Sat, Jan 3, 2026
Volume 11, Issue 2 (Spring 2025)
Caspian J Neurol Sci 2025, 11(2): 132-139 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Saadat P, Hamzehpour R, Karimi F, Ebrahimi P, Haji Ahmadi M. Fluoxetine Versus Citalopram in Improving Post-stroke Motor Function: A Comparative Single-blind Clinical Trial. Caspian J Neurol Sci 2025; 11 (2) :132-139
URL: http://cjns.gums.ac.ir/article-1-723-en.html
URL: http://cjns.gums.ac.ir/article-1-723-en.html
1- Clinical Research Development Unit, Rouhani Hospital, University of Babol Medical Sciences Babol, Iran. , sepanta1968@yahoo.com
2- Social Determinants of Health Research Center, Babol University of Medical Sciences, Babol, Iran.
3- Student Research Committee, Babol University of Medical Sciences, Babol, Iran.
4- Social Determinants of Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran.
2- Social Determinants of Health Research Center, Babol University of Medical Sciences, Babol, Iran.
3- Student Research Committee, Babol University of Medical Sciences, Babol, Iran.
4- Social Determinants of Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran.
Full-Text [PDF 1076 kb]
(281 Downloads)
| Abstract (HTML) (1430 Views)
Full-Text: (646 Views)
Introduction
After cardiovascular disease and cancer, stroke is the third greatest cause of death across the globe. Annually, roughly 33% of stroke victims are disabled [1]. There are two types of strokes, ischemic strokes (blockage of blood flow to the brain) and hemorrhagic strokes (sudden bleeding in the brain) [2, 3]. Ischemic stroke accounts for almost 80% of all strokes [4]. Risk factors for stroke include age, gender, blood pressure, smoking, high lipid profile and diabetes [5, 6]. Age, especially age above 55 and high blood pressure are among the highest risk factors for the situations above [7, 8]. CT scans and MRIs are used to diagnose strokes [9]. The treatment of these patients is thrombolytic medications, such as antiplatelets, plasminogen activators, and thrombolytic agents [10, 11].
Serotonin is a neurotransmitter that modulates motor and other brain functions [12]. Research in the laboratory has examined the effects of therapeutic medications on neurotransmitters and consequently, their impact on brain functional recovery [13]. These medications include selective serotonin reuptake inhibitors (SSRIs) like fluoxetine and citalopram, paroxetine and sertraline, as well as serotonin/norepinephrine reuptake inhibitors (SNRIs) like venlafaxine [14-16]. When this neurotransmitter (Serotonin) is produced from platelets, it promotes platelet aggregation. Therefore, the concept that medications that block this neurotransmitter, termed SSRIs or SNRIs, might be connected with the restoration of cerebral blood flow in the continuation of cerebral ischemic stroke is supported [17].
In animal research, fluoxetine has demonstrated positive long-term benefits on brain function recovery and infarct volume reduction [18]. In human investigations, it causes decreased stroke recurrence and improved patient sensorimotor performance [19, 20]. In animal experiments, citalopram has shown neurogenesis effects. This medication also enhances somatosensory function following a stroke [21]. Using the NIH stroke scale/score, clinical investigations have demonstrated that citalopram reduces the risk of vascular events and improves motor function [22].
In light of the variations between fluoxetine and citalopram and the difficulties we confront in patients with ischemic stroke, this study aimed to explore the impact of fluoxetine and citalopram on improving motor function following ischemic stroke.
Materials and Methods
Study design
This research was a placebo-controlled, single-blind clinical trial conducted in northern Iran. This study was done on individuals diagnosed with ischemic stroke who were hospitalized between January 2021 and July 2022.
Inclusion and exclusion criteria
The patients had a computerized tomography (CT) scan at the time of referral and, if necessary, a magnetic resonance imaging (MRI), the findings of which were validated by a neurologist to diagnose an ischemic stroke. The inclusion criteria were as follows: Aged above 18 years, developed hemiparesis or hemiplegia following the first ischemic stroke that occurred during the previous 24 hours and a score equal and higher than 2 for the motor components of NIHSS (sum of the score of motor impairment in the upper and lower limbs). The exclusion criteria were as follows: Patients admitted to the ICU with an ischemic stroke and loss of consciousness from the onset; history of psychiatric disorders in patients, mood disorders (which were evaluated using screening for mood disorders before patient discharge using diagnostic and statistical manual of mental disorders, 5th edition (DSM-5) criteria; having disorders such as aphasia, cognitive pathology, or any form of movement issue before stroke; pregnancy and breastfeeding; patients receiving psychiatric medications; existence of any kind therapeutic contraindications, including renal failure (glomerular filtration rate below 30), abnormal liver function tests, hyponatremia, and a prolonged QT interval; the emergence of any significant adverse effects of the medicine during therapy, such as agitation, hypertension, or serotonin syndrome symptoms; patients who received thrombolytic drugs; and Patients who did not complete the 3-month treatment period. [11] Patients under treatment with one drug as a standard treatment for ischemic stroke (daily Clopidogrel 75 mg or Aspirin 80 mg)
Sample size
Based on a 95% confidence interval (CI) with 80% power and standard deviation difference equal to 5 with a range of 5 units, 30 percipients were calculated for each group. Due to data collection restrictions (time limit, single-center and non-referral of certain patients included in the research), the sample size for each group was decreased from 30 to 20 participants.
Interventional therapy and blinding
To examine the effects of two kinds of selective serotonin inhibitors, long-acting and short-acting, which have differing pharmacodynamics, 20 patients were separated into three groups: A) Fluoxetine, B) Citalopram and C) Placebo. In this trial, citalopram (20 mg pill from Abidi Co.), fluoxetine (20 mg capsule from Arya Co.), and the placebo were disguised as starch. These medications were put into capsules of the same color and size (capsule size: 3) in the hospital’s clean room. The average weight of capsules filled with fluoxetine (group A) was 10±2 mg (the initial therapeutic dose) and 20±2 mg. The average weight of the capsules filled with citalopram (group B) was 10±2 mg and 20±2 mg (the initial therapeutic dose) and the average of placebo capsules (group C) was 20±2 mg; these capsules were packaged in white cans. They were packaged in identical containers, so the patient was not informed of the medication they received.
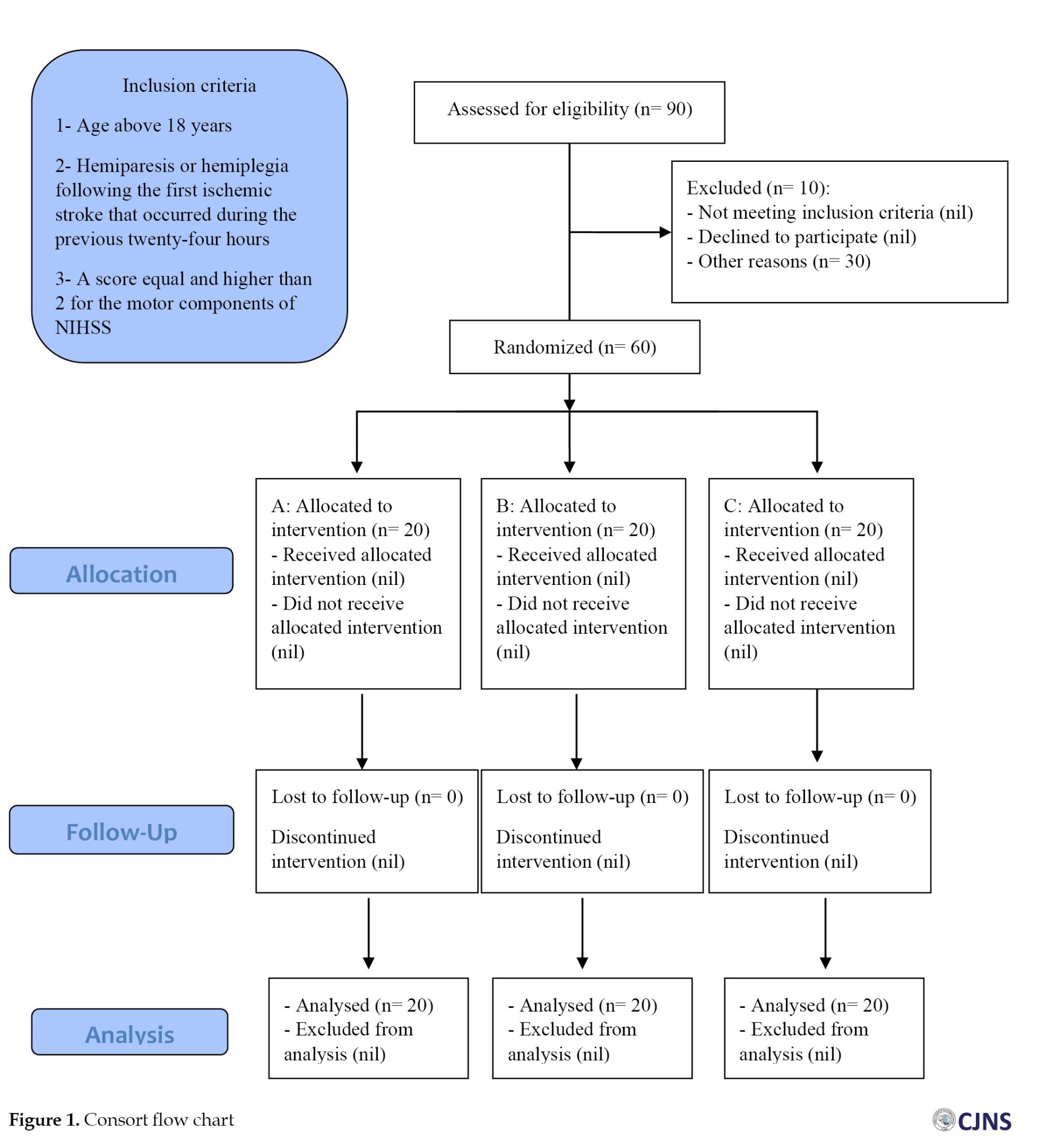
Considering that most patients were old, group A began with a starting dosage of 10 mg fluoxetine orally daily, subsequently raised to a maximum dose of 20 mg daily. In contrast, group B started with a starting dose of 20 mg daily. A daily dose of 5 mg citalopram was administered orally and according to the findings of the drug’s impact the dose was gradually raised until it reached the maximum amount of 20 mg daily. It should be noted that in addition to treatment with fluoxetine and citalopram, they also received standard treatment for ischemic stroke, including daily clopidogrel 75 mg and Aspirin 80 mg. For group C, a placebo consisting of a starch mixture was placed into the capsule. The treatment period for the patients was three months, and the medications were administered within the first week following the stroke with the patients’ informed agreement (and, if required, the assent of their first-degree relatives). All patients got the conventional stroke treatment protocol and one hour of physiotherapy. In line with the patient’s motor impairment, they were followed up in the hospital twice a week, and the technique used to accomplish this (Figure 1). Additionally, underlying diseases were matched among the treatment groups. Also, there was no investigation of the infarct volume in the patients.
Study instruments
NIHSS was utilized in this investigation to measure motor impairment in patients [23]. This scale has 11 components (for each item, scores range from 0 to 4). A score of 0 on each item indicates normal performance in that ability, whereas a score of 1 or more shows impairment. This scale was used to evaluate the motor score of the upper and lower limbs on the first, 30th, 60th and 90th days. In this study, the researcher assessed the NIHSS criterion without blinding himself or herself to the treatment allocation.
Statistical analysis
In addition to analyzing the data using the statistical program SPSS software, version 22.0, the data’s normality was also examined. Quantitative data are presented in this study as Mean±SD, whereas qualitative data are presented as frequency and percentage. To explore the link between qualitative and quantitative variables, the chi-square test and independent t-test were employed, respectively. The statistical test of repeated measurements was used to examine the effect of medications at various time intervals. In contrast, the general linear model test investigated the link between medicines and their impact at a particular period. In addition to the above-described instances, the paired t-test has also been used to explore the effects of medications across two distinct periods. In this study, the significance level was <0.05.
Results
In this interventional trial, 60 patients were evaluated to determine the efficacy of fluoxetine and citalopram compared to the control group on improving motor function following an ischemic stroke, based on the inclusion and exclusion criteria.
This research’s oldest and youngest patients were 91 and 33, respectively. Although there was a roughly 4-year age difference between the individuals getting the serotonin inhibitor medicine and those receiving the placebo, the age distribution across the three treatment groups was identical (P=0.274) (fluoxetine: 69.37±10.77, citalopram: 68.50±11.20, placebo: 73.90±11.69). The placebo (9, 45.0%) and fluoxetine (8, 40.0%) group had the most females, whereas the citalopram group contained the most males (14,70%). However, there was no significant correlation between gender and treatment groups (P=0.747).
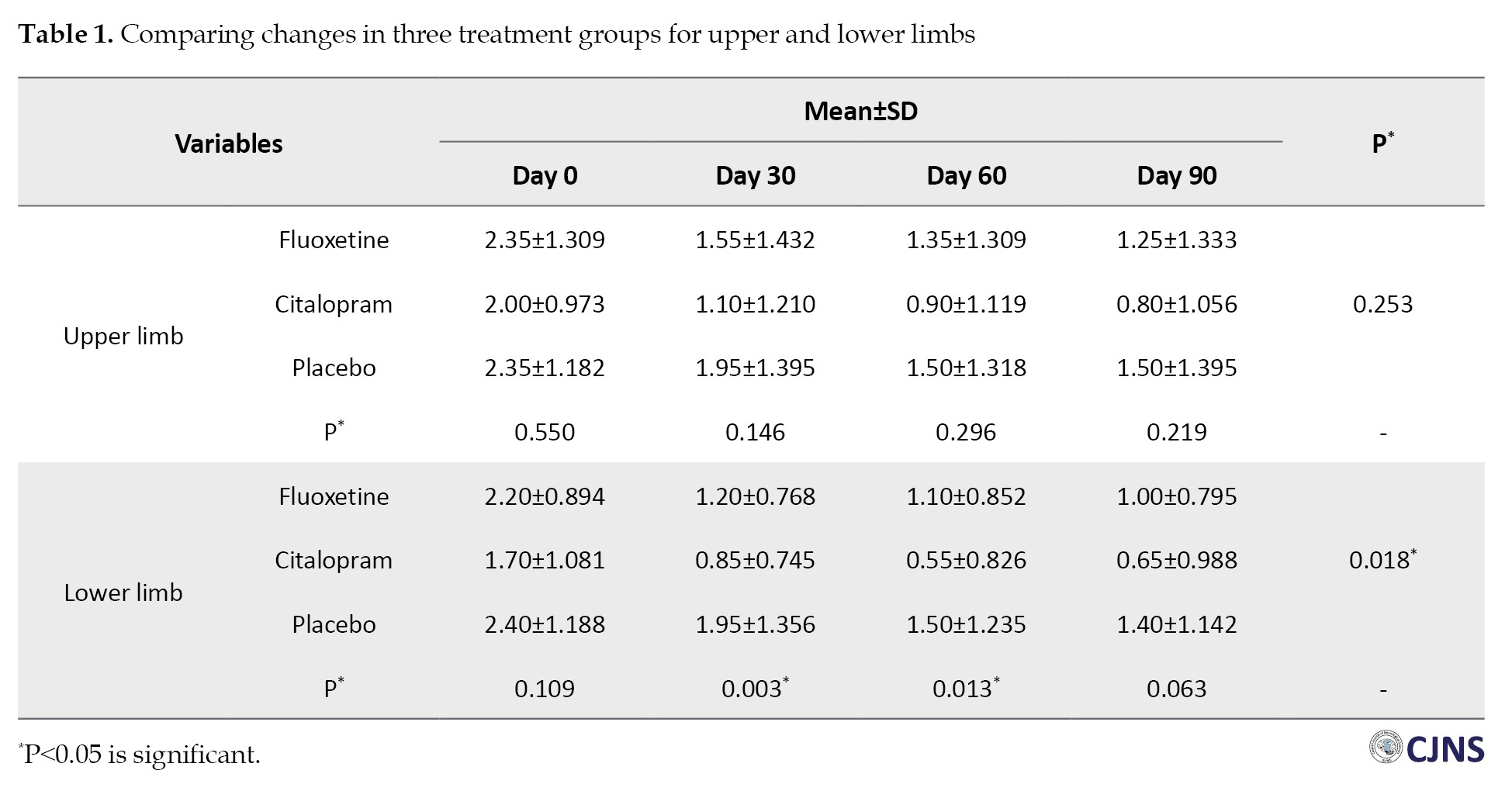
Using the NIHSS instrument, we assessed the influence of medications on patients’ mobility status. There was a significant association between the three groups over the various periods of the research in the lower limb but not in the upper limb (P=0.018) (Table 1).
In addition, citalopram, like fluoxetine, was effective on patients’ mobility status at the beginning of the research compared to all other times (P<0.001). Also, considering we used the minimum therapeutic doses of citalopram and fluoxetine, the studied patients had no side effects.
Discussion
In this single-blind clinical trial, we compared the efficacy of fluoxetine and citalopram in improving motor function following an ischemic stroke.
In the current study and in the time intervals from the beginning of the intervention to the following 90 days, the investigated drugs had a positive effect on the reduction of the average NIHSS score in the lower limb; this reduction was greater for fluoxetine than for citalopram and greater than for placebo. Statistically, this difference was significant. In the same investigation on the state of upper limb motor function, although there was a difference as previously, such that the difference from the beginning of the trial to the 90th day was more significant for citalopram than for fluoxetine and placebo, no significant difference was detected. In the research of medications administered over time, there was no significant difference between them for improving upper limb motor function. In the lower limbs, a separate scenario exists. One and two months following the beginning of the trial, there was a significant difference between these three medications in terms of the improvement of the NIHSS motor score in each of the given periods. Citalopram is more effective than fluoxetine during both periods.
In a meta-analysis by Elsnhory et al. done on 7165 patients, fluoxetine was found beneficial in enhancing motor function based on the NIHSS comparison. However, this effect takes time and its impact is transient [24]. Also, in the study of Liu et al. which was included in the previous meta-analysis, it was stated that the effect of fluoxetine in studies with smaller sample sizes was able to improve motor function as measured by the NIHSS scale. Still, in studies with larger sample sizes, this significance decreases. In addition, they note that the increased risk of seizures, hyponatremia, and bone fractures in stroke patients associated with the use of fluoxetine should be considered [25]. In the research of the mechanism of this treatment, investigations have revealed the anti-inflammatory and antioxidant effects of this drug on the protection of neurons in these patients, as well as the increased expression of brain-derived neurotrophic factor (BDNF), anti-apoptotic and increased expression of hemeoxygenase-1 (HO-1) [26-30]. In terms of the efficacy of this treatment on stroke patients, the studies mentioned above are comparable to ours. Nevertheless, further research is required to determine the drug’s long-term or short-term effects and inclusion criteria. It is essential to evaluate the advantages and risks of this medication. Fluoxetine should not be administered to stroke patients who do not have mood issues. Although this medicine improved the Fugl-Meyer motor scale or Barthel index, it failed to meet the modified Rankin scale and NIHSS requirements [25].
Regarding citalopram, a meta-analysis suggests that SSRIs help enhance the motor function of stroke patients. However, this result was only observed in the sub-analysis, including citalopram (not fluoxetine). In addition, they noted that it is preferable to focus more on the effect of this medicine in randomized control trials [31]. In intervention research by Savadi Oskouie et al. administering 20 mg of this medicine vs placebo for three months produced satisfactory results in terms of safety and tolerability for the recovery of motor function in stroke patients [32]. Also, in a trial by Acler et al. the treatment of 10 mg of this medicine for at least four months improved the NIHSS score of stroke patients [33]. The theorized mechanism of action of citalopram is the drug’s influence on apoptotic indicators and its ability to inhibit the production of inflammatory mediators [34-36]. The research mentioned above on citalopram is comparable to our concept.
In a similar study conducted by Asadollahi et al. a 90-day study of functional improvement with the Fugl-Meyer motor scale and with citalopram, fluoxetine, and placebo drugs in patients following ischemic stroke concluded that no significant difference between citalopram and fluoxetine, but both medicines can improve motor function in comparison to placebo [37]. Using the NIHSS scale, there was no significant difference in the averages of these three medications in the time preceding the study, as was the case in our study; thus, this study is comparable to ours.
The administration of two medications, citalopram, and fluoxetine, to individuals suffering from mobility difficulties after a stroke still requires investigation. The indicated mechanisms are based on animal studies, and further clinical research is necessary to study them further. Patients’ conditions must be evaluated before prescribing fluoxetine and citalopram, and it is best to begin treatment in those with mood disorders and lower limb movement problems. The length and onset of pharmacological effects require further research.
This study also has limitations. The spread of the coronavirus, limited access to hospitalized patients suffering from cerebral ischemia stroke, and no investigation of the infarct volume in the patients are the limitations of this study.
Conclusion
Citalopram and fluoxetine are more successful in treating lower limb movement problems in stroke patients than upper limb movement disorders. Compared to the commencement of the trial and the 90th day, administering these two medications one and two months after the study’s inception provided greater results.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Babol University of Medical Sciences, Babol, Iran (Code: IR.MUBABOL.REC.1399.279) and approved the Ethics Clinical Trial of Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20210307050617N).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization: Payam Saadat, Romina Hamzehpour and Pouyan Ebrahimi; Methodology: Mahmoud Haji Ahmadi; Investigation: Payam Saadat, Fatemeh Karimi and Pouyan Ebrahimi; Writing the original draft: Pouyan Ebrahimi; Writing, review, and editing: Pouyan Ebrahimi, Payam Saadat, Fatemeh Karimi, and Romina Hamzehpour; Funding acquisition: Payam Saadat; Resources: Payam Saadat and Romina Hamzehpour; Supervision: Payam Saadat and Pouyan Ebrahimi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors appreciate the assistance of the Clinical Research Development Unit of Rouhani Hospital.
References
After cardiovascular disease and cancer, stroke is the third greatest cause of death across the globe. Annually, roughly 33% of stroke victims are disabled [1]. There are two types of strokes, ischemic strokes (blockage of blood flow to the brain) and hemorrhagic strokes (sudden bleeding in the brain) [2, 3]. Ischemic stroke accounts for almost 80% of all strokes [4]. Risk factors for stroke include age, gender, blood pressure, smoking, high lipid profile and diabetes [5, 6]. Age, especially age above 55 and high blood pressure are among the highest risk factors for the situations above [7, 8]. CT scans and MRIs are used to diagnose strokes [9]. The treatment of these patients is thrombolytic medications, such as antiplatelets, plasminogen activators, and thrombolytic agents [10, 11].
Serotonin is a neurotransmitter that modulates motor and other brain functions [12]. Research in the laboratory has examined the effects of therapeutic medications on neurotransmitters and consequently, their impact on brain functional recovery [13]. These medications include selective serotonin reuptake inhibitors (SSRIs) like fluoxetine and citalopram, paroxetine and sertraline, as well as serotonin/norepinephrine reuptake inhibitors (SNRIs) like venlafaxine [14-16]. When this neurotransmitter (Serotonin) is produced from platelets, it promotes platelet aggregation. Therefore, the concept that medications that block this neurotransmitter, termed SSRIs or SNRIs, might be connected with the restoration of cerebral blood flow in the continuation of cerebral ischemic stroke is supported [17].
In animal research, fluoxetine has demonstrated positive long-term benefits on brain function recovery and infarct volume reduction [18]. In human investigations, it causes decreased stroke recurrence and improved patient sensorimotor performance [19, 20]. In animal experiments, citalopram has shown neurogenesis effects. This medication also enhances somatosensory function following a stroke [21]. Using the NIH stroke scale/score, clinical investigations have demonstrated that citalopram reduces the risk of vascular events and improves motor function [22].
In light of the variations between fluoxetine and citalopram and the difficulties we confront in patients with ischemic stroke, this study aimed to explore the impact of fluoxetine and citalopram on improving motor function following ischemic stroke.
Materials and Methods
Study design
This research was a placebo-controlled, single-blind clinical trial conducted in northern Iran. This study was done on individuals diagnosed with ischemic stroke who were hospitalized between January 2021 and July 2022.
Inclusion and exclusion criteria
The patients had a computerized tomography (CT) scan at the time of referral and, if necessary, a magnetic resonance imaging (MRI), the findings of which were validated by a neurologist to diagnose an ischemic stroke. The inclusion criteria were as follows: Aged above 18 years, developed hemiparesis or hemiplegia following the first ischemic stroke that occurred during the previous 24 hours and a score equal and higher than 2 for the motor components of NIHSS (sum of the score of motor impairment in the upper and lower limbs). The exclusion criteria were as follows: Patients admitted to the ICU with an ischemic stroke and loss of consciousness from the onset; history of psychiatric disorders in patients, mood disorders (which were evaluated using screening for mood disorders before patient discharge using diagnostic and statistical manual of mental disorders, 5th edition (DSM-5) criteria; having disorders such as aphasia, cognitive pathology, or any form of movement issue before stroke; pregnancy and breastfeeding; patients receiving psychiatric medications; existence of any kind therapeutic contraindications, including renal failure (glomerular filtration rate below 30), abnormal liver function tests, hyponatremia, and a prolonged QT interval; the emergence of any significant adverse effects of the medicine during therapy, such as agitation, hypertension, or serotonin syndrome symptoms; patients who received thrombolytic drugs; and Patients who did not complete the 3-month treatment period. [11] Patients under treatment with one drug as a standard treatment for ischemic stroke (daily Clopidogrel 75 mg or Aspirin 80 mg)
Sample size
Based on a 95% confidence interval (CI) with 80% power and standard deviation difference equal to 5 with a range of 5 units, 30 percipients were calculated for each group. Due to data collection restrictions (time limit, single-center and non-referral of certain patients included in the research), the sample size for each group was decreased from 30 to 20 participants.
Interventional therapy and blinding
To examine the effects of two kinds of selective serotonin inhibitors, long-acting and short-acting, which have differing pharmacodynamics, 20 patients were separated into three groups: A) Fluoxetine, B) Citalopram and C) Placebo. In this trial, citalopram (20 mg pill from Abidi Co.), fluoxetine (20 mg capsule from Arya Co.), and the placebo were disguised as starch. These medications were put into capsules of the same color and size (capsule size: 3) in the hospital’s clean room. The average weight of capsules filled with fluoxetine (group A) was 10±2 mg (the initial therapeutic dose) and 20±2 mg. The average weight of the capsules filled with citalopram (group B) was 10±2 mg and 20±2 mg (the initial therapeutic dose) and the average of placebo capsules (group C) was 20±2 mg; these capsules were packaged in white cans. They were packaged in identical containers, so the patient was not informed of the medication they received.
Considering that most patients were old, group A began with a starting dosage of 10 mg fluoxetine orally daily, subsequently raised to a maximum dose of 20 mg daily. In contrast, group B started with a starting dose of 20 mg daily. A daily dose of 5 mg citalopram was administered orally and according to the findings of the drug’s impact the dose was gradually raised until it reached the maximum amount of 20 mg daily. It should be noted that in addition to treatment with fluoxetine and citalopram, they also received standard treatment for ischemic stroke, including daily clopidogrel 75 mg and Aspirin 80 mg. For group C, a placebo consisting of a starch mixture was placed into the capsule. The treatment period for the patients was three months, and the medications were administered within the first week following the stroke with the patients’ informed agreement (and, if required, the assent of their first-degree relatives). All patients got the conventional stroke treatment protocol and one hour of physiotherapy. In line with the patient’s motor impairment, they were followed up in the hospital twice a week, and the technique used to accomplish this (Figure 1). Additionally, underlying diseases were matched among the treatment groups. Also, there was no investigation of the infarct volume in the patients.
Study instruments
NIHSS was utilized in this investigation to measure motor impairment in patients [23]. This scale has 11 components (for each item, scores range from 0 to 4). A score of 0 on each item indicates normal performance in that ability, whereas a score of 1 or more shows impairment. This scale was used to evaluate the motor score of the upper and lower limbs on the first, 30th, 60th and 90th days. In this study, the researcher assessed the NIHSS criterion without blinding himself or herself to the treatment allocation.
Statistical analysis
In addition to analyzing the data using the statistical program SPSS software, version 22.0, the data’s normality was also examined. Quantitative data are presented in this study as Mean±SD, whereas qualitative data are presented as frequency and percentage. To explore the link between qualitative and quantitative variables, the chi-square test and independent t-test were employed, respectively. The statistical test of repeated measurements was used to examine the effect of medications at various time intervals. In contrast, the general linear model test investigated the link between medicines and their impact at a particular period. In addition to the above-described instances, the paired t-test has also been used to explore the effects of medications across two distinct periods. In this study, the significance level was <0.05.
Results
In this interventional trial, 60 patients were evaluated to determine the efficacy of fluoxetine and citalopram compared to the control group on improving motor function following an ischemic stroke, based on the inclusion and exclusion criteria.
This research’s oldest and youngest patients were 91 and 33, respectively. Although there was a roughly 4-year age difference between the individuals getting the serotonin inhibitor medicine and those receiving the placebo, the age distribution across the three treatment groups was identical (P=0.274) (fluoxetine: 69.37±10.77, citalopram: 68.50±11.20, placebo: 73.90±11.69). The placebo (9, 45.0%) and fluoxetine (8, 40.0%) group had the most females, whereas the citalopram group contained the most males (14,70%). However, there was no significant correlation between gender and treatment groups (P=0.747).
Using the NIHSS instrument, we assessed the influence of medications on patients’ mobility status. There was a significant association between the three groups over the various periods of the research in the lower limb but not in the upper limb (P=0.018) (Table 1).

In addition, citalopram, like fluoxetine, was effective on patients’ mobility status at the beginning of the research compared to all other times (P<0.001). Also, considering we used the minimum therapeutic doses of citalopram and fluoxetine, the studied patients had no side effects.
Discussion
In this single-blind clinical trial, we compared the efficacy of fluoxetine and citalopram in improving motor function following an ischemic stroke.
In the current study and in the time intervals from the beginning of the intervention to the following 90 days, the investigated drugs had a positive effect on the reduction of the average NIHSS score in the lower limb; this reduction was greater for fluoxetine than for citalopram and greater than for placebo. Statistically, this difference was significant. In the same investigation on the state of upper limb motor function, although there was a difference as previously, such that the difference from the beginning of the trial to the 90th day was more significant for citalopram than for fluoxetine and placebo, no significant difference was detected. In the research of medications administered over time, there was no significant difference between them for improving upper limb motor function. In the lower limbs, a separate scenario exists. One and two months following the beginning of the trial, there was a significant difference between these three medications in terms of the improvement of the NIHSS motor score in each of the given periods. Citalopram is more effective than fluoxetine during both periods.
In a meta-analysis by Elsnhory et al. done on 7165 patients, fluoxetine was found beneficial in enhancing motor function based on the NIHSS comparison. However, this effect takes time and its impact is transient [24]. Also, in the study of Liu et al. which was included in the previous meta-analysis, it was stated that the effect of fluoxetine in studies with smaller sample sizes was able to improve motor function as measured by the NIHSS scale. Still, in studies with larger sample sizes, this significance decreases. In addition, they note that the increased risk of seizures, hyponatremia, and bone fractures in stroke patients associated with the use of fluoxetine should be considered [25]. In the research of the mechanism of this treatment, investigations have revealed the anti-inflammatory and antioxidant effects of this drug on the protection of neurons in these patients, as well as the increased expression of brain-derived neurotrophic factor (BDNF), anti-apoptotic and increased expression of hemeoxygenase-1 (HO-1) [26-30]. In terms of the efficacy of this treatment on stroke patients, the studies mentioned above are comparable to ours. Nevertheless, further research is required to determine the drug’s long-term or short-term effects and inclusion criteria. It is essential to evaluate the advantages and risks of this medication. Fluoxetine should not be administered to stroke patients who do not have mood issues. Although this medicine improved the Fugl-Meyer motor scale or Barthel index, it failed to meet the modified Rankin scale and NIHSS requirements [25].
Regarding citalopram, a meta-analysis suggests that SSRIs help enhance the motor function of stroke patients. However, this result was only observed in the sub-analysis, including citalopram (not fluoxetine). In addition, they noted that it is preferable to focus more on the effect of this medicine in randomized control trials [31]. In intervention research by Savadi Oskouie et al. administering 20 mg of this medicine vs placebo for three months produced satisfactory results in terms of safety and tolerability for the recovery of motor function in stroke patients [32]. Also, in a trial by Acler et al. the treatment of 10 mg of this medicine for at least four months improved the NIHSS score of stroke patients [33]. The theorized mechanism of action of citalopram is the drug’s influence on apoptotic indicators and its ability to inhibit the production of inflammatory mediators [34-36]. The research mentioned above on citalopram is comparable to our concept.
In a similar study conducted by Asadollahi et al. a 90-day study of functional improvement with the Fugl-Meyer motor scale and with citalopram, fluoxetine, and placebo drugs in patients following ischemic stroke concluded that no significant difference between citalopram and fluoxetine, but both medicines can improve motor function in comparison to placebo [37]. Using the NIHSS scale, there was no significant difference in the averages of these three medications in the time preceding the study, as was the case in our study; thus, this study is comparable to ours.
The administration of two medications, citalopram, and fluoxetine, to individuals suffering from mobility difficulties after a stroke still requires investigation. The indicated mechanisms are based on animal studies, and further clinical research is necessary to study them further. Patients’ conditions must be evaluated before prescribing fluoxetine and citalopram, and it is best to begin treatment in those with mood disorders and lower limb movement problems. The length and onset of pharmacological effects require further research.
This study also has limitations. The spread of the coronavirus, limited access to hospitalized patients suffering from cerebral ischemia stroke, and no investigation of the infarct volume in the patients are the limitations of this study.
Conclusion
Citalopram and fluoxetine are more successful in treating lower limb movement problems in stroke patients than upper limb movement disorders. Compared to the commencement of the trial and the 90th day, administering these two medications one and two months after the study’s inception provided greater results.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Babol University of Medical Sciences, Babol, Iran (Code: IR.MUBABOL.REC.1399.279) and approved the Ethics Clinical Trial of Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20210307050617N).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization: Payam Saadat, Romina Hamzehpour and Pouyan Ebrahimi; Methodology: Mahmoud Haji Ahmadi; Investigation: Payam Saadat, Fatemeh Karimi and Pouyan Ebrahimi; Writing the original draft: Pouyan Ebrahimi; Writing, review, and editing: Pouyan Ebrahimi, Payam Saadat, Fatemeh Karimi, and Romina Hamzehpour; Funding acquisition: Payam Saadat; Resources: Payam Saadat and Romina Hamzehpour; Supervision: Payam Saadat and Pouyan Ebrahimi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors appreciate the assistance of the Clinical Research Development Unit of Rouhani Hospital.
References
- Abbasi V, Fattahzadeh-Ardalani G, Safarnejad P, Aslanian R. Albumin impact on clinical practice and complications of ischemic stroke in patients with stroke. Int J Basic Clin Pharmacol. 2016; 5(5):2114-7. [DOI:10.18203/2319-2003.ijbcp20163246]
- Raichle ME. The pathophysiology of brain ischemia. Ann Neurol. 1983; 13(1):2-10. [DOI:10.1002/ana.410130103] [PMID]
- Hou K, Xu D, Li F, Chen S, Li Y. The progress of neuronal autophagy in cerebral ischemia stroke: Mechanisms, roles and research methods. J Neurol Sci. 2019; 400:72-82. [DOI:10.1016/j.jns.2019.03.015] [PMID]
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991; 337(8756):1521-6. [DOI:10.1016/0140-6736(91)93206-O] [PMID]
- O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the interstroke study): A case-control study. Lancet. 2010; 376(9735):112-23. [DOI:10.1016/S0140-6736(10)60834-3] [PMID]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet. 2006; 367(9524):1747-57. [DOI:10.1016/S0140-6736(06)68770-9] [PMID]
- Caplan LR. Stroke: A clinical approach. Philadelphia: Elsevier/Saunders; 2009. [Link]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003; 289(19):2560-72. [DOI:10.1001/jama.289.19.2560] [PMID]
- Merino JG, Warach S. Imaging of acute stroke. Nat Rev Neurol. 2010; 6(10):560-71. [DOI:10.1038/nrneurol.2010.129] [PMID]
- International Stroke Trial Collaborative Group. The international stroke trial (IST): A randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet. 1997; 349(9065):1569-81. [DOI:10.1016/S0140-6736(97)04011-7]
- Lee GA, Lin TN, Chen CY, Mau SY, Huang WZ, Kao YC, et al. Interleukin 15 blockade protects the brain from cerebral ischemia-reperfusion injury. Brain Behav Immun. 2018; 73:562-70. [DOI:10.1016/j.bbi.2018.06.021] [PMID]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009; 60:355-66. [DOI:10.1146/annurev.med.60.042307.110802] [PMID] [PMCID]
- Farner L, Wagle J, Engedal K, Flekkøy KM, Wyller TB, Fure B. Depressive symptoms in stroke patients: a 13 month follow-up study of patients referred to a rehabilitation unit. J Affect Disord. 2010; 127(1-3):211-8. [DOI:10.1016/j.jad.2010.05.025] [PMID]
- Gałecki P, Mossakowska-Wójcik J, Talarowska M. The anti-inflammatory mechanism of antidepressants-SSRIs, SNRIs. Prog Neuropsychopharmacol Biol Psychiatry. 2018; 80(Pt C):291-4. [DOI:10.1016/j.pnpbp.2017.03.016] [PMID]
- Buller KM, Wixey JA, Reinebrant HE. Disruption of the serotonergic system after neonatal hypoxia-ischemia in a rodent model. Neurol Res Int. 2012; 2012:650382. [DOI:10.1155/2012/650382] [PMID] [PMCID]
- Gaur V, Kumar A. Protective effect of desipramine, venlafaxine and trazodone against experimental animal model of transient global ischemia: Possible involvement of NO-cGMP pathway. Brain Res. 2010; 1353:204-12. [DOI:10.1016/j.brainres.2010.07.004] [PMID]
- Wgner A, Montero D, Mårtensson B, Siwers B, Asberg M. Effects of fluoxetine treatment of platelet 3H-imipramine binding, 5-HT uptake and 5-HT content in major depressive disorder. J Affect Disord. 1990; 20(2):101-13. [DOI:10.1016/0165-0327(90)90123-P] [PMID]
- Lee JY, Lee HE, Kang SR, Choi HY, Ryu JH, Yune TY. Fluoxetine inhibits transient global ischemia-induced hippocampal neuronal death and memory impairment by preventing blood-brain barrier disruption. Neuropharmacology. 2014; 79:161-71. [DOI:10.1016/j.neuropharm.2013.11.011] [PMID]
- He Y, Cai Z, Zeng S, Chen S, Tang B, Liang Y, et al. Effect of fluoxetine on three-year recurrence in acute ischemic stroke: A randomized controlled clinical study. Clin Neurol Neurosurg. 2018; 168:1-6. [DOI:10.1016/j.clineuro.2018.02.029] [PMID]
- Pariente J, Loubinoux I, Carel C, Albucher JF, Leger A, Manelfe C, et al. Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann Neurol. 2001; 50(6):718-29. [DOI:10.1002/ana.1257] [PMID]
- Espinera AR, Ogle ME, Gu X, Wei L. Citalopram enhances neurovascular regeneration and sensorimotor functional recovery after ischemic stroke in mice. Neuroscience. 2013; 247:1-11. [DOI:10.1016/j.neuroscience.2013.04.011] [PMID] [PMCID]
- Kraglund KL, Mortensen JK, Grove EL, Johnsen SP, Andersen G. TALOS: A multicenter, randomized, double-blind, placebo-controlled trial to test the effects of citalopram in patients with acute stroke. Int J Stroke. 2015; 10(6):985-7. [DOI:10.1111/ijs.12485] [PMID]
- Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke. 1994; 25(11):2220-6. [DOI:10.1161/01.STR.25.11.2220] [PMID]
- Elsnhory A, Hasan MT, Hagrass AI, Hanbal A, Fathy A, Ahmed E, et al. Recovery in stroke patients treated with fluoxetine versus placebo: A pooled analysis of 7,165 patients. Neurologist. 2023; 28(2):104-16. [DOI:10.1097/NRL.0000000000000451] [PMID]
- Liu G, Yang X, Xue T, Chen S, Wu X, Yan Z, et al. Is Fluoxetine Good for Subacute Stroke? A Meta-Analysis Evidenced From Randomized Controlled Trials. Front Neurol. 2021; 12:633781. [DOI:10.3389/fneur.2021.633781] [PMID] [PMCID]
- Lim CM, Kim SW, Park JY, Kim C, Yoon SH, Lee JK. Fluoxetine affords robust neuroprotection in the postischemic brain via its anti-inflammatory effect. J Neurosci Res. 2009; 87(4):1037-45. [DOI:10.1002/jnr.21899] [PMID]
- Kim DH, Li H, Yoo KY, Lee BH, Hwang IK, Won MH. Effects of fluoxetine on ischemic cells and expressions in BDNF and some antioxidants in the gerbil hippocampal CA1 region induced by transient ischemia. Exp Neurol. 2007; 204(2):748-58. [DOI:10.1016/j.expneurol.2007.01.008] [PMID]
- Xu F, Zhang G, Yin J, Zhang Q, Ge MY, Peng L, et al. Fluoxetine mitigating late-stage cognition and neurobehavior impairment induced by cerebral ischemia reperfusion injury through inhibiting ERS-mediated neurons apoptosis in the hippocampus. Behav Brain Res. 2019; 370:111952. [DOI:10.1016/j.bbr.2019.111952] [PMID]
- Hu Q, Liu L, Zhou L, Lu H, Wang J, Chen X, et al. Effect of fluoxetine on HIF-1α- Netrin/VEGF cascade, angiogenesis and neuroprotection in a rat model of transient middle cerebral artery occlusion. Exp Neurol. 2020;329:113312. [DOI:10.1016/j.expneurol.2020.113312] [PMID]
- Shin TK, Kang MS, Lee HY, Seo MS, Kim SG, Kim CD, et al. Fluoxetine and sertraline attenuate postischemic brain injury in mice. Korean J Physiol Pharmacol. 2009; 13(3):257-63. [DOI:10.4196/kjpp.2009.13.3.257] [PMID] [PMCID]
- Kalbouneh HM, Toubasi AA, Albustanji FH, Obaid YY, Al-Harasis LM. Safety and efficacy of SSRIs in improving poststroke recovery: A systematic review and meta-analysis. J Am Heart Assoc. 2022; 11(13):e025868. [DOI:10.1161/JAHA.122.025868] [PMID] [PMCID]
- Savadi Oskouie D, Sharifipour E, Sadeghi Bazargani H, Hashemilar M, Nikanfar M, Ghazanfari Amlashi S, et al. Efficacy of citalopram on acute ischemic stroke outcome: A randomized clinical trial. Neurorehabilitation and Neural Repair. 2017; 31(7):638-47. [DOI:10.1177/1545968317704902] [PMID]
- Acler M, Robol E, Fiaschi A, Manganotti P. A double blind placebo RCT to investigate the effects of serotonergic modulation on brain excitability and motor recovery in stroke patients. J Neurol. 2009; 256(7):1152-8. [DOI:10.1007/s00415-009-5093-7] [PMID]
- Gupta S, Upadhayay D, Sharma U, Jagannathan NR, Gupta YK. Citalopram attenuated neurobehavioral, biochemical, and metabolic alterations in transient middle cerebral artery occlusion model of stroke in male Wistar rats. J Neurosci Res. 2018; 96(7):1277-93. [DOI:10.1002/jnr.24226] [PMID]
- Nakata N, Kato H, Kogure K. Protective effects of serotonin reuptake inhibitors, citalopram and clomipramine, against hippocampal CA1 neuronal damage following transient ischemia in the gerbil. Brain Res. 1992; 590(1-2):48-52. [DOI:10.1016/0006-8993(92)91080-X] [PMID]
- Dhami KS, Churchward MA, Baker GB, Todd KG. Fluoxetine and citalopram decrease microglial release of glutamate and D-serine to promote cortical neuronal viability following ischemic insult. Mol Cell Neurosci. 2013; 56:365-74. [DOI:10.1016/j.mcn.2013.07.006] [PMID]
- Asadollahi M, Ramezani M, Khanmoradi Z, Karimialavijeh E. The efficacy comparison of citalopram, fluoxetine, and placebo on motor recovery after ischemic stroke: A double-blind placebo-controlled randomized controlled trial. Clin Rehabil. 2018; 32(8):1069-75. [DOI:10.1177/0269215518777791] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2024/06/15 | Accepted: 2025/01/27 | Published: 2025/04/1
Received: 2024/06/15 | Accepted: 2025/01/27 | Published: 2025/04/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |