Fri, Sep 26, 2025
Volume 11, Issue 3 (Summer 2025)
Caspian J Neurol Sci 2025, 11(3): 203-212 |
Back to browse issues page
Ethics code: IR.SHAHED.REC.1400.035
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Amini H, Karami M. Preliminary of Cognitive and Systemic Disorders in Rats Due to Chronic Exposure to Aluminum Chloride. Caspian J Neurol Sci 2025; 11 (3) :203-212
URL: http://cjns.gums.ac.ir/article-1-754-en.html
URL: http://cjns.gums.ac.ir/article-1-754-en.html
Preliminary of Cognitive and Systemic Disorders in Rats Due to Chronic Exposure to Aluminum Chloride
1- Department of Biology, Faculty of Basic Sciences, Shahed University, Tehran, Iran.
2- Department of Biology, Faculty of Basic Sciences, Shahed University, Tehran, Iran. & Neurophysiology Research Center, Shahed University, Tehran, Iran. ,karami@shahed.ac.ir
2- Department of Biology, Faculty of Basic Sciences, Shahed University, Tehran, Iran. & Neurophysiology Research Center, Shahed University, Tehran, Iran. ,
Keywords: Aluminum chloride (Al), Drinking water, Alzheimer disease (AD), Cognitive impairment (CI), Liver dysfunction, Rat
Full-Text [PDF 1426 kb]
(110 Downloads)
| Abstract (HTML) (282 Views)
Full-Text: (80 Views)
Introduction
Alzheimer disease (AD) is a serious neurological problem that is characterized by decreased awareness, loss of daily activities, and neuropsychiatric symptoms [1]. Reduced synthesis of the neurotransmitter acetylcholine, disruption of neuronal connections, buildup of amyloid β (Aβ) plaques, and neurofibrillary tangles of hyperphosphorylated tau proteins in the hippocampus and forebrain lead to AD. Studies show that Aβ and Aβ precursor proteins play an important role in the pathogenesis of early-onset AD. Millions of people worldwide suffer from AD, and it is the most important basis of age-related memory loss [2]. AD is known as a multifactorial disorder that can be attributed to genetic factors (familial AD) and environmental factors (sporadic AD), such as inflammation, high levels of free radicals, and accumulation of heavy metals (HMs) in the brain [3]. Aluminum is the third most abundant element on earth [4] and is widely used in human life [5]. It has been shown that there is a relationship between exposure to high levels of HMs such as aluminum and the risk of AD [6, 7]. Because its serum level is higher in patients with severe AD than in healthy people [8], this metal has direct and active access to sensitive brain areas, such as the hippocampus [9]. It may accumulate in the brain and cause AD [10]. Another study has argued that it probably affects the pathogenesis of AD in the brain by increasing the production of amyloid-forming protein and thereby increasing the deposition of Aβ [11]. It has been stated that this metal damages neurons by increasing oxidative stress [12]. Another immunohistochemical study has shown that aluminum chloride (Al)-induced neurodegeneration causes memory deficits [13]. A study using the Morris blue maze behavioral test has previously demonstrated the effect of Al on spatial memory [14], namely that this substance changes the shape of the nucleus and cytoplasm of hippocampal cells in the hippocampal tissue [15].
An important metal involved in the pathogenesis of AD is aluminum, which enhances the production of free radicals involved in oxidative stress reactions, leading to tissue damage [11]. The mechanism of this HM in inducing AD is not fully understood. However, most researchers have stated its capacity to intensify oxidative stress events [3] and, as a result, increase reactive oxygen species, which lead to oxidation and cell death [16]. Because these problems may affect the body, there is an idea that internal organs are also damaged in AD. The effect of Al on the liver has also been reported [17, 18].
Regarding the mechanism, some have considered using Al to cause its accumulation in the liver tissue and its tissue change [19]. Others believe the liver is essential to energy metabolism through its various mitochondria. Al blocks this metabolism through a possible effect on the enzymatic activity of the electron transport chain, causing damage to mitochondrial function, apoptosis, and cell necrosis [20]. Since all mechanistic aspects are not yet clear, this study aimed to investigate the cognitive and hepatic effects of Al using a minimally invasive method (in drinking water) over two and four weeks (chronic) in Wistar rats. Cognitive deficits were investigated in the novelty-seeking (NS) behavioral model, which determines the impairment of early animal memory retrieval. After confirming the cognitive impairment (CI) with histological and serological studies of the liver, we provide new information about the mechanism involved.
Materials and Methods
Animal subject
We purchased male Wistar rats (about 250 g) from the Pasteur Institute of Iran, Tehran, Iran. We followed the ethics for the use and care of animals.
Grouping of rats
Using a randomized design, the rats were divided into control and experimental animals (8 in each group). We first used a wider range of volumetric concentrations of Al in drinking water (10, 50, and 100 mg/kg) in a 2-week pilot study, and it was shown that animals experienced at least 50% mortality at 100 mg/kg compared with animals receiving regular drinking water. Therefore, we conducted the main study at lower concentrations (10 and 50 mg/kg).
We had several treatment durations (2 weeks and 4 weeks), each matching with a control group. Different volumetric concentrations of Al in drinking water (10 and 50 mg/kg as) were used in each treatment period. Controls received only water during the procedures. A total of 48 rats were used in this research.
Materials used
Hydrated aluminum chloride (with 6 water molecules) was purchased from Merck, Germany, and used in different volumetric concentrations of Al in drinking water (10 and 50 mg/kg). Ketamine (10%) and xylazine (8%) were bought from the Iran Veterinary Organization (Tehran, Iran), and other materials were provided as follows: Hematoxylin and eosin (H & E) (F-Arman Co., Iran), cresyl violet (Merck, Germany), and Entellan (Merck, Germany).
NS device
Spatial learning memory was used to measure the effects of oral Al intake on cognitive processes in rats using the NS paradigm. This paradigm was conducted over three days and included three phases (familiarization, confinement, and testing). In this method, a subset of spatial conditioning, the animal is bound on one side instead of conditioning (second stage). During two binding sessions (morning and evening with a minimum time interval of 6 hours), the rats stay on the same side for 30 minutes. During familiarization and test days, the animal can access all device parts for only 10 minutes.
The device used for this test was a wooden box with dimensions of 30×60×30 cm. It had two compartments that were separated by a gliding door. The compartments were white. However, they had different black geometric patterns and floors to provide differences in spatial and textural cues.
Familiarization stage
Each rat has an adaptation period with the instrument same as the first day. The rat was put in the device while the changeable wall was 12 cm above the floor of the tool; it moved freely throughout the device for 10 minutes. The EthoVision system, which was located 120 cm above the apparatus, documented all behaviors, signs, and stop times.
Confining stage
This stage was done after the familiarization day in two sessions with an interval of six hours. The animal was confined twice for 30 minutes in one part of the box, once in the morning and once in the evening, with a time break of 6 hours. At this time, the removable wall was closed.
Testing
This stage was ended on the third day. Same as the first day, the animal was entered the box with the open sliding wall, and the animal could move freely in the box for 10 minutes. Behaviors, movements, and signs of the animal were documented by EthoVision. It should be noted that the device was cleaned entirely at the end of each test.
NS behavioral signs
Rodents naturally tend to explore new environments. Searching for new environments in rodents is exploring new situations with unknown stimuli. It is an exploratory behavior and includes standing (rearing), sniffing, cleaning (grooming), and compartment entering. To quantify the new search time, we subtracted the time spent in the apparatus compartments on the familiarization and test days to obtain stops in the novel part (which the animal did not see during the confining phase).
Experimental procedures
Blood and tissue samples
Immediately after completing the behavioral procedure, the rats were sedated by intraperitoneal (IP) injection of ketamine and xylazine, and blood samples were taken transcardially under deep anesthesia. It should be noted that for anesthesia with ketamine and xylazine, the weight of the animal was first measured with an animal scale. Then, the anesthetic material was used as an IP injection. After blood collection, the animal was euthanized with carbon dioxide inhalation, and then the brain was rapidly separated from the skull on ice, and a part of the liver was dissected and placed in 10% formalin.
Tissue preparation
After 72 hours, hippocampus and liver tissue samples were handled and paraffinized. Slices (3-4 μm) were then cut with a microtome (Leica, Italy), fixed on poly-L-lysine slides, and put in xylene for 15-30 minutes. The slides were placed in descending alcohols (from 96% alcohol, 80% alcohol, 70% alcohol, and 50% alcohol, 3 minutes each) and then washed in phosphate-buffered saline (PBS) to follow the staining.
Hematoxylin & eosin staining: The liver samples were placed in hematoxylin dye (20%) for about 20 minutes, washed in PBS, and immersed in eosin for 5 minutes. They were removed, washed with graded alcohols, placed in xylenes for 5-10 min, and mounted by Entellan glue (Merck, Germany).
Cresyl violet staining: To prepare cresyl dye, 0.1 g of cresyl violet (Merck, Germany) was poured into 100 mL of distilled water, placed on a magnetic shaker for 30 minutes, and then cleaned with filter paper. The brain samples were exposed to xylene for 15-30 minutes and then immersed in 96% alcohol, 70% alcohol, and 50% alcohol for 5 minutes each. They were then exposed to distilled water and PBS. Then, we added the dye to the slides (15 minutes) and finally put them in distilled water. Then, the slides were placed in 70%, 80%, and 96% alcohol, respectively, for 1-3 minutes each, and eventually, the slides were placed in xylene (5-10 minutes), and Entellan glue was added at the end.
Blood serum preparation
After taking blood from the heart of an anesthetized animal, the blood samples were located in the laboratory for about 30 minutes to allow the blood to clot. The collected samples were centrifuged at 3000 rpm and 10-15 minutes. Then, the serum was poured into Eppendorf tubes with sample pipettes and placed in a freezer at -80 °C (it took about 2 weeks to complete the serological analysis).
Analysis of findings
The findings were analyzed using SPSS software, version 22. After the Kolmogorov-Smirnov test and data normality confirmation, one-way variance analysis (ANOVA) and Tukey post hoc test (to check the difference between groups) were used. The tissue slides were also quantitatively analyzed using ImageJ software, version 1.41 (free, Java).
Results
Oral administration of Al with different concentrations (10 and 50 mg/kg) in different time durations and the evaluation of new environment search behavior
The rats were placed in the NS box and evaluated using a 3-day program with three stages. They were first exposed to different doses of AlCl3 (Al) in drinking water (as volume concentration) for various periods (2 weeks and 4 weeks). The control groups were given ordinary drinking water during the same procedures.
We subtracted the time spent in the apparatus compartments on the familiarization and test days to obtain stops in the novel part. The findings show that rats significantly stopped on the novel side, the unconfined part (Figure 1).
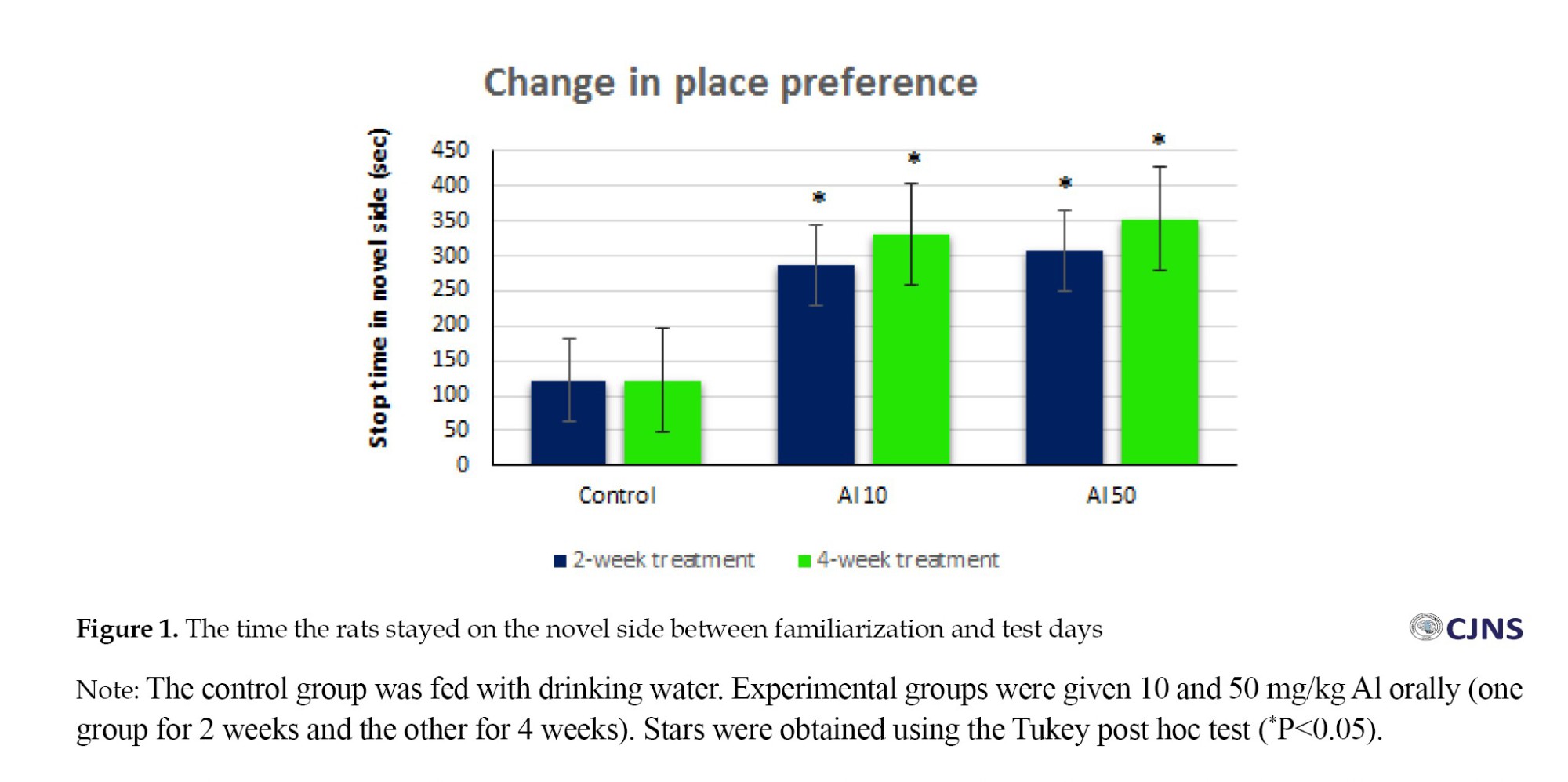
Oral consumption of Al with different concentrations (10 and 50 mg/kg) in 2-week and 4-week durations and behavioral signs in the NS test
The behavioral signs of each rat on the test day were counted in each part of the box for 10 minutes. The number of the same behavioral signs related to the familiarization day was calculated and subtracted, and the result was obtained. The results of oral consumption of Al with different concentrations (10 and 50 mg/kg) in 2-week and 4-week intervals on the behavior of standing (rearing) in the NS model showed that the doses used had no statistically significant effect on standing behavior compared to the control group (Figure 2).

Oral administration of Al with different concentrations (10 and 50 mg/kg) in 2-week and 4-week durations and sniffing behavior in the seeking device
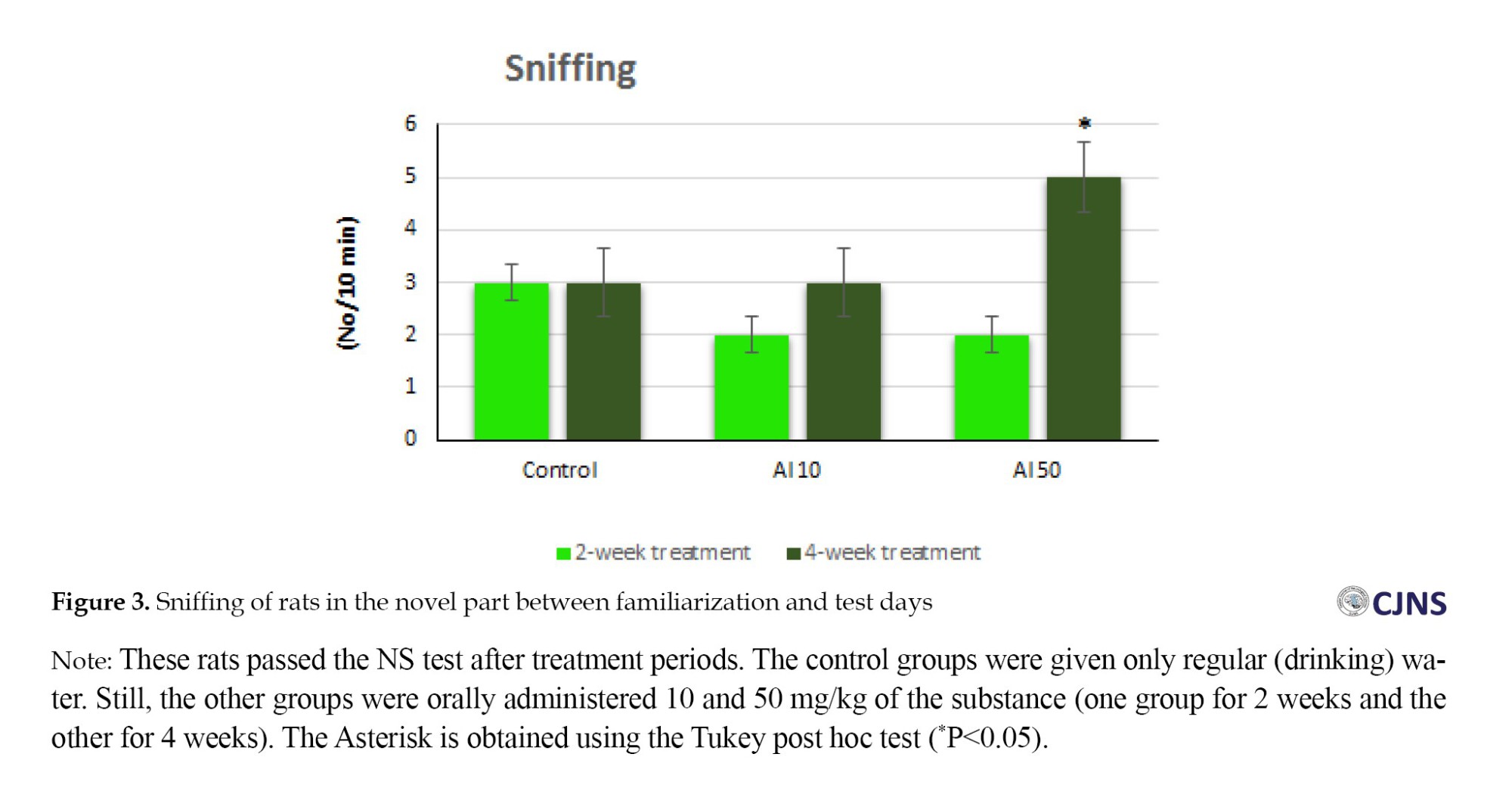
The statistical analysis of the data showed that the oral administration of aluminum chloride at a dose of 50 mg/kg for 4 weeks has a statistically significant effect on sniffing behavior compared to the control group. The rats (50 mg/kg dose for 4 weeks) showed a significant change (Figure 3).
Oral intake of Al with different concentrations (10 and 50 mg/kg) in 2-week and 4-week durations and grooming behavior in the device
The statistical analysis of the findings shows that the oral administration of Al at a dose of 10 mg/kg for 4 weeks significantly affects grooming behavior compared to the control group. Rats in the dose group of 10 mg/kg for 4 weeks showed a significant difference (Figure 4).

Oral intake of Al with different concentrations (10 and 50 mg/kg) in 2-week and 4-week durations and locomotor activity (compartment entering) in the device
The statistical analysis of the results shows that the oral administration had no significant effect on locomotor behavior compared to the control group. Therefore, the rats did not differ significantly in this regard (not shown).
Al oral intake with different concentrations (10 and 50 mg/kg) in 2-week and 4-week durations and lipid profile
The statistical analysis of the findings shows that the oral administration of Al with the doses and durations shown in Figure 5 has no significant effect on high-density lipoprotein (HDL) and low-density lipoprotein (LDL) compared to the control group. However, the administration of this substance significantly affected cholesterol (CHOL) in the group that received a dose of 50 mg/kg for 4 weeks. Statistical analysis for the group receiving Al (10 mg/kg) for 2 weeks did not significantly affect triglyceride (TG). Still, a significant difference was shown in the rest of the groups (Figure 5).

Al oral intake with different concentrations (10 and 50 mg/kg) in 2-week and 4-week durations and liver enzymes
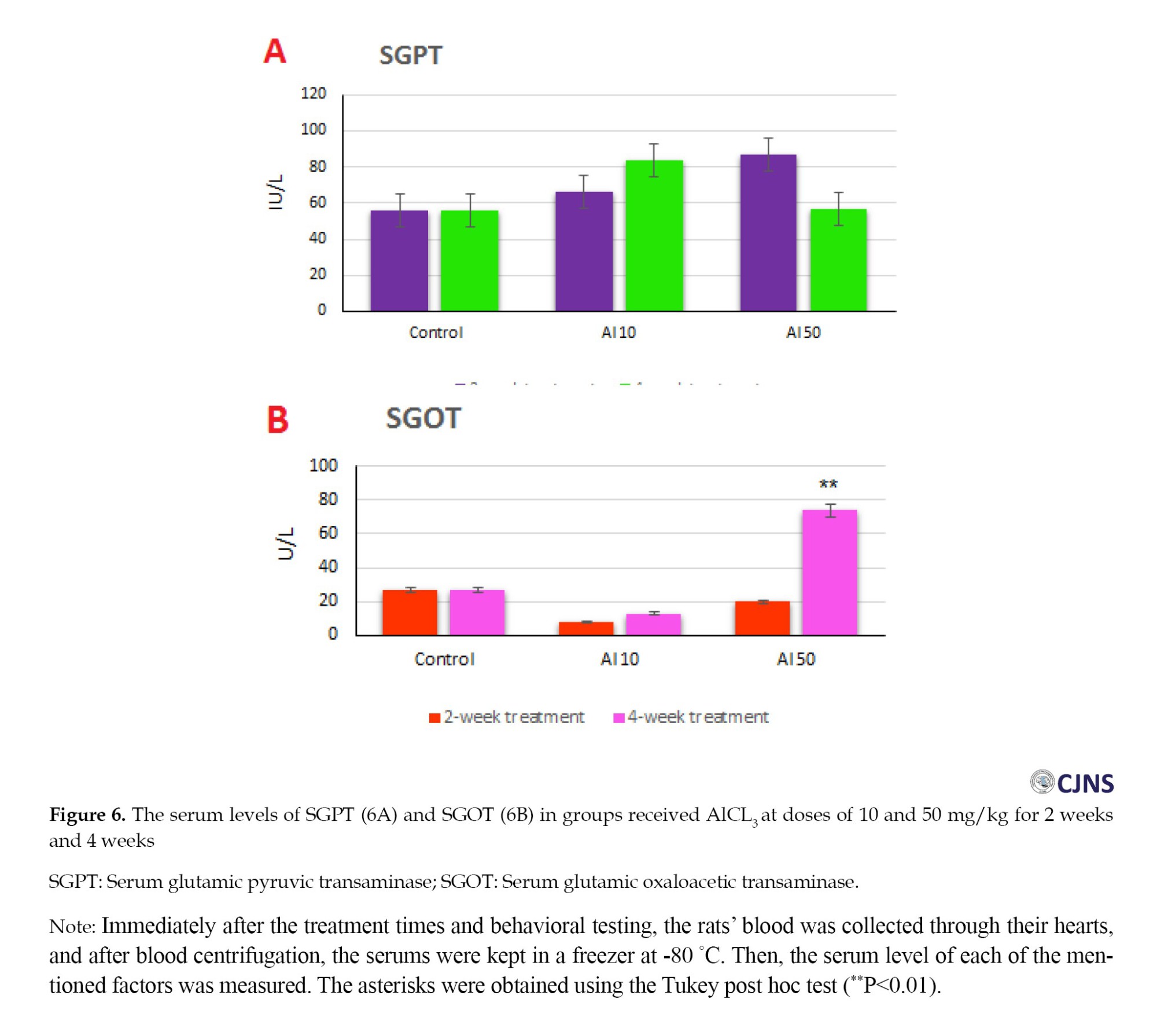
The statistical analysis of the results showed that the oral administration of Al with the doses and durations shown in Figure 6A had no significant effect on serum glutamic pyruvic transaminase (SGPT) or alanine transaminase (ALT) compared to the control group. Furthermore, the analysis of the data presented that the oral administration of Al with the doses and durations shown in Figure 6B had a significant effect on serum glutamic oxaloacetic transaminase (SGOT) or aspartate transaminase (AST) only in the group that received a dose of 50 mg/kg for four weeks. However, no significant effect was shown in other groups.
Histological evidence of oral administration of AL with doses of 10 and 50 mg/kg for 2 and 4 weeks on the hippocampus
Histological examinations with cresyl violet staining showed that CA1 (CA: Cornu ammonis) neurons were not seriously damaged in any groups. CA1 tissue damage was relatively low compared to the control group but was not statistically significant (not shown).
Histological examinations of the liver with hematoxylin-eosin staining under light microscopy with different magnifications
The data showed that the liver tissue was not damaged much in any group. No tissue damage was observed in the hepatic sinusoids and vascular network. Hepatocytes did not show cell necrosis (not shown).
Discussion
This research aimed to induce an AD-like model in rats through oral administration of Al, which is a less invasive and cost-effective way to research CI with a new idea. The idea was to study the effects of this impairment on the central nervous system (hippocampus) and the internal system (liver) to elucidate further the mechanisms involved.
Different doses of Al in inducing an AD-like model through NS tests, providing evidence of cognitive deficits (impaired memory retrieval)
As mentioned earlier, AD is a neurological disease that causes memory impairment. This disease shows neurological and behavioral changes in the patient. HMs such as aluminum are among the toxic environmental factors that cause AD development and progression. Many laboratory and clinical studies have reported the critical role of this HM in the pathogenesis of AD. It has been previously postulated that continuous exposure to it causes apoptosis in brain areas, especially the hippocampus. In other words, this metal can mimic the mechanisms responsible for AD pathophysiology in laboratory animals [21]. This AD modeling was less invasive and cost-effective. Many researchers induce AD by gavage (invasive! concerning animal care and welfare). We must remember that the animals did not sign a contract to participate in the research program. In the present study, Al was given to animals orally in drinking water. This method is less invasive than the gavage method to create an AD-like model in animals. Alternatively, we could have ignored oral administration and used direct injection of amyloid beta (Aβ) protein into the brain nuclei. But again, we must say that it is costly. We used specific doses of Al according to the conceptualization of the present experimental study on dose response. We had a wider range of Al, such as a 100 mg/kg dose, but it showed LD50. Researchers previously used a dose of 300 mg/kg Al for 28 days to induce AD [21]. Another researcher has used this substance in doses of 8.3 mg/kg and 32 mg/kg as gavage for 60 days [22]. However, we used volumetric concentrations of Al in drinking water (10 mg/kg and 50 mg/kg) for different durations (2 to 4 weeks). The results of the present behavioral experiments on Al-treated rats showed that the animals stopped significantly in the novel part of the box (Figure 1), indicating that the animal’s retrieval memory was impaired and, as others have suggested [23], it means that it was difficult to retrieve previous experience information [23].
Regarding the behavioral signs, the standing and moving showed no significant difference in any groups. However, regarding sniffing behavior, only in the group that received 50 mg/kg for 4 weeks there was a significant difference compared to the control group (Figure 3). In fact, the group administered Al in terms of dose and duration showed a significant difference in this behavior. Other groups did not show this notable difference. Regarding the grooming behavior, only the group that received a dose of 50 mg/kg of Al for both durations showed a significant difference compared to the control group. The rest of the groups did not show any significant difference (Figure 4). These results are also very interesting for the induction of an AD-like model because in Alzheimer’s patients, personal health issues are also affected by this disease, and grooming reflects this symptom.
Different doses of Al and lipid profile and liver enzymes
According to previous research, animals exposed to Al at a 50 mg/kg dose for 8 weeks increased serum CHOL and TG levels [24]. In this study, which used different doses and durations to create an AD-like model in rats, CHOL showed a significant increase only in the group that received Al 50 mg/kg in 4 weeks, while the other groups did not show a significant difference. Regarding TG, three groups (dose 50 for 2 weeks, dose 10 for 4 weeks, dose 50 for 4 weeks) showed a very substantial increase compared to the control group. Regarding LDL and HDL, previous studies have shown that after receiving Al at a dose of 100 mg/kg for 28 days by gavage, LDL increased and HDL decreased [18]. However, in the present study, no statistical difference was observed between the groups compared to the control group.
Toxic damage to the liver is associated with the release of certain marker enzymes that enter the bloodstream. In fact, in this study, like previous studies [18], liver damage was evaluated by measuring specific liver enzymes (SGOT, SGPT, or AST and ALT). In the present study, no statistical difference was observed regarding SGPT in any of the groups compared to the control group. However, SGOT showed a substantial increase only in the group that received the dose of 50 mg/kg for 4 weeks. These two enzymes are used to diagnose damaged liver cells and are reliable indicators of liver function found in higher concentrations in the cytoplasm. The increase of liver enzymes in the plasma after receiving Al can lead to cell destruction and changes in the permeability of the liver cell membrane—the release of these enzymes in the bloodstream results from the destruction of liver cells [17]. Therefore, in this study, we see functional damage to the liver.
Different doses of Al and hippocampus and liver tissue
Histological examination was assessed using cresyl violet (hippocampus) and hematoxylin-eosin staining (liver). In previous histological studies [15] using H & E staining, Al injection has been shown to cause damage to CA1 neurons. In another study [17], in which Al was injected at a dose of 90 mg/kg by gavage, H & E staining of liver tissue showed central venous congestion, hepatocyte destruction, inflammatory cell infiltration, and sinus enlargement. The present study observed no significant tissue structure changes in the hippocampus and liver. Of course, using the cresyl method is much more reliable than H & E for CA1. Therefore, we suggest that this method be used in future research. In previous research [25], it has been postulated that hippocampal cresyl staining in Al-treated groups demonstrates a decrease in the number of cells in this area. The present study observed no meaningful statistical changes in these cells. In the current study, memory impairment, especially spatial memory, was affected in the early stages (based on the results of behavioral studies). The liver tissue in the group administered a dose of 50 mg/kg is in the early stages of tissue damage according to the significant increase in SGOT enzyme. Still, histological examinations did not show cell necrosis changes in hepatocytes. As a result, enzyme changes indicate liver function damage rather than liver destruction. Our other suggestion is to conduct more detailed molecular investigations on the tissues to express a more definitive opinion in future studies. Since tissue changes are observed relatively, it is better to provide a more definitive opinion about these findings later by doing more research.
Conclusion
Chronic consumption of Al in drinking water appears to be effective in causing cognitive impairment and liver dysfunction, and the present study provided preliminary evidence of these cases.
Further suggestions for future research
Our suggestion is to conduct molecular studies for further mechanistic investigation of AD.
The study can continue on other memory elements, signaling pathways, metabolic, and vital factors.
Ethical Considerations
Compliance with ethical guidelines
All ethical guidelines were followed, and the Local Ethic Committee of Shahed University, Tehran, Iran, approved the study (Code: IR.SHAHED.REC.1400.035).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, study design, data analysis and writing the original draft: Manizheh Karami: Investigations: Haniyeh Amini; Review, editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank Shahed University, Tehran, Iran, for supporting this research.
References
Alzheimer disease (AD) is a serious neurological problem that is characterized by decreased awareness, loss of daily activities, and neuropsychiatric symptoms [1]. Reduced synthesis of the neurotransmitter acetylcholine, disruption of neuronal connections, buildup of amyloid β (Aβ) plaques, and neurofibrillary tangles of hyperphosphorylated tau proteins in the hippocampus and forebrain lead to AD. Studies show that Aβ and Aβ precursor proteins play an important role in the pathogenesis of early-onset AD. Millions of people worldwide suffer from AD, and it is the most important basis of age-related memory loss [2]. AD is known as a multifactorial disorder that can be attributed to genetic factors (familial AD) and environmental factors (sporadic AD), such as inflammation, high levels of free radicals, and accumulation of heavy metals (HMs) in the brain [3]. Aluminum is the third most abundant element on earth [4] and is widely used in human life [5]. It has been shown that there is a relationship between exposure to high levels of HMs such as aluminum and the risk of AD [6, 7]. Because its serum level is higher in patients with severe AD than in healthy people [8], this metal has direct and active access to sensitive brain areas, such as the hippocampus [9]. It may accumulate in the brain and cause AD [10]. Another study has argued that it probably affects the pathogenesis of AD in the brain by increasing the production of amyloid-forming protein and thereby increasing the deposition of Aβ [11]. It has been stated that this metal damages neurons by increasing oxidative stress [12]. Another immunohistochemical study has shown that aluminum chloride (Al)-induced neurodegeneration causes memory deficits [13]. A study using the Morris blue maze behavioral test has previously demonstrated the effect of Al on spatial memory [14], namely that this substance changes the shape of the nucleus and cytoplasm of hippocampal cells in the hippocampal tissue [15].
An important metal involved in the pathogenesis of AD is aluminum, which enhances the production of free radicals involved in oxidative stress reactions, leading to tissue damage [11]. The mechanism of this HM in inducing AD is not fully understood. However, most researchers have stated its capacity to intensify oxidative stress events [3] and, as a result, increase reactive oxygen species, which lead to oxidation and cell death [16]. Because these problems may affect the body, there is an idea that internal organs are also damaged in AD. The effect of Al on the liver has also been reported [17, 18].
Regarding the mechanism, some have considered using Al to cause its accumulation in the liver tissue and its tissue change [19]. Others believe the liver is essential to energy metabolism through its various mitochondria. Al blocks this metabolism through a possible effect on the enzymatic activity of the electron transport chain, causing damage to mitochondrial function, apoptosis, and cell necrosis [20]. Since all mechanistic aspects are not yet clear, this study aimed to investigate the cognitive and hepatic effects of Al using a minimally invasive method (in drinking water) over two and four weeks (chronic) in Wistar rats. Cognitive deficits were investigated in the novelty-seeking (NS) behavioral model, which determines the impairment of early animal memory retrieval. After confirming the cognitive impairment (CI) with histological and serological studies of the liver, we provide new information about the mechanism involved.
Materials and Methods
Animal subject
We purchased male Wistar rats (about 250 g) from the Pasteur Institute of Iran, Tehran, Iran. We followed the ethics for the use and care of animals.
Grouping of rats
Using a randomized design, the rats were divided into control and experimental animals (8 in each group). We first used a wider range of volumetric concentrations of Al in drinking water (10, 50, and 100 mg/kg) in a 2-week pilot study, and it was shown that animals experienced at least 50% mortality at 100 mg/kg compared with animals receiving regular drinking water. Therefore, we conducted the main study at lower concentrations (10 and 50 mg/kg).
We had several treatment durations (2 weeks and 4 weeks), each matching with a control group. Different volumetric concentrations of Al in drinking water (10 and 50 mg/kg as) were used in each treatment period. Controls received only water during the procedures. A total of 48 rats were used in this research.
Materials used
Hydrated aluminum chloride (with 6 water molecules) was purchased from Merck, Germany, and used in different volumetric concentrations of Al in drinking water (10 and 50 mg/kg). Ketamine (10%) and xylazine (8%) were bought from the Iran Veterinary Organization (Tehran, Iran), and other materials were provided as follows: Hematoxylin and eosin (H & E) (F-Arman Co., Iran), cresyl violet (Merck, Germany), and Entellan (Merck, Germany).
NS device
Spatial learning memory was used to measure the effects of oral Al intake on cognitive processes in rats using the NS paradigm. This paradigm was conducted over three days and included three phases (familiarization, confinement, and testing). In this method, a subset of spatial conditioning, the animal is bound on one side instead of conditioning (second stage). During two binding sessions (morning and evening with a minimum time interval of 6 hours), the rats stay on the same side for 30 minutes. During familiarization and test days, the animal can access all device parts for only 10 minutes.
The device used for this test was a wooden box with dimensions of 30×60×30 cm. It had two compartments that were separated by a gliding door. The compartments were white. However, they had different black geometric patterns and floors to provide differences in spatial and textural cues.
Familiarization stage
Each rat has an adaptation period with the instrument same as the first day. The rat was put in the device while the changeable wall was 12 cm above the floor of the tool; it moved freely throughout the device for 10 minutes. The EthoVision system, which was located 120 cm above the apparatus, documented all behaviors, signs, and stop times.
Confining stage
This stage was done after the familiarization day in two sessions with an interval of six hours. The animal was confined twice for 30 minutes in one part of the box, once in the morning and once in the evening, with a time break of 6 hours. At this time, the removable wall was closed.
Testing
This stage was ended on the third day. Same as the first day, the animal was entered the box with the open sliding wall, and the animal could move freely in the box for 10 minutes. Behaviors, movements, and signs of the animal were documented by EthoVision. It should be noted that the device was cleaned entirely at the end of each test.
NS behavioral signs
Rodents naturally tend to explore new environments. Searching for new environments in rodents is exploring new situations with unknown stimuli. It is an exploratory behavior and includes standing (rearing), sniffing, cleaning (grooming), and compartment entering. To quantify the new search time, we subtracted the time spent in the apparatus compartments on the familiarization and test days to obtain stops in the novel part (which the animal did not see during the confining phase).
Experimental procedures
Blood and tissue samples
Immediately after completing the behavioral procedure, the rats were sedated by intraperitoneal (IP) injection of ketamine and xylazine, and blood samples were taken transcardially under deep anesthesia. It should be noted that for anesthesia with ketamine and xylazine, the weight of the animal was first measured with an animal scale. Then, the anesthetic material was used as an IP injection. After blood collection, the animal was euthanized with carbon dioxide inhalation, and then the brain was rapidly separated from the skull on ice, and a part of the liver was dissected and placed in 10% formalin.
Tissue preparation
After 72 hours, hippocampus and liver tissue samples were handled and paraffinized. Slices (3-4 μm) were then cut with a microtome (Leica, Italy), fixed on poly-L-lysine slides, and put in xylene for 15-30 minutes. The slides were placed in descending alcohols (from 96% alcohol, 80% alcohol, 70% alcohol, and 50% alcohol, 3 minutes each) and then washed in phosphate-buffered saline (PBS) to follow the staining.
Hematoxylin & eosin staining: The liver samples were placed in hematoxylin dye (20%) for about 20 minutes, washed in PBS, and immersed in eosin for 5 minutes. They were removed, washed with graded alcohols, placed in xylenes for 5-10 min, and mounted by Entellan glue (Merck, Germany).
Cresyl violet staining: To prepare cresyl dye, 0.1 g of cresyl violet (Merck, Germany) was poured into 100 mL of distilled water, placed on a magnetic shaker for 30 minutes, and then cleaned with filter paper. The brain samples were exposed to xylene for 15-30 minutes and then immersed in 96% alcohol, 70% alcohol, and 50% alcohol for 5 minutes each. They were then exposed to distilled water and PBS. Then, we added the dye to the slides (15 minutes) and finally put them in distilled water. Then, the slides were placed in 70%, 80%, and 96% alcohol, respectively, for 1-3 minutes each, and eventually, the slides were placed in xylene (5-10 minutes), and Entellan glue was added at the end.
Blood serum preparation
After taking blood from the heart of an anesthetized animal, the blood samples were located in the laboratory for about 30 minutes to allow the blood to clot. The collected samples were centrifuged at 3000 rpm and 10-15 minutes. Then, the serum was poured into Eppendorf tubes with sample pipettes and placed in a freezer at -80 °C (it took about 2 weeks to complete the serological analysis).
Analysis of findings
The findings were analyzed using SPSS software, version 22. After the Kolmogorov-Smirnov test and data normality confirmation, one-way variance analysis (ANOVA) and Tukey post hoc test (to check the difference between groups) were used. The tissue slides were also quantitatively analyzed using ImageJ software, version 1.41 (free, Java).
Results
Oral administration of Al with different concentrations (10 and 50 mg/kg) in different time durations and the evaluation of new environment search behavior
The rats were placed in the NS box and evaluated using a 3-day program with three stages. They were first exposed to different doses of AlCl3 (Al) in drinking water (as volume concentration) for various periods (2 weeks and 4 weeks). The control groups were given ordinary drinking water during the same procedures.
We subtracted the time spent in the apparatus compartments on the familiarization and test days to obtain stops in the novel part. The findings show that rats significantly stopped on the novel side, the unconfined part (Figure 1).

Oral consumption of Al with different concentrations (10 and 50 mg/kg) in 2-week and 4-week durations and behavioral signs in the NS test
The behavioral signs of each rat on the test day were counted in each part of the box for 10 minutes. The number of the same behavioral signs related to the familiarization day was calculated and subtracted, and the result was obtained. The results of oral consumption of Al with different concentrations (10 and 50 mg/kg) in 2-week and 4-week intervals on the behavior of standing (rearing) in the NS model showed that the doses used had no statistically significant effect on standing behavior compared to the control group (Figure 2).

Oral administration of Al with different concentrations (10 and 50 mg/kg) in 2-week and 4-week durations and sniffing behavior in the seeking device
The statistical analysis of the data showed that the oral administration of aluminum chloride at a dose of 50 mg/kg for 4 weeks has a statistically significant effect on sniffing behavior compared to the control group. The rats (50 mg/kg dose for 4 weeks) showed a significant change (Figure 3).

Oral intake of Al with different concentrations (10 and 50 mg/kg) in 2-week and 4-week durations and grooming behavior in the device
The statistical analysis of the findings shows that the oral administration of Al at a dose of 10 mg/kg for 4 weeks significantly affects grooming behavior compared to the control group. Rats in the dose group of 10 mg/kg for 4 weeks showed a significant difference (Figure 4).

Oral intake of Al with different concentrations (10 and 50 mg/kg) in 2-week and 4-week durations and locomotor activity (compartment entering) in the device
The statistical analysis of the results shows that the oral administration had no significant effect on locomotor behavior compared to the control group. Therefore, the rats did not differ significantly in this regard (not shown).
Al oral intake with different concentrations (10 and 50 mg/kg) in 2-week and 4-week durations and lipid profile
The statistical analysis of the findings shows that the oral administration of Al with the doses and durations shown in Figure 5 has no significant effect on high-density lipoprotein (HDL) and low-density lipoprotein (LDL) compared to the control group. However, the administration of this substance significantly affected cholesterol (CHOL) in the group that received a dose of 50 mg/kg for 4 weeks. Statistical analysis for the group receiving Al (10 mg/kg) for 2 weeks did not significantly affect triglyceride (TG). Still, a significant difference was shown in the rest of the groups (Figure 5).

Al oral intake with different concentrations (10 and 50 mg/kg) in 2-week and 4-week durations and liver enzymes
The statistical analysis of the results showed that the oral administration of Al with the doses and durations shown in Figure 6A had no significant effect on serum glutamic pyruvic transaminase (SGPT) or alanine transaminase (ALT) compared to the control group. Furthermore, the analysis of the data presented that the oral administration of Al with the doses and durations shown in Figure 6B had a significant effect on serum glutamic oxaloacetic transaminase (SGOT) or aspartate transaminase (AST) only in the group that received a dose of 50 mg/kg for four weeks. However, no significant effect was shown in other groups.

Histological evidence of oral administration of AL with doses of 10 and 50 mg/kg for 2 and 4 weeks on the hippocampus
Histological examinations with cresyl violet staining showed that CA1 (CA: Cornu ammonis) neurons were not seriously damaged in any groups. CA1 tissue damage was relatively low compared to the control group but was not statistically significant (not shown).
Histological examinations of the liver with hematoxylin-eosin staining under light microscopy with different magnifications
The data showed that the liver tissue was not damaged much in any group. No tissue damage was observed in the hepatic sinusoids and vascular network. Hepatocytes did not show cell necrosis (not shown).
Discussion
This research aimed to induce an AD-like model in rats through oral administration of Al, which is a less invasive and cost-effective way to research CI with a new idea. The idea was to study the effects of this impairment on the central nervous system (hippocampus) and the internal system (liver) to elucidate further the mechanisms involved.
Different doses of Al in inducing an AD-like model through NS tests, providing evidence of cognitive deficits (impaired memory retrieval)
As mentioned earlier, AD is a neurological disease that causes memory impairment. This disease shows neurological and behavioral changes in the patient. HMs such as aluminum are among the toxic environmental factors that cause AD development and progression. Many laboratory and clinical studies have reported the critical role of this HM in the pathogenesis of AD. It has been previously postulated that continuous exposure to it causes apoptosis in brain areas, especially the hippocampus. In other words, this metal can mimic the mechanisms responsible for AD pathophysiology in laboratory animals [21]. This AD modeling was less invasive and cost-effective. Many researchers induce AD by gavage (invasive! concerning animal care and welfare). We must remember that the animals did not sign a contract to participate in the research program. In the present study, Al was given to animals orally in drinking water. This method is less invasive than the gavage method to create an AD-like model in animals. Alternatively, we could have ignored oral administration and used direct injection of amyloid beta (Aβ) protein into the brain nuclei. But again, we must say that it is costly. We used specific doses of Al according to the conceptualization of the present experimental study on dose response. We had a wider range of Al, such as a 100 mg/kg dose, but it showed LD50. Researchers previously used a dose of 300 mg/kg Al for 28 days to induce AD [21]. Another researcher has used this substance in doses of 8.3 mg/kg and 32 mg/kg as gavage for 60 days [22]. However, we used volumetric concentrations of Al in drinking water (10 mg/kg and 50 mg/kg) for different durations (2 to 4 weeks). The results of the present behavioral experiments on Al-treated rats showed that the animals stopped significantly in the novel part of the box (Figure 1), indicating that the animal’s retrieval memory was impaired and, as others have suggested [23], it means that it was difficult to retrieve previous experience information [23].
Regarding the behavioral signs, the standing and moving showed no significant difference in any groups. However, regarding sniffing behavior, only in the group that received 50 mg/kg for 4 weeks there was a significant difference compared to the control group (Figure 3). In fact, the group administered Al in terms of dose and duration showed a significant difference in this behavior. Other groups did not show this notable difference. Regarding the grooming behavior, only the group that received a dose of 50 mg/kg of Al for both durations showed a significant difference compared to the control group. The rest of the groups did not show any significant difference (Figure 4). These results are also very interesting for the induction of an AD-like model because in Alzheimer’s patients, personal health issues are also affected by this disease, and grooming reflects this symptom.
Different doses of Al and lipid profile and liver enzymes
According to previous research, animals exposed to Al at a 50 mg/kg dose for 8 weeks increased serum CHOL and TG levels [24]. In this study, which used different doses and durations to create an AD-like model in rats, CHOL showed a significant increase only in the group that received Al 50 mg/kg in 4 weeks, while the other groups did not show a significant difference. Regarding TG, three groups (dose 50 for 2 weeks, dose 10 for 4 weeks, dose 50 for 4 weeks) showed a very substantial increase compared to the control group. Regarding LDL and HDL, previous studies have shown that after receiving Al at a dose of 100 mg/kg for 28 days by gavage, LDL increased and HDL decreased [18]. However, in the present study, no statistical difference was observed between the groups compared to the control group.
Toxic damage to the liver is associated with the release of certain marker enzymes that enter the bloodstream. In fact, in this study, like previous studies [18], liver damage was evaluated by measuring specific liver enzymes (SGOT, SGPT, or AST and ALT). In the present study, no statistical difference was observed regarding SGPT in any of the groups compared to the control group. However, SGOT showed a substantial increase only in the group that received the dose of 50 mg/kg for 4 weeks. These two enzymes are used to diagnose damaged liver cells and are reliable indicators of liver function found in higher concentrations in the cytoplasm. The increase of liver enzymes in the plasma after receiving Al can lead to cell destruction and changes in the permeability of the liver cell membrane—the release of these enzymes in the bloodstream results from the destruction of liver cells [17]. Therefore, in this study, we see functional damage to the liver.
Different doses of Al and hippocampus and liver tissue
Histological examination was assessed using cresyl violet (hippocampus) and hematoxylin-eosin staining (liver). In previous histological studies [15] using H & E staining, Al injection has been shown to cause damage to CA1 neurons. In another study [17], in which Al was injected at a dose of 90 mg/kg by gavage, H & E staining of liver tissue showed central venous congestion, hepatocyte destruction, inflammatory cell infiltration, and sinus enlargement. The present study observed no significant tissue structure changes in the hippocampus and liver. Of course, using the cresyl method is much more reliable than H & E for CA1. Therefore, we suggest that this method be used in future research. In previous research [25], it has been postulated that hippocampal cresyl staining in Al-treated groups demonstrates a decrease in the number of cells in this area. The present study observed no meaningful statistical changes in these cells. In the current study, memory impairment, especially spatial memory, was affected in the early stages (based on the results of behavioral studies). The liver tissue in the group administered a dose of 50 mg/kg is in the early stages of tissue damage according to the significant increase in SGOT enzyme. Still, histological examinations did not show cell necrosis changes in hepatocytes. As a result, enzyme changes indicate liver function damage rather than liver destruction. Our other suggestion is to conduct more detailed molecular investigations on the tissues to express a more definitive opinion in future studies. Since tissue changes are observed relatively, it is better to provide a more definitive opinion about these findings later by doing more research.
Conclusion
Chronic consumption of Al in drinking water appears to be effective in causing cognitive impairment and liver dysfunction, and the present study provided preliminary evidence of these cases.
Further suggestions for future research
Our suggestion is to conduct molecular studies for further mechanistic investigation of AD.
The study can continue on other memory elements, signaling pathways, metabolic, and vital factors.
Ethical Considerations
Compliance with ethical guidelines
All ethical guidelines were followed, and the Local Ethic Committee of Shahed University, Tehran, Iran, approved the study (Code: IR.SHAHED.REC.1400.035).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, study design, data analysis and writing the original draft: Manizheh Karami: Investigations: Haniyeh Amini; Review, editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank Shahed University, Tehran, Iran, for supporting this research.
References
- Porsteinsson AP, Isaacson RS, Knox S, Sabbagh MN, Rubino I. Diagnosis of early alzheimer's disease: Clinical practice in 2021. J Prev Alzheimers Dis. 2021; 8(3):371-86. [DOI:10.14283/jpad.2021.23.] [PMID]
- Nichols E, Szoeke CE, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, et al. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019; 18(1):88-106. [DOI:10.1016/ S1474-4422(18)30403-4]
- Kadhim A, Ben Slima A, Alneamah G, Makni M. Assessment of histopathological alterations and oxidative stress in the liver and kidney of male rats following exposure to aluminum chloride. J Toxicol. 2024; 2024:3997463. [DOI:10.1155/2024/3997463] [PMID] [PMCID]
- Exley C. A biogeochemical cycle for aluminium? J Inorg Biochem. 2003; 97(1):1-7. [DOI:10.1016/S0162-0134(03)00274-5] [PMID]
- Gándara MF. Aluminium: The metal of choice. Mater Tehnol. 2013; 47(3):261-5. [Link]
- Montazeri A, Akhlaghi M, Barahimi AR, Jahan Abad AJ, Jabbari R. [The role of metals in degenerative diseases of the central nervous system (Persian)]. Shefaye Khatam. 2020; 8(2):130-146. [DOI:10.29252/shefa.8.2.130]
- Igbokwe IO, Igwenagu E, Igbokwe NA. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip Toxicol. 2019; 12(2):45-70. [DOI:10.2478/intox-2019-0007] [PMID] [PMCID]
- Tabatabai SM. [The level of aluminum in the serum of Alzheimer’s patients with the severity of the disease and its comparison with healthy people (Persian)]. J Ilam Univ Med Sci. 2016; 24(1):126-32. [DOI:10.18869/acadpub.sjimu.24.1.126]
- Elmorsy E, Elsharkawy E, Alhumaydhi FA, Salama M. The protective effect of Indian Catechu methanolic extract against aluminum chloride-induced neurotoxicity, A rodent model of Alzheimer's disease. Heliyon. 2021; 7(2):e06269. [DOI:10.1016/j.heliyon.2021.e06269] [PMID] [PMCID]
- Walton JR. Evidence that Ingested Aluminum additives contained in processed foods and alum-treated drinking water are a major risk factor for Alzheimer’s disease. Curr Inorg Chem. 2012; 2(1):19-39. [DOI:10.2174/1877944111202010019]
- Vishala T, Pitchaiah G, Pravadha D, Annapurna A. Effect of plain and fortified amla fruit powder on aluminum-induced Alzheimer’s disease in Wistar rats. Pharmacogn Res. 2019; 11(4):406-9. [DOI:10.4103/pr.pr_17_17]
- Yin S, Ran Q, Yang J, Zhao Y, Li C. Nootropic effect of neferine on aluminium chloride-induced Alzheimer's disease in experimental models. J Biochem Mol Toxicol. 2020; 34(2):e22429. [DOI:10.1002/jbt.22429] [PMID]
- Justin-Thenmozhi A, Dhivya Bharathi M, Kiruthika R, Manivasagam T, Borah A, Essa MM. Attenuation of aluminum chloride-induced neuroinflammation and caspase activation through the AKT/GSK-3β pathway by hesperidin in wistar rats. Neurotox Res. 2018; 34(3):463-76. [DOI:10.1007/s12640-018-9904-4] [PMID]
- Bitra VR, Rapaka D, Mathala N, Akula A. Effect of wheat grass powder on aluminum induced Alzheimer's disease in Wistar rats. Asian Pac J Trop Med. 2014; 7S1:S278-81. [DOI:10.1016/S1995-7645(14)60246-7] [PMID]
- Liaquat L, Sadir S, Batool Z, Tabassum S, Shahzad S, Afzal A, et al. Acute aluminum chloride toxicity revisited: Study on DNA damage and histopathological, biochemical and neurochemical alterations in rat brain. Life Sci. 2019; 217:202-11. [DOI:10.1016/j.lfs.2018.12.009] [PMID]
- Esparza JL, Gómez M, Domingo JL. Role of melatonin in aluminum-related neurodegenerative disorders: A review. Biol Trace Elem Res. 2019; 188(1):60-67. [DOI:/10.1007/s12011-018-1372-4] [PMID]
- Hamza NM, Yasir SM, Hussain KAM. Biological effects of aqueous extract of Laurus noboilis L. leaves on some histological and immunological parameters in male rat liver affected by aluminum chloride. Arch Razi Instit. 2021; 76(6):1745-53. [DOI:10.22092/ari.2021.356361.1827]
- Ghosh S, Gaur A, Sengupta T, Banerjee M, Nayak P. Effect of aluminium on lipid profile and atherogenic index in prepubertal and young adult female rats: A pilot study. Indian J Physiol Pharmacol. 2023; 67(2):92-9. [DOI:10.25259/IJPP_338_2022]
- Xu F, Liu Y, Zhao H, Yu K, Song M, Zhu Y, et al. Aluminum chloride caused liver dysfunction and mitochondrial energy metabolism disorder in rat. J Inorg Biochem. 2017; 174:55-62. [DOI:10.1016/j.jinorgbio.2017.04.016] [PMID]
- Hosseini SM, Hejazian LB, Amani R, Siahchehreh Badeli N. Geraniol attenuates oxidative stress, bioaccumulation, serological and histopathological changes during aluminum chloride-hepatopancreatic toxicity in male Wistar rats. Environ Sci Pollut Res Int. 2020; 27(16):20076-89. [DOI:10.1007/s11356-020-08128-1] [PMID]
- Hosseini-Sharifabad A, Rabbani M, Seyed-Yousefi Y, Safavi M. Magnesium increases the protective effect of citicoline on aluminum chloride-induced cognitive impairment. Clin Psychopharmacol Neurosci. 2020; 18(2):241-8. [DOI:10.9758/cpn.2020.18.2.241] [PMID] [PMCID]
- Fernandes RM, Corrêa MG, Aragão WAB, Nascimento PC, Cartágenes SC, Rodrigues CA, et al. Preclinical evidences of aluminum-induced neurotoxicity in hippocampus and pre-frontal cortex of rats exposed to low doses. Ecotoxicol Environ Saf. 2020; 206:111139. [DOI:10.1016/j.ecoenv.2020.111139] [PMID]
- Geravand S, Karami M, Sahraei H, Rahimi F. Protective effects of L-arginine on Alzheimer's disease: Modulating hippocampal nitric oxide levels and memory deficits in aluminum chloride-induced rat model. Eur J Pharmacol. 2023; 958:176030. [DOI:10.1016/j.ejphar.2023.176030] [PMID]
- Joël NND, Laure NJ, Enyong OJ. Effect of Autranellacongolensis on lipid profile of rats’ Brain with experimentally induced Alzheimer’s disease. J Food Res. 2020; 9:60. [DOI:10.5539/jfr.v9n4p60]
- Mehpara Farhat S, Mahboob A, Ahmed T. Oral exposure to aluminum leads to reduced nicotinic acetylcholine receptor gene expression, severe neurodegeneration and impaired hippocampus dependent learning in mice. Drug Chem Toxicol. 2021; 44(3):310-8. [DOI:10.1080/01480545.2019.1587452] [PMID]
Type of Study: Research |
Subject:
General
Received: 2025/02/22 | Accepted: 2025/03/21 | Published: 2025/07/1
Received: 2025/02/22 | Accepted: 2025/03/21 | Published: 2025/07/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






