Sun, Feb 1, 2026
Volume 11, Issue 4 (Autumn 2025)
Caspian J Neurol Sci 2025, 11(4): 338-343 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Niryana I W, Marleen M, Daniswara Maliawan M G, Priyambodo A, Putra M B, Prakoso D T et al . Stent-assisted Coiling for a Wide-neck Anterior Communicating Artery Aneurysm: A Case Report. Caspian J Neurol Sci 2025; 11 (4) :338-343
URL: http://cjns.gums.ac.ir/article-1-739-en.html
URL: http://cjns.gums.ac.ir/article-1-739-en.html
I Wayan Niryana *1 

 , Marleen Marleen2
, Marleen Marleen2 
 , Made Gemma Daniswara Maliawan2
, Made Gemma Daniswara Maliawan2 

 , Affan Priyambodo2
, Affan Priyambodo2 

 , Made Bhuwana Putra2
, Made Bhuwana Putra2 

 , Dicky Teguh Prakoso2
, Dicky Teguh Prakoso2 

 , Christopher Lauren2
, Christopher Lauren2 




 , Marleen Marleen2
, Marleen Marleen2 
 , Made Gemma Daniswara Maliawan2
, Made Gemma Daniswara Maliawan2 

 , Affan Priyambodo2
, Affan Priyambodo2 

 , Made Bhuwana Putra2
, Made Bhuwana Putra2 

 , Dicky Teguh Prakoso2
, Dicky Teguh Prakoso2 

 , Christopher Lauren2
, Christopher Lauren2 


1- Neurosurgery Division, Department of Surgery, Faculty of Medicine, Prof. Dr. IGNG Ngoerah General Hospital, Udayana University, Bali, Indonesia , niryanawayan@gmail.com
2- Neurosurgery Division, Department of Surgery, Faculty of Medicine, Prof. Dr. IGNG Ngoerah General Hospital, Udayana University, Bali, Indonesia
2- Neurosurgery Division, Department of Surgery, Faculty of Medicine, Prof. Dr. IGNG Ngoerah General Hospital, Udayana University, Bali, Indonesia
Keywords: Anterior communicating artery (AComA) aneurysm, Stent-assisted coiling (SAC), Wide-neck aneurysm
Full-Text [PDF 1867 kb]
(254 Downloads)
| Abstract (HTML) (573 Views)
Full-Text: (141 Views)
Introduction
Anterior communicating artery (AComA) aneurysms are a frequent etiology of subarachnoid hemorrhage. They present considerable challenges for endovascular management due to their intricate anatomical features and the critical need to preserve parent and perforating vessel patency [1]. The optimal treatment strategy, especially for wide-necked aneurysms, remains under active investigation and clinical debate [2, 3]. Endovascular techniques have been introduced to enhance occlusion efficacy while minimizing procedural risks [2–7]. This report outlines a detailed procedural description and discussion, supplemented by an extensive literature review, on applying stent-assisted coiling (SAC) to treat wide-neck AComA aneurysms.
Case Presentation
A 42-year-old male was referred to our institution following the incidental discovery of an unruptured anterior communicating artery (AComA) aneurysm. He reported intermittent, mild frontal headaches without any neurological deficits. The headache was non-pulsatile and not associated with aura, nausea, vomiting, or photophobia, making a direct link to the aneurysm unlikely. Primary headache syndromes, such as tension-type headaches or other benign etiologies, were considered more probable.
Given the confirmed presence of an intracranial aneurysm, digital subtraction angiography (DSA) was performed for further morphological and anatomical characterization. Although CT angiography (CTA) and MR angiography (MRA) are commonly used in initial evaluations, DSA remains the gold standard for detailed vascular assessment. In this case, DSA was indicated to delineate the aneurysm’s dimensions, projection, and relationship with adjacent vascular structures—key determinants in therapeutic decision-making.
The DSA revealed a wide-neck, superolaterally projecting AComA aneurysm (neck: 3.6 mm; dome width: 3.5 mm; dome height: 3.1 mm) with an aspect ratio of 1.1 (Figure 1). Additional findings included a tortuous AComA complex with a hypoplastic right A1 segment and a recurrent artery of Heubner originating near the aneurysm dome, increasing procedural complexity.
Despite the absence of hemorrhage or neurological symptoms directly attributable to the aneurysm, treatment was considered due to several factors. AComA aneurysms are associated with higher rupture risk than those in other locations, even when smaller than 7 mm. The aneurysm’s wide neck also posed a potential for progressive growth and future rupture. Furthermore, the anatomical variation involving the hypoplastic A1 segment raised concerns about compromised collateral circulation should a rupture occur. Considering these risks and the patient’s relatively young age, endovascular intervention using a SAC technique with the jailing method was planned. Dual antiplatelet therapy (aspirin 80 mg and clopidogrel 75 mg) was initiated one week before the procedure to mitigate thromboembolic complications.
Interventional procedure
Right femoral artery access was obtained using an 8-French sheath. A 6-French Neuron Max guiding catheter (Penumbra), coupled with a 5F vertebral catheter and a 0.035”/260 cm Terumo guidewire, was navigated coaxially to the C1 segment of the right internal carotid artery (ICA). Following diagnostic angiography to determine optimal working angles, a 6-French Sofia (MicroVention) intermediate catheter was advanced to the C4 segment of the ICA. A 1.7F Excelsior SL 10 (Stryker Neurovascular) microcatheter and a 0.014” Synchro microwire were introduced into the ipsilateral A2 segment for stent deployment. Simultaneously, a 1.9F Vasco 10 (Balt) microcatheter and a Synchro microwave were used to access the contralateral A2 segment and repositioned into the aneurysm dome for coil delivery.
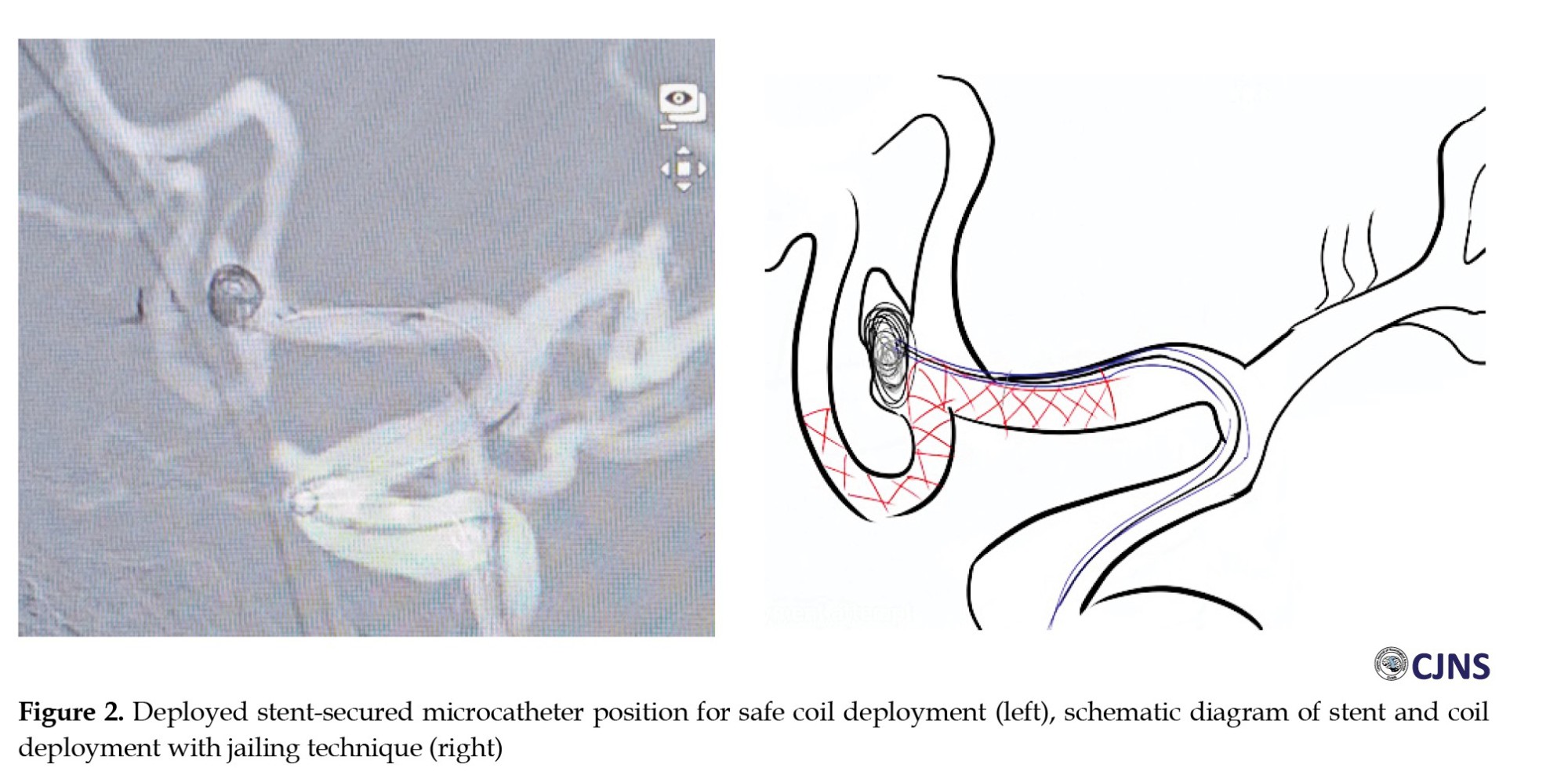
A Neuroform Atlas (Stryker) 3×24 mm laser-cut stent was deployed from A2 to A1 via the SL 10 microcatheter. Through the jailed Vasco microcatheter, a Target Ultra 3.5 mm × 8 cm (Stryker) framing coil was deployed and detached, followed by a Microplex 2 mm × 6 cm (MicroVention) filling coil (Figure 2). Intraoperative heparin was administered to maintain an activated clotting time (ACT) above 200 seconds. Final angiography confirmed a complete aneurysm occlusion (Raymond-Roy occlusion classification 1, Figure 3).
Follow-up and outcome
The patient remained neurologically intact throughout a 3-day postoperative hospitalization. Dual antiplatelet therapy was continued for 3 months, followed by a transition to monotherapy. One-year follow-up demonstrated stable occlusion with no evidence of aneurysm recurrence, hemorrhagic complications, or new neurological deficits. The patient resumed daily activities without limitation.
Discussion
Aneurysms of the AComA are among the most common cerebral aneurysms, which account for approximately 40% of all treated aneurysms [8]. AComA aneurysms usually occur at the junction of the dominant A1 and AComA (81.3%), with only 18.7% occurring in the middle of the AComA [1]. The AcomA complex has intricate anatomic architecture, diverse anatomical variations, numerous pivotal perforators, and a complicated regional flow dynamic that softens the challenging surgical approach [9]. In our case, the endovascular intervention was chosen over microsurgical clipping because of the convoluted AComA complex with a superolateral projection of the dome, which may hinder the evaluation of the perforators and the angioarchitecture of the recurrent artery which encircles the aneurysm, making it difficult to be preserved with the clipping procedure.
In coil embolization of wide-neck AComA aneurysms, maintaining the patency of the surrounding vessels, including perforators, is crucial for successful endovascular treatment [10]. Various techniques have been developed, including balloon-assisted coiling (BAC), SAC, or double catheter coiling (DCC) technique [1]. Some considerations must be taken in choosing the instrument to achieve the best occlusion rate with acceptable or least complication. BAC may be less associated with hemorrhagic complications than SAC since no dual antiplatelet treatment was needed. Nevertheless, some disadvantages include limited coil packing, especially at the neck, the risk of vascular stretch and subsequent intimal injury while manipulating the balloon, and the risk of coil displacement or protrusion to the parent artery after balloon deflation, in which stent deployment is eventually needed as a rescue measure [11]. There is also a possibility of an anchored microcatheter to the aneurysm by the balloon, which, as the coiling progresses, may impede the microcatheter movement, causing elevated pressure on the wall of the aneurysm [12]. DCC, on the other hand, while it may be a feasible strategy, is correlated with a low occlusion rate. A greater number of coils may also be needed to achieve appropriate occlusion, as in this technique, two coils are deployed as an initial framing coil, and a subsequent filling coil is made into both coil frames. There is also a risk of coil breakage, stretching, or interlocking during coil insertion with this technique and lower. Compared to BAC and SAC, DCC provides limited neck protection and the lowest complete occlusion rates [11].
With the drawback of the necessity of antiplatelet administration, SAC provides better occlusion rates than BAC or DCC. The stent may also alter the intra-aneurysmal flow and prompt orifice endothelialization, which helps in the progression of occlusion [11]. Lawson et al. described the occlusion rate as 18.5 times higher in the SAC compared to the group without stent involvement [13]. SAC is considered more suitable for aneurysms with a lower dome-to-neck ratio [11].
Several stent models are available and may be used to assist coil embolization. Among the stent models, we chose Neuroform Atlas (Stryker), an open-cell laser cut stent, for its advantage of better vessel apposition than the closed-cell stent [14]. This will facilitate deployment, especially in tortuous vessels, as in our case. Compared to other stents, Neuroform Atlas has less metal content and may be guided with a low-profile catheter [15]. Stents are often placed via the dominant A1. There are several ways to utilize stent placement in coil embolization: dominant A1 to ipsilateral A2, dominant A1 to contralateral A2, dominant A1 to contralateral A1, dominant A1 to aneurysm (waffle-cone technique or new stents pCONus [Phenox] and PulseRider [Cerenovus]), and dominant A1 to both A2 (double stenting). A single-stent deployment may not adequately cover the neck, causing coil protrusion, while double stenting may increase thromboembolism risk due to poor wall apposition [4, 10].
Single stenting in wide-neck AComA aneurysms has high periprocedural complication rates (4.8%-11%) and moderate complete occlusion rates (43.2%-44% initially, 72.7%-86.9% later) [7, 16, 17]. Recanalization and retreatment rates are 8.3%-13.1% and 3.4%-8.1%, respectively. Double stenting, like X, Y, and T, shows higher complete occlusion rates (85.8%-95.7%) and complication rates (6.7%-17.5%). Recanalization and retreatment rates are 0%-7.8% and 0%-2.4% [18].
While double stenting may provide complete coverage of the aneurysm neck, a single well-placed stent may offer a complete embolization without parent artery coil protrusion and less cost than the double stenting counterpart. In our case, the stent was placed in the ipsilateral A2 since the aneurysm neck leaned more on the ipsilateral side, with a higher risk of coil protrusion toward the abovementioned side. The stent was also placed to cover some parts of the contralateral side of the neck to maximize the neck coverage (Figure 2). In the stent-assisted procedure, the jailing technique is considered more straightforward than the trans-cell technique as the microcatheter may not pass the stent or be stuck in the struts [19]; the jailing technique was utilized in this case.
Conclusion
SAC embolization with jailing technique is a feasible and reliable technique in managing a wide-neck AComA aneurysm.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were conducted in compliance with the ethical guidelines of the Declaration of Helsinki 2013.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, writing, review, editing, and investigation: All authors; Writing the original draft, resources and supervision I Wayan Niryana.
Conflict of interest
The authors declared no conflict of interest.
References
Anterior communicating artery (AComA) aneurysms are a frequent etiology of subarachnoid hemorrhage. They present considerable challenges for endovascular management due to their intricate anatomical features and the critical need to preserve parent and perforating vessel patency [1]. The optimal treatment strategy, especially for wide-necked aneurysms, remains under active investigation and clinical debate [2, 3]. Endovascular techniques have been introduced to enhance occlusion efficacy while minimizing procedural risks [2–7]. This report outlines a detailed procedural description and discussion, supplemented by an extensive literature review, on applying stent-assisted coiling (SAC) to treat wide-neck AComA aneurysms.
Case Presentation
A 42-year-old male was referred to our institution following the incidental discovery of an unruptured anterior communicating artery (AComA) aneurysm. He reported intermittent, mild frontal headaches without any neurological deficits. The headache was non-pulsatile and not associated with aura, nausea, vomiting, or photophobia, making a direct link to the aneurysm unlikely. Primary headache syndromes, such as tension-type headaches or other benign etiologies, were considered more probable.
Given the confirmed presence of an intracranial aneurysm, digital subtraction angiography (DSA) was performed for further morphological and anatomical characterization. Although CT angiography (CTA) and MR angiography (MRA) are commonly used in initial evaluations, DSA remains the gold standard for detailed vascular assessment. In this case, DSA was indicated to delineate the aneurysm’s dimensions, projection, and relationship with adjacent vascular structures—key determinants in therapeutic decision-making.
The DSA revealed a wide-neck, superolaterally projecting AComA aneurysm (neck: 3.6 mm; dome width: 3.5 mm; dome height: 3.1 mm) with an aspect ratio of 1.1 (Figure 1). Additional findings included a tortuous AComA complex with a hypoplastic right A1 segment and a recurrent artery of Heubner originating near the aneurysm dome, increasing procedural complexity.
Despite the absence of hemorrhage or neurological symptoms directly attributable to the aneurysm, treatment was considered due to several factors. AComA aneurysms are associated with higher rupture risk than those in other locations, even when smaller than 7 mm. The aneurysm’s wide neck also posed a potential for progressive growth and future rupture. Furthermore, the anatomical variation involving the hypoplastic A1 segment raised concerns about compromised collateral circulation should a rupture occur. Considering these risks and the patient’s relatively young age, endovascular intervention using a SAC technique with the jailing method was planned. Dual antiplatelet therapy (aspirin 80 mg and clopidogrel 75 mg) was initiated one week before the procedure to mitigate thromboembolic complications.
Interventional procedure
Right femoral artery access was obtained using an 8-French sheath. A 6-French Neuron Max guiding catheter (Penumbra), coupled with a 5F vertebral catheter and a 0.035”/260 cm Terumo guidewire, was navigated coaxially to the C1 segment of the right internal carotid artery (ICA). Following diagnostic angiography to determine optimal working angles, a 6-French Sofia (MicroVention) intermediate catheter was advanced to the C4 segment of the ICA. A 1.7F Excelsior SL 10 (Stryker Neurovascular) microcatheter and a 0.014” Synchro microwire were introduced into the ipsilateral A2 segment for stent deployment. Simultaneously, a 1.9F Vasco 10 (Balt) microcatheter and a Synchro microwave were used to access the contralateral A2 segment and repositioned into the aneurysm dome for coil delivery.
A Neuroform Atlas (Stryker) 3×24 mm laser-cut stent was deployed from A2 to A1 via the SL 10 microcatheter. Through the jailed Vasco microcatheter, a Target Ultra 3.5 mm × 8 cm (Stryker) framing coil was deployed and detached, followed by a Microplex 2 mm × 6 cm (MicroVention) filling coil (Figure 2). Intraoperative heparin was administered to maintain an activated clotting time (ACT) above 200 seconds. Final angiography confirmed a complete aneurysm occlusion (Raymond-Roy occlusion classification 1, Figure 3).
Follow-up and outcome
The patient remained neurologically intact throughout a 3-day postoperative hospitalization. Dual antiplatelet therapy was continued for 3 months, followed by a transition to monotherapy. One-year follow-up demonstrated stable occlusion with no evidence of aneurysm recurrence, hemorrhagic complications, or new neurological deficits. The patient resumed daily activities without limitation.
Discussion
Aneurysms of the AComA are among the most common cerebral aneurysms, which account for approximately 40% of all treated aneurysms [8]. AComA aneurysms usually occur at the junction of the dominant A1 and AComA (81.3%), with only 18.7% occurring in the middle of the AComA [1]. The AcomA complex has intricate anatomic architecture, diverse anatomical variations, numerous pivotal perforators, and a complicated regional flow dynamic that softens the challenging surgical approach [9]. In our case, the endovascular intervention was chosen over microsurgical clipping because of the convoluted AComA complex with a superolateral projection of the dome, which may hinder the evaluation of the perforators and the angioarchitecture of the recurrent artery which encircles the aneurysm, making it difficult to be preserved with the clipping procedure.
In coil embolization of wide-neck AComA aneurysms, maintaining the patency of the surrounding vessels, including perforators, is crucial for successful endovascular treatment [10]. Various techniques have been developed, including balloon-assisted coiling (BAC), SAC, or double catheter coiling (DCC) technique [1]. Some considerations must be taken in choosing the instrument to achieve the best occlusion rate with acceptable or least complication. BAC may be less associated with hemorrhagic complications than SAC since no dual antiplatelet treatment was needed. Nevertheless, some disadvantages include limited coil packing, especially at the neck, the risk of vascular stretch and subsequent intimal injury while manipulating the balloon, and the risk of coil displacement or protrusion to the parent artery after balloon deflation, in which stent deployment is eventually needed as a rescue measure [11]. There is also a possibility of an anchored microcatheter to the aneurysm by the balloon, which, as the coiling progresses, may impede the microcatheter movement, causing elevated pressure on the wall of the aneurysm [12]. DCC, on the other hand, while it may be a feasible strategy, is correlated with a low occlusion rate. A greater number of coils may also be needed to achieve appropriate occlusion, as in this technique, two coils are deployed as an initial framing coil, and a subsequent filling coil is made into both coil frames. There is also a risk of coil breakage, stretching, or interlocking during coil insertion with this technique and lower. Compared to BAC and SAC, DCC provides limited neck protection and the lowest complete occlusion rates [11].
With the drawback of the necessity of antiplatelet administration, SAC provides better occlusion rates than BAC or DCC. The stent may also alter the intra-aneurysmal flow and prompt orifice endothelialization, which helps in the progression of occlusion [11]. Lawson et al. described the occlusion rate as 18.5 times higher in the SAC compared to the group without stent involvement [13]. SAC is considered more suitable for aneurysms with a lower dome-to-neck ratio [11].
Several stent models are available and may be used to assist coil embolization. Among the stent models, we chose Neuroform Atlas (Stryker), an open-cell laser cut stent, for its advantage of better vessel apposition than the closed-cell stent [14]. This will facilitate deployment, especially in tortuous vessels, as in our case. Compared to other stents, Neuroform Atlas has less metal content and may be guided with a low-profile catheter [15]. Stents are often placed via the dominant A1. There are several ways to utilize stent placement in coil embolization: dominant A1 to ipsilateral A2, dominant A1 to contralateral A2, dominant A1 to contralateral A1, dominant A1 to aneurysm (waffle-cone technique or new stents pCONus [Phenox] and PulseRider [Cerenovus]), and dominant A1 to both A2 (double stenting). A single-stent deployment may not adequately cover the neck, causing coil protrusion, while double stenting may increase thromboembolism risk due to poor wall apposition [4, 10].
Single stenting in wide-neck AComA aneurysms has high periprocedural complication rates (4.8%-11%) and moderate complete occlusion rates (43.2%-44% initially, 72.7%-86.9% later) [7, 16, 17]. Recanalization and retreatment rates are 8.3%-13.1% and 3.4%-8.1%, respectively. Double stenting, like X, Y, and T, shows higher complete occlusion rates (85.8%-95.7%) and complication rates (6.7%-17.5%). Recanalization and retreatment rates are 0%-7.8% and 0%-2.4% [18].
While double stenting may provide complete coverage of the aneurysm neck, a single well-placed stent may offer a complete embolization without parent artery coil protrusion and less cost than the double stenting counterpart. In our case, the stent was placed in the ipsilateral A2 since the aneurysm neck leaned more on the ipsilateral side, with a higher risk of coil protrusion toward the abovementioned side. The stent was also placed to cover some parts of the contralateral side of the neck to maximize the neck coverage (Figure 2). In the stent-assisted procedure, the jailing technique is considered more straightforward than the trans-cell technique as the microcatheter may not pass the stent or be stuck in the struts [19]; the jailing technique was utilized in this case.
Conclusion
SAC embolization with jailing technique is a feasible and reliable technique in managing a wide-neck AComA aneurysm.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were conducted in compliance with the ethical guidelines of the Declaration of Helsinki 2013.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, writing, review, editing, and investigation: All authors; Writing the original draft, resources and supervision I Wayan Niryana.
Conflict of interest
The authors declared no conflict of interest.
References
- Agrawal A, Kato Y, Chen L, Karagiozov K, Yoneda M, Imizu S, Sano H, Kanno T. Anterior communicating artery aneurysms: an overview. Minim Invasive Neurosurg. 2008; 51(3):131-5. [DOI:10.1055/s-2008-1073169] [PMID]
- Chung J, Lim YC, Suh SH, Shim YS, Kim YB, Joo JY, et al. Stent-assisted coil embolization of ruptured wide-necked aneurysms in the acute period: Incidence of and risk factors for periprocedural complications. J Neurosurg. 2014; 121(1):4-11. [DOI:10.3171/2014.4.JNS131662] [PMID]
- Lee YJ, Kim DJ, Suh SH, Lee SK, Kim J, Kim DI. Stent-assisted coil embolization of intracranial wide-necked aneurysms. Neuroradiology. 2005; 47(9):680-9. [DOI:10.1007/s00234-005-1402-8] [PMID]
- Shapiro M, Becske T, Sahlein D, Babb J, Nelson PK. Stent-supported aneurysm coiling: A literature survey of treatment and follow-up. AJNR Am J Neuroradiol. 2012; 33(1):159-63. [DOI:10.3174/ajnr.A2719] [PMID]
- Bodily KD, Cloft HJ, Lanzino G, Fiorella DJ, White PM, Kallmes DF. Stent-assisted coiling in acutely ruptured intracranial aneurysms: A qualitative, systematic literature review. AJNR Am J Neuroradiol. 2011; 32(7):1232-6. [DOI:10.3174/ajnr.A2478] [PMID]
- Peterson E, Hanak B, Morton R, Osbun JW, Levitt MR, Kim LJ. Are aneurysms treated with balloon-assisted coiling and stent-assisted coiling different? Morphological analysis of 113 unruptured wide-necked aneurysms treated with adjunctive devices. Neurosurgery. 2014; 75(2):145-51; quiz 151. [DOI:10.1227/NEU.0000000000000366] [PMID]
- Kitahara T, Hatano T, Hayase M, Hattori E, Miyakoshi A, Nakamura T. Jailed double-microcatheter technique following horizontal stenting for coil embolization of intracranial wide-necked bifurcation aneurysms: A technical report of two cases. Interv Neuroradiol. 2017; 23(2):117-22.[DOI:10.1177/1591019916685080] [PMID]
- Moon JS, Choi CH, Lee TH, Ko JK. Result of coiling versus clipping of unruptured anterior communicating artery aneurysms treated by a hybrid vascular neurosurgeon. J Cerebrovasc Endovasc Neurosurg. 2020; 22(4):225-36. [DOI:10.7461/jcen.2020.E2020.06.005] [PMID]
- Lee SH, Park JS. Outcome of ruptured anterior communicating artery aneurysm treatment compared between surgical clipping and endovascular coiling: A single-center analysis. Medicine (United States). 2022; 101(38):e30754. [DOI:10.1097/MD.0000000000030754] [PMID]
- Lodi YM, Latorre JG, El-Zammar Z, Swarnkar A, Deshaies E, Fessler RD. Stent assisted coiling of the ruptured wide necked intracranial aneurysm. J Neurointerv Surg. 2012; 4(4):281-6. [DOI:10.1136/neurintsurg-2011-010035] [PMID]
- Chung EJ, Shin YS, Lee CH, Song JH, Park JE. Comparison of clinical and radiologic outcomes among stent-assisted, double-catheter, and balloon-assisted coil embolization of wide neck aneurysms. Acta Neurochir (Wien). 2014; 156(7):1289-95.[DOI:10.1007/s00701-014-2104-y] [PMID]
- Aletich VA, Debrun GM, Misra M, Charbel F, Ausman JI. The remodeling technique of balloon-assisted Guglielmi detachable coil placement in wide-necked aneurysms: Experience at the University of Illinois at Chicago. J Neurosurg. 2000; 93(3):388-96. [DOI:10.3171/jns.2000.93.3.0388] [PMID]
- Lawson MF, Newman WC, Chi YY, Mocco JD, Hoh BL. Stent-associated flow remodeling causes further occlusion of incompletely coiled aneurysms. Neurosurgery. 2011; 69(3):598-603. [DOI:10.1227/NEU.0b013e3182181c2b] [PMID]
- Strittmatter C, Meyer L, Broocks G, Alexandrou M, Politi M, Boutchakova M, et al. Procedural outcome following stent-assisted coiling for wide-necked aneurysms using three different stent models: A single-center experience. J Clin Med. 2022; 11(12):3469. [DOI:10.3390/jcm11123469] [PMID]
- Nakajo T, Terada T, Tsumoto T, Matsuda Y, Matsumoto H, Nakayama S, et al. Stent-assisted coil embolization of ruptured aneurysms in the acute stage: Advantages and disadvantages. J Neuroendovasc Ther. 2023; 17(10):209-16. [DOI:10.5797/jnet.oa.2023-0028] [PMID]
- Labeyrie PE, Gory B, Aguilar-Perez M, Pomero E, Biondi A, Riva R, et al. The pCONus device for treatment of complex wide-neck anterior communicating artery aneurysms. World Neurosurg. 2017; 101:498-505. [DOI:10.1016/j.wneu.2017.02.045] [PMID]
- Kiyosue H, Tanoue S, Okahara M, Hori Y, Nakamura T, Nagatomi H, et al. Anatomic features predictive of complete aneurysm occlusion can be determined with three-dimensional digital subtraction angiography. AJNR Am J Neuroradiol. 2002; 23(7):1206-13. [PMID]
- Cloft HJ, Joseph GJ, Tong FC, Goldstein JH, Dion JE. Use of three-dimensional guglielmi detachable coils in the treatment of wide-necked cerebral aneurysms. AJNR Am J Neuroradiol. 2000; 21(7):1312-4. [PMID]
- Hanaoka Y, Koyama JI, Yamazaki D, Ogiwara T, Ito K, Horiuchi T. Passability and impassability of microcatheters through the neuroform atlas stent during the trans-cell approach: An experimental evaluation. World Neurosurg. 2020; 141:e474-83. [DOI:10.1016/j.wneu.2020.05.215] [PMID]
Type of Study: case report |
Subject:
Special
Received: 2024/08/21 | Accepted: 2025/04/3 | Published: 2025/10/26
Received: 2024/08/21 | Accepted: 2025/04/3 | Published: 2025/10/26
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |