Tue, Oct 28, 2025
Volume 11, Issue 3 (Summer 2025)
Caspian J Neurol Sci 2025, 11(3): 223-229 |
Back to browse issues page
Ethics code: 2249/UN14.2.2.VII.14/LT/2024
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Niryana I W, Awyono S, Susanto J I, Satyarsa A B S. Endoscopic Clot Evacuation of Spontaneous Intracerebral Hemorrhage: Optimizing Functional Recovery. Caspian J Neurol Sci 2025; 11 (3) :223-229
URL: http://cjns.gums.ac.ir/article-1-762-en.html
URL: http://cjns.gums.ac.ir/article-1-762-en.html
1- Neurosurgery Division, Department of Surgery, Faculty of Medicine, Prof. Dr. IGNG General Hospital, Udayana University, Bali, Indonesia. , niryanawayan@gmail.com
2- Neurosurgery Division, Department of Surgery, Faculty of Medicine, Prof. Dr. IGNG General Hospital, Udayana University, Bali, Indonesia.
2- Neurosurgery Division, Department of Surgery, Faculty of Medicine, Prof. Dr. IGNG General Hospital, Udayana University, Bali, Indonesia.
Keywords: Intracranial hemorrhages, Endoscopy, Minimally invasive surgical procedures, Treatment outcome
Full-Text [PDF 1040 kb]
(196 Downloads)
| Abstract (HTML) (479 Views)
Full-Text: (96 Views)
Introduction
Despite the significant improvement in knowledge of medical and surgical therapies in patients with spontaneous intracerebral hemorrhage (sICH), patient management has remained a challenging issue. Ongoing research and development of various management modalities, both conservative and surgical, continue to offer hope for improving patient survival rates [1-3].
Although many studies have identified factors that influence patient prognosis, a standard has yet to be established because there are still areas for improvement in the available evidence [4]. Surgical intervention in patients with sICH is a concern, especially regarding the proper indications. The decision to perform surgery is very resonant and must be adjusted to the individual patient’s condition [5]. The basic concept of surgery is to decompress and control the source of bleeding. Decompression can be done by removing the clot surgically, either by using the conventional craniotomy approach, which is more invasive with a higher risk of complication, or by a minimally invasive technique using endoscopic, which offers a more simple approach with smaller incisions but still provides proper visualization. This technique has the advantages of lower risk of tissue trauma, shorter operating time, and faster recovery time. However, it also has limitations, especially in the accessibility of complex and large bleeding areas. Proper patient selection is essential for the success of surgery, especially in minimally invasive techniques [3, 5]. Factors such as location, size of bleeding, and general condition of the patient must be carefully considered to determine the most appropriate approach. Therefore, based on the explanation above, we want to learn more about sICH surgery using endoscopic techniques and evaluate the prognosis of patients after the procedure.
Materials and Methods
Study population and design
This is a retrospective study on patients diagnosed with supratentorial sICH who received endoscopic clot evacuation at Prof. Dr. I.G.N.G. Ngoerah General Hospital from January 2021 to June 2024. sICH was diagnosed as an acute neurological deficit based on head computed tomography (CT) without any history of trauma or surgery.
The following data were collected in a computerized database: Age, sex, initial Glasgow coma scale (GCS) score, the volume of the clot, and postoperative modified Rankin scale (mRS).
The inclusion criteria were as follows: 1) Diagnosed with supratentorial sICH, 2) Age of 17 years or older, and 3) Underwent surgery to evacuate the blood clot using endoscopic. The exclusion criteria were as follows: 1) Having two or more surgeries related to an intracerebral hemorrhage (ICH), 2) Being treated surgically without endoscopic, 3) Patients that were not evaluated on third-month postoperative, and 4) Patients surgically treated in another hospital then were referred to our hospital for further treatment.
Statistical analysis
All statistical analyses were done using SPSS software, version 27. Continuous variables are mentioned as mean with standard deviation or median with 25th and 75th percentiles, according to distribution manner. Using backward binary logistics, bivariate analysis for categorical variables was analyzed by the chi-square to determine variables for multivariate analysis of mRS. P≤0.05 were considered significant.
Results
Between January 2021 and June 2024, 43 patients were surgically treated for supratentorial sICH using endoscopic. Five patients were excluded after we performed the patient selection, making the total sample 38 patients.
Univariate analysis
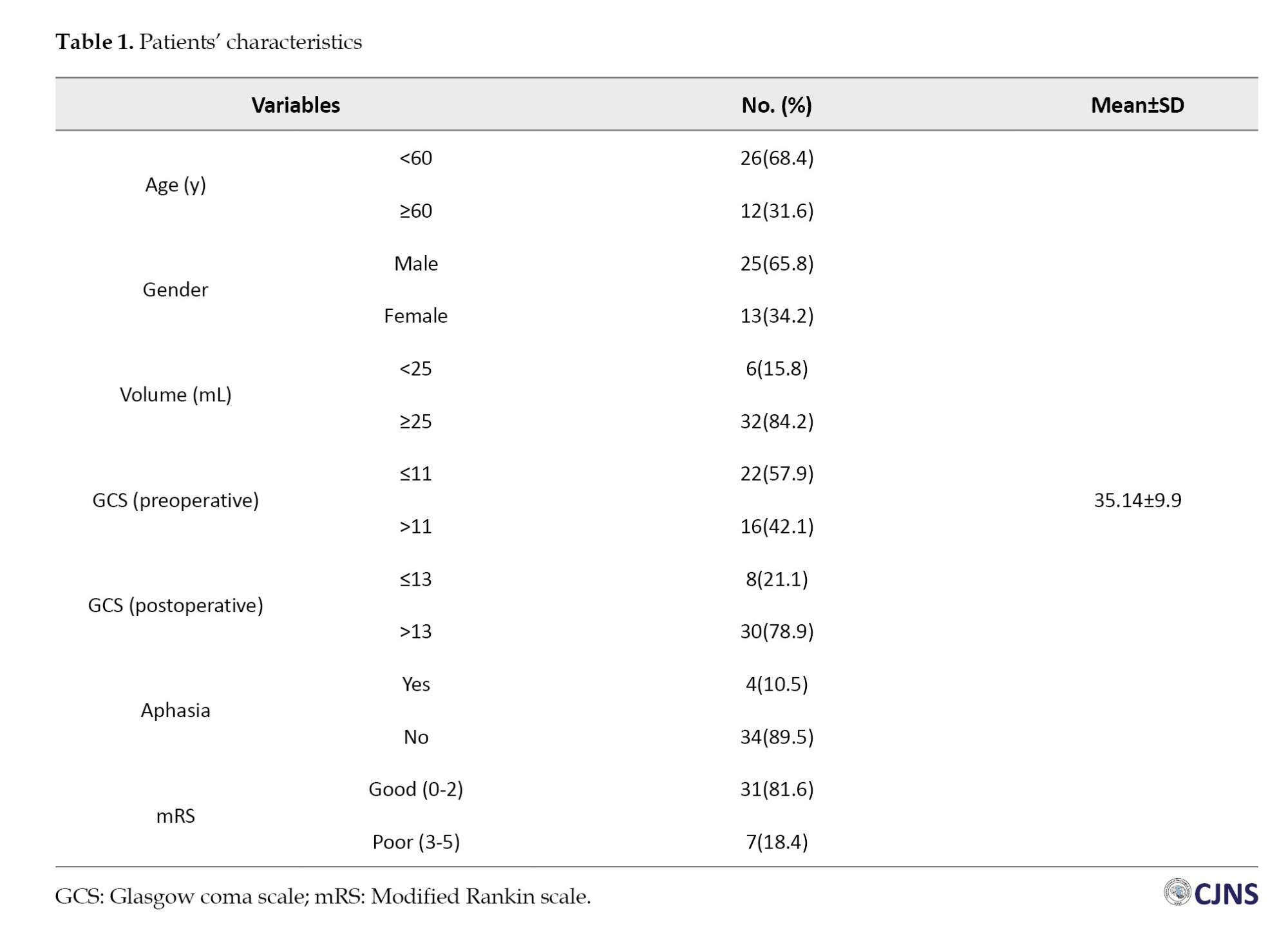
Based on our univariate analysis, sample characteristic data were obtained, as shown in Table 1. Based on age, patients were 20-30 years old, and the median value was 55 years. The mean ICH volume was 35.14±9.9 mL. The preop GCS of the sample ranged from 5 to 15, while the postop GCS ranged from 3 to 15. Based on the mRS, 18.4% had moderate to severe mRS, and 81.6% had mild mRS. Based on gender, the majority were male (61.3%). Most patients (89.5%) were not aphasic.
Multivariate analysis
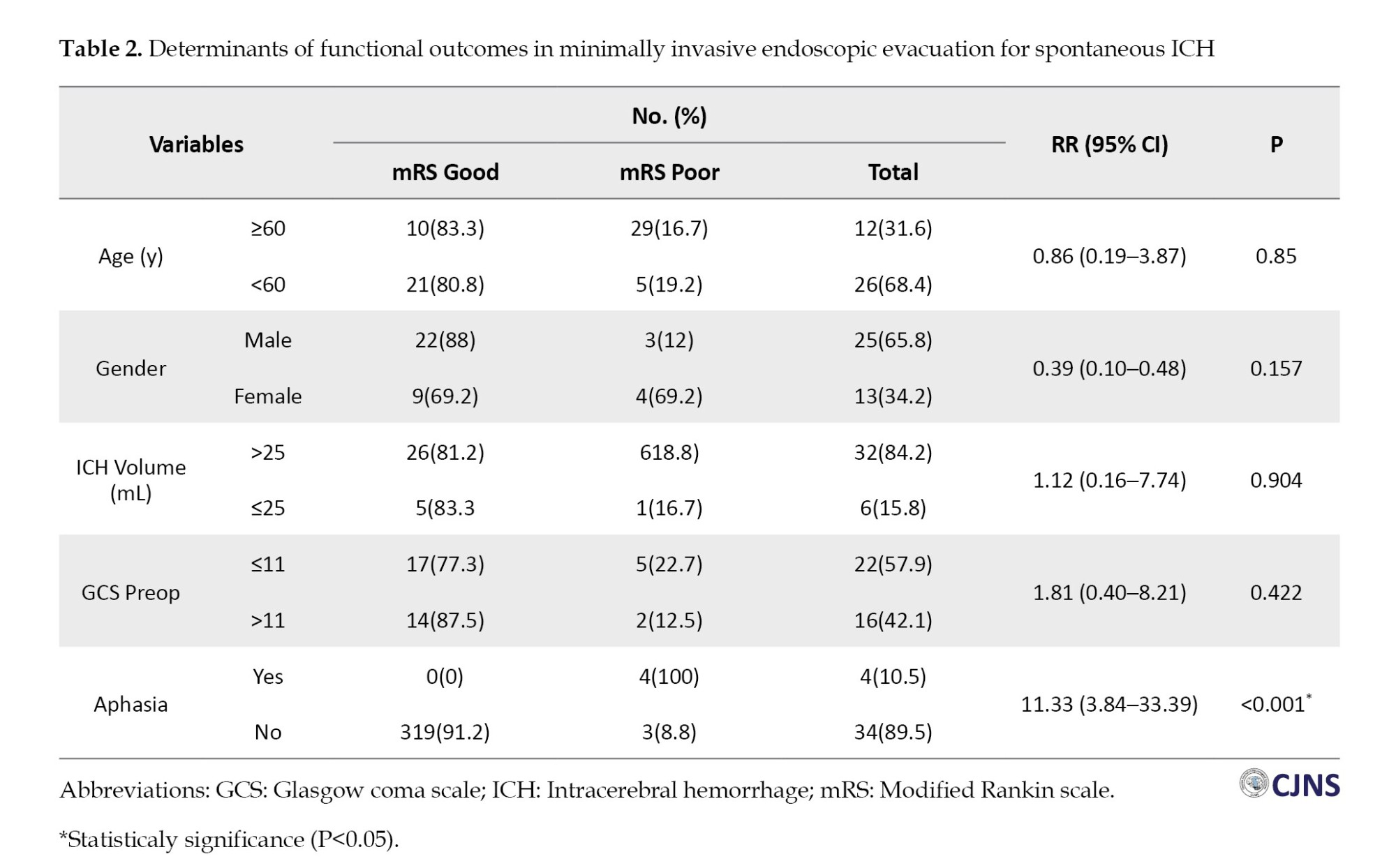
Based on multivariate analysis, there was no significant difference between mRS and preop GCS, with a P=0.422. Likewise, the mRS variable with postop GCS showed a significant difference with a P<0.001. Based on cross-tabulation analysis, significant results were obtained on the relationship between aphasia and mRS with a P<0.001. The age variable (P=0.850), gender (P=0.157), and ICH volume (P=0.346) did not show significant relationships with mRS.
Multivariate analysis determined the relative risk (RR) of severe mRS outcomes associated with each factor. Statistical significance was assessed using P, with a threshold set at P<0.05. Aphasia was associated with an RR of 11.33 (95% CI, 3.84%, 33.39%) for moderate to severe mRS outcomes (Table 2).
Discussion
Based on age, most samples were under 60, with 26 samples (68.4%). According to earlier research using retrospective cohort studies on samples older than 18, this outcome is dependable. The study found that the median age of 319 patients with sICH was 69 [1]. Akhtar’s study on 1660 patients revealed that the sample age ranged from 41.5 to 58 years, which differs from our findings [2].
Most samples were male, with 25 samples (65.8%). According to earlier research by Abulhasan (2023), men comprised 60% of the patients [1]. The study by Akthar also revealed that men comprised the majority of the sample [2].
Regarding ICH volume, most samples had a volume (25 mL) total of 32(84.2%). The majority of samples had an ICH volume of less than 30 mL, according to Rathor’s analysis. A hematoma volume of fewer than 30 mL is associated with an excellent functional result. Just 32(29.6%) of the survivors attained functional independence at discharge, while the bulk of 76(70.4%) were functionally dependent [6].
Based on preoperative GCS, most samples came with a GCS below 11, with 22 samples (57.9%). According to postoperative GCS, most samples (n=30, 78.9%) had GCS >13. The mean GCS score was considerably higher among survivors, per Rathor’s assessment of 160 samples (8.5±0.5 vs 12.8±0.4; P<0.001) [6].
Relationships between functional outcomes and patient characteristics
Previous research has shown a substantial correlation between bad outcomes and increasing age. The age threshold of >51 years was used to distinguish between patients with favorable mRS and unfavorable mRS. Several studies that found similar mean ages were in agreement with these findings. However, according to that study, some European studies revealed higher mean ages. Longer life expectancies in developed countries than in developing nations may cause variations in mean ages across research. Furthermore, at earlier ages, patients in poor nations are more likely to have ICH risk factors such as uncontrolled hypertension and diabetes mellitus [7-9].
According to previous findings, there was no discernible gender difference in the functional outcome. Several researchers corroborated this finding. Ganti et al., on the other hand, discovered that a high ICH score and a bad functional outcome were linked to the female gender [10]. According to some research, women often experience a more severe inflammatory response in some illnesses, which can have a negative impact on their prognosis. According to some studies, societal or non-physiological variables may account for the disparities in gender outcomes following ICH. Women are likelier to live alone in their senior years because they often outlive men. Additionally, women might not have as much access to healthcare and rehabilitation programs [7, 11].
Most patients in this study demonstrated good functional outcomes. These results are consistent with a previous study utilizing propensity score analysis of the external compression of lateral ventricles (ECL) group, which indicated that avoiding ECL might have contributed to favorable outcomes in 18.8% of cases and reduced mortality rates by 50% [12]. However, when bad outcomes were considered, rather than merely mortality, the maximally treated group’s results at 12 months were comparable to the sample group’s at 3 months (mRS score ≥4, 55% vs 50%, respectively) [13]. These findings contradict Akhtar’s study, which revealed that 673 individuals (40.5%) had an adverse outcome at day 90. The indigenous population had a 49.2% unfavorable outcome at day 90 (mRS 4–6), compared to 44.4% for Africans, 39.0% for South Asians, 35.3% for Far Easterners, and 7.7% for Caucasians (P<0.001) [2]. An unfavorable functional result with an mRS score of 4–6 was 50% at a median of 3. One month following the ICH, the 90-day mortality rates were 16% and 22%, respectively, according to a prior study by Abulhasan. The corresponding negative results for ICH scores 3 and 4 were 73% and 86%, respectively. The most frequent primary causes of death were medical complications (11%), refractory cerebral edema (21%), and the direct effects or advancement of ICH (54%) [1]. Likewise, prior research revealed that the 30-day mortality rate for ICH score 3 was 65.72%, whereas for ICH score 4, it was 85%. A retrospective study of 554 individuals by the second group revealed a 30-day mortality rate for ICH scores 3 and 4, 48.8% and 70.6%, respectively [14].
Another study, using the ASTRAL score (developed initially from a Swiss population), predicted poor outcomes (mRS score >2) at three months, one year, and five years post-stroke in a Chinese registry, with a C-statistic of ≥0.81, indicating strong predictive accuracy. Similarly, the IScore demonstrated strong predictive performance for poor outcomes or death in Korean patients (C-statistic=0.81) and the Chinese registry (C-statistic=0.82) [15]. Most ICH research has concentrated on 30-day and early in-hospital mortality. Patients who survive the initial shock, however, will suffer from severe disabilities. Around the world, the mRS is used to describe recovery following a stroke, and it has occasionally been employed in ICH research to assess recovery at 90 days to 1 year. According to prior research, 59.5% of patients had an mRS of 0–3 (excellent outcome) 90 days following ICH. A mRS of 0–2 was attained by 51% of participants at 3 months and 56% after 1 year in a smaller study with 243 individuals. In a broader trial of 3255 Chinese patients, 49% had an mRS of 0%–2% at 90 days and 53% at 1 year [16].
Based on bivariate analysis, a significant relationship was found between mRS and aphasia (P<0.001), while other variables such as age, gender, ICH, and preoperative GCS did not show significant results. Studies explicitly explaining the connection between mRS and aphasia are still scarce and do not demonstrate a clear correlation. Aphasia is a language comprehension or formulation issue brought on by injury to the language cortical centers. Language-related brain lesions typically found in the dominant hemisphere are the underlying cause of aphasia. ICH problems are one of the main indicators of early death and unfavorable results. Specialized neurocritical centers are essential for treating patients and enhancing their results [14].
Importantly, even after adjusting for the National Institutes of Health stroke scale (NIHSS) score, aphasia was an independent predictor linked to 1.22 more days of hospitalization than in patients without aphasia, while hemiparesis was unrelated to length of stay. In one study, billing codes and a non-standardized and invalidated proxy for aphasia likely led to underreporting (12%) of cases. However, other studies have observed a higher length of stay among aphasics [9]. A prior study sought to ascertain the prevalence of symptomatic sICH associated with this treatment and to evaluate the factors that contribute to it and the outcomes of patients. According to the study’s 6-month follow-up, 9 patients (60%) with sICH passed away, compared to 18% of those without sICH (P<0.001). Comparing patients with and without sICH, only one (7%) had a favorable functional result, which is characterized as an mRS score of 0 to 2 [17-19].
Nowadays, endoscopic evacuation, stereotactic aspiration, traditional craniotomy, and conservative treatment are the main choices for treating sICH. Indeed, there are two main issues with treating people with ICH. When compared to the craniotomy procedure, neuro-endoscopy is a less invasive therapy option that could be more effective. While the neuro-endoscopy group had a higher rate of good recovery than the craniotomy group (P<0.0001), the neuro-endoscopy group’s operation time (P<0.0001), intraoperative blood loss volume (P<0.0001), hematoma evacuation rate (P=0.0002), complications (P<0.0001), hospitalization days (P=0.004), and mortality (P<0.0001) were significantly different from the craniotomy (C) group [17].
The focus of therapeutic approaches for acute ICH has been stopping the spread of bleeding, reducing the volume of clots in intraventricular and parenchymal hematomas, and addressing inflammation and perihematomal edema. The success of hemostatic therapy using platelet transfusion and other clotting complexes, quick blood pressure lowering, and hematoma volume reduction utilizing minimally invasive procedures have been the subject of large randomized controlled trials that have influenced therapeutic standards [20].
When ICH is surgically evacuated, neuro-endoscopic surgery is linked to a markedly lower rate of complications and mortality. Following neuro-endoscopy, there was also a statistically significant decrease in the chance of a poor functional outcome. The three primary consequences of ICH are rebleeding lung infection and cerebral infection. In this study, the incidence of postoperative complications was significantly lower in the observation group than in the control group. The following analysis of the primary causes was done in line with the idea of minimally invasive surgery. Neuroendoscopic surgery provides outside lighting with sufficient brightness to inspect the deep hematoma; therefore, it can circumvent key brain functional regions. Furthermore, this technique uses smaller incisions with less brain tissue injury and brief operative time to lower the infection [17].
Conclusion
In conclusion, this study found that patients with sICH surgically treated with endoscopic clot evacuation had a good functional outcome. Several factors contributing to worse outcomes in patients are preoperative GCS and the aphasia status of the patients. This study supports previous studies that found that minimally invasive clot evacuation using endoscopic means gives good functional outcomes for the patient. However, patient selection has an essential role in decision-making.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Faculty of Medicine, Udayana University, Bali, Indonesia (Code: 2249/UN14.2.2.VII.14/LT/2024). The consent to participate was waived due to the retrospective design.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, resources, and supervision: I Wayan Niryana; Writing the original draft: I Wayan Niryana, Steven Awyono, and Jevon Indra Sutanto; Investigation, review, and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors want to express their gratitude to all operating theatre scrub nurses and neurocritical care nurses of Prof. Dr. I.G.N.G. Ngoerah Hospital, who assisted in managing the patient.
References
Despite the significant improvement in knowledge of medical and surgical therapies in patients with spontaneous intracerebral hemorrhage (sICH), patient management has remained a challenging issue. Ongoing research and development of various management modalities, both conservative and surgical, continue to offer hope for improving patient survival rates [1-3].
Although many studies have identified factors that influence patient prognosis, a standard has yet to be established because there are still areas for improvement in the available evidence [4]. Surgical intervention in patients with sICH is a concern, especially regarding the proper indications. The decision to perform surgery is very resonant and must be adjusted to the individual patient’s condition [5]. The basic concept of surgery is to decompress and control the source of bleeding. Decompression can be done by removing the clot surgically, either by using the conventional craniotomy approach, which is more invasive with a higher risk of complication, or by a minimally invasive technique using endoscopic, which offers a more simple approach with smaller incisions but still provides proper visualization. This technique has the advantages of lower risk of tissue trauma, shorter operating time, and faster recovery time. However, it also has limitations, especially in the accessibility of complex and large bleeding areas. Proper patient selection is essential for the success of surgery, especially in minimally invasive techniques [3, 5]. Factors such as location, size of bleeding, and general condition of the patient must be carefully considered to determine the most appropriate approach. Therefore, based on the explanation above, we want to learn more about sICH surgery using endoscopic techniques and evaluate the prognosis of patients after the procedure.
Materials and Methods
Study population and design
This is a retrospective study on patients diagnosed with supratentorial sICH who received endoscopic clot evacuation at Prof. Dr. I.G.N.G. Ngoerah General Hospital from January 2021 to June 2024. sICH was diagnosed as an acute neurological deficit based on head computed tomography (CT) without any history of trauma or surgery.
The following data were collected in a computerized database: Age, sex, initial Glasgow coma scale (GCS) score, the volume of the clot, and postoperative modified Rankin scale (mRS).
The inclusion criteria were as follows: 1) Diagnosed with supratentorial sICH, 2) Age of 17 years or older, and 3) Underwent surgery to evacuate the blood clot using endoscopic. The exclusion criteria were as follows: 1) Having two or more surgeries related to an intracerebral hemorrhage (ICH), 2) Being treated surgically without endoscopic, 3) Patients that were not evaluated on third-month postoperative, and 4) Patients surgically treated in another hospital then were referred to our hospital for further treatment.
Statistical analysis
All statistical analyses were done using SPSS software, version 27. Continuous variables are mentioned as mean with standard deviation or median with 25th and 75th percentiles, according to distribution manner. Using backward binary logistics, bivariate analysis for categorical variables was analyzed by the chi-square to determine variables for multivariate analysis of mRS. P≤0.05 were considered significant.
Results
Between January 2021 and June 2024, 43 patients were surgically treated for supratentorial sICH using endoscopic. Five patients were excluded after we performed the patient selection, making the total sample 38 patients.
Univariate analysis
Based on our univariate analysis, sample characteristic data were obtained, as shown in Table 1. Based on age, patients were 20-30 years old, and the median value was 55 years. The mean ICH volume was 35.14±9.9 mL. The preop GCS of the sample ranged from 5 to 15, while the postop GCS ranged from 3 to 15. Based on the mRS, 18.4% had moderate to severe mRS, and 81.6% had mild mRS. Based on gender, the majority were male (61.3%). Most patients (89.5%) were not aphasic.

Multivariate analysis
Based on multivariate analysis, there was no significant difference between mRS and preop GCS, with a P=0.422. Likewise, the mRS variable with postop GCS showed a significant difference with a P<0.001. Based on cross-tabulation analysis, significant results were obtained on the relationship between aphasia and mRS with a P<0.001. The age variable (P=0.850), gender (P=0.157), and ICH volume (P=0.346) did not show significant relationships with mRS.
Multivariate analysis determined the relative risk (RR) of severe mRS outcomes associated with each factor. Statistical significance was assessed using P, with a threshold set at P<0.05. Aphasia was associated with an RR of 11.33 (95% CI, 3.84%, 33.39%) for moderate to severe mRS outcomes (Table 2).

Discussion
Based on age, most samples were under 60, with 26 samples (68.4%). According to earlier research using retrospective cohort studies on samples older than 18, this outcome is dependable. The study found that the median age of 319 patients with sICH was 69 [1]. Akhtar’s study on 1660 patients revealed that the sample age ranged from 41.5 to 58 years, which differs from our findings [2].
Most samples were male, with 25 samples (65.8%). According to earlier research by Abulhasan (2023), men comprised 60% of the patients [1]. The study by Akthar also revealed that men comprised the majority of the sample [2].
Regarding ICH volume, most samples had a volume (25 mL) total of 32(84.2%). The majority of samples had an ICH volume of less than 30 mL, according to Rathor’s analysis. A hematoma volume of fewer than 30 mL is associated with an excellent functional result. Just 32(29.6%) of the survivors attained functional independence at discharge, while the bulk of 76(70.4%) were functionally dependent [6].
Based on preoperative GCS, most samples came with a GCS below 11, with 22 samples (57.9%). According to postoperative GCS, most samples (n=30, 78.9%) had GCS >13. The mean GCS score was considerably higher among survivors, per Rathor’s assessment of 160 samples (8.5±0.5 vs 12.8±0.4; P<0.001) [6].
Relationships between functional outcomes and patient characteristics
Previous research has shown a substantial correlation between bad outcomes and increasing age. The age threshold of >51 years was used to distinguish between patients with favorable mRS and unfavorable mRS. Several studies that found similar mean ages were in agreement with these findings. However, according to that study, some European studies revealed higher mean ages. Longer life expectancies in developed countries than in developing nations may cause variations in mean ages across research. Furthermore, at earlier ages, patients in poor nations are more likely to have ICH risk factors such as uncontrolled hypertension and diabetes mellitus [7-9].
According to previous findings, there was no discernible gender difference in the functional outcome. Several researchers corroborated this finding. Ganti et al., on the other hand, discovered that a high ICH score and a bad functional outcome were linked to the female gender [10]. According to some research, women often experience a more severe inflammatory response in some illnesses, which can have a negative impact on their prognosis. According to some studies, societal or non-physiological variables may account for the disparities in gender outcomes following ICH. Women are likelier to live alone in their senior years because they often outlive men. Additionally, women might not have as much access to healthcare and rehabilitation programs [7, 11].
Most patients in this study demonstrated good functional outcomes. These results are consistent with a previous study utilizing propensity score analysis of the external compression of lateral ventricles (ECL) group, which indicated that avoiding ECL might have contributed to favorable outcomes in 18.8% of cases and reduced mortality rates by 50% [12]. However, when bad outcomes were considered, rather than merely mortality, the maximally treated group’s results at 12 months were comparable to the sample group’s at 3 months (mRS score ≥4, 55% vs 50%, respectively) [13]. These findings contradict Akhtar’s study, which revealed that 673 individuals (40.5%) had an adverse outcome at day 90. The indigenous population had a 49.2% unfavorable outcome at day 90 (mRS 4–6), compared to 44.4% for Africans, 39.0% for South Asians, 35.3% for Far Easterners, and 7.7% for Caucasians (P<0.001) [2]. An unfavorable functional result with an mRS score of 4–6 was 50% at a median of 3. One month following the ICH, the 90-day mortality rates were 16% and 22%, respectively, according to a prior study by Abulhasan. The corresponding negative results for ICH scores 3 and 4 were 73% and 86%, respectively. The most frequent primary causes of death were medical complications (11%), refractory cerebral edema (21%), and the direct effects or advancement of ICH (54%) [1]. Likewise, prior research revealed that the 30-day mortality rate for ICH score 3 was 65.72%, whereas for ICH score 4, it was 85%. A retrospective study of 554 individuals by the second group revealed a 30-day mortality rate for ICH scores 3 and 4, 48.8% and 70.6%, respectively [14].
Another study, using the ASTRAL score (developed initially from a Swiss population), predicted poor outcomes (mRS score >2) at three months, one year, and five years post-stroke in a Chinese registry, with a C-statistic of ≥0.81, indicating strong predictive accuracy. Similarly, the IScore demonstrated strong predictive performance for poor outcomes or death in Korean patients (C-statistic=0.81) and the Chinese registry (C-statistic=0.82) [15]. Most ICH research has concentrated on 30-day and early in-hospital mortality. Patients who survive the initial shock, however, will suffer from severe disabilities. Around the world, the mRS is used to describe recovery following a stroke, and it has occasionally been employed in ICH research to assess recovery at 90 days to 1 year. According to prior research, 59.5% of patients had an mRS of 0–3 (excellent outcome) 90 days following ICH. A mRS of 0–2 was attained by 51% of participants at 3 months and 56% after 1 year in a smaller study with 243 individuals. In a broader trial of 3255 Chinese patients, 49% had an mRS of 0%–2% at 90 days and 53% at 1 year [16].
Based on bivariate analysis, a significant relationship was found between mRS and aphasia (P<0.001), while other variables such as age, gender, ICH, and preoperative GCS did not show significant results. Studies explicitly explaining the connection between mRS and aphasia are still scarce and do not demonstrate a clear correlation. Aphasia is a language comprehension or formulation issue brought on by injury to the language cortical centers. Language-related brain lesions typically found in the dominant hemisphere are the underlying cause of aphasia. ICH problems are one of the main indicators of early death and unfavorable results. Specialized neurocritical centers are essential for treating patients and enhancing their results [14].
Importantly, even after adjusting for the National Institutes of Health stroke scale (NIHSS) score, aphasia was an independent predictor linked to 1.22 more days of hospitalization than in patients without aphasia, while hemiparesis was unrelated to length of stay. In one study, billing codes and a non-standardized and invalidated proxy for aphasia likely led to underreporting (12%) of cases. However, other studies have observed a higher length of stay among aphasics [9]. A prior study sought to ascertain the prevalence of symptomatic sICH associated with this treatment and to evaluate the factors that contribute to it and the outcomes of patients. According to the study’s 6-month follow-up, 9 patients (60%) with sICH passed away, compared to 18% of those without sICH (P<0.001). Comparing patients with and without sICH, only one (7%) had a favorable functional result, which is characterized as an mRS score of 0 to 2 [17-19].
Nowadays, endoscopic evacuation, stereotactic aspiration, traditional craniotomy, and conservative treatment are the main choices for treating sICH. Indeed, there are two main issues with treating people with ICH. When compared to the craniotomy procedure, neuro-endoscopy is a less invasive therapy option that could be more effective. While the neuro-endoscopy group had a higher rate of good recovery than the craniotomy group (P<0.0001), the neuro-endoscopy group’s operation time (P<0.0001), intraoperative blood loss volume (P<0.0001), hematoma evacuation rate (P=0.0002), complications (P<0.0001), hospitalization days (P=0.004), and mortality (P<0.0001) were significantly different from the craniotomy (C) group [17].
The focus of therapeutic approaches for acute ICH has been stopping the spread of bleeding, reducing the volume of clots in intraventricular and parenchymal hematomas, and addressing inflammation and perihematomal edema. The success of hemostatic therapy using platelet transfusion and other clotting complexes, quick blood pressure lowering, and hematoma volume reduction utilizing minimally invasive procedures have been the subject of large randomized controlled trials that have influenced therapeutic standards [20].
When ICH is surgically evacuated, neuro-endoscopic surgery is linked to a markedly lower rate of complications and mortality. Following neuro-endoscopy, there was also a statistically significant decrease in the chance of a poor functional outcome. The three primary consequences of ICH are rebleeding lung infection and cerebral infection. In this study, the incidence of postoperative complications was significantly lower in the observation group than in the control group. The following analysis of the primary causes was done in line with the idea of minimally invasive surgery. Neuroendoscopic surgery provides outside lighting with sufficient brightness to inspect the deep hematoma; therefore, it can circumvent key brain functional regions. Furthermore, this technique uses smaller incisions with less brain tissue injury and brief operative time to lower the infection [17].
Conclusion
In conclusion, this study found that patients with sICH surgically treated with endoscopic clot evacuation had a good functional outcome. Several factors contributing to worse outcomes in patients are preoperative GCS and the aphasia status of the patients. This study supports previous studies that found that minimally invasive clot evacuation using endoscopic means gives good functional outcomes for the patient. However, patient selection has an essential role in decision-making.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Faculty of Medicine, Udayana University, Bali, Indonesia (Code: 2249/UN14.2.2.VII.14/LT/2024). The consent to participate was waived due to the retrospective design.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, resources, and supervision: I Wayan Niryana; Writing the original draft: I Wayan Niryana, Steven Awyono, and Jevon Indra Sutanto; Investigation, review, and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors want to express their gratitude to all operating theatre scrub nurses and neurocritical care nurses of Prof. Dr. I.G.N.G. Ngoerah Hospital, who assisted in managing the patient.
References
- Abulhasan YB, Teitelbaum J, Al-Ramadhani K, Morrison KT, Angle MR. Functional outcomes and mortality in patients with intracerebral hemorrhage after intensive medical and surgical support. Neurology. 2023; 100(19):e1985-95. [DOI:10.1212/WNL.0000000000207132] [PMID] [PMCID]
- Akhtar N, Kate M, Kamran S, Joseph S, Morgan D, Uy R, et al. Short-term functional outcomes of patients with acute intracerebral hemorrhage in the native and expatriate population. Front Neurol. 2024; 15:1384985. [DOI:10.3389/fneur.2024.1384985] [PMID] [PMCID]
- Rennert RC, Tringale K, Steinberg JA, Warnke P, Konety I, Sand LA, et al. Surgical management of spontaneous intracerebral hemorrhage: Insights from randomized controlled trials. Neurosurg Rev. 2020; 43(3):999-1006. [DOI:10.1007/s10143-019-01115-2] [PMID]
- Godoy DA, Piñero G, Di Napoli M. Predicting mortality in spontaneous intracerebral hemorrhage: Can modification to original score improve the prediction? Stroke. 2006; 37(4):1038-44. [DOI:10.1161/01.STR.0000206441.79646.49] [PMID]
- Xu X, Zheng Y, Chen X, Li F, Zhang H, Ge X. Comparison of endoscopic evacuation, stereotactic aspiration and craniotomy for the treatment of supratentorial hypertensive intracerebral haemorrhage: Study protocol for a randomised controlled trial. Trials. 2017; 18(1):296. [DOI:10.1186/s13063-017-2041-1] [PMID] [PMCID]
- Rathor MY, Rani MF, Jamalludin AR, Amran M, Shahrin TC, Shah A. Prediction of functional outcome in patients with primary intracerebral hemorrhage by clinical-computed tomographic correlations. J Res Med Sci. 2012; 17(11):1056-62. [PMID]
- Amer HA, El-Jaafary SIM, Sadek HMAE, Fouad AM, Mohammed SS. Clinical and paraclinical predictors of early neurological deterioration and poor outcome in spontaneous intracerebral hemorrhage. Egypt J Neurol Psychiatr Neurosurg. 2023; 59(1):74. [DOI:10.1186/s41983-023-00675-x] [PMID] [PMCID]
- Kim TJ, Lee JS, Yoon JS, Oh MS, Kim JW, Jung KH, et al. Impact of the dedicated neurointensivists on the outcome in patients with ischemic stroke based on the linked big data for stroke in Korea. J Korean Med Sci. 2020; 35(21):e135. [DOI:10.3346/jkms.2020.35.e135] [PMID] [PMCID]
- Falcone GJ, Biffi A, Brouwers HB, Anderson CD, Battey TW, Ayres AM, et al. Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA Neurol. 2013; 70(8):988-94. [DOI:10.1001/jamaneurol.2013.98] [PMID] [PMCID]
- Ganti L, Jain A, Yerragondu N, Jain M, Bellolio MF, Gilmore RM, et al. Female gender remains an independent risk factor for poor outcome after acute nontraumatic intracerebral hemorrhage. Neurol Res Int. 2013; 2013:219097. [DOI:10.1155/2013/219097] [PMID] [PMCID]
- Craen A, Mangal R, Stead TG, Ganti L. Gender differences in outcomes after non-traumatic intracerebral hemorrhage. Cureus. 2019; 11(10):e5818. [DOI:10.7759/cureus.5818]
- Øie LR, Madsbu MA, Solheim O, Jakola AS, Giannadakis C, Vorhaug A, et al. Functional outcome and survival following spontaneous intracerebral hemorrhage: A retrospective population-based study. Brain Behav. 2018; 8(10):e01113. [DOI:10.1002/brb3.1113] [PMID] [PMCID]
- Sembill JA, Gerner ST, Volbers B, Bobinger T, Lücking H, Kloska SP, et al. Severity assessment in maximally treated ICH patients: The max-ICH score. Neurology. 2017; 89(5):423-31. [DOI:10.1212/WNL.0000000000004174] [PMID]
- McCracken DJ, Lovasik BP, McCracken CE, Frerich JM, McDougal ME, Ratcliff JJ, et al. The intracerebral hemorrhage score: A self-fulfilling prophecy? Neurosurgery. 2019; 84(3):741-8. [DOI:10.1093/neuros/nyy193] [PMID]
- Rempe DA. Predicting outcomes after transient ischemic attack and stroke. Continuum. 2014; 20(2 Cerebrovascular Disease):412-28. [DOI:10.1212/01.CON.0000446110.97667.58] [PMID] [PMCID]
- Wang W, Lu J, Wang C, Wang Y, Li H, Zhao X. Prognostic value of ICH score and ICH-GS score in Chinese intracerebral hemorrhage patients: Analysis from the China national stroke registry (CNSR). Plos One. 2013; 8(10):e77421. [DOI:10.1371/journal.pone.0077421] [PMID] [PMCID]
- Amaral S, Duloquin G, Béjot Y. Symptomatic intracranial hemorrhage after ischemic stroke treated with bridging revascularization Therapy. Life. 2023; 13(7):1593. [DOI:10.3390/life13071593] [PMID] [PMCID]
- Du X, Lin X, Wang C, Zhou K, Wei Y, Tian X. Endoscopic surgery versus craniotomy in the treatment of spontaneous intracerebral hematoma: a systematic review and meta-analysis. Chin Neurosurg J. 2022; 8(1):36. [DOI:10.1186/s41016-022-00304-1] [PMID] [PMCID]
- Boehme AK, Martin-Schild S, Marshall RS, Lazar RM. Effect of aphasia on acute stroke outcomes. Neurology. 2016; 87(22):2348-54. [DOI:10.1212/WNL.0000000000003297] [PMID] [PMCID]
- Al-Kawaz MN, Hanley DF, Ziai W. Advances in therapeutic approaches for spontaneous intracerebral hemorrhage. Neurother J Am Soc Exp Neurother. 2020; 17(4):1757-67. [DOI:10.1007/s13311-020-00902-w] [PMID] [PMCID]
Type of Study: Research |
Subject:
Special
Received: 2025/03/22 | Accepted: 2025/03/29 | Published: 2025/07/1
Received: 2025/03/22 | Accepted: 2025/03/29 | Published: 2025/07/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






