Sat, Jan 3, 2026
Volume 11, Issue 1 (Winter 2025)
Caspian J Neurol Sci 2025, 11(1): 87-94 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ehtiatkar Z, Saberi A, Ghorbani Shirkouhi S, Hosseininezhad M, Yousefzadeh-Chabok S, Moadabi Y, et al . Nano-curcumin Effects on Ischemic Stroke Clinical Outcomes and Interleukin-6 Levels: Pilot Randomized Clinical Trial. Caspian J Neurol Sci 2025; 11 (1) :87-94
URL: http://cjns.gums.ac.ir/article-1-768-en.html
URL: http://cjns.gums.ac.ir/article-1-768-en.html
Zeinab Ehtiatkar1 

 , Alia Saberi1
, Alia Saberi1 

 , Samaneh Ghorbani Shirkouhi1
, Samaneh Ghorbani Shirkouhi1 

 , Mozaffar Hosseininezhad1
, Mozaffar Hosseininezhad1 

 , Shahrokh Yousefzadeh-Chabok2
, Shahrokh Yousefzadeh-Chabok2 

 , Yaser Moadabi1
, Yaser Moadabi1 

 , Ashkan Karimi1
, Ashkan Karimi1 

 , Haniyeh Shadrou Gharani1
, Haniyeh Shadrou Gharani1 

 , Sasan Andalib *3
, Sasan Andalib *3 




 , Alia Saberi1
, Alia Saberi1 

 , Samaneh Ghorbani Shirkouhi1
, Samaneh Ghorbani Shirkouhi1 

 , Mozaffar Hosseininezhad1
, Mozaffar Hosseininezhad1 

 , Shahrokh Yousefzadeh-Chabok2
, Shahrokh Yousefzadeh-Chabok2 

 , Yaser Moadabi1
, Yaser Moadabi1 

 , Ashkan Karimi1
, Ashkan Karimi1 

 , Haniyeh Shadrou Gharani1
, Haniyeh Shadrou Gharani1 

 , Sasan Andalib *3
, Sasan Andalib *3 


1- Neuroscience Research Center, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Road Trauma Research Center, Trauma Institute, Guilan University of Medical Sciences, Rasht, Iran.
3- Research Unit of Neurology, Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark. ,sasan.andalib@health.sdu.dk
2- Road Trauma Research Center, Trauma Institute, Guilan University of Medical Sciences, Rasht, Iran.
3- Research Unit of Neurology, Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark. ,
Full-Text [PDF 1491 kb]
(544 Downloads)
| Abstract (HTML) (880 Views)
Full-Text: (758 Views)
Introduction
Stroke is classified into two main types: Ischemic and hemorrhagic. Ischemic stroke can result from a thrombotic or embolic event that reduces blood flow to the brain. Penumbra refers to the poorly perfusion tissue around the ischemic core, where blood flow is low for electrical activity but enough to maintain ion channels. However, this region is exposed to various harmful metabolic processes, including excitotoxicity, oxidative stress and inflammatory response, which spread from the core to adjacent tissues, leading to the expansion of the ischemic core and subsequent clinical consequences [1].
Significant increases in interleukin-6 (IL-6) levels have been demonstrated in patients with stroke shortly following the ischemic event [2]. Elevated IL-6 levels in the early phase of ischemic stroke can be related to infarct size, neurological deterioration, and poor outcome [3]. On the other hand, IL-6 can induce the liberation of prostaglandin E2 (PGE2) in the brain [4]. PGE2 activates the hypothalamus, giving rise to an increase in body temperature [5]. Fever has been reported to be associated with poor outcomes following stroke [6].
Reactive oxygen species (ROS) serve a crucial role in human physiological processes. Nevertheless, ROS overproduction occurs in ischemic stroke, an important mediator of ischemic damage [7]. Anti-ROS treatments have been used in ischemic stroke [7]. Curcumin is an extract from the Curcuma longa (turmeric) root. Turmeric extract contains three curcuminoids: Curcumin, desmethoxycurcumin, and bisdemethoxycurcumin. Curcumin is turmeric’s most active component [8]. It exerts a preventive effect on stroke by reducing oxidative stress and improving vascular endothelial function [9]. Curcumin is believed to be a potential antioxidant and anti-inflammatory agent [10]. It reduces lipid peroxidation and inhibits the expression of nuclear factor-κB (NF-κB) and tumor necrosis factor-alpha (TNF-α) [10].
Curcumin is lipophilic and can cross the blood-brain barrier [11, 12]. It reduces brain TNF-α and IL-6 levels in the animal model of stroke [13]. Jiang et al. [14] demonstrated that a single curcumin dose (1 and 2 mg/kg, IV) 30 minutes following focal cerebral ischemia in rats would reduce mortality, infarct volume, the brain’s water content, and neurological deficits.
As curcumin is lipophilic, the oral curcumin absorption is very low. However, nano-curcumin is encapsulated in the hydrophobic part of curcumin nano-micelles. These spherical nano-micelles are 10 nm in size and increase the water curcumin solubility, and their bioavailability after oral use is significantly higher than that of a simple powder form of common curcumin [15].
Previous findings have demonstrated curcumin’s anti-inflammatory effects. Given this substance’s low absorption and rapid metabolism, we decided to investigate the improvement of ischemic stroke symptoms after treatment with nano-curcumin, which is more stable and soluble than curcumin.
Materials and Methods
This study is a double-blind, randomized clinical trial pilot study on ischemic stroke patients hospitalized at Neurology Ward, Poursina Hospital, Guilan University of Medical Sciences, Rasht City, Iran. Forty acute ischemic stroke patients confirmed by computed tomography (CT) scan of the brain with symptoms no more than a week were included in the study. Informed consent was obtained from these subjects.
With blocks of four, using a randomization process, the patients were allocated to nano-curcumin and control groups, with 20 subjects in each group. In addition to routine antiplatelet, anti-lipid and antihypertensive medications, which were given to both groups for possible stroke prophylaxis, the curcumin group received an 80-mg curcumin capsule in the form of nano-micelles (Sina Curcumin®, Nano Sina Elixir Co., Iran) daily for 1 month. The controls received a placebo of curcumin (capsule containing polysorbate 80 produced by Nano Sina Elixir Co., Iran) for 1 month. The patients and their evaluator were blind to the treatment or placebo.
Patients were followed for one month, and the National Institutes of Health Stroke Scale (NIHSS) [16, 17] and modified Rankin scale (mRS) [18] were used for the assessment of the neurological deficits of the patients. These questionnaires were completed at three time points: baseline (before the treatment, starting point for assessing the variables), one week and one month after the treatment.
A blood sample was collected from the patients in three stages. IL-6 serum levels were assessed at the baseline and one month after the treatment using an ELISA kit in the two groups.
The prothrombin time (PT), partial thromboplastin time (PTT), clotting time (CT), and bleeding time (BT) were assessed in the two groups at the baseline and after one week.
The volume of the infarct area was also evaluated using brain CT scans one day and one week after the stroke. A brain CT scan was also performed if the patient’s symptoms worsened with a suspicion of hemorrhagic stroke.
The study’s inclusion and exclusion criteria are tabulated in Table 1.
A per-protocol approach was used in data analysis. The data were analyzed using SPSS statistical software, version 22. Quantitative variables in the two groups were examined by an independent t-test when the data were normally distributed and by the Mann-Whitney U test in case of a non-normal distribution. Qualitative variables in the two groups were compared using the chi-square test. A repeated measure analysis of variance (ANOVA) was used to compare the results at different follow-up times.
Results
As shown in Table 2, half of the patients were men, and half were women in the curcumin group.
In the control group, 60% of the patients were women and 40% were men. Also, the Mean±SD of the age of the patients participating in the present study was 73.45±9.87 and 67.35±11 years in the curcumin group and the controls, respectively. In terms of the location of the stroke, most of the infarcts in both the curcumin and control groups were in the right superior and inferior middle cerebral arteries (MCA) (n=6, 30%). No significant differences in the stroke location were seen between the two curcumin groups (P=0.990) (Table 2).
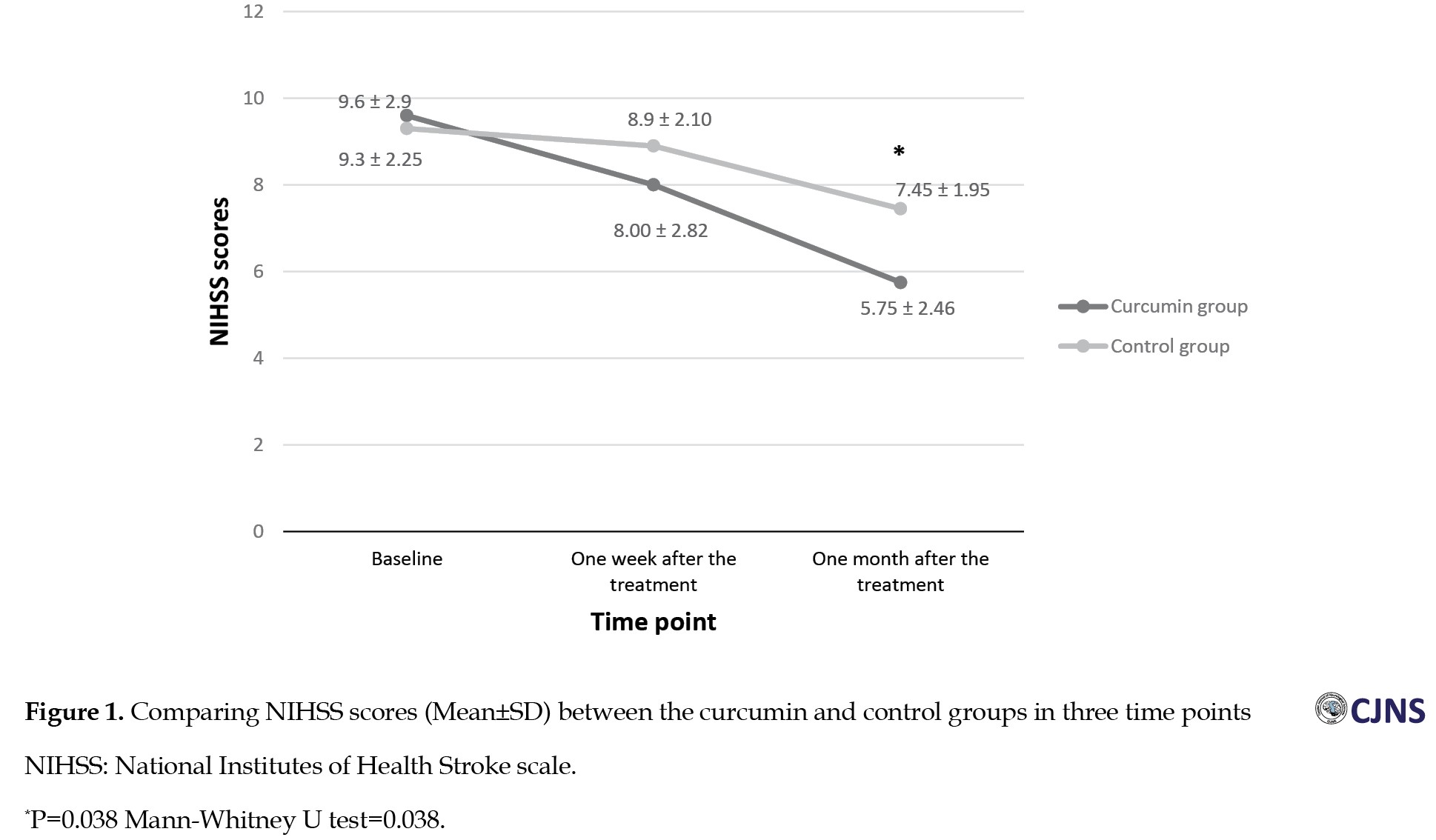
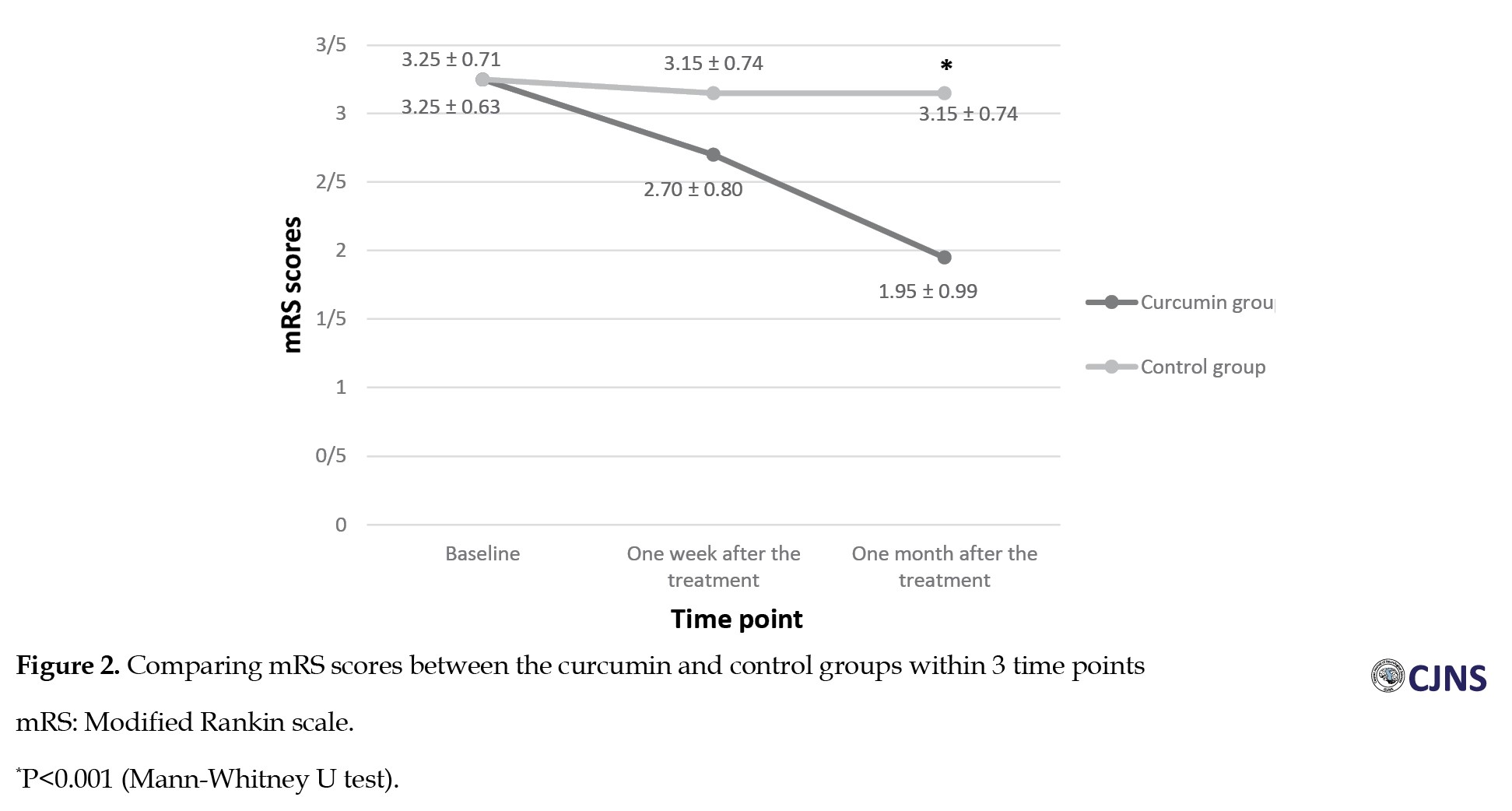
The comparison of the NIHSS scores between the two groups showed no significant difference at the baseline and one week after the treatment (P>0.05), while a significant difference was seen between the two groups one month after the treatment. The NIHSS scores after one month in the curcumin group were significantly lower than those in the control group (5.75±2 vs 7.45±1.95, P=0.038) (Figure 1). The comparison of the mRS scores indicated no significant differences between the two groups at the baseline and one week following the treatment. However, one month after the treatment, mRS scores in the curcumin group were significantly low compared with those in the control group (1.95±0.99 vs 3.15±0.74, P<0.001) (Figure 2). The comparison of the volume of the infarct at the baseline (43.10±15.61 vs 37±12.51) and one week after the treatment (44.25±16.83 vs 42.90±13.17) showed no significant difference between the curcumin and the control groups (P>0.05) (Table 3).
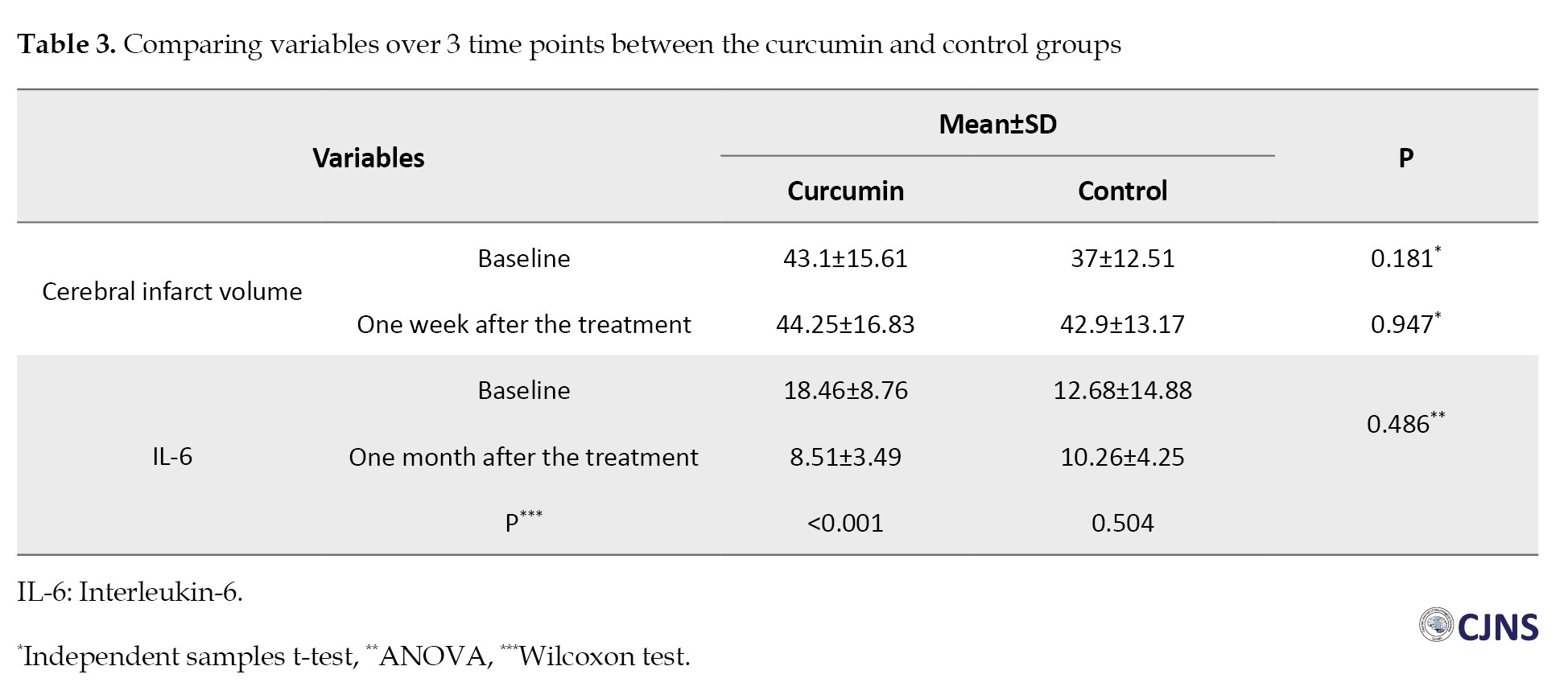
One month after the treatment, evaluation of IL-6 serum levels demonstrated no significant differences between the two groups (P=0.486). There was a significant difference in IL-6 serum levels at the baseline and one month after the treatment in the curcumin group (P<0.001). Nonetheless, no significant difference was seen among the controls (P=0.504) (Table 3).
As shown in Table 4, the results of comparing changes in the variables at the baseline and one week after the treatment, as well as at the baseline and one month after the treatment, illustrated a significant difference between the two groups.
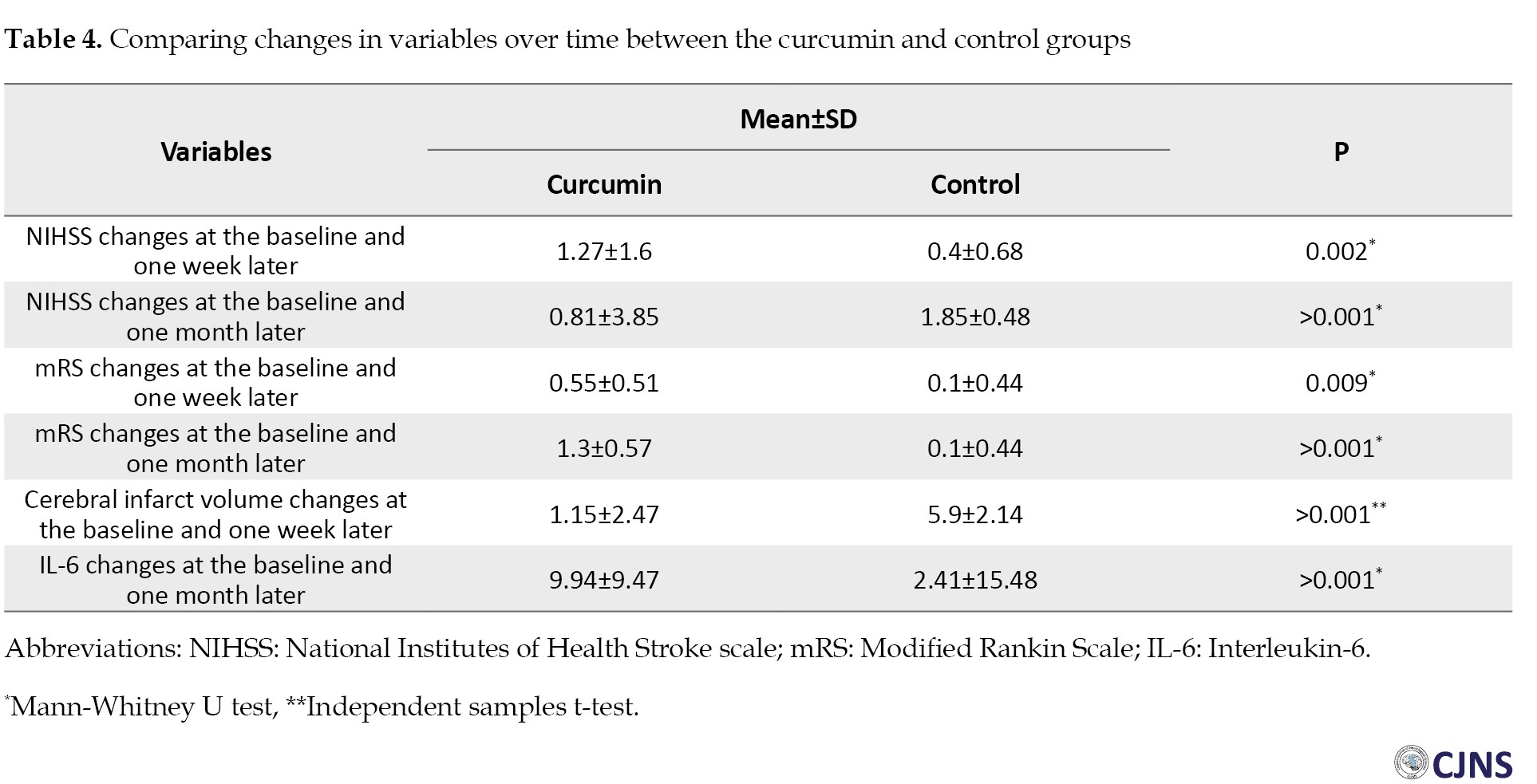
The changes in the curcumin group were significantly greater than those in the control group (P<0.05) (Table 4).
The coagulation parameters were within normal limits in the two groups. The Mann-Whitney U test results indicated no significant difference between the curcumin group and the controls at the baseline and one week after the treatment in PT (P=0.98, P=0.56, respectively), PTT (P=0.18, P=0.49, respectively), CT (P=0.65, P=0.10, respectively) and BT (P=0.77, P=0.98, respectively) parameters.
Discussion
In this double-blind, randomized clinical trial pilot study, the effect of nano-curcumin (Sina Curcumin®, Nano Sina Elixir Co., Iran) was evaluated on clinical symptoms and IL-6 serum levels in ischemic stroke patients. Nano-curcumin in ischemic stroke patients in the acute phase of the disease significantly reduced NIHSS and mRS scores one month after the treatment compared to those of the controls.
After one month, it was observed that nano-curcumin could reduce IL-6 serum levels in the patients compared with those in the controls. Abdolahi et al. [19] demonstrated the effect of nano-curcumin on reducing the IL-6 serum levels of subjects afflicted with migraine. In the Zhang et al. [20] investigation, the stroke models of rats received curcumin (25 mg/kg). IL-6 and TNF-α decreased significantly in the brain region after using curcumin. Zahedi et al. [21] studied the effect of Curcumin C3 Complex® on 62 patients with traumatic brain injury for 7 consecutive days. Compared with baseline, serum levels of IL-6 and TNF-α were significantly reduced in the treated patients. Dolati et al. [22] evaluated the nano-curcumin anti-inflammatory effect in 50 relapsing-remitting multiple sclerosis patients for 6 months. The results indicated significant down-regulation in the levels of expression of IL-6, TNF-α and other inflammatory factors. A clinical trial by Ringman et al. [23] indicated no significant difference in MMSE scores in the patients with Alzheimer's disease who were treated with two doses of 2 g/d and 4 g/d of curcumin C3 Complex® compared with those in the placebo group.
NIHSS scores were evaluated in the present study at the baseline, one week after the treatment and one month later. The results showed that NIHSS scores decreased significantly over time in both groups, and the comparison showed no significant difference in NIHSS scores between the curcumin and control groups at the baseline and one week after the treatment. However, one month after the treatment, the NIHSS scores in the curcumin group were significantly lower than in the controls. This result means that the clinical symptoms improved in the curcumin group more than in the control group [24, 25].
Also, mRS scores were assessed at the baseline, one week following the treatment, and one month later. A comparison of mRS scores illustrated no significant difference between the two groups at the baseline and one week after the treatment. However, one month after the treatment, in the curcumin group, the mRS scores were significantly low compared with those in the controls. Also, an intragroup comparison of mRS scores showed that in the curcumin group, mRS scores decreased significantly over time, while no significant difference was observed in the control group. mRS is used to assess disability after a stroke. This outcome means that functional independence increased in the curcumin group more than in the control group [24, 26].
Using the CT scan, the cerebral infarct volume in the patients was compared at the baseline and one week after the treatment. The results indicated a significant increase in the cerebral infarct volume in the controls within one week, while this observed increase was not significant in the curcumin group. Preclinical studies suggested that decreased volume infarction may reflect curcumin’s protective and anti-inflammatory effects against the damaged brain [27, 28].
Anti-inflammatory and neuroprotective effects of nano-curcumin have been shown in various preclinical and clinical studies. The nano-curcumin indicated a much higher aqueous diffusion than the free form, which leads to improved systemic availability. Also, nano-encapsulation of curcumin may improve circulation and retention of medicine in the body. This mechanism reduces and maintains the threshold level of curcumin [29]. This pilot study’s findings demonstrated the nano-curcumin effect on improving NIHSS and mRS scores and reducing infarct volume and serum levels of IL-6.
Conclusion
In this pilot study, we examined the effect of nano-curcumin on stroke clinical symptoms and serum IL-6 levels in ischemic stroke patients. The present study showed nano-curcumin’s protective and anti-inflammatory effects against the damaged brain. Future studies with a larger sample size should evaluate if nano-curcumin could be a promising treatment for improving stroke clinical symptoms in ischemic stroke patients.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Guilan University of Medical Sciences, Rasht, Iran (Code: IR.GUMS.REC.1398.222) and was registered by the Iranian Clinical Trials Registration System (IRCT) (Code: IRCT20091108002680N3).
Funding
This study was financially supported by the Research and Technology Vice-Chancellorship of Guilan University of Medical Sciences, Guilan, Iran.
Authors contributions
All authors have contributed to this study and preparation of this article. All authors approved the final version of the article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors appreciate the support of the Medical Staff at Poursina Clinical Research Development Unit, Poursina Hospital and Guilan University of Medical Sciences, Rasht, Iran.
References
Stroke is classified into two main types: Ischemic and hemorrhagic. Ischemic stroke can result from a thrombotic or embolic event that reduces blood flow to the brain. Penumbra refers to the poorly perfusion tissue around the ischemic core, where blood flow is low for electrical activity but enough to maintain ion channels. However, this region is exposed to various harmful metabolic processes, including excitotoxicity, oxidative stress and inflammatory response, which spread from the core to adjacent tissues, leading to the expansion of the ischemic core and subsequent clinical consequences [1].
Significant increases in interleukin-6 (IL-6) levels have been demonstrated in patients with stroke shortly following the ischemic event [2]. Elevated IL-6 levels in the early phase of ischemic stroke can be related to infarct size, neurological deterioration, and poor outcome [3]. On the other hand, IL-6 can induce the liberation of prostaglandin E2 (PGE2) in the brain [4]. PGE2 activates the hypothalamus, giving rise to an increase in body temperature [5]. Fever has been reported to be associated with poor outcomes following stroke [6].
Reactive oxygen species (ROS) serve a crucial role in human physiological processes. Nevertheless, ROS overproduction occurs in ischemic stroke, an important mediator of ischemic damage [7]. Anti-ROS treatments have been used in ischemic stroke [7]. Curcumin is an extract from the Curcuma longa (turmeric) root. Turmeric extract contains three curcuminoids: Curcumin, desmethoxycurcumin, and bisdemethoxycurcumin. Curcumin is turmeric’s most active component [8]. It exerts a preventive effect on stroke by reducing oxidative stress and improving vascular endothelial function [9]. Curcumin is believed to be a potential antioxidant and anti-inflammatory agent [10]. It reduces lipid peroxidation and inhibits the expression of nuclear factor-κB (NF-κB) and tumor necrosis factor-alpha (TNF-α) [10].
Curcumin is lipophilic and can cross the blood-brain barrier [11, 12]. It reduces brain TNF-α and IL-6 levels in the animal model of stroke [13]. Jiang et al. [14] demonstrated that a single curcumin dose (1 and 2 mg/kg, IV) 30 minutes following focal cerebral ischemia in rats would reduce mortality, infarct volume, the brain’s water content, and neurological deficits.
As curcumin is lipophilic, the oral curcumin absorption is very low. However, nano-curcumin is encapsulated in the hydrophobic part of curcumin nano-micelles. These spherical nano-micelles are 10 nm in size and increase the water curcumin solubility, and their bioavailability after oral use is significantly higher than that of a simple powder form of common curcumin [15].
Previous findings have demonstrated curcumin’s anti-inflammatory effects. Given this substance’s low absorption and rapid metabolism, we decided to investigate the improvement of ischemic stroke symptoms after treatment with nano-curcumin, which is more stable and soluble than curcumin.
Materials and Methods
This study is a double-blind, randomized clinical trial pilot study on ischemic stroke patients hospitalized at Neurology Ward, Poursina Hospital, Guilan University of Medical Sciences, Rasht City, Iran. Forty acute ischemic stroke patients confirmed by computed tomography (CT) scan of the brain with symptoms no more than a week were included in the study. Informed consent was obtained from these subjects.
With blocks of four, using a randomization process, the patients were allocated to nano-curcumin and control groups, with 20 subjects in each group. In addition to routine antiplatelet, anti-lipid and antihypertensive medications, which were given to both groups for possible stroke prophylaxis, the curcumin group received an 80-mg curcumin capsule in the form of nano-micelles (Sina Curcumin®, Nano Sina Elixir Co., Iran) daily for 1 month. The controls received a placebo of curcumin (capsule containing polysorbate 80 produced by Nano Sina Elixir Co., Iran) for 1 month. The patients and their evaluator were blind to the treatment or placebo.
Patients were followed for one month, and the National Institutes of Health Stroke Scale (NIHSS) [16, 17] and modified Rankin scale (mRS) [18] were used for the assessment of the neurological deficits of the patients. These questionnaires were completed at three time points: baseline (before the treatment, starting point for assessing the variables), one week and one month after the treatment.
A blood sample was collected from the patients in three stages. IL-6 serum levels were assessed at the baseline and one month after the treatment using an ELISA kit in the two groups.
The prothrombin time (PT), partial thromboplastin time (PTT), clotting time (CT), and bleeding time (BT) were assessed in the two groups at the baseline and after one week.
The volume of the infarct area was also evaluated using brain CT scans one day and one week after the stroke. A brain CT scan was also performed if the patient’s symptoms worsened with a suspicion of hemorrhagic stroke.
The study’s inclusion and exclusion criteria are tabulated in Table 1.

A per-protocol approach was used in data analysis. The data were analyzed using SPSS statistical software, version 22. Quantitative variables in the two groups were examined by an independent t-test when the data were normally distributed and by the Mann-Whitney U test in case of a non-normal distribution. Qualitative variables in the two groups were compared using the chi-square test. A repeated measure analysis of variance (ANOVA) was used to compare the results at different follow-up times.
Results
As shown in Table 2, half of the patients were men, and half were women in the curcumin group.

In the control group, 60% of the patients were women and 40% were men. Also, the Mean±SD of the age of the patients participating in the present study was 73.45±9.87 and 67.35±11 years in the curcumin group and the controls, respectively. In terms of the location of the stroke, most of the infarcts in both the curcumin and control groups were in the right superior and inferior middle cerebral arteries (MCA) (n=6, 30%). No significant differences in the stroke location were seen between the two curcumin groups (P=0.990) (Table 2).
The comparison of the NIHSS scores between the two groups showed no significant difference at the baseline and one week after the treatment (P>0.05), while a significant difference was seen between the two groups one month after the treatment. The NIHSS scores after one month in the curcumin group were significantly lower than those in the control group (5.75±2 vs 7.45±1.95, P=0.038) (Figure 1). The comparison of the mRS scores indicated no significant differences between the two groups at the baseline and one week following the treatment. However, one month after the treatment, mRS scores in the curcumin group were significantly low compared with those in the control group (1.95±0.99 vs 3.15±0.74, P<0.001) (Figure 2). The comparison of the volume of the infarct at the baseline (43.10±15.61 vs 37±12.51) and one week after the treatment (44.25±16.83 vs 42.90±13.17) showed no significant difference between the curcumin and the control groups (P>0.05) (Table 3).

One month after the treatment, evaluation of IL-6 serum levels demonstrated no significant differences between the two groups (P=0.486). There was a significant difference in IL-6 serum levels at the baseline and one month after the treatment in the curcumin group (P<0.001). Nonetheless, no significant difference was seen among the controls (P=0.504) (Table 3).
As shown in Table 4, the results of comparing changes in the variables at the baseline and one week after the treatment, as well as at the baseline and one month after the treatment, illustrated a significant difference between the two groups.

The changes in the curcumin group were significantly greater than those in the control group (P<0.05) (Table 4).
The coagulation parameters were within normal limits in the two groups. The Mann-Whitney U test results indicated no significant difference between the curcumin group and the controls at the baseline and one week after the treatment in PT (P=0.98, P=0.56, respectively), PTT (P=0.18, P=0.49, respectively), CT (P=0.65, P=0.10, respectively) and BT (P=0.77, P=0.98, respectively) parameters.
Discussion
In this double-blind, randomized clinical trial pilot study, the effect of nano-curcumin (Sina Curcumin®, Nano Sina Elixir Co., Iran) was evaluated on clinical symptoms and IL-6 serum levels in ischemic stroke patients. Nano-curcumin in ischemic stroke patients in the acute phase of the disease significantly reduced NIHSS and mRS scores one month after the treatment compared to those of the controls.
After one month, it was observed that nano-curcumin could reduce IL-6 serum levels in the patients compared with those in the controls. Abdolahi et al. [19] demonstrated the effect of nano-curcumin on reducing the IL-6 serum levels of subjects afflicted with migraine. In the Zhang et al. [20] investigation, the stroke models of rats received curcumin (25 mg/kg). IL-6 and TNF-α decreased significantly in the brain region after using curcumin. Zahedi et al. [21] studied the effect of Curcumin C3 Complex® on 62 patients with traumatic brain injury for 7 consecutive days. Compared with baseline, serum levels of IL-6 and TNF-α were significantly reduced in the treated patients. Dolati et al. [22] evaluated the nano-curcumin anti-inflammatory effect in 50 relapsing-remitting multiple sclerosis patients for 6 months. The results indicated significant down-regulation in the levels of expression of IL-6, TNF-α and other inflammatory factors. A clinical trial by Ringman et al. [23] indicated no significant difference in MMSE scores in the patients with Alzheimer's disease who were treated with two doses of 2 g/d and 4 g/d of curcumin C3 Complex® compared with those in the placebo group.
NIHSS scores were evaluated in the present study at the baseline, one week after the treatment and one month later. The results showed that NIHSS scores decreased significantly over time in both groups, and the comparison showed no significant difference in NIHSS scores between the curcumin and control groups at the baseline and one week after the treatment. However, one month after the treatment, the NIHSS scores in the curcumin group were significantly lower than in the controls. This result means that the clinical symptoms improved in the curcumin group more than in the control group [24, 25].
Also, mRS scores were assessed at the baseline, one week following the treatment, and one month later. A comparison of mRS scores illustrated no significant difference between the two groups at the baseline and one week after the treatment. However, one month after the treatment, in the curcumin group, the mRS scores were significantly low compared with those in the controls. Also, an intragroup comparison of mRS scores showed that in the curcumin group, mRS scores decreased significantly over time, while no significant difference was observed in the control group. mRS is used to assess disability after a stroke. This outcome means that functional independence increased in the curcumin group more than in the control group [24, 26].
Using the CT scan, the cerebral infarct volume in the patients was compared at the baseline and one week after the treatment. The results indicated a significant increase in the cerebral infarct volume in the controls within one week, while this observed increase was not significant in the curcumin group. Preclinical studies suggested that decreased volume infarction may reflect curcumin’s protective and anti-inflammatory effects against the damaged brain [27, 28].
Anti-inflammatory and neuroprotective effects of nano-curcumin have been shown in various preclinical and clinical studies. The nano-curcumin indicated a much higher aqueous diffusion than the free form, which leads to improved systemic availability. Also, nano-encapsulation of curcumin may improve circulation and retention of medicine in the body. This mechanism reduces and maintains the threshold level of curcumin [29]. This pilot study’s findings demonstrated the nano-curcumin effect on improving NIHSS and mRS scores and reducing infarct volume and serum levels of IL-6.
Conclusion
In this pilot study, we examined the effect of nano-curcumin on stroke clinical symptoms and serum IL-6 levels in ischemic stroke patients. The present study showed nano-curcumin’s protective and anti-inflammatory effects against the damaged brain. Future studies with a larger sample size should evaluate if nano-curcumin could be a promising treatment for improving stroke clinical symptoms in ischemic stroke patients.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Guilan University of Medical Sciences, Rasht, Iran (Code: IR.GUMS.REC.1398.222) and was registered by the Iranian Clinical Trials Registration System (IRCT) (Code: IRCT20091108002680N3).
Funding
This study was financially supported by the Research and Technology Vice-Chancellorship of Guilan University of Medical Sciences, Guilan, Iran.
Authors contributions
All authors have contributed to this study and preparation of this article. All authors approved the final version of the article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors appreciate the support of the Medical Staff at Poursina Clinical Research Development Unit, Poursina Hospital and Guilan University of Medical Sciences, Rasht, Iran.
References
- Ramos-Cabrer P, Campos F, Sobrino T, Castillo J. Targeting the ischemic penumbra. Stroke. 2011; 42(1 Suppl):S7-11. [DOI:10.1161/STROKEAHA.110.596684] [PMID]
- Aref HMA, Fahmy NA, Khalil SH, Ahmed MF, ElSadek A, Abdulghani MO. Role of interleukin-6 in ischemic stroke outcome. Egypt J Neurol Psychiatry Neurosurg. 2020; 56:1-7. [DOI:10.1186/s41983-019-0121-8]
- Zhu H, Hu S, Li Y, Sun Y, Xiong X, Hu X, et al. Interleukins and Ischemic Stroke. Front Immunol. 2022; 13:828447. [DOI:10.3389/fimmu.2022.828447] [PMID]
- Wrotek SE, Kozak WE, Hess DC, Fagan SC. Treatment of fever after stroke: Conflicting evidence. Pharmacotherapy. 2011; 31(11):1085-91. [DOI:10.1592/phco.31.11.1085] [PMID]
- Rotondo D, Abul HT, Milton AS, Davidson J. Pyrogenic immunomodulators increase the level of prostaglandin E2 in the blood simultaneously with the onset of fever. Eur J Pharmacol. 1988; 154(2):145-52. [DOI:10.1016/0014-2999(88)90091-X] [PMID]
- Phipps MS, Desai RA, Wira C, Bravata DM. Epidemiology and outcomes of fever burden among patients with acute ischemic stroke. Stroke. 2011; 42(12):3357-62. [DOI:10.1161/STROKEAHA.111.621425] [PMID]
- Li W, Yang S. Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies. Brain Circ. 2016; 2(4):153-63. [DOI:10.4103/2394-8108.195279] [PMID]
- Chearwae W, Anuchapreeda S, Nandigama K, Ambudkar SV, Limtrakul P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem Pharmacol. 2004; 68(10):2043-52. [DOI:10.1016/j.bcp.2004.07.009] [PMID]
- Lan C, Chen X, Zhang Y, Wang W, Wang WE, Liu Y, et al. Curcumin prevents strokes in stroke-prone spontaneously hypertensive rats by improving vascular endothelial function. BMC Cardiovasc Disord. 2018; 18(1):43. [DOI:10.1186/s12872-018-0768-6] [PMID]
- Ciuca MD, Racovita RC. Curcumin: Overview of extraction methods, health benefits, and encapsulation and delivery using microemulsions and nanoemulsions. Int J Mol Sci. 2023; 24(10):8874. [DOI:10.3390/ijms24108874] [PMID]
- Mishra S, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann Indian Acad Neurol. 2008; 11(1):13-9. [DOI:10.4103/0972-2327.40220] [PMID]
- Barbara R, Belletti D, Pederzoli F, Masoni M, Keller J, Ballestrazzi A, et al. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates. Int J Pharm. 2017; 526(1-2):413-24. [DOI:10.1016/j.ijpharm.2017.05.015] [PMID]
- Miao Y, Zhao S, Gao Y, Wang R, Wu Q, Wu H, et al. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain Res Bull. 2016; 121:9-15. [DOI:10.1016/j.brainresbull.2015.11.019] [PMID]
- Jiang J, Wang W, Sun YJ, Hu M, Li F, Zhu DY. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood-brain barrier damage. Eur J Pharmacol. 2007; 561(1-3):54-62. [DOI:10.1016/j.ejphar.2006.12.028] [PMID]
- Rahimi HR, Nedaeinia R, Sepehri Shamloo A, Nikdoust S, Kazemi Oskuee R. Novel delivery system for natural products: Nano-curcumin formulations. Avicenna J Phytomed. 2016; 6(4):383-98. [PMID]
- Kazemnejad-Leili E, Rezaei S, Hosseini-Nejad M, Bakhshayesh-Eghbali B, Saberi A, Keshavarz P. The applicability, concurrent validity and internal consistency reliability of the Persian version of the National Institutes of Health stroke scale (NIHSS): Evidences for gender differences. Caspian J Neurol Sci 2016; 2(1):18-28. [DOI:10.18869/acadpub.cjns.2.4.18]
- Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS). J Physiother. 2014; 60(1):61. [DOI:10.1016/j.jphys.2013.12.012] [PMID]
- Quinn TJ, Taylor-Rowan M, Coyte A, Clark AB, Musgrave SD, Metcalf AK, et al. Pre-stroke modified Rankin scale: Evaluation of validity, prognostic accuracy, and association with treatment. Front Neurol. 2017; 8:275. [DOI:10.3389/fneur.2017.00275] [PMID]
- Abdolahi M, Sarraf P, Javanbakht MH, Honarvar NM, Hatami M, Soveyd N, et al. A novel combination of ω-3 fatty acids and nano-curcumin modulates interleukin-6 gene expression and high sensitivity C-reactive protein serum levels in patients with migraine: A randomized clinical trial study. CNS Neurol Disord Drug Targets. 2018; 17(6):430-8. [DOI:10.2174/1871527317666180625101643] [PMID]
- Zhang Y, Yan Y, Cao Y, Yang Y, Zhao Q, Jing R, et al. Potential therapeutic and protective effect of curcumin against stroke in the male albino stroke-induced model rats. Life Sci. 2017; 183:45-9. [DOI:10.1016/j.lfs.2017.06.023] [PMID]
- Zahedi H, Hosseinzadeh-Attar MJ, Shadnoush M, Sahebkar A, Barkhidarian B, Sadeghi O, et al. Effects of curcuminoids on inflammatory and oxidative stress biomarkers and clinical outcomes in critically ill patients: A randomized double-blind placebo-controlled trial. Phytother Res. 2021; 35(8):4605-15. [DOI:10.1002/ptr.7179] [PMID]
- Dolati S, Ahmadi M, Aghebti-Maleki L, Nikmaram A, Marofi F, Rikhtegar R, et al. Nanocurcumin is a potential novel therapy for multiple sclerosis by influencing inflammatory mediators. Pharmacol Rep. 2018; 70(6):1158-67.[DOI:10.1016/j.pharep.2018.05.008] [PMID]
- Ringman JM, Frautschy SA, Teng E, Begum AN, Bardens J, Beigi M, et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther. 2012; 4(5):43. [DOI:10.1186/alzrt146] [PMID]
- Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006; 5(7):603-12. [DOI:10.1016/S1474-4422(06)70495-1] [PMID]
- Lyden PD, Lu M, Levine SR, Brott TG, Broderick J; NINDS rtPA Stroke Study Group. A modified National Institutes of Health Stroke Scale for use in stroke clinical trials: Preliminary reliability and validity. Stroke. 2001; 32(6):1310-7. [DOI:10.1161/01.STR.32.6.1310] [PMID]
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. stroke. 1988; 19(5):604-7. [DOI:10.1161/01.STR.19.5.604] [PMID]
- Rathore P, Dohare P, Varma S, Ray A, Sharma U, Jagannathan NR, et al. Curcuma oil: Reduces early accumulation of oxidative product and is anti-apoptogenic in transient focal ischemia in rat brain. Neurochem Res. 2008; 33(9):1672-82. [DOI:10.1007/s11064-007-9515-6] [PMID]
- Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004; 74(8):969-85. [DOI:10.1016/j.lfs.2003.06.042] [PMID]
- Flora G, Gupta D, Tiwari A. Nanocurcumin: A promising therapeutic advancement over native curcumin. Crit Rev Ther Drug Carrier Syst. 2013; 30(4):331-68. [DOI:10.1615/CritRevTherDrugCarrierSyst.2013007236] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2025/01/3 | Accepted: 2025/01/30 | Published: 2025/01/12
Received: 2025/01/3 | Accepted: 2025/01/30 | Published: 2025/01/12
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |