Tue, Dec 30, 2025
Volume 10, Issue 4 (Autumn 2024)
Caspian J Neurol Sci 2024, 10(4): 335-340 |
Back to browse issues page
Ethics code: (IR.GUMS.REC.1400.416
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sedighinejad A, Kousha M, Soltanipour S, Vakili E, Naderi N, Biazar G et al . Association Between Early Exposure to General Anesthesia and Autism Spectrum Disorder: A Case Control Study. Caspian J Neurol Sci 2024; 10 (4) :335-340
URL: http://cjns.gums.ac.ir/article-1-729-en.html
URL: http://cjns.gums.ac.ir/article-1-729-en.html
Abbas Sedighinejad1 

 , Maryam Kousha2
, Maryam Kousha2 
 , Soheil Soltanipour *3
, Soheil Soltanipour *3 

 , Erfan Vakili1
, Erfan Vakili1 
 , Novin Naderi1
, Novin Naderi1 
 , Gelareh Biazar1
, Gelareh Biazar1 

 , Mahin Tayefeh Ashrafiyeh1
, Mahin Tayefeh Ashrafiyeh1 




 , Maryam Kousha2
, Maryam Kousha2 
 , Soheil Soltanipour *3
, Soheil Soltanipour *3 

 , Erfan Vakili1
, Erfan Vakili1 
 , Novin Naderi1
, Novin Naderi1 
 , Gelareh Biazar1
, Gelareh Biazar1 

 , Mahin Tayefeh Ashrafiyeh1
, Mahin Tayefeh Ashrafiyeh1 


1- Department of Anesthesiology, Anesthesiology Research Center, Alzahra Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Psychiatry, School of Medicine, Kavosh Cognitive Behavior Sciences and Addiction Research Center, Guilan University of Medical Sciences, Rasht, Iran.
3- Department of Community Medicine, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran. ,ssoltanipour@yahoo.com
2- Department of Psychiatry, School of Medicine, Kavosh Cognitive Behavior Sciences and Addiction Research Center, Guilan University of Medical Sciences, Rasht, Iran.
3- Department of Community Medicine, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran. ,
Full-Text [PDF 963 kb]
(542 Downloads)
| Abstract (HTML) (1091 Views)
Full-Text: (409 Views)
Introduction
Autism spectrum disorder (ASD) refers to a broad range of conditions characterized by challenges with social skills and a narrow range of restricted and repetitive behaviors [1, 2]. To this day, the etiology of ASD has been the topic of several research studies and is still unknown. During the past three decades, the prevalence of ASD, as a significant public health concern, has increased worldwide. Although public awareness has been raised and diagnostic criteria have been revised, the effects of environmental risk factors cannot be ignored [2]. According to the epidemiologic studies about the prenatal and perinatal risk factors of ASD, inconsistent results have been reported [3].
Interestingly, among the predisposing factors, the association between delivery mode and ASD has been frequently demonstrated as children born by Cesarian section (CS) under general anesthesia (GA) had a higher risk of ASD development compared to those delivered vaginally. However, the causal mechanism has not been established. Anesthesia, sex and history of miscarriage may influence the association between CS delivery and ASD [4-6]. Some well-planned previous studies supported this finding.
On the other hand, recent studies have focused on the association between early GA exposure and behavioral and cognitive disorders. Studies have suggested that exposure of the premature brain of the fetus to anesthetics causes histopathologic damage, leading to neurodevelopment disorders [7, 8]. A combination of these supporting evidence backs the hypothesis of GA-related neurotoxicity at early exposure to anesthetic agents. Two cohort studies notably demonstrated that children who were born by CS with GA were at higher risk of ASD, which emphasizes that GA-related neurotoxicity could be a potent risk factor for ASD development [9, 10]. On the contrary, some human studies reported different results. Considering the discrepancy among findings of various studies and very limited studies in our country about GA neurotoxicity in the developing brain, this study was planned. The present study explored whether GA exposure to the developing brain before age 4 is associated with ADS.
Materials and Methods
This case-control study was performed in a pediatric psychology clinic of an academic hospital affiliated with Guilan University of Medical Sciences from December 2021 to April 2024. One experienced pediatric psychiatrist confirmed the diagnosis of ASD based on DSM-5 criteria. Firstly, residents of anesthesiology screened all the medical records of the children who were diagnosed with ASD cases.
The inclusion criteria were known cases of ASD, aged between 5 and 18, having a sister or brother as the healthy control group and born via normal vaginal delivery.
The exclusion criteria were children with congenital heart disease or any other severe physical disease, those whose first diagnosis was other psychiatric diseases, or those who lacked a healthy sister or brother.
Neonate variables for both cases and the control group included gender, age and term or preterm. Parents were questioned about the child’s history of receiving GA for any diagnostic or surgery procedure before four years old, the age of exposure, birth status (term or preterm), and single or multi-exposure. Finally, the data were compared between the cases and the healthy control group.
After sorting out the children’s medical files, the anesthesiology resident called the parents and explained the purpose of his contact. When they agreed to participate, a checklist was completed through a 10-minute interview.
According to the odds ratio (OR=1.52; 95% CI, 1.18%, 1.94%) calculated in the study of Chien et al. [10], 40% frequency of cesarean operation, 5% error and 80% power for the volume estimation study, the sample was calculated using OpenEpi software, Version 3, open-source calculator. A total of 270 children in two case and control groups were studied separately regarding age, sex, birth age, birth weight, history of anesthesia, and type of surgery.
Statistical analysis
The collected data were analyzed using SPSS software, version 21. The Mann-Whitney U and chi-square tests were used. To evaluate all associations, we used the logistic regression model. Statistical significance was considered as P<0.05.
Results
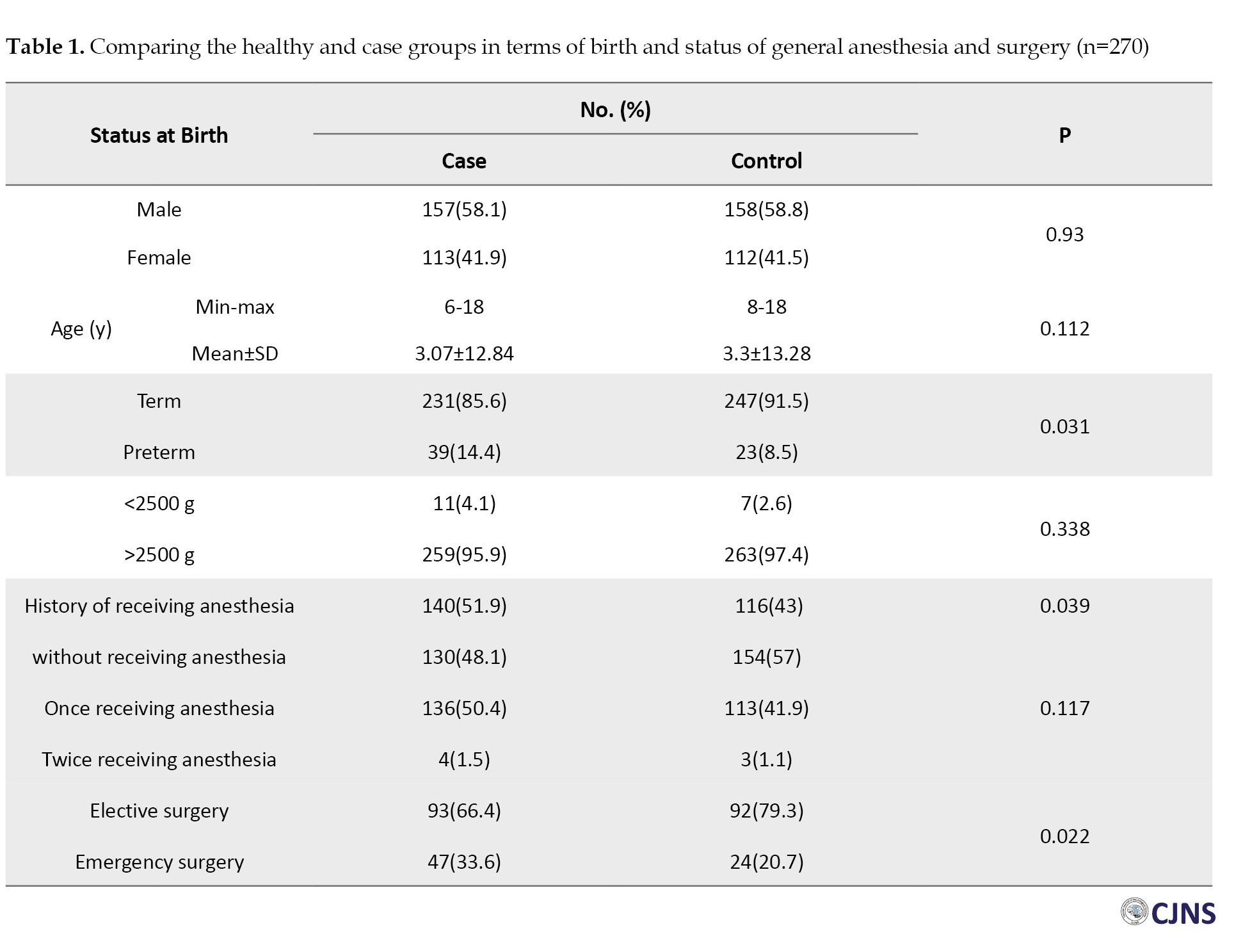
The data of 540 cases and controls were analyzed. Comparing the two groups, a significant difference was observed in terms of birth status (term/preterm) (P=0.031), history of receiving anesthesia under 4 years (P=0.039), age of receiving anesthesia (P=0.021), and type of surgery requiring anesthesia (elective/emergency) (P=0.022) (Table 1).
Multivariate analysis using logistic regression showed that only the variable of receiving anesthesia has a significant role in the incidence of autism behavioral disorders (P=0.043). So, children who had a history of receiving anesthesia were 1.47 times more likely to have autism (Table 2).

Discussion
Annually, a large number of young children undergo surgeries or diagnostic procedures that require GA. Unfortunately, despite the universal focus and interest in the subject of GA-related neurotoxicity in young children, the issue has not adequately received attention in Iran. Despite controversial results, the potential risk of GA-related neurotoxicity exists definitely [11]. To the best of our knowledge, this study is among the limited studies in our country on the effect of GA neurotoxicity in the developing brain. Because a sibling analysis was performed, adjusting familial and genetic confounding factors was possible. Our results showed the association between early exposure to anesthesia and ASD, which supported the studies conducted by Chien et al. and Huberman Samuel et al. who reported that CS under GA was positively correlated with the development of ASD [10, 12]. Recently, we explored the association between GA in the developing brain and ADHD [13]. In contrast, Chung reported that anesthetics-related neurotoxicity induced neurodegeneration but not ASD [14]. Laporta et al. also assessed the hypothesis that early exposure of children to procedures requiring GA was correlated to an increased risk of ASD in later life and found opposite results [15]. Wen-RO KO found no relationship between exposure to GA before the age of 2 and the development of ASD [16].
Although the issue has been confirmed in animal studies [17], controversial findings in human studies have been reported. Several factors could explain these discrepancies among human studies. It is well known that the results of these studies were affected by families’ economic and social status. The studied populations differ in terms of age, diagnostic criteria, times and the method of data collection, according to medical documents or interviews, which are influential factors. The results of a face-to-face conversation, phone calls and sending emails could not be the same. Also, the length of follow-ups, anesthetic dosage, timing, single or multiple exposures and duration of exposure were different. In contrast to animal studies, human studies could not provide complete control over experimental conditions. The diagnostic tools are also different. The “child behavioral checklist” has a high sensitivity, while the “strengths and difficulties questionnaire” is short and has better specificity. The study’s nature is a retrospective or large cohort, with long-term follow-ups or nested with a population-based dataset [18].
In contrast to animal studies, complete control experimental conditions could not be provided. Therefore, recently evaluating some specific biomarkers and neuroimaging data has been recommended [19-21]. Overall, and based on the current literature and FDA warnings in humans, it is wise to avoid any unnecessary exposure to GA before 4 [22], as the time from pregnancy to 4 years had been considered unsafe [23]. To achieve this goal, the anesthesiologist’s knowledge is insufficient, and physicians of several fields, such as pediatrics and surgeons, should also be aware of this risk [24, 25]. We performed another study to assess the knowledge and performance of GUMS faculty members towards the issue of anesthesia neurotoxicity, which showed disappointing results [24]. Studies have also shown that parents should be informed to cooperate better [26, 27]. In this regard, it is very crucial to avoid misunderstanding. Deprivation of the child from anesthesia benefits, including prevention of pain and anxiety, is not ethically and legally accepted, and severe pain and perioperative fear could be much more harmful than the risk of GA [28].
Conclusion
This study supported the association between ASD and early exposure to GA. The history of receiving anesthesia under age 4 was significantly associated with the later development of ASD. Further prospective well-planned cohort studies are welcome to confirm these findings.
Study limitations
Our lack of knowledge of the harmful events around birth, such as hypoxia during delivery or neonatal jaundice, can be one of the limitations of this study.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by thics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1400.416). Informed consent was obtained from all parents who agreed to participate.
Funding
This study was extracted from the PhD dissertation of Erfan Vakili’s approved by Research Council of Gilan University of Medical Sciences (Code: 1400081006).
Authors contributions
Conceptualization and study design: Abbas Sedighinejad and Maryam Kousha; Data analysis and interpretatio: Soheil Soltanipour, Erfan Vakili and Novin Naderi; Provision of study material or patients: Erfan Vakili and Mahin Tayefeh Ashrafiyeh; Critical revision: Gelareh Biazar and Abbas Sedighinejad; Statistical expertise and final approval: Soheil Soltanipour.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the Anesthesiology Research Center and Guilan University of Medical Sciences staff for collaborating in this study.
References
Autism spectrum disorder (ASD) refers to a broad range of conditions characterized by challenges with social skills and a narrow range of restricted and repetitive behaviors [1, 2]. To this day, the etiology of ASD has been the topic of several research studies and is still unknown. During the past three decades, the prevalence of ASD, as a significant public health concern, has increased worldwide. Although public awareness has been raised and diagnostic criteria have been revised, the effects of environmental risk factors cannot be ignored [2]. According to the epidemiologic studies about the prenatal and perinatal risk factors of ASD, inconsistent results have been reported [3].
Interestingly, among the predisposing factors, the association between delivery mode and ASD has been frequently demonstrated as children born by Cesarian section (CS) under general anesthesia (GA) had a higher risk of ASD development compared to those delivered vaginally. However, the causal mechanism has not been established. Anesthesia, sex and history of miscarriage may influence the association between CS delivery and ASD [4-6]. Some well-planned previous studies supported this finding.
On the other hand, recent studies have focused on the association between early GA exposure and behavioral and cognitive disorders. Studies have suggested that exposure of the premature brain of the fetus to anesthetics causes histopathologic damage, leading to neurodevelopment disorders [7, 8]. A combination of these supporting evidence backs the hypothesis of GA-related neurotoxicity at early exposure to anesthetic agents. Two cohort studies notably demonstrated that children who were born by CS with GA were at higher risk of ASD, which emphasizes that GA-related neurotoxicity could be a potent risk factor for ASD development [9, 10]. On the contrary, some human studies reported different results. Considering the discrepancy among findings of various studies and very limited studies in our country about GA neurotoxicity in the developing brain, this study was planned. The present study explored whether GA exposure to the developing brain before age 4 is associated with ADS.
Materials and Methods
This case-control study was performed in a pediatric psychology clinic of an academic hospital affiliated with Guilan University of Medical Sciences from December 2021 to April 2024. One experienced pediatric psychiatrist confirmed the diagnosis of ASD based on DSM-5 criteria. Firstly, residents of anesthesiology screened all the medical records of the children who were diagnosed with ASD cases.
The inclusion criteria were known cases of ASD, aged between 5 and 18, having a sister or brother as the healthy control group and born via normal vaginal delivery.
The exclusion criteria were children with congenital heart disease or any other severe physical disease, those whose first diagnosis was other psychiatric diseases, or those who lacked a healthy sister or brother.
Neonate variables for both cases and the control group included gender, age and term or preterm. Parents were questioned about the child’s history of receiving GA for any diagnostic or surgery procedure before four years old, the age of exposure, birth status (term or preterm), and single or multi-exposure. Finally, the data were compared between the cases and the healthy control group.
After sorting out the children’s medical files, the anesthesiology resident called the parents and explained the purpose of his contact. When they agreed to participate, a checklist was completed through a 10-minute interview.
According to the odds ratio (OR=1.52; 95% CI, 1.18%, 1.94%) calculated in the study of Chien et al. [10], 40% frequency of cesarean operation, 5% error and 80% power for the volume estimation study, the sample was calculated using OpenEpi software, Version 3, open-source calculator. A total of 270 children in two case and control groups were studied separately regarding age, sex, birth age, birth weight, history of anesthesia, and type of surgery.
Statistical analysis
The collected data were analyzed using SPSS software, version 21. The Mann-Whitney U and chi-square tests were used. To evaluate all associations, we used the logistic regression model. Statistical significance was considered as P<0.05.
Results
The data of 540 cases and controls were analyzed. Comparing the two groups, a significant difference was observed in terms of birth status (term/preterm) (P=0.031), history of receiving anesthesia under 4 years (P=0.039), age of receiving anesthesia (P=0.021), and type of surgery requiring anesthesia (elective/emergency) (P=0.022) (Table 1).

Multivariate analysis using logistic regression showed that only the variable of receiving anesthesia has a significant role in the incidence of autism behavioral disorders (P=0.043). So, children who had a history of receiving anesthesia were 1.47 times more likely to have autism (Table 2).

Discussion
Annually, a large number of young children undergo surgeries or diagnostic procedures that require GA. Unfortunately, despite the universal focus and interest in the subject of GA-related neurotoxicity in young children, the issue has not adequately received attention in Iran. Despite controversial results, the potential risk of GA-related neurotoxicity exists definitely [11]. To the best of our knowledge, this study is among the limited studies in our country on the effect of GA neurotoxicity in the developing brain. Because a sibling analysis was performed, adjusting familial and genetic confounding factors was possible. Our results showed the association between early exposure to anesthesia and ASD, which supported the studies conducted by Chien et al. and Huberman Samuel et al. who reported that CS under GA was positively correlated with the development of ASD [10, 12]. Recently, we explored the association between GA in the developing brain and ADHD [13]. In contrast, Chung reported that anesthetics-related neurotoxicity induced neurodegeneration but not ASD [14]. Laporta et al. also assessed the hypothesis that early exposure of children to procedures requiring GA was correlated to an increased risk of ASD in later life and found opposite results [15]. Wen-RO KO found no relationship between exposure to GA before the age of 2 and the development of ASD [16].
Although the issue has been confirmed in animal studies [17], controversial findings in human studies have been reported. Several factors could explain these discrepancies among human studies. It is well known that the results of these studies were affected by families’ economic and social status. The studied populations differ in terms of age, diagnostic criteria, times and the method of data collection, according to medical documents or interviews, which are influential factors. The results of a face-to-face conversation, phone calls and sending emails could not be the same. Also, the length of follow-ups, anesthetic dosage, timing, single or multiple exposures and duration of exposure were different. In contrast to animal studies, human studies could not provide complete control over experimental conditions. The diagnostic tools are also different. The “child behavioral checklist” has a high sensitivity, while the “strengths and difficulties questionnaire” is short and has better specificity. The study’s nature is a retrospective or large cohort, with long-term follow-ups or nested with a population-based dataset [18].
In contrast to animal studies, complete control experimental conditions could not be provided. Therefore, recently evaluating some specific biomarkers and neuroimaging data has been recommended [19-21]. Overall, and based on the current literature and FDA warnings in humans, it is wise to avoid any unnecessary exposure to GA before 4 [22], as the time from pregnancy to 4 years had been considered unsafe [23]. To achieve this goal, the anesthesiologist’s knowledge is insufficient, and physicians of several fields, such as pediatrics and surgeons, should also be aware of this risk [24, 25]. We performed another study to assess the knowledge and performance of GUMS faculty members towards the issue of anesthesia neurotoxicity, which showed disappointing results [24]. Studies have also shown that parents should be informed to cooperate better [26, 27]. In this regard, it is very crucial to avoid misunderstanding. Deprivation of the child from anesthesia benefits, including prevention of pain and anxiety, is not ethically and legally accepted, and severe pain and perioperative fear could be much more harmful than the risk of GA [28].
Conclusion
This study supported the association between ASD and early exposure to GA. The history of receiving anesthesia under age 4 was significantly associated with the later development of ASD. Further prospective well-planned cohort studies are welcome to confirm these findings.
Study limitations
Our lack of knowledge of the harmful events around birth, such as hypoxia during delivery or neonatal jaundice, can be one of the limitations of this study.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by thics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1400.416). Informed consent was obtained from all parents who agreed to participate.
Funding
This study was extracted from the PhD dissertation of Erfan Vakili’s approved by Research Council of Gilan University of Medical Sciences (Code: 1400081006).
Authors contributions
Conceptualization and study design: Abbas Sedighinejad and Maryam Kousha; Data analysis and interpretatio: Soheil Soltanipour, Erfan Vakili and Novin Naderi; Provision of study material or patients: Erfan Vakili and Mahin Tayefeh Ashrafiyeh; Critical revision: Gelareh Biazar and Abbas Sedighinejad; Statistical expertise and final approval: Soheil Soltanipour.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the Anesthesiology Research Center and Guilan University of Medical Sciences staff for collaborating in this study.
References
- Hodges H, Fealko C, Soares N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr. 2020; 9(Suppl 1):S55-65. [DOI:10.21037/tp.2019.09.09] [PMID] [PMCID]
- van 't Hof M, Tisseur C, van Berckelear-Onnes I, van Nieuwenhuyzen A, Daniels AM, Deen M, et al. Age at autism spectrum disorder diagnosis: A systematic review and meta-analysis from 2012 to 2019. Autism. 2021; 25(4):862-73. [DOI:10.1177/1362361320971107] [PMID]
- Yang Y, Lin J, Lu X, Xun G, Wu R, Li Y, et al. Anesthesia, sex and miscarriage history may influence the association between cesarean delivery and autism spectrum disorder. BMC Pediatr. 2021; 21(1):62. [DOI:10.1186/s12887-021-02518-1] [PMID] [PMCID]
- Andoy Galvan JA, Ramalingam PN, Patil SS, Bin Shobri MAS, Chinna K, Sahrir MS, et al. Mode of delivery, order of birth, parental age gap and autism spectrum disorder among Malaysian children: A case-control study. Heliyon. 2020; 6(10):e05068. [DOI:10.1016/j.heliyon.2020.e05068] [PMID] [PMCID]
- Al-Zalabani AH, Al-Jabree AH, Zeidan ZA. Is cesarean section delivery associated with autism spectrum disorder? Neurosciences. 2019; 24(1):11-5. [DOI:10.17712/nsj.2019.1.20180303] [PMID] [PMCID]
- Gregory SG, Anthopolos R, Osgood CE, Grotegut CA, Miranda ML. Association of autism with induced or augmented childbirth in North Carolina Birth Record (1990-1998) and Education Research (1997-2007) databases. JAMA Pediatr. 2013; 167(10):959-66. [DOI:10.1001/jamapediatrics.2013.2904] [PMID]
- Ing C, Jackson WM, Zaccariello MJ, Goldberg TE, McCann ME, Grobler A, et al. Prospectively assessed neurodevelopmental outcomes in studies of anaesthetic neurotoxicity in children: A systematic review and meta-analysis. Br J Anaesth. 2021; 126(2):433-44. [DOI:10.1016/j.bja.2020.10.022] [PMID] [PMCID]
- Wu L, Zhao H, Weng H, Ma D. Lasting effects of general anesthetics on the brain in the young and elderly: "Mixed picture" of neurotoxicity, neuroprotection and cognitive impairment. J Anesth. 2019; 33(2):321-35. [DOI:10.1007/s00540-019-02623-7] [PMID] [PMCID]
- Curran EA, Cryan JF, Kenny LC, Dinan TG, Kearney PM, Khashan AS. Obstetrical mode of delivery and childhood behavior and psychological development in a British cohort. J Autism Dev Disord. 2016; 46(2):603-14. [DOI:10.1007/s10803-015-2616-1] [PMID]
- Chien LN, Lin HC, Shao YH, Chiou ST, Chiou HY. Risk of autism associated with general anesthesia during cesarean delivery: A population-based birth-cohort analysis. J Autism Dev Disord. 2015; 45(4):932-42. [DOI:10.1007/s10803-014-2247-y] [PMID]
- Bellinger DC, Calderon J. Neurotoxicity of general anesthetics in children: Evidence and uncertainties. Curr Opin Pediatr. 2019; 31(2):267-73. [DOI:10.1097/MOP.0000000000000737] [PMID]
- Huberman Samuel M, Meiri G, Dinstein I, Flusser H, Michaelovski A, Bashiri A, et al. Exposure to general anesthesia may contribute to the association between cesarean delivery and autism spectrum disorder. J Autism Dev Disord. 2019; 49(8):3127-35. [DOI:10.1007/s10803-019-04034-9] [PMID]
- Sedighinejad A, Soltanipour S, Saberi A, Kousha M, Bidabadi E, Biazar G, et al. Risk of attention deficit hyper activity disorder after early exposure to general anesthesia; A case control study. Iranian Journal of Pediatrics. 2020; 30(3):e99976. [DOI:10.5812/ijp.99976]
- Chung W, Park S, Hong J, Park S, Lee S, Heo J, et al. Sevoflurane exposure during the neonatal period induces long-term memory impairment but not autism-like behaviors. Paediatr Anaesth. 2015; 25(10):1033-45. [DOI:10.1111/pan.12694] [PMID]
- Laporta ML, Sprung J, Fejedelem CA, Henning DT, Weaver AL, Hanson AC, et al. Association between exposure of children to general anesthesia and autism spectrum disorder. J Autism Dev Disord. 2022; 52(10):4301-10. [DOI:10.1007/s10803-021-05305-0] [PMID] [PMCID]
- Ko WR, Huang JY, Chiang YC, Nfor ON, Ko PC, Jan SR, et al. Risk of autistic disorder after exposure to general anaesthesia and surgery: A nationwide, retrospective matched cohort study. Eur J Anaesthesiol. 2015; 32(5):303-10. [DOI:10.1097/EJA.0000000000000130] [PMID]
- Slikker Jr W, Han X, Liu F, Zhang X, Gu Q, Liu S, et al. Identifying potential biomarkers, mechanisms and protective strategies for general anesthetic-induced neurotoxicity in the developing nonhuman primate. FASEB J. 2018; 32(S1):691.8. [DOI:10.1096/fasebj.2018.32.1_supplement.691.8]
- Goodman R, Ford T, Simmons H, Gatward R, Meltzer H. Using the strengths and difficulties questionnaire (sdq) to screen for child psychiatric disorders in a community sample. Br J Psychiatry. 2000; 177:534-9. [DOI:10.1192/bjp.177.6.534] [PMID]
- Wang C, Han X, Liu F, Patterson TA, Hanig JP, Paule MG, et al. Lipid profiling as an effective approach for identifying biomarkers/adverse events associated with pediatric anesthesia. Toxicol Appl Pharmacol. 2018; 354:191-5. [DOI:10.1016/j.taap.2018.03.017] [PMID]
- Imam SZ, He Z, Cuevas E, Rosas-Hernandez H, Lantz SM, Sarkar S, et al. Changes in the metabolome and microRNA levels in biological fluids might represent biomarkers of neurotoxicity: A trimethyltin study. Exp Biol Med. 2018; 243(3):228-36. [DOI:10.1177/1535370217739859] [PMID] [PMCID]
- Hung CC, Liu YH, Huang CC, Chou CY, Chen CM, Duann JR, et al. Effects of early ketamine exposure on cerebral gray matter volume and functional connectivity. Sci Rep. 2020; 10(1):15488. [DOI:10.1038/s41598-020-72320-z] [PMID] [PMCID]
- Food and Drug Administration. FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. Maryland: Food and Drug Administration; 2017.
- Walkden GJ, Gill H, Davies NM, Peters AE, Wright I, Pickering AE. Early childhood general anesthesia and neurodevelopmental outcomes in the avon longitudinal study of parents and children birth cohort. Anesthesiology. 2020; 133(5):1007-20. [DOI:10.1097/ALN.0000000000003522] [PMID]
- Sedighinejad A, Soltanipour S, Rimaz S, Biazar G, Chaibakhsh Y, Badri Kouhi M. General anesthesia-related neurotoxicity in the developing brain and current knowledge and practice of physicians at Guilan academic hospitals. Anesth Pain Med. 2019; 9(4):e92366. [DOI:10.5812/aapm.92366] [PMID] [PMCID]
- Ward CG, Hines SJ, Maxwell LG, McGowan FX, Sun LS. Neurotoxicity, general anesthesia in young children, and a survey of current pediatric anesthesia practice at US teaching institutions. Paediatr Anaesth. 2016; 26(1):60-5. [DOI:10.1111/pan.12814] [PMID]
- Nemergut ME, Aganga D, Flick RP. Anesthetic neurotoxicity: What to tell the parents? Paediatr Anaesth. 2014; 24(1):120-6. [DOI:10.1111/pan.12325] [PMID]
- Rosenblatt A, Kremer M, Paun O, Swanson B, Hamilton R, Schwartz A. Parental decision-making for surgery and anesthesia in young children. West J Nurs Res. 2022; 44(10):904-911. [DOI:10.1177/01939459211021622] [PMID]
- Oubenyahya H, Bouhabba N. General anesthesia in the management of early childhood caries: An overview. J Dent Anesth Pain Med. 2019; 19(6):313-22. [DOI:10.17245/jdapm.2019.19.6.313] [PMID] [PMCID]
Type of Study: Research |
Subject:
Special
Received: 2024/07/17 | Accepted: 2024/07/24 | Published: 2024/10/1
Received: 2024/07/17 | Accepted: 2024/07/24 | Published: 2024/10/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



