Sat, Jan 31, 2026
Volume 11, Issue 3 (Summer 2025)
Caspian J Neurol Sci 2025, 11(3): 198-202 |
Back to browse issues page
Ethics code: 0
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yadegari S. Non-arteritic Anterior Ischemic Optic Neuropathy MRI Findings Compared With Optic Neuritis: A Narrative Review. Caspian J Neurol Sci 2025; 11 (3) :198-202
URL: http://cjns.gums.ac.ir/article-1-761-en.html
URL: http://cjns.gums.ac.ir/article-1-761-en.html
Department of Neuro-ophthalmology and Strabismus, Farabi Eye Hospital, Tehran University of Medical Sciences, Tehran, Iran. & Eye Research Center, Farabi Eye Hospital, Tehran University of Medical Sciences, Tehran, Iran. , yadegarisamira@yahoo.com
Keywords: Non-arteritic anterior ischemic optic neuropathy (NAION), Magnetic resonance imaging (MRI), Orbit, Optic neuritis
Full-Text [PDF 995 kb]
(580 Downloads)
| Abstract (HTML) (827 Views)
Full-Text: (487 Views)
Introduction
Non-arteritic anterior ischemic optic neuropathy (NAION) is the second most common optic neuropathy in adults [1]. Optic neuritis (ON) is another common cause of acute optic neuropathy, which often occurs under the age of 50 and could be related to demyelinating disorders. Clinical discrimination of these two optic neuropathies can sometimes be very challenging. The diagnostic dilemma increases when no cerebrovascular risk factors favor NAION, and brain magnetic resonance imaging (MRI) appears normal. Previous studies have shown that the simultaneous presence of white matter hyperintensities in T2 brain MRI with a demyelinating pattern can guide the clinician to diagnose ON. At the same time, NAION is more likely to occur in white matter lesions with the features of microangiopathic and cerebrovascular small vessel disease [2-5].
Early differentiation between the two entities is critical for effective management, prognosis, and future treatment planning. Despite advances in MRI techniques and well-described MRI characteristics of ON, its role in NAION has been less considered. MRI criteria for differentiating one from the other have not been described in the medical literature.
Herein, we aimed to review the current studies considering MRI characteristics that help to differentiate NAION from ON. We searched all studies that used the keywords to compare MRI findings in AION and ON. PubMed, Scopus, and Google Scholar search engines between 1990 and December 2024 were searched, and their outcomes were evaluated, subtracted, summarized, and then categorized.
Current evidence
NAION and ON could present with acute vision loss, but the pathophysiology of vision loss differs in each one. ON is immune-mediated and related to inflammatory and or demyelinating mechanisms. NAION is presumed to be caused by a circulatory insufficiency or an interruption of the blood flow to the optic nerve [1]. MRI characteristics of ON have been well described in the literature, with enhancement and signal tau inversion recovery (STIR). The optic nerve signal abnormalities often involve long segments of the intra-orbital nerve with occasional intracranial extension [6]. In contrast, the MRI characteristics of NAION have been less described. Recently, some studies have compared MRI parameters between ON and NAION patients and suggested that MRI may be useful in distinguishing equivocal cases. Table 1 summarizes these findings.
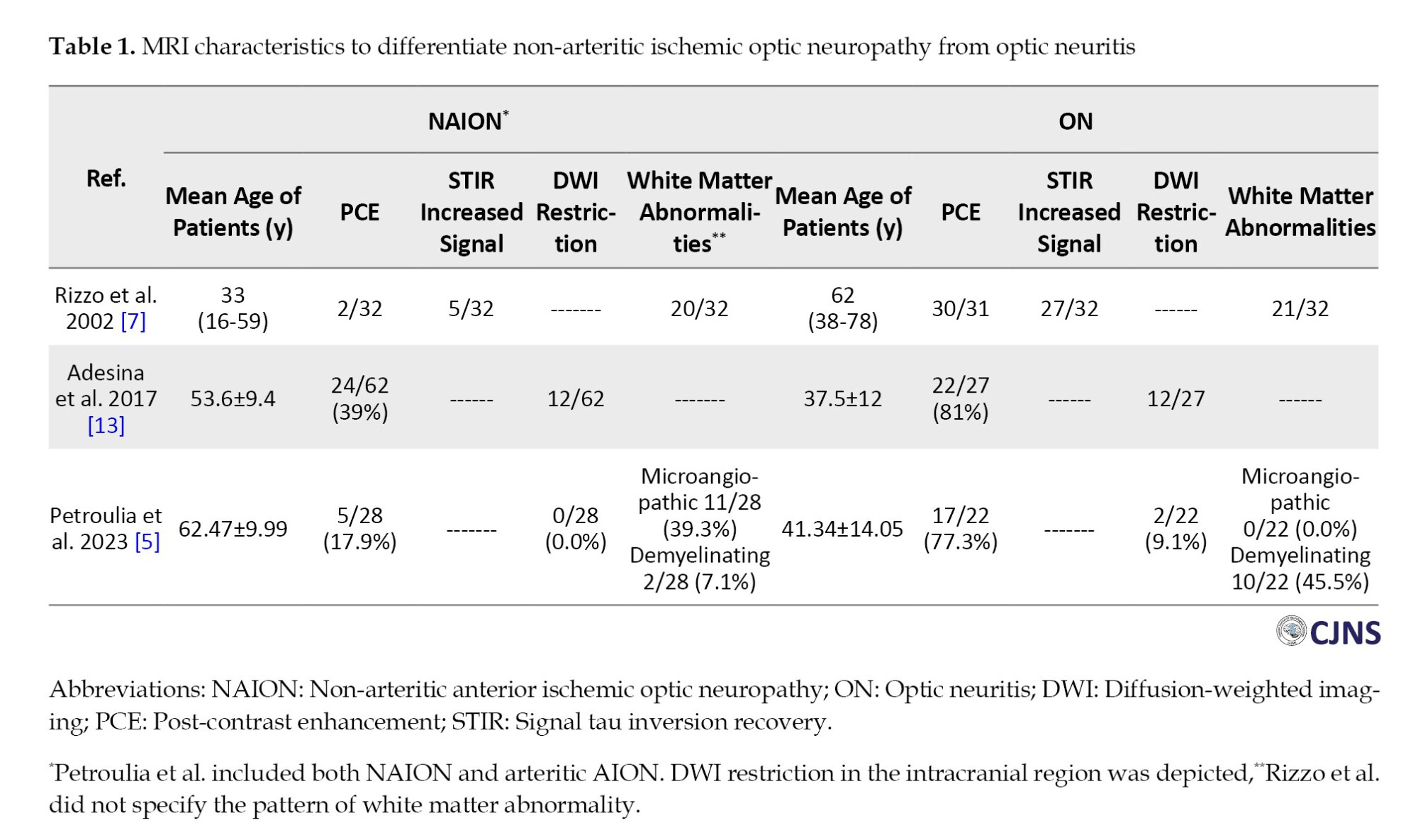
Rizzo et al. first compared the MRI characteristics of NAION and ON in 2002 [7]. After excluding cases with giant cell arteritis and using a 1.5-Tesla scanner, they reported post-contrast enhancement (PCE) and abnormal STIR signal of optic nerve in 98% and 84% cases of ON, respectively (n=32). In addition, gadolinium enhancement and increased STIR signal were observed when coincidences were in the same region, except for one patient. In 56% of ONs, lesions occupied more than two-thirds of the intra-orbital optic nerve. By contrast, abnormal scans were seen in only 5 (out of 32) cases of NAION, with all 5 cases showing abnormal STIR signal, and only two patients had PCE. The authors concluded that MRI of the optic nerve helps to distinguish visual loss caused by ON from NAION. Consequently, the finding of PCE in 2 (out of 32) of NAIONs was argued by Lee AG [8]. He reported the enhancement of optic nerve in arteritic AION (A-AION) previously [9] and suggested an alternative diagnosis for those two cases of NAION with optic nerve enhancement. Then, authors replied that their observed enhancement in NAIONs was slight and subtle [10].
In 2017, Remond et al. reported focal areas of enhancement in the optic nerve head of all A-AION (15/15) and 7/15 patients with NAION in a prospective survey called “central bright spot sign.” This difference was statistically significant, and they concluded that patients without this sign always had a non-arteritic pathophysiology [11]. Recently, in a systematic review of orbital MRI findings in patients with giant cell arteritis and ocular manifestations, the most prevalent regions of enhancement were optic nerve sheath (53%), intraconal fat (25%), and optic nerve/chiasm (14%). MRI has been suggested as an adjunct diagnostic tool [12].
Several studies evaluated diffusion-weighted imaging (DWI) in ischemic optic neuropathies; however, they were non-comparative or had insufficient patients. In 2017, a comparative study conducted by Adesina et al. retrospectively evaluated MRI of NAION and ON patients (n=62 and 27, respectively) [13]. They found positive DWI signal (focal restriction) in 19% of NAIONs and 44% of ONs (P=0.02) and PCE in 39% and 81% of NA-AIONs and ONs, respectively (P<0.001). PCE in NAION patients was more frequent at the optic disc (32%) compared with the intra-orbital segment of the optic nerve (7%), and was bilateral in three patients. The authors suggested that the characteristics of PCE and DWI may help differentiate these two entities. However, they noted that PCE and DWI signals of the optic disc alone did not discriminate between NAION and ON. Positive findings in both DWI and PCE at the intra-orbital segment were predictive of ON over NAION.
Petroulina et al. included both non-arteritic and arteritic AION patients (n=28) and ON (n=22) in a retrospective study [5]. They evaluated MRI findings in different locations of optic nerve and found that PCE of optic nerve was more frequent in ON (77.3% in ON versus 17.9% in AION, P<0.001), while PCE of optic nerve head, bright spot sign, was more frequent in AION than ON (60.7% versus 22.7%, P=0.01). In addition, DWI restriction at different locations (intra-orbital, intraconal, prechiasmal, optic nerve sheath, and perineural fat tissue) was not significantly different between AION and ON. They conducted a model for testing the diagnostic accuracy of parameters to predict the diagnosis and found that DWI added no further useful data. However, the distribution of cerebral T2-hyperintensities, the presence and location of optic nerve enhancement, and the ‘central bright spot’ sign had good accuracy for the discrimination of AION from ON.
The imaging findings at the optic nerve head in NAION patients in Adesina et al. and Petroulia et al. might be related to the lamina cribrosa (LC) structure. Figure 1 shows a high-resolution (3-Tesla) orbital MRI of one of our patients diagnosed with NAION. The observed enhancement in the optic nerve head could be attributable to LC. Lamina cribrosa is a mesh-like structure at the optic nerve head that allows retinal ganglion cell axons to pass through the sclera. LC enhancement in NAION could result from edema following the ischemic insult. In the brain, ion (Na+, Cl-) channels are damaged quickly after ischemia, and there is an intracellular cytotoxic edema. Then, extracellular ionic and vasogenic edema (extravasation of plasma protein) occurs [14]. As the optic nerve is a part of the central nervous system, a similar mechanism may be involved in the pathophysiology of NAION, which could explain the extravasation of contrast and subsequent enhancement of ischemic edematous LC. This finding contrasts with enhancing patterns of optic neuritis, in which the optic nerve short or long segment or chiasma enhancement was often seen [6].

Conclusion
When the clinical diagnosis of NAION and ON is doubtful in patients with acute vision loss, MRI findings may aid in proper diagnosis. The presence and location of optic nerve enhancement seem to be the most important factors. Intra-orbital enhancement may be in favor of ON. However, if there is any enhancement in AION, it will be observed in the head of the optic nerve. However, further prospective studies are necessary to confirm the insufficient existing evidence. LC enhancement in high-resolution orbital MRI needs further surveys. Future studies of NAIONs could expand the insights into the underlying pathophysiology and treatment options regarding high-resolution MRI.
Study limitations
As the diagnosis of NAION is mainly clinical, data regarding MRI findings in AION are scant. The comparative studies of MRIs in AION and ON were retrospective. Some included brain MRI, which may not be sufficient for detailed orbital evaluation, and some patients had received steroid pulse before their MRI studies. Finally, arteritic and non-arteritic AIONs, which may have different MRI findings, are not separated in one study.
Ethical Considerations
Compliance with ethical guidelines
We presented a balanced overview of existing research to avoid bias and selective reporting. To avoid misleading conclusions, we ensured all referenced studies, including their limitations, are correctly cited.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The author declared no conflict of interest.
Acknowledgements
The author would like to thank Sara Kamali Zonouzi for her assistance in the literature review during this research.
References
Non-arteritic anterior ischemic optic neuropathy (NAION) is the second most common optic neuropathy in adults [1]. Optic neuritis (ON) is another common cause of acute optic neuropathy, which often occurs under the age of 50 and could be related to demyelinating disorders. Clinical discrimination of these two optic neuropathies can sometimes be very challenging. The diagnostic dilemma increases when no cerebrovascular risk factors favor NAION, and brain magnetic resonance imaging (MRI) appears normal. Previous studies have shown that the simultaneous presence of white matter hyperintensities in T2 brain MRI with a demyelinating pattern can guide the clinician to diagnose ON. At the same time, NAION is more likely to occur in white matter lesions with the features of microangiopathic and cerebrovascular small vessel disease [2-5].
Early differentiation between the two entities is critical for effective management, prognosis, and future treatment planning. Despite advances in MRI techniques and well-described MRI characteristics of ON, its role in NAION has been less considered. MRI criteria for differentiating one from the other have not been described in the medical literature.
Herein, we aimed to review the current studies considering MRI characteristics that help to differentiate NAION from ON. We searched all studies that used the keywords to compare MRI findings in AION and ON. PubMed, Scopus, and Google Scholar search engines between 1990 and December 2024 were searched, and their outcomes were evaluated, subtracted, summarized, and then categorized.
Current evidence
NAION and ON could present with acute vision loss, but the pathophysiology of vision loss differs in each one. ON is immune-mediated and related to inflammatory and or demyelinating mechanisms. NAION is presumed to be caused by a circulatory insufficiency or an interruption of the blood flow to the optic nerve [1]. MRI characteristics of ON have been well described in the literature, with enhancement and signal tau inversion recovery (STIR). The optic nerve signal abnormalities often involve long segments of the intra-orbital nerve with occasional intracranial extension [6]. In contrast, the MRI characteristics of NAION have been less described. Recently, some studies have compared MRI parameters between ON and NAION patients and suggested that MRI may be useful in distinguishing equivocal cases. Table 1 summarizes these findings.

Rizzo et al. first compared the MRI characteristics of NAION and ON in 2002 [7]. After excluding cases with giant cell arteritis and using a 1.5-Tesla scanner, they reported post-contrast enhancement (PCE) and abnormal STIR signal of optic nerve in 98% and 84% cases of ON, respectively (n=32). In addition, gadolinium enhancement and increased STIR signal were observed when coincidences were in the same region, except for one patient. In 56% of ONs, lesions occupied more than two-thirds of the intra-orbital optic nerve. By contrast, abnormal scans were seen in only 5 (out of 32) cases of NAION, with all 5 cases showing abnormal STIR signal, and only two patients had PCE. The authors concluded that MRI of the optic nerve helps to distinguish visual loss caused by ON from NAION. Consequently, the finding of PCE in 2 (out of 32) of NAIONs was argued by Lee AG [8]. He reported the enhancement of optic nerve in arteritic AION (A-AION) previously [9] and suggested an alternative diagnosis for those two cases of NAION with optic nerve enhancement. Then, authors replied that their observed enhancement in NAIONs was slight and subtle [10].
In 2017, Remond et al. reported focal areas of enhancement in the optic nerve head of all A-AION (15/15) and 7/15 patients with NAION in a prospective survey called “central bright spot sign.” This difference was statistically significant, and they concluded that patients without this sign always had a non-arteritic pathophysiology [11]. Recently, in a systematic review of orbital MRI findings in patients with giant cell arteritis and ocular manifestations, the most prevalent regions of enhancement were optic nerve sheath (53%), intraconal fat (25%), and optic nerve/chiasm (14%). MRI has been suggested as an adjunct diagnostic tool [12].
Several studies evaluated diffusion-weighted imaging (DWI) in ischemic optic neuropathies; however, they were non-comparative or had insufficient patients. In 2017, a comparative study conducted by Adesina et al. retrospectively evaluated MRI of NAION and ON patients (n=62 and 27, respectively) [13]. They found positive DWI signal (focal restriction) in 19% of NAIONs and 44% of ONs (P=0.02) and PCE in 39% and 81% of NA-AIONs and ONs, respectively (P<0.001). PCE in NAION patients was more frequent at the optic disc (32%) compared with the intra-orbital segment of the optic nerve (7%), and was bilateral in three patients. The authors suggested that the characteristics of PCE and DWI may help differentiate these two entities. However, they noted that PCE and DWI signals of the optic disc alone did not discriminate between NAION and ON. Positive findings in both DWI and PCE at the intra-orbital segment were predictive of ON over NAION.
Petroulina et al. included both non-arteritic and arteritic AION patients (n=28) and ON (n=22) in a retrospective study [5]. They evaluated MRI findings in different locations of optic nerve and found that PCE of optic nerve was more frequent in ON (77.3% in ON versus 17.9% in AION, P<0.001), while PCE of optic nerve head, bright spot sign, was more frequent in AION than ON (60.7% versus 22.7%, P=0.01). In addition, DWI restriction at different locations (intra-orbital, intraconal, prechiasmal, optic nerve sheath, and perineural fat tissue) was not significantly different between AION and ON. They conducted a model for testing the diagnostic accuracy of parameters to predict the diagnosis and found that DWI added no further useful data. However, the distribution of cerebral T2-hyperintensities, the presence and location of optic nerve enhancement, and the ‘central bright spot’ sign had good accuracy for the discrimination of AION from ON.
The imaging findings at the optic nerve head in NAION patients in Adesina et al. and Petroulia et al. might be related to the lamina cribrosa (LC) structure. Figure 1 shows a high-resolution (3-Tesla) orbital MRI of one of our patients diagnosed with NAION. The observed enhancement in the optic nerve head could be attributable to LC. Lamina cribrosa is a mesh-like structure at the optic nerve head that allows retinal ganglion cell axons to pass through the sclera. LC enhancement in NAION could result from edema following the ischemic insult. In the brain, ion (Na+, Cl-) channels are damaged quickly after ischemia, and there is an intracellular cytotoxic edema. Then, extracellular ionic and vasogenic edema (extravasation of plasma protein) occurs [14]. As the optic nerve is a part of the central nervous system, a similar mechanism may be involved in the pathophysiology of NAION, which could explain the extravasation of contrast and subsequent enhancement of ischemic edematous LC. This finding contrasts with enhancing patterns of optic neuritis, in which the optic nerve short or long segment or chiasma enhancement was often seen [6].

Conclusion
When the clinical diagnosis of NAION and ON is doubtful in patients with acute vision loss, MRI findings may aid in proper diagnosis. The presence and location of optic nerve enhancement seem to be the most important factors. Intra-orbital enhancement may be in favor of ON. However, if there is any enhancement in AION, it will be observed in the head of the optic nerve. However, further prospective studies are necessary to confirm the insufficient existing evidence. LC enhancement in high-resolution orbital MRI needs further surveys. Future studies of NAIONs could expand the insights into the underlying pathophysiology and treatment options regarding high-resolution MRI.
Study limitations
As the diagnosis of NAION is mainly clinical, data regarding MRI findings in AION are scant. The comparative studies of MRIs in AION and ON were retrospective. Some included brain MRI, which may not be sufficient for detailed orbital evaluation, and some patients had received steroid pulse before their MRI studies. Finally, arteritic and non-arteritic AIONs, which may have different MRI findings, are not separated in one study.
Ethical Considerations
Compliance with ethical guidelines
We presented a balanced overview of existing research to avoid bias and selective reporting. To avoid misleading conclusions, we ensured all referenced studies, including their limitations, are correctly cited.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The author declared no conflict of interest.
Acknowledgements
The author would like to thank Sara Kamali Zonouzi for her assistance in the literature review during this research.
References
- Miller NR, Arnold AC. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye. 2015; 29(1):65-79. [DOI:10.1038/eye.2014.144] [PMID] [PMCID]
- Arnold AC, Hepler RS, Hamilton DR, Lufkin RB. Magnetic resonance imaging of the brain in nonarteritic ischemic optic neuropathy. J Neuroophthalmol 1995; 15(3):158-60. [DOI:10.1097/00041327-199509000-00006]
- Kim MS, Jeong HY, Cho KH, Oh SW, Byun SJ, Woo SJ, et al. Nonarteritic anterior ischemic optic neuropathy is associated with cerebral small vessel disease. Plos One. 2019; 14(11):e0225322. [DOI:10.1371/journal.pone.0225322] [PMID] [PMCID]
- Arnold AC. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2003; 23(2):157-63. [DOI:10.1097/00041327-200306000-00012] [PMID]
- Petroulia VD, Brügger D, Hoepner R, Vicini R, Winklehner A, Abegg M, et al. MRI signs helpful in the differentiation of patients with anterior ischaemic optic neuropathy and optic neuritis. Br J Ophthalmol. 2023; 107(1):121-6. [DOI:10.1136/bjophthalmol-2021-319537] [PMID]
- Winter A, Chwalisz B. MRI Characteristics of NMO, MOG and MS Related Optic Neuritis. Semin Ophthalmol. 2020; 35(7-8):333-342. [DOI:10.1080/08820538.2020.1866027] [PMID]
- Rizzo JF 3rd, Andreoli CM, Rabinov JD. Use of magnetic resonance imaging to differentiate optic neuritis and nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2002; 109(9):1679-84. [DOI:10.1016/S0161-6420(02)01148-X] [PMID]
- Lee AG. MRI to differentiate causes of optic nerve disease. Ophthalmology. 2003; 110(9):1862-3. [DOI:10.1016/S0161-6420(03)00851-0] [PMID]
- Lee AG, Eggenberger ER, Kaufman DI, Manrique C. Optic nerve enhancement on magnetic resonance imaging in arteritic ischemic optic neuropathy. J Neuroophthalmol. 1999; 19(4):235-7. [DOI:10.1097/00041327-199912000-00005] [PMID]
- Rizzo JF, Andreoli CM, Rabinov JD. Magnetic resonance imaging to differentiate causes of optic nerve disease: Author reply. Ophthalmology. 2003; 110(9):1863. [DOI:10.1016/S0161-6420(03)00852-2]
- Remond P, Attyé A, Lecler A, Lamalle L, Boudiaf N, Aptel F, et al. The central bright spot sign: A potential new MR imaging sign for the early diagnosis of anterior ischemic optic neuropathy due to giant cell arteritis. AJNR Am J Neuroradiol. 2017; 38(7):1411-5. [DOI:10.3174/ajnr.A5205] [PMID] [PMCID]
- Guggenberger KV, Pavlou A, Cao Q, Bhatt IJ, Cui QN, Bley TA, et al. Orbital magnetic resonance imaging of giant cell arteritis with ocular manifestations: A systematic review and individual participant data meta-analysis. Eur Radiol. 2023; 33(11):7913-22. [DOI:10.1007/s00330-023-09770-2] [PMID] [PMCID]
- Adesina OO, Scott McNally J, Salzman KL, Katz BJ, Warner JEA, McFadden M, et al. Diffusion-weighted imaging and post-contrast enhancement in differentiating optic neuritis and non-arteritic anterior optic neuropathy. Neuroophthalmology. 2017; 42(2):90-98. [DOI:10.1080/01658107.2017.1356856] [PMID] [PMCID]
- Liebeskind DS, Jüttler E, Shapovalov Y, Yegin A, Landen J, Jauch EC. Cerebral edema associated with large hemispheric infarction. Stroke. 2019; 50(9):2619-25. [DOI:10.1161/STROKEAHA.118.024766] [PMID]
Type of Study: case report |
Subject:
Special
Received: 2025/03/18 | Accepted: 2025/06/23 | Published: 2025/07/1
Received: 2025/03/18 | Accepted: 2025/06/23 | Published: 2025/07/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






