure 2).
Relevant publications were found according to criteria, including researcher details, year of publication (2014-2024), research objectives, sample demographics and size, experimental methodology, and important findings. The literature study revealed that phytochemicals derived from herbs has antiepileptic properties. This facilitates the selection of plant-based phytochemicals/bioactive chemicals/bioactive compounds for investigators seeking phytochemical treatments for epilepsy. Despite the growing body of knowledge on natural medicine, its constituents remain intricate, including alkaloids, cardiac glycosides, coumarins, flavonoids, quinones, saponins, terpenes, and volatile oils. An investigation was performed to investigate the antiepileptic efficacy of phytochemicals enriched in Acalypha fruticosa extract in rats [18, 19].
Discussion
The A. fruticosa crude extract at dosages ranging from 30 to 300 mg/kg was assessed for its effects on maximal electroshock (MES), pentylenetetrazol (PTZ), and isoniazid (INH)-caused epilepsy in mouse. In comparison to diazepam-treated animals using the MES approach, the extract significantly safeguarded the mouse against electroshock-induced convulsions in a dose-dependent manner, exhibiting enhanced efficacy at 300 mg/kg. The extract efficiently inhibited seizures in mice compared to phenobarbitone sodium using the PTZ method, whereas it prolonged the onset of seizures in a dose-dependent manner using the INH methodology but failed to prevent mortality [20]. The existence of antioxidant ingredients, such as flavonoids may account for significant and dosage-dependent antiepileptic activity. In PTZ, bicuculline, and picrotoxin treatments, Achyranthes aspera extract at dosages of 5–10 mg/kg demonstrated a substantial elevation in seizure level relative to saline-treated mouse; nevertheless, the extract lacked any sort of immunity against MES-stimulated seizures [21]. Moreover, A. aspera treatment at 5–10 mg/kg elevated GABA concentrations within the cortex and hippocampus relative to the control group, as determined by HPLC analysis [21]. The antiepileptic properties of A. aspera extract are presumed to be enhanced through the participation of GABAergic neurotransmission. Numerous compounds have been identified as possessing antiepileptic properties, primarily through mechanisms that regulate synaptic and receptor processes (such as GABA, 5-HT, Glu, and NMDAR), immune responses (such as CD3, IL-1β, IL-1, IL-2, IL-4, IL-6, and IL-10, CD4, TNF-α, IgG, and IgA) [22], ion channels (for instance, Na+, Ca2+, and K+), glial cell functions (such as proliferation and K+ uptake) [23], as well as mitochondrial disorders and oxidative stress (such as apoptosis, oxidative markers, and Ca2+ accumulation) [22].
Selective mechanism of phytochemicals on epilepsy
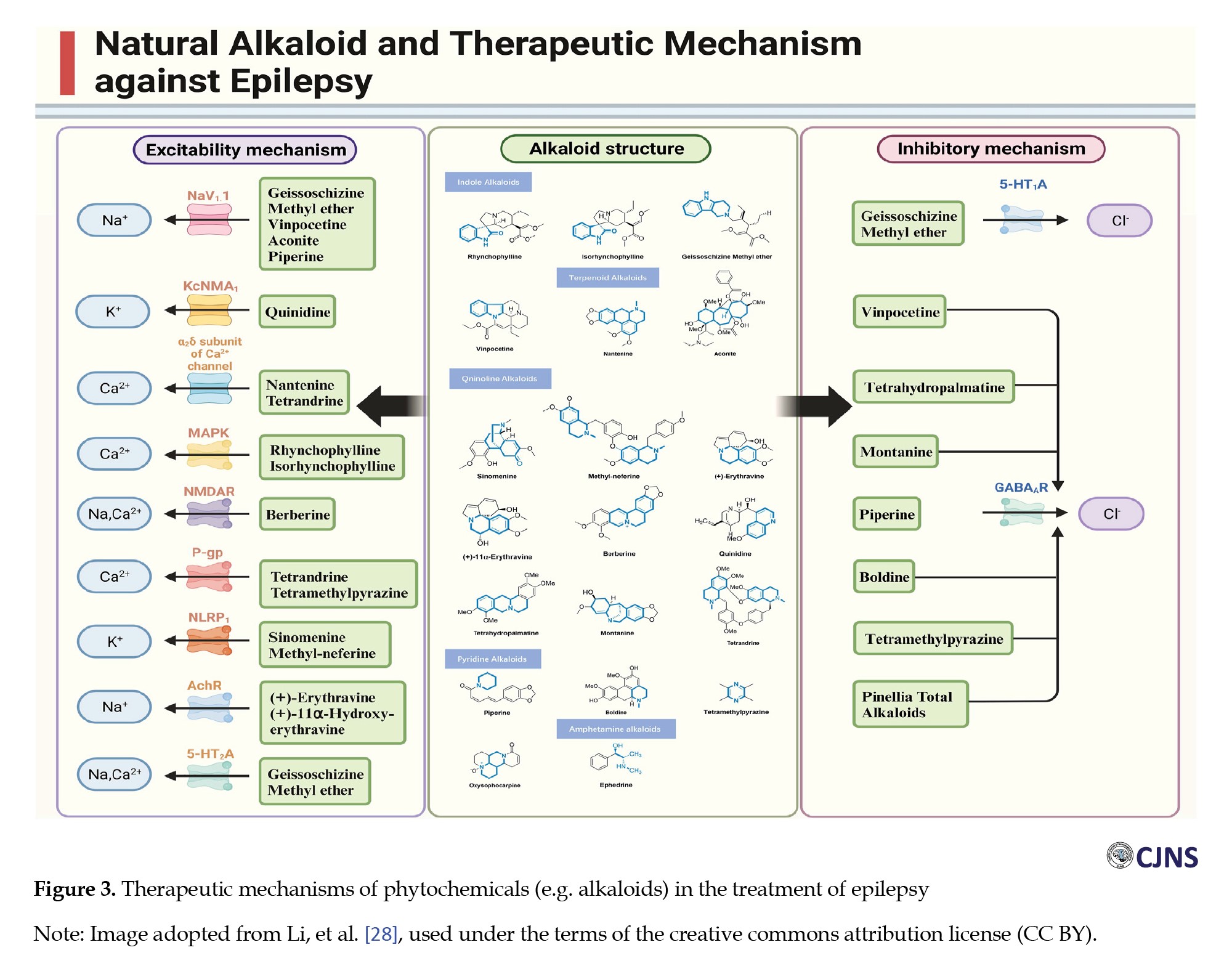
Phytochemicals offer a multi-faceted strategy for epilepsy management by influencing neurotransmitters, ion channels, oxidative stress, inflammation, and mitochondrial activity [23] (Figure 3).
Discussion
The A. fruticosa crude extract at dosages ranging from 30 to 300 mg/kg was assessed for its effects on maximal electroshock (MES), pentylenetetrazol (PTZ), and isoniazid (INH)-caused epilepsy in mouse. In comparison to diazepam-treated animals using the MES approach, the extract significantly safeguarded the mouse against electroshock-induced convulsions in a dose-dependent manner, exhibiting enhanced efficacy at 300 mg/kg. The extract efficiently inhibited seizures in mice compared to phenobarbitone sodium using the PTZ method, whereas it prolonged the onset of seizures in a dose-dependent manner using the INH methodology but failed to prevent mortality [20]. The existence of antioxidant ingredients, such as flavonoids may account for significant and dosage-dependent antiepileptic activity. In PTZ, bicuculline, and picrotoxin treatments, Achyranthes aspera extract at dosages of 5–10 mg/kg demonstrated a substantial elevation in seizure level relative to saline-treated mouse; nevertheless, the extract lacked any sort of immunity against MES-stimulated seizures [21]. Moreover, A. aspera treatment at 5–10 mg/kg elevated GABA concentrations within the cortex and hippocampus relative to the control group, as determined by HPLC analysis [21]. The antiepileptic properties of A. aspera extract are presumed to be enhanced through the participation of GABAergic neurotransmission. Numerous compounds have been identified as possessing antiepileptic properties, primarily through mechanisms that regulate synaptic and receptor processes (such as GABA, 5-HT, Glu, and NMDAR), immune responses (such as CD3, IL-1β, IL-1, IL-2, IL-4, IL-6, and IL-10, CD4, TNF-α, IgG, and IgA) [22], ion channels (for instance, Na+, Ca2+, and K+), glial cell functions (such as proliferation and K+ uptake) [23], as well as mitochondrial disorders and oxidative stress (such as apoptosis, oxidative markers, and Ca2+ accumulation) [22].
Selective mechanism of phytochemicals on epilepsy
Phytochemicals offer a multi-faceted strategy for epilepsy management by influencing neurotransmitters, ion channels, oxidative stress, inflammation, and mitochondrial activity [23] (Figure 3).
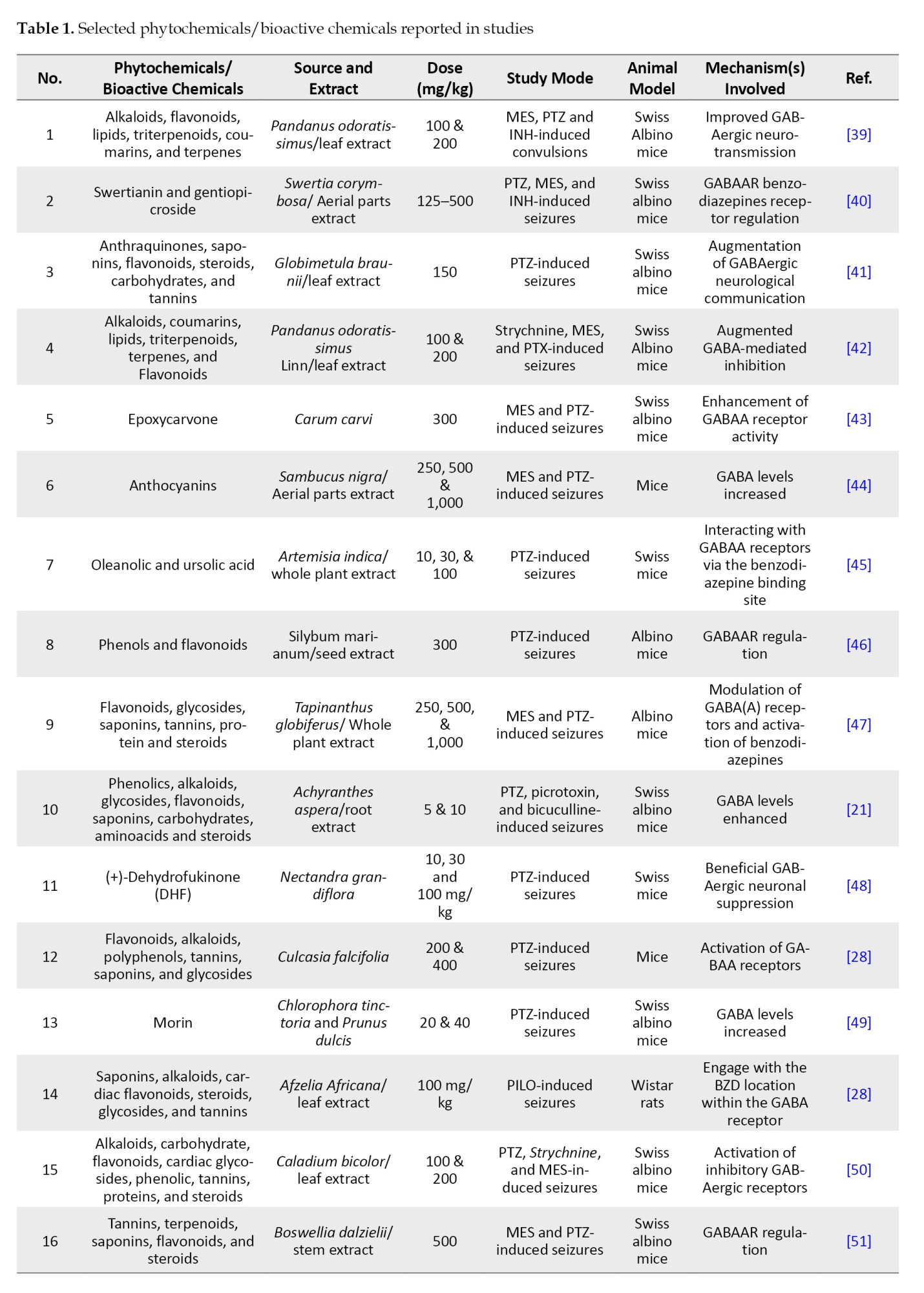
Their potential as alternative or supplementary therapy to traditional antiepileptic medications (AEDs) underscores the necessity for additional study and clinical validation. Table 1 presents plant-based phytochemicals reported recently (2014-2024) with antiepileptic activity through various mechanisms studied by in-vitro and in-vivo studied.
Authorized pharmaceuticals and therapeutic targets
Epilepsy impacts individuals across all age groups, particularly those aged 65 and older. About 25% to 35% of individuals continue to exhibit resistance to existing AEDs [24]. Treatment modalities encompass surgical intervention, ketogenic diet, transcranial direct current activation, vagus nerve activation, deep brain activation, and transcranial magnetic activation. Each method has limitations, including accessibility, cost, and efficacy rates. Currently, pharmacological agents are the predominant therapeutic modality due to their accessibility, high efficacy, and economic viability [25]. During the latter part of the 19th century, potassium bromide (KBr) and herbal remedies were employed to manage epilepsy. Phenobarbital, a GABAA receptor agonist, received regulatory approval for the management of epilepsy around 1912 during the 20th century [26]. Between 1850 and 1960, five medications were predominantly utilized, followed by an additional eight by 1980 [27]. In the modern era, advances in neuroscience have led to the development of more than twenty medications for the management of epilepsy. Investigators are now examining the cost-effectiveness and exceptional efficacy of various medications, with multiple pharmacological and therapeutic interventions undergoing clinical trials [28].
Concurrent utilization of phytochemicals with modern antiepileptic medications
Pharmacological treatment continues to be the primary method for managing epilepsy. The effectiveness of conventional therapies in managing epilepsy is evident, but they are accompanied by numerous known side effects, including anorexia, impaired liver function, dizziness, headaches, leukopenia, cognitive impairment, and diminished quality of life [29]. Particularly for pediatric patients, the physical harm caused by Western medicine is far greater. Conversely, natural medications exhibit minimal toxicity and side effects, resulting in reduced unpleasantness for patients compared to Western pharmaceuticals [30]. The integration of traditional Chinese and Western therapy offers promising options for epilepsy patients who are unresponsive to Western treatments [31]. Furthermore, traditional medicine and its recommendations can significantly enhance the effectiveness of conventional medicine while concurrently mitigating the adverse effects associated with its use. Chinese herbal medicines in the treatment of conditioned tonic improve patients’ anti-epileptic and anti-convulsive conditions, mitigate damage to nerves in epilepsy, and facilitate recovery from the illness [32].
Nobiletin and clonazepam effectively diminish epilepsy severity through suppressing seizure-stimulated elevations in apoptosis protein synthesis, restoring the Glu/GABA equilibrium, and modifying GAD 65 and GABAA [33]. They additionally enhance PI3K/Akt signaling. Overall systemic subcutaneous treatment with UMB at 150 mg/kg can increase the risk of EMS in rats. The combination of valproate/phenobarbital with UMB merits consideration for refractory epileptics. Naringin, when combined with phenytoin, has demonstrated neuroprotective effects against seizures and enhanced the acquired reduction responses in a PTZ-stimulated kindling paradigm [34]. This combination improves neurochemical balance by augmenting GABA and dopamine concentrations, reducing MDA and Glu levels, and increasing antioxidant activity. Gastrodin, in conjunction with carbamazepine, can enhance treatment outcomes and rectify electroencephalogram anomalies in epilepsy patients, demonstrating substantial clinical effectiveness and reduced morbidity [35, 36].
Prescriptions are increasingly utilized in medical care, as well as their therapeutic efficacy is acknowledged by a greater number of patients. The optimal combination of two pharmaceuticals can diminish toxicity as well as augment efficacy, whilst offering a more agreeable therapy experience. Tongqiao Dingxian soup, Ziziphi spinosae decoction, and polyester phlegm soup were documented to reduce the transcription of NMDAR1 and Glu [37]. The integration of different forms of medication has improved therapeutic efficacy while minimizing toxicity and adverse effects, including ligustrazine hydrochloride injections, tranquillizers as well as antiepileptic medications, gastrodin injection, and wild jujube seed decoction [38].
Conclusion
Epilepsy represents a multifaceted disorder impacting the nervous, immunological, and metabolic systems, which can be managed with phytochemical remedies, such as alkaloids, flavonoids, terpenoids etc. These phytochemical remedies have been beneficial in managing psychiatric diseases and enhancing the release of neurotransmitters, rectifying ion channel imbalances, mitigating inflammatory responses, alleviating oxidative stress, repairing mitochondrial damage, and addressing glycogen metabolism abnormalities. The integration of plant-based phytochemicals/bioactive compounds and conventional therapy has demonstrated encouraging outcomes in the treatment of epilepsy, as it mitigates potential adverse effects and enhances overall treatment effectiveness. For certain patients, a holistic treatment strategy is a viable and efficacious alternative. Nonetheless, the study possesses shortcomings, including its emphasis on animal models and its questionable relevance to human subjects. The etiology of epilepsy is intricate, and an example generated by a single pharmacological agent cannot fully replicate its pathophysiology. Future research should integrate clinical experience with theoretical frameworks for the management of epilepsy. Gene therapy, an emerging therapeutic approach, warrants consideration, as the hypothesis that alterations within a single gene can precipitate epilepsy require additional validation. Moreover, brain stem cell transplantation has demonstrated favorable results in the domain of epilepsy. Briefly, the integration of phytochemical medicine and conventional medicine in the treatment of epilepsy remains a prospective and beneficial strategy. Future studies should prioritize the development of more complete epilepsy models, as well as the exploration of gene therapy and brain stem cell transplantation to enhance outcomes.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict interests.
Acknowledgements
All authors thank Saveetha Institute of Medical and Technical Science (SIMATS), Thandalam, India, for their constant support.



Authorized pharmaceuticals and therapeutic targets
Epilepsy impacts individuals across all age groups, particularly those aged 65 and older. About 25% to 35% of individuals continue to exhibit resistance to existing AEDs [24]. Treatment modalities encompass surgical intervention, ketogenic diet, transcranial direct current activation, vagus nerve activation, deep brain activation, and transcranial magnetic activation. Each method has limitations, including accessibility, cost, and efficacy rates. Currently, pharmacological agents are the predominant therapeutic modality due to their accessibility, high efficacy, and economic viability [25]. During the latter part of the 19th century, potassium bromide (KBr) and herbal remedies were employed to manage epilepsy. Phenobarbital, a GABAA receptor agonist, received regulatory approval for the management of epilepsy around 1912 during the 20th century [26]. Between 1850 and 1960, five medications were predominantly utilized, followed by an additional eight by 1980 [27]. In the modern era, advances in neuroscience have led to the development of more than twenty medications for the management of epilepsy. Investigators are now examining the cost-effectiveness and exceptional efficacy of various medications, with multiple pharmacological and therapeutic interventions undergoing clinical trials [28].
Concurrent utilization of phytochemicals with modern antiepileptic medications
Pharmacological treatment continues to be the primary method for managing epilepsy. The effectiveness of conventional therapies in managing epilepsy is evident, but they are accompanied by numerous known side effects, including anorexia, impaired liver function, dizziness, headaches, leukopenia, cognitive impairment, and diminished quality of life [29]. Particularly for pediatric patients, the physical harm caused by Western medicine is far greater. Conversely, natural medications exhibit minimal toxicity and side effects, resulting in reduced unpleasantness for patients compared to Western pharmaceuticals [30]. The integration of traditional Chinese and Western therapy offers promising options for epilepsy patients who are unresponsive to Western treatments [31]. Furthermore, traditional medicine and its recommendations can significantly enhance the effectiveness of conventional medicine while concurrently mitigating the adverse effects associated with its use. Chinese herbal medicines in the treatment of conditioned tonic improve patients’ anti-epileptic and anti-convulsive conditions, mitigate damage to nerves in epilepsy, and facilitate recovery from the illness [32].
Nobiletin and clonazepam effectively diminish epilepsy severity through suppressing seizure-stimulated elevations in apoptosis protein synthesis, restoring the Glu/GABA equilibrium, and modifying GAD 65 and GABAA [33]. They additionally enhance PI3K/Akt signaling. Overall systemic subcutaneous treatment with UMB at 150 mg/kg can increase the risk of EMS in rats. The combination of valproate/phenobarbital with UMB merits consideration for refractory epileptics. Naringin, when combined with phenytoin, has demonstrated neuroprotective effects against seizures and enhanced the acquired reduction responses in a PTZ-stimulated kindling paradigm [34]. This combination improves neurochemical balance by augmenting GABA and dopamine concentrations, reducing MDA and Glu levels, and increasing antioxidant activity. Gastrodin, in conjunction with carbamazepine, can enhance treatment outcomes and rectify electroencephalogram anomalies in epilepsy patients, demonstrating substantial clinical effectiveness and reduced morbidity [35, 36].
Prescriptions are increasingly utilized in medical care, as well as their therapeutic efficacy is acknowledged by a greater number of patients. The optimal combination of two pharmaceuticals can diminish toxicity as well as augment efficacy, whilst offering a more agreeable therapy experience. Tongqiao Dingxian soup, Ziziphi spinosae decoction, and polyester phlegm soup were documented to reduce the transcription of NMDAR1 and Glu [37]. The integration of different forms of medication has improved therapeutic efficacy while minimizing toxicity and adverse effects, including ligustrazine hydrochloride injections, tranquillizers as well as antiepileptic medications, gastrodin injection, and wild jujube seed decoction [38].
Conclusion
Epilepsy represents a multifaceted disorder impacting the nervous, immunological, and metabolic systems, which can be managed with phytochemical remedies, such as alkaloids, flavonoids, terpenoids etc. These phytochemical remedies have been beneficial in managing psychiatric diseases and enhancing the release of neurotransmitters, rectifying ion channel imbalances, mitigating inflammatory responses, alleviating oxidative stress, repairing mitochondrial damage, and addressing glycogen metabolism abnormalities. The integration of plant-based phytochemicals/bioactive compounds and conventional therapy has demonstrated encouraging outcomes in the treatment of epilepsy, as it mitigates potential adverse effects and enhances overall treatment effectiveness. For certain patients, a holistic treatment strategy is a viable and efficacious alternative. Nonetheless, the study possesses shortcomings, including its emphasis on animal models and its questionable relevance to human subjects. The etiology of epilepsy is intricate, and an example generated by a single pharmacological agent cannot fully replicate its pathophysiology. Future research should integrate clinical experience with theoretical frameworks for the management of epilepsy. Gene therapy, an emerging therapeutic approach, warrants consideration, as the hypothesis that alterations within a single gene can precipitate epilepsy require additional validation. Moreover, brain stem cell transplantation has demonstrated favorable results in the domain of epilepsy. Briefly, the integration of phytochemical medicine and conventional medicine in the treatment of epilepsy remains a prospective and beneficial strategy. Future studies should prioritize the development of more complete epilepsy models, as well as the exploration of gene therapy and brain stem cell transplantation to enhance outcomes.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict interests.
Acknowledgements
All authors thank Saveetha Institute of Medical and Technical Science (SIMATS), Thandalam, India, for their constant support.
References
- Bonilla-Jaime H, Zeleke H, Rojas A, Espinosa-Garcia C. Sleep disruption worsens seizures: Neuroinflammation as a potential mechanistic link. Int J Mol Sci. 2021; 22(22):12531. [DOI:10.3390/ijms222212531] [PMID]
- Koepp MJ, Caciagli L, Pressler RM, Lehnertz K, Beniczky S. Reflex seizures, traits, and epilepsies: From physiology to pathology. Lancet Neurol. 2016; 15(1):92-105. [DOI:10.1016/S1474-4422(15)00219-7] [PMID]
- Specchio N, Wirrell EC, Scheffer IE, Nabbout R, Riney K, Samia P, et al. International league against epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022; 63(6):1398-442. [DOI:10.1111/epi.17241] [PMID]
- Giourou E, Stavropoulou-Deli A, Giannakopoulou A, Kostopoulos GK, Koutroumanidis M. Introduction to epilepsy and related brain disorders. In: Voros N, Antonopoulos C, editors. cyberphysical systems for epilepsy and related brain disorders. Cham: Springer; 2015. [DOI:10.1007/978-3-319-20049-1_2]
- Anzellotti F, Dono F, Evangelista G, Di Pietro M, Carrarini C, Russo M, et al. Psychogenic non-epileptic seizures and pseudo-refractory epilepsy, a management challenge. Front Neurol. 2020; 11:461. [DOI:10.3389/fneur.2020.00461] [PMID]
- Kalra S, Bhatia S, Harrasi AA, Mohan S, Sachdeva H, Sharma D, et al. Ethnopharmacological perspective for treatment of epilepsy: An updated review. Scientifica (Cairo). 2024; 2024:8052659. [DOI:10.1155/2024/8052659] [PMID]
- Rajinikanth V, Mohan R, Narayanan M. Deep learning and features fusion for colorectal cancer detection from histopathology images. Paper presented at: 2024 9th International Conference on Communication and Electronics Systems (ICCES). 16-18 December 2024; Coimbatore, India. [DOI:10.1109/ICCES63552.2024.10859542]
- Narayanan M, Mohan R, Rajinikanth V. Solar cell defect detection using deep-learning segmentation with two-fold training. Paper presented at: 2024 9th International Conference on Communication and Electronics Systems (ICCES). 16-18 December 2024; Coimbatore, India. [DOI:10.1109/ICCES63552.2024.10860045]
- Mohan R, Narayanan M, Rajinikanth V. Cancer region segmentation in pre-processed breast ultrasound image using VGG16 based UNet/SegNet. Paper presented at: 2024 9th International Conference on Communication and Electronics Systems (ICCES). 16-18 December 2024; Coimbatore, India. [DOI:10.1109/ICCES63552.2024.10859846]
- Wei F, Yan LM, Su T, He N, Lin ZJ, Wang J, et al. Ion channel genes and epilepsy: functional alteration, pathogenic potential, and mechanism of epilepsy. Neurosci Bull. 2017; 33(4):455-77. [DOI:10.1007/s12264-017-0134-1] [PMID]
- Brust P, Deuther-Conrad W, Donat C, Barthel H, Riss P, Paterson L, et al. Preclinical and clinical aspects of nicotinic acetylcholine receptor imaging. In: Dierckx RA, Otte A, de Vries EF, van Waarde A, Lammertsma AA, editors. PET and SPECT of neurobiological systems. Cham: Springer; 2021. [DOI:10.1007/978-3-030-53176-8_18]
- Billakota S, Devinsky O, Kim KW. Why we urgently need improved epilepsy therapies for adult patients. Neuropharmacology. 2020; 170:107855. [DOI:10.1016/j.neuropharm.2019.107855] [PMID]
- Narayanan M. Evaluation of antibacterial (antibiofilm) activity potential of ZnONPs coated on wound dressing cloth. Dubai Med J. 2024; 7(3):149–59. [DOI:10.18502/dmj.v7i3.17731]
- Al-Worafi YM. Herbal medicines safety issues. In: Drug safety in developing countries: Achievements and challenges. Amsterdam: Elsevier; 2020. [DOI:10.1016/B978-0-12-819837-7.00014-5]
- Sivalingam AM, Pandian A. Identification and characterization of silver nanoparticles from Erythrina indica and its antioxidant and Uropathogenic antimicrobial properties. Microb Pathog. 2024; 190:106635. [DOI:10.1016/j.micpath.2024.106635] [PMID]
- Sen S, Chakraborty R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: Importance, challenges and future. J Tradit Complement Med. 2016; 7(2):234-44. [DOI:10.1016/j.jtcme.2016.05.006] [PMID]
- Mohanasundari C, Anbalagan S, Srinivasan K, Chinnathambi A, Salmen SH, Meganathan V, et al. Evaluation of antibacterial efficacy of various solvent extracts of Evolvulus alsinoides and Mucuna pruriens against multidrug resistant (MDR) pathogenic bacteria. Appl Nanosci. 2023; 13(2):1425-35. [DOI:10.1007/s13204-021-02052-7]
- Waris A, Ullah A, Asim M, Ullah R, Rajdoula MR, Bello ST, et al. Phytotherapeutic options for the treatment of epilepsy: pharmacology, targets, and mechanism of action. Front Pharmacol. 2024; 15:1403232. [DOI:10.3389/fphar.2024.1403232] [PMID]
- Ng YH, Jamil SNH, Sarian MN, Ahmed QU, Latip J, Lam SD, Feroz SR. Antiseizure medications: Advancements, challenges, and prospects in drug development. Curr Neuropharmacol. 2025; 23(8):879-906. [DOI:10.2174/011570159X323666241029171256] [PMID]
- Birhan YS. Medicinal plants utilized in the management of epilepsy in Ethiopia: Ethnobotany, pharmacology and phytochemistry. Chin Med. 2022; 17(1):129. [DOI:10.1186/s13020-022-00686-5] [PMID]
- Gawande DY, Druzhilovsky D, Gupta RC, Poroikov V, Goel RK. Anticonvulsant activity and acute neurotoxic profile of Achyranthes aspera Linn. J Ethnopharmacol. 2017; 202:97-102. [DOI:10.1016/j.jep.2017.03.018] [PMID]
- Li D, Yu S, Long Y, Shi A, Deng J, Ma Y, et al. Tryptophan metabolism: Mechanism-oriented therapy for neurological and psychiatric disorders. Front Immunol. 2022; 13:985378. [DOI:10.3389/fimmu.2022.985378] [PMID]
- Grabarczyk M, Justyńska W, Czpakowska J, Smolińska E, Bielenin A, Glabinski A, et al. Role of plant phytochemicals: resveratrol, curcumin, luteolin and quercetin in demyelination, neurodegeneration, and epilepsy. Antioxidants (Basel). 2024; 13(11):1364. [DOI:10.3390/antiox13111364] [PMID]
- Perucca E, Perucca P, White HS, Wirrell EC. Drug resistance in epilepsy. Lancet Neurol. 2023; 22(8):723-34. [DOI:10.1016/S1474-4422(23)00151-5] [PMID]
- Tambuyzer E, Vandendriessche B, Austin CP, Brooks PJ, Larsson K, Miller Needleman KI, et al. Therapies for rare diseases: therapeutic modalities, progress and challenges ahead. Nat Rev Drug Discov. 2020; 19(2):93-111. [DOI:10.1038/s41573-019-0049-9] [PMID]
- Richardson RJ, Petrou S, Bryson A. Established and emerging GABAA receptor pharmacotherapy for epilepsy. Front Pharmacol. 2024; 15:1341472. [DOI:10.3389/fphar.2024.1341472] [PMID]
- Malerba F, Orsenigo L. The evolution of the pharmaceutical industry. Bus His. 2015; 57(5), 664–87. [DOI:10.1080/00076791.2014.975119]
- Li S, Lin X, Duan L. Harnessing the power of natural alkaloids: The emergent role in epilepsy therapy. Front Pharmacol. 2024; 15:1418555. [DOI:10.3389/fphar.2024.1418555] [PMID]
- Greer D, Liu MT, Maroney M. Side effects of antiseizure medications. In: Side effects of drugs Annual. Amsterdam: Elsevier; 2023.[DOI:10.1016/bs.seda.2023.07.001]
- Predescu IA, Jîjie AR, Pătraşcu D, Pasc AL, Piroş EL, Trandafirescu C, et al. Unveiling the complexities of medications, substance abuse, and plants for recreational and narcotic purposes: An in-depth analysis. Pharmacy (Basel). 2025; 13(1):7. [DOI:10.3390/pharmacy13010007] [PMID]
- Anand A, Shrivastava A, Singh K, Barik R, Gayakwad D, Jailani S, et al. Neuroprotective efficacy and complementary treatment with medicinal herbs: A comprehensive review of recent therapeutic approaches in epilepsy management. CNS Neurol Disord Drug Targets. 2025; 24(1):60-73. [DOI:10.2174/0118715273332140240724093837] [PMID]
- Ali NH, Al-Kuraishy HM, Al-Gareeb AI, Alnaaim SA, Alexiou A, Papadakis M, et al. Autophagy and autophagy signaling in epilepsy: Possible role of autophagy activator. Mol Med. 2023; 29(1):142. [DOI:10.1186/s10020-023-00742-2] [PMID]
- Sharifian M, Hassanpour S. Antiepileptic effect of nobiletin in PTZ-induced catamenial seizures in the rat. J Basic Clin. Pathophysiol. 2025; 13(1):44-9. [DOI: 10.22070/jbcp.2025.20006.1193]
- Rehman MU, Wali AF, Ahmad A, Shakeel S, Rasool S, Ali R, et al. Neuroprotective strategies for neurological disorders by natural products: An update. Curr Neuropharmacol. 2019; 17(3):247-67. [DOI:10.2174/1570159X16666180911124605] [PMID]
- Singh S, Singh TG, Rehni AK. An insight into molecular mechanisms and novel therapeutic approaches in epileptogenesis. CNS Neurol Disord Drug Targets. 2020; 19(10):750-79. [DOI:10.2174/1871527319666200910153827] [PMID]
- Perumalsamy H, Balusamy SR, Sukweenadhi J, Nag S, MubarakAli D, El-Agamy Farh M, et al. A comprehensive review on Moringa oleifera nanoparticles: Importance of polyphenols in nanoparticle synthesis, nanoparticle efficacy and their applications. J Nanobiotechnology. 2024; 22(1):71. [DOI:10.1186/s12951-024-02332-8] [PMID]
- He LY, Hu MB, Li RL, Zhao R, Fan LH, He L, et al. Natural medicines for the treatment of epilepsy: Bioactive components, pharmacology and mechanism. Front Pharmacol. 2021; 12:604040. [DOI:10.3389/fphar.2021.604040] [PMID]
- Patel CN, Shakeel A, Mall R, Alawi KM, Ozerov IV, Zhavoronkov A, et al. Strategies for redesigning withdrawn drugs to enhance therapeutic efficacy and safety: A review. WIREs Comput Mol Sci 2025; 15(4): e70004. [DOI:10.1002/wcms.70004]
- Govindu S, Adikay S. Evaluation of antiepileptic activity of chloroform extract of Acalypha fruticosa in mice. Pharmacognosy Res. 2014; 6(2):108-12. [DOI:10.4103/0974-8490.128970] [PMID]
- Mahendran G, Thamotharan G, Sengottuvelu S, Bai VN. Evaluation of anticonvulsant, sedative, anxiolytic, and phytochemical profile of the methanol extract from the aerial parts of Swertia corymbosa (Griseb.) wight ex C.B. Clarke. Biomed Res Int. 2014; 2014:542385. [DOI:10.1155/2014/542385] [PMID]
- Aliyu MM, Musa AI, Kamal MJ, Mohammed MG. Phytochemical screening and anticonvulsant studies of ethyl acetate fraction of Globimetula braunii on laboratory animals. Asian Pac J Trop Biomed. 2014; 4(4):285-9. [DOI:10.12980/APJTB.4.2014C925] [PMID]
- Adkar PP, Jadhav PP, Ambavade SD, Shelke TT, Bhaskar VH. Protective effect of leaf extract of Pandanus odoratissimus Linn on experimental model of epilepsy. Int J Nutr Pharm Neurol Dis. 2014; 4(2):81-7. [Link]
- Salgado PR, da Fonsêca DV, Braga RM, de Melo CG, Andrade LN, de Almeida RN, et al. Comparative anticonvulsant study of epoxycarvone stereoisomers. Molecules. 2015; 20(11):19660-73. [DOI:10.3390/molecules201119649] [PMID]
- Ataee R, Falahati A, Ebrahimzadeh MA, Shokrzadeh M. Anticonvulsant activities of Sambucus nigra. Eur Rev Med Pharmacol Sci. 2016; 20(14):3123-6. [PMID]
- Kazmi I, Afzal M, Gupta G, Anwar F. Antiepileptic potential of ursolic acid stearoyl glucoside by GABA receptor stimulation. CNS Neurosci Ther. 2012; 18(9):799-800. [DOI:10.1111/j.1755-5949.2012.00369.x] [PMID]
- Waqar H, Khan HM, Anjum AA. Antiepileptic potential of Silybum marianum seeds in pentylenetetrazol-induced kindled mice. Bangladesh J Pharm. 2016; 11(3):603-9. [DOI:10.3329/bjp.v11i3.26181]
- Abubakar K, Adebisi IM, Ugwah-Oguejiofor JC, Idris G, Idris B, MsheliaH. Phytochemical screening and anticonvulsant activity of the residual aqueous fraction of Tapinanthus globiferus growing on Ficus glums. Herb Med. 2016; 2:2. [DOI:10.21767/2472-0151.100013]
- Garlet QI, Pires LDC, Milanesi LH, Marafiga JR, Baldisserotto B, Mello CF, et al. (+)-Dehydrofukinone modulates membrane potential and delays seizure onset by GABAa receptor-mediated mechanism in mice. Toxicol Appl Pharmacol. 2017; 332:52-63. [DOI:10.1016/j.taap.2017.07.010] [PMID]
- Kandhare AD, Mukherjee AA, Bodhankar SL. Anti-epileptic effect of morin against experimental pentylenetetrazol-induced seizures via modulating brain monoamines and oxidative stress. Asian Pac J Trop Biomed. 2018; 8(7):352-9. [DOI:10.4103/2221-1691.237078]
- Akhigbemen AM, Ozolua RI, Bafor EE, Okwuofu EO. Evaluation of some neuropharmacological effects of Caladium bicolor aiton (araceae) leaf extracts in mice. Metab Brain Dis. 2019; 34(2):537-44. [DOI:10.1007/s11011-019-0390-z] [PMID]
- Medugu AN, Yakubu J, Medugu UN, Marte HI, Tata FY, Balami VM. Phytochemical and anti-epileptic studies of ethanol extract of Boswellia dalzielii (Frankincense Tree) Stem Bark. Eur J Med Plants. 2020; 31(8):94-100. [DOI:10.9734/ejmp/2020/v31i830262]
- Adebayo MA, Akinpelu LA, Okwuofu EO, Ibia DE, Lawson-Jack AF, Igbe I. Anticonvulsant, antiamnesic and anxiolytic activities of methanol leaf extract of Bambusa vulgaris (Poaceae) in mice. J Afr Assoc Physiol. 2020;8(2):149-57.[Link]
- Uruaka CI, Georgwill O. Evaluation of the anticonvulsant, hypnotic and anxiolytic-like effects of methanol seed extract of Dennettia tripetala in mice. J Afr Assoc Physiol Sci. 2020; 8(1):41-9. [Link]
- Kandeda AK, Taiwe GS, Ayissi REM, Moutchida C. An aqueous extract of Canarium schweinfurthii attenuates seizures and potentiates sleep in mice: Evidence for involvement of GABA pathway. Biomed Pharmacother. 2021; 142:111973. [DOI:10.1016/j.biopha.2021.111973] [PMID]
- Zehra T, Sarfaraz S, Ikram R. Dose dependent anticonvulsant activity of Morus nigra in strychnine induced seizures model. Pak J Pharm Sci. 2021; 34(6):2167-71. [PMID]
- Fokoua AR, Ndjenda MK 2nd, Kaptué Wuyt A, Tatsinkou Bomba FD, Dongmo AK, Chouna R, et al. Anticonvulsant effects of the aqueous and methanol extracts from the stem bark of Psychotria camptopus Verdc. (Rubiacaea) in rats. J Ethnopharmacol. 2021; 272:113955. [DOI:10.1016/j.jep.2021.113955] [PMID]
- Loshali A, Joshi BC, Sundriyal A, Uniyal S. Antiepileptic effects of antioxidant potent extract from Urtica dioica Linn. root on pentylenetetrazole and maximal electroshock induced seizure models. Heliyon. 2021; 7(2):e06195. [DOI:10.1016/j.heliyon.2021.e06195] [PMID]
- Kediso TE, Tolessa T, Getachew F, Makonnen E, Seifu D. Effect of 70% ethanol extract and its solvent fractions of Artemisia afra (Jacq. Ex Willd.) against Pentylenetetrazole-Induced Seizure in mice. Evid Based Complement Alternat Med. 2021; 2021:6690965. [DOI:10.1155/2021/6690965] [PMID]
- Fisseha N, Shibeshi W, Bisrat D. Evaluation of anticonvulsant activity of 80% methanolic root bark extract and solvent fractions of pentas schimperiana (A. Rich.) Vatke (Rubiaceae) in Swiss Albino Mice. Adv Pharmacol Pharm Sci. 2021; 2021:6689879. [DOI:10.1155/2021/6689879] [PMID]
- Obese E, Biney RP, Henneh IT, Adakudugu EA, Anokwah D, Agyemang LS, et al. The anticonvulsant effect of hydroethanolic leaf extract of calotropis procera (Ait) R. Br. (Apocynaceae). Neural Plast. 2021; 2021:5566890.[DOI:10.1155/2021/5566890] [PMID]
- Ajibade MA, Akhigbemen AM, Okolie NP, Ozolua RI. Methanol leaf extract of Paullinia pinnata exerts sleep-enhancing and anticonvulsant effects via a mechanism involving the GABAergic pathway. Epilepsy Res. 2022; 183:106943. [DOI:10.1016/j.eplepsyres.2022.106943] [PMID]
- Parmar H, Gupta SK, Shrivastava SK. Anti-convulsant activity of aqueous extract of aerial parts of sarcostemma acidum W. and A. NeuroQuantology. 2022; 20(20):1730-3. [Link]
- Nwachukwu EO, Akuodor GC, Oyindamola JO, Chilaka KC, Ilo CE, Obi E, Unekwe PC. Evaluation of the anticonvulsant properties of the ethanol extract of Detarium senegalense leaves in mice. Int J Biol Pharm Sci Arch. 2022; 3(2):67-73. [DOI:10.53771/ijbpsa.2022.3.2.0049]
- da Silva AW, Ferreira MKA, Pereira LR, Rebouças EL, Coutinho MR, Dos J, et al. Combretum lanceolatum extract reverses anxiety and seizure behavior in adult zebrafish through GABAergic neurotransmission: An in vivo and in silico study. J Biomol Struct Dyn. 2022; 40(20):9801-14. [DOI:10.1080/07391102.2021.1935322] [PMID]
- Fisseha N, Hammeso WW, Nureye D. Anticonvulsant activity of hydro alcoholic extract and solvent fractions of Biophytum umbraculum Welw. Syn (Oxalidaceae) root in mice. J Exp Pharmacol. 2022; 14:291-9. [DOI:10.2147/JEP.S374890] [PMID]
- Chiroma SS, Nazifi AB, Jamilu Y, Musa A, Bichi, LA, Chiroma AM. Anticonvulsant activity and mechanism of actions of fractions of Ipomoea asarifolia (Desr)(Convolvulaceae) ethanol leaf extract. Bull Natl Res Cent. 2022; 46:150. [DOI:10.1186/s42269-022-00839-4]
- Celestine UO, Jude AI, Asogwa FK, Maduabuchi OR, Nnamdi NS. Anticonvulsant activity of methanol extract of harungana madagascariensis leaf on mice model of isoniazid-induced siezure. J Adv Med Pharm Sci. 2022; 24(8):34-41.[DOI:10.9734/jamps/2022/v24i8572]
- Aghaei I, Rostampour M, Shabani M, Naderi N, Motamedi F, Babaei P, et al. Palmitoylethanolamide attenuates PTZ-induced seizures through CB1 and CB2 receptors. Epilepsy Res. 2015; 117:23-8. [DOI:10.1016/j.eplepsyres.2015.08.010] [PMID]
- Sarfo A, Abotsi WK, Ekuadzi E, Woode E. Ceiba pentandra (L.) Gaertn hydroethanolic leaf extract exhibits anticonvulsant properties in mouse models. Phytomedicine Plus. 2022; 2(2):100263. [DOI:10.1016/j.phyplu.2022.100263]
- Liu KM, Huang Y, Wan PP, Lu YH, Zhou N, Li JJ, et al. Ursolic acid protects neurons in temporal lobe epilepsy and cognitive impairment by repressing inflammation and oxidation. Front Pharmacol. 2022 ; 13:877898. [DOI:10.3389/fphar.2022.877898] [PMID]
- Offiah RO, Okolo KO, Ifeoma AN. Preliminary phytochemical, GC-MS analysis and antiepileptic studies on the methanol leaf extract of Bixa orellana (bixaceae). Igbinedion Univ Okada J Pharm Sci. 2023; 2(1):13-26. [Link]
- Usman S, Ahmad MM, Tahir A, Umar AK. Exploring the antiepileptic potential of Amaranthus spinosus: an experimental study in albino mice. Sci Pharm. 2023; 2(3):204-10. [DOI:10.58920/sciphar02030106]
- Ugwah-Oguejiofor CJ, Amuda MB, Abubakar K, Ugwah OM, Ofokansi MN, Mshelia HE. An experimental evaluation of anticonvulsant activity of aqueous extract of Caralluma dalzielii NE Brown. Phytomedicine Plus. 2023; 3(1):100401.[DOI:10.1016/j.phyplu.2022.100401]
- Ukwubile CA, Ikpefan EO, Dibal MY, Umeano VA, Menkiti DN, Kaosi CC, et al Pharmacognostic profiles, evaluation of analgesic, anti-inflammatory and anticonvulsant activities of Newbouldia laevis (P. Beauv.) Seem. ex Bureau leaf and root extracts in Wistar rats. J Ethnopharmacol. 2023; 314:116632. [DOI:10.1016/j.jep.2023.116632] [PMID]
- Adassi MB, Ngoupaye GT, Yassi FB, Foutsop AF, Kom TD, Ngo Bum E. Revealing the most effective anticonvulsant part of Malvaviscus arboreus Dill. Ex Cav. and its acute and sub-acute toxicity. J Ethnopharmacol. 2023; 303:115995. [DOI:10.1016/j.jep.2022.115995] [PMID]
- Foutsop AF, Ateufack G, Adassi BM, Yassi FB, Kom TD, Noungoua CM, et al. The aqueous lyophilisate of Alchemilla Kiwuensis Engl. (Rosaceae) displays antiepileptogenic and antiepileptic effects on PTZ-induced kindling in rats: Evidence of modulation of glutamatergic and GABAergic pathways coupled to antioxidant properties. Neurochem Res. 2023; 48(10):3228-48. [DOI:10.1007/s11064-023-03982-0] [PMID]
Type of Study: Review |
Subject:
Special
Received: 2025/03/31 | Accepted: 2025/08/23 | Published: 2025/10/26
Received: 2025/03/31 | Accepted: 2025/08/23 | Published: 2025/10/26
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |