Mon, Sep 29, 2025
Volume 11, Issue 2 (Spring 2025)
Caspian J Neurol Sci 2025, 11(2): 101-114 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hatami H, Nasiri M J, Eyvani K, Fatthy M. The Neurological Effects of Ribavirin in Crimean-congo Hemorrhagic Fever Symptoms: A Systematic Review. Caspian J Neurol Sci 2025; 11 (2) :101-114
URL: http://cjns.gums.ac.ir/article-1-755-en.html
URL: http://cjns.gums.ac.ir/article-1-755-en.html
1- Department of Public Health, School of Public Health and Safety and Environmental and Occupational Hazards Control Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran .
2- Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Neurology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Public Health, School of Public Health and Safety and Environmental and Occupational Hazards Control Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,fathi.mobina78@gmail.com
2- Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Neurology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Public Health, School of Public Health and Safety and Environmental and Occupational Hazards Control Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,
Keywords: Ribavirin, Crimean-Congo hemorrhagic fever, Nervous system diseases, encephalopathy, Neurotoxicity syndromes, Antiviral agent
Full-Text [PDF 1653 kb]
(226 Downloads)
| Abstract (HTML) (819 Views)
Full-Text: (366 Views)
Introduction
In various parts of Africa, Europe and Asia, domestic and wild animals and birds carry the viruses that cause Crimean-Congo hemorrhagic fever (CCHF) [1]. Researchers have discovered CCHF for the first time in Crimea in 1944. In 1956, the virus was found in the blood of a feverish patient in Africa [2]. In 1967, Simpson et al. described a virus different from the one isolated in 1956, and they also noted that this type of Congo virus was comparable to virus strains from Bulgaria and central Asia [3]. The virus has been identified as a genus of Nairoviruses belonging to the Bunyaviridae family. CCHF causes lethal acute diseases with hemorrhagic symptoms [4].
The mortality rate of CCHF disease is between 20% to 35%. As a re-emerging disease, this illness is severe, febrile, hemorrhagic, and occasionally fatal. Hyalomma tick bites or direct contact with the blood or secretions of infected individuals or animals can spread this virus [5]. The four stages of the disease’s clinical course are hemorrhagic, incubation, pre-hemorrhagic, and healing. Pre-hemorrhagic phase symptoms include fever, headache, chills, nausea, vomiting, hyperemia, anathemas, rheumatic pain, and lumbar pain. It is crucial to make a diagnosis to manage the disease effectively. Petechiae, ecchymosis, hematomas, or major hemorrhages are the signs of the brief and quickly deteriorating hemorrhagic phase. After 15 to 20 days from the beginning of the disease, a patient enters the convalescence phase, which is marked by overall weakness, fatigability, poor appetite, and nausea [6]. Additionally, a study shows that a rare complication such as cerebral hemorrhage in CCHF patients can cause various neurological symptoms such as confusion, seizures, coma, behavioral disorders, hemiplegia, peripheral mononeuritis with upper limb paralysis, and headache [7]. The severity of the headache, which is very similar to migraine headaches, is probably related to vasodilatation, the release of inflammatory cytokines, and, as a result, damage to the vascular endothelium [8].
Ribavirin is a broad-spectrum antiviral nucleoside analog that is one of the most used therapies in treating CCHF. Clinically, the efficacy of ribavirin has been manifested by a reduction of viral load and mortality rate. However, treatment with this drug has raised some neurological concerns, mainly when administered in very high doses or for extended periods. These neurological manifestations can vary from mild symptoms such as headaches, dizziness and confusion to more serious complications involving seizures, encephalopathy, or even neuropsychiatric disturbances. The exact underlying pathophysiology for ribavirin-induced neurotoxicity has not been well understood to date; it is postulated to involve mitochondrial dysfunction because ribavirin can inhibit mitochondrial polymerases and decrease cellular production of ATP, leading to neuronal insult. Also, ribavirin may affect the neurotransmitter metabolic process, especially interference in gamma-aminobutyric acid pathways, thereby showing the development of seizure and neuropsychiatric symptoms. Also, interference of ribavirin with the immune system could facilitate neuroinflammation, which increases neurological complications. These risks underscore the need for close neurological monitoring of CCHF patients receiving ribavirin, including careful vigilance regarding dose adjustments and treatment duration. Further research is essential to define the exact mechanisms of ribavirin’s neurotoxicity and formulate therapeutic strategies that minimize neurological risks without compromising its antiviral efficacy [9, 10, 11].
Immune cells and endothelial cells are possible targets in CCHF. The virus induces endothelial cells to generate proinflammatory cytokines. This process causes vasodilatation, increased vascular permeability, hypotension, multiple organ failure, shock, and death in severe circumstances. Apoptosis of lymphocytes, hemophagocytosis, decreased antibody response and partial activation of dendritic cells and macrophages are other ways CCHF may suppress the immune system [12].
Although numerous research studies have been performed to treat CCHF patients with immunoglobulins (Ig) obtained from vaccinated horses [13] and serum collected from convalescing CCHF patients [14], prompt supportive care, including the infusion of blood products, no clinical trials examining the latter therapies have yet been documented [15, 16].
The objectives of the current investigation encompass assessing the advisability of prophylactic administration after exposure to the CCHF virus and examining the impact of ribavirin administration in correlation with both the severity of illness and the timing of medication initiation relative to the onset of symptoms.
Materials and Methods
Search strategy
We used PubMed, Scopus, Web of Science and Google Scholar databases without language restriction or publication status to conduct a thorough search. The search includes articles published until June 2024. The following search criteria were entered into an advanced search using the keywords: CCHF, ribavirin, neurological diseases, immunoglobulin, hospitalization and mortality.
Data extraction
Two authors independently reviewed all titles and abstracts found during the search following inclusion criteria. All possibly qualifying studies, or ones whose eligibility has not been defined, have been carefully assessed. After this step, the full-text publications were thoroughly evaluated, and any differences of opinion were resolved. For each study, data such as the name of the first author, the country, the publication year of the articles, the type of study, average age of patients, gender and nationality of the patients, dosage of ribavirin, and method of administration (oral or intravenous), adverse events due to ribavirin administration, duration of hospitalization, and the mortality rate due to the use of ribavirin drug, have been extracted.
Inclusion criteria
We selected these articles: Studies report primary data on CCHF epidemiology, clinical characteristics, risk factors, or outcomes; studies published in peer-reviewed journals; studies available in English; and studies conducted on human subjects.
Standard definitions for CCHF include clinical definition, which refers to the set of signs and symptoms characteristic of the disease. They include fever, headache, muscle pain, dizziness, and bleeding. The epidemiological definition of CCHF considers the exposure history and risk factors associated with the disease. This definition includes factors such as a history of tick bites, contact with infected animals or their tissues/fluids, or residing in areas where CCHF is endemic or has been reported. A possible case definition is used to identify individuals who exhibit clinical signs and symptoms suggestive of CCHF and have a history of potential exposure or risk factors. Probable case definition indicates CCHF more strongly than a possible case. It may include additional criteria such as laboratory findings, radiological evidence, or clinical progression that strongly supports a CCHF diagnosis but falls short of definitive confirmation. A definite or clear case definition is used when there is laboratory confirmation of CCHF infection. It requires the detection of CCHF virus-specific antibodies or genetic material (RNA) in the patient’s blood sample using validated laboratory tests like enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR).
Exclusion criteria
The exclusion criteria of our articles included studies with insufficient or duplicated data, studies that focused on animal models or in vitro experiments, case reports, letters, editorials, and conference abstracts.
Neurological effects of ribavirin in CCHF
Data from trials reporting any neurological side effects or complications related to ribavirin administration during or after treatment were tabulated to ascertain the neurological impact of ribavirin among patients with CCHF. These findings fell into the spectrum of headache, dizziness, confusion, and more severe manifestations such as seizures and encephalopathy.
Considering the studies for inclusion, we emphasized the detailed accounts of neurological symptoms and their relationship to the given ribavirin dosage and route of administration taken, orally or via intravenous administration. We also examined whether pre-existing conditions, such as renal impairment or the protracted administration of ribavirin, impacted neurological complications’ development and or severity. Data on neurological adverse effects thus extracted were then evaluated, along with other adverse events, regarding the overall safety profile of ribavirin in CCHF treatment.
Types of studies
We included randomized control trials, quasi-RCTs, non-randomized controlled studies, case-control studies, and cohort studies, which assessed the efficacy of ribavirin administration on mortality and length of hospital stay.
Types of participants
Adults or children of any age with a confirmed diagnosis of CCHF by laboratory test (Ig) or PCR were chosen as participants.
Types of interventions
IV or oral ribavirin compared with supportive care or other interventions have been considered.
Types of outcome measures
The primary outcome was comparing the mortality rate between ribavirin and no-ribavirin groups. The secondary results compared the length of hospital stay (days) among ribavirin and control groups, serious adverse events, or withdrawal of treatment due to serious adverse events.
Quality assessment
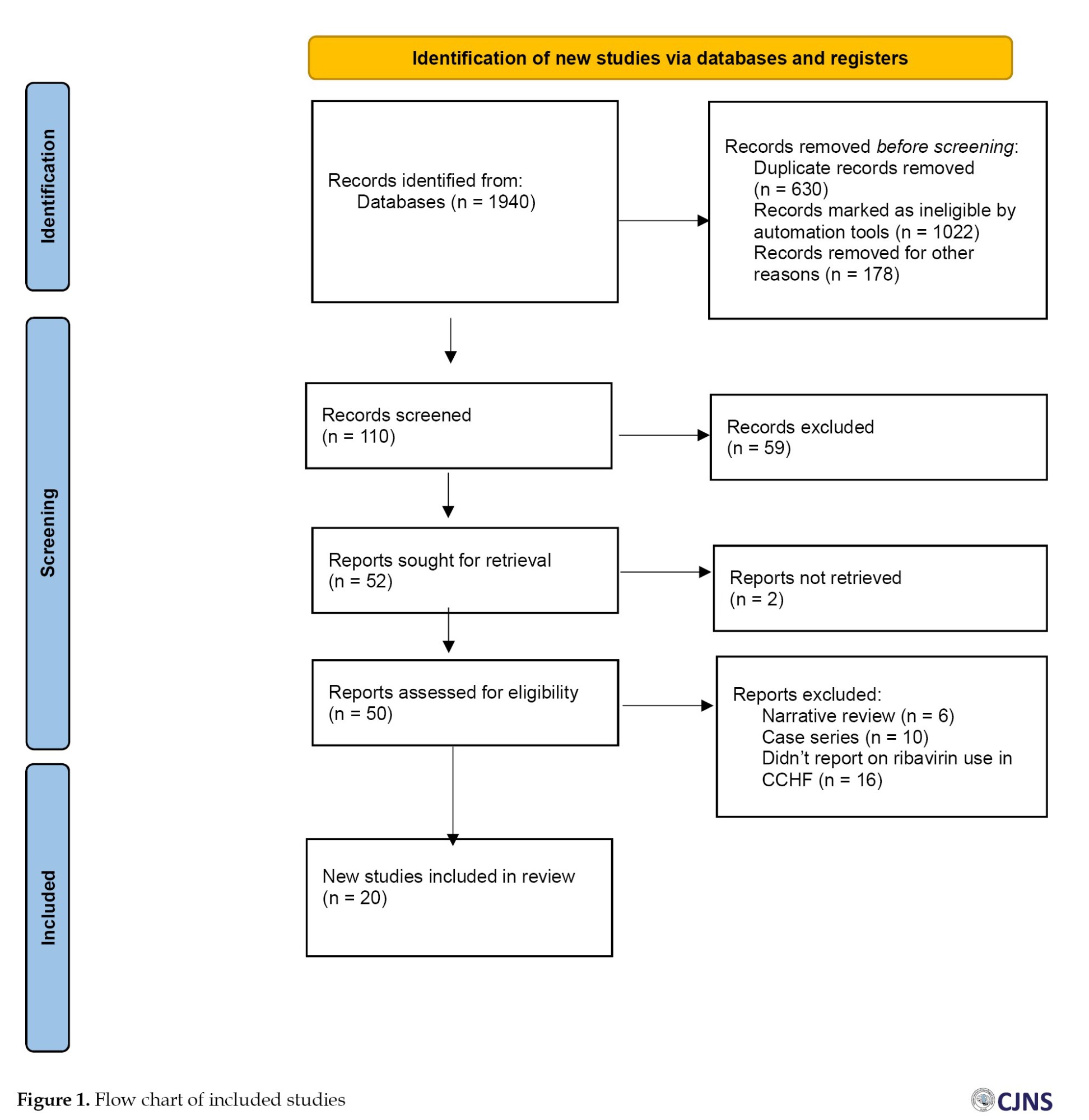
We have used the Newcastle-Ottawa Scale (NOS) [12] to check the quality of the papers. The PRISMA flow chart for the systematic review is displayed in Figure 1.
Results
Search results
A total of 1940 references were found throughout our search. After examining all titles and abstracts and manually deleting 630 duplicates, two reviewers assessed the remaining 110 articles. We thoroughly evaluated all the publications before excluding 58 for reasons such as non-English articles, reviews irrelevant to this meta-analysis, unclear or insufficient data, and low quality. Twenty publications were eligible and included in the meta-analysis following a full-text screening of the remaining (Figure 1).
Quality assessment
We primarily assessed the methodological quality of included studies using the NOS developed by the Cochrane Collaboration. Two independent reviewers have evaluated the Risk of bias in the included studies. Randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting are all factors to be considered when determining the risk of bias (Table 1).
Study design
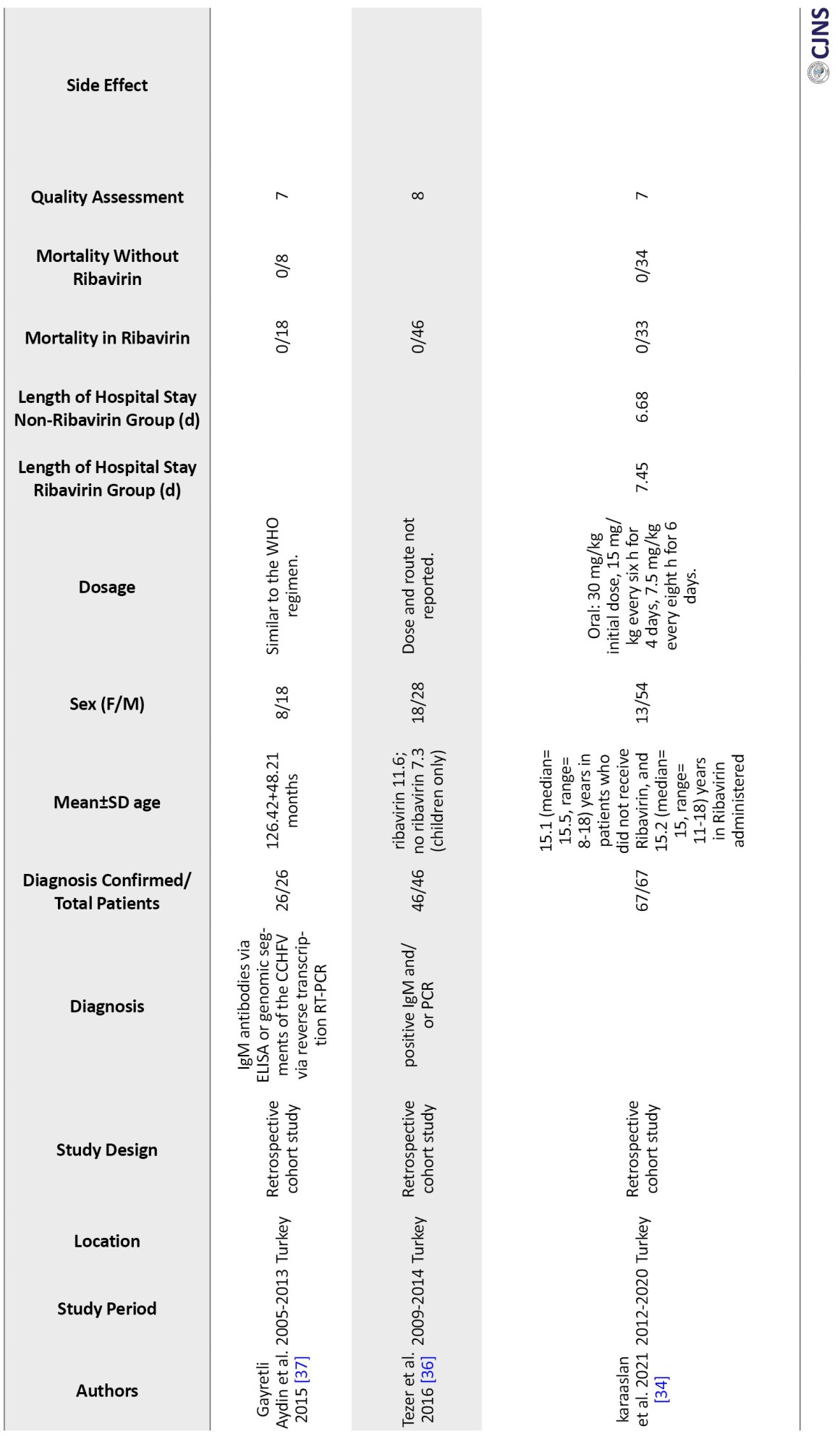
One mixed prospective and retrospective cohort study [17], one matched cohort study [18], one case-control study [19], one cohort with a historical control [20], and 16 observational studies were included in our study. Studies were conducted in Iran [21-23], Pakistan [24], Turkey, and Russia [17-20, 25-37].
Characteristics data of participants
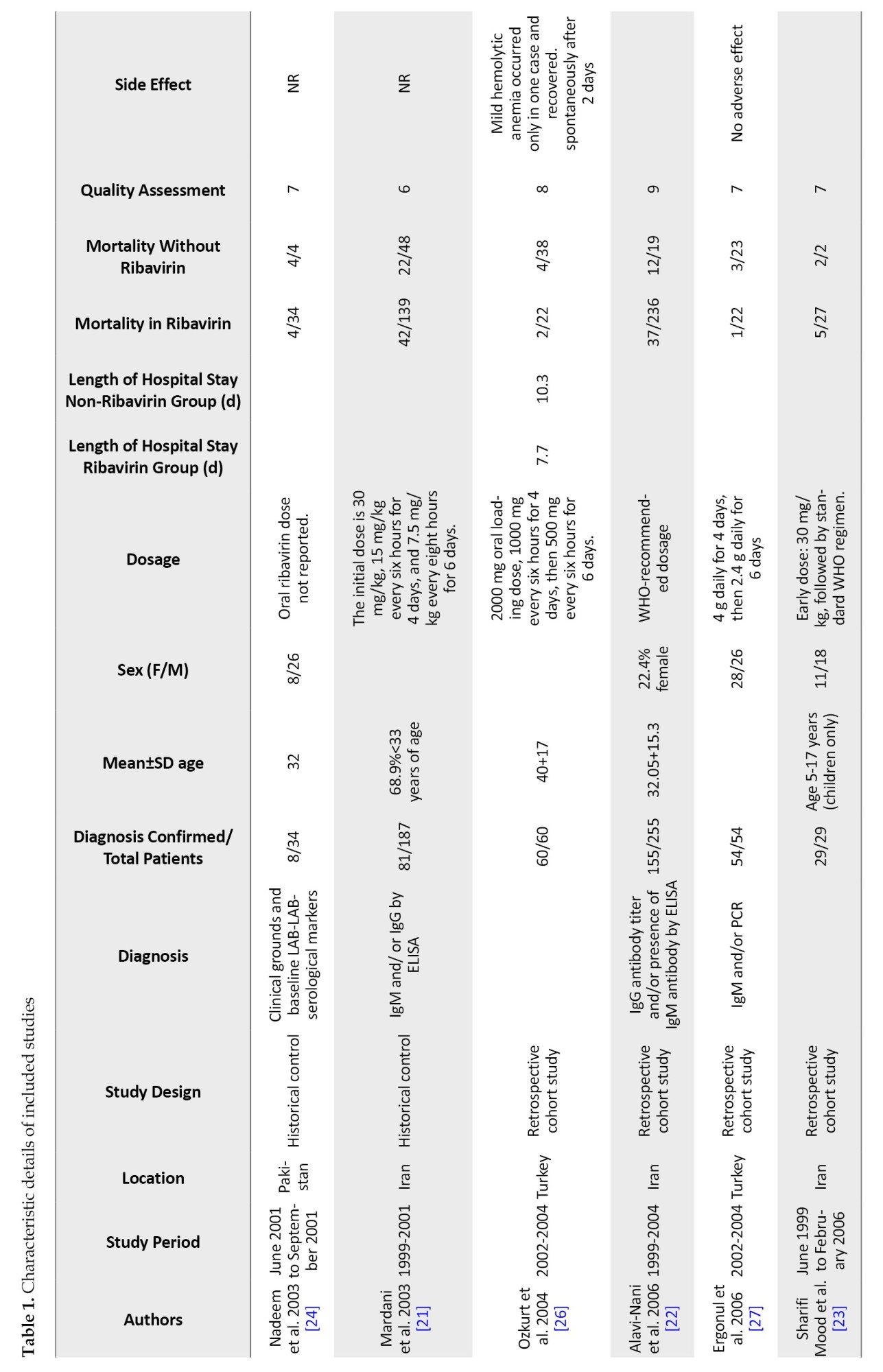
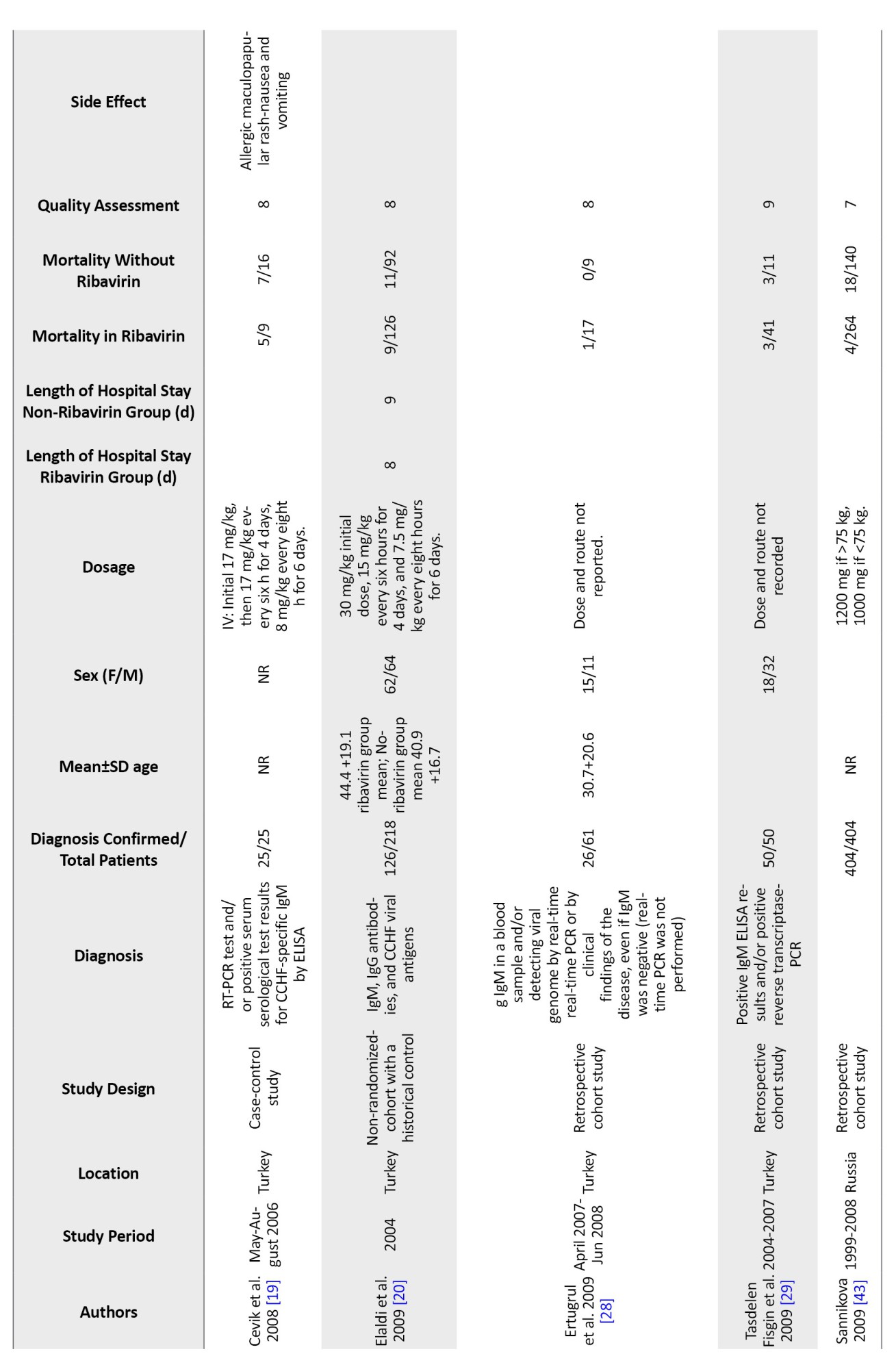
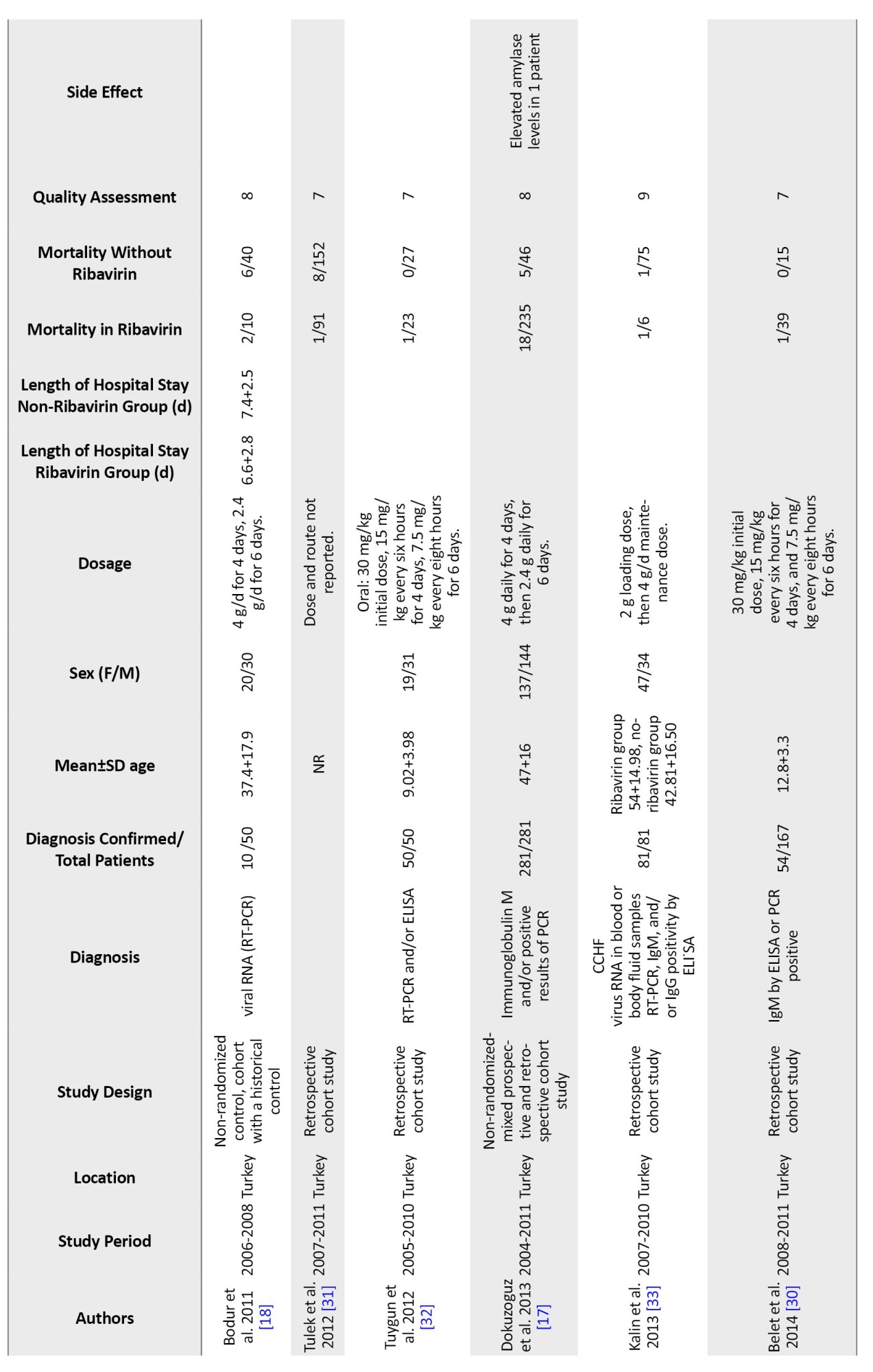
Table 1 displays the main features of the included studies. The precise number of adolescents could not be determined because they were not mentioned in the included studies. However, only children were included in six studies [23, 32, 37].
In 15 studies, IgM and IgG antibodies were used to confirm the diagnosis of CCHF using either Ig ELISA or PCR. In comparison, no information was recorded in four studies. Only one study confirmed the diagnosis of CCHF based on clinical judgment and baseline laboratory data, plus serological markers [24].
In 9 studies, oral ribavirin was given with the World Health Organization (WHO) recommended dosage (30 mg/kg as an initial loading dose, followed by 15 mg/kg every 6 hours for four days, and then 7.5 mg/kg every 8 hours for six days), while different dosages were used in six studies and were not reported in four others. Except for Cevic, all studies included in this analysis used oral ribavirin. The characteristics of the included studies in the Table are described in full detail [19] (Table 1).
Mortality in ribavirin group and non-ribavirin group
Twenty included studies comparing mortality in CCHF patients who received ribavirin with controls who did not receive ribavirin. The results show a significant difference between the studies, so the mortality rate in patients who took ribavirin was reported from 0% to 55% and from %0 to 100% in the control group. In the studies where patients received ribavirin, the mortality rate was 0% in 3 studies [34, 36, 37] and less than 10% in 10 other studies [17, 18, 23, 27, 28, 30, 32, 37]. Additionally, in 6 studies, the death rate of patients who had received ribavirin was between 12% to 30% [21-24, 33, 38]. However, Çevik et al.’s study reports a mortality rate of 55% in CCHF patients who received ribavirin [19]. The mortality rate in the 19 control groups that did not receive ribavirin was 0% in 5 studies [28, 30, 32, 34, 37] and between 5% and 63% in 12 studies [17-19, 21-23, 27, 31, 33, 37, 38]. However, two studies showed a 100% mortality rate in the control group [23, 24]. These two groups had very small sample sizes. Due to the high variability in the results, conducting studies with a large group population is necessary.
Length of stay in hospital
Among the included studies, 4 compare the length of stay of CCHF patients in the hospital who received ribavirin with the control group who did not. These studies show that patients who received ribavirin were hospitalized for an average of 6 to 8 days, while the control group was hospitalized for about 6 to 10 days [18, 19, 26, 34].
Laboratory diagnosis and clinical symptoms of CCHF
Eleven studies from the included articles examine the clinical symptoms and laboratory tests of CCHF patients. These studies show that the most common clinical symptoms observed in these patients include fever, bleeding (oral, nasal, GI tract, vaginal, gingival) and myalgia [22, 23, 30]. Also, symptoms such as leukopenia, thrombocytopenia, maculopapular rash, splenomegaly, hepatomegaly, facial hyperemia, headache, fatigue, nausea and vomiting are seen in CCHF patients [20, 23, 28, 32, 37]. Laboratory tests can be beneficial in diagnosing CCHF and also in follow-up. These tests include aspartate transaminase (AST), alanine transaminase (ALT), prothrombin time (PT), partial thromboplastin time (PTT), platelet (PLT), creatine kinase (CK), lactate dehydrogenase (LDH), creatine phosphokinase (CPK), and WBC. Various studies have shown that performing daily tests can help us diagnose the effect of the ribavirin drug on CCHF patients and predict the prognosis of the disease. [18, 20, 28-30, 36, 37].
Neurological complications caused by CCHF
Öztoprak et al. observed that patients with CCHF suffer from arthralgia, myalgia, fever, and headache [38]. Blood disorders such as thrombocytopenia (100% of patients), leukopenia (72% of patients), prolonged PT, PTT (88% of patients), and anemia (72% of patients) are seen in most CCHF patients and cause hematuria, purpura, petechiae, and ecchymosis. Therefore, the neurological complications seen in CCHF patients are probably the result of blood disorders and cerebral hemorrhage [7]. Additionally, a study on the relationship between platelet count, the severity of headaches and the length of hospitalization showed that people with a severe headache have a longer hospitalization and significantly lower amounts of platelets than people with a moderate headache. This study suggests a relationship between blood factors and clinical complications [8].
Neurological effects of ribavirin in CCHF
Headache, dizziness, or confusion, as well as encephalopathy and seizures, have been reported as neurological side effects attributed to ribavirin therapy by several studies conducted for CCHF treatment. Such neurological complications can be due to their impact on the central nervous system (CNS), with higher risks when there is renal impairment or if given in higher doses. At times, encephalopathy is associated with or following the administration of ribavirin and raised some questions about the safety of administering higher dosage for a more extended period [39].
However, evidence on the neurological effects of ribavirin directs in different directions. Whereas some sources report that neurological side effects due to ribavirin are infrequent and primarily manageable, other studies show several serious risks, mainly where ribavirin is used in patients with pre-existing conditions that may worsen its adverse side effects. Notably, the benefits of ribavirin in reducing CCHF mortality remain highly controversial; much research needs to be done to understand its safety profile, especially regarding neurological outcomes [39].
Discussion
In this article, we considered 20 original studies that investigated the effects of ribavirin on CCHF patients and a control group that did not take ribavirin.
Three studies investigated ribavirin’s effects on CCHF patients under 18. The studies show that the mortality rate is the same in the two groups of CCHF patients who received ribavirin and the control group who did not. One of the reasons for low mortality at this age is the low cytokine production in children. Since cytokines cause inflammation, their low production can improve the prognosis of the disease [34, 36, 37]. Ertem et al.’s study on the effects of ribavirin in adult CCHF patients shows that patients who receive ribavirin are significantly different from CCHF patients who receive ribavirin in terms of laboratory parameters (AST, ALT, PT, PTT, CPK, PLT, LDH). They had not consumed and only received blood products such as FFP (fresh frozen plasma) [40].
Antiviral use has been extended to treat viral infections such as CCHF, but the potential neurological side effects of therapy with ribavirin have thus attracted concern among medical scientists. Studies have documented that a wide range of neurological symptoms may be precipitated by the administration of ribavirin, ranging from mild headache and vertigo to confusion, encephalopathy, and seizures [39].
It appears that these side effects are dose-dependent but could also be exacerbated in patients with underlying renal impairment or other pre-existing conditions, suggesting that the risk of ribavirin-induced neurotoxicity in certain populations is high.
Neurological symptoms among patients undergoing ribavirin treatment may be attributed to its CNS effect, which can precipitate changes in mental status, bizarre behaviors and seizures in extreme cases. Of importance is the development of encephalopathy, severe brain dysfunction, which both the virus and ribavirin can cause, making it somewhat challenging to determine the actual cause of such symptoms. The same complexity of cerebral hemorrhaging that may be part of the development of CCHF was also mentioned in numerous studies, complicating distinguishing drug-induced versus disease-induced neurological problems [41].
While the therapeutic advantages of mortality reduction have been identified, the mixed outcomes from efficacy, and the associated risks of serious neurological side effects, there have been various debates regarding its usage. For example, early treatment of ribavirin has shown a reduction in mortality and length of hospital stay, but on other occasions, it is no different from treatment outcome.
However, other reports argue that the side effects, particularly those affecting the nervous system, may outweigh the benefits, especially in patients already vulnerable to such complications. The current evidence is inconclusive and high-quality randomized controlled trials are needed to explore ribavirin’s overall risk-benefit ratio further. With the uncertainties already inherent, close monitoring by health practitioners in patients undergoing ribavirin treatment is essential for early signs of neurological deterioration, especially in those patients with predispositions that put them at higher risk for such adverse effects. Clinicians may wish to consider alternative antiviral therapies or supportive care for patients who develop severe neurotoxic symptoms in such cases [41].
Most of the included articles show mortality in CCHF patients who received ribavirin and the control group who did not receive ribavirin. Many deaths are caused by sinus bradycardia, blood pressure, decreased heart rate, and disturbances in laboratory parameters such as hemoglobin level, platelet count, leukocyte count, AST, ALT, PT, PTT, and CK in these patients [30]. Also, a small number of patients were intubated due to breathing problems and bleeding. Studies show that reducing viral load, AST, and ALT levels increases PLT and the death rates between the two groups are not significantly different [17, 28, 39, 41]. So, treating CCHF patients with ribavirin will not have much effect, and most supportive therapies should be done based on clinical symptoms.
Several studies have shown that ribavirin effectively treats CCHF patients and reduces mortality [21, 23]. Daily tests taken from CCHF patients showed that liver and hematological complications of this patient, such as increased AST and ALT and decreased PLT, improved faster than CCHF patients who did not receive ribavirin.
As a result, with the improvement of coagulation factors, the possibility of bleeding in various organs, such as the brain, and the development of neurological complications, including coma, behavioral disorders, convulsion, hemiplegia, and mortality can be reduced through the use of ribavirin [7, 23, 30]. Tignor et al.’s study on rats suffering from CCHF showed that using ribavirin (50 mg/kg, single dose) reduces the amount of virus in the liver and blood so that despite viremia, viruses are in the brain. The heart and spleen were not isolated, so none of the mice treated with ribavirin were killed. However, over time, the effects of a single dose of ribavirin (50 mg/kg) decreased, so it was necessary to conduct multiple tests and use high doses of ribavirin [42].
Some studies show the harmful effects of ribavirin on CCHF patients, so the mortality rate is higher in the ribavirin treatment group than in the control group. A study by Kalin et al. and Tuygun et al. showed in CCHF patients that ribavirin treatment had a higher mortality rate than a group of CCHF patients who received only supportive care (red blood cells, plasma suspensions, and platelets). However, the exact mechanism of ribavirin in patients with CCHF is still unknown and these studies have been conducted with a small statistical population [32, 33].
Conclusion
Ribavirin is one of the few antiviral treatments that has shown potential to decrease mortality and improve outcomes in CCHF and, therefore, remains essential in managing CCHF. The drug is associated with a myriad of neurological side effects ranging from mild symptoms, such as headaches and dizziness, to severe manifestations, including encephalopathy and seizures. Adverse effects are more pronounced in patients with pre-existing conditions, including renal impairment and higher dosages or longer treatment courses.
Though it is evident that therapeutic benefits attributed to ribavirin reduction of viral load and mortality are indicated in some studies, the efficacy has so far remained inconsistent, and its neurotoxic risks require caution. The detailed underlying mechanisms of these neurological effects are not fully comprehended but may involve mitochondrial dysfunction, neurotransmitter interference and immune-mediated neuroinflammation.
Future studies should be directed at the elucidation of the mechanisms of neurotoxicity induced by ribavirin and the development of safer therapeutic alternatives. Clinicians should know early signs of neurological complications and adjust ribavirin therapy based on individual risk profiles. While ribavirin is paramount in managing CCHF, its use should be weighed against a thorough assessment of risks and benefits to achieve optimum outcomes.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Department of Public Health, School of Public Health and Safety, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.PHNS.REC.1401.116)
Funding
This study was extracted from MPH thesis of Mobina Fathi, approved by Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors contributions
Literature review, editing and manuscript: Hossein Hatami; Conceptualization and methodology: Mohammad Javad Nasiri and Mobina Fathi; Data collection and writing the original draft: Kimia Eyvani and Mobina Fathi; Data analysis, interpretation, visualization, project supervision and critical revision: Mobina Fathi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors are thankful to Shahid Beheshti University of Medical Sciences, Tehran, Iran and people who guide them through conducting this research.
References
In various parts of Africa, Europe and Asia, domestic and wild animals and birds carry the viruses that cause Crimean-Congo hemorrhagic fever (CCHF) [1]. Researchers have discovered CCHF for the first time in Crimea in 1944. In 1956, the virus was found in the blood of a feverish patient in Africa [2]. In 1967, Simpson et al. described a virus different from the one isolated in 1956, and they also noted that this type of Congo virus was comparable to virus strains from Bulgaria and central Asia [3]. The virus has been identified as a genus of Nairoviruses belonging to the Bunyaviridae family. CCHF causes lethal acute diseases with hemorrhagic symptoms [4].
The mortality rate of CCHF disease is between 20% to 35%. As a re-emerging disease, this illness is severe, febrile, hemorrhagic, and occasionally fatal. Hyalomma tick bites or direct contact with the blood or secretions of infected individuals or animals can spread this virus [5]. The four stages of the disease’s clinical course are hemorrhagic, incubation, pre-hemorrhagic, and healing. Pre-hemorrhagic phase symptoms include fever, headache, chills, nausea, vomiting, hyperemia, anathemas, rheumatic pain, and lumbar pain. It is crucial to make a diagnosis to manage the disease effectively. Petechiae, ecchymosis, hematomas, or major hemorrhages are the signs of the brief and quickly deteriorating hemorrhagic phase. After 15 to 20 days from the beginning of the disease, a patient enters the convalescence phase, which is marked by overall weakness, fatigability, poor appetite, and nausea [6]. Additionally, a study shows that a rare complication such as cerebral hemorrhage in CCHF patients can cause various neurological symptoms such as confusion, seizures, coma, behavioral disorders, hemiplegia, peripheral mononeuritis with upper limb paralysis, and headache [7]. The severity of the headache, which is very similar to migraine headaches, is probably related to vasodilatation, the release of inflammatory cytokines, and, as a result, damage to the vascular endothelium [8].
Ribavirin is a broad-spectrum antiviral nucleoside analog that is one of the most used therapies in treating CCHF. Clinically, the efficacy of ribavirin has been manifested by a reduction of viral load and mortality rate. However, treatment with this drug has raised some neurological concerns, mainly when administered in very high doses or for extended periods. These neurological manifestations can vary from mild symptoms such as headaches, dizziness and confusion to more serious complications involving seizures, encephalopathy, or even neuropsychiatric disturbances. The exact underlying pathophysiology for ribavirin-induced neurotoxicity has not been well understood to date; it is postulated to involve mitochondrial dysfunction because ribavirin can inhibit mitochondrial polymerases and decrease cellular production of ATP, leading to neuronal insult. Also, ribavirin may affect the neurotransmitter metabolic process, especially interference in gamma-aminobutyric acid pathways, thereby showing the development of seizure and neuropsychiatric symptoms. Also, interference of ribavirin with the immune system could facilitate neuroinflammation, which increases neurological complications. These risks underscore the need for close neurological monitoring of CCHF patients receiving ribavirin, including careful vigilance regarding dose adjustments and treatment duration. Further research is essential to define the exact mechanisms of ribavirin’s neurotoxicity and formulate therapeutic strategies that minimize neurological risks without compromising its antiviral efficacy [9, 10, 11].
Immune cells and endothelial cells are possible targets in CCHF. The virus induces endothelial cells to generate proinflammatory cytokines. This process causes vasodilatation, increased vascular permeability, hypotension, multiple organ failure, shock, and death in severe circumstances. Apoptosis of lymphocytes, hemophagocytosis, decreased antibody response and partial activation of dendritic cells and macrophages are other ways CCHF may suppress the immune system [12].
Although numerous research studies have been performed to treat CCHF patients with immunoglobulins (Ig) obtained from vaccinated horses [13] and serum collected from convalescing CCHF patients [14], prompt supportive care, including the infusion of blood products, no clinical trials examining the latter therapies have yet been documented [15, 16].
The objectives of the current investigation encompass assessing the advisability of prophylactic administration after exposure to the CCHF virus and examining the impact of ribavirin administration in correlation with both the severity of illness and the timing of medication initiation relative to the onset of symptoms.
Materials and Methods
Search strategy
We used PubMed, Scopus, Web of Science and Google Scholar databases without language restriction or publication status to conduct a thorough search. The search includes articles published until June 2024. The following search criteria were entered into an advanced search using the keywords: CCHF, ribavirin, neurological diseases, immunoglobulin, hospitalization and mortality.
Data extraction
Two authors independently reviewed all titles and abstracts found during the search following inclusion criteria. All possibly qualifying studies, or ones whose eligibility has not been defined, have been carefully assessed. After this step, the full-text publications were thoroughly evaluated, and any differences of opinion were resolved. For each study, data such as the name of the first author, the country, the publication year of the articles, the type of study, average age of patients, gender and nationality of the patients, dosage of ribavirin, and method of administration (oral or intravenous), adverse events due to ribavirin administration, duration of hospitalization, and the mortality rate due to the use of ribavirin drug, have been extracted.
Inclusion criteria
We selected these articles: Studies report primary data on CCHF epidemiology, clinical characteristics, risk factors, or outcomes; studies published in peer-reviewed journals; studies available in English; and studies conducted on human subjects.
Standard definitions for CCHF include clinical definition, which refers to the set of signs and symptoms characteristic of the disease. They include fever, headache, muscle pain, dizziness, and bleeding. The epidemiological definition of CCHF considers the exposure history and risk factors associated with the disease. This definition includes factors such as a history of tick bites, contact with infected animals or their tissues/fluids, or residing in areas where CCHF is endemic or has been reported. A possible case definition is used to identify individuals who exhibit clinical signs and symptoms suggestive of CCHF and have a history of potential exposure or risk factors. Probable case definition indicates CCHF more strongly than a possible case. It may include additional criteria such as laboratory findings, radiological evidence, or clinical progression that strongly supports a CCHF diagnosis but falls short of definitive confirmation. A definite or clear case definition is used when there is laboratory confirmation of CCHF infection. It requires the detection of CCHF virus-specific antibodies or genetic material (RNA) in the patient’s blood sample using validated laboratory tests like enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR).
Exclusion criteria
The exclusion criteria of our articles included studies with insufficient or duplicated data, studies that focused on animal models or in vitro experiments, case reports, letters, editorials, and conference abstracts.
Neurological effects of ribavirin in CCHF
Data from trials reporting any neurological side effects or complications related to ribavirin administration during or after treatment were tabulated to ascertain the neurological impact of ribavirin among patients with CCHF. These findings fell into the spectrum of headache, dizziness, confusion, and more severe manifestations such as seizures and encephalopathy.
Considering the studies for inclusion, we emphasized the detailed accounts of neurological symptoms and their relationship to the given ribavirin dosage and route of administration taken, orally or via intravenous administration. We also examined whether pre-existing conditions, such as renal impairment or the protracted administration of ribavirin, impacted neurological complications’ development and or severity. Data on neurological adverse effects thus extracted were then evaluated, along with other adverse events, regarding the overall safety profile of ribavirin in CCHF treatment.
Types of studies
We included randomized control trials, quasi-RCTs, non-randomized controlled studies, case-control studies, and cohort studies, which assessed the efficacy of ribavirin administration on mortality and length of hospital stay.
Types of participants
Adults or children of any age with a confirmed diagnosis of CCHF by laboratory test (Ig) or PCR were chosen as participants.
Types of interventions
IV or oral ribavirin compared with supportive care or other interventions have been considered.
Types of outcome measures
The primary outcome was comparing the mortality rate between ribavirin and no-ribavirin groups. The secondary results compared the length of hospital stay (days) among ribavirin and control groups, serious adverse events, or withdrawal of treatment due to serious adverse events.
Quality assessment
We have used the Newcastle-Ottawa Scale (NOS) [12] to check the quality of the papers. The PRISMA flow chart for the systematic review is displayed in Figure 1.
Results
Search results
A total of 1940 references were found throughout our search. After examining all titles and abstracts and manually deleting 630 duplicates, two reviewers assessed the remaining 110 articles. We thoroughly evaluated all the publications before excluding 58 for reasons such as non-English articles, reviews irrelevant to this meta-analysis, unclear or insufficient data, and low quality. Twenty publications were eligible and included in the meta-analysis following a full-text screening of the remaining (Figure 1).
Quality assessment
We primarily assessed the methodological quality of included studies using the NOS developed by the Cochrane Collaboration. Two independent reviewers have evaluated the Risk of bias in the included studies. Randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting are all factors to be considered when determining the risk of bias (Table 1).




Study design
One mixed prospective and retrospective cohort study [17], one matched cohort study [18], one case-control study [19], one cohort with a historical control [20], and 16 observational studies were included in our study. Studies were conducted in Iran [21-23], Pakistan [24], Turkey, and Russia [17-20, 25-37].
Characteristics data of participants
Table 1 displays the main features of the included studies. The precise number of adolescents could not be determined because they were not mentioned in the included studies. However, only children were included in six studies [23, 32, 37].
In 15 studies, IgM and IgG antibodies were used to confirm the diagnosis of CCHF using either Ig ELISA or PCR. In comparison, no information was recorded in four studies. Only one study confirmed the diagnosis of CCHF based on clinical judgment and baseline laboratory data, plus serological markers [24].
In 9 studies, oral ribavirin was given with the World Health Organization (WHO) recommended dosage (30 mg/kg as an initial loading dose, followed by 15 mg/kg every 6 hours for four days, and then 7.5 mg/kg every 8 hours for six days), while different dosages were used in six studies and were not reported in four others. Except for Cevic, all studies included in this analysis used oral ribavirin. The characteristics of the included studies in the Table are described in full detail [19] (Table 1).
Mortality in ribavirin group and non-ribavirin group
Twenty included studies comparing mortality in CCHF patients who received ribavirin with controls who did not receive ribavirin. The results show a significant difference between the studies, so the mortality rate in patients who took ribavirin was reported from 0% to 55% and from %0 to 100% in the control group. In the studies where patients received ribavirin, the mortality rate was 0% in 3 studies [34, 36, 37] and less than 10% in 10 other studies [17, 18, 23, 27, 28, 30, 32, 37]. Additionally, in 6 studies, the death rate of patients who had received ribavirin was between 12% to 30% [21-24, 33, 38]. However, Çevik et al.’s study reports a mortality rate of 55% in CCHF patients who received ribavirin [19]. The mortality rate in the 19 control groups that did not receive ribavirin was 0% in 5 studies [28, 30, 32, 34, 37] and between 5% and 63% in 12 studies [17-19, 21-23, 27, 31, 33, 37, 38]. However, two studies showed a 100% mortality rate in the control group [23, 24]. These two groups had very small sample sizes. Due to the high variability in the results, conducting studies with a large group population is necessary.
Length of stay in hospital
Among the included studies, 4 compare the length of stay of CCHF patients in the hospital who received ribavirin with the control group who did not. These studies show that patients who received ribavirin were hospitalized for an average of 6 to 8 days, while the control group was hospitalized for about 6 to 10 days [18, 19, 26, 34].
Laboratory diagnosis and clinical symptoms of CCHF
Eleven studies from the included articles examine the clinical symptoms and laboratory tests of CCHF patients. These studies show that the most common clinical symptoms observed in these patients include fever, bleeding (oral, nasal, GI tract, vaginal, gingival) and myalgia [22, 23, 30]. Also, symptoms such as leukopenia, thrombocytopenia, maculopapular rash, splenomegaly, hepatomegaly, facial hyperemia, headache, fatigue, nausea and vomiting are seen in CCHF patients [20, 23, 28, 32, 37]. Laboratory tests can be beneficial in diagnosing CCHF and also in follow-up. These tests include aspartate transaminase (AST), alanine transaminase (ALT), prothrombin time (PT), partial thromboplastin time (PTT), platelet (PLT), creatine kinase (CK), lactate dehydrogenase (LDH), creatine phosphokinase (CPK), and WBC. Various studies have shown that performing daily tests can help us diagnose the effect of the ribavirin drug on CCHF patients and predict the prognosis of the disease. [18, 20, 28-30, 36, 37].
Neurological complications caused by CCHF
Öztoprak et al. observed that patients with CCHF suffer from arthralgia, myalgia, fever, and headache [38]. Blood disorders such as thrombocytopenia (100% of patients), leukopenia (72% of patients), prolonged PT, PTT (88% of patients), and anemia (72% of patients) are seen in most CCHF patients and cause hematuria, purpura, petechiae, and ecchymosis. Therefore, the neurological complications seen in CCHF patients are probably the result of blood disorders and cerebral hemorrhage [7]. Additionally, a study on the relationship between platelet count, the severity of headaches and the length of hospitalization showed that people with a severe headache have a longer hospitalization and significantly lower amounts of platelets than people with a moderate headache. This study suggests a relationship between blood factors and clinical complications [8].
Neurological effects of ribavirin in CCHF
Headache, dizziness, or confusion, as well as encephalopathy and seizures, have been reported as neurological side effects attributed to ribavirin therapy by several studies conducted for CCHF treatment. Such neurological complications can be due to their impact on the central nervous system (CNS), with higher risks when there is renal impairment or if given in higher doses. At times, encephalopathy is associated with or following the administration of ribavirin and raised some questions about the safety of administering higher dosage for a more extended period [39].
However, evidence on the neurological effects of ribavirin directs in different directions. Whereas some sources report that neurological side effects due to ribavirin are infrequent and primarily manageable, other studies show several serious risks, mainly where ribavirin is used in patients with pre-existing conditions that may worsen its adverse side effects. Notably, the benefits of ribavirin in reducing CCHF mortality remain highly controversial; much research needs to be done to understand its safety profile, especially regarding neurological outcomes [39].
Discussion
In this article, we considered 20 original studies that investigated the effects of ribavirin on CCHF patients and a control group that did not take ribavirin.
Three studies investigated ribavirin’s effects on CCHF patients under 18. The studies show that the mortality rate is the same in the two groups of CCHF patients who received ribavirin and the control group who did not. One of the reasons for low mortality at this age is the low cytokine production in children. Since cytokines cause inflammation, their low production can improve the prognosis of the disease [34, 36, 37]. Ertem et al.’s study on the effects of ribavirin in adult CCHF patients shows that patients who receive ribavirin are significantly different from CCHF patients who receive ribavirin in terms of laboratory parameters (AST, ALT, PT, PTT, CPK, PLT, LDH). They had not consumed and only received blood products such as FFP (fresh frozen plasma) [40].
Antiviral use has been extended to treat viral infections such as CCHF, but the potential neurological side effects of therapy with ribavirin have thus attracted concern among medical scientists. Studies have documented that a wide range of neurological symptoms may be precipitated by the administration of ribavirin, ranging from mild headache and vertigo to confusion, encephalopathy, and seizures [39].
It appears that these side effects are dose-dependent but could also be exacerbated in patients with underlying renal impairment or other pre-existing conditions, suggesting that the risk of ribavirin-induced neurotoxicity in certain populations is high.
Neurological symptoms among patients undergoing ribavirin treatment may be attributed to its CNS effect, which can precipitate changes in mental status, bizarre behaviors and seizures in extreme cases. Of importance is the development of encephalopathy, severe brain dysfunction, which both the virus and ribavirin can cause, making it somewhat challenging to determine the actual cause of such symptoms. The same complexity of cerebral hemorrhaging that may be part of the development of CCHF was also mentioned in numerous studies, complicating distinguishing drug-induced versus disease-induced neurological problems [41].
While the therapeutic advantages of mortality reduction have been identified, the mixed outcomes from efficacy, and the associated risks of serious neurological side effects, there have been various debates regarding its usage. For example, early treatment of ribavirin has shown a reduction in mortality and length of hospital stay, but on other occasions, it is no different from treatment outcome.
However, other reports argue that the side effects, particularly those affecting the nervous system, may outweigh the benefits, especially in patients already vulnerable to such complications. The current evidence is inconclusive and high-quality randomized controlled trials are needed to explore ribavirin’s overall risk-benefit ratio further. With the uncertainties already inherent, close monitoring by health practitioners in patients undergoing ribavirin treatment is essential for early signs of neurological deterioration, especially in those patients with predispositions that put them at higher risk for such adverse effects. Clinicians may wish to consider alternative antiviral therapies or supportive care for patients who develop severe neurotoxic symptoms in such cases [41].
Most of the included articles show mortality in CCHF patients who received ribavirin and the control group who did not receive ribavirin. Many deaths are caused by sinus bradycardia, blood pressure, decreased heart rate, and disturbances in laboratory parameters such as hemoglobin level, platelet count, leukocyte count, AST, ALT, PT, PTT, and CK in these patients [30]. Also, a small number of patients were intubated due to breathing problems and bleeding. Studies show that reducing viral load, AST, and ALT levels increases PLT and the death rates between the two groups are not significantly different [17, 28, 39, 41]. So, treating CCHF patients with ribavirin will not have much effect, and most supportive therapies should be done based on clinical symptoms.
Several studies have shown that ribavirin effectively treats CCHF patients and reduces mortality [21, 23]. Daily tests taken from CCHF patients showed that liver and hematological complications of this patient, such as increased AST and ALT and decreased PLT, improved faster than CCHF patients who did not receive ribavirin.
As a result, with the improvement of coagulation factors, the possibility of bleeding in various organs, such as the brain, and the development of neurological complications, including coma, behavioral disorders, convulsion, hemiplegia, and mortality can be reduced through the use of ribavirin [7, 23, 30]. Tignor et al.’s study on rats suffering from CCHF showed that using ribavirin (50 mg/kg, single dose) reduces the amount of virus in the liver and blood so that despite viremia, viruses are in the brain. The heart and spleen were not isolated, so none of the mice treated with ribavirin were killed. However, over time, the effects of a single dose of ribavirin (50 mg/kg) decreased, so it was necessary to conduct multiple tests and use high doses of ribavirin [42].
Some studies show the harmful effects of ribavirin on CCHF patients, so the mortality rate is higher in the ribavirin treatment group than in the control group. A study by Kalin et al. and Tuygun et al. showed in CCHF patients that ribavirin treatment had a higher mortality rate than a group of CCHF patients who received only supportive care (red blood cells, plasma suspensions, and platelets). However, the exact mechanism of ribavirin in patients with CCHF is still unknown and these studies have been conducted with a small statistical population [32, 33].
Conclusion
Ribavirin is one of the few antiviral treatments that has shown potential to decrease mortality and improve outcomes in CCHF and, therefore, remains essential in managing CCHF. The drug is associated with a myriad of neurological side effects ranging from mild symptoms, such as headaches and dizziness, to severe manifestations, including encephalopathy and seizures. Adverse effects are more pronounced in patients with pre-existing conditions, including renal impairment and higher dosages or longer treatment courses.
Though it is evident that therapeutic benefits attributed to ribavirin reduction of viral load and mortality are indicated in some studies, the efficacy has so far remained inconsistent, and its neurotoxic risks require caution. The detailed underlying mechanisms of these neurological effects are not fully comprehended but may involve mitochondrial dysfunction, neurotransmitter interference and immune-mediated neuroinflammation.
Future studies should be directed at the elucidation of the mechanisms of neurotoxicity induced by ribavirin and the development of safer therapeutic alternatives. Clinicians should know early signs of neurological complications and adjust ribavirin therapy based on individual risk profiles. While ribavirin is paramount in managing CCHF, its use should be weighed against a thorough assessment of risks and benefits to achieve optimum outcomes.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Department of Public Health, School of Public Health and Safety, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.PHNS.REC.1401.116)
Funding
This study was extracted from MPH thesis of Mobina Fathi, approved by Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors contributions
Literature review, editing and manuscript: Hossein Hatami; Conceptualization and methodology: Mohammad Javad Nasiri and Mobina Fathi; Data collection and writing the original draft: Kimia Eyvani and Mobina Fathi; Data analysis, interpretation, visualization, project supervision and critical revision: Mobina Fathi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors are thankful to Shahid Beheshti University of Medical Sciences, Tehran, Iran and people who guide them through conducting this research.
References
- Çeviker SA, Yılmaz M, Uyar C, Demiray EKD. Bibliometric analysis of scientific research on Crimean-Congo hemorrhagic fever in Turkey. Demiroğlu Bilim Üniv Florence Nightingale Tıp Dergisi. 2021; 7(2):097-102. [DOI:10.5606/fng.btd.2021.25064]
- Fillâtre P, Revest M, Tattevin P. Crimean-congo hemorrhagic fever: An update. Med Mal Infect. 2019; 49(8):574-85. [DOI:10.1016/j.medmal.2019.09.005] [PMID]
- Shayan S, Bokaean M, Shahrivar MR, Chinikar S. Crimean-Congo Hemorrhagic Fever. Lab Med. 2015; 46(3):180-9. [DOI:10.1309/LMN1P2FRZ7BKZSCO] [PMID]
- Ergönül Ö, Keske Ş, Çeldir MG, Kara İA, Pshenichnaya N, Abuova G, et al. Systematic review and meta-analysis of postexposure prophylaxis for crimean-congo hemorrhagic fever virus among healthcare workers. Emerg Infect Dis. 2018; 24(9):1642-8. [DOI:10.3201/eid2409.171709] [PMID] [PMCID]
- Jüni P, Loke Y, Pigott T, Ramsay C, Regidor D, Rothstein H, et al. Risk of bias in non-randomized studies of interventions (ROBINS-I): Detailed guidance. BMJ. 2016; 355:i4919. [DOI:10.1136/bmj.i4919]
- Fabara SP, Ortiz JF, Smith DW, Parwani J, Srikanth S, Varghese T, et al. Crimean-congo hemorrhagic fever beyond ribavirin: A systematic review. Cureus. 2021; 13(9):e17842. [DOI:10.7759/cureus.17842]
- Kouhpayeh H. An overview of complications and mortality of Crimean-Congo hemorrhagic fever. Int J Infect. 2019; 6(2):e91707. [DOI:10.5812/iji.91707]
- Aksoy D, Barut H, Duygu F, Çevik B, Kurt S, Sümbül O. Characteristics of headache and its relationship with disease severity in patients with Crimean-Congo hemorrhagic fever. Agri. 2018; 30(1):12-7. [DOI:10.5505/agri.2017.76259] [PMID]
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013; 100(1):159-89. [DOI:10.1016/j.antiviral.2013.07.006] [PMID]
- Ceylan B, Calıca A, Ak O, Akkoyunlu Y, Turhan V. Ribavirin is not effective against Crimean-Congo hemorrhagic fever: Observations from the Turkish experience. Int J Infect Dis. 2013; 17(10):e799-801. [DOI:10.1016/j.ijid.2013.02.030] [PMID] [PMCID]
- Schulz A. Crimean-Congo hemorrhagic fever orthonairovirus (CCHFV): Surveillance studies among different livestock in sub-Saharan Africa and the molecular characterization of Hyalomma ticks serving as main reservoir and vector [doctoral dissertation]. Hannover: University of Veterinary Medicine Hannover; 2020. [Link]
- Akıncı E, Bodur H, Leblebicioglu H. Pathogenesis of Crimean-Congo hemorrhagic fever. Vector Borne Zoonotic Dis. 2013; 13(7):429-37. [DOI:10.1089/vbz.2012.1061] [PMID]
- Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979; 15(4):307-417. [DOI:10.1093/jmedent/15.4.307] [PMID]
- Vassilenko SM, Vassilev TL, Bozadjiev LG, Bineva IL, Kazarov GZ. Specific intravenous immunoglobulin for Crimean-Congo haemorrhagic fever. Lancet. 1990; 335(8692):791-2. [DOI:10.1016/0140-6736(90)90906-L] [PMID]
- Johnsen E, Jørgensen HA. Effectiveness of second generation antipsychotics: A systematic review of randomized trials. BMC Psychiatry. 2008; 8:31. [DOI:10.1186/1471-244X-8-31] [PMID] [PMCID]
- Maltezou HC, Andonova L, Andraghetti R, Bouloy M, Ergonul O, Jongejan F, et al. Crimean-Congo hemorrhagic fever in Europe: current situation calls for preparedness. Euro Surveill. 2010; 15(10):19504. [DOI:10.2807/ese.15.10.19504-en] [PMID]
- Dokuzoguz B, Celikbas AK, Gök ŞE, Baykam N, Eroglu MN, Ergönül Ö. Severity scoring index for Crimean-Congo hemorrhagic fever and the impact of ribavirin and corticosteroids on fatality. Clin Infect Dis. 2013; 57(9):1270-4. [DOI:10.1093/cid/cit527] [PMID]
- Bodur H, Erbay A, Akıncı E, Öngürü P, Bayazıt N, Eren SS, et al. Effect of oral ribavirin treatment on the viral load and disease progression in Crimean-Congo hemorrhagic fever. Int J Infect Dis. 2011; 15(1):e44-7. [DOI:10.1016/j.ijid.2010.09.009] [PMID]
- Cevik MA, Elaldi N, Akinci E, Ongürü P, Erbay A, Buzgan T, et al. A preliminary study to evaluate the effect of intravenous ribavirin treatment on survival rates in Crimean-Congo hemorrhagic fever. J Infect. 2008; 57(4):350-1. [DOI:10.1016/j.jinf.2008.07.007] [PMID]
- Elaldi N, Bodur H, Ascioglu S, Celikbas A, Ozkurt Z, Vahaboglu H, et al. Efficacy of oral ribavirin treatment in Crimean-Congo haemorrhagic fever: A quasi-experimental study from Turkey. J Infect. 2009; 58(3):238-44. [DOI:10.1016/j.jinf.2009.01.014] [PMID]
- Mardani M, Jahromi MK, Naieni KH, Zeinali M. The efficacy of oral ribavirin in the treatment of crimean-congo hemorrhagic fever in Iran. Clin Infect Dis. 2003; 36(12):1613-8. [DOI:10.1086/375058] [PMID]
- Alavi-Naini R, Moghtaderi A, Koohpayeh HR, Sharifi-Mood B, Naderi M, Metanat M, et al. Crimean-Congo hemorrhagic fever in Southeast of Iran. J Infect. 2006; 52(5):378-82. [DOI:10.1016/j.jinf.2005.07.015] [PMID]
- Sharifi Mood B, Alavi-Naini R, Metanat M, Rakhshani F. Ribavirin: An effective drug for treatment of children with Crimean Congo hemorrhagic fever: A seven years experience. Pak J Biolo Sci. 2006; 9(8):1598-600. [DOI:10.3923/pjbs.2006.1598.1600]
- Nadeem M, Ali N, Anwar M, Hussain I, Mohammad T, Hayee A. A comparision of clinical diagnosis & Serological diagnosis in an epidemic of Crimean-Congo Haemorrhagic Fever. Pak J Med Sci. 2003; 19(4):247-52. [Link]
- Koksal I, Yilmaz G, Aksoy F, Aydin H, Yavuz I, Iskender S, et al. The efficacy of ribavirin in the treatment of Crimean-Congo hemorrhagic fever in Eastern Black Sea region in Turkey. J Clin Virol. 2010; 47(1):65-8. [DOI:10.1016/j.jcv.2009.11.007] [PMID]
- Ozkurt Z, Kiki I, Erol S, Erdem F, Yilmaz N, Parlak M, et al. Crimean-Congo hemorrhagic fever in Eastern Turkey: Clinical features, risk factors and efficacy of ribavirin therapy. J Infect. 2006; 52(3):207-15. [DOI:10.1016/j.jinf.2005.05.003] [PMID]
- Ergonul O, Celikbas A, Baykam N, Eren S, Dokuzoguz B. Analysis of risk-factors among patients with Crimean-Congo haemorrhagic fever virus infection: Severity criteria revisited. Clin Microbiol Infect. 2006; 12(6):551-4. [DOI:10.1111/j.1469-0691.2006.01445.x] [PMID]
- Ertugrul B, Uyar Y, Yavas K, Turan C, Oncu S, Saylak O, et al. An outbreak of Crimean-Congo hemorrhagic fever in western Anatolia, Turkey. Int J Infect Dis. 2009; 13(6):e431-6. [DOI:10.1016/j.ijid.2009.02.011] [PMID]
- Tasdelen Fisgin N, Ergonul O, Doganci L, Tulek N. The role of ribavirin in the therapy of Crimean-Congo hemorrhagic fever: Early use is promising. Eur J Clin Microbiol Infect Dis. 2009; 28(8):929-33. [DOI:10.1007/s10096-009-0728-2] [PMID]
- Belet N, Top A, Terzi O, Arslan HN, Baysal K, Sensoy G. Evaluation of children with Crimean-Congo hemorrhagic fever in the central Blacksea region. Pediatr Infect Dis J. 2014; 33(8):e194-7. [DOI:10.1097/INF.0000000000000281] [PMID]
- Tulek N, Ozturk B, Bulut C, Tuncer Ertem G, Erdinc F, Altun S. The evaluation of ribavirin use in patients with Crimean-Congo haemorrhagic fever. Clin Microbiol Infect. 2012; 18.
- Tuygun N, Tanir G, Caglayik DY, Uyar Y, Korukluoglu G, Cenesiz F. Pediatric cases of Crimean-Congo hemorrhagic fever in Turkey. Pediatr Int. 2012; 54(3):402-6. [DOI:10.1111/j.1442-200X.2011.03549.x] [PMID]
- Kalın G, Metan G, Demiraslan H, Doganay M. Do we really need ribavirin in the treatment of crimean-congo hemorrhagic Fever? J Chemother. 2014; 26(3):146-9. [DOI:10.1179/1973947813Y.0000000123] [PMID]
- Karaaslan E, Çetin Ş. [Evaluation of efficacy of ribavirin on laboratory test and severity score in crimean-congo hemorrhagic fever in children (Turkish)]. Mikrobiyol Bul. 2021; 55(2):180-93. [DOI:10.5578/mb.20219905] [PMID]
- Gayretli Aydin ZG, Yesilbas O, Reis GP, Guven B. The first pediatric case of hemophagocytic lymphohistiocytosis secondary to Crimean-Congo haemorrhagic fever successfully treated with therapeutic plasma exchange accompanying ribavirin and intravenous immunoglobulin. J Clin Apher. 2021; 36(5):780-4. [DOI:10.1002/jca.21915] [PMID]
- Tezer H, Ozkaya-Parlakay A, Gulhan B, Kanik-Yuksek S. Ribavirin use in pediatric patients with Crimean Congo Hemorrhagic Fever: Is it really necessary? Braz J Infect Dis. 2016; 20(2):222-3. [DOI:10.1016/j.bjid.2015.11.012] [PMID] [PMCID]
- Gayretli Aydin ZG, Tanir G, Metin O, Aydin Teke T, Bayhan GI, Oz FN, et al. Transient sinus bradycardia during the course of Crimean-Congo hemorrhagic fever in children. Ticks Tick Borne Dis. 2015; 6(2):185-8. [DOI:10.1016/j.ttbdis.2014.12.003] [PMID]
- Öztoprak B, Öztoprak İ, Engin A. Is the brain spared in Crimean-Congo haemorrhagic fever? An MR-SWI study to reveal CNS involvement. Eur Radiol. 2018; 28(9):3893-901. [DOI:10.1007/s00330-018-5310-9] [PMID]
- Soares-Weiser K, Thomas S, Thomson G, Garner P. Ribavirin for Crimean-Congo hemorrhagic fever: Systematic review and meta-analysis. BMC Infect Dis. 2010; 10:207.[DOI:10.1186/1471-2334-10-207] [PMID] [PMCID]
- Ertem G, Sönmezer MÇ, Temoçin F, Ataman Hatipoğlu Ç, Tülek N, Oral B. The efficacy of oral ribavirin on clinical and laboratory parameters inCrimean-Congo hemorrhagic fever: An observational study from Turkey. Turk J Med Sci. 2016; 46(5):1407-14. [DOI:10.3906/sag-1506-92] [PMID]
- Espy N, Pérez-Sautu U, Ramírez de Arellano E, Negredo A, Wiley MR, Bavari S, et al. Ribavirin had demonstrable effects on the Crimean-Congo hemorrhagic fever virus (CCHFV) population and load in a patient with CCHF infection. J Infect Dis. 2018; 217(12):1952-6. [DOI:10.1093/infdis/jiy163] [PMID]
- Tignor GH, Hanham CA. Ribavirin efficacy in an in vivo model of Crimean-Congo hemorrhagic fever virus (CCHF) infection. Antiviral Res. 1993; 22(4):309-25. [DOI:10.1016/0166-3542(93)90040-P] [PMID]
- Sannikova IV. Crimean-Congo haemorrhagic fever: Clinico-pathogenic aspects and optimisation of treatment [doctoral dissertation]. Moscow: Stavropol State Medical University, 2009.
Type of Study: Review |
Subject:
General
Received: 2024/10/21 | Accepted: 2025/01/27 | Published: 2025/04/1
Received: 2024/10/21 | Accepted: 2025/01/27 | Published: 2025/04/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |