Sun, Feb 1, 2026
Volume 11, Issue 2 (Spring 2025)
Caspian J Neurol Sci 2025, 11(2): 140-162 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Udodi P S, Ndubuisi K B, Mofunanya U C, Onuorah P C, Odunwa U P, Ndinojuo S C, et al . Ameliorative Effects of Silymarin on Methamphetamine-Induced Neurobehavioral and Histopathological Alterations in Adolescent Wistar Rats. Caspian J Neurol Sci 2025; 11 (2) :140-162
URL: http://cjns.gums.ac.ir/article-1-744-en.html
URL: http://cjns.gums.ac.ir/article-1-744-en.html
Princewill Sopuluchukwu Udodi *1 

 , Kelechi Blessed Ndubuisi2
, Kelechi Blessed Ndubuisi2 

 , Uchenna Confidence Mofunanya2
, Uchenna Confidence Mofunanya2 

 , Promise Chukwuebuka Onuorah2
, Promise Chukwuebuka Onuorah2 

 , Ukamaka Peace Odunwa2
, Ukamaka Peace Odunwa2 

 , Sylvia Chinaecherem Ndinojuo2
, Sylvia Chinaecherem Ndinojuo2 

 , Damian Nnabuihe Ezejindu2
, Damian Nnabuihe Ezejindu2 
 , Roseline Ebube Udodi2
, Roseline Ebube Udodi2 




 , Kelechi Blessed Ndubuisi2
, Kelechi Blessed Ndubuisi2 

 , Uchenna Confidence Mofunanya2
, Uchenna Confidence Mofunanya2 

 , Promise Chukwuebuka Onuorah2
, Promise Chukwuebuka Onuorah2 

 , Ukamaka Peace Odunwa2
, Ukamaka Peace Odunwa2 

 , Sylvia Chinaecherem Ndinojuo2
, Sylvia Chinaecherem Ndinojuo2 

 , Damian Nnabuihe Ezejindu2
, Damian Nnabuihe Ezejindu2 
 , Roseline Ebube Udodi2
, Roseline Ebube Udodi2 


1- Department of Anatomy, Faculty of Basic Medical Sciences, Nnamdi Azikiwe University, Awka, Nigeria. , ps.udodi@unizik.edu.ng
2- Department of Anatomy, Faculty of Basic Medical Sciences, Nnamdi Azikiwe University, Awka, Nigeria.
2- Department of Anatomy, Faculty of Basic Medical Sciences, Nnamdi Azikiwe University, Awka, Nigeria.
Full-Text [PDF 9287 kb]
(648 Downloads)
| Abstract (HTML) (1294 Views)
Full-Text: (733 Views)
Introduction
Methamphetamine exposure levels may rise as a result of the emergence of new methamphetamine consumption techniques that are more accessible in our local surroundings [1]. Methamphetamine can be injected intramuscularly or intravenously. It can be absorbed by insufflation, smoking, or ingestion. The effects of drug use are particularly sensitive and noticeable during adolescence and chronic methamphetamine use is more prevalent and begins earlier in life among teenagers [2, 3]. Teenagers mainly misuse synthetic psychostimulants for recreational purposes, as they are extremely addictive. Actually, with over 35 million users worldwide, methamphetamine is the second most popular illegal substance in the world [4, 5]. According to estimates from the United Nations Office on Drug and Crime (UNODC) [6], methamphetamine use is on the rise in Nigeria, with a 32% increase in cases of methamphetamine use and a 55% increase in cases of methamphetamine use disorder [6]. There seems to be a noticeable increase in methamphetamine usage in the southeast of Nigeria, which could account for the majority of methamphetamine use disorders [7].
The transitional phase between childhood and adulthood, known as adolescence, is crucial for brain development and growth. In humans, it is believed to last between the ages of 12 and 18 and in rats, between postnatal (PND) days 28 and 42 [8]. Teenagers are susceptible to the harmful effects of illegal drugs. Studies on animals have shown that exposure to illegal drugs throughout adolescence changes the brain’s development in ways that last for years. Adolescent learning, memory, attention, behavioral activities, and possible addiction could all be affected as a consequence [9, 10].
Methamphetamine use among teenagers is common, and the detrimental effects it has on memory and learning (cognition) in our community are well documented. However, substances that may lessen these effects have not been studied. Some publications claim that if herbs and other sources of nutrition preserve the neural system, diseases associated with cognitive deficits may be preventable [11, 12]. Given the abundance of herbs and herbal products found in Africa, we are optimistic about preventing mental illness in the future. Silybum marianum (L.) Gaertner, an annual or biennial plant in the Asteraceae family, is the source of silymarin. It is also known by several names, including milk thistle, blessed milk thistle, Marian thistle, Mary thistle and Saint Mary’s thistle. The plant is now common worldwide, previously found only in southern Europe and Asia [13]. This herb is a challenging weed being cultivated for its medicinal qualities and is one of the most essential medical crops in the world. Since its earliest usage as a medication more than 2000 years ago, milk thistle has been chiefly used to treat liver conditions such as cirrhosis and hepatitis and protect the liver from toxins [14]. The extracts from this plant have also been found to have a wide range of pharmacological characteristics, such as anti-inflammatory and antifibrotic effects [15]. The major element behind milk thistle’s pharmacological effects is the presence of a class of compounds called flavonolignans, sometimes called silymarin.
Treatment options for methamphetamine use disorder currently consist of behavioral and synthetic pharmaceutical approaches, both of which are usually ineffective. Research toward developing medications to treat methamphetamine use disorder has been prioritized by the National Institute on Drug Abuse (NIDA) [16]. The inherent limitations and inconsistent efficacy of prior synthetic agents have contributed to the need for the development of novel protective agents with efficient modes of action, particularly those derived from medicinal plants (due to their alleged reduced side effects compared to synthetic drugs). Although silymarin has been proven to have protective effects against oxidative stress and neurotoxicity, less attention has been paid to its neurobehavioral recovery effects on methamphetamine-induced deficits in adolescents experiencing a developmental period. This study thus fills that gap by investigating behavioral and molecular changes in adolescent Wistar rats.
Therefore, the current study hopes to understand the various neurobehavioral, oxidative stress and histopathological effects that silymarin has on the neurotoxicity of methamphetamine in adolescent rats. This study aims to establish how the findings from silymarin effectiveness on antioxidative and neuroprotective properties in methamphetamine-exposed adolescent rats improve the functional and structural aspects in these animals by combining behavioral results with molecular and histological analyses.
Materials and Methods
Study materials
The dried seeds of S. marianum (L.) Gaertner were supplied by Biokoma Dried Herbs (Jumia Store, Sangotedo, Lagos State, Nigeria) in the summer of 2022. These seeds were verified at the Biological Science Department of Nnamdi Azikiwe University using the authentication code AU106. A sizable representative sample of the plant material (500 g) was crushed and sieved to obtain a particle size of 0.4 mm. Wianowska and Wis’niewski described an effective three-stage extraction process using accurately weighted material pieces [17].
Methamphetamine was obtained under government approval and stored under government-required conditions, and usage was logged as prescribed by national legislation. The substance was stored in a dry and cool environment.
Experimental animals
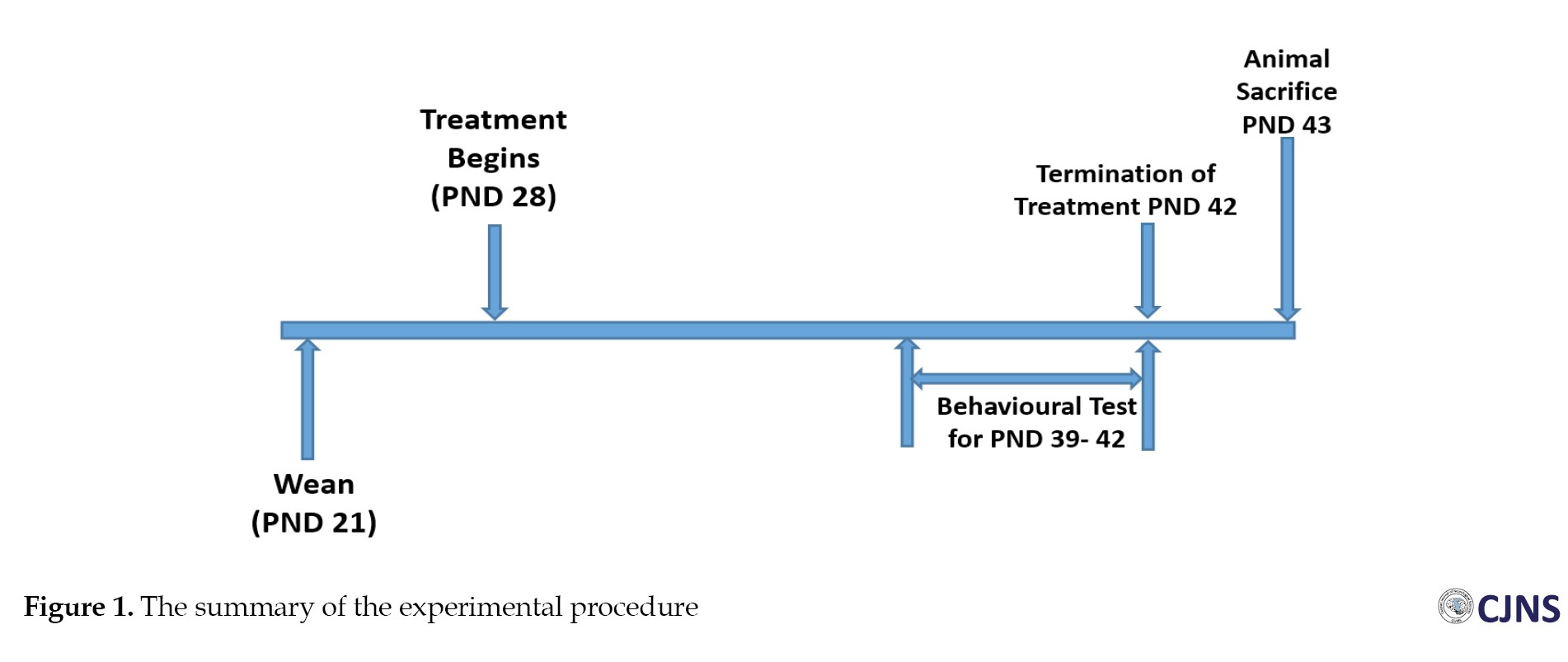
Male and female Wistar rats aged 70 to 80 days, weighing between 159 to 205 g, were raised in our colony in breeding cages with 10 male and 20 female rats each; female rats were single-housed at the first sign of pregnancy with a vaginal plug. The young rats were weaned on PND day 21 and kept in a different cage from their parents. The experiment was conducted from PND 28 to 42 using only 40 male offspring. Routine experiments were performed with the animals in groups of four per Perspex cage in a designated pathogen-free environment; wire gauze was necessary for cross-ventilation. The animals were kept in a controlled environment under a 12-hour day/night photoperiod, with a temperature range of 25–28 °C and relative humidity of 60–80%, at the anatomy lab in Nnamdi Azikiwe University’s Nnewi Campus’ College of Health Sciences. The animals regularly received water and guinea feed pellets (from Agro Feed Mill Nigeria Ltd.). The Faculty of Basic Medical Sciences, College of Health Sciences, Nnamdi Azikiwe University Nnewi Campus approved the treatment of all the animals, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals [18].
Acute toxicity test (LD50) of methamphetamine and silymarin
Using the methodology outlined in the organization for economic co-operation and development’s guideline for testing chemicals [19], the acute test was conducted for silymarin and methamphetamine. The lethal dose of 50% (LD50) for silymarin was 500 mg/kg and 10 mg/kg for methamphetamine.
Experimental design
A total of 40 young Wistar rats were used in this study. Four groups with 10 male animals each were formed. The groups were given the designations A (control [CTRL]), B (methamphetamine [METH]), C (silymarin [SILY]), and D (METH+SILY). The animals in each of the following groups received the following treatments: A (vehicle), B (methamphetamine), C (silymarin), and D (methamphetamine and silymarin) from PND 28 to PND 42. The treatment had a pH of 9.2 and was administered at room temperature. The oral dosages for silymarin and methamphetamine were 385 mg/kg and 5 mg/kg, respectively. Sesame oil was used to dilute the methamphetamine and silymarin and it also served as the vehicle for the control group. The oral administration was done using a flexible 16-gauge feeding plastic tubing oral bulb-tipped gavage needle of about 2 inches in length.
Animal sacrifice or euthanasia
Through intramuscular injection, ketamine (55 mg/kg) was used to put the animals to sleep. The animals were anesthetized 24 hours after the last day of treatment, then decapitated, and had an occipitofrontal incision made to remove the brain. They were also cardiac-perfused with heparinized phosphate-buffered saline (PBS). The brain tissues of four animals in each group were immediately prepared for biochemical analysis through homogenization to identify oxidative markers of interest. In contrast, six brain tissues in each group were fixed for 48 hours in 10% neutral buffered formalin to section the three fixed brain tissues for hematoxylin and eosin (H&E) and bielschowsky silver (BS) stain. Three other fixed brain tissues in each group were embedded in cryoprotectants for immunohistochemical investigations.
Behavioral function test
Before sacrifice, the behavioral test started at PND 39 and ended at PND 42 (later in adolescence). Forty Wistar rats in all (10 per treatment group) were subjected to behavioral tests in the following order: PND 40 was used for the elevated plus maze test (EPM), PND 41 was used for the tail suspension test, and PND 42 and 43 were used for the Morris water maze test. On each day of the behavioral test, the tests were administered a few hours before the treatment.
EPM test (anxiety-related behavior)
The EPM test on the PND 39 was used to assess animal anxiety-related behavior. Two oppositely positioned closed arms, two oppositely positioned open arms, and a center region comprise the “+”-shaped maze that makes up the EPM device. The maze is elevated above the ground. The subjects have 5 minutes to navigate the maze freely during this test. A surveillance camera positioned above the labyrinth recorded their motions, and their activities in the video were subsequently examined on a laptop. Time spent in the open or closed arms was used as an index for preference of either arm and used to measure anxiety-like behavior.
Consequently, increased closed-arm preference indicates elevated anxiety. Anxiety was measured directly by using entries and time spent in closed arms [20]. The amount of time spent in open arms and the entries were utilized as direct indicators of anxiety; in other words, less open-arm avoidance corresponds to lower anxiety levels or anti-anxiety behavior [20]. The study used risk-assessment behavior as an inverse measure of anxiety. Specifically, the frequency and duration of head-dips, which include moving the head downward toward the floor while on the open arms, were found to be associated with lower levels of anxiety [21].
Tail suspension test
On the PND 40, the tail suspension test was utilized to assess behaviors associated with depression. Throughout the experiment, the Wistar rats were kept from escaping or clinging to adjacent surfaces by being suspended by their tails from a metal hook that protruded from the chamber ceiling. Immobility is the main result. The rats attempted to escape in almost all cases during the first 2 minutes of the 6-minute test, so the immobility period was measured during the last four minutes. A measure of “depression-like” behavior was determined by keeping scores of each animal’s total immobility time [22]. An animal’s total immobility time is the time it hangs without movement.
Morris water maze test
From PND 41 to PND 42, the animal’s spatial learning and memory were assessed using the Morris water maze. The procedure was followed as directed by Joca et al. [23]. The apparatus comprises a large circular pool, or tank, about 6 feet in diameter and 3 feet deep. The interior of the tank was painted white, while its exterior was painted brown. It was filled with about 25 °C tap water under bright lighting. The maze was separated into 4 virtual quadrants, with a platform measuring 24 cm in height and 10 cm in circumference in one of the pool’s quadrants. During training, the platform was one inch above the water.
The Wistar rat learned and used this platform as the most effective way to get out of the water. The experimenter led the animals who had difficulty finding the platform during training, and they were given 3 s to remain there. Before the test session, the platform was submerged in the quadrant during the training session because the animals needed to be retrained to locate the submerged platform hidden in one of the four quadrants. The test animal was placed in a different quadrant for each trial, while the platform was kept in the same quadrant. Each test session had three trials lasting 60 s each, with an average inter-trial delay of 10 s. The animal was trained and ready for the test, and the escape platform was placed an inch below the water’s surface. Non-toxic white tempera paint was added to make the water opaque. One day was all needed to finish the training test session for opaque and transparent materials. If the animal had not clambered onto the platform after 60 s of the trial’s termination, it was removed from the water and placed on it. Before the subsequent trial began, the animal remained on the platform for 20 min during the intertrial. When all three tests were completed, the animal was removed and placed under a lamp to warm. The interval between each session was 1 hour. The escape latency was manually recorded using a stopwatch.
Analysis of oxidative markers
For the evaluation of antioxidants like glutathione (GSH) and superoxide dismutase (SOD), as well as lipid peroxidative indicators like malondialdehyde (MDA), the Biochemistry Department at Nnamdi Azikiwe University, Nnewi Campus, was engaged. One gram of each animal tissue was mixed with 10 mL of 0.9% normal saline and homogenized at room temperature. After that, each sample was centrifuged for 20 minutes at 3000 rpm at room temperature. The supernatants were separated using a spectrophotometer, and the levels of MDA, GSH and SOD were measured in accordance with the instructions provided by Ahmed Amar et al. [24].
Tissue processing H&E and BS stain
After being prepared according to Feldman and Wolfe [25], the tissues were sectioned and stained with H&E and BS stain, and three of the tissues were examined. After the fixation, excess fixatives were removed by rinsing them under tap water for an entire night. The fixed tissues underwent dehydration to eliminate water and other substances. After dehydration, tissues were cleared with xylene for two hours. This action was followed by two variations of two hours of infiltration at 60 °C molten paraffin wax. The paraffin wax was sectioned when it had cooled [25].
Hematoxylin and eosin staining methods
The H&E staining approach was used to identify necrotic entities with an eosinophilic appearance and histomorphological changes [26]. Coronal sections containing the appropriate brain tissue were mounted on slides, dried overnight, rehydrated and stained with H&E in accordance with the guidelines provided by Granado et al. [27, 28]. Photomicrographs were taken during the microscopy process using an eyepiece camera attached to an Olympus microscope (Tokyo, Japan).
Bielschowsky sliver staining method
BS stain is a useful technique that we utilized to demonstrate nerve fibers. Visible staining was observed in the dendrites, axons, neurofibrils and senile plaques of the central nervous system. This approach is widely used in the study of Alzheimer disease (AD). Coronal sections containing the appropriate brain tissue were put on slides, dried overnight, rehydrated, and stained by BS stain BS [29].
Stereological quantification of neurons in the brain
H&E-stained neurons in the amygdala and hippocampus were counted unilaterally in every fourth segment of the tissues for each group (n¼4-6). The degeneration of neurons in the selected brain tissues was assessed by counting H&E neurons in each experimental group (n¼4-6) [30]. An experienced observer unaware of the treatment circumstances employed the optical fractionator, Stereo Investigator program (MicroBrightField Bioscience, Colchester, VT), as described in some studies [31, 32]. The brain tissues’ shapes were illustrated at low power (×2) in accordance with recognized anatomic landmarks. At the same time, the number of cells was counted at higher power (×20 for the hippocampus and ×100 for the amygdala). H&E-labeled cell bodies could easily be differentiated from terminal degeneration in the hippocampus and amygdala at these magnifications. To avoid double counting, neurons were only counted in the focal plane, where their nuclei were most visible. The count includes several neurons with extremely low H&E signals.
Myelin basic protein (MBP) and oligodendrocyte transcription factor (OLIG2) expressions
The three fixed tissues were used for immunohistochemistry staining after being embedded in a cryoprotectant. SuperFrost microscope slides obtained serial coronal sections (20 μm). OLIG2 and MBP immunohistochemistry were performed on free-floating sections using standard avidin-biotin immunocytochemical protocols [33, 34]. The slides were cleaned three times for five minutes each in PBS and then blocked. Following application to the slides, the primary antibodies (OLIG2 and MBP) were incubated at a concentration of 1:1000 for a whole night. The following day, slides were incubated with a secondary antibody (goat anti-mouse) at a concentration of 1:250 for two hours after being washed in PBS three times for five minutes each. The VECTASHIELD mounting media was used to cover the slides.
After that, pictures were taken at a magnification of ×20 using an Olympus Ix-81 microscope and the integrated optical density was computed using SlideBook software, version 6.0. After obtaining all photomicrographs, the average intensity of MBP and OLIG2 immunoreactivity in the cornu ammonis 3 (CA3) and basolateral amygdala (BLA) was evaluated [35].
Quantitative assessment of immunoreactive expressions
An optical microscope equipped with a Leica DFC290 HD video camera was used to capture images of the tissue sections obtained with an ×4 lens and the expression levels of OLIG2, MBP and BS staining in the brain were measured. Using an image analysis system (analytical imaging station), the staining area in the brain tissue was calculated as a proportion of the tissue’s pixels that displayed staining (stained area) relative to all pixels in the brain tissue of interest (scanned area). This is called a proportional stained area [36, 37].
Data analysis
The data were analyzed using SPSS software, version 27.0.1. We could determine the Mean±SE. Values within a group and between groups were compared using an independent samples t-test and a one-way analysis of variance (ANOVA), respectively. The post hoc analysis of Turkey was utilized to determine differences in the experimental parameters between the groups. The data were presented as Mean±SEM and were considered statistically significant when P<0.05, P<0.01 and P<0.001 were obtained.
Results
Neurobehavioral test
The tests included EPM, tail suspension and Morris water maze tests. The duration spent in the closed and open arms is displayed in Table 1.
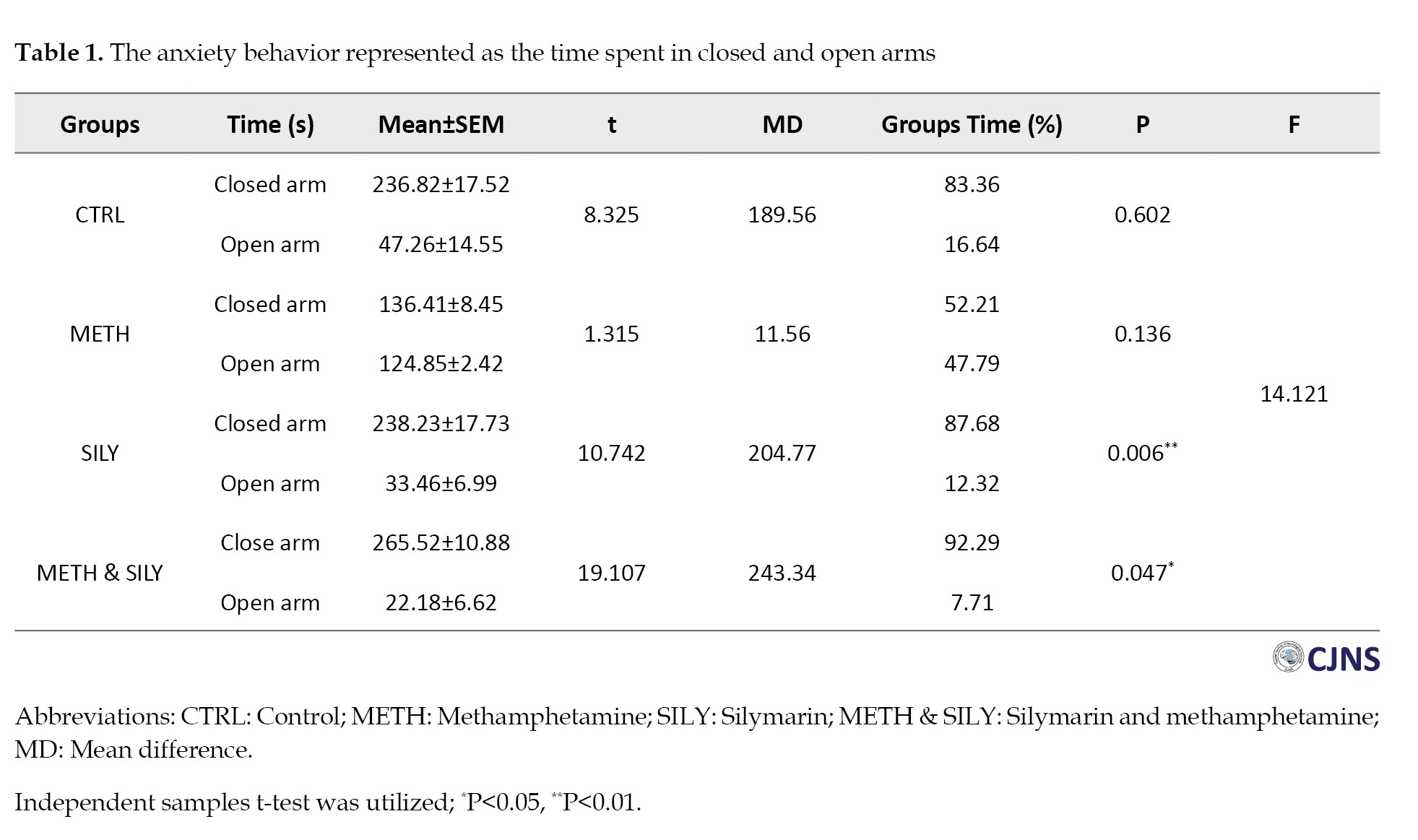
It is used as an index for measuring the animal groups’ anxiety levels during the EPM test. The independent samples t-test was used to analyze the data and the results were considered significant at P<0.05 and P<0.01, represented as * and **, respectively. The mean of the closed and open arms in SILY and METH & SILY groups varied significantly, according to the independent samples t-test. Furthermore, Table 1 shows no significant difference between the CTRL and METH groups’ closed and open arm means.
The risk assessment for each animal group is shown in Table 2 above.
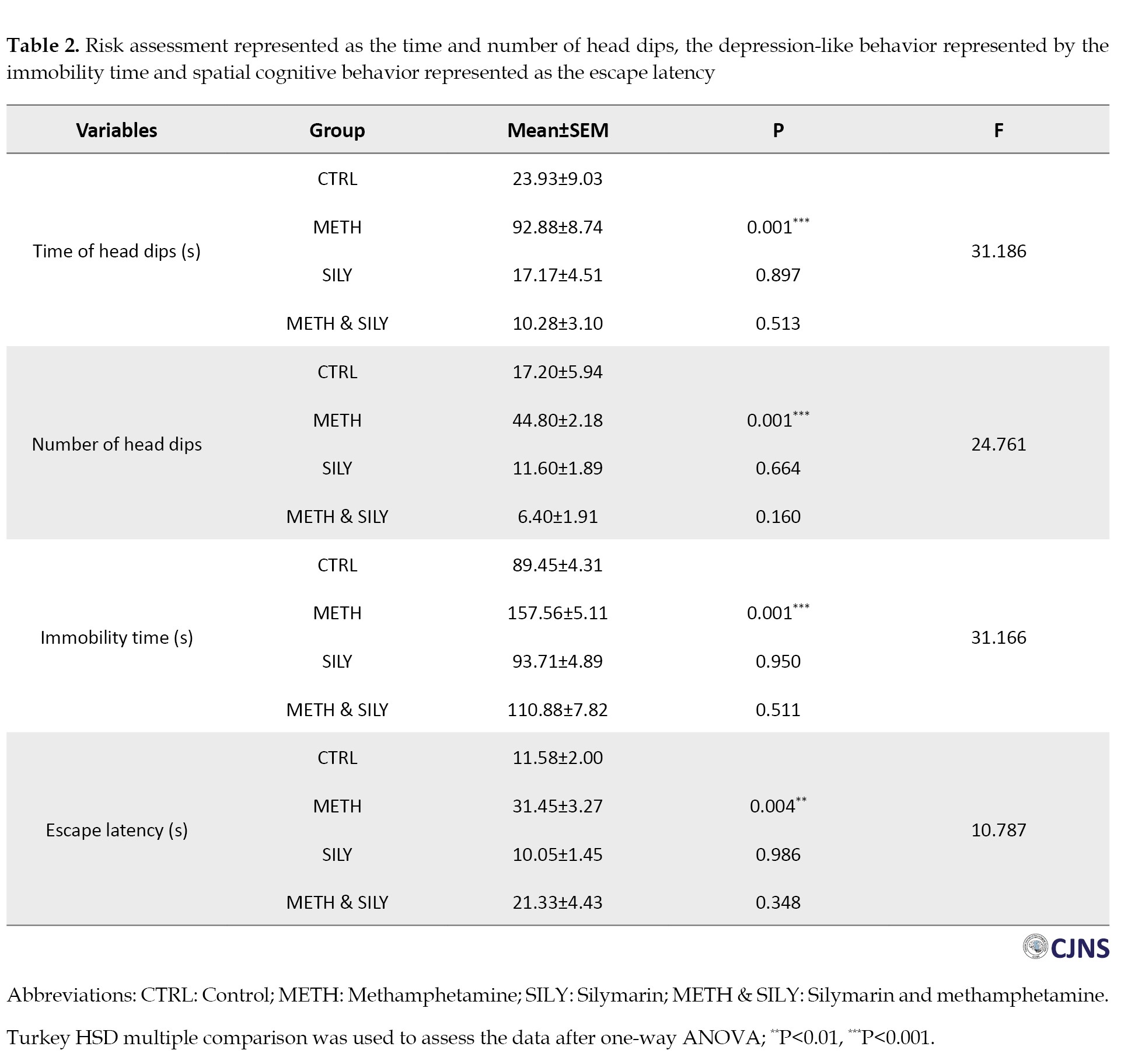
In comparison to the CTRL group, the METH group shows a significant increase in both the duration and frequency of head dips. In contrast, the SILY and METH & SILY groups show nonsignificant differences in the duration and frequency of head dips done by the animals throughout a five-minute test.
The immobility time for each animal group is also shown above. A one-way ANOVA and a Turkey HSD multiple comparisons were used to analyze the data, and the results were considered significant at P<0.001, which is represented as ***. When compared to the CTRL group, the immobility times of the SILY and METH & SILY groups show nonsignificant differences. Still, the immobility time of the METH group animals shows a significant increase.
The observed reduction in anxiety-like behaviors and improvement in spatial learning in the SILY and METH & SILY groups directly support the hypothesis that silymarin mitigates methamphetamine-induced neurobehavioral deficits, aligning with its proposed neuroprotective role.
Table 2 presents the time the animals take to locate the hidden platform. The difference in the time it took the animals in the SILY and METH & SILY groups to locate the hidden platform was not statistically significant when compared to the CTRL group. Still, there was a statistically significant increase in the time the animals took in the METH group to locate the hidden platform. The observed reduction in anxiety-like behaviors and improvement in spatial learning in the SILY and METH & SILY groups directly support the hypothesis that silymarin mitigates methamphetamine-induced neurobehavioral deficits, aligning with its proposed neuroprotective role.
Biomarkers of oxidative stress
Data were analyzed using one-way ANOVA followed by Turkey HSD multiple comparisons, and data were considered significant at P<0.01, represented as **.
Table 3 presents the data for some oxidative stress markers.
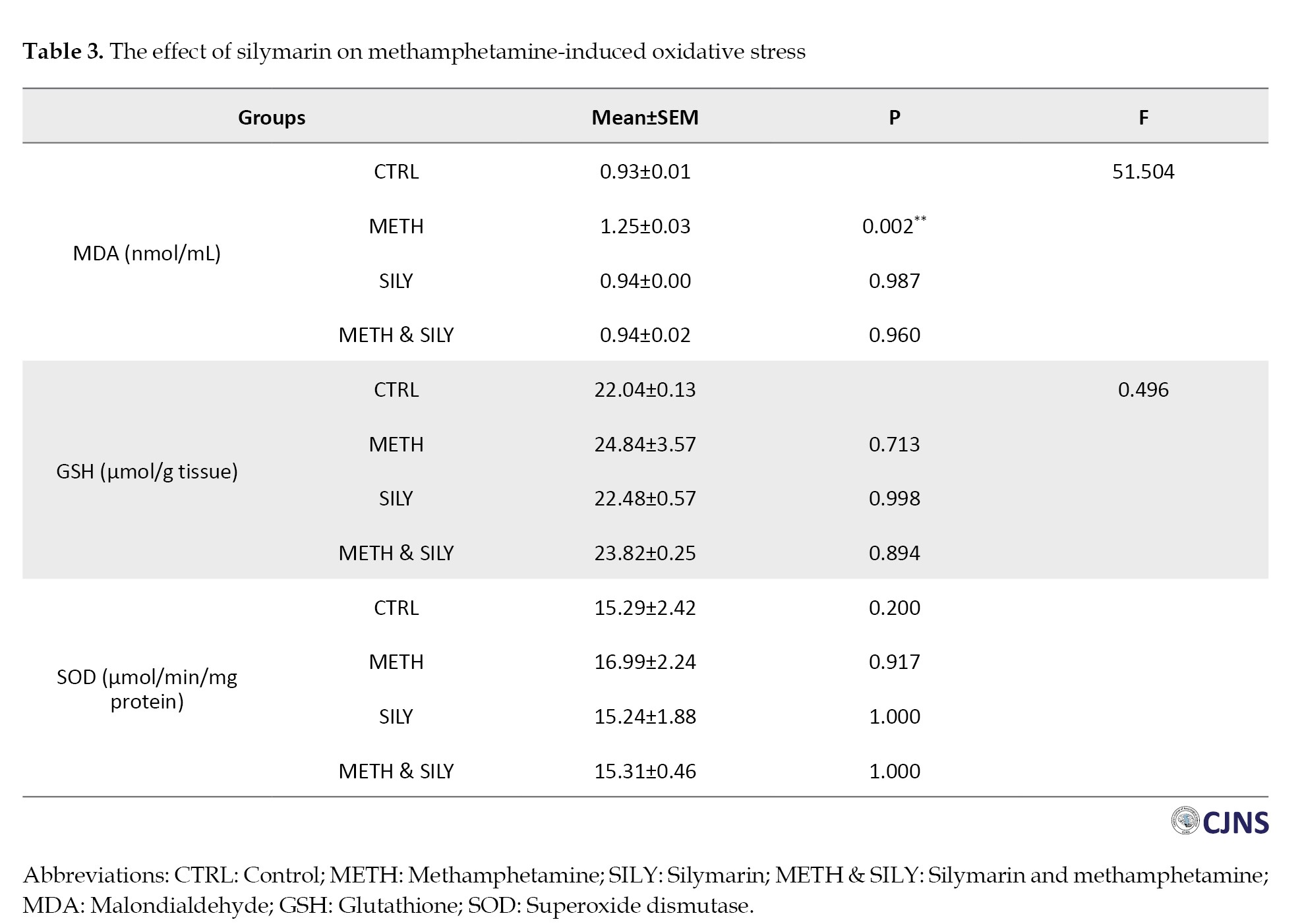
The results were evaluated after a one-way ANOVA and a Turkey HSD multiple comparison. The data were considered significant at P<0.01, represented as **. In contrast to the CTRL group, the MDA shows a significant increase in the METH group, but other group differences were not statistically significant. Comparing the experimental groups METH, SILY and METH & SILY to the control group, the differences in GSH and SOD representation were not statistically significant.
The significant reduction in MDA levels and stabilization of SOD and GSH levels in the METH & SILY group indicates that silymarin effectively counters methamphetamine-induced oxidative damage, a key mechanism underlying the observed behavioral and histological improvements.
Histological expressions
H&E stain and BS stain for neurofibrillary tangle and senile plaque
Table 4 presents the data for different H&E-sensitive cells.
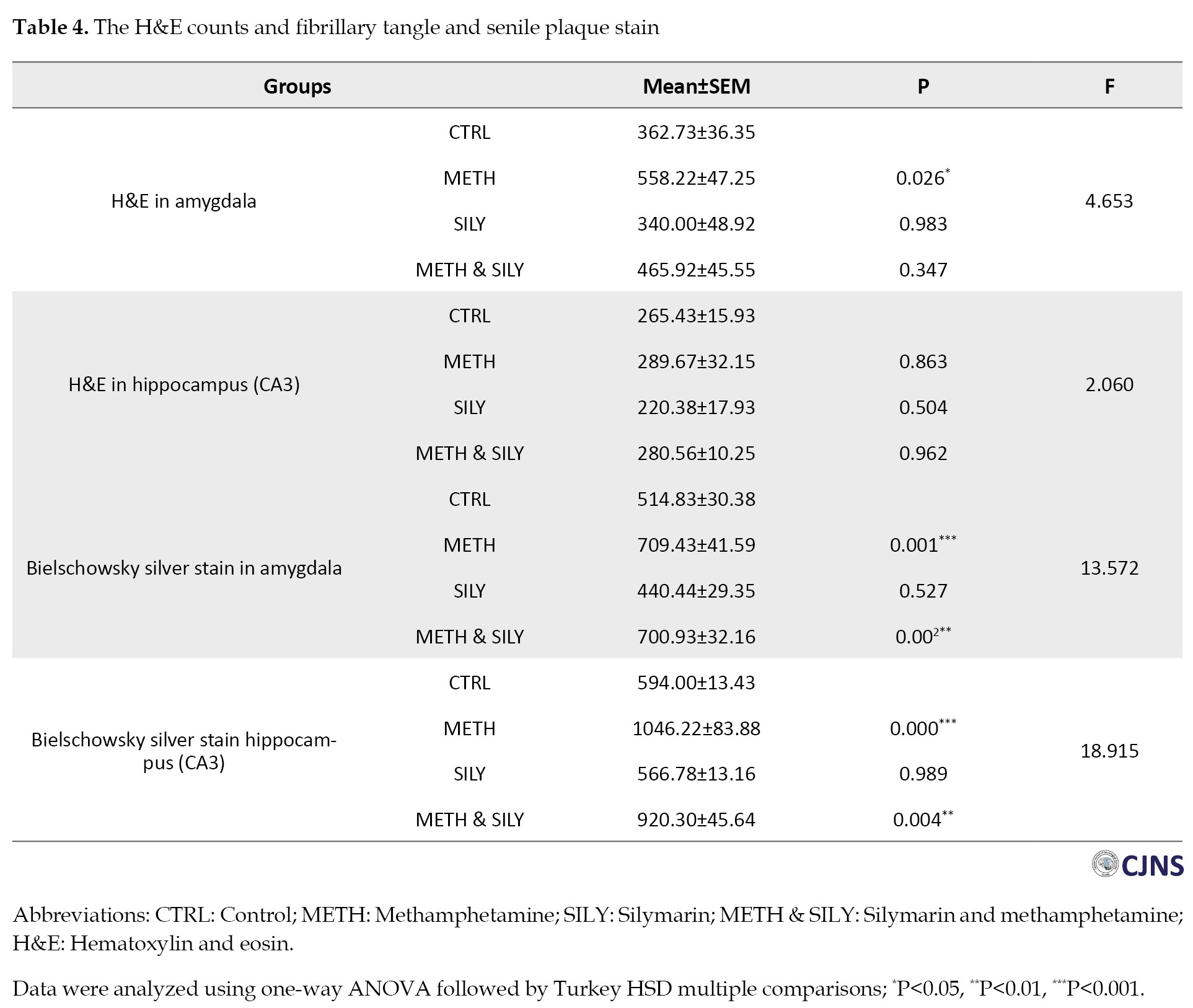
A one-way ANOVA was used to evaluate the data, and then a Turkey HSD multiple comparison was conducted. The METH group shows a significant increase in amygdala H&E-sensitive cells, while the remaining amygdala groups showed nonsignificant changes compared to the CTRL group. Comparing the experimental groups METH, SILY, and METH & SILY to the CTRL group, the differences in the hippocampal representations were not statistically significant.
The data for different structures that are sensitive to BS stain are shown in Table 4. The data were analyzed using a one-way ANOVA and a Turkey HSD multiple comparison. The data were considered significant at P<0.01, P<0.001, represented as ** and ***, respectively. The METH and METH & SILY groups showed a significant increase in the BS-sensitive structures of the amygdala and hippocampus. In contrast, the SILY group difference in these tissues was not statistically significant compared to the CTRL group.
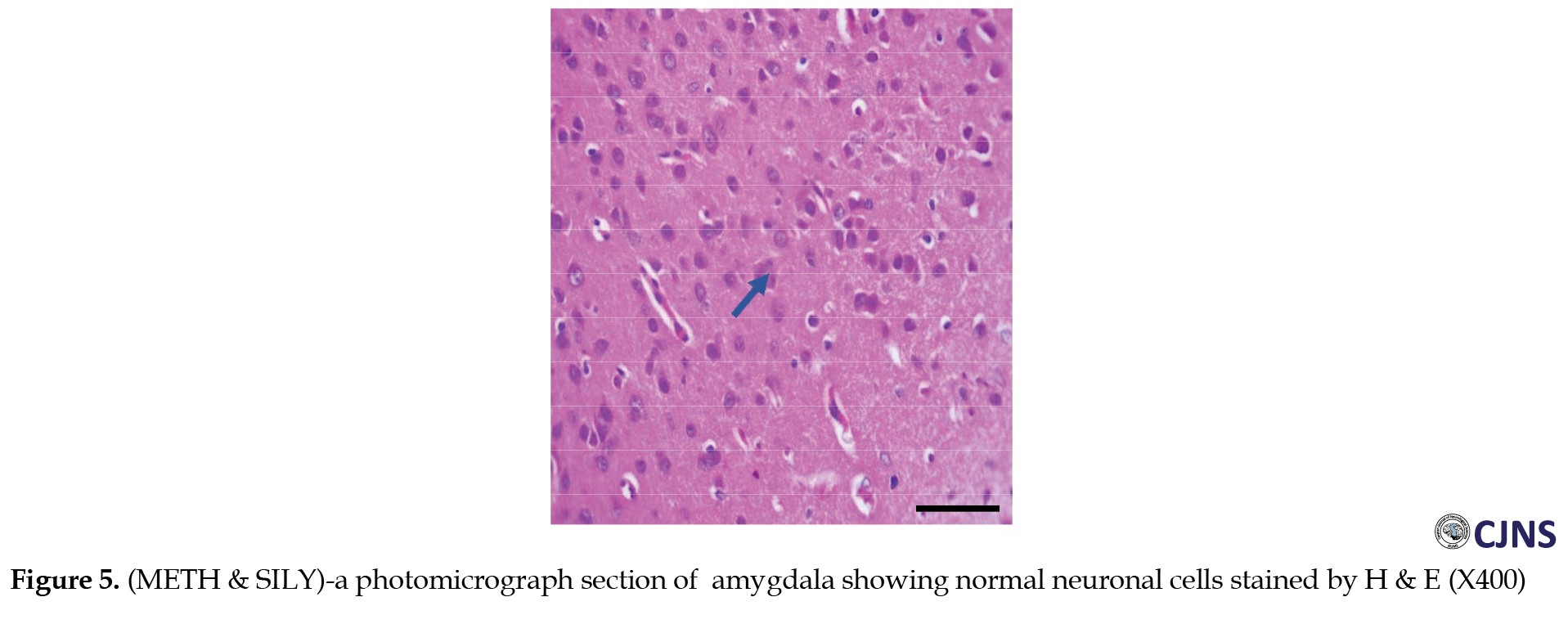
Amygdala H&E stain
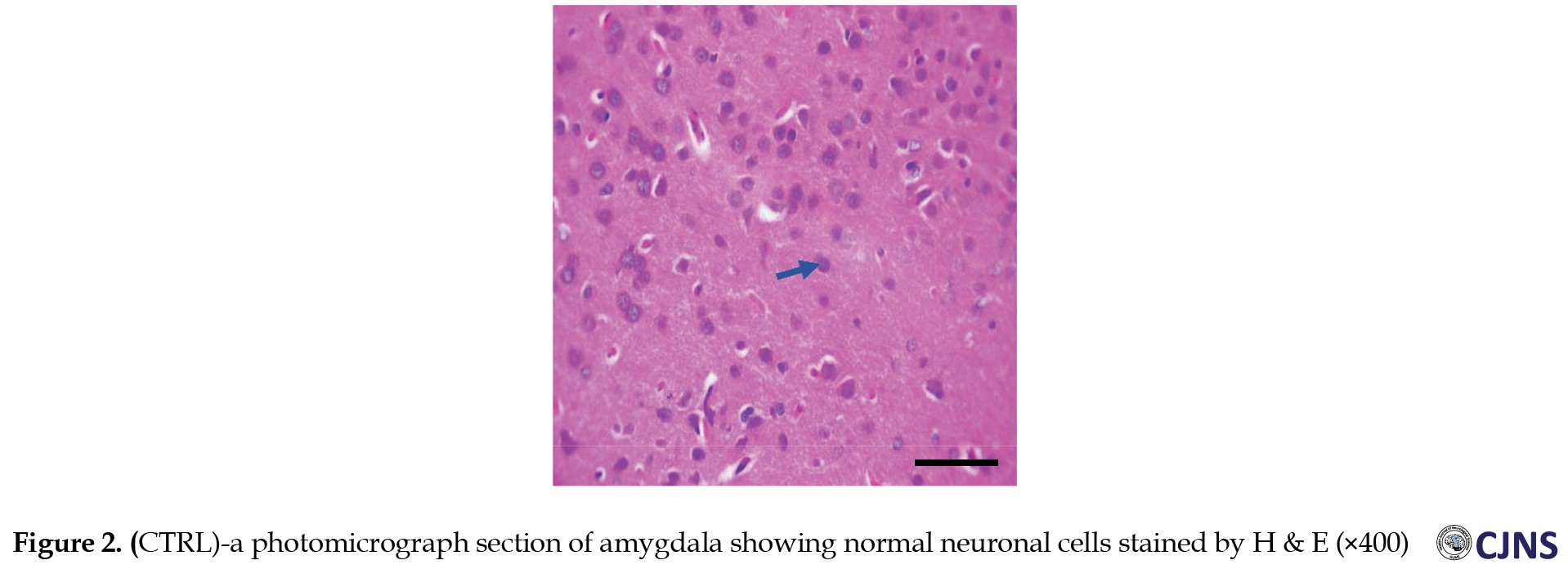
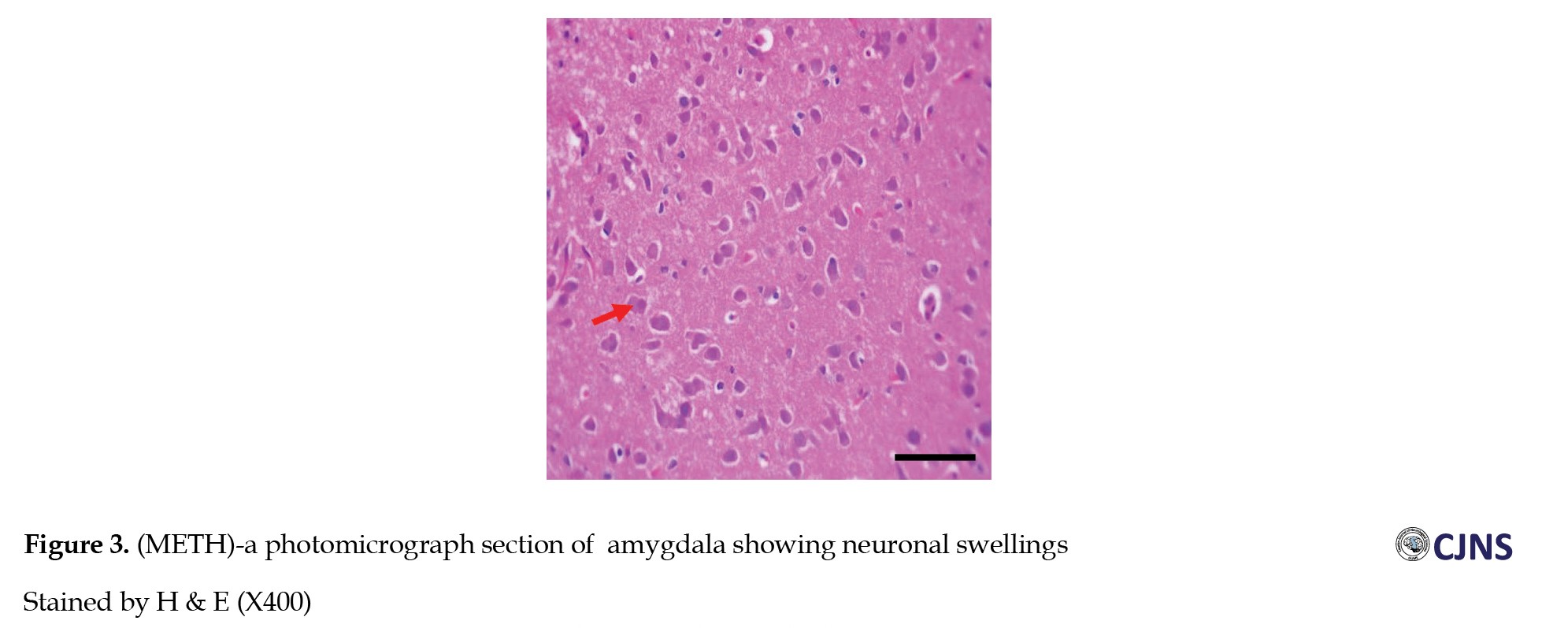
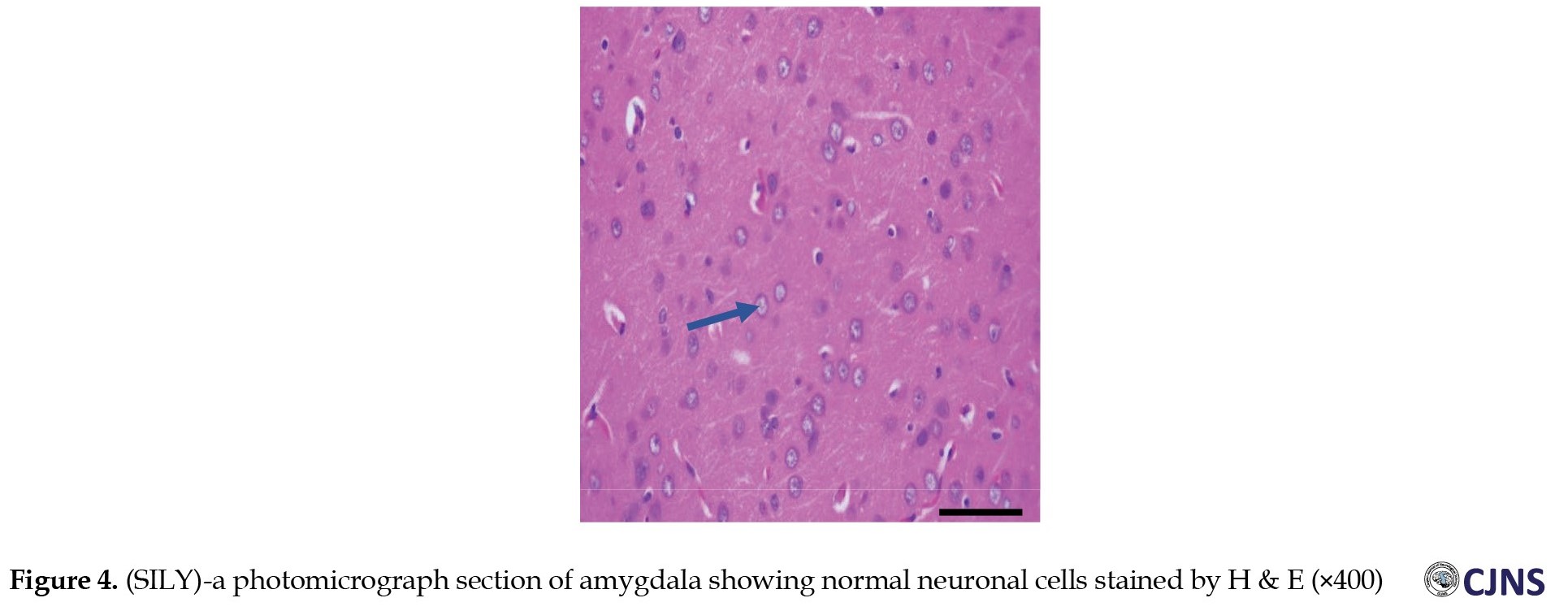
The H&E-sensitive amygdala cells in all the groups of Wistar rats treated are shown in the photomicrograph section (Figures 1, 2, 3, 4 and 5). The METH group shows micrographs of swollen cells, whereas CTRL, SILY and METH & SILY groups show micrographs of normal cell architecture (×400 scale bar, 50 µm).
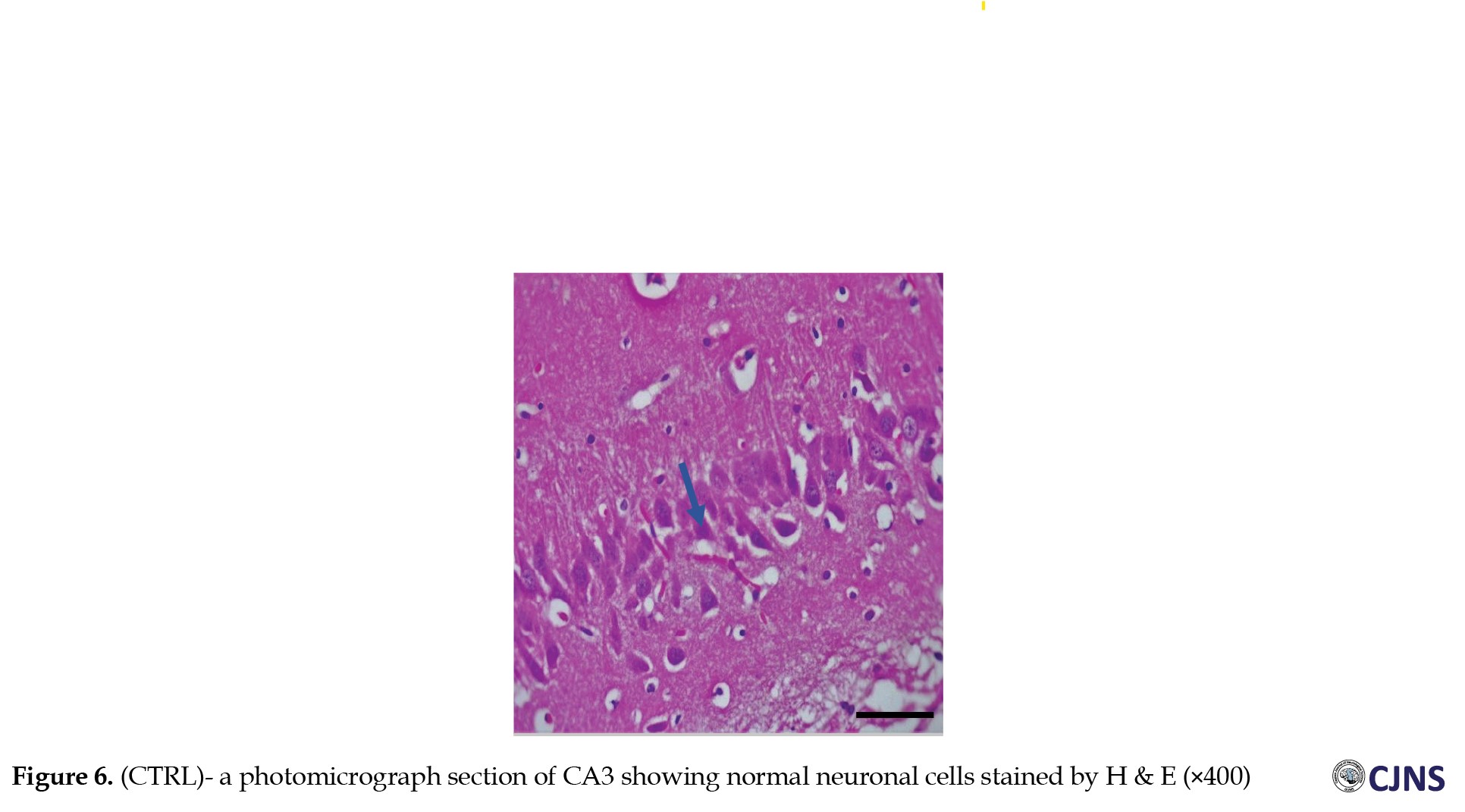
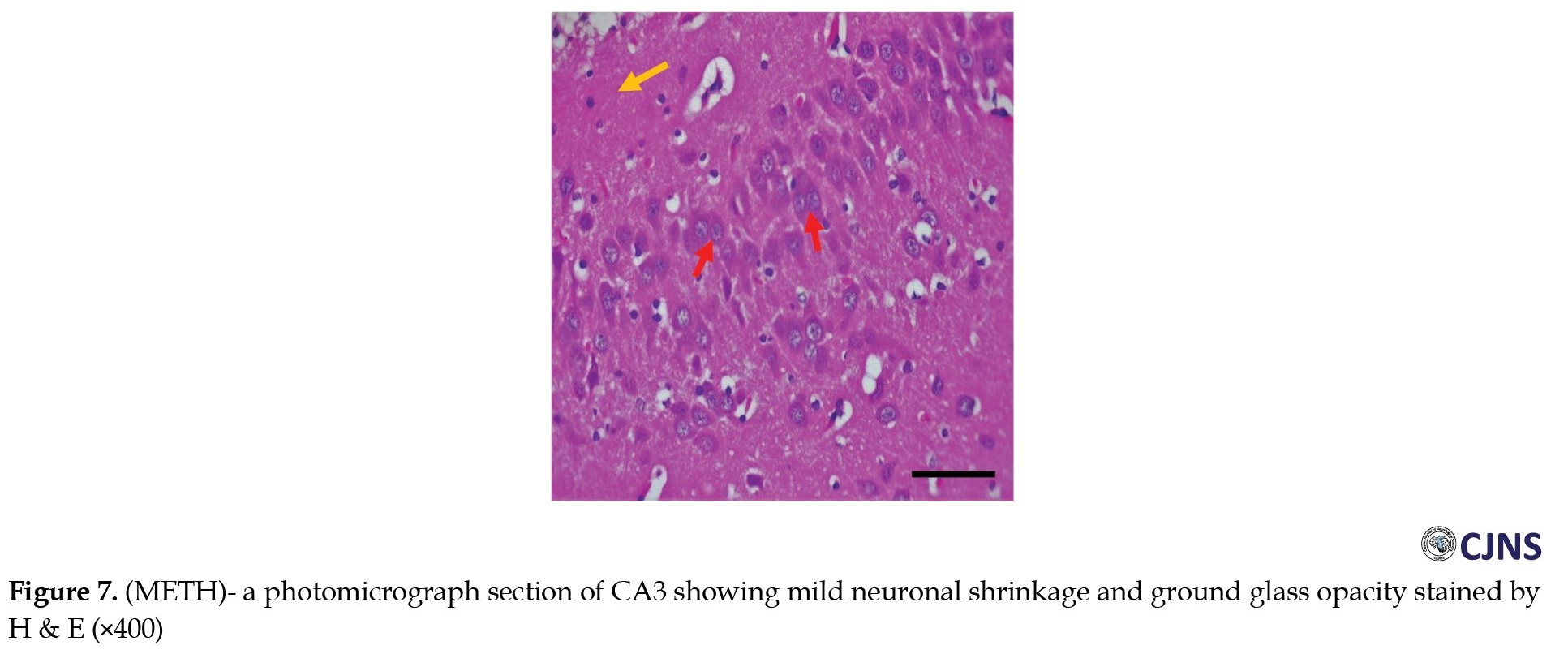
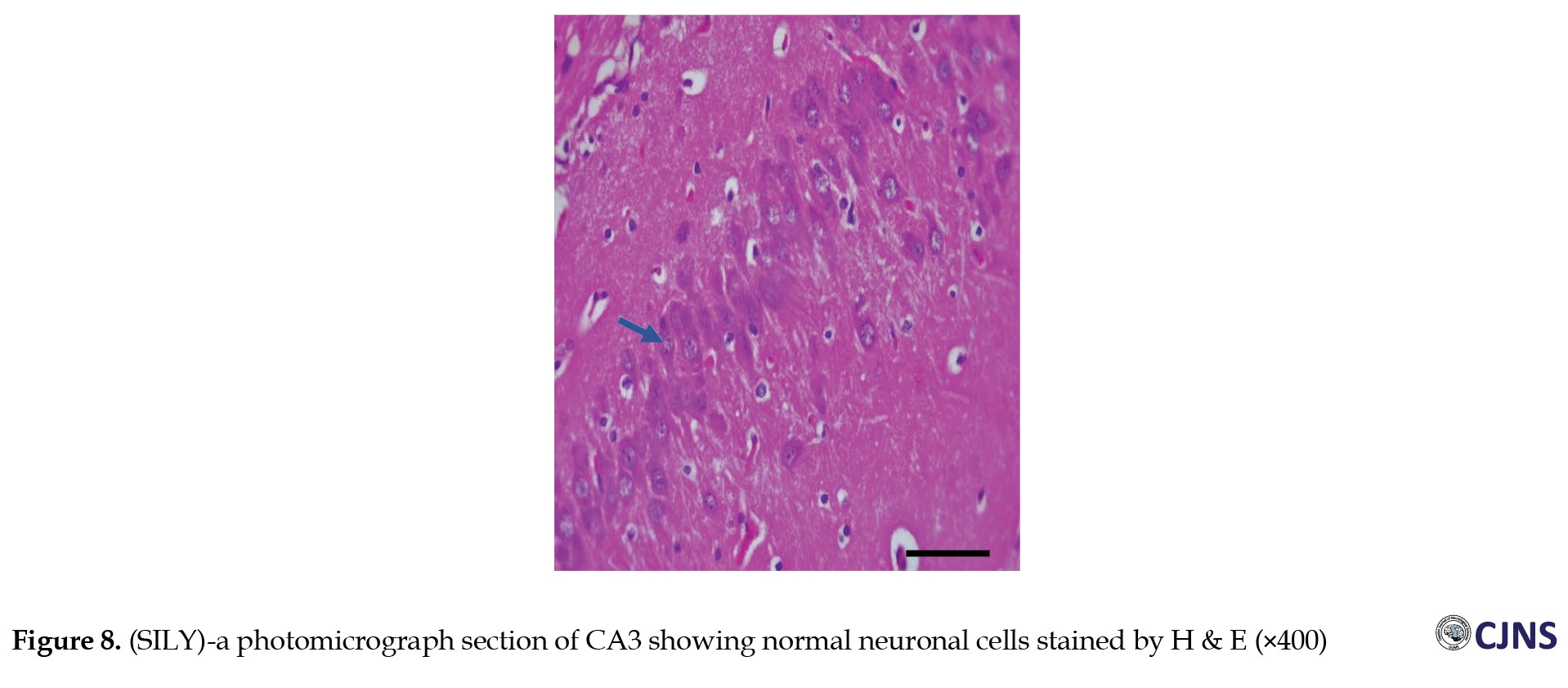
CA3 H&E stain
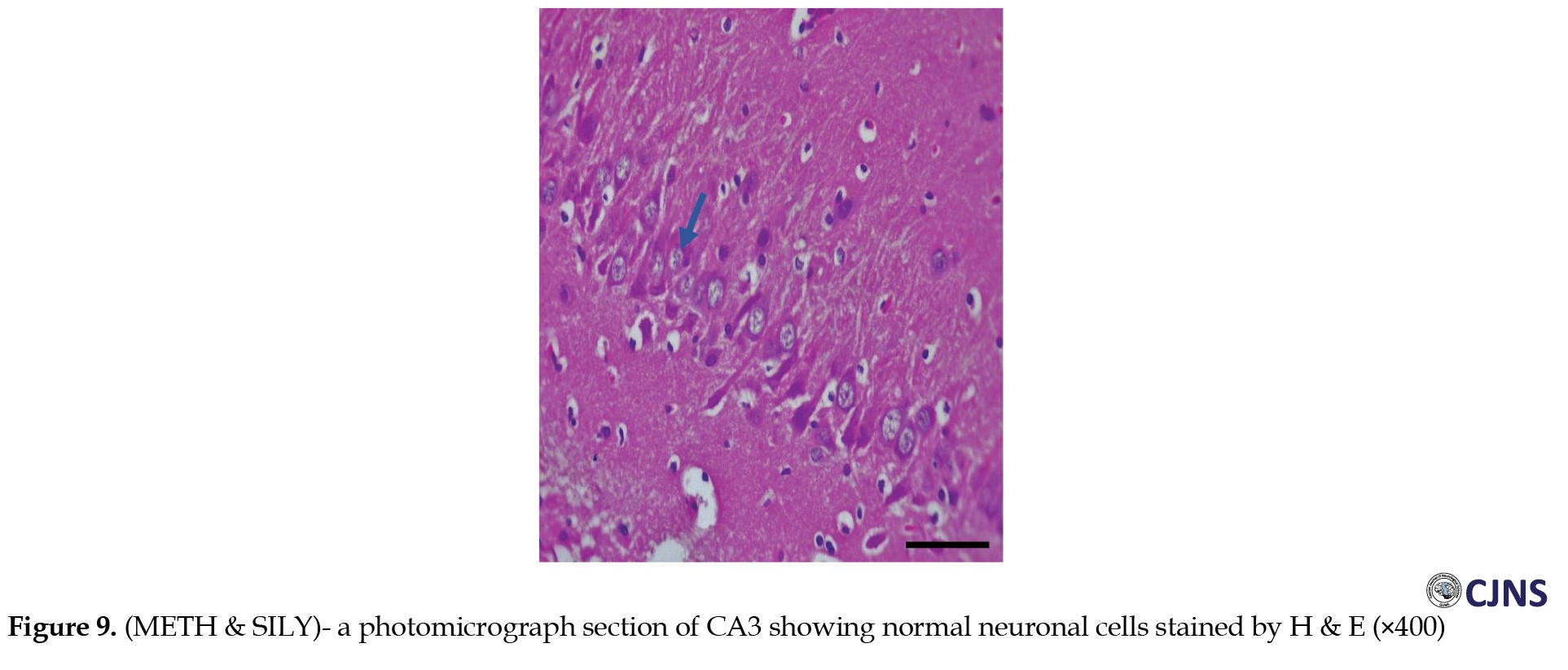
Figures 6, 7, 8 and 9 show the tissue and cellular architecture of the hippocampal CA3 region in all treated groups. While the METH group demonstrates mild neuronal shrinkage and ground glass opacity, CTRL, SILY, and METH & SILY groups show normal H&E-sensitive hippocampus cells and tissue in their micrographs (×400 scale bar, 50 µm).
Amygdala BS stain
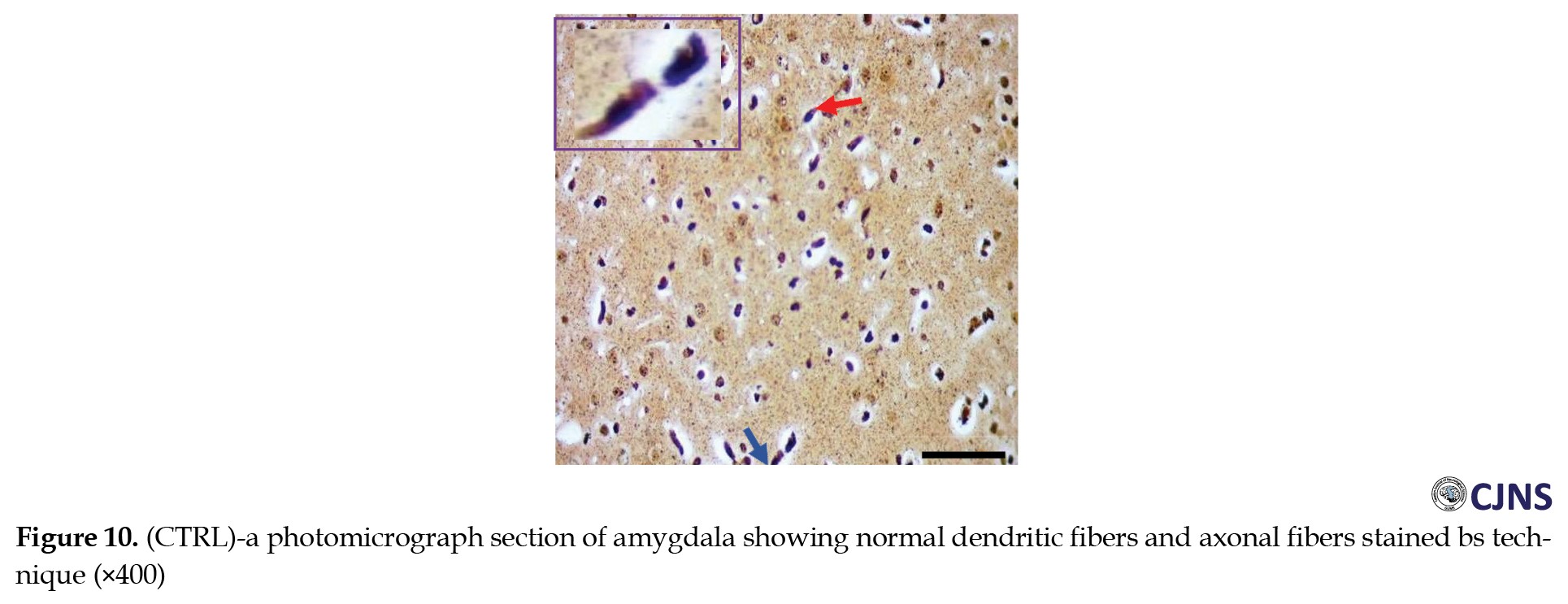
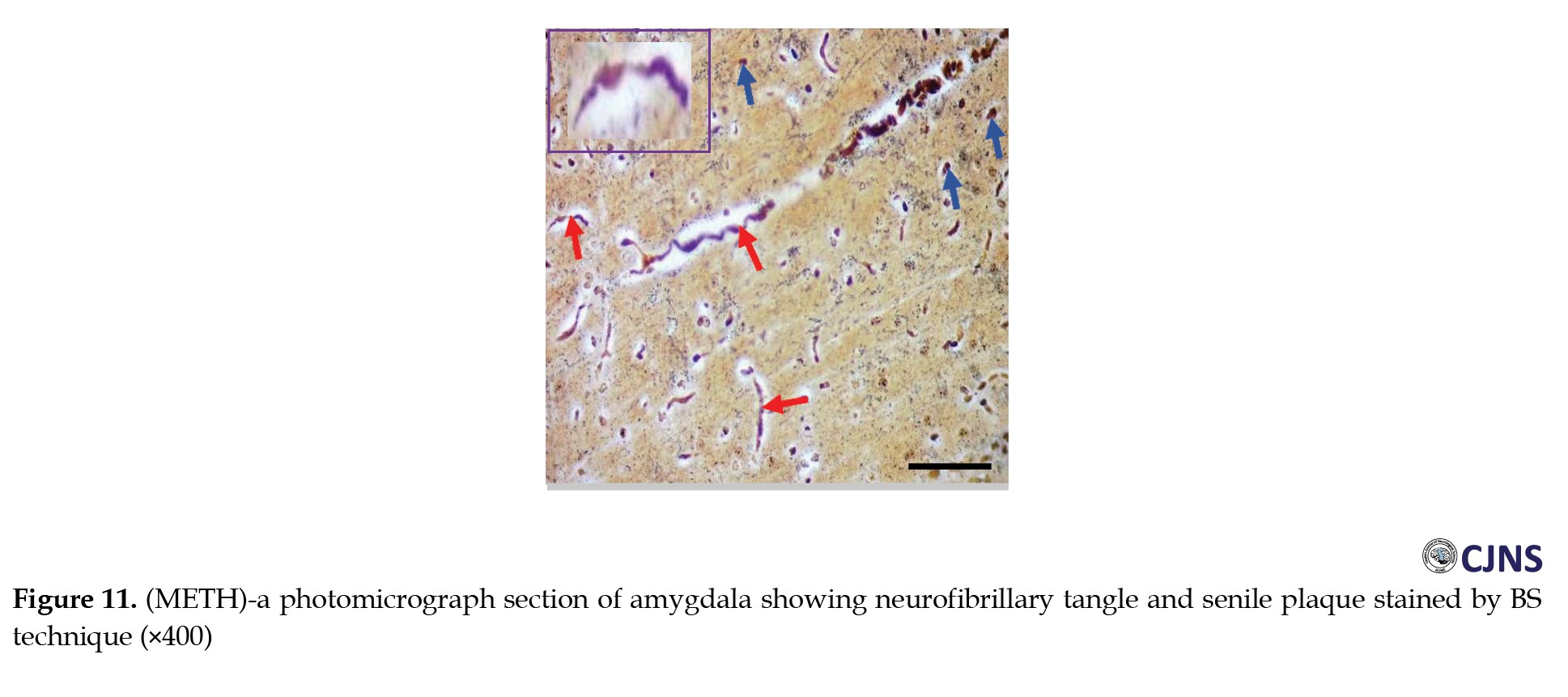
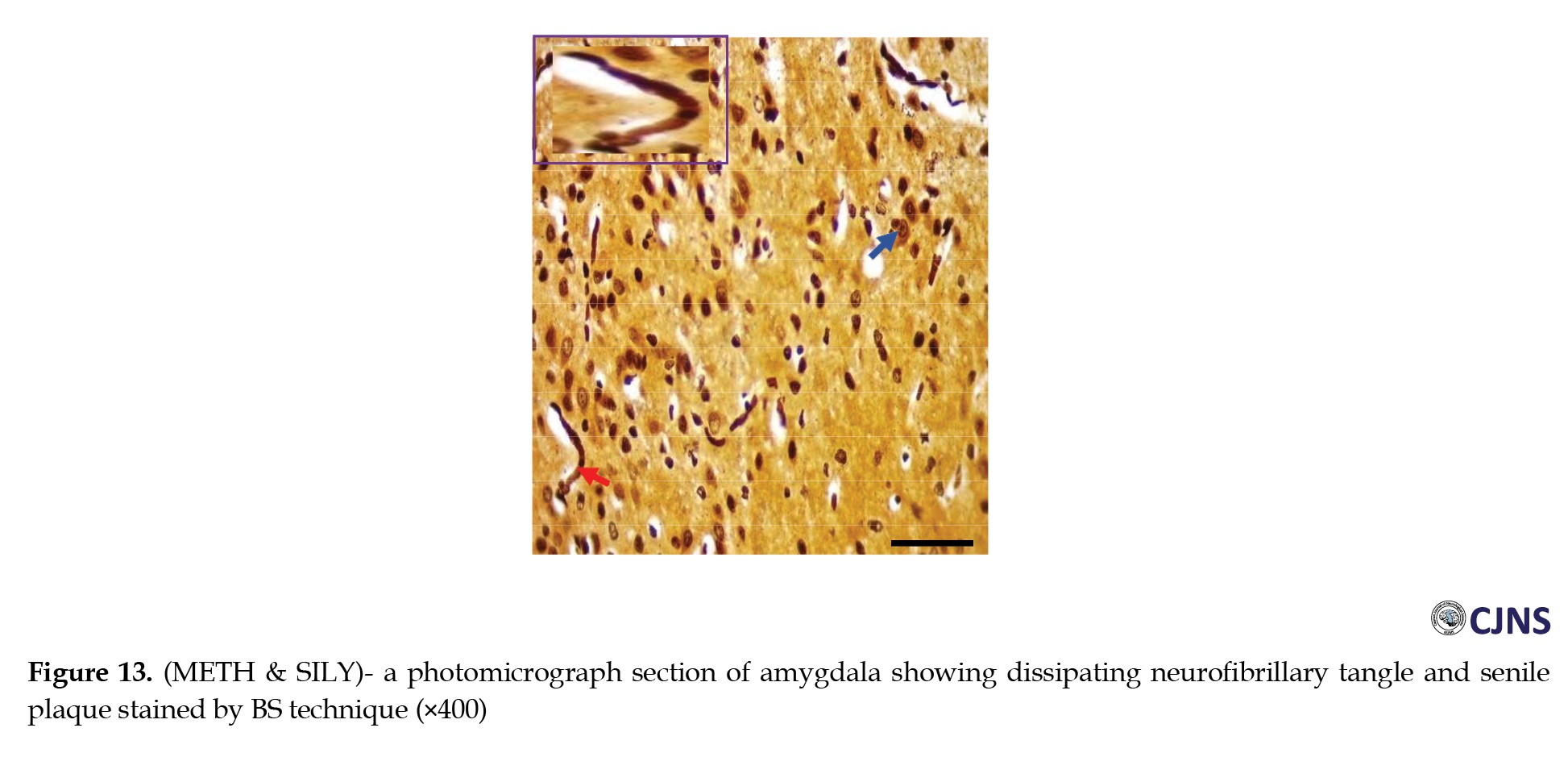
The count of proteins sensitive to BS stain in the amygdala tissues of animals belonging to all the groups is displayed in the photomicrographs shown in Figures 10, 11, 12 and 13. Normal dendritic and axonal fibers are shown in CTRL and SILY groups. Still, neurofibrillary tangle and senile plaque are seen in the METH group and gradually disappear in METH & SILY group (×400 scale bar, 50 µm).
CA3 BS stain
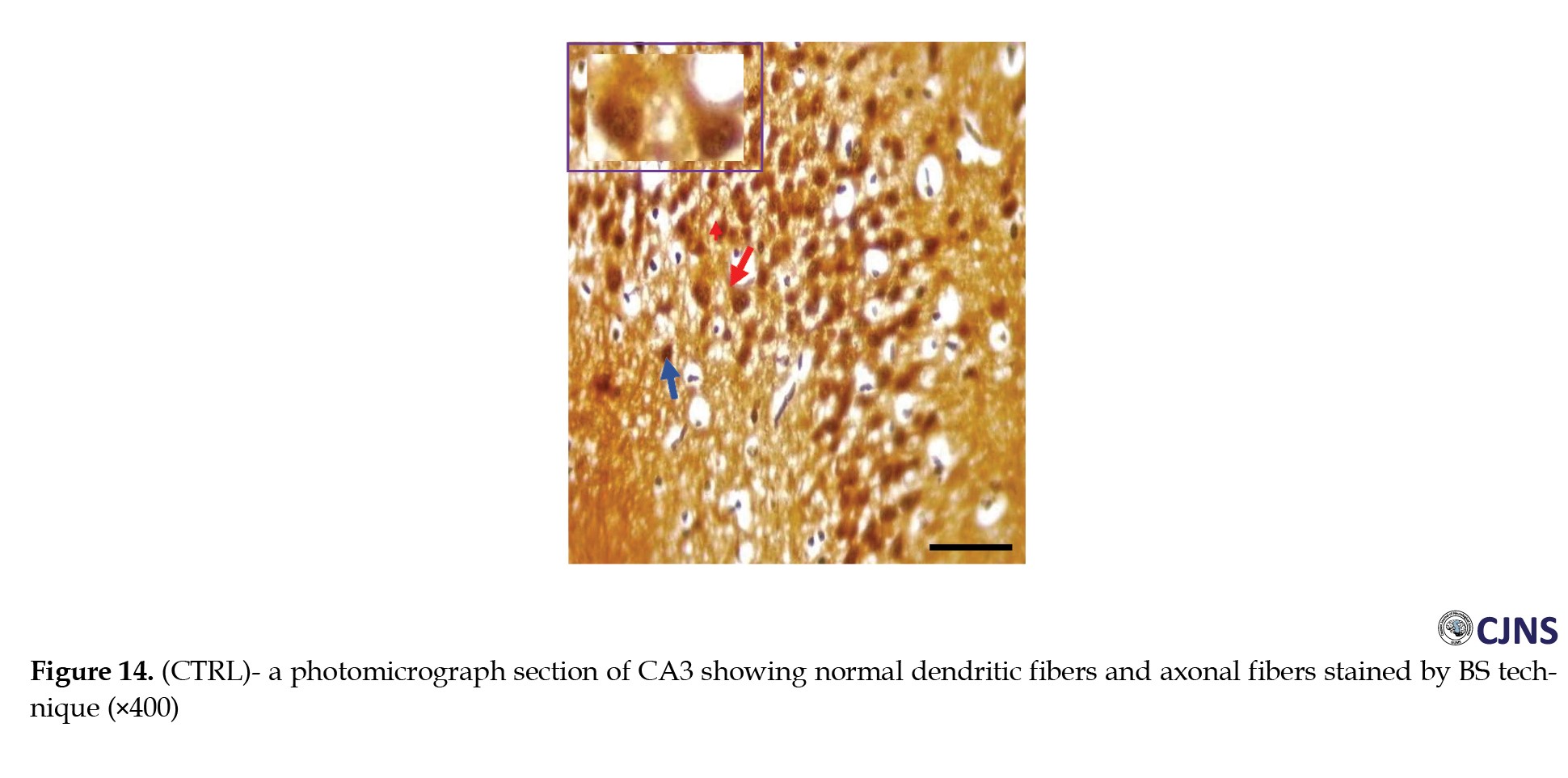
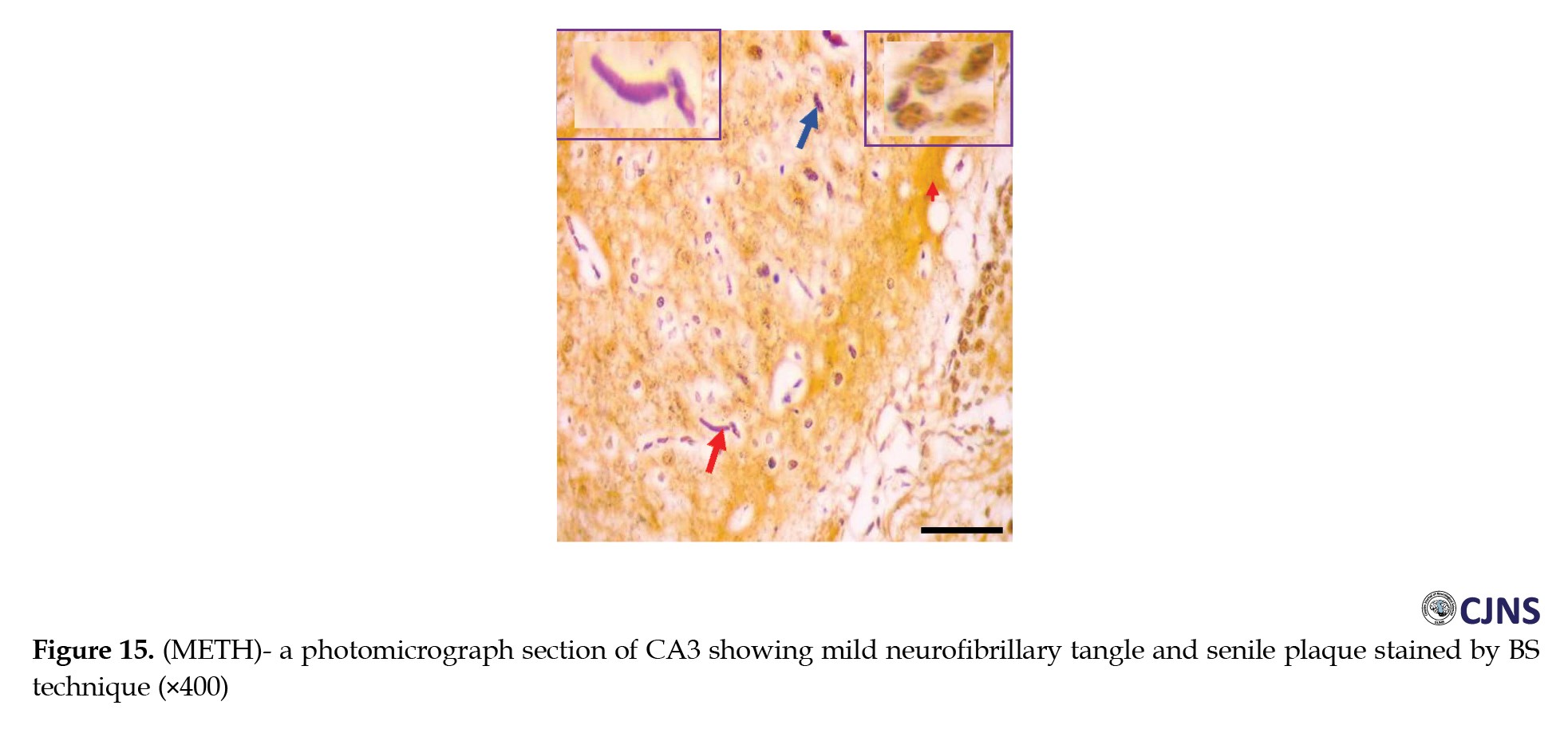
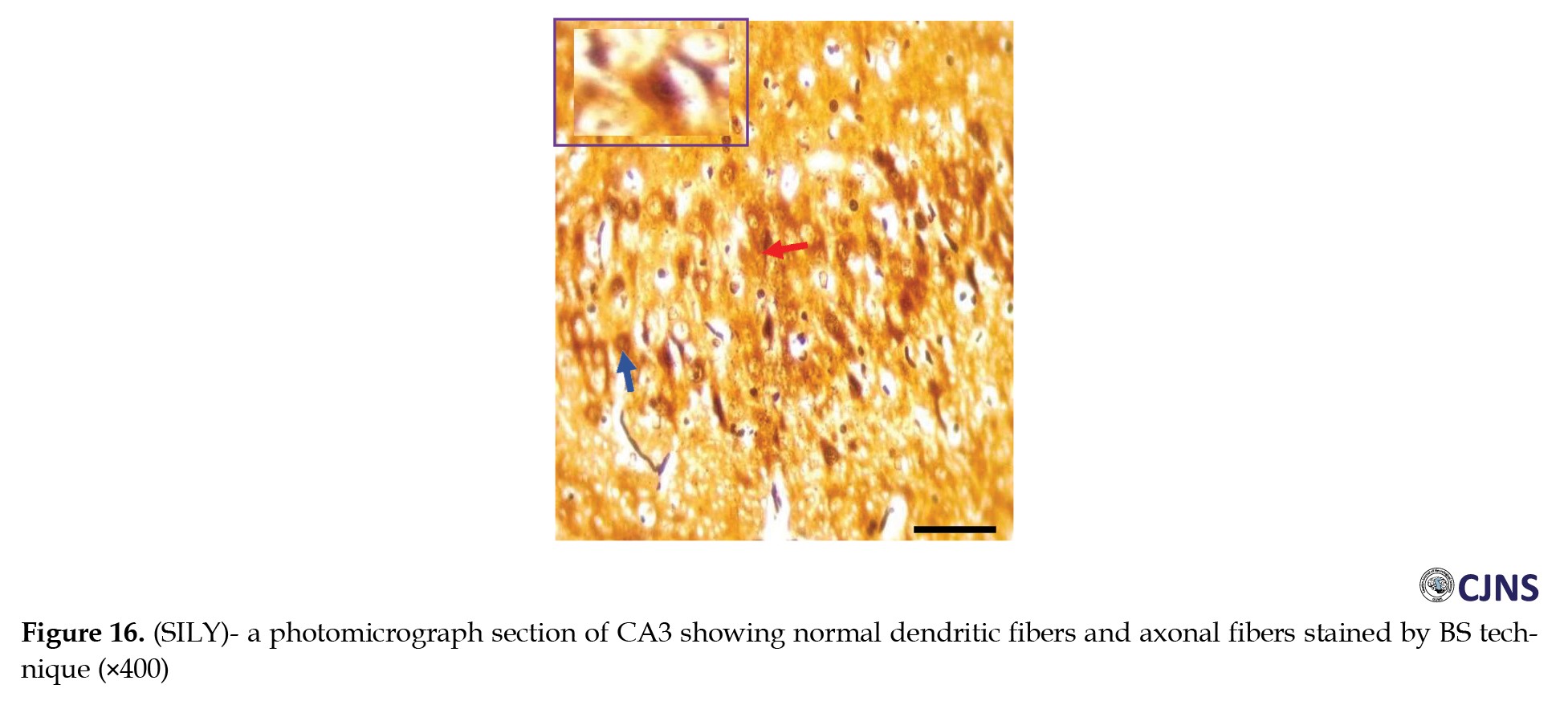
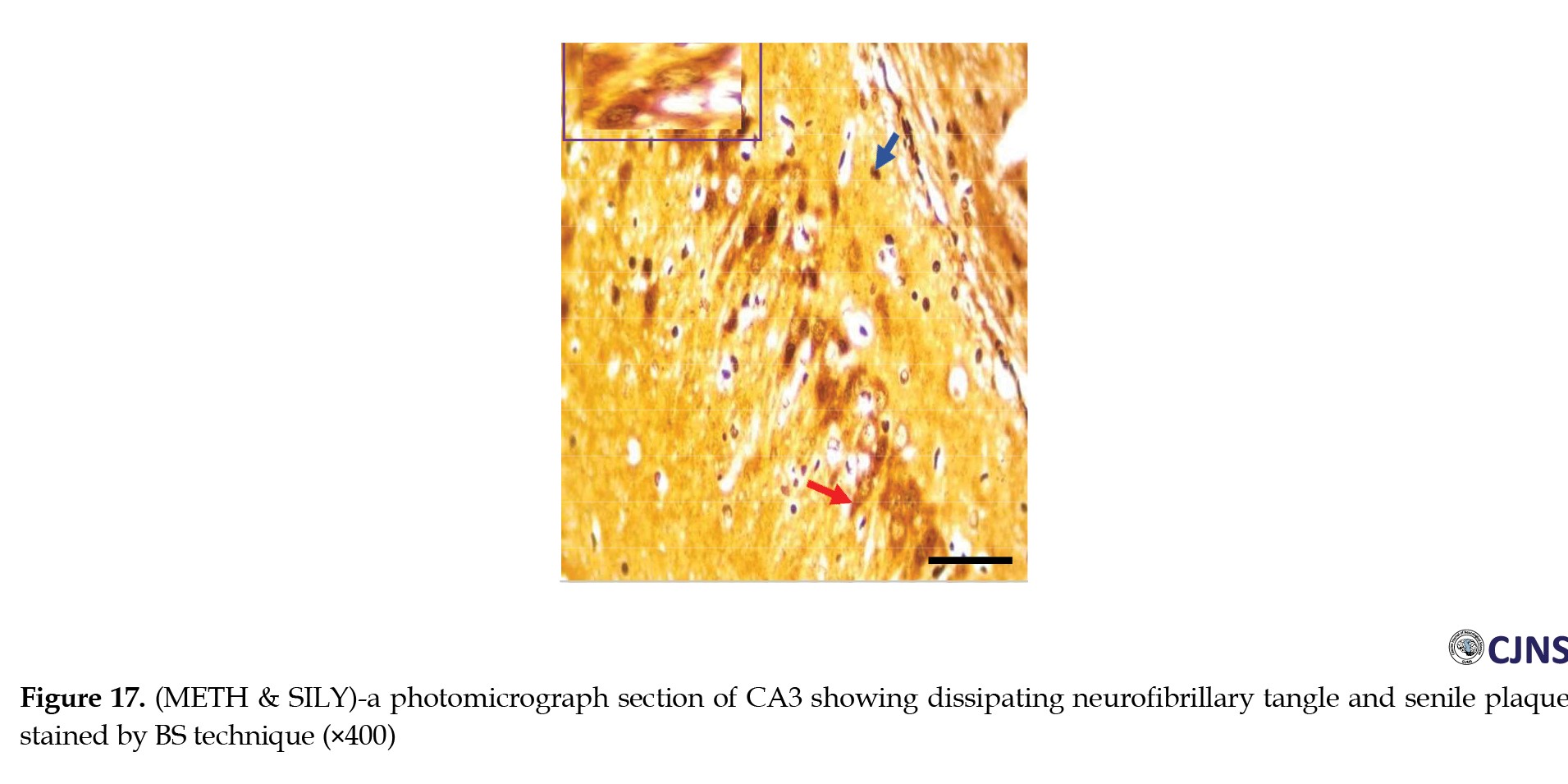
In the CA3 region of the hippocampus tissues of all the groups, photomicrographs shown in Figures 14, 15, 16, 17, 18, 19, 20 and 21 demonstrate the count of proteins sensitive to the BS stain. CTRL and SILY groups showed the count of normal dendritic and axonal fibers. In contrast, the METH group shows conspicuous neurofibrillary tangle and senile plaque, and METH & SILY group shows dissipating neurofibrillary tangle and senile plaque (×400 scale bar, 50 µm).
Immunohistochemical expressions
MBP and OLIG2 expression
The data for different MBP expressions are shown in Table 5.
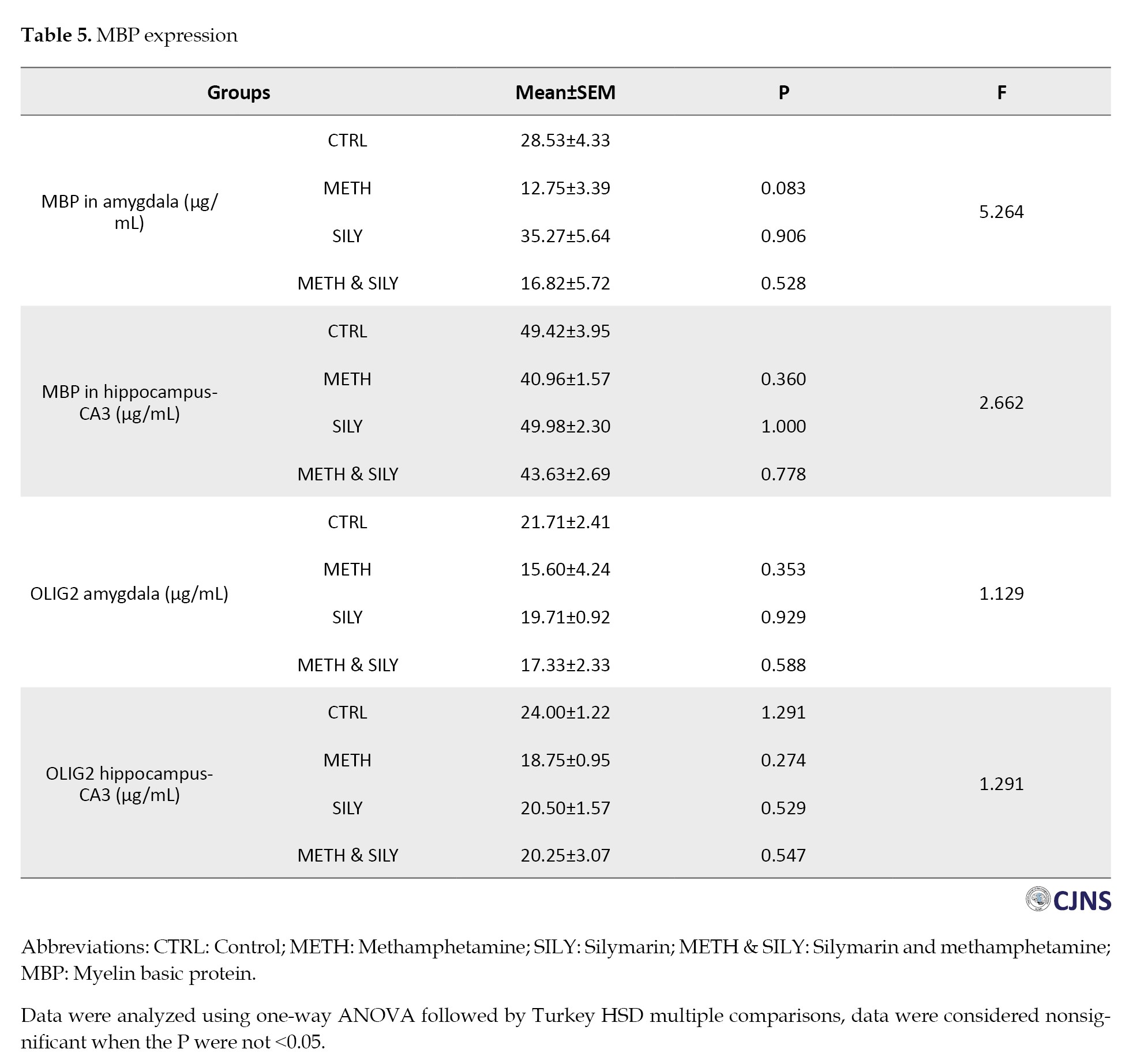
A one-way ANOVA and a Turkey HSD multiple comparisons were used to analyze the data and the results were considered nonsignificant when the P were not <0.05. When comparing the experimental groups to their control group (group A), the amygdala and hippocampal MBP expressions show a statistically nonsignificant difference.
Table 5 presents different expressions of OLIG2. The results were analyzed after a one-way ANOVA and a Turkey HSD multiple comparison. The data were considered nonsignificant when the P were not <0.05. When comparing the experimental groups to their control group, the differences in the amygdala and hippocampus are statistically not significant.
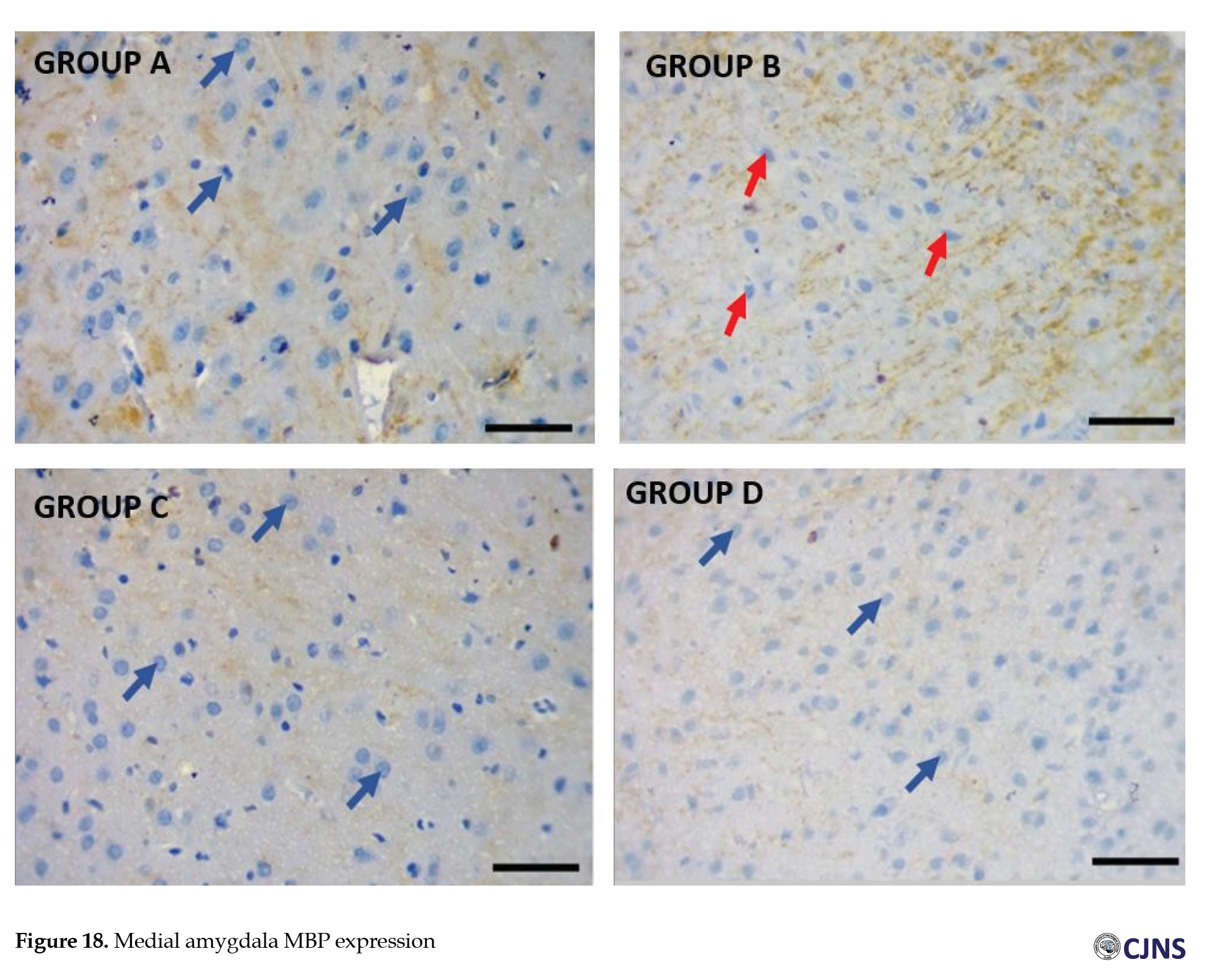
Amygdala MBP expression
CTRL and SILY (A and C) groups show normal immunoreactivity and expression of MBP; METH (B) group shows the lowest expression of MBP, followed by METH & SILY (D) group (×100 scale bar, 100 µm).
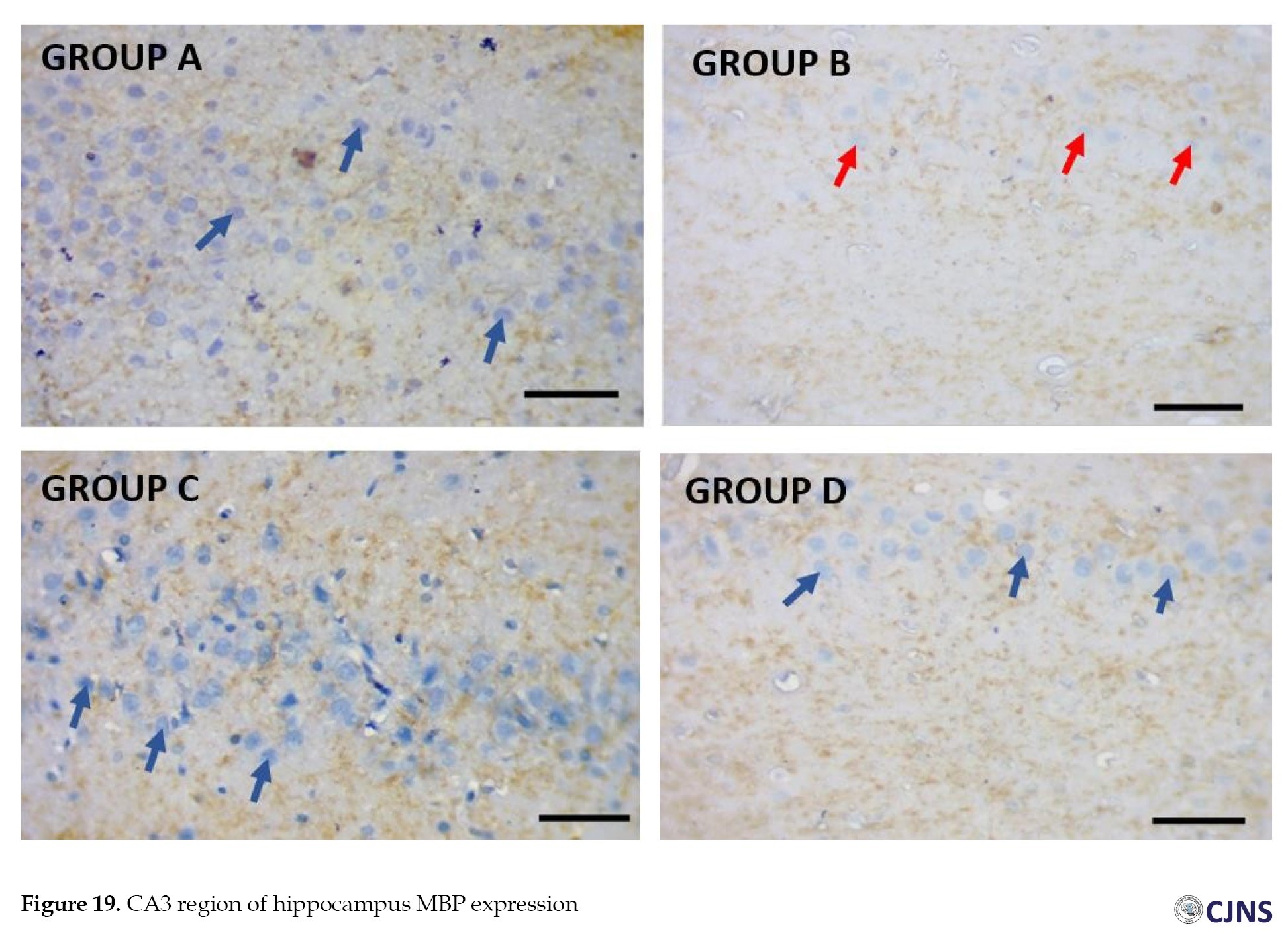
Hippocampus MBP expression
CTRL and SILY (A and C) groups show normal immunoreactivity and expression of MBP. In contrast, METH (B) group shows the lowest expression of MBP, followed by METH & SILY (D) group (×100 scale bar, 100 µm).
OLIG2 expression
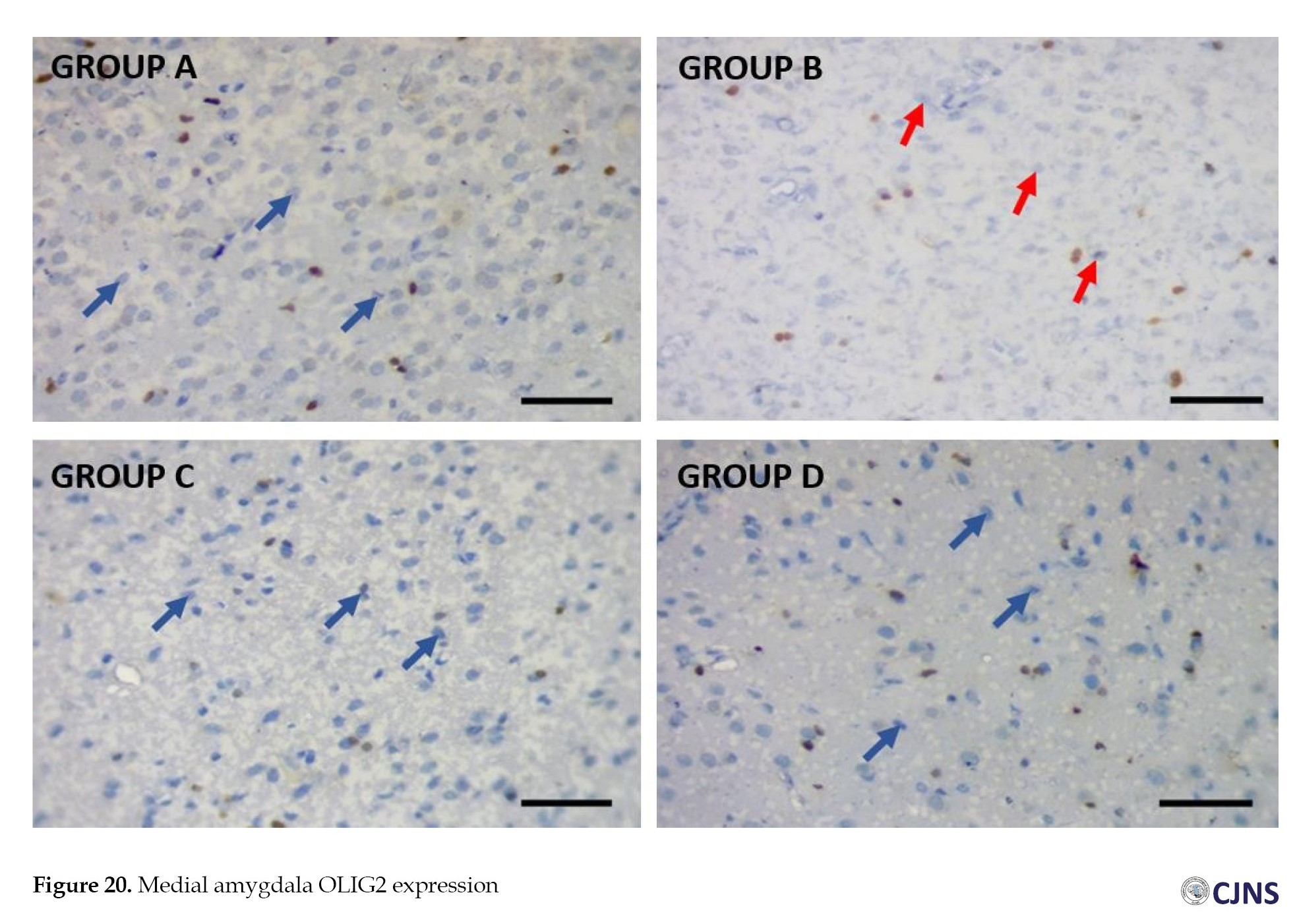
Amygdala OLIG2 expression
CTRL and SILY (A and C) groups show normal OLIG2 expression. In contrast, METH (B) group shows the lowest expression of Olig 2, followed by METH & SILY (D) group (×100 scale bar, 100 µm).
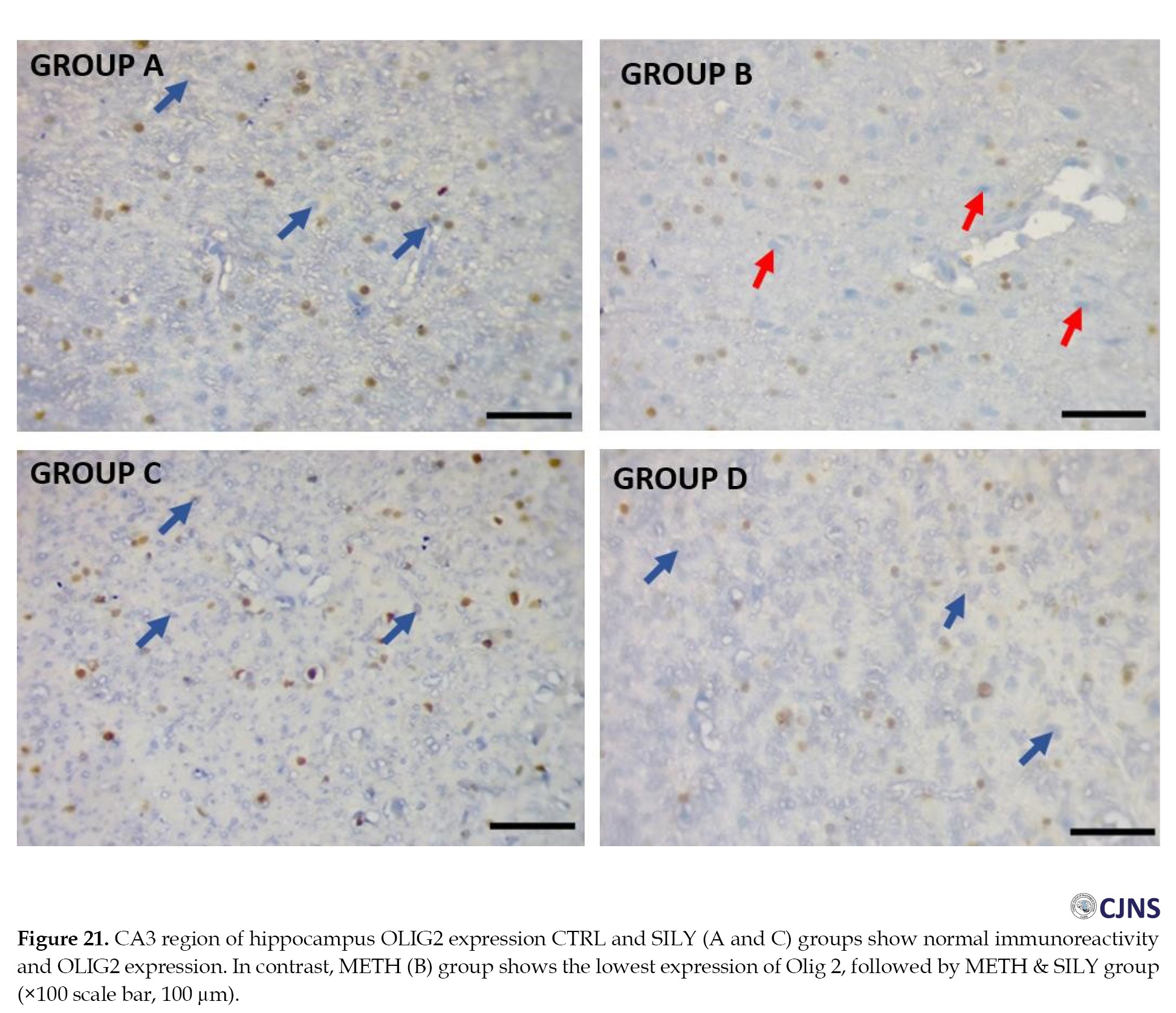
Hippocampus (CA3) OLIG2 expression
Discussion
According to a growing literature of studies, methamphetamine addiction may be associated with emotional and cognitive impairments. Based on a meta-analysis of 17 cross-sectional studies, subjects who had taken methamphetamine showed significantly lower cognitive scores than those who did not abuse drugs [38]. Although they are seeking treatment
for their addiction, people who use methamphetamine for a long time display aggressive, hostile, irritable, depressive, and aggressive behaviors when they first discontinue the use of the substance [39]. A few studies have suggested that certain neuronal deficits may be avoided if there has previously been neuronal protection with medications derived from plants [11, 40]. The current study extends our understanding of the neuroprotective implication of silymarin on adolescent mice in a vulnerable stage of development exposed to neurotoxic compounds, including methamphetamine, during this phase. Our results provide a broad-based demonstration of the effects of silymarin in reversing methamphetamine-induced neurotoxicity as supported by a combination of behavioral analysis with elaborate histological and molecular tests.
Recent studies have looked into the behavioral and cognitive effects of methamphetamine exposure in adolescent rodents. Joca et al. [23] utilized a model of the heightened rates of depression among human adolescent methamphetamine users to show how much exposure to the substance during early adolescence increases depressive-like behavior, as we showed in the tail suspension test. The benefits of using silymarin as a neuroprotectant in this study, however, suggest that it may be possible to reduce the methamphetamine-induced depression and suicidal thoughts that are common in adolescents [23, 41] and adults [42]. A recent study by Buck et al. [43] found that early adolescent exposure to methamphetamine decreased anxiety-like behavior in the open field test. These results align with our EPM test findings, in which the animals spent significant time in the open arm. However, as shown in numerous scientific papers [44, 45], silymarin utilized in our investigation mitigates the decreased anxiety behaviors induced by methamphetamine. Behavioral, biochemical, and histopathological assessments are used simultaneously in the present study to provide a complete picture of silymarin’s neuroprotective potential. Unlike previous research, where these and other experiments provided evidence concerning the effect of a specific intervention on a single result [43-45], in this study, we detailed how molecular changes are associated with functional improvement. This combined approach enhances the validation of silymarin’s therapeutic benefit, which has various applications.
Wistar rats exposed to methamphetamine during adolescence have impaired spatial learning in the Morris water maze and sequential learning in the Cincinnati water maze. These effects do not appear in pre-adolescence or adulthood, indicating that adolescence is a time when methamphetamine-induced cognitive impairments are more likely to occur [46, 47]. If effective neuroprotectants are not used, this impairment continues until adulthood [23, 48]. However, our research found that the active phytochemical molecule from S. marianum (L.) Gaertner seeds alleviated the cognitive impairments caused by methamphetamine, similar to what was shown in a previous study [49] about the cognitive impairment caused by amyloid-beta. Silymarin could reduce cognitive impairment by inhibiting oxidative damage to the brain in terms of lipid peroxidation and antioxidant levels [49]. Our results agree with the results obtained by Lu et al. [49], where the authors reported that the major component of silymarin, silibinin, abolished the memory deficits in an Alzheimer model due to the downregulation of oxidative stress. Likewise, Raza et al. [44] have shown the beneficial impacts of silymarin on behavioral activities after ischemia-induced brain damage. These studies corroborate silymarin’s antioxidant and neurotrophic actions, as found in the present investigation.
The enhancements in anxiety and cognitive behaviors found in the present study by measuring the efficacy of silymarin through the EPM and Morris water maze tasks are in concordance with the hypothesized neuroprotective effects of silymarin. Such behavioral changes indicate their effectiveness and provide biochemical and histological results.
It has been demonstrated that silibinin, one of the silymarin compounds, is a potent antioxidant that reduces lipid peroxidation while maintaining levels of SOD and GSH [50]. This study, however, confirmed the antioxidative characteristics of silymarin and postulated that the primary mechanism responsible for its neuroprotective effectiveness was the decrease in GSH, SOD, and MDA synthesis following an aberrant increase in these substances caused by methamphetamine [50, 51]. These phenomena show the significant involvement of oxidative stress with normalizing MDA and GSH levels in methamphetamine-induced neurotoxicity. Consequently, since silymarin antagonized these alterations, its application confirmed the aforementioned behavioral and cellular recovery evidenced by antioxidant properties.
Thus, the balance of neurobehavioral tests, histological studies, and oxidative stress markers in the current work allows for a comprehensive approach to defining the mechanisms of the neuroprotective action of silymarin. Unlike prior studies, which have mostly examined the individual effects, this integration yields a profound assessment of its therapeutic use.
According to Rothman et al., dopamine, serotonin, and norepinephrine are the main neurotransmitters released by methamphetamine [52]. The amygdala and hippocampus regions of the animal group exposed only to methamphetamine showed signs of swelling and necrotic degradation of neuronal cells, which is likely related to these neurotransmitters, just as previously reported by Panenka et al. [53] in the mesolimbic region of the brain. The use of silymarin prevented the deleterious effects of methamphetamine on animal groups that were exposed to the substances. This silymarin finding is in line with the outcomes of the Dashti-Khavidaki et al. [54] study, which showed that perirenal fat infiltration, pelvic inflammation, interstitial inflammation, and nephrotoxic drug-induced glomerular and tubular necrosis were all decreased by dose-dependent exposure to silymarin. It also prevented ischemia and reperfusion following renal injury. Increased cell density is caused by the activation and migration of microglia cells to the brain’s injured areas in the METH group of H&E expression [31, 55], a process that silymarin prevented in the METH & SILY group. The capacity of silymarin to inhibit and stop microglia from proliferating has not been well documented [56, 57]. Wang et al. [58] reported that silymarin inhibited lipopolysaccharide-induced dopaminergic neurodegeneration in an animal model of Parkinson disease by blocking microglia activation. However, other studies by Hou et al. [56] and Tsai et al. [57] described silymarin’s mechanism of action as blocking inducible nitric oxide synthase production in cellular models to prevent microglia cell activation. Senile plaques and extracellular neurofibrillary tangles, which are indicators of AD, are expressed in this study as a result of methamphetamine exposure [59–61]. As seen in our investigation, treatment with silymarin has reduced these pathological features [43, 49, 50]. The protection of neurons and decreased neurofibrillary tangles and senile plaques observed in silymarin groups reflect its antioxidizing and anti-inflammatory effects. These observations have histological implications as to its potential for treating neurodegenerative and neurotoxic situations.
In the METH group, exposure to methamphetamine induces demyelination and oligodendrocyte disintegration [62], as evidenced by a decrease in the expression of OLIG2 [63] and MBPs [64]. In AD, there is increased buildup of amyloid-beta due to myelin disintegration and a decrease in MBP expression [64]. These changes were inhibited in animal groups exposed to silymarin [63]. Oxidative stress, which is frequently associated with axonal shrinkage, may be the cause of the aberrations in MBP and OLIG2 expression seen in the METH group [65]. Silymarin, on the other hand, was successful in reducing the adverse effects of methamphetamine on oligodendrocytes and the myelin sheath by preventing oxidative stress [49, 63].
In contrast to prior research that has closely described silymarin’s antioxidant and neuroprotective actions, the present work considers the capacity of silymarin to ameliorate methamphetamine-caused abnormalities in neurobehavioral performance and histopathology in adolescent subjects. Of particular interest here are the findings on silymarin for its effects on microglia activation and neurofibrillary tangles, which have received insignificant attention from previous investigations in adolescent models of methamphetamine exposure. The present evidence points to silymarin as a possible therapeutic target for ameliorating the neurotoxic consequences of methamphetamine, especially in adolescents for whom the brain is at its most vulnerable state. The results help continue the clinical trials about the effectiveness of silymarin substances in mental disorders caused by substance use disorders.
Despite the rich data obtained in this study regarding the beneficial effects of silymarin on methamphetamine-induced neurotoxicity, it has several limitations. These limitations include the use of adolescent Wistar rats, which, although it has helped evaluate methamphetamine-induced neurotoxicity, may not be the best model given the physiological and behavioral characteristics of human adolescents. Also, a short period of treatment, 14 days, only hinders the ability to assess the long-term neuroprotective efficacies of silymarin or even the delayed neurotoxic effects of methamphetamine. Moreover, only one dose of silymarin was considered in this study, which may not be sufficient to estimate all the features of the silymarin dose-response relationship and provide an adequate therapeutic and safe dose.
However, more research is needed to show the long-term effectiveness and non-toxicity of silymarin in chronic methamphetamine models. Further studies should also aim to dissect both its neurochemical relationship with differential neurotransmitter systems and its molecular neurobiology to decipher how it exerts neurotrophic effects in adolescent populations. The application of these findings is uniquely relevant to translational neuroscience because it demonstrates that silymarin may present interesting therapeutic potential to manage neurotoxicity in adolescents with methamphetamine use disorder. This finding underlines the need for additional clinical research concerning its uses.
Conclusion
This study reveals that silymarin possesses the ability to reduce methamphetamine-induced impaired behavioral alterations, oxidative stress and histopathological alterations in adolescent rats. Through these findings connecting functional recovery with biochemical and structural changes, the current studies also provide a framework for silymarin’s neuroprotective effects and demonstrate its application for protecting against methamphetamine neurotoxicity in susceptible groups.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Nnamdi Azikiwe University Nnewi, Awka, Nigeria (Code: UNIZIK/FBM/EA/2023/033).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization: Princewill Sopuluchukwu Udodi, Kelechi Blessed Ndubuisi and Roseline Ebube Udodi; Methodology: Damian Nnabuihe Ezejindu, Roseline Ebube Udodi, Uchenna Confidence Mofunanya and Promise Chukwuebuka Onuorah; Investigation: Princewill Sopuluchukwu Udodi, Kelechi Blessed Ndubuisi, Uchenna Confidence Mofunanya, Promise Chukwuebuka Onuorah, Ukamaka Peace Odunwa, Sylvia Chinaecherem Ndinojuo and Roseline Ebube Udodi; Writing the original draft: Kelechi Blessed Ndubuisi, Uchenna Confidence Mofunanya, Promise Chukwuebuka Onuorah, Ukamaka Peace Odunwa and Sylvia Chinaecherem Ndinojuo; Review, editing and supervision: Princewill Sopuluchukwu Udodi and Damian Nnabuihe Ezejindu; Funding acquisition and resources: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Methamphetamine exposure levels may rise as a result of the emergence of new methamphetamine consumption techniques that are more accessible in our local surroundings [1]. Methamphetamine can be injected intramuscularly or intravenously. It can be absorbed by insufflation, smoking, or ingestion. The effects of drug use are particularly sensitive and noticeable during adolescence and chronic methamphetamine use is more prevalent and begins earlier in life among teenagers [2, 3]. Teenagers mainly misuse synthetic psychostimulants for recreational purposes, as they are extremely addictive. Actually, with over 35 million users worldwide, methamphetamine is the second most popular illegal substance in the world [4, 5]. According to estimates from the United Nations Office on Drug and Crime (UNODC) [6], methamphetamine use is on the rise in Nigeria, with a 32% increase in cases of methamphetamine use and a 55% increase in cases of methamphetamine use disorder [6]. There seems to be a noticeable increase in methamphetamine usage in the southeast of Nigeria, which could account for the majority of methamphetamine use disorders [7].
The transitional phase between childhood and adulthood, known as adolescence, is crucial for brain development and growth. In humans, it is believed to last between the ages of 12 and 18 and in rats, between postnatal (PND) days 28 and 42 [8]. Teenagers are susceptible to the harmful effects of illegal drugs. Studies on animals have shown that exposure to illegal drugs throughout adolescence changes the brain’s development in ways that last for years. Adolescent learning, memory, attention, behavioral activities, and possible addiction could all be affected as a consequence [9, 10].
Methamphetamine use among teenagers is common, and the detrimental effects it has on memory and learning (cognition) in our community are well documented. However, substances that may lessen these effects have not been studied. Some publications claim that if herbs and other sources of nutrition preserve the neural system, diseases associated with cognitive deficits may be preventable [11, 12]. Given the abundance of herbs and herbal products found in Africa, we are optimistic about preventing mental illness in the future. Silybum marianum (L.) Gaertner, an annual or biennial plant in the Asteraceae family, is the source of silymarin. It is also known by several names, including milk thistle, blessed milk thistle, Marian thistle, Mary thistle and Saint Mary’s thistle. The plant is now common worldwide, previously found only in southern Europe and Asia [13]. This herb is a challenging weed being cultivated for its medicinal qualities and is one of the most essential medical crops in the world. Since its earliest usage as a medication more than 2000 years ago, milk thistle has been chiefly used to treat liver conditions such as cirrhosis and hepatitis and protect the liver from toxins [14]. The extracts from this plant have also been found to have a wide range of pharmacological characteristics, such as anti-inflammatory and antifibrotic effects [15]. The major element behind milk thistle’s pharmacological effects is the presence of a class of compounds called flavonolignans, sometimes called silymarin.
Treatment options for methamphetamine use disorder currently consist of behavioral and synthetic pharmaceutical approaches, both of which are usually ineffective. Research toward developing medications to treat methamphetamine use disorder has been prioritized by the National Institute on Drug Abuse (NIDA) [16]. The inherent limitations and inconsistent efficacy of prior synthetic agents have contributed to the need for the development of novel protective agents with efficient modes of action, particularly those derived from medicinal plants (due to their alleged reduced side effects compared to synthetic drugs). Although silymarin has been proven to have protective effects against oxidative stress and neurotoxicity, less attention has been paid to its neurobehavioral recovery effects on methamphetamine-induced deficits in adolescents experiencing a developmental period. This study thus fills that gap by investigating behavioral and molecular changes in adolescent Wistar rats.
Therefore, the current study hopes to understand the various neurobehavioral, oxidative stress and histopathological effects that silymarin has on the neurotoxicity of methamphetamine in adolescent rats. This study aims to establish how the findings from silymarin effectiveness on antioxidative and neuroprotective properties in methamphetamine-exposed adolescent rats improve the functional and structural aspects in these animals by combining behavioral results with molecular and histological analyses.
Materials and Methods
Study materials
The dried seeds of S. marianum (L.) Gaertner were supplied by Biokoma Dried Herbs (Jumia Store, Sangotedo, Lagos State, Nigeria) in the summer of 2022. These seeds were verified at the Biological Science Department of Nnamdi Azikiwe University using the authentication code AU106. A sizable representative sample of the plant material (500 g) was crushed and sieved to obtain a particle size of 0.4 mm. Wianowska and Wis’niewski described an effective three-stage extraction process using accurately weighted material pieces [17].
Methamphetamine was obtained under government approval and stored under government-required conditions, and usage was logged as prescribed by national legislation. The substance was stored in a dry and cool environment.
Experimental animals
Male and female Wistar rats aged 70 to 80 days, weighing between 159 to 205 g, were raised in our colony in breeding cages with 10 male and 20 female rats each; female rats were single-housed at the first sign of pregnancy with a vaginal plug. The young rats were weaned on PND day 21 and kept in a different cage from their parents. The experiment was conducted from PND 28 to 42 using only 40 male offspring. Routine experiments were performed with the animals in groups of four per Perspex cage in a designated pathogen-free environment; wire gauze was necessary for cross-ventilation. The animals were kept in a controlled environment under a 12-hour day/night photoperiod, with a temperature range of 25–28 °C and relative humidity of 60–80%, at the anatomy lab in Nnamdi Azikiwe University’s Nnewi Campus’ College of Health Sciences. The animals regularly received water and guinea feed pellets (from Agro Feed Mill Nigeria Ltd.). The Faculty of Basic Medical Sciences, College of Health Sciences, Nnamdi Azikiwe University Nnewi Campus approved the treatment of all the animals, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals [18].
Acute toxicity test (LD50) of methamphetamine and silymarin
Using the methodology outlined in the organization for economic co-operation and development’s guideline for testing chemicals [19], the acute test was conducted for silymarin and methamphetamine. The lethal dose of 50% (LD50) for silymarin was 500 mg/kg and 10 mg/kg for methamphetamine.
Experimental design
A total of 40 young Wistar rats were used in this study. Four groups with 10 male animals each were formed. The groups were given the designations A (control [CTRL]), B (methamphetamine [METH]), C (silymarin [SILY]), and D (METH+SILY). The animals in each of the following groups received the following treatments: A (vehicle), B (methamphetamine), C (silymarin), and D (methamphetamine and silymarin) from PND 28 to PND 42. The treatment had a pH of 9.2 and was administered at room temperature. The oral dosages for silymarin and methamphetamine were 385 mg/kg and 5 mg/kg, respectively. Sesame oil was used to dilute the methamphetamine and silymarin and it also served as the vehicle for the control group. The oral administration was done using a flexible 16-gauge feeding plastic tubing oral bulb-tipped gavage needle of about 2 inches in length.
Animal sacrifice or euthanasia
Through intramuscular injection, ketamine (55 mg/kg) was used to put the animals to sleep. The animals were anesthetized 24 hours after the last day of treatment, then decapitated, and had an occipitofrontal incision made to remove the brain. They were also cardiac-perfused with heparinized phosphate-buffered saline (PBS). The brain tissues of four animals in each group were immediately prepared for biochemical analysis through homogenization to identify oxidative markers of interest. In contrast, six brain tissues in each group were fixed for 48 hours in 10% neutral buffered formalin to section the three fixed brain tissues for hematoxylin and eosin (H&E) and bielschowsky silver (BS) stain. Three other fixed brain tissues in each group were embedded in cryoprotectants for immunohistochemical investigations.
Behavioral function test
Before sacrifice, the behavioral test started at PND 39 and ended at PND 42 (later in adolescence). Forty Wistar rats in all (10 per treatment group) were subjected to behavioral tests in the following order: PND 40 was used for the elevated plus maze test (EPM), PND 41 was used for the tail suspension test, and PND 42 and 43 were used for the Morris water maze test. On each day of the behavioral test, the tests were administered a few hours before the treatment.
EPM test (anxiety-related behavior)
The EPM test on the PND 39 was used to assess animal anxiety-related behavior. Two oppositely positioned closed arms, two oppositely positioned open arms, and a center region comprise the “+”-shaped maze that makes up the EPM device. The maze is elevated above the ground. The subjects have 5 minutes to navigate the maze freely during this test. A surveillance camera positioned above the labyrinth recorded their motions, and their activities in the video were subsequently examined on a laptop. Time spent in the open or closed arms was used as an index for preference of either arm and used to measure anxiety-like behavior.
Consequently, increased closed-arm preference indicates elevated anxiety. Anxiety was measured directly by using entries and time spent in closed arms [20]. The amount of time spent in open arms and the entries were utilized as direct indicators of anxiety; in other words, less open-arm avoidance corresponds to lower anxiety levels or anti-anxiety behavior [20]. The study used risk-assessment behavior as an inverse measure of anxiety. Specifically, the frequency and duration of head-dips, which include moving the head downward toward the floor while on the open arms, were found to be associated with lower levels of anxiety [21].
Tail suspension test
On the PND 40, the tail suspension test was utilized to assess behaviors associated with depression. Throughout the experiment, the Wistar rats were kept from escaping or clinging to adjacent surfaces by being suspended by their tails from a metal hook that protruded from the chamber ceiling. Immobility is the main result. The rats attempted to escape in almost all cases during the first 2 minutes of the 6-minute test, so the immobility period was measured during the last four minutes. A measure of “depression-like” behavior was determined by keeping scores of each animal’s total immobility time [22]. An animal’s total immobility time is the time it hangs without movement.
Morris water maze test
From PND 41 to PND 42, the animal’s spatial learning and memory were assessed using the Morris water maze. The procedure was followed as directed by Joca et al. [23]. The apparatus comprises a large circular pool, or tank, about 6 feet in diameter and 3 feet deep. The interior of the tank was painted white, while its exterior was painted brown. It was filled with about 25 °C tap water under bright lighting. The maze was separated into 4 virtual quadrants, with a platform measuring 24 cm in height and 10 cm in circumference in one of the pool’s quadrants. During training, the platform was one inch above the water.
The Wistar rat learned and used this platform as the most effective way to get out of the water. The experimenter led the animals who had difficulty finding the platform during training, and they were given 3 s to remain there. Before the test session, the platform was submerged in the quadrant during the training session because the animals needed to be retrained to locate the submerged platform hidden in one of the four quadrants. The test animal was placed in a different quadrant for each trial, while the platform was kept in the same quadrant. Each test session had three trials lasting 60 s each, with an average inter-trial delay of 10 s. The animal was trained and ready for the test, and the escape platform was placed an inch below the water’s surface. Non-toxic white tempera paint was added to make the water opaque. One day was all needed to finish the training test session for opaque and transparent materials. If the animal had not clambered onto the platform after 60 s of the trial’s termination, it was removed from the water and placed on it. Before the subsequent trial began, the animal remained on the platform for 20 min during the intertrial. When all three tests were completed, the animal was removed and placed under a lamp to warm. The interval between each session was 1 hour. The escape latency was manually recorded using a stopwatch.
Analysis of oxidative markers
For the evaluation of antioxidants like glutathione (GSH) and superoxide dismutase (SOD), as well as lipid peroxidative indicators like malondialdehyde (MDA), the Biochemistry Department at Nnamdi Azikiwe University, Nnewi Campus, was engaged. One gram of each animal tissue was mixed with 10 mL of 0.9% normal saline and homogenized at room temperature. After that, each sample was centrifuged for 20 minutes at 3000 rpm at room temperature. The supernatants were separated using a spectrophotometer, and the levels of MDA, GSH and SOD were measured in accordance with the instructions provided by Ahmed Amar et al. [24].
Tissue processing H&E and BS stain
After being prepared according to Feldman and Wolfe [25], the tissues were sectioned and stained with H&E and BS stain, and three of the tissues were examined. After the fixation, excess fixatives were removed by rinsing them under tap water for an entire night. The fixed tissues underwent dehydration to eliminate water and other substances. After dehydration, tissues were cleared with xylene for two hours. This action was followed by two variations of two hours of infiltration at 60 °C molten paraffin wax. The paraffin wax was sectioned when it had cooled [25].
Hematoxylin and eosin staining methods
The H&E staining approach was used to identify necrotic entities with an eosinophilic appearance and histomorphological changes [26]. Coronal sections containing the appropriate brain tissue were mounted on slides, dried overnight, rehydrated and stained with H&E in accordance with the guidelines provided by Granado et al. [27, 28]. Photomicrographs were taken during the microscopy process using an eyepiece camera attached to an Olympus microscope (Tokyo, Japan).
Bielschowsky sliver staining method
BS stain is a useful technique that we utilized to demonstrate nerve fibers. Visible staining was observed in the dendrites, axons, neurofibrils and senile plaques of the central nervous system. This approach is widely used in the study of Alzheimer disease (AD). Coronal sections containing the appropriate brain tissue were put on slides, dried overnight, rehydrated, and stained by BS stain BS [29].
Stereological quantification of neurons in the brain
H&E-stained neurons in the amygdala and hippocampus were counted unilaterally in every fourth segment of the tissues for each group (n¼4-6). The degeneration of neurons in the selected brain tissues was assessed by counting H&E neurons in each experimental group (n¼4-6) [30]. An experienced observer unaware of the treatment circumstances employed the optical fractionator, Stereo Investigator program (MicroBrightField Bioscience, Colchester, VT), as described in some studies [31, 32]. The brain tissues’ shapes were illustrated at low power (×2) in accordance with recognized anatomic landmarks. At the same time, the number of cells was counted at higher power (×20 for the hippocampus and ×100 for the amygdala). H&E-labeled cell bodies could easily be differentiated from terminal degeneration in the hippocampus and amygdala at these magnifications. To avoid double counting, neurons were only counted in the focal plane, where their nuclei were most visible. The count includes several neurons with extremely low H&E signals.
Myelin basic protein (MBP) and oligodendrocyte transcription factor (OLIG2) expressions
The three fixed tissues were used for immunohistochemistry staining after being embedded in a cryoprotectant. SuperFrost microscope slides obtained serial coronal sections (20 μm). OLIG2 and MBP immunohistochemistry were performed on free-floating sections using standard avidin-biotin immunocytochemical protocols [33, 34]. The slides were cleaned three times for five minutes each in PBS and then blocked. Following application to the slides, the primary antibodies (OLIG2 and MBP) were incubated at a concentration of 1:1000 for a whole night. The following day, slides were incubated with a secondary antibody (goat anti-mouse) at a concentration of 1:250 for two hours after being washed in PBS three times for five minutes each. The VECTASHIELD mounting media was used to cover the slides.
After that, pictures were taken at a magnification of ×20 using an Olympus Ix-81 microscope and the integrated optical density was computed using SlideBook software, version 6.0. After obtaining all photomicrographs, the average intensity of MBP and OLIG2 immunoreactivity in the cornu ammonis 3 (CA3) and basolateral amygdala (BLA) was evaluated [35].
Quantitative assessment of immunoreactive expressions
An optical microscope equipped with a Leica DFC290 HD video camera was used to capture images of the tissue sections obtained with an ×4 lens and the expression levels of OLIG2, MBP and BS staining in the brain were measured. Using an image analysis system (analytical imaging station), the staining area in the brain tissue was calculated as a proportion of the tissue’s pixels that displayed staining (stained area) relative to all pixels in the brain tissue of interest (scanned area). This is called a proportional stained area [36, 37].
Data analysis
The data were analyzed using SPSS software, version 27.0.1. We could determine the Mean±SE. Values within a group and between groups were compared using an independent samples t-test and a one-way analysis of variance (ANOVA), respectively. The post hoc analysis of Turkey was utilized to determine differences in the experimental parameters between the groups. The data were presented as Mean±SEM and were considered statistically significant when P<0.05, P<0.01 and P<0.001 were obtained.
Results
Neurobehavioral test
The tests included EPM, tail suspension and Morris water maze tests. The duration spent in the closed and open arms is displayed in Table 1.

It is used as an index for measuring the animal groups’ anxiety levels during the EPM test. The independent samples t-test was used to analyze the data and the results were considered significant at P<0.05 and P<0.01, represented as * and **, respectively. The mean of the closed and open arms in SILY and METH & SILY groups varied significantly, according to the independent samples t-test. Furthermore, Table 1 shows no significant difference between the CTRL and METH groups’ closed and open arm means.
The risk assessment for each animal group is shown in Table 2 above.

In comparison to the CTRL group, the METH group shows a significant increase in both the duration and frequency of head dips. In contrast, the SILY and METH & SILY groups show nonsignificant differences in the duration and frequency of head dips done by the animals throughout a five-minute test.
The immobility time for each animal group is also shown above. A one-way ANOVA and a Turkey HSD multiple comparisons were used to analyze the data, and the results were considered significant at P<0.001, which is represented as ***. When compared to the CTRL group, the immobility times of the SILY and METH & SILY groups show nonsignificant differences. Still, the immobility time of the METH group animals shows a significant increase.
The observed reduction in anxiety-like behaviors and improvement in spatial learning in the SILY and METH & SILY groups directly support the hypothesis that silymarin mitigates methamphetamine-induced neurobehavioral deficits, aligning with its proposed neuroprotective role.
Table 2 presents the time the animals take to locate the hidden platform. The difference in the time it took the animals in the SILY and METH & SILY groups to locate the hidden platform was not statistically significant when compared to the CTRL group. Still, there was a statistically significant increase in the time the animals took in the METH group to locate the hidden platform. The observed reduction in anxiety-like behaviors and improvement in spatial learning in the SILY and METH & SILY groups directly support the hypothesis that silymarin mitigates methamphetamine-induced neurobehavioral deficits, aligning with its proposed neuroprotective role.
Biomarkers of oxidative stress
Data were analyzed using one-way ANOVA followed by Turkey HSD multiple comparisons, and data were considered significant at P<0.01, represented as **.
Table 3 presents the data for some oxidative stress markers.

The results were evaluated after a one-way ANOVA and a Turkey HSD multiple comparison. The data were considered significant at P<0.01, represented as **. In contrast to the CTRL group, the MDA shows a significant increase in the METH group, but other group differences were not statistically significant. Comparing the experimental groups METH, SILY and METH & SILY to the control group, the differences in GSH and SOD representation were not statistically significant.
The significant reduction in MDA levels and stabilization of SOD and GSH levels in the METH & SILY group indicates that silymarin effectively counters methamphetamine-induced oxidative damage, a key mechanism underlying the observed behavioral and histological improvements.
Histological expressions
H&E stain and BS stain for neurofibrillary tangle and senile plaque
Table 4 presents the data for different H&E-sensitive cells.

A one-way ANOVA was used to evaluate the data, and then a Turkey HSD multiple comparison was conducted. The METH group shows a significant increase in amygdala H&E-sensitive cells, while the remaining amygdala groups showed nonsignificant changes compared to the CTRL group. Comparing the experimental groups METH, SILY, and METH & SILY to the CTRL group, the differences in the hippocampal representations were not statistically significant.
The data for different structures that are sensitive to BS stain are shown in Table 4. The data were analyzed using a one-way ANOVA and a Turkey HSD multiple comparison. The data were considered significant at P<0.01, P<0.001, represented as ** and ***, respectively. The METH and METH & SILY groups showed a significant increase in the BS-sensitive structures of the amygdala and hippocampus. In contrast, the SILY group difference in these tissues was not statistically significant compared to the CTRL group.
Amygdala H&E stain
The H&E-sensitive amygdala cells in all the groups of Wistar rats treated are shown in the photomicrograph section (Figures 1, 2, 3, 4 and 5). The METH group shows micrographs of swollen cells, whereas CTRL, SILY and METH & SILY groups show micrographs of normal cell architecture (×400 scale bar, 50 µm).
CA3 H&E stain
Figures 6, 7, 8 and 9 show the tissue and cellular architecture of the hippocampal CA3 region in all treated groups. While the METH group demonstrates mild neuronal shrinkage and ground glass opacity, CTRL, SILY, and METH & SILY groups show normal H&E-sensitive hippocampus cells and tissue in their micrographs (×400 scale bar, 50 µm).
Amygdala BS stain
The count of proteins sensitive to BS stain in the amygdala tissues of animals belonging to all the groups is displayed in the photomicrographs shown in Figures 10, 11, 12 and 13. Normal dendritic and axonal fibers are shown in CTRL and SILY groups. Still, neurofibrillary tangle and senile plaque are seen in the METH group and gradually disappear in METH & SILY group (×400 scale bar, 50 µm).
CA3 BS stain
In the CA3 region of the hippocampus tissues of all the groups, photomicrographs shown in Figures 14, 15, 16, 17, 18, 19, 20 and 21 demonstrate the count of proteins sensitive to the BS stain. CTRL and SILY groups showed the count of normal dendritic and axonal fibers. In contrast, the METH group shows conspicuous neurofibrillary tangle and senile plaque, and METH & SILY group shows dissipating neurofibrillary tangle and senile plaque (×400 scale bar, 50 µm).
Immunohistochemical expressions
MBP and OLIG2 expression
The data for different MBP expressions are shown in Table 5.

A one-way ANOVA and a Turkey HSD multiple comparisons were used to analyze the data and the results were considered nonsignificant when the P were not <0.05. When comparing the experimental groups to their control group (group A), the amygdala and hippocampal MBP expressions show a statistically nonsignificant difference.
Table 5 presents different expressions of OLIG2. The results were analyzed after a one-way ANOVA and a Turkey HSD multiple comparison. The data were considered nonsignificant when the P were not <0.05. When comparing the experimental groups to their control group, the differences in the amygdala and hippocampus are statistically not significant.
Amygdala MBP expression
CTRL and SILY (A and C) groups show normal immunoreactivity and expression of MBP; METH (B) group shows the lowest expression of MBP, followed by METH & SILY (D) group (×100 scale bar, 100 µm).
Hippocampus MBP expression
CTRL and SILY (A and C) groups show normal immunoreactivity and expression of MBP. In contrast, METH (B) group shows the lowest expression of MBP, followed by METH & SILY (D) group (×100 scale bar, 100 µm).
OLIG2 expression
Amygdala OLIG2 expression
CTRL and SILY (A and C) groups show normal OLIG2 expression. In contrast, METH (B) group shows the lowest expression of Olig 2, followed by METH & SILY (D) group (×100 scale bar, 100 µm).
Hippocampus (CA3) OLIG2 expression
Discussion
According to a growing literature of studies, methamphetamine addiction may be associated with emotional and cognitive impairments. Based on a meta-analysis of 17 cross-sectional studies, subjects who had taken methamphetamine showed significantly lower cognitive scores than those who did not abuse drugs [38]. Although they are seeking treatment
for their addiction, people who use methamphetamine for a long time display aggressive, hostile, irritable, depressive, and aggressive behaviors when they first discontinue the use of the substance [39]. A few studies have suggested that certain neuronal deficits may be avoided if there has previously been neuronal protection with medications derived from plants [11, 40]. The current study extends our understanding of the neuroprotective implication of silymarin on adolescent mice in a vulnerable stage of development exposed to neurotoxic compounds, including methamphetamine, during this phase. Our results provide a broad-based demonstration of the effects of silymarin in reversing methamphetamine-induced neurotoxicity as supported by a combination of behavioral analysis with elaborate histological and molecular tests.
Recent studies have looked into the behavioral and cognitive effects of methamphetamine exposure in adolescent rodents. Joca et al. [23] utilized a model of the heightened rates of depression among human adolescent methamphetamine users to show how much exposure to the substance during early adolescence increases depressive-like behavior, as we showed in the tail suspension test. The benefits of using silymarin as a neuroprotectant in this study, however, suggest that it may be possible to reduce the methamphetamine-induced depression and suicidal thoughts that are common in adolescents [23, 41] and adults [42]. A recent study by Buck et al. [43] found that early adolescent exposure to methamphetamine decreased anxiety-like behavior in the open field test. These results align with our EPM test findings, in which the animals spent significant time in the open arm. However, as shown in numerous scientific papers [44, 45], silymarin utilized in our investigation mitigates the decreased anxiety behaviors induced by methamphetamine. Behavioral, biochemical, and histopathological assessments are used simultaneously in the present study to provide a complete picture of silymarin’s neuroprotective potential. Unlike previous research, where these and other experiments provided evidence concerning the effect of a specific intervention on a single result [43-45], in this study, we detailed how molecular changes are associated with functional improvement. This combined approach enhances the validation of silymarin’s therapeutic benefit, which has various applications.
Wistar rats exposed to methamphetamine during adolescence have impaired spatial learning in the Morris water maze and sequential learning in the Cincinnati water maze. These effects do not appear in pre-adolescence or adulthood, indicating that adolescence is a time when methamphetamine-induced cognitive impairments are more likely to occur [46, 47]. If effective neuroprotectants are not used, this impairment continues until adulthood [23, 48]. However, our research found that the active phytochemical molecule from S. marianum (L.) Gaertner seeds alleviated the cognitive impairments caused by methamphetamine, similar to what was shown in a previous study [49] about the cognitive impairment caused by amyloid-beta. Silymarin could reduce cognitive impairment by inhibiting oxidative damage to the brain in terms of lipid peroxidation and antioxidant levels [49]. Our results agree with the results obtained by Lu et al. [49], where the authors reported that the major component of silymarin, silibinin, abolished the memory deficits in an Alzheimer model due to the downregulation of oxidative stress. Likewise, Raza et al. [44] have shown the beneficial impacts of silymarin on behavioral activities after ischemia-induced brain damage. These studies corroborate silymarin’s antioxidant and neurotrophic actions, as found in the present investigation.
The enhancements in anxiety and cognitive behaviors found in the present study by measuring the efficacy of silymarin through the EPM and Morris water maze tasks are in concordance with the hypothesized neuroprotective effects of silymarin. Such behavioral changes indicate their effectiveness and provide biochemical and histological results.
It has been demonstrated that silibinin, one of the silymarin compounds, is a potent antioxidant that reduces lipid peroxidation while maintaining levels of SOD and GSH [50]. This study, however, confirmed the antioxidative characteristics of silymarin and postulated that the primary mechanism responsible for its neuroprotective effectiveness was the decrease in GSH, SOD, and MDA synthesis following an aberrant increase in these substances caused by methamphetamine [50, 51]. These phenomena show the significant involvement of oxidative stress with normalizing MDA and GSH levels in methamphetamine-induced neurotoxicity. Consequently, since silymarin antagonized these alterations, its application confirmed the aforementioned behavioral and cellular recovery evidenced by antioxidant properties.
Thus, the balance of neurobehavioral tests, histological studies, and oxidative stress markers in the current work allows for a comprehensive approach to defining the mechanisms of the neuroprotective action of silymarin. Unlike prior studies, which have mostly examined the individual effects, this integration yields a profound assessment of its therapeutic use.
According to Rothman et al., dopamine, serotonin, and norepinephrine are the main neurotransmitters released by methamphetamine [52]. The amygdala and hippocampus regions of the animal group exposed only to methamphetamine showed signs of swelling and necrotic degradation of neuronal cells, which is likely related to these neurotransmitters, just as previously reported by Panenka et al. [53] in the mesolimbic region of the brain. The use of silymarin prevented the deleterious effects of methamphetamine on animal groups that were exposed to the substances. This silymarin finding is in line with the outcomes of the Dashti-Khavidaki et al. [54] study, which showed that perirenal fat infiltration, pelvic inflammation, interstitial inflammation, and nephrotoxic drug-induced glomerular and tubular necrosis were all decreased by dose-dependent exposure to silymarin. It also prevented ischemia and reperfusion following renal injury. Increased cell density is caused by the activation and migration of microglia cells to the brain’s injured areas in the METH group of H&E expression [31, 55], a process that silymarin prevented in the METH & SILY group. The capacity of silymarin to inhibit and stop microglia from proliferating has not been well documented [56, 57]. Wang et al. [58] reported that silymarin inhibited lipopolysaccharide-induced dopaminergic neurodegeneration in an animal model of Parkinson disease by blocking microglia activation. However, other studies by Hou et al. [56] and Tsai et al. [57] described silymarin’s mechanism of action as blocking inducible nitric oxide synthase production in cellular models to prevent microglia cell activation. Senile plaques and extracellular neurofibrillary tangles, which are indicators of AD, are expressed in this study as a result of methamphetamine exposure [59–61]. As seen in our investigation, treatment with silymarin has reduced these pathological features [43, 49, 50]. The protection of neurons and decreased neurofibrillary tangles and senile plaques observed in silymarin groups reflect its antioxidizing and anti-inflammatory effects. These observations have histological implications as to its potential for treating neurodegenerative and neurotoxic situations.
In the METH group, exposure to methamphetamine induces demyelination and oligodendrocyte disintegration [62], as evidenced by a decrease in the expression of OLIG2 [63] and MBPs [64]. In AD, there is increased buildup of amyloid-beta due to myelin disintegration and a decrease in MBP expression [64]. These changes were inhibited in animal groups exposed to silymarin [63]. Oxidative stress, which is frequently associated with axonal shrinkage, may be the cause of the aberrations in MBP and OLIG2 expression seen in the METH group [65]. Silymarin, on the other hand, was successful in reducing the adverse effects of methamphetamine on oligodendrocytes and the myelin sheath by preventing oxidative stress [49, 63].
In contrast to prior research that has closely described silymarin’s antioxidant and neuroprotective actions, the present work considers the capacity of silymarin to ameliorate methamphetamine-caused abnormalities in neurobehavioral performance and histopathology in adolescent subjects. Of particular interest here are the findings on silymarin for its effects on microglia activation and neurofibrillary tangles, which have received insignificant attention from previous investigations in adolescent models of methamphetamine exposure. The present evidence points to silymarin as a possible therapeutic target for ameliorating the neurotoxic consequences of methamphetamine, especially in adolescents for whom the brain is at its most vulnerable state. The results help continue the clinical trials about the effectiveness of silymarin substances in mental disorders caused by substance use disorders.
Despite the rich data obtained in this study regarding the beneficial effects of silymarin on methamphetamine-induced neurotoxicity, it has several limitations. These limitations include the use of adolescent Wistar rats, which, although it has helped evaluate methamphetamine-induced neurotoxicity, may not be the best model given the physiological and behavioral characteristics of human adolescents. Also, a short period of treatment, 14 days, only hinders the ability to assess the long-term neuroprotective efficacies of silymarin or even the delayed neurotoxic effects of methamphetamine. Moreover, only one dose of silymarin was considered in this study, which may not be sufficient to estimate all the features of the silymarin dose-response relationship and provide an adequate therapeutic and safe dose.
However, more research is needed to show the long-term effectiveness and non-toxicity of silymarin in chronic methamphetamine models. Further studies should also aim to dissect both its neurochemical relationship with differential neurotransmitter systems and its molecular neurobiology to decipher how it exerts neurotrophic effects in adolescent populations. The application of these findings is uniquely relevant to translational neuroscience because it demonstrates that silymarin may present interesting therapeutic potential to manage neurotoxicity in adolescents with methamphetamine use disorder. This finding underlines the need for additional clinical research concerning its uses.
Conclusion
This study reveals that silymarin possesses the ability to reduce methamphetamine-induced impaired behavioral alterations, oxidative stress and histopathological alterations in adolescent rats. Through these findings connecting functional recovery with biochemical and structural changes, the current studies also provide a framework for silymarin’s neuroprotective effects and demonstrate its application for protecting against methamphetamine neurotoxicity in susceptible groups.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Nnamdi Azikiwe University Nnewi, Awka, Nigeria (Code: UNIZIK/FBM/EA/2023/033).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization: Princewill Sopuluchukwu Udodi, Kelechi Blessed Ndubuisi and Roseline Ebube Udodi; Methodology: Damian Nnabuihe Ezejindu, Roseline Ebube Udodi, Uchenna Confidence Mofunanya and Promise Chukwuebuka Onuorah; Investigation: Princewill Sopuluchukwu Udodi, Kelechi Blessed Ndubuisi, Uchenna Confidence Mofunanya, Promise Chukwuebuka Onuorah, Ukamaka Peace Odunwa, Sylvia Chinaecherem Ndinojuo and Roseline Ebube Udodi; Writing the original draft: Kelechi Blessed Ndubuisi, Uchenna Confidence Mofunanya, Promise Chukwuebuka Onuorah, Ukamaka Peace Odunwa and Sylvia Chinaecherem Ndinojuo; Review, editing and supervision: Princewill Sopuluchukwu Udodi and Damian Nnabuihe Ezejindu; Funding acquisition and resources: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Procyshyn RM, Bezchlibnyk-Butler KZ, Kim DD. Clinical handbook of psychotropic drugs. Göttingen: Hogrefe Publishing GmbH; 2023. [DOI:10.1027/00561-000]
- Spear LP. Consequences of adolescent use of alcohol and other drugs: Studies using rodent models. Neurosci Biobehav Rev. 2016; 70:228-243. [DOI:10.1016/j.neubiorev.2016.07.026] [PMID] [PMCID]
- Canton H. United Nations Office on Drugs and Crime- UNODC. London: Routledge; 2017. [Link]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: Classical and emerging mechanisms. Ann N Y Acad Sci. 2010; 1187:101-21. [DOI:10.1111/j.1749-6632.2009.05141.x] [PMID] [PMCID]
- Mehrjerdi ZA, Abarashi Z, Noroozi A, Arshad L, Zarghami M. Correlates of shared methamphetamine injection among methamphetamine-injecting treatment seekers: The first report from Iran. Int J STD AIDS. 2014; 25(6):420-7. [DOI:10.1177/0956462413512806] [PMID]
- UNODC. Executive summary conclusions and policy implications. Vienna: UNODC; 2018. [Link]
- Dumbili EW, Ebuenyi ID. Methamphetamine (Mkpulummiri) use in eastern Nigeria: A new addition to drug users’ repertoire. African J Drug Alcohol Stud. 2022; 20(1):79-89. [DOI:10.4314/ajdas.v20i1.6]
- England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: A neglected element in the electronic cigarette debate. Am J Prev Med. 2015; 49(2):286-93. [DOI:10.1016/j.amepre.2015.01.015] [PMID] [PMCID]
- Gould TJ, Leach PT. Cellular, molecular, and genetic substrates underlying the impact of nicotine on learning. Neurobiol Learn Mem. 2014; 107:108-32. [DOI:10.1016/j.nlm.2013.08.004] [PMID] [PMCID]
- England LJ, Aagaard K, Bloch M, Conway K, Cosgrove K, Grana R, et al. Developmental toxicity of nicotine: A transdisciplinary synthesis and implications for emerging tobacco products. Neurosci Biobehav Rev. 2017; 72:176-89. [DOI:10.1016/j.neubiorev.2016.11.013] [PMID] [PMCID]
- Kris-Etherton PM, Grieger JA, Etherton TD. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot Essent Fatty Acids. 2009; 81(2-3):99-104. [DOI:10.1016/j.plefa.2009.05.011] [PMID]
- Barnard ND, Bush AI, Ceccarelli A, Cooper J, de Jager CA, Erickson KI, et al. Dietary and lifestyle guidelines for the prevention of Alzheimer's disease. Neurobiol Aging. 2014; 35(Suppl 2):S74-8. [DOI:10.1016/j.neurobiolaging.2014.03.033] [PMID]
- Marmouzi I, Bouyahya A, Ezzat SM, El Jemli M, Kharbach M. The food plant Silybum marianum (L.) Gaertn.: Phytochemistry, ethnopharmacology and clinical evidence. J Ethnopharmacol. 2021; 265:113303. [DOI:10.1016/j.jep.2020.113303] [PMID]
- Salehi M, Hassanloo T, Mehrabian S, Farahmand S. Effects of Silybum marianum (L.) Gaertner seeds extract on dermatophytes and saprophytes fungi in vitro compared to clotrimazole. J Pharm Sci. 2011; 16:203-10. [Link]
- Lee JI, Hsu BH, Wu D, Barrett JS. Separation and characterization of silybin, isosilybin, silydianin and silychristin in milk thistle extract by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A. 2006; 1116(1-2):57-68. [DOI:10.1016/j.chroma.2006.03.053] [PMID]
- National Institute on Drug Abuse [NIDA]. Methamphetamine [Internet]? 2025 [Updated 2025 March 5] Available from: [Link]
- Wianowska D, Wiśniewski M. Simplified procedure of silymarin extraction from silybum marianum L. Gaertner. J Chromatogr Sci. 2015; 53(2):366-72. [DOI:10.1093/chromsci/bmu049] [PMID]
- National Research Council. Guide for the care and use of laboratory animals. Washington: The National Academies Press; 2011. [Link]
- Organisation for Economic Co-operation and Development (OECD). Guidelines for the testing of chemicals [Internet]. 2025 [Updated 2025 March 5] Available from: [Link]
- Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav. 2007; 86(2):209-19. [DOI:10.1016/j.pbb.2006.07.033] [PMID] [PMCID]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005; 29(8):1193-205. [DOI:10.1016/j.neubiorev.2005.04.017] [PMID]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology. 1985; 85(3):367-70. [DOI:10.1007/BF00428203] [PMID]
- Joca L, Zuloaga DG, Raber J, Siegel JA. Long-term effects of early adolescent methamphetamine exposure on depression-like behavior and the hypothalamic vasopressin system in mice. Dev Neurosci. 2014; 36(2):108-18. [DOI:10.1159/000360001] [PMID] [PMCID]
- Ahmed Amar SA, Eryilmaz R, Demir H, Aykan S, Demir C. Determination of oxidative stress levels and some antioxidant enzyme activities in prostate cancer. Aging Male. 2019; 22(3):198-206. [DOI:10.1080/13685538.2018.1488955] [PMID]
- Feldman AT, Wolfe D. Tissue processing and hematoxylin and eosin staining. Methods Mol Biol. 2014; 1180:31-43. [DOI:10.1007/978-1-4939-1050-2_3] [PMID]
- Astier A. The different types of necrosis and their histological identifications. Tehran: Educating in Medicine & Pharmacy; 2020. [Link]
- Granado N, Ares-Santos S, Oliva I, O'Shea E, Martin ED, Colado MI, et al. Dopamine D2-receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiol Dis. 2011; 42(3):391-403. [DOI:10.1016/j.nbd.2011.01.033] [PMID]
- Granado N, Lastres-Becker I, Ares-Santos S, Oliva I, Martin E, Cuadrado A,et al. Nrf2 deficiency potentiates methamphetamine-induced dopaminergic axonal damage and gliosis in the striatum. Glia. 2011; 59(12):1850-63. [DOI:10.1002/glia.21229] [PMID]
- Intorcia AJ, Filon JR, Hoffman B, Serrano GE, Sue LI, Beach GT. A modification of the Bielschowsky silver stain for Alzheimer neuritic plaques: Suppression of artifactual staining by pretreatment with oxidizing agents. bioRxiv. [Unpublished]. [DOI:10.1101/570093. Published 2019]
- de Olmos S, Bender C, de Olmos JS, Lorenzo A. Neurodegeneration and prolonged immediate early gene expression throughout cortical areas of the rat brain following acute administration of dizocilpine. Neuroscience. 2009; 164(3):1347-59. [DOI:10.1016/j.neuroscience.2009.09.022] [PMID]
- Ares-Santos S, Granado N, Oliva I, O'Shea E, Martin ED, Colado MI, et al. Dopamine D(1) receptor deletion strongly reduces neurotoxic effects of methamphetamine. Neurobiol Dis. 2012; 45(2):810-20. [DOI:10.1016/j.nbd.2011.11.005] [PMID]
- Espadas I, Darmopil S, Vergaño-Vera E, Ortiz O, Oliva I, Vicario-Abejón C, et al. L-DOPA-induced increase in TH-immunoreactive striatal neurons in parkinsonian mice: Insights into regulation and function. Neurobiol Dis. 2012; 48(3):271-81. [DOI:10.1016/j.nbd.2012.07.012] [PMID]
- Granado N, Escobedo I, O'Shea E, Colado I, Moratalla R. Early loss of dopaminergic terminals in striosomes after MDMA administration to mice. Synapse. 2008; 62(1):80-4. [DOI:10.1002/syn.20466] [PMID]
- Ortiz O, Delgado-García JM, Espadas I, Bahí A, Trullas R, Dreyer JL, et al. Associative learning and CA3-CA1 synaptic plasticity are impaired in D1R null, Drd1a-/-mice and in hippocampal siRNA silenced Drd1a mice. J Neurosci. 2010; 30(37):12288-300. [DOI:10.1523/JNEUROSCI.2655-10.2010] [PMID] [PMCID]
- Aliaghaei A, Meymand AZ, Boroujeni ME, Khodagoli F, Meftahi GH, Hadipour MM, et al. Neuro-restorative effect of sertoli cell transplants in a rat model of amyloid beta toxicity. Behav Brain Res. 2019; 367:158-65. [DOI:10.1016/j.bbr.2019.03.030] [PMID]
- Darmopil S, Muñetón-Gómez VC, de Ceballos ML, Bernson M, Moratalla R. Tyrosine hydroxylase cells appearing in the mouse striatum after dopamine denervation are likely to be projection neurones regulated by L-DOPA. Eur J Neurosci. 2008; 27(3):580-92. [DOI:10.1111/j.1460-9568.2008.06040.x] [PMID]
- Darmopil S, Martín AB, De Diego IR, Ares S, Moratalla R. Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol Psychiatry. 2009; 66(6):603-13. [DOI:10.1016/j.biopsych.2009.04.025] [PMID]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychol Rev. 2007; 17(3):275-97. [DOI:10.1007/s11065-007-9031-0] [PMID]
- Payer DE, Lieberman MD, London ED. Neural correlates of affect processing and aggression in methamphetamine dependence. Arch Gen Psychiatry. 2011; 68(3):271-82. [DOI:10.1001/archgenpsychiatry.2010.154] [PMID] [PMCID]
- Nnama N, Ezejindu DN, Udodi PS, Agulanna AE, Enemuo IC, Okafor EC. Ameliorative effects of palm oil on sniper induced toxicity on the cerebral cortex of adult male Wistar rats. World Journal of Advanced Research and Reviews. 2023; 19(2):809–15. [DOI:10.30574/wjarr.2023.19.2.1496]
- Rawson RA, Gonzales R, Obert JL, McCann MJ, Brethen P. Methamphetamine use among treatment-seeking adolescents in Southern California: Participant characteristics and treatment response. J Subst Abuse Treat. 2005; 29(2):67-74. [DOI:10.1016/j.jsat.2005.04.001] [PMID]
- Murata N, Murakami K, Ozawa Y, Kinoshita N, Irie K, Shirasawa T, et al. Silymarin attenuated the amyloid β plaque burden and improved behavioral abnormalities in an Alzheimer's disease mouse model. Biosci Biotechnol Biochem. 2010; 74(11):2299-306. [DOI:10.1271/bbb.100524] [PMID]
- Buck JM, Morris AS, Weber SJ, Raber J, Siegel JA. Effects of adolescent methamphetamine and nicotine exposure on behavioral performance and MAP-2 immunoreactivity in the nucleus accumbens of adolescent mice. Behav Brain Res. 2017; 323:78-85. [DOI:10.1016/j.bbr.2017.01.010] [PMID] [PMCID]
- Raza SS, Khan MM, Ashafaq M, Ahmad A, Khuwaja G, Khan A, et al. Silymarin protects neurons from oxidative stress associated damages in focal cerebral ischemia: A behavioral, biochemical and immunohistological study in Wistar rats. J Neurol Sci. 2011; 309(1-2):45-54. [DOI:10.1016/j.jns.2011.07.035] [PMID]
- Muley MM, Thakare VN, Patil RR, Kshirsagar AD, Naik SR. Silymarin improves the behavioural, biochemical and histoarchitecture alterations in focal ischemic rats: A comparative evaluation with piracetam and protocatachuic acid. Pharmacol Biochem Behav. 2012; 102(2):286-93. [DOI:10.1016/j.pbb.2012.05.004] [PMID]
- Vorhees CV, Reed TM, Morford LL, Fukumura M, Wood SL, Brown CA, et al. Periadolescent rats (P41-50) exhibit increased susceptibility to D-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (P21-30 or P31-40) or adult rats (P51-60). Neurotoxicol Teratol. 2005; 27(1):117-34. [DOI:10.1016/j.ntt.2004.09.005] [PMID]
- Siegel JA, Park BS, Raber J. Methamphetamine exposure during brain development alters the brain acetylcholine system in adolescent mice. J Neurochem. 2011; 119(1):89-99. [DOI:10.1111/j.1471-4159.2011.07418.x] [PMID] [PMCID]
- Ye T, Pozos H, Phillips TJ, Izquierdo A. Long-term effects of exposure to methamphetamine in adolescent rats. Drug Alcohol Depend. 2014; 138:17-23. [DOI:10.1016/j.drugalcdep.2014.02.021] [PMID] [PMCID]
- Lu P, Mamiya T, Lu LL, Mouri A, Zou L, Nagai T, et al. Silibinin prevents amyloid beta peptide-induced memory impairment and oxidative stress in mice. Br J Pharmacol. 2009; 157(7):1270-7. [DOI:10.1111/j.1476-5381.2009.00295.x] [PMID] [PMCID]
- Yin F, Liu J, Ji X, Wang Y, Zidichouski J, Zhang J. Silibinin: A novel inhibitor of Aβ aggregation. Neurochem Int. 2011; 58(3):399-403. [DOI:10.1016/j.neuint.2010.12.017] [PMID]
- Toklu HZ, Tunali Akbay T, Velioglu-Ogunc A, Ercan F, Gedik N, Keyer-Uysal M, et al. Silymarin, the antioxidant component of Silybum marianum, prevents sepsis-induced acute lung and brain injury. J Surg Res. 2008; 145(2):214-22. [DOI:10.1016/j.jss.2007.03.072] [PMID]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001; 39(1):32-41. [DOI:10.1002/1098-2396(20010101)39:13.0.CO;2-3] [PMID]
- Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, et al. Methamphetamine use: A comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013; 129(3):167-79. [DOI:10.1016/j.drugalcdep.2012.11.016] [PMID]
- Dashti-Khavidaki S, Shahbazi F, Khalili H, Lessan-Pezeshki M. Potential renoprotective effects of silymarin against nephrotoxic drugs: A review of literature. J Pharm Pharm Sci. 2012; 15(1):112-23. [DOI:10.18433/J3F88S] [PMID]
- Granado M, Fernández N, Monge L, Figueras JC, Carreño-Tarragona G, Amor S,et al. Effects of coronary ischemia-reperfusion in a rat model of early overnutrition. Role of angiotensin receptors. Plos One. 2013; 8(2):e54984. [DOI:10.1371/journal.pone.0054984] [PMID] [PMCID]
- Hou YC, Liou KT, Chern CM, Wang YH, Liao JF, Chang S, et al. Preventive effect of silymarin in cerebral ischemia-reperfusion-induced brain injury in rats possibly through impairing NF-κB and STAT-1 activation. Phytomedicine. 2010; 17(12):963-73. [DOI:10.1016/j.phymed.2010.03.012] [PMID]
- Tsai MJ, Liao JF, Lin DY, Huang MC, Liou DY, Yang HC, et al. Silymarin protects spinal cord and cortical cells against oxidative stress and lipopolysaccharide stimulation. Neurochem Int. 2010; 57(8):867-75. [DOI:10.1016/j.neuint.2010.09.005] [PMID]
- Wang MJ, Lin WW, Chen HL, Chang YH, Ou HC, Kuo JS, et al. Silymarin protects dopaminergic neurons against lipopolysaccharide-induced neurotoxicity by inhibiting microglia activation. Eur J Neurosci. 2002; 16(11):2103-12. [DOI:10.1046/j.1460-9568.2002.02290.x] [PMID]
- Mayeux R. Epidemiology of neurodegeneration. Annu Rev Neurosci. 2003; 26:81-104. [DOI:10.1146/annurev.neuro.26.043002.094919] [PMID]
- Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011; 70(4):532-40. [DOI:10.1002/ana.22615] [PMID] [PMCID]
- Schnabel J. Amyloid: Little proteins, big clues. Nature. 2011; 475(7355):S12-4. [DOI:10.1038/475S12a] [PMID]
- Yang L, Guo Y, Huang M, Wu X, Li X, Chen G, et al. Thioredoxin-1 protects spinal cord from demyelination induced by methamphetamine through suppressing endoplasmic reticulum stress and inflammation. Front Neurol. 2018; 9:49. [DOI:10.3389/fneur.2018.00049] [PMID] [PMCID]
- Aboelwafa HR, El-Kott AF, Abd-Ella EM, Yousef HN. The possible neuroprotective effect of silymarin against aluminum chloride-prompted alzheimer's-like disease in rats. Brain Sci. 2020; 10(9):628. [DOI:10.3390/brainsci10090628] [PMID] [PMCID]
- Papuć E, Rejdak K. The role of myelin damage in Alzheimer's disease pathology. Arch Med Sci. 2018; 16(2):345-51. [DOI:10.5114/aoms.2018.76863] [PMID] [PMCID]
- Zhu YJ, Zeng T, Zhu YB, Yu SF, Wang QS, Zhang LP, et al. Effects of acrylamide on the nervous tissue antioxidant system and sciatic nerve electrophysiology in the rat. Neurochem Res. 2008; 33(11):2310-7. [DOI:10.1007/s11064-008-9730-9] [PMID]
Type of Study: Research |
Subject:
General
Received: 2024/09/6 | Accepted: 2025/01/2 | Published: 2025/04/1
Received: 2024/09/6 | Accepted: 2025/01/2 | Published: 2025/04/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |