Tue, Sep 30, 2025
Volume 11, Issue 2 (Spring 2025)
Caspian J Neurol Sci 2025, 11(2): 172-179 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Azarnia S, Ezzati K, Saberi A, Moaddabi Y, Eghbali B B. Unihemispheric Dual-site Anodal tDCS for Lower Limb Motor Function in Chronic Stroke Patients: A Randomized Clinical Trial. Caspian J Neurol Sci 2025; 11 (2) :172-179
URL: http://cjns.gums.ac.ir/article-1-753-en.html
URL: http://cjns.gums.ac.ir/article-1-753-en.html
1- Department of Physiotherapy, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Physiotherapy, Neuroscience Research Center, School of Medicine, Trauma Institute, Poorsina Hospital, Guilan University of Medical Sciences, Rasht, Iran. ,Ez_kamran@yahoo.com
3- Department of Neurology, Neuroscience Research Center, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Guilan, Iran
4- Department of Neurology, Faculty of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
5- Department of Physiotherapy, Neuroscience Research Center, School of Medicine, Trauma Institute, Poorsina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Physiotherapy, Neuroscience Research Center, School of Medicine, Trauma Institute, Poorsina Hospital, Guilan University of Medical Sciences, Rasht, Iran. ,
3- Department of Neurology, Neuroscience Research Center, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Guilan, Iran
4- Department of Neurology, Faculty of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
5- Department of Physiotherapy, Neuroscience Research Center, School of Medicine, Trauma Institute, Poorsina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
Full-Text [PDF 1361 kb]
(211 Downloads)
| Abstract (HTML) (694 Views)
Full-Text: (234 Views)
Introduction
Stroke is a major global health issue, ranking among the top causes of mortality and long-term disability [1]. Stroke can severely impair motor function in the lower limbs, causing problems with walking, balance, and activities of daily living [2]. After stroke, impaired movement control and limb weakness reduce muscle force production and affect coordination between the limbs during movement [2]. Stroke-related physical disability is a significant burden on healthcare systems and carers, affecting patients’ independence and quality of life. As a result, regaining lower limb function is a key objective of rehabilitation following a stroke [3]. Conventional rehabilitation strategies focus mainly on physical therapy. Nowadays, neurorehabilitation methods are considered a new and effective approach to the treatment of stroke patients. One of these methods is transcranial direct current therapy (tDCS), which uses a direct, weak current (1 to 2 mA) to change cortical excitability [4]. The current is applied to the scalp through two electrodes. tDCS affects motor learning by exploiting neuroplasticity’s effect and increasing rehabilitation’s effectiveness [5]. Many studies have shown the effectiveness of tDCS in improving motor performance. In recent years, researchers have looked for ways to increase the effectiveness of tDCS. One of the most essential parameters of brain stimulation is the location of the electrode. Anodal tDCS of the primary motor cortex (M1) is a routine stimulation that improves motor performance and learning. In addition to M1, other cortical areas functionally related to M1, such as the dorsolateral prefrontal cortices (DLPFC), have also been stimulated to alter corticospinal excitability [6]. The results showed that uni-hemispheric dual-site anodal tDCSM1-DLPFC resulted in greater motor cortex excitability compared with stimulation of the primary motor cortex alone [7]. Vaseghi et al. (2015) indicated that stimulating M1 and DLPFC bilaterally in healthy subjects increased cortical excitability 1.5-fold [8]. Most studies have focused on upper limb function [9–11]. Therefore, this study investigated the effects of unihemispheric dual-site anodal tDCSM1-DLPFC on improving lower limb motor function.
This study aims to assess the effectiveness of uni-hemispheric dual-site anodal tDCS targeting M1 and DLPFC in improving lower limb motor function. Secondary objectives include evaluating the impact of tDCS on various aspects of motor performance, such as muscle strength, coordination, and balance in the affected lower limb.
Materials and Methods
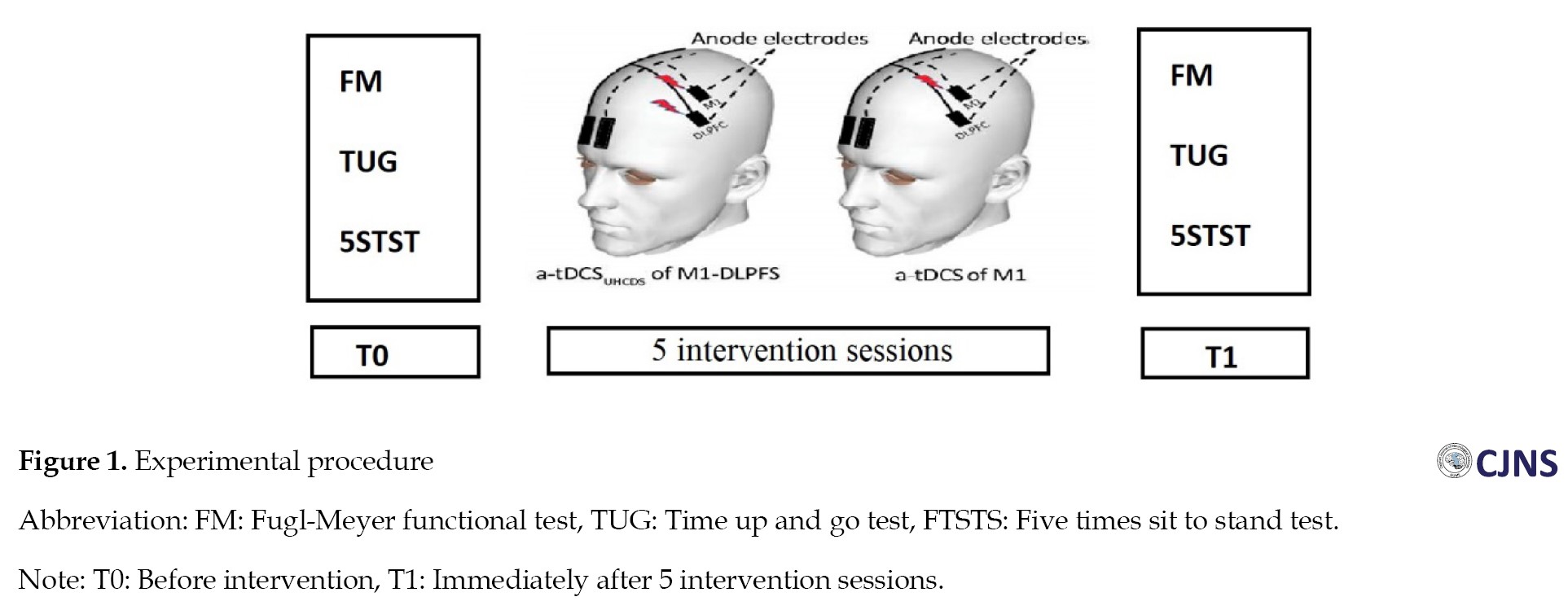
The trial was a double-blind, randomized, controlled clinical trial. Eighteen patients with chronic cerebral infarction (six months post-stroke) were enrolled. The mini-mental status examination (MMSE) was used to assess the patient’s cognitive status. All patients received 5 intervention sessions on 5 consecutive days. The patient’s functional status was evaluated and recorded before intervention (T0), immediately after 5 intervention sessions (T1) and by Fugl-Meyer functional test (FM), time up and go test (TUG), and five times sit to stand test (FTSTS) (Figure 1). The Inclusion criteria were as follows:
1) First stroke [5]; 2) Patients over 40 years of age; 3) Stroke caused by involvement of the anterior cerebral artery, diagnosed by a neurologist; 4) Patients without chronic neurological disorders, including Parkinson disease, Alzheimer disease, schizophrenia, radiculopathy, and musculoskeletal disorders—especially those affecting lower limb movement—diagnosed by neurologists and physiotherapists; 5) Participants with knee flexor spasticity of 1 or more on the modified Ashworth scale (MMAS); 6) No history of brain tumor; 7) Verbal communication skills with the therapist; 8) Not taking any medication that alters a person’s cognitive state; 9) Not having a heart condition and a pacemaker; 10) No history of seizures or previous brain surgery; 11) Patients without significant cognitive and memory deficits will be assessed using the Farsi translation of the MMSE, which requires a minimum score of 23 out of 30 [9].
The exclusion criteria were as follows:
1) Non-cooperation of the patient in the post-intervention tests [10]; 2) Demonstration of scalp sensitivity to stimulation by the patient; 3) People withdraw from the research for any reason, not wanting to continue the research; 4) Extreme fatigue means the person cannot continue the test.
Randomization
Both participants and raters were unaware of group assignments. Randomization was conducted via the Randomisation.com website. The patients were divided into two experimental groups, 1 and 2, through a computer-generated randomization block. In experiment group 1, participants underwent 5 consecutive sessions of active anodal tDCS targeting the M1-DLPFC. In contrast, experiment group 2 included 5 consecutive sessions that combined active tDCS at M1 with sham treatment at DLPFC. After the initial assessment, patients were allocated to treatment and control groups using a randomized block design.
Transcranial direct current stimulation (tDCS) parameters
Direct current stimulation was delivered via two saline-soaked electrodes using two single-channel tDCS devices. The electrodes were positioned according to the international 10-20 system used in electroencephalography. In both groups, active electrodes were placed on M1 at C3/C4 and DLPFC at F3/F4, based on the targeted hemisphere. Reference electrodes were positioned over the supraorbital area of the non-targeted side [11]. A constant current of 1 mA was applied for 20 minutes, consistent with previous research [12]. In the sham group (experiment group 2), stimulation was turned off after 30 seconds, specifically in the DLPFC region. A standard 5×7 cm² electrode was used as the reference electrode, while a 4×4 cm² active electrode was placed on the M1 and DLPFC areas to target motor cortex excitability and enhance corticospinal tract excitability.
Outcome measures
The primary outcomes were FM, TUG and 5TSST, which were assessed using a questionnaire and timer. The FM was used to assess 5 domains of motor function, sensory function, joint mobility, balance, and pain in stroke patients [13].
TUG was used to assess the individual’s mobility and to evaluate static and dynamic balance. The 5TSST was employed to evaluate the functional strength of the lower limbs and transfer movements, balance and fall risk in the patients.
Data analysis utilized SPSS software, version 26 (IBM SPSS Statistics for Windows, version 26, IBM Corp, Armonk, NY, USA). Continuous variables are presented as Mean±SD. The Shapiro-Wilk test was employed to evaluate the normality of the quantitative data, indicating that the data adhered to a normal distribution. The non-parametric Mann-Whitney U-test and Wilcoxon signed-rank test were applied for comparisons between and within groups.
A P<0.05 was deemed statistically significant. The sample size was determined using G*Power software, version 3.1, based on an effect size (d=2.0) reported in the Klomjai study, with a power of 0.90 and α=0.05. To account for possible dropouts, a 20% buffer was added to the calculations [14].
Results
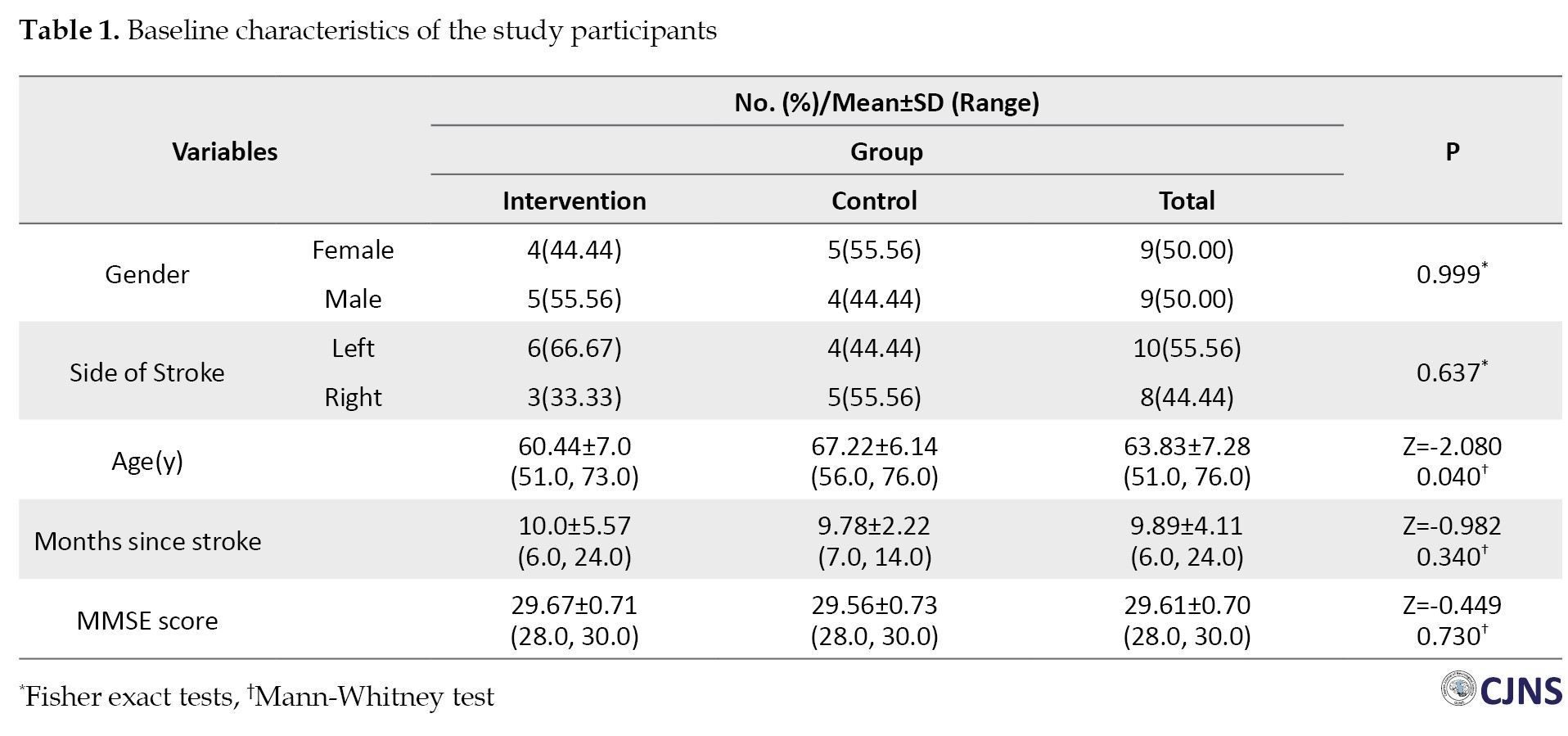
Eighteen stroke patients (9 women and 9 men) with a mean age of 60.94±6.92 years participated in the study. The average time since stroke onset was 34.28±8.91 weeks. Table 1 shows no statistically significant differences in demographic characteristics, comorbidities, and spasticity levels between the two study groups.
This study evaluated the average scores of motor function, mobility and functional strength of the lower limbs both at baseline and following the intervention in M1.
Comparisons of the effect of the intervention on motor function, PROM and joint pain
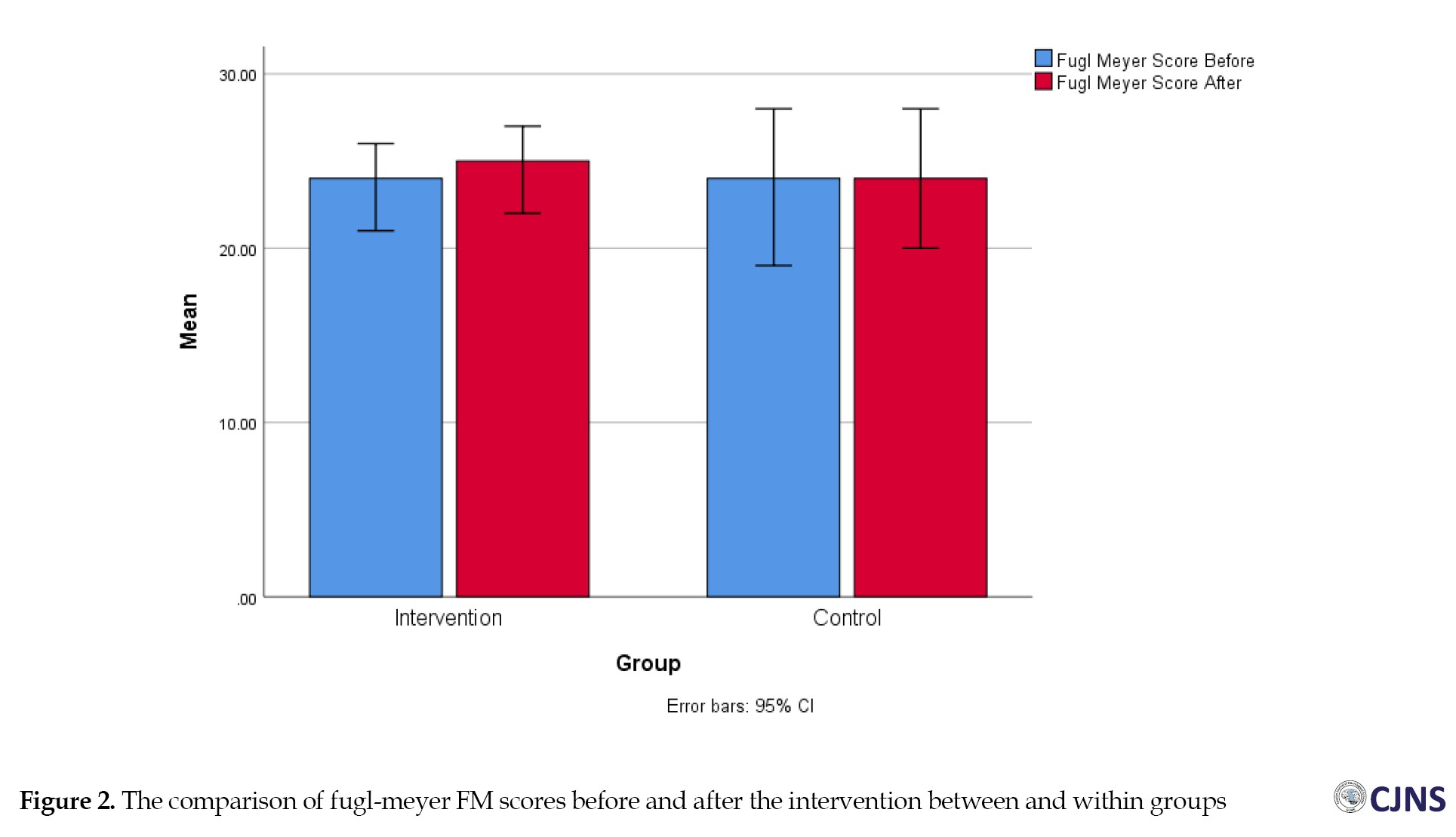
The results showed that in the between-group comparison, motor performance, passive range of motion and joint pain were not statistically significantly different in the two treatment and control groups before the intervention (P=0.66, P=0.73, P=0.66). There was no significant change after the intervention. The changes in motor performance, passive range of motion, and joint pain were not statistically significant (P=0.54, P=0.29, P=0.34) (Figure 2). When comparing within groups, there was a statistical difference in motor performance after the intervention compared to before in the treatment group (P=0.02). At the same time, there was no statistical difference in the control group (P=0.10). Both groups exhibited significant changes in passive range of motion and joint pain after the intervention compared to their status before.
Between-group and within-group comparison of the effect of the intervention on mobility and functional strength of the lower limbs in chronic stroke patients
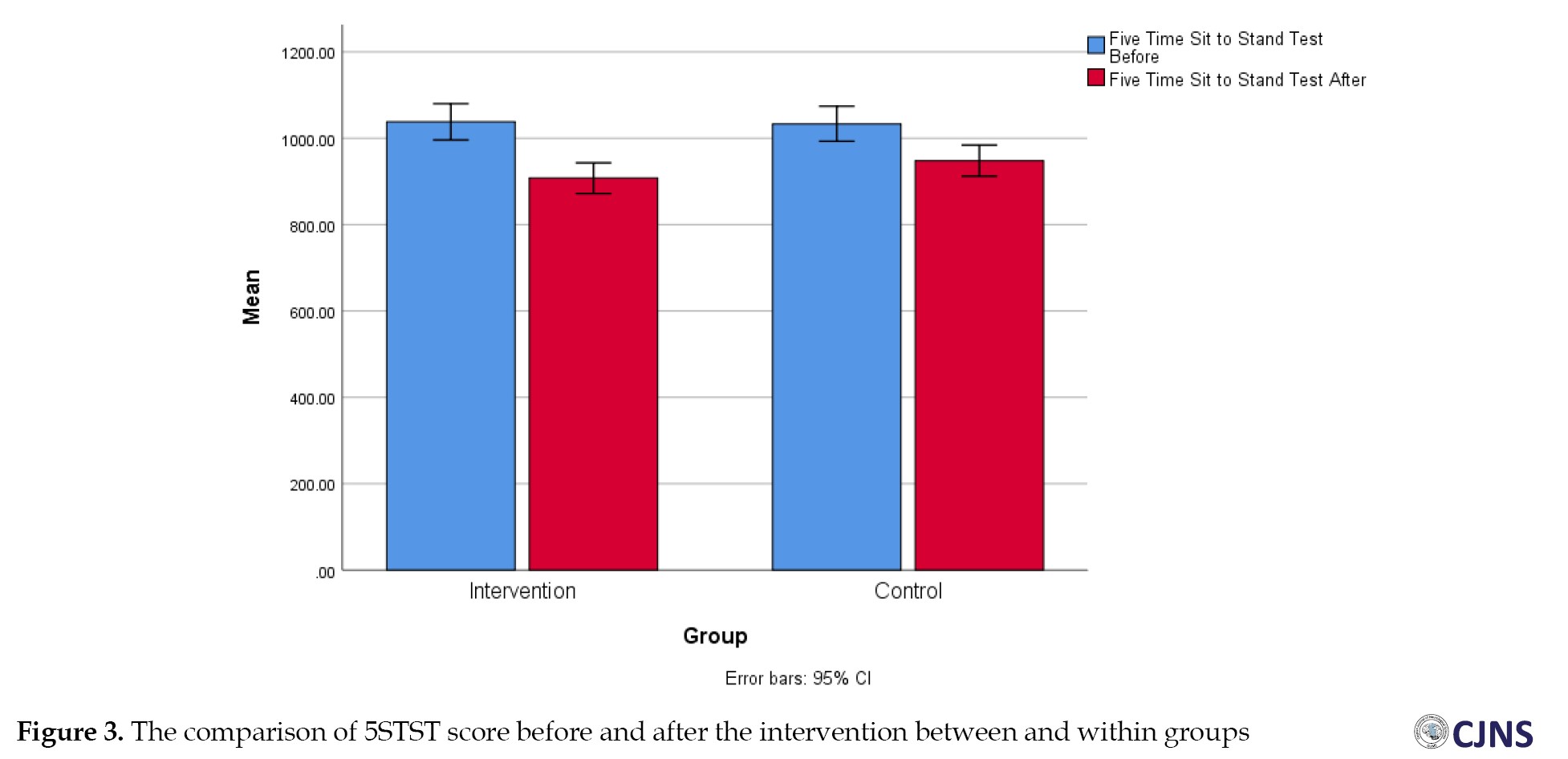
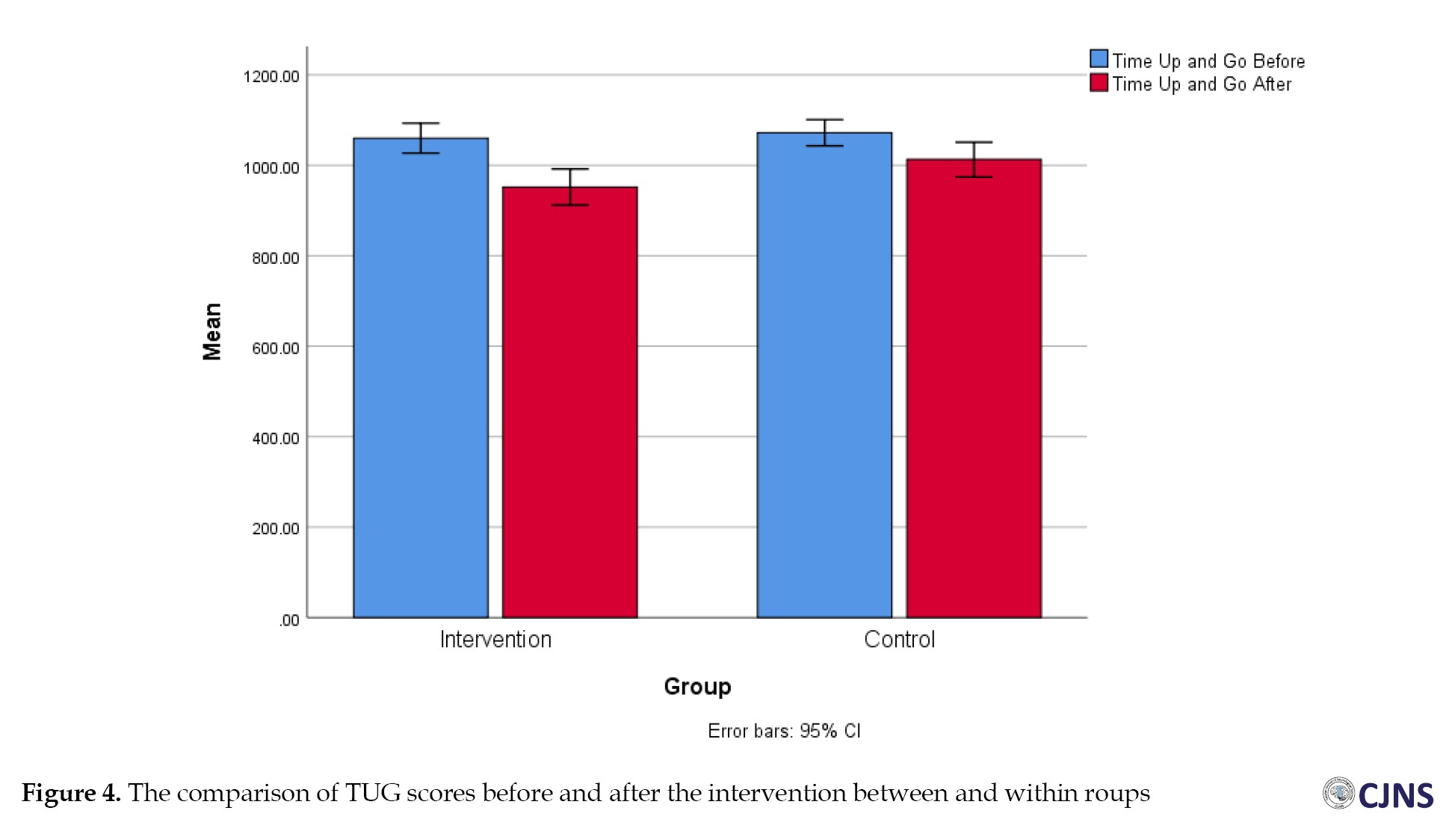
Before the intervention, the results for mobility TUG and functional strength five times sit to stand test (5STST) showed no statistically significant difference between the treatment and control groups (P=0.60). However, after the intervention, A notable difference was found between the two groups (P=0.04 and P=0.07), with the treatment group demonstrating a greater decrease in time than the control group. Additionally, significant differences were observed between the two groups in both TUG and 5STST (Figures 3 and 4).
Discussion
This study evaluated the effects of two tDCS protocols, two-point versus one-point stimulation, on motor performance and mobility in chronic stroke patients. Our findings align with existing literature, which generally supports the positive impact of tDCS on motor function. By focusing on comparing the two-point and one-point tDCS configurations, we explored how targeting different areas of the motor cortex may influence treatment outcomes. This direct comparison is novel in stroke rehabilitation research and could provide insights into identifying the configuration that best enhances patient motor and functional outcomes. We selected sensitive assessment tools, such as the FM score, TUG and 5STST, to effectively evaluate the impact of tDCS on mobility and functional strength. This choice was critical in capturing meaningful changes post-intervention and allows for comparisons with a broader body of tDCS literature.
The trial results demonstrated no difference between FM test scores, passive range of motion, and joint pain, 5STST, after stimulation of two points and one point. However, TUG showed statistically significant changes, aligning with the findings of Klomjai et al. [14], who investigated the effects of one session of a-tDCS targeting M1 on lower limb motor performance in patients with subacute stroke. Azarnia et al. [15] studied the effect of stimulating two points and one point on the levels of brain metabolites and showed no change in the levels of metabolites. Prathum et al. [16] examined the intervention’s effect on the motor performance of patients’ upper and lower limbs and reported positive intervention results. In their study, the patients were split into control and intervention groups. The control group received no stimulation. However, in our study, the control group received stimulation. One point and the intervention group received two points of stimulation. Dumont et al. [17] studied the effects of treadmill and primary motor cortex electrical stimulation on static postural oscillations in stroke patients, and the results showed that the patients’ anterior-posterior oscillations decreased after one session of stimulation and training. Our results were consistent with their findings.
In our study, both groups received brain stimulation; the results were better than before. One of the reasons for the difference in the effect on organ function when using tDCS is the attention to the type of involvement in the brain. It is said that stroke patients with subcortical participation may benefit more from the effects of tDCS stimulation than those with cortical involvement. Edward et al. [18] showed that tDCS before robotic therapy improved motor performance in chronic stroke patients. Motor learning improves movement skills through repetition and practice, which is associated with long-term neurological changes. Therefore, the long-term effects of tDCS, together with repetition and training, may improve patients’ motor performance.
In another study [18], the intervention was carried out over 36 sessions, of which 5 stimulation sessions were used in the current study. Perhaps the cumulative effect of more stimulation sessions effectively improves motor performance. Other trials that have shown positive results from using tDCS in enhancing patients’ motor performance have used electrical stimulation in combination with other treatments [16, 19-22]. Despite these findings, several limitations should be considered. The limited sample size of 18 participants restricts the generalizability of the findings and may impact the statistical power. Consequently, larger studies are necessary to validate these findings. In addition, although the intervention consisted of 5 tDCS sessions within a rehabilitation program, this limited frequency may not adequately reflect the potential cumulative effects of tDCS. The study groups were designed to examine only the impact of brain stimulation. Because brain stimulation has a stimulating effect on the brain, combining two-point brain stimulation with exercise therapy may produce effective results, which we suggest be addressed in future studies. Finally, the absence of extended follow-up assessments restricts our understanding of the sustainability of tDCS-related improvements in motor function and mobility.
Conclusion
This study underscores the potential of tDCS as a viable intervention for enhancing motor performance and mobility in chronic stroke patients. While both one-point and two-point tDCS stimulation protocols were evaluated, only the treatment group exhibited statistically significant improvements in motor function following the intervention, demonstrated by enhanced performance on the FM score and notable mobility improvements in the TUG and 5STST.
Despite the lack of significant differences between the two stimulation techniques in certain assessed areas, the findings align with existing literature highlighting the beneficial effects of tDCS on motor rehabilitation. However, the study’s limitations, such as the small sample size, short intervention duration, and absence of long-term follow-up, indicate the need for further research to confirm these results and optimize treatment protocols. Future investigations should also explore the synergistic effects of tDCS combined with other rehabilitation strategies to maximize patient outcomes, particularly in those with different types of brain involvement. Overall, this research adds to the growing body of evidence supporting tDCS as a promising adjunct therapy in stroke rehabilitation.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Guilan University of Medical Science, Rasht, Iran (Code: IRGUMSREC.1401.408) and approved the Ethics Clinical Trial of Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20211030052912N3). All patients signed an informed consent form.
Funding
This project was financially supported by Poursina Neuroscience Research Center, Guilan University of Medical Sciences, Rasht, Iran.
Authors contributions
Conceptualization and methodology: Babak Bakhshayesh and Somaye Azarnia; Data collection and writing the original draft: Somaye Azarnia; Formal analysis, writing, review and editing: Kamran Ezzati; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors want to thank the patients who cooperated in the implementation of the study.
References
Stroke is a major global health issue, ranking among the top causes of mortality and long-term disability [1]. Stroke can severely impair motor function in the lower limbs, causing problems with walking, balance, and activities of daily living [2]. After stroke, impaired movement control and limb weakness reduce muscle force production and affect coordination between the limbs during movement [2]. Stroke-related physical disability is a significant burden on healthcare systems and carers, affecting patients’ independence and quality of life. As a result, regaining lower limb function is a key objective of rehabilitation following a stroke [3]. Conventional rehabilitation strategies focus mainly on physical therapy. Nowadays, neurorehabilitation methods are considered a new and effective approach to the treatment of stroke patients. One of these methods is transcranial direct current therapy (tDCS), which uses a direct, weak current (1 to 2 mA) to change cortical excitability [4]. The current is applied to the scalp through two electrodes. tDCS affects motor learning by exploiting neuroplasticity’s effect and increasing rehabilitation’s effectiveness [5]. Many studies have shown the effectiveness of tDCS in improving motor performance. In recent years, researchers have looked for ways to increase the effectiveness of tDCS. One of the most essential parameters of brain stimulation is the location of the electrode. Anodal tDCS of the primary motor cortex (M1) is a routine stimulation that improves motor performance and learning. In addition to M1, other cortical areas functionally related to M1, such as the dorsolateral prefrontal cortices (DLPFC), have also been stimulated to alter corticospinal excitability [6]. The results showed that uni-hemispheric dual-site anodal tDCSM1-DLPFC resulted in greater motor cortex excitability compared with stimulation of the primary motor cortex alone [7]. Vaseghi et al. (2015) indicated that stimulating M1 and DLPFC bilaterally in healthy subjects increased cortical excitability 1.5-fold [8]. Most studies have focused on upper limb function [9–11]. Therefore, this study investigated the effects of unihemispheric dual-site anodal tDCSM1-DLPFC on improving lower limb motor function.
This study aims to assess the effectiveness of uni-hemispheric dual-site anodal tDCS targeting M1 and DLPFC in improving lower limb motor function. Secondary objectives include evaluating the impact of tDCS on various aspects of motor performance, such as muscle strength, coordination, and balance in the affected lower limb.
Materials and Methods
The trial was a double-blind, randomized, controlled clinical trial. Eighteen patients with chronic cerebral infarction (six months post-stroke) were enrolled. The mini-mental status examination (MMSE) was used to assess the patient’s cognitive status. All patients received 5 intervention sessions on 5 consecutive days. The patient’s functional status was evaluated and recorded before intervention (T0), immediately after 5 intervention sessions (T1) and by Fugl-Meyer functional test (FM), time up and go test (TUG), and five times sit to stand test (FTSTS) (Figure 1). The Inclusion criteria were as follows:
1) First stroke [5]; 2) Patients over 40 years of age; 3) Stroke caused by involvement of the anterior cerebral artery, diagnosed by a neurologist; 4) Patients without chronic neurological disorders, including Parkinson disease, Alzheimer disease, schizophrenia, radiculopathy, and musculoskeletal disorders—especially those affecting lower limb movement—diagnosed by neurologists and physiotherapists; 5) Participants with knee flexor spasticity of 1 or more on the modified Ashworth scale (MMAS); 6) No history of brain tumor; 7) Verbal communication skills with the therapist; 8) Not taking any medication that alters a person’s cognitive state; 9) Not having a heart condition and a pacemaker; 10) No history of seizures or previous brain surgery; 11) Patients without significant cognitive and memory deficits will be assessed using the Farsi translation of the MMSE, which requires a minimum score of 23 out of 30 [9].
The exclusion criteria were as follows:
1) Non-cooperation of the patient in the post-intervention tests [10]; 2) Demonstration of scalp sensitivity to stimulation by the patient; 3) People withdraw from the research for any reason, not wanting to continue the research; 4) Extreme fatigue means the person cannot continue the test.
Randomization
Both participants and raters were unaware of group assignments. Randomization was conducted via the Randomisation.com website. The patients were divided into two experimental groups, 1 and 2, through a computer-generated randomization block. In experiment group 1, participants underwent 5 consecutive sessions of active anodal tDCS targeting the M1-DLPFC. In contrast, experiment group 2 included 5 consecutive sessions that combined active tDCS at M1 with sham treatment at DLPFC. After the initial assessment, patients were allocated to treatment and control groups using a randomized block design.
Transcranial direct current stimulation (tDCS) parameters
Direct current stimulation was delivered via two saline-soaked electrodes using two single-channel tDCS devices. The electrodes were positioned according to the international 10-20 system used in electroencephalography. In both groups, active electrodes were placed on M1 at C3/C4 and DLPFC at F3/F4, based on the targeted hemisphere. Reference electrodes were positioned over the supraorbital area of the non-targeted side [11]. A constant current of 1 mA was applied for 20 minutes, consistent with previous research [12]. In the sham group (experiment group 2), stimulation was turned off after 30 seconds, specifically in the DLPFC region. A standard 5×7 cm² electrode was used as the reference electrode, while a 4×4 cm² active electrode was placed on the M1 and DLPFC areas to target motor cortex excitability and enhance corticospinal tract excitability.
Outcome measures
The primary outcomes were FM, TUG and 5TSST, which were assessed using a questionnaire and timer. The FM was used to assess 5 domains of motor function, sensory function, joint mobility, balance, and pain in stroke patients [13].
TUG was used to assess the individual’s mobility and to evaluate static and dynamic balance. The 5TSST was employed to evaluate the functional strength of the lower limbs and transfer movements, balance and fall risk in the patients.
Data analysis utilized SPSS software, version 26 (IBM SPSS Statistics for Windows, version 26, IBM Corp, Armonk, NY, USA). Continuous variables are presented as Mean±SD. The Shapiro-Wilk test was employed to evaluate the normality of the quantitative data, indicating that the data adhered to a normal distribution. The non-parametric Mann-Whitney U-test and Wilcoxon signed-rank test were applied for comparisons between and within groups.
A P<0.05 was deemed statistically significant. The sample size was determined using G*Power software, version 3.1, based on an effect size (d=2.0) reported in the Klomjai study, with a power of 0.90 and α=0.05. To account for possible dropouts, a 20% buffer was added to the calculations [14].
Results
Eighteen stroke patients (9 women and 9 men) with a mean age of 60.94±6.92 years participated in the study. The average time since stroke onset was 34.28±8.91 weeks. Table 1 shows no statistically significant differences in demographic characteristics, comorbidities, and spasticity levels between the two study groups.

This study evaluated the average scores of motor function, mobility and functional strength of the lower limbs both at baseline and following the intervention in M1.
Comparisons of the effect of the intervention on motor function, PROM and joint pain
The results showed that in the between-group comparison, motor performance, passive range of motion and joint pain were not statistically significantly different in the two treatment and control groups before the intervention (P=0.66, P=0.73, P=0.66). There was no significant change after the intervention. The changes in motor performance, passive range of motion, and joint pain were not statistically significant (P=0.54, P=0.29, P=0.34) (Figure 2). When comparing within groups, there was a statistical difference in motor performance after the intervention compared to before in the treatment group (P=0.02). At the same time, there was no statistical difference in the control group (P=0.10). Both groups exhibited significant changes in passive range of motion and joint pain after the intervention compared to their status before.
Between-group and within-group comparison of the effect of the intervention on mobility and functional strength of the lower limbs in chronic stroke patients
Before the intervention, the results for mobility TUG and functional strength five times sit to stand test (5STST) showed no statistically significant difference between the treatment and control groups (P=0.60). However, after the intervention, A notable difference was found between the two groups (P=0.04 and P=0.07), with the treatment group demonstrating a greater decrease in time than the control group. Additionally, significant differences were observed between the two groups in both TUG and 5STST (Figures 3 and 4).
Discussion
This study evaluated the effects of two tDCS protocols, two-point versus one-point stimulation, on motor performance and mobility in chronic stroke patients. Our findings align with existing literature, which generally supports the positive impact of tDCS on motor function. By focusing on comparing the two-point and one-point tDCS configurations, we explored how targeting different areas of the motor cortex may influence treatment outcomes. This direct comparison is novel in stroke rehabilitation research and could provide insights into identifying the configuration that best enhances patient motor and functional outcomes. We selected sensitive assessment tools, such as the FM score, TUG and 5STST, to effectively evaluate the impact of tDCS on mobility and functional strength. This choice was critical in capturing meaningful changes post-intervention and allows for comparisons with a broader body of tDCS literature.
The trial results demonstrated no difference between FM test scores, passive range of motion, and joint pain, 5STST, after stimulation of two points and one point. However, TUG showed statistically significant changes, aligning with the findings of Klomjai et al. [14], who investigated the effects of one session of a-tDCS targeting M1 on lower limb motor performance in patients with subacute stroke. Azarnia et al. [15] studied the effect of stimulating two points and one point on the levels of brain metabolites and showed no change in the levels of metabolites. Prathum et al. [16] examined the intervention’s effect on the motor performance of patients’ upper and lower limbs and reported positive intervention results. In their study, the patients were split into control and intervention groups. The control group received no stimulation. However, in our study, the control group received stimulation. One point and the intervention group received two points of stimulation. Dumont et al. [17] studied the effects of treadmill and primary motor cortex electrical stimulation on static postural oscillations in stroke patients, and the results showed that the patients’ anterior-posterior oscillations decreased after one session of stimulation and training. Our results were consistent with their findings.
In our study, both groups received brain stimulation; the results were better than before. One of the reasons for the difference in the effect on organ function when using tDCS is the attention to the type of involvement in the brain. It is said that stroke patients with subcortical participation may benefit more from the effects of tDCS stimulation than those with cortical involvement. Edward et al. [18] showed that tDCS before robotic therapy improved motor performance in chronic stroke patients. Motor learning improves movement skills through repetition and practice, which is associated with long-term neurological changes. Therefore, the long-term effects of tDCS, together with repetition and training, may improve patients’ motor performance.
In another study [18], the intervention was carried out over 36 sessions, of which 5 stimulation sessions were used in the current study. Perhaps the cumulative effect of more stimulation sessions effectively improves motor performance. Other trials that have shown positive results from using tDCS in enhancing patients’ motor performance have used electrical stimulation in combination with other treatments [16, 19-22]. Despite these findings, several limitations should be considered. The limited sample size of 18 participants restricts the generalizability of the findings and may impact the statistical power. Consequently, larger studies are necessary to validate these findings. In addition, although the intervention consisted of 5 tDCS sessions within a rehabilitation program, this limited frequency may not adequately reflect the potential cumulative effects of tDCS. The study groups were designed to examine only the impact of brain stimulation. Because brain stimulation has a stimulating effect on the brain, combining two-point brain stimulation with exercise therapy may produce effective results, which we suggest be addressed in future studies. Finally, the absence of extended follow-up assessments restricts our understanding of the sustainability of tDCS-related improvements in motor function and mobility.
Conclusion
This study underscores the potential of tDCS as a viable intervention for enhancing motor performance and mobility in chronic stroke patients. While both one-point and two-point tDCS stimulation protocols were evaluated, only the treatment group exhibited statistically significant improvements in motor function following the intervention, demonstrated by enhanced performance on the FM score and notable mobility improvements in the TUG and 5STST.
Despite the lack of significant differences between the two stimulation techniques in certain assessed areas, the findings align with existing literature highlighting the beneficial effects of tDCS on motor rehabilitation. However, the study’s limitations, such as the small sample size, short intervention duration, and absence of long-term follow-up, indicate the need for further research to confirm these results and optimize treatment protocols. Future investigations should also explore the synergistic effects of tDCS combined with other rehabilitation strategies to maximize patient outcomes, particularly in those with different types of brain involvement. Overall, this research adds to the growing body of evidence supporting tDCS as a promising adjunct therapy in stroke rehabilitation.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Guilan University of Medical Science, Rasht, Iran (Code: IRGUMSREC.1401.408) and approved the Ethics Clinical Trial of Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20211030052912N3). All patients signed an informed consent form.
Funding
This project was financially supported by Poursina Neuroscience Research Center, Guilan University of Medical Sciences, Rasht, Iran.
Authors contributions
Conceptualization and methodology: Babak Bakhshayesh and Somaye Azarnia; Data collection and writing the original draft: Somaye Azarnia; Formal analysis, writing, review and editing: Kamran Ezzati; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors want to thank the patients who cooperated in the implementation of the study.
References
- Kuklina EV, Tong X, George MG, Bansil P. Epidemiology and prevention of stroke: A worldwide perspective. Expert Rev Neurother. 2012; 12(2):199-208. [DOI:10.1586/ern.11.99] [PMID] [PMCID]
- Kim YW. Update on stroke rehabilitation in motor impairment. Brain Neurorehabil. 2022; 15(2):e12. [DOI:10.12786/bn.2022.15.e12] [PMID] [PMCID]
- Belagaje SR. Stroke rehabilitation. continuum (minneap minn). 2017; 23(1, Cerebrovascular Disease):238-253. [DOI:10.1212/CON.0000000000000423] [PMID]
- Paulus W. Transcranial direct current stimulation (tDCS). Suppl Clin Neurophysiol. 2003; 56:249-54. [DOI:10.1016/S1567-424X(09)70229-6] [PMID]
- Kang J, Cai E, Han J, Tong Z, Li X, Sokhadze EM, et al. Transcranial direct current stimulation (tDCS) can modulate EEG complexity of children with autism spectrum disorder. Front Neurosci. 2018; 12:201. [DOI:10.3389/fnins.2018.00201] [PMID] [PMCID]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005; 22(2):495-504. [DOI:10.1111/j.1460-9568.2005.04233.x] [PMID] [PMCID]
- Lindenberg R, Nachtigall L, Meinzer M, Sieg MM, Flöel A. Differential effects of dual and unihemispheric motor cortex stimulation in older adults. J Neurosci. 2013; 33(21):9176-83. [DOI:10.1523/JNEUROSCI.0055-13.2013] [PMID] [PMCID]
- Vaseghi B, Zoghi M, Jaberzadeh S. The effects of anodal-tDCS on corticospinal excitability enhancement and its after-effects: conventional vs. unihemispheric concurrent dual-site stimulation. Front Hum Neurosci. 2015; 9:533. [DOI:10.3389/fnhum.2015.00533] [PMID] [PMCID]
- Ansari NN, Naghdi S, Hasson S, Valizadeh L, Jalaie S. Validation of a mini-mental state examination (MMSE) for the Persian population: A pilot study. Appl Neuropsychol. 2010; 17(3):190-5. [DOI:10.1080/09084282.2010.499773] [PMID]
- Di Lazzaro V, Dileone M, Capone F, Pellegrino G, Ranieri F, Musumeci G, et al. Immediate and late modulation of interhemipheric imbalance with bilateral transcranial direct current stimulation in acute stroke. Brain Stimul. 2014; 7(6):841-8. [DOI:10.1016/j.brs.2014.10.001] [PMID]
- van der Zijden JP, van Eijsden P, de Graaf RA, Dijkhuizen RM. 1H/13C MR spectroscopic imaging of regionally specific metabolic alterations after experimental stroke. Brain. 2008; 131(Pt 8):2209-19. [DOI:10.1093/brain/awn139] [PMID]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010; 75(24):2176-84. [DOI:10.1212/WNL.0b013e318202013a] [PMID] [PMCID]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000; 527(Pt 3):633-9. [DOI:10.1111/j.1469-7793.2000.t01-1-00633.x] [PMID] [PMCID]
- Klomjai W, Aneksan B, Pheungphrarattanatrai A, Chantanachai T, Choowong N, Bunleukhet S, et al. Effect of single-session dual-tDCS before physical therapy on lower-limb performance in sub-acute stroke patients: A randomized sham-controlled crossover study. Ann Phys Rehabil Med. 2018; 61(5):286-91. [DOI:10.1016/j.rehab.2018.04.005] [PMID]
- Azarnia S, Ezzati K, Saberi A, Naghdi S, Abdollahi I, Jaberzadeh S. The effect of uni-hemispheric dual-site anodal tDCS on brain metabolic changes in stroke patients: A randomized clinical trial. Brain Sci. 2023; 13(7):1100. [DOI:10.3390/brainsci13071100] [PMID] [PMCID]
- Prathum T, Piriyaprasarth P, Aneksan B, Hiengkaew V, Pankhaew T, Vachalathiti R, et al. Effects of home-based dual-hemispheric transcranial direct current stimulation combined with exercise on upper and lower limb motor performance in patients with chronic stroke. Disabil Rehabil. 2022; 44(15):3868-79. [DOI:10.1080/09638288.2021.1891464] [PMID]
- Dumont AJL, Cimolin V, Parreira RB, Armbrust D, Fonseca DRP, Fonseca AL, et al. Effects of transcranial direct current stimulation combined with treadmill training on kinematics and spatiotemporal gait variables in stroke survivors: A randomized, triple-blind, sham-controlled study. Brain Sci. 2022; 13(1):11. [DOI:10.3390/brainsci13010011] [PMID] [PMCID]
- Edwards DJ, Cortes M, Rykman-Peltz A, Chang J, Elder J, Thickbroom G, et al. Clinical improvement with intensive robot-assisted arm training in chronic stroke is unchanged by supplementary tDCS. Restor Neurol Neurosci. 2019; 37(2):167-80. [DOI:10.3233/RNN-180869] [PMID]
- Lee YS, Yang HS, Jeong CJ, Yoo YD, Jeong SH, Jeon OK, et al. The effects of transcranial direct current stimulation on functional movement performance and balance of the lower extremities. J Phys Ther Sci. 2012; 24(12):1215-8. [DOI:10.1589/jpts.24.1215]
- Xu Z, Shen B, Xiao S, Zhang C, Zhan J, Li J, et al. The effect of transcranial direct current stimulation on lower-limb endurance performance: A systematic review. Bioengineering. 2024; 11(11):1088. [DOI:10.3390/bioengineering11111088] [PMID] [PMCID]
- Klomjai W, Aneksan B. A randomized sham-controlled trial on the effects of dual-tDCS "during" physical therapy on lower limb performance in sub-acute stroke and a comparison to the previous study using a "before" stimulation protocol. BMC Sports Sci Med Rehabil. 2022; 14(1):68. [DOI:10.1186/s13102-022-00463-9] [PMID] [PMCID]
- Rodríguez-Ugarte M, Iáñez E, Ortiz M, Azorín JM. Improving real-time lower limb motor imagery detection using tDCS and an exoskeleton. Front Neurosci. 2018; 12:757. [DOI:10.3389/fnins.2018.00757] [PMID] [PMCID]
Type of Study: Research |
Subject:
Special
Received: 2024/10/16 | Accepted: 2025/02/27 | Published: 2025/04/1
Received: 2024/10/16 | Accepted: 2025/02/27 | Published: 2025/04/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |