Wed, Dec 31, 2025
Volume 10, Issue 4 (Autumn 2024)
Caspian J Neurol Sci 2024, 10(4): 347-353 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rimaz S, Emir Alavi C, Soltanipour S, Sedighinejad A, Einollahzadeh R, Haghighi M, et al . Efficacy and Safety of Cisatracurium in Electroconvulsive Therapy: A Randomized, Single-blind, Clinical Trial. Caspian J Neurol Sci 2024; 10 (4) :347-353
URL: http://cjns.gums.ac.ir/article-1-730-en.html
URL: http://cjns.gums.ac.ir/article-1-730-en.html
Siamak Rimaz1 

 , Cyrus Emir Alavi2
, Cyrus Emir Alavi2 

 , Soheil Soltanipour3
, Soheil Soltanipour3 

 , Abbas Sedighinejad1
, Abbas Sedighinejad1 

 , Reihaneh Einollahzadeh1
, Reihaneh Einollahzadeh1 

 , Mohammad Haghighi1
, Mohammad Haghighi1 

 , Ali Pourramzani *4
, Ali Pourramzani *4 

 , Gelareh Biazar1
, Gelareh Biazar1 




 , Cyrus Emir Alavi2
, Cyrus Emir Alavi2 

 , Soheil Soltanipour3
, Soheil Soltanipour3 

 , Abbas Sedighinejad1
, Abbas Sedighinejad1 

 , Reihaneh Einollahzadeh1
, Reihaneh Einollahzadeh1 

 , Mohammad Haghighi1
, Mohammad Haghighi1 

 , Ali Pourramzani *4
, Ali Pourramzani *4 

 , Gelareh Biazar1
, Gelareh Biazar1 


1- Department of Anesthesiology, Anesthesiology Research Center, Faculty of Medicine, Alzahra Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Anesthesiology, Neuroscience Research Center, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
3- Department of Community Medicine, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Psychiatry, Kavosh Cognitive Behavior Sciences and Addiction Research Center, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran. ,dr_pourramzani@yahoo.com
2- Department of Anesthesiology, Neuroscience Research Center, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
3- Department of Community Medicine, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Psychiatry, Kavosh Cognitive Behavior Sciences and Addiction Research Center, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran. ,
Full-Text [PDF 1125 kb]
(408 Downloads)
| Abstract (HTML) (953 Views)
Full-Text: (278 Views)
Introduction
Despite the significant progress that has occurred in pharmacological and non-pharmacological treatment options in psychiatry, electroconvulsive therapy (ECT) has remained a lifesaving treatment for refractory or the need for an immediate response in psychiatric patients. ECT is performed safely in children, older people, and pregnant women [1, 2]. However, social disparities and stigma still hinder the widespread use of ECT [3]. For 30 years, ECT was performed without anesthesia. Since then, this treatment method has been performed under general anesthesia and receiving muscle relaxants [4]. The side effects of ECT without using muscle relaxants include neuromuscular damage, tooth fracture, tongue damage, joint dislocation, and severe muscle contractions or bone fractures, which become more problematic in some conditions, such as osteoporosis [5]. ECT under anesthesia is associated with fewer physical and psychological complications and is well accepted today [6]. In general anesthesia for ECT is prescribed to act as a hypnotic agent and a muscle relaxant. According to studies, the duration of seizures is important in its effectiveness and is affected by anesthetics and muscle relaxants. For example, when a longer seizure is needed, etomidate, methohexital, or the combination of low doses of short-acting narcotics with propofol are used [7]. The characteristics of a suitable anesthetic agent in ECT are that it effectively reduces hyperdynamic responses to shock, prevents severe movements, has the least interference with convulsive movements, and is associated with rapid recovery. Since anesthetic drugs have a dose-dependent effect on the anticonvulsant properties, the minimum effective dose should be considered in these patients [7]. Although succinylcholine is commonly used in ECT, managing patients when it is contraindicated has always been challenging [8, 9]. The anesthesiologist may accept the risk of side effects of succinylcholine or prescribe non-depolarizing muscle relaxants (NDMRs) without accurate dosing, which may result in prolonged apnea or inadequate relaxation, or the procedure is performed without muscle relaxants, which can result in unbearable muscle pain, joint dislocation, and tooth fracture. On the other hand, succinylcholine may not be available in Iran for long periods due to sanctions. Cisatracurium is an isomer of atracurium. This drug has largely replaced it in the clinic with minimal release of histamine [10]. Therefore, considering the importance of maintaining the continuity of the treatment process of ECT patients, cisatracurium was selected as an alternative to succinylcholine in the present study.
Materials and Methods
After the approval of the study protocol by the Research Ethics Committee of Guilan University of Medical Sciences (GUMS), this single-blind clinical trial was performed at Shafa Hospital, Northern Iran, from July 2023 to March 2024. This academic hospital is a referral center for all types of psychiatric diseases.
The inclusion criteria were psychiatric patient candidates for ECT, aged 18-70 years, and class I & II based on the American Society of Anesthesiologists.
The exclusion criteria were patients with difficult mask ventilation criteria, Mallampati classification III or IV, body mass index (BMI) ≥30 kg/m2, severely limited mandibular protrusion, a history of snoring, any contraindication for cisatracurium or succinylcholine and the need for any urgent intervention such as tracheal intubation.
Randomization and blinding
A nurse who was not engaged in the study process allocated the patients into groups of succinylcholine and cisatracurium. It was a single-blind study due to obvious differences in the characteristics of two muscle relaxants.
Anesthesia management and the procedure
The patients underwent a standard preoperative visit, and all the considerations of general anesthesia were followed. The patients received atropine sulfate (0.01 mg/kg IM) 30 minutes before ECT. After admission, standard monitoring was performed, and an intravenous assessment was secured. Anesthesia was induced by propofol (1 mg/kg IV) followed by 0.1 mg/kg cisatracurium (50 mg/mL, Caspian Tamin Co, Iran) for the cisatracurium group and 0.5 mg/kg succinylcholine (500 mg/10 mL, Caspian Tamin Co, Iran) for the succinylcholine group. Before anesthesia induction, the patient was pre-oxygenated via a facial mask, and after complete unconsciousness, active hyperventilation at a rate of 40-50 breaths per minute was performed, and a mouth guard and bi-temporal electrodes were placed. Then, a grand-mal seizure was induced with the following characteristics (70-120 V, 800 mA of direct current, and a duration of 100 ms to 6 s). With the end of the seizure movements, active ventilation was started until the patient’s effective breathing returned. In group cisatracurium, the patient was ventilated 4 minutes before stimulation and 30 seconds before seizure induction; a bolus dose of propofol (0.2 mg/kg) was administrated. At the end of the procedure, intravenous atropine 0.02 mg/kg and neostigmine 0.04 mg/kg were administrated to reverse the effects of muscle relaxant.
Measurement point times and outcomes
Heart rate (HR) and mean arterial pressure (MAP) were recorded at four measurement point times: Before induction of anesthesia (T0), one minute after the seizure (T1), 15 minutes after induction of anesthesia (T2), and in the recovery ward (T3). The seizure duration was recorded according to clinical observation of colonic movements by a stopwatch. The patients were transferred to the recovery ward after regaining full consciousness and in stable hemodynamic status. They were discharged according to the Aldrete scoring criteria, a reliable scoring system determining when the patient can safely be discharged from the recovery unit. The duration of seizure, the time to return spontaneous breathing, and the time of discharge (recovery) were compared between the two groups. The anesthesiologist was prepared to manage any unexpected event from the patient’s admission until discharge from the recovery ward.
Statistical analysis
SPSS software, version 21, was used to analyze the gathered data, and the chi-square test, Fisher exact test, t-test, and repeated measurement analysis of variance test were applied. The parametric data were presented as Mean±SD, and nonparametric data as median (range). P<0.05 was considered significant.
Results
The data of 62 ECT patients were analyzed. The mean age of patients in the succinylcholine group was 43.41±11.26, and in the cisatracurium group was 44.29±12.54 years. As shown in Table 1, in terms of demographic data, no significant difference was observed between the two groups (P>0.05).

Seizure duration was longer in the succinylcholine group compared to the cisatracurium group (19.41±2.21 vs 18.9±2.15 s), but the difference was not significant (P=0.356). The time to return to spontaneous breathing was significantly longer in the cisatracurium group compared to the succinylcholine group (19.06±4.95 vs 11.74±2.63 s; P=0.0001). Furthermore, the recovery time was significantly longer in the cisatracurium group compared to the succinylcholine group (29.25±5.4 vs 20.32±2.94 min; P=0.0001) (Table 2).

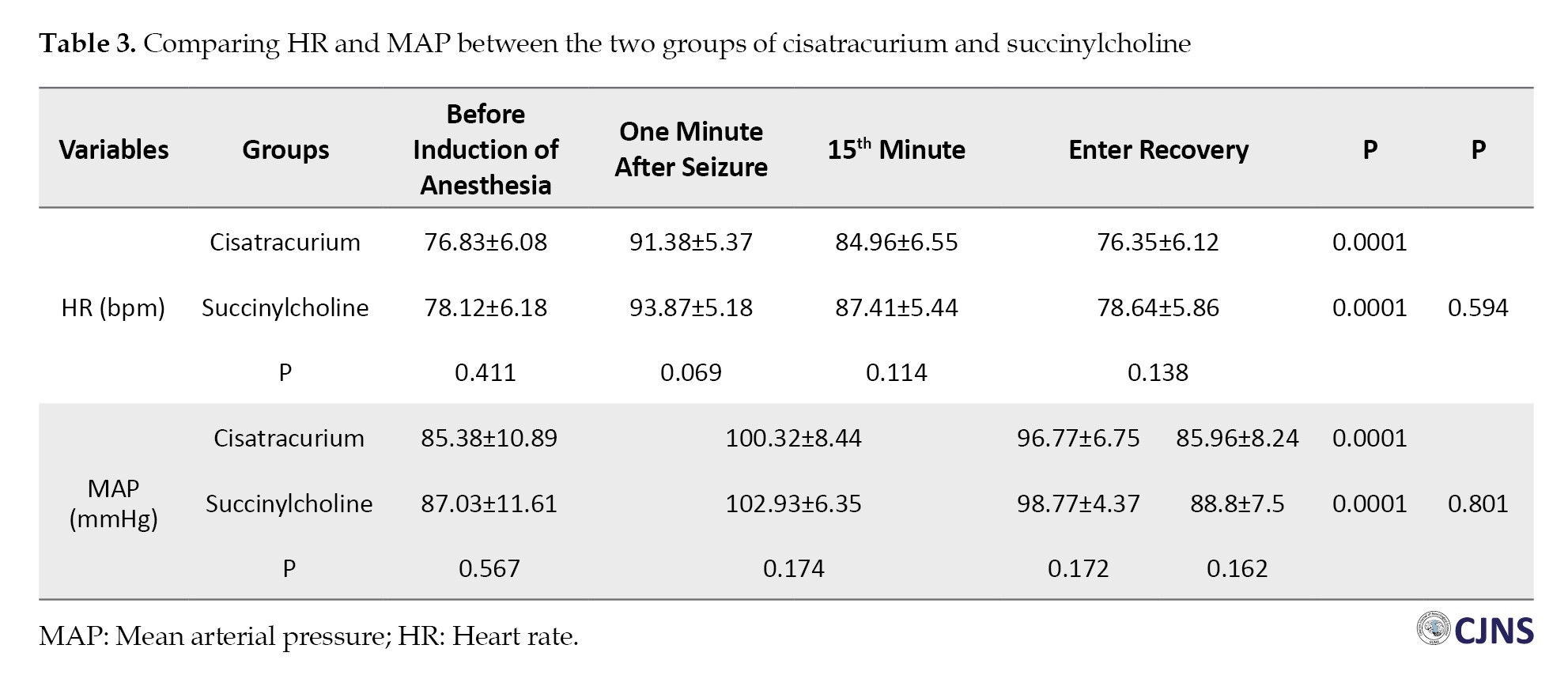
Hemodynamic parameters, including HR and MAP, were also compared between the two groups. In each group, the trend of changes was significant from T0 (before induction of anesthesia) to T3 (recovery ward) (P=0.0001). However, when comparing the two groups, the difference was not significant (P>0.05) (Table 3).
All patients passed the ECT process safely without any serious complications, and Table 4 shows no significant difference between the two groups regarding the side effects (P>0.05).

Discussion
Following the promising results obtained from the study to investigate the safety and efficacy of NDMRs as a substitute alternative for succinylcholine in ECT patients, the present study was planned [11]. In addition, recent studies recommended replacing atracurium with cisatracurium due to the marked advantages [12]. In the present study, cisatracurium was compared with succinylcholine in terms of efficacy, safety, and clinical outcomes. The results of this study demonstrated no significant difference in the duration of seizures in the two groups. Considering that one of the consequences of effective treatment responses is the duration of seizures, this result is practical and valuable. Also, no significant difference was observed in the incidence of complications between the two groups. In this research, the hemodynamic parameters were compared between the two groups at different time points, showing the advantage of cisatracurium in hemodynamic stability compared to atracurium. This finding is justified according to the pharmacokinetics and pharmacodynamics of the cisatracurium. The time of return to effective breathing and patients’ consciousness and recovery times were significantly longer in the cisatracurium group, which was expected. Because succinylcholine is a short-acting relaxant, while cisatracurium is a medium-acting relaxant. This result is consistent with the results of the atracurium study [11]. The results obtained in the comparative studies between succinylcholine and NDMRs are the interpretation of why succinylcholine is the relaxant of choice in ECT because it is proportional to the duration of the procedure. In addition, considering the high number and special conditions of psychiatric patients, it is necessary to have a proper turnover because long fasting and long waiting times are not tolerable for them. In the following, limited literature on the use of NDMRs instead of succinylcholine for ECT are mentioned. Kaur et al. described a case of catatonia scheduled for ECT. The risk of life-threatening hyperkalemia as a result of long-term immobilization led them to avoide succinylcholine. They used I-gel and choose atracurium (15 mg) as muscle relaxant [13]. Nazemoraaya et al. performed two clinical trials on ECT patients, comparing succinylcholine with cisatracurium. They reported that cisatracurium could be a safe alternative to succinylcholine during ECT. It caused less elevation in serum potassium, much fewer complications, and a more stable hemodynamic status [14, 15]. Hoshi et al. compared rocuronium combined with sugammadex with succinylcholine during ECT. They concluded that rocuronium-sugammadex could be accepted as an alternative to succinylcholine for muscle relaxation in ECT patients [16]. Although this combination is used successfully, the high price should be noted [9]. It is much more important in low-income countries. Rocuronium and sugammadex are not widely available in Iran. Mivacurium is also suggested in ECT patients [17]; however, histamine release is one of its side effects, and also, like succinylcholine, mivacurium is contraindicated in pseudo cholinesterase deficiency [18]. Liu et al. described a general anesthetic method for ECT patients by using bispectral index, supraglottic devices, and replacing succinylcholine with cisatracurium, and they found promising results [19]. Takazawa et al. demonstrated that rocuronium and sugammadex were better options than succinylcholine for ECT, which had fewer side effects. They emphasized the conditions when succinylcholine is contraindicated [20]. In this study, ventilation was performed only with a facial mask, and other methods, such as a laryngeal mask or tracheal tube, were not used to secure the airway, which is the difference and strength of this research. The patients were managed in a simple manner, leading to a higher turnover, while effective ventilation was maintained throughout the procedure. The studies that, for the 1st time, discussed the issue of replacement of succinylcholine with NDMRs with intermediate duration of action, had concerns regarding airway safety. In general, taking into account the NDMRs that have been introduced as safe alternatives for succinylcholine and considering their advantages and disadvantages and of course the pharmaceutical facilities in the country, it seems that the two NDMRs, atracurium and cisatracurium are suitable choices. Although the main goal of this study was to find a suitable alternative to succinylcholine in case of drug contraindication, this goal was also extended to the specific conditions of our country. In fact, when we face to the shortage of succinylcholine due to drug sanctions, the treating process will not be disturbed. Overall, consistent with previous studies, we also found that cisatracurium can be used safely in ECT patients. However, this drug cannot be a substitute for succinylcholine because if the drug is widely used, the turnover of the ECT department will be very low due to the longer time spend for each patient and the need for the reverse of the effect of NDMRs at the end of the procedure.
We hope that the promising results of this study can contribute to the safe management of ECT patients without interrupting the treatment process. Even though the safety of succinylcholine alternatives in ECT has been demonstrated, many questions and uncertainties have remained unanswered. The optimal dosage of NDMRs, which provides appropriate depth of anesthesia and relaxation with minimal impact on seizure duration, is unclear. Also, patients with compromised airways or difficult mask ventilation criteria have not been assessed. Maybe this patient with this manifestation is one of the contraindications for succinylcholine.
Conclusion
This study revealed that cisatracurium can be used as an effective and safe alternative when succinylcholine is contraindicated. Thus, the treatment process of psychiatric patients will not be disturbed.
Study limitations
First, the seizure duration was evaluated based on the researcher’s observation. However, according to valid references, the results could not be significantly affected. Second, the patient himself could not decide to participate. Third, the non-entry of high-risk cases with underlying diseases was another limitation. Perhaps patients who met difficult mask ventilation criteria or other exclusion criteria needed to receive an alternative to succinylcholine
Study strengths
So far, the studies of replacing succinylcholine with other muscle relaxants in ECT patients have mostly been in case reports, and clinical trials are scarce and have numerous limitations. This research is precious in the form of a clinical trial with practical results.
Study suggestions
Considering that the safety and utility of cisatracurium in electroshock patients have been shown as the initial steps to achieve more applicable results, future research in this field with other doses and with limited exclusion criteria is welcome. Also, based on the promising results of two recent clinical trials conducted in the same center, planning a clinical trial to compare these two drugs can obtain much more practical information.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1402.078) and was registered in the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT 20110425006280N14). Moreover, informed consent was obtained from the patients’ legal guardians.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization: Siamak Rimaz and Abbas Sedighinejad; Methodology: Soheil Soltanipour; Investigation and resources: Mohammad Haghighi and Ali Pourramzani; Data collection: Reihaneh Einollahzadeh; Writing the original draft: Cyrus Emir Alavi; Review and editing: Gelareh Biazar and Mohammad Haghighi; Supervision: Siamak Rimaz.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the Anesthesiology Research Center staff, Batool Montazeri, and Samira Mirzababaee, for collaborating on this study.
References
Despite the significant progress that has occurred in pharmacological and non-pharmacological treatment options in psychiatry, electroconvulsive therapy (ECT) has remained a lifesaving treatment for refractory or the need for an immediate response in psychiatric patients. ECT is performed safely in children, older people, and pregnant women [1, 2]. However, social disparities and stigma still hinder the widespread use of ECT [3]. For 30 years, ECT was performed without anesthesia. Since then, this treatment method has been performed under general anesthesia and receiving muscle relaxants [4]. The side effects of ECT without using muscle relaxants include neuromuscular damage, tooth fracture, tongue damage, joint dislocation, and severe muscle contractions or bone fractures, which become more problematic in some conditions, such as osteoporosis [5]. ECT under anesthesia is associated with fewer physical and psychological complications and is well accepted today [6]. In general anesthesia for ECT is prescribed to act as a hypnotic agent and a muscle relaxant. According to studies, the duration of seizures is important in its effectiveness and is affected by anesthetics and muscle relaxants. For example, when a longer seizure is needed, etomidate, methohexital, or the combination of low doses of short-acting narcotics with propofol are used [7]. The characteristics of a suitable anesthetic agent in ECT are that it effectively reduces hyperdynamic responses to shock, prevents severe movements, has the least interference with convulsive movements, and is associated with rapid recovery. Since anesthetic drugs have a dose-dependent effect on the anticonvulsant properties, the minimum effective dose should be considered in these patients [7]. Although succinylcholine is commonly used in ECT, managing patients when it is contraindicated has always been challenging [8, 9]. The anesthesiologist may accept the risk of side effects of succinylcholine or prescribe non-depolarizing muscle relaxants (NDMRs) without accurate dosing, which may result in prolonged apnea or inadequate relaxation, or the procedure is performed without muscle relaxants, which can result in unbearable muscle pain, joint dislocation, and tooth fracture. On the other hand, succinylcholine may not be available in Iran for long periods due to sanctions. Cisatracurium is an isomer of atracurium. This drug has largely replaced it in the clinic with minimal release of histamine [10]. Therefore, considering the importance of maintaining the continuity of the treatment process of ECT patients, cisatracurium was selected as an alternative to succinylcholine in the present study.
Materials and Methods
After the approval of the study protocol by the Research Ethics Committee of Guilan University of Medical Sciences (GUMS), this single-blind clinical trial was performed at Shafa Hospital, Northern Iran, from July 2023 to March 2024. This academic hospital is a referral center for all types of psychiatric diseases.
The inclusion criteria were psychiatric patient candidates for ECT, aged 18-70 years, and class I & II based on the American Society of Anesthesiologists.
The exclusion criteria were patients with difficult mask ventilation criteria, Mallampati classification III or IV, body mass index (BMI) ≥30 kg/m2, severely limited mandibular protrusion, a history of snoring, any contraindication for cisatracurium or succinylcholine and the need for any urgent intervention such as tracheal intubation.
Randomization and blinding
A nurse who was not engaged in the study process allocated the patients into groups of succinylcholine and cisatracurium. It was a single-blind study due to obvious differences in the characteristics of two muscle relaxants.
Anesthesia management and the procedure
The patients underwent a standard preoperative visit, and all the considerations of general anesthesia were followed. The patients received atropine sulfate (0.01 mg/kg IM) 30 minutes before ECT. After admission, standard monitoring was performed, and an intravenous assessment was secured. Anesthesia was induced by propofol (1 mg/kg IV) followed by 0.1 mg/kg cisatracurium (50 mg/mL, Caspian Tamin Co, Iran) for the cisatracurium group and 0.5 mg/kg succinylcholine (500 mg/10 mL, Caspian Tamin Co, Iran) for the succinylcholine group. Before anesthesia induction, the patient was pre-oxygenated via a facial mask, and after complete unconsciousness, active hyperventilation at a rate of 40-50 breaths per minute was performed, and a mouth guard and bi-temporal electrodes were placed. Then, a grand-mal seizure was induced with the following characteristics (70-120 V, 800 mA of direct current, and a duration of 100 ms to 6 s). With the end of the seizure movements, active ventilation was started until the patient’s effective breathing returned. In group cisatracurium, the patient was ventilated 4 minutes before stimulation and 30 seconds before seizure induction; a bolus dose of propofol (0.2 mg/kg) was administrated. At the end of the procedure, intravenous atropine 0.02 mg/kg and neostigmine 0.04 mg/kg were administrated to reverse the effects of muscle relaxant.
Measurement point times and outcomes
Heart rate (HR) and mean arterial pressure (MAP) were recorded at four measurement point times: Before induction of anesthesia (T0), one minute after the seizure (T1), 15 minutes after induction of anesthesia (T2), and in the recovery ward (T3). The seizure duration was recorded according to clinical observation of colonic movements by a stopwatch. The patients were transferred to the recovery ward after regaining full consciousness and in stable hemodynamic status. They were discharged according to the Aldrete scoring criteria, a reliable scoring system determining when the patient can safely be discharged from the recovery unit. The duration of seizure, the time to return spontaneous breathing, and the time of discharge (recovery) were compared between the two groups. The anesthesiologist was prepared to manage any unexpected event from the patient’s admission until discharge from the recovery ward.
Statistical analysis
SPSS software, version 21, was used to analyze the gathered data, and the chi-square test, Fisher exact test, t-test, and repeated measurement analysis of variance test were applied. The parametric data were presented as Mean±SD, and nonparametric data as median (range). P<0.05 was considered significant.
Results
The data of 62 ECT patients were analyzed. The mean age of patients in the succinylcholine group was 43.41±11.26, and in the cisatracurium group was 44.29±12.54 years. As shown in Table 1, in terms of demographic data, no significant difference was observed between the two groups (P>0.05).

Seizure duration was longer in the succinylcholine group compared to the cisatracurium group (19.41±2.21 vs 18.9±2.15 s), but the difference was not significant (P=0.356). The time to return to spontaneous breathing was significantly longer in the cisatracurium group compared to the succinylcholine group (19.06±4.95 vs 11.74±2.63 s; P=0.0001). Furthermore, the recovery time was significantly longer in the cisatracurium group compared to the succinylcholine group (29.25±5.4 vs 20.32±2.94 min; P=0.0001) (Table 2).

Hemodynamic parameters, including HR and MAP, were also compared between the two groups. In each group, the trend of changes was significant from T0 (before induction of anesthesia) to T3 (recovery ward) (P=0.0001). However, when comparing the two groups, the difference was not significant (P>0.05) (Table 3).

All patients passed the ECT process safely without any serious complications, and Table 4 shows no significant difference between the two groups regarding the side effects (P>0.05).

Discussion
Following the promising results obtained from the study to investigate the safety and efficacy of NDMRs as a substitute alternative for succinylcholine in ECT patients, the present study was planned [11]. In addition, recent studies recommended replacing atracurium with cisatracurium due to the marked advantages [12]. In the present study, cisatracurium was compared with succinylcholine in terms of efficacy, safety, and clinical outcomes. The results of this study demonstrated no significant difference in the duration of seizures in the two groups. Considering that one of the consequences of effective treatment responses is the duration of seizures, this result is practical and valuable. Also, no significant difference was observed in the incidence of complications between the two groups. In this research, the hemodynamic parameters were compared between the two groups at different time points, showing the advantage of cisatracurium in hemodynamic stability compared to atracurium. This finding is justified according to the pharmacokinetics and pharmacodynamics of the cisatracurium. The time of return to effective breathing and patients’ consciousness and recovery times were significantly longer in the cisatracurium group, which was expected. Because succinylcholine is a short-acting relaxant, while cisatracurium is a medium-acting relaxant. This result is consistent with the results of the atracurium study [11]. The results obtained in the comparative studies between succinylcholine and NDMRs are the interpretation of why succinylcholine is the relaxant of choice in ECT because it is proportional to the duration of the procedure. In addition, considering the high number and special conditions of psychiatric patients, it is necessary to have a proper turnover because long fasting and long waiting times are not tolerable for them. In the following, limited literature on the use of NDMRs instead of succinylcholine for ECT are mentioned. Kaur et al. described a case of catatonia scheduled for ECT. The risk of life-threatening hyperkalemia as a result of long-term immobilization led them to avoide succinylcholine. They used I-gel and choose atracurium (15 mg) as muscle relaxant [13]. Nazemoraaya et al. performed two clinical trials on ECT patients, comparing succinylcholine with cisatracurium. They reported that cisatracurium could be a safe alternative to succinylcholine during ECT. It caused less elevation in serum potassium, much fewer complications, and a more stable hemodynamic status [14, 15]. Hoshi et al. compared rocuronium combined with sugammadex with succinylcholine during ECT. They concluded that rocuronium-sugammadex could be accepted as an alternative to succinylcholine for muscle relaxation in ECT patients [16]. Although this combination is used successfully, the high price should be noted [9]. It is much more important in low-income countries. Rocuronium and sugammadex are not widely available in Iran. Mivacurium is also suggested in ECT patients [17]; however, histamine release is one of its side effects, and also, like succinylcholine, mivacurium is contraindicated in pseudo cholinesterase deficiency [18]. Liu et al. described a general anesthetic method for ECT patients by using bispectral index, supraglottic devices, and replacing succinylcholine with cisatracurium, and they found promising results [19]. Takazawa et al. demonstrated that rocuronium and sugammadex were better options than succinylcholine for ECT, which had fewer side effects. They emphasized the conditions when succinylcholine is contraindicated [20]. In this study, ventilation was performed only with a facial mask, and other methods, such as a laryngeal mask or tracheal tube, were not used to secure the airway, which is the difference and strength of this research. The patients were managed in a simple manner, leading to a higher turnover, while effective ventilation was maintained throughout the procedure. The studies that, for the 1st time, discussed the issue of replacement of succinylcholine with NDMRs with intermediate duration of action, had concerns regarding airway safety. In general, taking into account the NDMRs that have been introduced as safe alternatives for succinylcholine and considering their advantages and disadvantages and of course the pharmaceutical facilities in the country, it seems that the two NDMRs, atracurium and cisatracurium are suitable choices. Although the main goal of this study was to find a suitable alternative to succinylcholine in case of drug contraindication, this goal was also extended to the specific conditions of our country. In fact, when we face to the shortage of succinylcholine due to drug sanctions, the treating process will not be disturbed. Overall, consistent with previous studies, we also found that cisatracurium can be used safely in ECT patients. However, this drug cannot be a substitute for succinylcholine because if the drug is widely used, the turnover of the ECT department will be very low due to the longer time spend for each patient and the need for the reverse of the effect of NDMRs at the end of the procedure.
We hope that the promising results of this study can contribute to the safe management of ECT patients without interrupting the treatment process. Even though the safety of succinylcholine alternatives in ECT has been demonstrated, many questions and uncertainties have remained unanswered. The optimal dosage of NDMRs, which provides appropriate depth of anesthesia and relaxation with minimal impact on seizure duration, is unclear. Also, patients with compromised airways or difficult mask ventilation criteria have not been assessed. Maybe this patient with this manifestation is one of the contraindications for succinylcholine.
Conclusion
This study revealed that cisatracurium can be used as an effective and safe alternative when succinylcholine is contraindicated. Thus, the treatment process of psychiatric patients will not be disturbed.
Study limitations
First, the seizure duration was evaluated based on the researcher’s observation. However, according to valid references, the results could not be significantly affected. Second, the patient himself could not decide to participate. Third, the non-entry of high-risk cases with underlying diseases was another limitation. Perhaps patients who met difficult mask ventilation criteria or other exclusion criteria needed to receive an alternative to succinylcholine
Study strengths
So far, the studies of replacing succinylcholine with other muscle relaxants in ECT patients have mostly been in case reports, and clinical trials are scarce and have numerous limitations. This research is precious in the form of a clinical trial with practical results.
Study suggestions
Considering that the safety and utility of cisatracurium in electroshock patients have been shown as the initial steps to achieve more applicable results, future research in this field with other doses and with limited exclusion criteria is welcome. Also, based on the promising results of two recent clinical trials conducted in the same center, planning a clinical trial to compare these two drugs can obtain much more practical information.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1402.078) and was registered in the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT 20110425006280N14). Moreover, informed consent was obtained from the patients’ legal guardians.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization: Siamak Rimaz and Abbas Sedighinejad; Methodology: Soheil Soltanipour; Investigation and resources: Mohammad Haghighi and Ali Pourramzani; Data collection: Reihaneh Einollahzadeh; Writing the original draft: Cyrus Emir Alavi; Review and editing: Gelareh Biazar and Mohammad Haghighi; Supervision: Siamak Rimaz.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the Anesthesiology Research Center staff, Batool Montazeri, and Samira Mirzababaee, for collaborating on this study.
References
- Kaliora SC, Zervas IM, Papadimitriou GN. [Electroconvulsive therapy: 80 years of use in psychiatry (Greek)]. Psychiatriki. 2018; 29(4):291-302. [DOI:10.22365/jpsych.2018.294.291] [PMID]
- Trevizol AP, Blumberger DM. An update on repetitive transcranial magnetic stimulation for the treatment of major depressive disorder. Clin Pharmacol Ther. 2019; 106(4):747-62. [DOI:10.1002/cpt.1550] [PMID]
- Ninke T, Groene P. Electroconvulsive therapy: Recent advances and anesthetic considerations. Curr Opin Anaesthesiol. 2023; 36(4):441-6. [PMID] [DOI:10.1097/ACO.0000000000001279]
- McCall WV, Kellner CH, Fink M. Convulsive therapy and the Journal of ECT: 30 years of publication and continuing. J ECT. 2014; 30(1):1-2. [PMID] [DOI:10.1097/YCT.0000000000000107]
- Yang HS, Joung KW. Electroconvulsive therapy and muscle relaxants. Anesth Pain Med (Seoul). 2023; 18(4):447-8.[DOI:10.17085/apm.23018] [PMID]
- Acero González ÁR, Guzmán Sabogal YR, Salamanca Dimas H, Páez Avendaño V, Pineda Carrascal E, Izquierdo Polanco J, et al. Clinical experience of electroconvulsive therapy with anaesthetic and muscle relaxant at the Clínica Universidad de La Sabana: 2009-2017. Rev Colomb Psiquiatr (Engl Ed). 2023; 52(2):101-6. [DOI:10.1016/j.rcpeng.2021.01.004] [PMID]
- Joung KW, Park DH, Jeong CY, Yang HS. Anesthetic care for electroconvulsive therapy. Anesth Pain Med (Seoul). 2022; 17(2):145-56. [DOI:10.17085/apm.22145] [PMID]
- Nakano M, Funayama M, Takata T, Wakisaka R, Koyama G, Koreki A, et al. Caution for psychiatrists: malignant hyperthermia risks with the anesthetic agent succinylcholine (Suxamethonium) during electroconvulsive therapy. BMC Psychiatry. 2024; 24(1):411. [DOI:10.1186/s12888-024-05846-5] [PMID]
- González A, Benavides J, Lema G. Anesthesia and electroconvulsive therapy: When succinylcholine is contraindicated. J ECT. 2013; 29(1):75-6. [DOI:10.1097/YCT.0b013e3182673228]
- Strawbridge AD, Khanna NR, Hauser JM. Cisatracurium. Treasure Islands: StatPearls; 2020. [Link]
- Sedighinejad A, Khoshrang H, Soltanipour S, Rezvani SM, Soleimani R, Haghighi M, et al. Atracurium as an alternative to succinylcholine in electroconvulsive therapy: A randomized clinical trial. Caspian J Neurol Sci. 2022; 8(3):163-70.[DOI:10.32598/CJNS.8.30.2]
- Bulteau S, Laforgue EJ, Chimot L, Dumont R, Loutrel O, Etcheverrigaray F, et al. Management of emergency electroconvulsive therapy in the intensive care unit for life-threatening psychiatric conditions: A case series. J ECT. 2018; 34(1):55-9. [DOI:10.1097/YCT.0000000000000451]
- Kaur M, Chhabra S, Bhatia P, Chouhan RS. Electroconvulsive therapy in a catatonia patient: Succinylcholine or no succinylcholine? Anesth Pain Med (Seoul). 2022; 17(4):454-5. [DOI:10.17085/apm.22202] [PMID]
- Nazemroaya B, Ghosouri A, Honarmand A, Hashemi ST. Comparison of hemodynamic changes and serum potassium levels in the use of succinylcholine and cisatracurium in electroconvulsive therapy. J Res Med Sci. 2021; 26:106. [DOI:10.4103/jrms.JRMS_951_19] [PMID]
- Nazemoraaya B, Moradi-Farsani D, Sadeghi-Vaghfi A. [Comparison of cisatracurium and succinylcholine on hemodynamic changes during and after electroconvulsive therapy (Persian)]. J Isfahan Med Sch. 2016; 34(395):963-70. [Link]
- Hoshi H, Kadoi Y, Kamiyama J, Nishida A, Saito H, Taguchi M, et al. Use of rocuronium-sugammadex, an alternative to succinylcholine, as a muscle relaxant during electroconvulsive therapy. J Anesth. 2011; 25:286-90. [DOI:10.1007/s00540-011-1095-6]
- Karunarathna I, Kusumarathna K, Gunarathna I, Karunathilake S, Siriwardana R, Walgama KH, et al. Advancements in electroconvulsive therapy (ECT) and anesthetic management: A comprehensive review [internet]. 2024. [Updated 2 November 2024]. Available from: [Link]
- Cornelius BW, Jacobs TM. Pseudocholinesterase deficiency considerations: A case study. Anesth Prog. 2020; 67(3):177-84. [DOI:10.2344/anpr-67-03-16]
- Liu CC, Qian XY, An JX, Yu ZL, Wu JP, Wen H, et al. Electroconvulsive therapy under general anesthesia with cisatracurium, laryngeal mask airways, and bispectral index. J ECT. 2016; 32(1):17-9. [DOI:10.1097/YCT.0000000000000251]
- Takazawa T, Suto T, Aihara M, Anzai T, Horiuchi T, Yamada MH, et al. Comparison between succinylcholine and rocuronium as neuromuscular blocking agents for electroconvulsive therapy in a patient with pseudocholinesterase deficiency. JA Clin Rep. 2015; 1:1-4. [DOI:10.1186/s40981-015-0009-2]
Type of Study: Research |
Subject:
Special
Received: 2024/07/13 | Accepted: 2024/07/24 | Published: 2024/10/5
Received: 2024/07/13 | Accepted: 2024/07/24 | Published: 2024/10/5
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



