Mon, Dec 29, 2025
Volume 11, Issue 3 (Summer 2025)
Caspian J Neurol Sci 2025, 11(3): 237-244 |
Back to browse issues page
Ethics code: IR.GUMS.REC.1399.615.
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khoshtarash H, Saberi A, Hatamian H, Ashraf A, Ahmadi S, Yazdanipour M A. The Association Between Recombinant Tissue Plasminogen Activator and Post-ischemic Stroke Seizure: A Retrospective Cohort Study. Caspian J Neurol Sci 2025; 11 (3) :237-244
URL: http://cjns.gums.ac.ir/article-1-780-en.html
URL: http://cjns.gums.ac.ir/article-1-780-en.html
Hanie Khoshtarash *1 

 , Alia Saberi2
, Alia Saberi2 

 , Hamidreza Hatamian2
, Hamidreza Hatamian2 

 , Ali Ashraf3
, Ali Ashraf3 

 , Somayeh Ahmadi4
, Somayeh Ahmadi4 

 , Mohammad Ali Yazdanipour5
, Mohammad Ali Yazdanipour5 




 , Alia Saberi2
, Alia Saberi2 

 , Hamidreza Hatamian2
, Hamidreza Hatamian2 

 , Ali Ashraf3
, Ali Ashraf3 

 , Somayeh Ahmadi4
, Somayeh Ahmadi4 

 , Mohammad Ali Yazdanipour5
, Mohammad Ali Yazdanipour5 


1- Department of Neurology, Neurosciences Research Center, Faculty of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran. , Haniekhoshtarash@yahoo.com
2- Department of Neurology, Neurosciences Research Center, Faculty of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
3- Clinical Research Development Unit, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Biostatistics, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5- Neuroscience Research Center, Trauma Institute, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Neurology, Neurosciences Research Center, Faculty of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
3- Clinical Research Development Unit, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Biostatistics, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5- Neuroscience Research Center, Trauma Institute, Guilan University of Medical Sciences, Rasht, Iran.
Full-Text [PDF 1673 kb]
(287 Downloads)
| Abstract (HTML) (498 Views)
Full-Text: (139 Views)
Introduction
Stroke has remained the reason for inpatient rehabilitation because it leads to significant disabilities and long-term complications such as seizures [1]. Post-stroke seizures (PSS) severely impact patients’ quality of life, increase morbidity, and place emotional and financial strain on both families and society at large [2]. These seizures are classified as early onset and late onset seizures; early onset seizure occurs within two weeks after the stroke. The highest reported rate is approximately 45%, most often occurring within the first 24 hours following cerebrovascular event [3]. If the seizure occurs after two weeks, it is classified as a late onset, which typically develops within the first year post-stroke [4, 5]. While all types of strokes carry an increased risk of seizure, hemorrhagic strokes are associated with a higher incidence compared to ischemic strokes [2].
Nevertheless, it is essential to emphasize that due to the higher incidence of ischemic stroke, the overall burden of post-stroke seizure is greater in this group [2]. Statistics show that seizure develops in 2%-14 % of patients who have had an ischemic stroke [6]. In ischemic stroke patients, factors such as the severity of the initial neurological deficit (measured by the National Institutes of Health Stroke scale [NIHSS]), persistent disability, larger or multiple brain lesions (based on Alberta stroke program early CT score [ASPECTS]), cortical damage, and hippocampus involvement are predictors of post-stroke seizure development [7].
Recombinant tissue plasminogen activator (r-tPA) is currently regarded as the standard thrombolytic treatment for eligible patients with acute ischemic stroke [8]. There is no doubt that r-tPA plays an effective role in these patients' treatment [9]. Still, apart from its thrombolytic capacity, r-tPA has known neurotoxic effects [10-12], including some that may give rise to epileptogenic tissue alterations [13]. Specifically, r-tPA may worsen brain injury by increasing glutamic acid release following ischemia, particularly if it crosses into brain tissue. It further stimulates leukocyte infiltration, activates microglial, and enhances free radical production in the ischemic brain [14]. More importantly, it activates matrix metalloproteases which contribute to the disruption of blood-brain barrier [15-17]. These processes can lead to cerebral edema, intracerebral hemorrhage, and hemorrhagic transformation (HT). Research also indicates that during the early phase following stroke onset, changes such as brain edema and cytotoxic injury, neurotransmitter imbalance, glutamate toxicity, and gliosis may trigger seizure activity [4, 18]. HT following stroke increases the risk of early post-stroke seizure, emphasizing the role of blood leakage in promoting epileptogenesis. This risk is heightened in the cortical region, where HT frequently coexists with edema, further stimulating cortical excitability [19]. These changes, especially when HT and edema are present, may serve as independent predictors of status epilepticus in patients with acute ischemic stroke [20]. This study seeks to clarify whether the use of r-tPA in acute ischemic stroke patients has any statistically significant association with the incidence of post-stroke seizures, thereby contributing to safer and more informed treatment decisions.
Materials and Methods
This retrospective cohort study was conducted on 100 patients with r-tPA treatment and 100 patients without r-tPA treatment, who were first admitted with acute ischemic stroke at an academic stroke center in the north of Iran in the years 2017-2020. To select patients, we searched all patients affected by acute ischemic stroke (AIS) in our center from 2017 to 2020, and then, according to exclusion criteria, we selected them randomly. Exclusion criteria involved any pre-existing epilepsy or seizures, the use of antiepileptic medication before receiving inpatient care, and the presence of other significant neurological disorders or metabolic abnormalities, which are risk factors for seizures and patients who are dead.
Data gathered for all patients included demographic information (age, gender), medical history (hypertension, diabetes, hyperlipidemia, cardiac disease), and lifestyle factors (smoking, alcohol, and opium use). Additional stroke-specific data were also recorded, such as the affected artery, side, and size of the infarct and ASPECTS and NIHSS scores upon admission. To differentiate early from late-onset seizure, a 2-week threshold following stroke onset, similar to the approach used in posttraumatic seizure, was applied [8]. The stroke management protocol at our center involves promoting non-contrast brain CT imaging and an assessment for potential eligibility for intravenous thrombolysis therapy. Information such as demographic factors, underlying disease and smoking, and alcohol or opium use was collected from the academic stroke center’s medical files. Infarct radiologic features were collected from the academic stroke center’s PACS (picture archiving and communication system). The occurrence and timing of PSS were obtained during the patient’s follow-up. This information was collected via phone interviews for those who did not attend the visit.
Statistical analysis
The sample size was determined using G*Power software, version 3.1, relying on the seizure frequencies of 19% in the r-tPA group and 6% in the non-r-tPA group reported by Nesselroth et al. [8]. To achieve a power of 0.80 and an alpha level of 0.05, it was established that a minimum of 100 cases is necessary in each group.
The data were arranged using Microsoft Excel, version 2013 (Microsoft Corporation, Redmond, WA, USA) and analyzed with IBM SPSS Statistics for Windows (version 26.0; IBM Corp., Armonk, NY, USA). Mean±SD is used for continuous variables, while numbers (percentages) are used for categorical variables. Normal distribution was assessed using the Shapiro–Wilk test. The chi-squared test or Fisher exact test was applied to categorical variables, and the independent t-test or Mann-Whitney U test was used for continuous variables in group comparisons. A multivariate regression model included variables with a significance level of P<0.20 or lower on initial univariate analysis. Logistic regression with control on accompanying parameters was conducted to evaluate the association between r-tPA and seizure; adjusted odds ratios are provided. A P≤0.05 was considered statistically significant.
Results
Of 200 patients, 100 received intravenous r-tPA, while 100 did not. Both groups were matched in terms of sex and major stroke risk factors, including diabetes, hypertension, hyperlipidemia, smoking, and substance use. Our result showed that patients with IV r-tPA therapy had a higher mean age (P=0.049), and this group had a higher rate of heart disease (P=0.027).
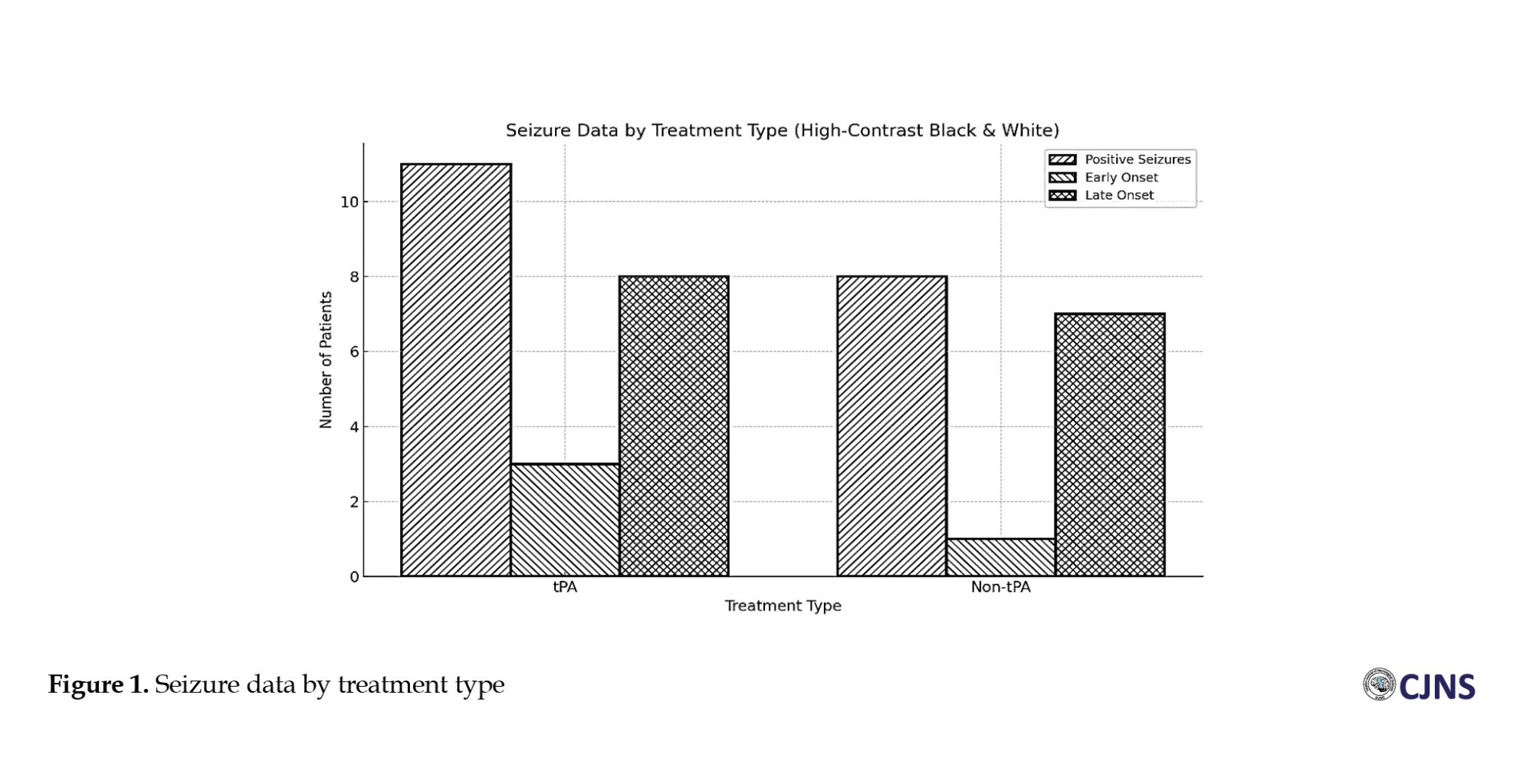
There was a statistically significant difference in the distribution of involved arteries between patients treated with and without r-tPA therapy (P=0.002). This study showed that the middle cerebral artery (MCA) trunk, superior MCA, and anterior cerebral artery were more involved in the r-tPA group.
The mean NIHSS score at admission was significantly higher in the r-tPA group (11.5) compared to the non-treated group (6.8) (P<0.001). Conversely, the non-treated group had higher ASPECTS scores (P=0.034), indicating less severe infarcts (Table 1).
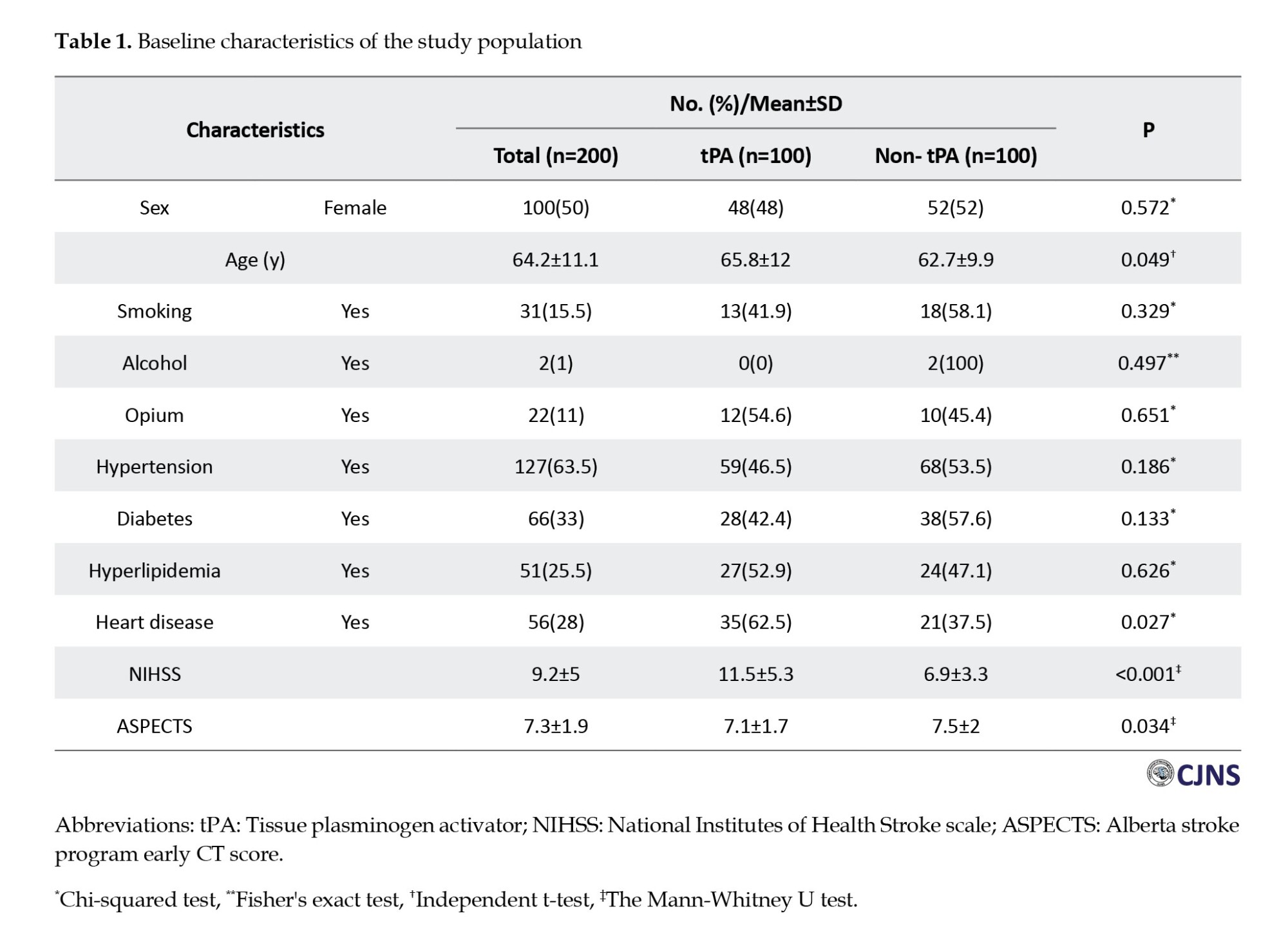
Figure 1 shows 19 patients (10%) who experienced seizures in our retrospective cohort study. Of these, 8(8%) were in the non-r-tPA group and 11(11%) in the r-tPA group. The majority of seizures were classified as late onset. Although the seizure rate was slightly higher in the r-tPA group, statistical analysis revealed no significant difference in seizure incidence between the r-tPA and non-r-tPA groups (P=0.469).
We found no statistical association between baseline parameters, including age, sex, smoking, alcohol or opium use, and underlying diseases and PSS in both groups (P>0.05).
However, our results indicated a statistically significant relationship between the lesion location and the risk of developing secondary seizures. Specifically, involvement of the MCA trunk was associated with a higher risk for post-stroke seizure in the non-r-tPA group (Table 2).
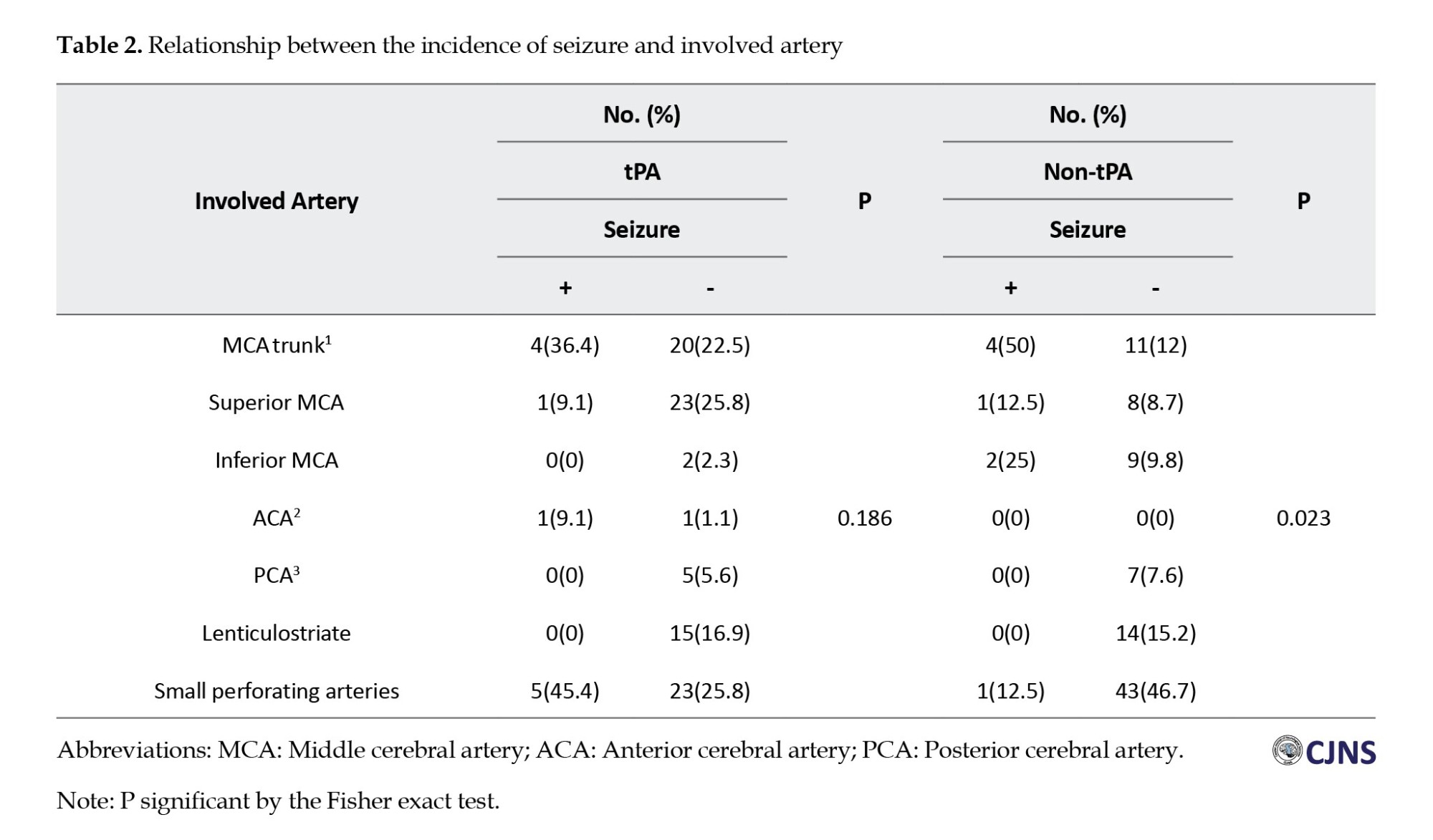
Furthermore, a significant association was absorbed between seizure incidence and stroke severity in both groups, as measured by the NIHSS score at admission. Patients with high-grade strokes (NIHSS ≥6) had a higher likelihood of developing PSS compared to those with mild strokes (NIHSS ≤5) (Table 3).
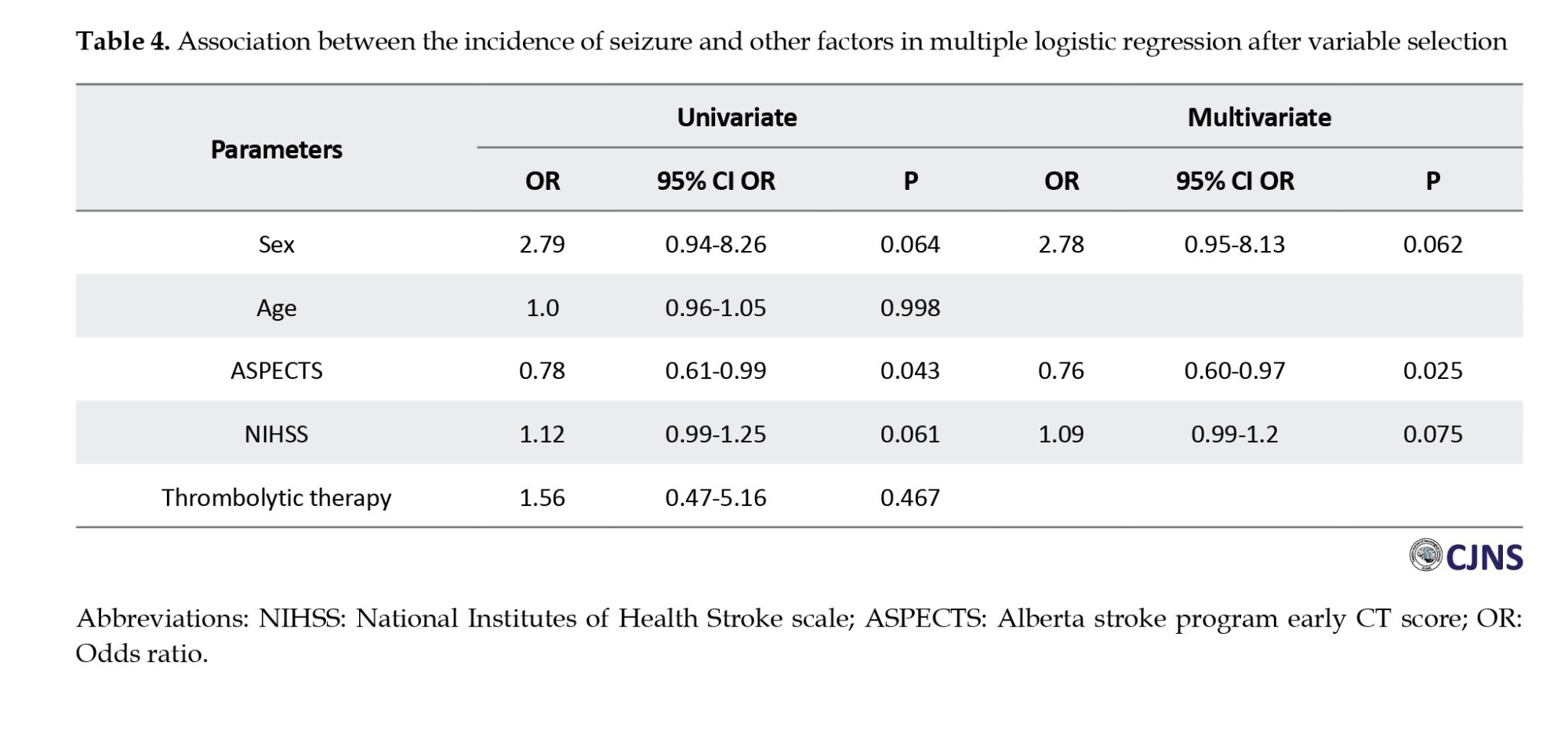
As in Table 4, this finding indicated that considering all dependent factors, r-tPA therapy does not increase the risk of post-stoke seizure. In addition, the only parameter associated with the risk of post-stoke seizure is the score of ASPECT, and by increasing the number of ASPECTS, the rate of seizure occurrence decreases by 24.
Discussion
The overall incidence of post-stroke seizure, according to our data, was 10, with rates of 11 in the r-tPA group and 8 in the non-r-tPA group. Our findings did not demonstrate a significant association between r-tPA administration and post-stroke seizure (P=469). This finding is consistent with previous studies [21-23]. However, the role of r-tpA in the development of PSS remains controversial, as some recent studies reported conflicting results. For instance, research by Brigo et al. [24] stroke localization, NIHSS and Alvarez et al. [25] indicates a higher risk of seizure following r-tPA administration. One proposed mechanism suggests that reperfusion injury, possibly mediated by free-radical, may provoke seizure even in the absence of hemorrhage [25]; in contrast, Nesselroth et al. reported that r-tPA was linked to a reduced risk of post-stroke seizure [8].
Also, we found no association between individual characteristics and PSS. However, some studies have suggested that a higher cholesterol level may protect against seizure [26, 27]. Possibly due to the anticonvulsant properties of cholesterol-derived compounds, such as neurosteroids [28]. However, our data do not support the proposed protective effect of hypercholesterolemia. Also, the Krakow study showed diabetes as a predictor of PSS [29]. It can be explained by the mechanism that acute seizures after AIS are thought to result from cellular biochemical dysfunction leading to electrically irritable tissue [30]. Acute ischemia elevates intracellular levels of calcium and sodium as well as extracellular glutamate in the peri-infarct region. These epileptogenic mechanisms may be further intensified by hyperglycemia in diabetic patients, a phenomenon also supported by experimental findings [29].
Regarding the type of treatment, we found that MCA trunk is more commonly involved in patients with PSS. Because it causes a broad cortex involvement, it is essential to note that many previous studies support the epileptogenic potential of cortical involvement. They confirm that participation of the cerebral cortex, such as in trauma [31] and tumor [32], is found to increase the risk of seizure. This is due to the role of the neocortex in seizure occurrence [33] and the limited number of patients with the involvement of any other arteries in this study; we cannot conclude them.
Also, according to our study regarding the type of treatment, patients with higher NIHSS scores have more chance for PSS (P=0.019). As another risk factor, we found a significant association between ASPECTS and seizure occurrence (odds ratio 0.76, P=025).
To summarize, r-tPA treatment did not affect the frequency of post-stroke seizure manifestation compared to patients who were not treated with r-tPA.
This study has several limitations, the most important being the small sample size. Although the participants were of varied ages, which may reduce some selection bias, the relatively limited sample may still influence the statistical power and generalizability of the results. Further research with larger cohorts is necessary to draw a more definitive conclusion.
Additionally, several potential biases must be acknowledged. Patients in the non-r-tPA group either had contraindications to r-tPA therapy or presented to the hospital more than 4.5 hours after symptom onset, resulting in prolonged cerebral ischemia. As a result, it was initially assumed that this group would include more severe and complex cases. However, findings from the study by Nesselroth et al. [8] showed a lower average NIHSS score in the non-r-tPA group. This inconsistency could be due to the inclusion of patients with milder strokes who were not eligible for r-tPA administration. Thus, selection bias may have been a confounding variable, potentially affecting the study’s findings.
Conclusion
Based on the findings of this retrospective cohort study, administration of r-tPA in patients with acute ischemic stroke does not appear to increase the risk of post-stroke seizures. The treatment remains a safe and effective therapeutic approach within the appropriate clinical timeframe. Continuous monitoring and further studies may help clarify the impact of additional risk factors on seizure development. In addition, it may not be necessary to prescribe seizure prophylactic drugs in these patients. Conducting a larger, multicenter study may address these limitations and further highlight the benefits of r-tPA in AIS.
Ethical Considerations
Compliance with ethical guidelines
All procedures and experimental protocols followed the relevant ethical guidelines and regulations. The study received approval from the Ethics Committee of the Vice Chancellor for Research at Guilan University of Medical Sciences (GUMS), Rasht, Iran (Code: IR.GUMS.REC.1399.615). All participants gave verbal informed consent via phone call to use their medical information, including details obtained from phone interviews, doctor visits, and previously recorded hospital data.
Funding
This study was extracted from the general medical doctorate thesis of Haniyeh Khoshtarash, approved by the Faculty of Medicine, Giulan University of Medical Sciences, Rasht, Iran (Registered No.: 2416).
Authors contributions
Conceptualization: Alia Saberi and Hamidreza Hatamian; Study design and analysis: Hanie Khoshtarash; Methodology: Mohammad Ali Yazdanipour and Somayeh Ahmadi; Supervision: Alia Saberi; Writing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors sincerely thank patients and the Clinical Research Development Units of Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran, for participating in this study.
References
Stroke has remained the reason for inpatient rehabilitation because it leads to significant disabilities and long-term complications such as seizures [1]. Post-stroke seizures (PSS) severely impact patients’ quality of life, increase morbidity, and place emotional and financial strain on both families and society at large [2]. These seizures are classified as early onset and late onset seizures; early onset seizure occurs within two weeks after the stroke. The highest reported rate is approximately 45%, most often occurring within the first 24 hours following cerebrovascular event [3]. If the seizure occurs after two weeks, it is classified as a late onset, which typically develops within the first year post-stroke [4, 5]. While all types of strokes carry an increased risk of seizure, hemorrhagic strokes are associated with a higher incidence compared to ischemic strokes [2].
Nevertheless, it is essential to emphasize that due to the higher incidence of ischemic stroke, the overall burden of post-stroke seizure is greater in this group [2]. Statistics show that seizure develops in 2%-14 % of patients who have had an ischemic stroke [6]. In ischemic stroke patients, factors such as the severity of the initial neurological deficit (measured by the National Institutes of Health Stroke scale [NIHSS]), persistent disability, larger or multiple brain lesions (based on Alberta stroke program early CT score [ASPECTS]), cortical damage, and hippocampus involvement are predictors of post-stroke seizure development [7].
Recombinant tissue plasminogen activator (r-tPA) is currently regarded as the standard thrombolytic treatment for eligible patients with acute ischemic stroke [8]. There is no doubt that r-tPA plays an effective role in these patients' treatment [9]. Still, apart from its thrombolytic capacity, r-tPA has known neurotoxic effects [10-12], including some that may give rise to epileptogenic tissue alterations [13]. Specifically, r-tPA may worsen brain injury by increasing glutamic acid release following ischemia, particularly if it crosses into brain tissue. It further stimulates leukocyte infiltration, activates microglial, and enhances free radical production in the ischemic brain [14]. More importantly, it activates matrix metalloproteases which contribute to the disruption of blood-brain barrier [15-17]. These processes can lead to cerebral edema, intracerebral hemorrhage, and hemorrhagic transformation (HT). Research also indicates that during the early phase following stroke onset, changes such as brain edema and cytotoxic injury, neurotransmitter imbalance, glutamate toxicity, and gliosis may trigger seizure activity [4, 18]. HT following stroke increases the risk of early post-stroke seizure, emphasizing the role of blood leakage in promoting epileptogenesis. This risk is heightened in the cortical region, where HT frequently coexists with edema, further stimulating cortical excitability [19]. These changes, especially when HT and edema are present, may serve as independent predictors of status epilepticus in patients with acute ischemic stroke [20]. This study seeks to clarify whether the use of r-tPA in acute ischemic stroke patients has any statistically significant association with the incidence of post-stroke seizures, thereby contributing to safer and more informed treatment decisions.
Materials and Methods
This retrospective cohort study was conducted on 100 patients with r-tPA treatment and 100 patients without r-tPA treatment, who were first admitted with acute ischemic stroke at an academic stroke center in the north of Iran in the years 2017-2020. To select patients, we searched all patients affected by acute ischemic stroke (AIS) in our center from 2017 to 2020, and then, according to exclusion criteria, we selected them randomly. Exclusion criteria involved any pre-existing epilepsy or seizures, the use of antiepileptic medication before receiving inpatient care, and the presence of other significant neurological disorders or metabolic abnormalities, which are risk factors for seizures and patients who are dead.
Data gathered for all patients included demographic information (age, gender), medical history (hypertension, diabetes, hyperlipidemia, cardiac disease), and lifestyle factors (smoking, alcohol, and opium use). Additional stroke-specific data were also recorded, such as the affected artery, side, and size of the infarct and ASPECTS and NIHSS scores upon admission. To differentiate early from late-onset seizure, a 2-week threshold following stroke onset, similar to the approach used in posttraumatic seizure, was applied [8]. The stroke management protocol at our center involves promoting non-contrast brain CT imaging and an assessment for potential eligibility for intravenous thrombolysis therapy. Information such as demographic factors, underlying disease and smoking, and alcohol or opium use was collected from the academic stroke center’s medical files. Infarct radiologic features were collected from the academic stroke center’s PACS (picture archiving and communication system). The occurrence and timing of PSS were obtained during the patient’s follow-up. This information was collected via phone interviews for those who did not attend the visit.
Statistical analysis
The sample size was determined using G*Power software, version 3.1, relying on the seizure frequencies of 19% in the r-tPA group and 6% in the non-r-tPA group reported by Nesselroth et al. [8]. To achieve a power of 0.80 and an alpha level of 0.05, it was established that a minimum of 100 cases is necessary in each group.
The data were arranged using Microsoft Excel, version 2013 (Microsoft Corporation, Redmond, WA, USA) and analyzed with IBM SPSS Statistics for Windows (version 26.0; IBM Corp., Armonk, NY, USA). Mean±SD is used for continuous variables, while numbers (percentages) are used for categorical variables. Normal distribution was assessed using the Shapiro–Wilk test. The chi-squared test or Fisher exact test was applied to categorical variables, and the independent t-test or Mann-Whitney U test was used for continuous variables in group comparisons. A multivariate regression model included variables with a significance level of P<0.20 or lower on initial univariate analysis. Logistic regression with control on accompanying parameters was conducted to evaluate the association between r-tPA and seizure; adjusted odds ratios are provided. A P≤0.05 was considered statistically significant.
Results
Of 200 patients, 100 received intravenous r-tPA, while 100 did not. Both groups were matched in terms of sex and major stroke risk factors, including diabetes, hypertension, hyperlipidemia, smoking, and substance use. Our result showed that patients with IV r-tPA therapy had a higher mean age (P=0.049), and this group had a higher rate of heart disease (P=0.027).
There was a statistically significant difference in the distribution of involved arteries between patients treated with and without r-tPA therapy (P=0.002). This study showed that the middle cerebral artery (MCA) trunk, superior MCA, and anterior cerebral artery were more involved in the r-tPA group.
The mean NIHSS score at admission was significantly higher in the r-tPA group (11.5) compared to the non-treated group (6.8) (P<0.001). Conversely, the non-treated group had higher ASPECTS scores (P=0.034), indicating less severe infarcts (Table 1).

Figure 1 shows 19 patients (10%) who experienced seizures in our retrospective cohort study. Of these, 8(8%) were in the non-r-tPA group and 11(11%) in the r-tPA group. The majority of seizures were classified as late onset. Although the seizure rate was slightly higher in the r-tPA group, statistical analysis revealed no significant difference in seizure incidence between the r-tPA and non-r-tPA groups (P=0.469).

We found no statistical association between baseline parameters, including age, sex, smoking, alcohol or opium use, and underlying diseases and PSS in both groups (P>0.05).
However, our results indicated a statistically significant relationship between the lesion location and the risk of developing secondary seizures. Specifically, involvement of the MCA trunk was associated with a higher risk for post-stroke seizure in the non-r-tPA group (Table 2).

Furthermore, a significant association was absorbed between seizure incidence and stroke severity in both groups, as measured by the NIHSS score at admission. Patients with high-grade strokes (NIHSS ≥6) had a higher likelihood of developing PSS compared to those with mild strokes (NIHSS ≤5) (Table 3).

As in Table 4, this finding indicated that considering all dependent factors, r-tPA therapy does not increase the risk of post-stoke seizure. In addition, the only parameter associated with the risk of post-stoke seizure is the score of ASPECT, and by increasing the number of ASPECTS, the rate of seizure occurrence decreases by 24.

Discussion
The overall incidence of post-stroke seizure, according to our data, was 10, with rates of 11 in the r-tPA group and 8 in the non-r-tPA group. Our findings did not demonstrate a significant association between r-tPA administration and post-stroke seizure (P=469). This finding is consistent with previous studies [21-23]. However, the role of r-tpA in the development of PSS remains controversial, as some recent studies reported conflicting results. For instance, research by Brigo et al. [24] stroke localization, NIHSS and Alvarez et al. [25] indicates a higher risk of seizure following r-tPA administration. One proposed mechanism suggests that reperfusion injury, possibly mediated by free-radical, may provoke seizure even in the absence of hemorrhage [25]; in contrast, Nesselroth et al. reported that r-tPA was linked to a reduced risk of post-stroke seizure [8].
Also, we found no association between individual characteristics and PSS. However, some studies have suggested that a higher cholesterol level may protect against seizure [26, 27]. Possibly due to the anticonvulsant properties of cholesterol-derived compounds, such as neurosteroids [28]. However, our data do not support the proposed protective effect of hypercholesterolemia. Also, the Krakow study showed diabetes as a predictor of PSS [29]. It can be explained by the mechanism that acute seizures after AIS are thought to result from cellular biochemical dysfunction leading to electrically irritable tissue [30]. Acute ischemia elevates intracellular levels of calcium and sodium as well as extracellular glutamate in the peri-infarct region. These epileptogenic mechanisms may be further intensified by hyperglycemia in diabetic patients, a phenomenon also supported by experimental findings [29].
Regarding the type of treatment, we found that MCA trunk is more commonly involved in patients with PSS. Because it causes a broad cortex involvement, it is essential to note that many previous studies support the epileptogenic potential of cortical involvement. They confirm that participation of the cerebral cortex, such as in trauma [31] and tumor [32], is found to increase the risk of seizure. This is due to the role of the neocortex in seizure occurrence [33] and the limited number of patients with the involvement of any other arteries in this study; we cannot conclude them.
Also, according to our study regarding the type of treatment, patients with higher NIHSS scores have more chance for PSS (P=0.019). As another risk factor, we found a significant association between ASPECTS and seizure occurrence (odds ratio 0.76, P=025).
To summarize, r-tPA treatment did not affect the frequency of post-stroke seizure manifestation compared to patients who were not treated with r-tPA.
This study has several limitations, the most important being the small sample size. Although the participants were of varied ages, which may reduce some selection bias, the relatively limited sample may still influence the statistical power and generalizability of the results. Further research with larger cohorts is necessary to draw a more definitive conclusion.
Additionally, several potential biases must be acknowledged. Patients in the non-r-tPA group either had contraindications to r-tPA therapy or presented to the hospital more than 4.5 hours after symptom onset, resulting in prolonged cerebral ischemia. As a result, it was initially assumed that this group would include more severe and complex cases. However, findings from the study by Nesselroth et al. [8] showed a lower average NIHSS score in the non-r-tPA group. This inconsistency could be due to the inclusion of patients with milder strokes who were not eligible for r-tPA administration. Thus, selection bias may have been a confounding variable, potentially affecting the study’s findings.
Conclusion
Based on the findings of this retrospective cohort study, administration of r-tPA in patients with acute ischemic stroke does not appear to increase the risk of post-stroke seizures. The treatment remains a safe and effective therapeutic approach within the appropriate clinical timeframe. Continuous monitoring and further studies may help clarify the impact of additional risk factors on seizure development. In addition, it may not be necessary to prescribe seizure prophylactic drugs in these patients. Conducting a larger, multicenter study may address these limitations and further highlight the benefits of r-tPA in AIS.
Ethical Considerations
Compliance with ethical guidelines
All procedures and experimental protocols followed the relevant ethical guidelines and regulations. The study received approval from the Ethics Committee of the Vice Chancellor for Research at Guilan University of Medical Sciences (GUMS), Rasht, Iran (Code: IR.GUMS.REC.1399.615). All participants gave verbal informed consent via phone call to use their medical information, including details obtained from phone interviews, doctor visits, and previously recorded hospital data.
Funding
This study was extracted from the general medical doctorate thesis of Haniyeh Khoshtarash, approved by the Faculty of Medicine, Giulan University of Medical Sciences, Rasht, Iran (Registered No.: 2416).
Authors contributions
Conceptualization: Alia Saberi and Hamidreza Hatamian; Study design and analysis: Hanie Khoshtarash; Methodology: Mohammad Ali Yazdanipour and Somayeh Ahmadi; Supervision: Alia Saberi; Writing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors sincerely thank patients and the Clinical Research Development Units of Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran, for participating in this study.
References
- Pande SD, Lwin MT, Kyaw KM, Khine AA, Thant AA, Win MM, et al. Post-stroke seizure-Do the locations, types and managements of stroke matter? Epilepsia Open. 2018; 3(3):392-8. [DOI:10.1002/epi4.12249] [PMID] [PMCID]
- Zhang C, Wang X, Wang Y, Zhang JG, Hu W, Ge M, Zhang K, et al. Risk factors for post-stroke seizures: A systematic review and meta-analysis. Epilepsy Res. 2014; 108(10):1806-16. [DOI:10.1016/j.eplepsyres.2014.09.030] [PMID]
- Berlet MH, Stambo GW, Kelley M, Van Epps K, Woeste T, Steffen D. Does modern ischemic stroke therapy in a large community-based dedicated stroke center improve clinical outcomes? A two-year retrospective study. J Stroke Cerebrovasc Dis. 2014; 23(5):869-78. [DOI:10.1016/j.jstrokecerebrovasdis.2013.07.016] [PMID]
- Myint PK, Staufenberg EF, Sabanathan K. Post-stroke seizure and post-stroke epilepsy. Postgrad Med J. 2006; 82(971):568-72. [DOI:10.1136/pgmj.2005.041426] [PMID] [PMCID]
- Olsen TS. Post-stroke epilepsy. Curr Atheroscler Rep. 2001; 3(4):340-4. [DOI:10.1007/s11883-001-0029-4] [PMID]
- Menon B, Shorvon SD. Ischaemic stroke in adults and epilepsy. Epilepsy Res. 2009; 87(1):1-11. [DOI:10.1016/j.eplepsyres.2009.08.007] [PMID]
- Browne TR, Holmes GL. Handbook of epilepsy. Burlington: Jones & Bartlett Learning; 2008. [Link]
- Nesselroth D, Gilad R, Namneh M, Avishay S, Eilam A. Estimation of seizures prevalence in ischemic strokes after thrombolytic therapy. Seizure. 2018; 62:91-94. [DOI:10.1016/j.seizure.2018.09.001] [PMID]
- Medcalf RL, Davis SM. Plasminogen activation and thrombolysis for ischemic stroke. Int J Stroke. 2012; 7(5):419-25. [DOI:10.1111/j.1747-4949.2012.00783.x] [PMID]
- Goto H, Fujisawa H, Oka F, Nomura S, Kajiwara K, Kato S, et al. Neurotoxic effects of exogenous recombinant tissue-type plasminogen activator on the normal rat brain. J Neurotrauma. 2007; 24(4):745-52. [DOI:10.1089/neu.2006.0183] [PMID]
- Lemarchant S, Docagne F, Emery E, Vivien D, Ali C, Rubio M. tPA in the injured central nervous system: Different scenarios starring the same actor? Neuropharmacology. 2012; 62(2):749-56. [DOI:10.1016/j.neuropharm.2011.10.020]
- Harston GW, Sutherland BA, Kennedy J, Buchan AM. The contribution of L-arginine to the neurotoxicity of recombinant tissue plasminogen activator following cerebral ischemia: a review of rtPA neurotoxicity. J Cereb Blood Flow Metab. 2010; 30(11):1804-16. [DOI:10.1038/jcbfm.2010.149] [PMID] [PMCID]
- Samson AL, Medcalf RL. Tissue-type plasminogen activator: A multifaceted modulator of neurotransmission and synaptic plasticity. Neuron. 2006; 50(5):673-8. [DOI:10.1016/j.neuron.2006.04.013] [PMID]
- Wang W, Li M, Chen Q, Wang J. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: Mechanisms, models, and biomarkers. Mol Neurobiol. 2015; 52(3):1572-9. [DOI:10.1007/s12035-014-8952-x] [PMID] [PMCID]
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004; 35(11 Suppl 1):2726-30. [DOI:10.1161/01.STR.0000143219.16695.af] [PMID]
- Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005; 36(9):1954-9. [DOI:10.1161/01.STR.0000177517.01203.eb] [PMID]
- Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribó M, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003; 107(4):598-603. [DOI:10.1161/01.CIR.0000046451.38849.90] [PMID]
- Saberi A, Ghayeghran AR, Kazemnejad E, Nasiri M, Kazemi S. Post-stroke seizure and its risk factors. J of Guilan Univ of Med Sci 2017; 26(104):1-13. [Link]
- Alberti A, Paciaroni M, Caso V, Venti M, Palmerini F, Agnelli G. Early seizures in patients with acute stroke: frequency, predictive factors, and effect on clinical outcome. Vasc Health Risk Manag. 2008; 4(3):715-20. [Link]
- Bateman BT, Claassen J, Willey JZ, Hirsch LJ, Mayer SA, Sacco RL, et al. Convulsive status epilepticus after ischemic stroke and intracerebral hemorrhage: Frequency, predictors, and impact on outcome in a large administrative dataset. Neurocrit Care. 2007; 7(3):187-93. [DOI:10.1007/s12028-007-0056-2] [PMID]
- Abbasi MH, Esmaeili S, Tabatabaee S, Sheibani F, Pirouz MS, Joghataei MT, et al. No concerns for post-stroke seizure or epilepsy with rTPA; A retrospective cohort study. [Unpublished]. [DOI:10.21203/rs.3.rs-1669033/v1]
- De Reuck J, Van Maele G. Acute ischemic stroke treatment and the occurrence of seizures. Clin Neurol Neurosurg. 2010; 112(4):328-31. [DOI:10.1016/j.clineuro.2010.01.004] [PMID]
- Lekoubou A, Fox J, Ssentongo P. Incidence and association of reperfusion therapies with poststroke seizures: A systematic review and meta-analysis. Stroke. 2020; 51(9):2715-23. [DOI:10.1161/STROKEAHA.119.028899] [PMID]
- Brigo F, Schneider M, Wagenpfeil G, Ragoschke-Schumm A, Fousse M, Holzhoffer C, et al. Intravenous thrombolysis with tPA and cortical involvement increase the risk of early poststroke seizures: Results of a case-control study. Epilepsy Behav. 2020; 104(Pt B):106312. [DOI:10.1016/j.yebeh.2019.04.056] [PMID]
- Alvarez V, Rossetti AO, Papavasileiou V, Michel P. Acute seizures in acute ischemic stroke: Does thrombolysis have a role to play? J Neurol. 2013; 260(1):55-61. [DOI:10.1007/s00415-012-6583-6] [PMID]
- Beghi E, D'Alessandro R, Beretta S, Consoli D, Crespi V, Delaj L, et al. Incidence and predictors of acute symptomatic seizures after stroke. Neurology. 2011; 77(20):1785-93. [DOI:10.1212/WNL.0b013e3182364878] [PMID]
- Devuyst G, Karapanayiotides T, Hottinger I, Van Melle G, Bogousslavsky J. Prodromal and early epileptic seizures in acute stroke: Does higher serum cholesterol protect? Neurology. 2003; 61(2):249-52. [DOI:10.1212/01.WNL.0000070410.68541.7E] [PMID]
- Biagini G, Panuccio G, Avoli M. Neurosteroids and epilepsy. Curr Opin Neurol. 2010; 23(2):170-6. [DOI:10.1097%2FWCO.0b013e32833735cf] [PMID] [PMCID]
- Krakow K, Sitzer M, Rosenow F, Steinmetz H, Foerch C; Arbeitsgruppe Schlaganfall Hessen. Predictors of acute poststroke seizures. Cerebrovasc Dis. 2010; 30(6):584-9. [DOI:10.1159/000319777] [PMID]
- Camilo O, Goldstein LB. Seizures and epilepsy after ischemic stroke. Stroke. 2004; 35(7):1769-75. [DOI:10.1161/01.STR.0000130989.17100.96] [PMID]
- Englander J, Bushnik T, Duong TT, Cifu DX, Zafonte R, Wright J, et al. Analyzing risk factors for late posttraumatic seizures: A prospective, multicenter investigation. Arch Phys Med Rehabil. 2003; 84(3):365-73. [DOI:10.1053/apmr.2003.50022] [PMID]
- van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: Epidemiology, mechanisms, and management. Lancet Neurol. 2007; 6(5):421-30. [DOI:10.1016/S1474-4422(07)70103-5] [PMID]
- Connors BW. Initiation of synchronized neuronal bursting in neocortex. Nature. 1984; 310(5979):685-7. [DOI:10.1038/310685a0] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2025/03/17 | Accepted: 2025/06/13 | Published: 2025/07/1
Received: 2025/03/17 | Accepted: 2025/06/13 | Published: 2025/07/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



