Tue, Dec 30, 2025
Volume 11, Issue 1 (Winter 2025)
Caspian J Neurol Sci 2025, 11(1): 51-57 |
Back to browse issues page
Ethics code: IR.GUMS.REC.1403.091
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Souri Z, Eghrari Gigloo M, Soltanipour S, Sarshad H, Biazar G. Investigating the Relationship Between General Anesthesia and Early Brain Atrophy: A Case-control Study. Caspian J Neurol Sci 2025; 11 (1) :51-57
URL: http://cjns.gums.ac.ir/article-1-740-en.html
URL: http://cjns.gums.ac.ir/article-1-740-en.html
1- Department of Radiology, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Anesthesiology, Anesthesiology Research Center, Alzahra hospital, Guilan University of Medical Sciences, Rasht, Iran.
3- Department of Community Medicine, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Anesthesiology, Anesthesiology Research Center, Alzahra hospital, Guilan University of Medical Sciences, Rasht, Iran. ,gelarehbiazar1386@gmail.com
2- Department of Anesthesiology, Anesthesiology Research Center, Alzahra hospital, Guilan University of Medical Sciences, Rasht, Iran.
3- Department of Community Medicine, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Anesthesiology, Anesthesiology Research Center, Alzahra hospital, Guilan University of Medical Sciences, Rasht, Iran. ,
Full-Text [PDF 1064 kb]
(357 Downloads)
| Abstract (HTML) (955 Views)
Full-Text: (406 Views)
Introduction
After age 50, the brain volume decreases until the age of 86 and then stops. However, the decrease in brain volume is not only influenced by age. Other factors are considered to accelerate this process. Brain atrophy is a common finding in older people that can be detected by magnetic resonance imaging (MRI). In some situations, the process of brain atrophy does not correspond to age and is revealed prematurely [1-5]. So far, the predisposing factors of this situation are not fully understood. Conditions such as higher and lower blood pressure than normal [6, 7], diabetes mellitus [8], multiple sclerosis [9, 10] and traumatic brain injury [11] are known to be the cause of brain atrophy. Some studies have found males to be more susceptible [12]. Recently, the neurotoxicity of anesthetic drugs has been highly focused on as an underlying cause of early brain atrophy. However, very little has yet been discovered about the potential effects of general anesthesia (GA) on brain structure. Notably, human evidence is limited [13]. A few studies have shown that anesthetics can reduce the brain’s gray matter volume. A recent review article reported a significant association between brain anesthetic exposure and decreased white matter volume [14].
In contrast, other volumetric analyses demonstrated that while various white matter volumes decreased during GA, some gray matter volumes increased and all these changes were transient [15]. Animal studies have established that exposure of the developing brain to anesthetics leads to permanent changes in the nervous structure. In human studies, however, many confounding factors and the influence of environmental and genetic factors should be considered [16]. Young et al. investigated the neurotoxic effects of anesthetic drugs on the developing brains of monkeys. Following the exposure to GA, they found a significant decrease in the white matter volume. The issue was confirmed in another animal study on mice [17, 18]. In general, the results of these studies do not recommend depriving people of analgesia and anesthesia, but based on the available evidence, it is recommended that non-emergency surgeries be postponed until the age of four years [19]. Brain atrophy causes numerous cognitive and behavioral problems. Studies have shown that early and progressive brain atrophy is associated with later dementia in an asymptomatic middle-aged individual [20]. Despite the importance of the topic, it has rarely been investigated in our country. Considering the limitations of the research and the conflicting results, this study was designed with the aim of whether GA can be one of the underlying factors to accelerate the process of brain atrophy or not?
Materials and Methods
After the approval of the Research Ethics Committee of the Guilan University of Medical Sciences, Rasht City, Iran, this case-control study was performed in Poursina and Razi hospitals affiliated with Guilan University of Medical Sciences from May 2024 to August 2024.
The inclusion criteria included patients between 40 and 65 years diagnosed with early brain atrophy based on the valid diagnostic criteria [21, 22]. The exclusion criteria were dissatisfaction with participating in the study and inability to communicate properly due to speech problems or differences in accent and language.
Diagnostic criteria for early brain atrophy
First, there is mild to moderate brain volume reduction, including the size of the ventricles, the size of the sulci, which are more commonly affected in the frontal and parietal lobes, the atrophy of the inner part of the temporal lobe in a mild form, as well as the hippocampal sulcus.
Second, there is an enlargement of the spaces around the vessels (Virchow-robin) in the basal ganglia near the anterior commissure, which is seen in the CT scan, the white matter of the centrum ovale (close to the vertex, which is seen in the MRI), the mesencephalon (seen on MRI).
Third, there are vascular wall changes, including elongation and tortuosity of the vascular wall, an increase in wall thickness, and calcification in the vessel wall.
Fourth, there are vascular changes, including ischemic changes of the white matter in the form of points and interconnected (microvascular ischemic change), lacunar infarcts and micro-hemorrhages.
Fifth, an iron accumulation is seen in MRI in the globus pallidus, putamen and dentate nuclei.
Sixth, there are calcifications in CT scans in the globus pallidus, pineal gland, choroid plexus (except in the Luschka foramen), cerebral falx and sometimes with bone changes in the cerebral falx [21].
The diagnosis was based on the interpretation of MRI images (1.5 Tesla MRI machine, Phillips Ingenico model, made in the Netherlands) without contrast protocol, T1 axial, T2 axial and coronal and sagittal, FLAIR axial, DWI axial, DC axial, exponential axial sequences and 5 mm thickness and 30 slices, which an expert radiologist confirmed.
Patients diagnosed with early brain atrophy according to the mentioned criteria were included as the case group, and those who did not meet the criteria were included as the control group. According to the study of Goettel et al. [1] a sample size of 27 cases in each group was considered sufficient. Then, a checklist containing items such as age, gender, education level, body mass index (BMI), habits, co-morbidities, history of receiving GA, age and number of times receiving GA was completed through a direct interview. Finally, the achieved data were compared between cases and control groups. First, the study’s purpose and the information’s confidentiality were explained to the patients, and informed consent was obtained. Patients diagnosed with early brain atrophy according to the mentioned criteria were included in the case group, and those who did not meet the criteria were included in the control group. Then, a checklist containing items such as age, gender, education level, BMI, habits, co-morbidities, history of receiving GA, age and number of times receiving GA was completed through a direct interview. Finally, the achieved data were compared between cases and control groups.
Statistical analysis
The collected data were analyzed using SPSS software, version 21. Two independent sample t-test and chi-square test were used to analyze the data. If the data did not follow a normal distribution, it was replaced by the equivalent non-parametric test. Logistic regression analysis was also used to control the effect of confounding variables and calculate the odds ratio. Statistical significance was considered as P<0.05.
Results
Finally, the data from 54 people were analyzed. As shown in Table 1, in terms of demographic data, no significant difference was observed between the two groups of case and control (P>0.05).
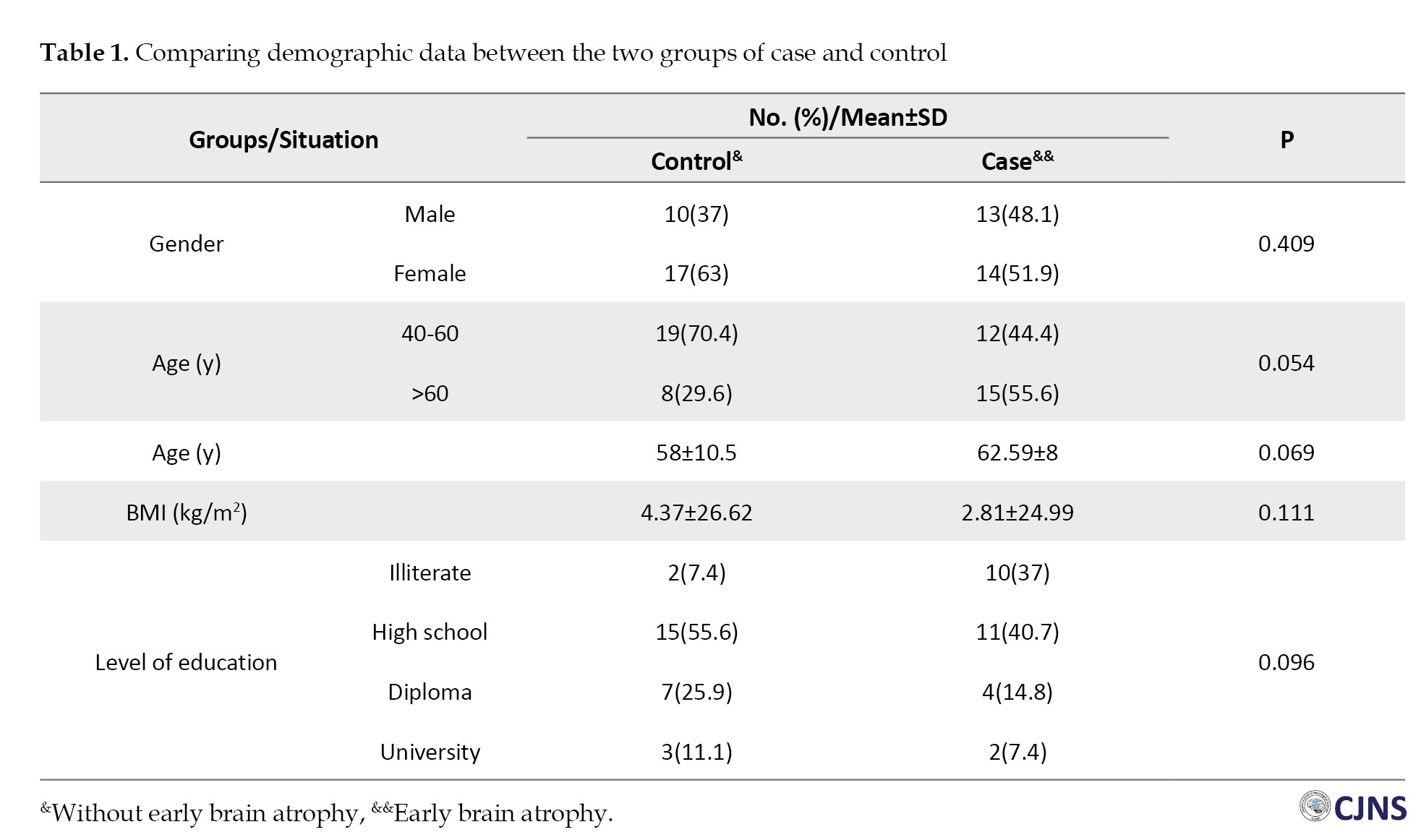
Their mean age was higher in the early brain atrophy group than in the control group, 62.59±8 vs 58±10.5 years; however, the difference was not significant (P=0.069). Two groups were also compared according to several risk factors: diabetes (P=0.054), hypertension (P=0.248), stroke (P=0.34) and alcohol consumption addiction (P=0.552). Also, no significant difference was observed. Although early brain atrophy was more common among diabetic patients, the difference was not statistically significant. Regarding the history of receiving GA as the main variable of the study, the difference between the two groups was not significant (P=0.78) (Table 2).

Finally, multivariate regression analysis found no correlation between the studied variables and early brain atrophy. In addition, the number of exposures to GA did not significantly affect the results (Table 3).

Discussion
Theories have been proposed regarding the underlying mechanisms of anesthetic drugs related to adverse effects on the brain. Shrinking of glial cells due to reduced activity [23, 24], the effect of GA on the glymphatic system, which is responsible for cleaning the nervous system [25, 26], the effect of anesthetic drugs on the microtubules system, which is part of the axonal structure and [27, 28], increased intracranial pressure due to the vasodilatory effects of anesthetic drugs have been suggested [29]. Based on the results of this research, there was no statistically significant difference between the demographic data of the two groups, including age, BMI, gender and education. Although diabetes, alcohol and addiction were more common in the brain atrophy group, the difference was not significant. The two groups did not differ in terms of concomitant diseases, drug abuse and alcohol consumption; no significant difference was observed in terms of the main variable, which was the history of receiving GA. The results of this study were contrary to previous research conducted at the same university. In two recent studies, the relationship between early exposure to GA and the development of attention deficit hyperactivity disorder and autism spectrum disorder was investigated [30, 31], and another study examined the relationship between anesthesia in childbirth and child behavioral disorders. The differences in these studies could justify this inconsistency. The most important question in this study was the history of receiving GA and the age of exposure was much more important than that. In the previous studies, parents of children with behavioral disorders were interviewed. The parent’s answer to the question of whether your child had received anesthesia for any reason in the first three years of birth is much more accurate compared to the answer of middle-aged or older people. This issue is one of the most influential factors in the difference between the results obtained from this research and our previous studies.
Another point is that according to Iran’s cultural and legal insight, people hardly provide an accurate history of alcohol and substance use, which is one of the significant differences between the results of this study and similar studies in some other countries. Walsh et al. (2021) described that preterm infants who were exposed to surgery under GA showed reduced white matter volume and neurodevelopmental impairment at the age of two years. They also reported that longer exposure to GA was associated with a greater decrease in white matter volume and poor cognitive outcomes [32]. Salaün et al. demonstrated that children who had received an early single GA procedure during infancy developed lasting reduction of the periaqueductal gray matter [33]. In a different study, Tang et al. used diffusion tensor imaging to investigate the white matter microstructure of healthy elderly subjects during GA. They found transient decreases throughout the brain. Other diffusion tensor imaging metrics, such as axial diffusivity, radial diffusivity, and mean diffusivity, increased during GA. Volumetric analysis showed an increase in gray matter volume and a decrease in white matter volume [15]. Block et al. in a structural neuroimaging study, compared two groups of children who had operations during infancy with the non-exposed group regarding white matter integrity. They reported that the two groups did not differ regarding gray matter, total intracranial, and cerebrospinal fluid volumes. However, the exposed group showed a significant decrease in integrity and volume of white matter [13]. Unlike animal studies, controversial findings have been reported in human works, which can be justified by the differences in the studied population and methodologies. The differences in socioeconomic and demographic data, the diagnostic tools and criteria and the data collection methods should be considered. Of course, the results of a direct interview are more reliable than phone calls or sending emails. In addition, in retrospective studies, we are unaware of the type and dose of anesthesia, time, single or multiple exposures, or duration of exposure. Also, unlike animal studies, the fully controlled situation in experimental studies cannot be considered in human studies.
Study suggestions
It is suggested that more studies should be performed to isolate the possible effects of surgery stimulation versus anesthesia. Furthermore, the effects of different surgical procedures, invasiveness, and anesthesia for nonsurgical procedures should be investigated.
Conclusion
Based on the study results, although co-morbidities such as diabetes and older age were more affected by early brain atrophy, no significant relationship was observed between receiving GA and premature brain atrophy. Due to the limited research and remarkable limitations of this study, further well-designed.
Study limitations
Considering the age of the patients who suffered from early brain atrophy, it was very likely that they could not provide accurate information about receiving GA in the first years of life. Indeed, they mainly reported the history of surgeries under GA in adulthood. The risk of forgetfulness is much more pronounced among people with brain atrophy. Another limitation of this study was the possibility of not providing a detailed history of alcoholism and addiction due to social stigma. Moreover, according to current studies, brain atrophy affects the patient’s ability to communicate, speak, and retain memory. The other limitation was the impossibility of examining all the risk factors of early brain atrophy, such as individuals’ nutritional status. Studies demonstrated that diet and daily consumption of some food categories are important in delaying and accelerating this process.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Ethics Research Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1403.091) and informed consent was obtained from all Patients who agreed to participate.
Funding
This study Was extracted from medical thesis of Mahsa Eghrari Gigloo, approved by Giulan University of Medical Sciences (Grand No.: 1488).
Authors contributions
Conceptualization and study design: Zobin Souri and Gelareh Biazar; Date analysis and data interpretation: Soheil Soltanipour and Hakimeh Sarshad; Provision of study material or patients: Mahsa Eghrari Gigloo; Statistical analysis: Soheil Soltanipour; Critical revision: Gelareh Biazar and Hakimeh Sarshad; Final approval: Zobin Souri and Gelareh Biazar.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank Anesthesiology Research Center members of Guilan University of Medical Sciences for their collaboration in this study.
References
After age 50, the brain volume decreases until the age of 86 and then stops. However, the decrease in brain volume is not only influenced by age. Other factors are considered to accelerate this process. Brain atrophy is a common finding in older people that can be detected by magnetic resonance imaging (MRI). In some situations, the process of brain atrophy does not correspond to age and is revealed prematurely [1-5]. So far, the predisposing factors of this situation are not fully understood. Conditions such as higher and lower blood pressure than normal [6, 7], diabetes mellitus [8], multiple sclerosis [9, 10] and traumatic brain injury [11] are known to be the cause of brain atrophy. Some studies have found males to be more susceptible [12]. Recently, the neurotoxicity of anesthetic drugs has been highly focused on as an underlying cause of early brain atrophy. However, very little has yet been discovered about the potential effects of general anesthesia (GA) on brain structure. Notably, human evidence is limited [13]. A few studies have shown that anesthetics can reduce the brain’s gray matter volume. A recent review article reported a significant association between brain anesthetic exposure and decreased white matter volume [14].
In contrast, other volumetric analyses demonstrated that while various white matter volumes decreased during GA, some gray matter volumes increased and all these changes were transient [15]. Animal studies have established that exposure of the developing brain to anesthetics leads to permanent changes in the nervous structure. In human studies, however, many confounding factors and the influence of environmental and genetic factors should be considered [16]. Young et al. investigated the neurotoxic effects of anesthetic drugs on the developing brains of monkeys. Following the exposure to GA, they found a significant decrease in the white matter volume. The issue was confirmed in another animal study on mice [17, 18]. In general, the results of these studies do not recommend depriving people of analgesia and anesthesia, but based on the available evidence, it is recommended that non-emergency surgeries be postponed until the age of four years [19]. Brain atrophy causes numerous cognitive and behavioral problems. Studies have shown that early and progressive brain atrophy is associated with later dementia in an asymptomatic middle-aged individual [20]. Despite the importance of the topic, it has rarely been investigated in our country. Considering the limitations of the research and the conflicting results, this study was designed with the aim of whether GA can be one of the underlying factors to accelerate the process of brain atrophy or not?
Materials and Methods
After the approval of the Research Ethics Committee of the Guilan University of Medical Sciences, Rasht City, Iran, this case-control study was performed in Poursina and Razi hospitals affiliated with Guilan University of Medical Sciences from May 2024 to August 2024.
The inclusion criteria included patients between 40 and 65 years diagnosed with early brain atrophy based on the valid diagnostic criteria [21, 22]. The exclusion criteria were dissatisfaction with participating in the study and inability to communicate properly due to speech problems or differences in accent and language.
Diagnostic criteria for early brain atrophy
First, there is mild to moderate brain volume reduction, including the size of the ventricles, the size of the sulci, which are more commonly affected in the frontal and parietal lobes, the atrophy of the inner part of the temporal lobe in a mild form, as well as the hippocampal sulcus.
Second, there is an enlargement of the spaces around the vessels (Virchow-robin) in the basal ganglia near the anterior commissure, which is seen in the CT scan, the white matter of the centrum ovale (close to the vertex, which is seen in the MRI), the mesencephalon (seen on MRI).
Third, there are vascular wall changes, including elongation and tortuosity of the vascular wall, an increase in wall thickness, and calcification in the vessel wall.
Fourth, there are vascular changes, including ischemic changes of the white matter in the form of points and interconnected (microvascular ischemic change), lacunar infarcts and micro-hemorrhages.
Fifth, an iron accumulation is seen in MRI in the globus pallidus, putamen and dentate nuclei.
Sixth, there are calcifications in CT scans in the globus pallidus, pineal gland, choroid plexus (except in the Luschka foramen), cerebral falx and sometimes with bone changes in the cerebral falx [21].
The diagnosis was based on the interpretation of MRI images (1.5 Tesla MRI machine, Phillips Ingenico model, made in the Netherlands) without contrast protocol, T1 axial, T2 axial and coronal and sagittal, FLAIR axial, DWI axial, DC axial, exponential axial sequences and 5 mm thickness and 30 slices, which an expert radiologist confirmed.
Patients diagnosed with early brain atrophy according to the mentioned criteria were included as the case group, and those who did not meet the criteria were included as the control group. According to the study of Goettel et al. [1] a sample size of 27 cases in each group was considered sufficient. Then, a checklist containing items such as age, gender, education level, body mass index (BMI), habits, co-morbidities, history of receiving GA, age and number of times receiving GA was completed through a direct interview. Finally, the achieved data were compared between cases and control groups. First, the study’s purpose and the information’s confidentiality were explained to the patients, and informed consent was obtained. Patients diagnosed with early brain atrophy according to the mentioned criteria were included in the case group, and those who did not meet the criteria were included in the control group. Then, a checklist containing items such as age, gender, education level, BMI, habits, co-morbidities, history of receiving GA, age and number of times receiving GA was completed through a direct interview. Finally, the achieved data were compared between cases and control groups.
Statistical analysis
The collected data were analyzed using SPSS software, version 21. Two independent sample t-test and chi-square test were used to analyze the data. If the data did not follow a normal distribution, it was replaced by the equivalent non-parametric test. Logistic regression analysis was also used to control the effect of confounding variables and calculate the odds ratio. Statistical significance was considered as P<0.05.
Results
Finally, the data from 54 people were analyzed. As shown in Table 1, in terms of demographic data, no significant difference was observed between the two groups of case and control (P>0.05).

Their mean age was higher in the early brain atrophy group than in the control group, 62.59±8 vs 58±10.5 years; however, the difference was not significant (P=0.069). Two groups were also compared according to several risk factors: diabetes (P=0.054), hypertension (P=0.248), stroke (P=0.34) and alcohol consumption addiction (P=0.552). Also, no significant difference was observed. Although early brain atrophy was more common among diabetic patients, the difference was not statistically significant. Regarding the history of receiving GA as the main variable of the study, the difference between the two groups was not significant (P=0.78) (Table 2).

Finally, multivariate regression analysis found no correlation between the studied variables and early brain atrophy. In addition, the number of exposures to GA did not significantly affect the results (Table 3).

Discussion
Theories have been proposed regarding the underlying mechanisms of anesthetic drugs related to adverse effects on the brain. Shrinking of glial cells due to reduced activity [23, 24], the effect of GA on the glymphatic system, which is responsible for cleaning the nervous system [25, 26], the effect of anesthetic drugs on the microtubules system, which is part of the axonal structure and [27, 28], increased intracranial pressure due to the vasodilatory effects of anesthetic drugs have been suggested [29]. Based on the results of this research, there was no statistically significant difference between the demographic data of the two groups, including age, BMI, gender and education. Although diabetes, alcohol and addiction were more common in the brain atrophy group, the difference was not significant. The two groups did not differ in terms of concomitant diseases, drug abuse and alcohol consumption; no significant difference was observed in terms of the main variable, which was the history of receiving GA. The results of this study were contrary to previous research conducted at the same university. In two recent studies, the relationship between early exposure to GA and the development of attention deficit hyperactivity disorder and autism spectrum disorder was investigated [30, 31], and another study examined the relationship between anesthesia in childbirth and child behavioral disorders. The differences in these studies could justify this inconsistency. The most important question in this study was the history of receiving GA and the age of exposure was much more important than that. In the previous studies, parents of children with behavioral disorders were interviewed. The parent’s answer to the question of whether your child had received anesthesia for any reason in the first three years of birth is much more accurate compared to the answer of middle-aged or older people. This issue is one of the most influential factors in the difference between the results obtained from this research and our previous studies.
Another point is that according to Iran’s cultural and legal insight, people hardly provide an accurate history of alcohol and substance use, which is one of the significant differences between the results of this study and similar studies in some other countries. Walsh et al. (2021) described that preterm infants who were exposed to surgery under GA showed reduced white matter volume and neurodevelopmental impairment at the age of two years. They also reported that longer exposure to GA was associated with a greater decrease in white matter volume and poor cognitive outcomes [32]. Salaün et al. demonstrated that children who had received an early single GA procedure during infancy developed lasting reduction of the periaqueductal gray matter [33]. In a different study, Tang et al. used diffusion tensor imaging to investigate the white matter microstructure of healthy elderly subjects during GA. They found transient decreases throughout the brain. Other diffusion tensor imaging metrics, such as axial diffusivity, radial diffusivity, and mean diffusivity, increased during GA. Volumetric analysis showed an increase in gray matter volume and a decrease in white matter volume [15]. Block et al. in a structural neuroimaging study, compared two groups of children who had operations during infancy with the non-exposed group regarding white matter integrity. They reported that the two groups did not differ regarding gray matter, total intracranial, and cerebrospinal fluid volumes. However, the exposed group showed a significant decrease in integrity and volume of white matter [13]. Unlike animal studies, controversial findings have been reported in human works, which can be justified by the differences in the studied population and methodologies. The differences in socioeconomic and demographic data, the diagnostic tools and criteria and the data collection methods should be considered. Of course, the results of a direct interview are more reliable than phone calls or sending emails. In addition, in retrospective studies, we are unaware of the type and dose of anesthesia, time, single or multiple exposures, or duration of exposure. Also, unlike animal studies, the fully controlled situation in experimental studies cannot be considered in human studies.
Study suggestions
It is suggested that more studies should be performed to isolate the possible effects of surgery stimulation versus anesthesia. Furthermore, the effects of different surgical procedures, invasiveness, and anesthesia for nonsurgical procedures should be investigated.
Conclusion
Based on the study results, although co-morbidities such as diabetes and older age were more affected by early brain atrophy, no significant relationship was observed between receiving GA and premature brain atrophy. Due to the limited research and remarkable limitations of this study, further well-designed.
Study limitations
Considering the age of the patients who suffered from early brain atrophy, it was very likely that they could not provide accurate information about receiving GA in the first years of life. Indeed, they mainly reported the history of surgeries under GA in adulthood. The risk of forgetfulness is much more pronounced among people with brain atrophy. Another limitation of this study was the possibility of not providing a detailed history of alcoholism and addiction due to social stigma. Moreover, according to current studies, brain atrophy affects the patient’s ability to communicate, speak, and retain memory. The other limitation was the impossibility of examining all the risk factors of early brain atrophy, such as individuals’ nutritional status. Studies demonstrated that diet and daily consumption of some food categories are important in delaying and accelerating this process.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Ethics Research Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1403.091) and informed consent was obtained from all Patients who agreed to participate.
Funding
This study Was extracted from medical thesis of Mahsa Eghrari Gigloo, approved by Giulan University of Medical Sciences (Grand No.: 1488).
Authors contributions
Conceptualization and study design: Zobin Souri and Gelareh Biazar; Date analysis and data interpretation: Soheil Soltanipour and Hakimeh Sarshad; Provision of study material or patients: Mahsa Eghrari Gigloo; Statistical analysis: Soheil Soltanipour; Critical revision: Gelareh Biazar and Hakimeh Sarshad; Final approval: Zobin Souri and Gelareh Biazar.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank Anesthesiology Research Center members of Guilan University of Medical Sciences for their collaboration in this study.
References
- Goettel N, Mistridis P, Berres M, Reinhardt J, Stippich C, Monsch AU, et al. Association between changes in cerebral grey matter volume and postoperative cognitive dysfunction in elderly patients: Study protocol for a prospective observational cohort study. BMC Anesthesiol. 2016; 16(1):118. [DOI:10.1186/s12871-016-0285-z] [PMID] [PMCID]
- Jack CR Jr, Shiung MM, Gunter JL, O'Brien PC, Weigand SD, Knopman DS, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004; 62(4):591-600. [DOI:10.1212/01.WNL.0000110315.26026.EF] [PMID] [PMCID]
- Jack CR Jr, Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005; 65(8):1227-31. [DOI:10.1212/01.wnl.0000180958.22678.91] [PMID] [PMCID]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007; 21(4):412-8. [DOI:10.1037/0894-4105.21.4.412] [PMID] [PMCID]
- Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, et al. Changes in premorbid brain volume predict Alzheimer's disease pathology. Neurology. 2003; 61(4):487-92. [DOI:10.1212/01.WNL.0000079053.77227.14] [PMID]
- Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology. 1999; 52(8):1687-9. [DOI:10.1212/WNL.52.8.1687] [PMID]
- Jochemsen HM, Muller M, Visseren FL, Scheltens P, Vincken KL, Mali WP, et al. Blood pressure and progression of brain atrophy: The SMART-MR study. JAMA Neurol. 2013; 70(8):1046-53. [DOI:10.1001/jamaneurol.2013.217] [PMID]
- Habes M, Jacobson AM, Braffett BH, Rashid T, Ryan CM, Shou H, et al. Patterns of regional brain atrophy and brain aging in middle- and older-aged adults with type 1 diabetes. JAMA Netw Open. 2023; 6(6):e2316182. [DOI:10.1001/jamanetworkopen.2023.16182] [PMID] [PMCID]
- Fallah Arzpeyma S, Janeshin S, Soofi Afshar N, Saberi A, Ghalyanchi Langroodi H, Ghaffari ME, et al. Brain MRI volumetric assessment of patients with multiple sclerosis: the volume of basal ganglia, thalamus, and posterior fossa. Basic Clin Neurosci. 2023; 14(6):741-52. [DOI:10.32598/bcn.2023.1324.4] [PMID] [PMCID]
- Lomer NB, Asalemi KA, Saberi A, Sarlak K. Predictors of multiple sclerosis progression: A systematic review of conventional magnetic resonance imaging studies. Plos One. 2024; 19(4):e0300415. [DOI:10.1371/journal.pone.0300415] [PMID] [PMCID]
- Angoa-Pérez M, Zagorac B, Anneken JH, Briggs DI, Winters AD, Greenberg JM, et al. Repetitive, mild traumatic brain injury results in a progressive white matter pathology, cognitive deterioration, and a transient gut microbiota dysbiosis. Sci Rep. 2020; 10(1):8949. [DOI:10.1038/s41598-020-65972-4] [PMID] [PMCID]
- Voskuhl RR, Patel K, Paul F, Gold SM, Scheel M, Kuchling J, et al. Sex differences in brain atrophy in multiple sclerosis. Biol Sex Differ. 2020; 11(1):49. [DOI:10.1186/s13293-020-00326-3] [PMID] [PMCID]
- Block RI, Magnotta VA, Bayman EO, Choi JY, Thomas JJ, Kimble KK. Are anesthesia and surgery during infancy associated with decreased white matter integrity and volume during childhood? Anesthesiology. 2017; 127(5):788-99. [DOI:10.1097/ALN.0000000000001808] [PMID]
- Wu Z, Yu W, Song Y, Zhao P. General anaesthesia, the developing brain, and cerebral white matter alterations: A narrative review. Br J Anaesth. 2023; 131(6):1022-9.[DOI:10.1016/j.bja.2023.09.008] [PMID]
- Tang CY, Wang VX, Lun MY, Mincer JS, Ng JC, Brallier JW, et al. Transient changes in white matter microstructure during general anesthesia. Plos One. 2021; 16(3):e0247678. [DOI:10.1371/journal.pone.0247678] [PMID] [PMCID]
- Kim J, Barcus R, Lipford ME, Yuan H, Ririe DG, Jung Y, et al. Effects of multiple anesthetic exposures on rhesus macaque brain development: A longitudinal structural MRI analysis. Cereb Cortex. 2024; 34(1):bhad463. [DOI:10.1093/cercor/bhad463] [PMID] [PMCID]
- Young JT, Vlasova RM, Howell BR, Knickmeyer RC, Morin E, Kuitchoua KI, et al. General anaesthesia during infancy reduces white matter micro-organisation in developing rhesus monkeys. Br J Anaesth. 2021; 126(4):845-53. [DOI:10.1016/j.bja.2020.12.029] [PMID] [PMCID]
- Wu Z, Xue H, Gao Q, Zhao P. Effects of early postnatal sevoflurane exposure on oligodendrocyte maturation and myelination in cerebral white matter of the rat. Biomed Pharmacother. 2020; 131:110733. [DOI:10.1016/j.biopha.2020.110733] [PMID]
- Niu Y, Yan J, Jiang H. Anesthesia and developing brain: What have we learned from recent studies. Front Mol Neurosci. 2022; 15:1017578. [DOI:10.3389/fnmol.2022.1017578] [PMID] [PMCID]
- O'Brien JT, Firbank MJ, Ritchie K, Wells K, Williams GB, Ritchie CW, et al. Association between midlife dementia risk factors and longitudinal brain atrophy: The PREVENT-Dementia study. J Neurol Neurosurg Psychiatry. 2020; 91(2):158-61. [DOI:10.1136/jnnp-2019-321652] [PMID]
- Dixon AK, Adam A, Schaefer-Prokop C, Gillard JH. Grainger & Allison’s diagnostic radiology: A textbook of medical imaging. Amsterdam: Elsevier; 2020. [Link]
- Singh S, Bajorek B. Defining 'elderly' in clinical practice guidelines for pharmacotherapy. Pharm Pract. 2014; 12(4):489. [DOI:10.4321/S1886-36552014000400007] [PMID] [PMCID]
- Mandl RC, Schnack HG, Zwiers MP, Kahn RS, Hulshoff Pol HE. Functional diffusion tensor imaging at 3 Tesla. Front Hum Neurosci. 2013; 7:817. [DOI:10.3389/fnhum.2013.00817] [PMID] [PMCID]
- Mandl RC, Schnack HG, Zwiers MP, van der Schaaf A, Kahn RS, Hulshoff Pol HE. Functional diffusion tensor imaging: measuring task-related fractional anisotropy changes in the human brain along white matter tracts. Plos One. 2008; 3(11):e3631. [DOI:10.1371/journal.pone.0003631] [PMID] [PMCID]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013; 342(6156):373-7. [DOI:10.1126/science.1241224] [PMID] [PMCID]
- Gao X, Ming J, Liu S, Lai B, Fang F, Cang J. Sevoflurane enhanced the clearance of Aβ1-40 in hippocampus under surgery via up-regulating AQP-4 expression in astrocyte. Life Sci. 2019; 221:143-51. [DOI:10.1016/j.lfs.2019.02.024] [PMID]
- Conde C, Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009; 10(5):319-32. [DOI:10.1038/nrn2631] [PMID]
- Lasser M, Tiber J, Lowery LA. The role of the microtubule cytoskeleton in neurodevelopmental disorders. Front Cell Neurosci. 2018; 12:165. [DOI:10.3389/fncel.2018.00165] [PMID] [PMCID]
- Kotani J, Sugioka S, Momota Y, Ueda Y. Effect of sevoflurane on intracranial pressure, sagittal sinus pressure, and the intracranial volume-pressure relation in cats. J Neurosurg Anesthesiol. 1992; 4(3):194-8. [DOI:10.1097/00008506-199207000-00008] [PMID]
- Sedighinejad A, Kousha M, Soltanipour S, Vakili E, Naderi N, Biazar G, et al. Association between early exposure to general anesthesia and autism spectrum disorder; A case control study. Caspian J Neurol Sci. 2024; 10(4):335-40. [DOI:10.32598/CJNS.10.39.506.1]
- Sedighnejad A, Soltanipour S, Saberi A, Kousha M, Bidabadi E, Biazar G, et al. Risk of attention deficit hyper activity disorder after early exposure to general anesthesia; A case control study. Iran J Pediatr. 2020; 30(3):e99976. [DOI:10.5812/ijp.99976]
- Walsh BH, Paul RA, Inder TE, Shimony JS, Smyser CD, Rogers CE. Surgery requiring general anesthesia in preterm infants is associated with altered brain volumes at term equivalent age and neurodevelopmental impairment. Pediatr Res. 2021; 89(5):1200-7. [DOI:10.1038/s41390-020-1030-3] [PMID] [PMCID]
- Salaün JP, Chagnot A, Cachia A, Poirel N, Datin-Dorrière V, Dujarrier C, et al. Consequences of general anesthesia in infancy on behavior and brain structure. Anesth Analg. 2023; 136(2):240-50. [DOI:10.1213/ANE.0000000000006233] [PMID]
Type of Study: Research |
Subject:
General
Received: 2024/03/22 | Accepted: 2024/05/14 | Published: 2025/01/1
Received: 2024/03/22 | Accepted: 2024/05/14 | Published: 2025/01/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






