Fri, Jan 30, 2026
Volume 10, Issue 4 (Autumn 2024)
Caspian J Neurol Sci 2024, 10(4): 247-265 |
Back to browse issues page
Ethics code: not applicable
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mosleh H, Ghayyem H, Sharifpour S, Eyvani K, Niknejad S, Davoodi A, et al et al . Amyloid-β Peptide-targeting Immunotherapy in Alzheimer Disease: A Systematic Review of Clinical Studies. Caspian J Neurol Sci 2024; 10 (4) :247-265
URL: http://cjns.gums.ac.ir/article-1-714-en.html
URL: http://cjns.gums.ac.ir/article-1-714-en.html
Haideh Mosleh1 

 , Hani Ghayyem2
, Hani Ghayyem2 

 , Sanaz Sharifpour3
, Sanaz Sharifpour3 

 , Kimia Eyvani4
, Kimia Eyvani4 

 , Sepideh Niknejad5
, Sepideh Niknejad5 

 , Ali Davoodi5
, Ali Davoodi5 

 , Ameneh Zarebidoki6
, Ameneh Zarebidoki6 

 , Sarina Ahmadian7
, Sarina Ahmadian7 

 , Kosar Kohandel8
, Kosar Kohandel8 

 , Amirreza Eghbaldoost9
, Amirreza Eghbaldoost9 

 , Mehraeen Jashni Pour10
, Mehraeen Jashni Pour10 

 , Sara Binaei11
, Sara Binaei11 

 , Ali Sanaye Abbasi4
, Ali Sanaye Abbasi4 

 , Mohammadreza Kosari12
, Mohammadreza Kosari12 

 , Ali Sanaye Abbasi13
, Ali Sanaye Abbasi13 
 , Mohammadreza Kosari *14
, Mohammadreza Kosari *14 



 , Hani Ghayyem2
, Hani Ghayyem2 

 , Sanaz Sharifpour3
, Sanaz Sharifpour3 

 , Kimia Eyvani4
, Kimia Eyvani4 

 , Sepideh Niknejad5
, Sepideh Niknejad5 

 , Ali Davoodi5
, Ali Davoodi5 

 , Ameneh Zarebidoki6
, Ameneh Zarebidoki6 

 , Sarina Ahmadian7
, Sarina Ahmadian7 

 , Kosar Kohandel8
, Kosar Kohandel8 

 , Amirreza Eghbaldoost9
, Amirreza Eghbaldoost9 

 , Mehraeen Jashni Pour10
, Mehraeen Jashni Pour10 

 , Sara Binaei11
, Sara Binaei11 

 , Ali Sanaye Abbasi4
, Ali Sanaye Abbasi4 

 , Mohammadreza Kosari12
, Mohammadreza Kosari12 

 , Ali Sanaye Abbasi13
, Ali Sanaye Abbasi13 
 , Mohammadreza Kosari *14
, Mohammadreza Kosari *14 

1- Hearing Disorder Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Internal Medicine, Faculty of Medicine, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran.
4- Student Research Committee, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
5- Noncommunicable Diseases Research Center, Fasa University of Medical Sciences, Fasa, Iran.
6- Student Research Committee, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
7- Department of Internal Medicine, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
8- Multiple Sclerosis Researcher Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran.
9- Student Research Committee, Golestan University of Medical Sciences, Gorgan, Iran.
10- Institue of Biology and Medicine, Taras Shevchenko National University of Kyiv, Kyiv, Ukraine.
11- Endocrinology and Metabolism Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran.
12- Department of Neurology, Tongji Medical College, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China.
13- Student Research Committee, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
14- Department of Neurology, Tongji Medical College, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China. ,Mh.reza.kosari@gmail.com
2- Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Internal Medicine, Faculty of Medicine, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran.
4- Student Research Committee, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
5- Noncommunicable Diseases Research Center, Fasa University of Medical Sciences, Fasa, Iran.
6- Student Research Committee, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
7- Department of Internal Medicine, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
8- Multiple Sclerosis Researcher Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran.
9- Student Research Committee, Golestan University of Medical Sciences, Gorgan, Iran.
10- Institue of Biology and Medicine, Taras Shevchenko National University of Kyiv, Kyiv, Ukraine.
11- Endocrinology and Metabolism Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran.
12- Department of Neurology, Tongji Medical College, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China.
13- Student Research Committee, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
14- Department of Neurology, Tongji Medical College, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China. ,
Keywords: Immunotherapy, Alzheimer disease, Amyloid-β peptide, Plaque, Amyloid, Cerebral amyloid angiopathy, Apolipoproteins E
Full-Text [PDF 3058 kb]
(718 Downloads)
| Abstract (HTML) (1637 Views)
Full-Text: (647 Views)
Introduction
Alzheimer disease (AD) is the most common form of dementia [1], with nearly 10 million new cases every year [2]. Cholinesterase inhibitors, N-methyl-D-aspartate receptor partial antagonists, and other medications used to treat secondary behavioral symptoms associated with AD are currently used to manage this disease [2]. However, no drug or other intervention can currently prevent, slow down, or stop AD progression [3]. Therefore, developing a clinically validated method to slow down the progression of AD is challenging but essential [4].
Although the exact mechanism of Aβ toxicity is still debated, interventions that prevent Aβ aggregation and deposition are among promising therapeutic strategies. In transgenic AD animal models, passive immunization with anti-Aβ antibodies and vaccination with Aβ42 or other Aβ fragments have led to the elimination of Aβ deposits and improved behavioral deficits. These preclinical observations prompted clinical trials, one of which was abruptly stopped when several patients developed adverse neuroinflammatory reactions. Although the exact biological mechanisms of vaccination-induced Aβ clearance from brain tissue have remained unclear, they may involve a combination of microglial Fc-receptor-mediated phagocytosis, the sequestration of Aβ in plasma with subsequent release of soluble Aβ from cerebrospinal fluid (CSF) into plasma, and antibody-mediated breakdown of Aβ fibrils [5]. In an AD animal model, antibody-induced Aβ clearance from brain parenchyma occurred within three days [6]. As a result, a multicenter trial was set up to test active immunization with a pre-aggregated synthetic Aβ42 preparation (AN1792) in mild-to-moderate AD [5]. Due to the continuous production and maturation of therapeutic antibodies and the need for less frequent vaccine administration, active immunotherapy offers long-term advantages over passive immunotherapy. Furthermore, in phase III clinical trials in patients with mild-to-moderate AD, passive immunotherapies with monoclonal antibodies, including bapineuzumab and solanezumab, failed to meet their primary endpoint criteria, such as an improvement in cognition and activities of daily living index [7].
Active immunization with pre-aggregated human Aβ42 effectively reduced the number of Aβ plaques in the transgenic mice [8, 9, 10, 11, 12] that expressed mutations associated with the dominantly inherited AD. Intriguingly, there was an inverse correlation between the levels of remaining amyloid load in the brain and serum anti-Aβ antibody titers. The amyloid plaques that remain after vaccination are poorly identifiable, even though collapsed “moth-eaten” plaques have been documented.
The link between Aβ clearance and the status of neurofibrillary tangles, tau phosphorylation, and aggregation has not been fully described in AN1792-immunized patients. Nevertheless, post-mortem studies [1, 13] and in vivo amyloid imaging [14, 15] show the removal of Aβ following immunotherapy in patients living with AD [16, 17]. Although side effects of Aβ immunization have been observed in clinical trials [13, 17-19], the removal of Aβ is associated with potentially beneficial effects on the disease pathology, such as reduced tau phosphorylation in the brain [16] and diminished tau protein levels in the CSF [20]. Aβ immunization also alleviated disease-related deterioration of neuronal processes [21]. Notably, however, patients with mild-to-moderate cognitive impairment participating in the study by Serrano-Pozo et al. [22] developed persistent, progressive dementia and subsequently died of AD despite immunization.
Since the Aβ immunotherapy was successful in transgenic AD mice, a clinical trial was conducted in AD patients using aggregated Aβ42 (AN1792) in an adjuvant formulated with QS-21. Aseptic meningoencephalitis occurred in a subset of patients (6%) in this study and the clinical trial was stopped. Instead of the six injections initially scheduled in this phase II study protocol, patients received only one to three injections of AN1792 [23]. Despite the interruption of this trial, modest but significant positive effects of immunization on select cognitive and functional outcome measures were documented. Further evaluation of the entire study cohort revealed limited cognitive benefits and “less worsening” of clinical outcome measures [23]. Similarly, the recently reported complications associated with bapineuzumab passive immunotherapy are attributed to the development of brain vasogenic edema in some participants carrying the apolipoprotein E ε4 allele (ApoE4), which resulted in their exclusion from the study. Despite these adverse events, the pathology of abnormal neurites and tau levels/phosphorylation are reduced in AN-1792-immunized AD patients [24].
This review summarizes the effects of Aβ immunotherapy on AD pathological features and clinical outcomes. The evidence indicates that immunization with Aβ42 has protective effects at the early stages of AD and significantly affects the pathogenesis of established AD. By clearing Aβ deposits in the brain, particularly in the frontal cortex, Aβ immunotherapy slows the decline of cognitive and functional capacities of AD patients.
Materials and Methods
This systematic review was conducted according to the 2009 Preferred reporting items for systematic reviews and meta-analyses (PRISMA) guideline [25].
Search strategy
PubMed, Scopus, Web of Science, Google Scholar, and Embase were systematically searched using relevant search terms to identify pertinent articles published before 2023. Only articles written in English were considered in this review. Preclinical studies using animal models were excluded. The specific search terms used to identify AD immunotherapy studies are listed in Table 1.






This systematic review includes original, investigational, and follow-up studies undertaken to assess the potential effects of Aβ vaccination in AD patients. Only articles focusing on adult human populations were considered for this review. We included original research articles published from 2002 until 2023 written in English involving AD patients. Finally, we only included studies investigating the outcomes of vaccination trials in AD.
Inclusion and exclusion criteria
All relevant articles considering AD vaccines, including AADvac1, AN-1792 and CAD106, were screened before including them in the final analysis. Non-human studies, systematic reviews, meta-analyses, non-English language articles and duplicate publications were excluded.
Study selection methodology
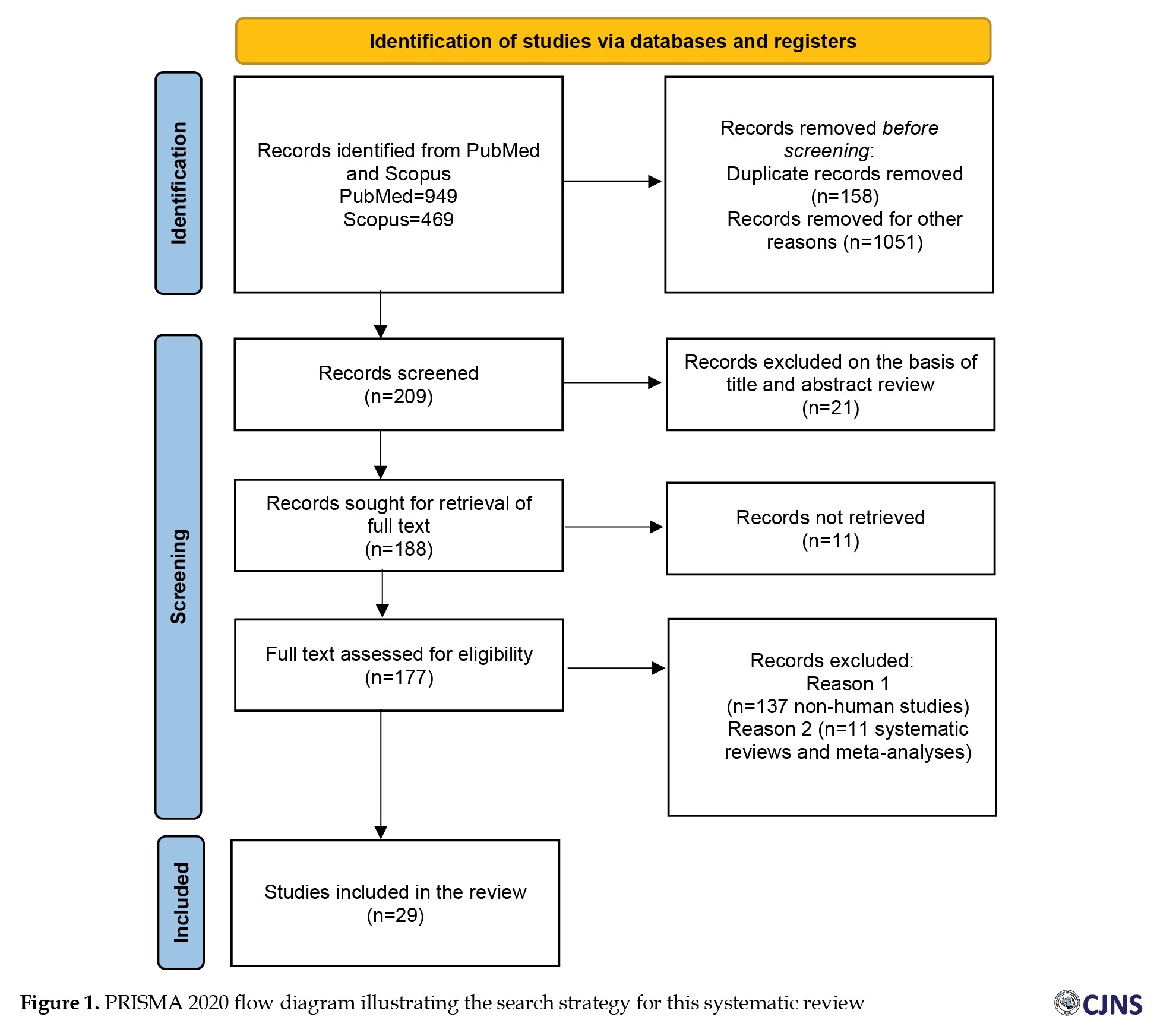
The studies included in this review were chosen following a three-step process illustrated in Figure 1. All duplicate publications were eliminated first. Then, based on the inclusion and exclusion criteria stated above, the titles and abstracts of articles were independently assessed by two reviewers to create a shortlist of eligible publications. Subsequently, the same reviewers evaluated the full text of the publications. Finally, a third reviewer evaluated the quality and accuracy of the selected data. Disagreements among the three reviewers were resolved through discussions until a consensus was reached. The Newcastle-Ottawa scale (NOS) was used to assess the quality of each included study. The eight evaluation criteria of the Ottawa checklist were utilized to assess the features of the studies, including selection, comparability and outcome. All studies were categorized into three groups based on their NOS scores: High quality (scores 7-9), moderate quality (scores 5-6) and low quality (scores 0-4).
Results
Demographic characteristics of the subjects
The review included 2725 AD patients in this review. The mean age of the patients was 75.69 years and 51% were female. The mean mini-mental state examination score (MMSE) score of AD patients was 21.24, while the control group had a mean MMSE score of 21.21.
Anti-Aβ immunization in AD
Most immunotherapies and vaccinations target Aβ to trigger a suitable immune response (anti-Aβ antibodies) that removes the Aβ deposits and enhances cognitive and functional capacities. AN1792 was the first vaccine used for active immunotherapy in AD, and it was used in 26 out of 29 studies we reviewed. AN-1792 includes aggregated human Aβ42 combined with a saponin-based adjuvant QS-21. The safety and tolerability of this vaccine were first demonstrated in a phase I study. Anti-Aβ42 antibodies from AN1792 recipients could distinguish between Aβ plaques located extracellularly and those in brain blood vessels [5, 26].
AADvac1 is another vaccine comprising synthetic peptides resembling the misfolded tau protein, with aluminum hydroxide and keyhole limpet hemocyanin as adjuvants. AADvac1 attenuated neurofibrillary pathology and insoluble tau deposits in the brains of immunized AD patients. IgG antibodies against tau were produced in 29 out of 30 treated older individuals with mild-to-moderate AD, demonstrating its strong immunogenicity in humans [27].
CAD106 is a second-generation vaccine for active Aβ immunotherapy that includes several copies of the Aβ1-6 peptide attached to a carrier that consists of 180 copies of the bacteriophage Q coat protein. It successfully induces the production of anti-Aβ antibodies without causing an Aβ-specific T-cell response [28]. The first human study of CAD106 also shows a favorable safety profile and an encouraging antibody response when administered with adjuvants alum (50 µg and 150 µg) and MF59 (squalene-based oil-in-water emulsion, 125 µL and 250 µL) [28]. CAD106 administeration without adjuvants at doses up to 150 µg generates consistent anti-Aβ antibody responses in otherwise healthy AD patients without significant safety concerns. The combination of alum adjuvant and CAD106 (450 μg) shows the optimum balance between tolerability and antibody response [7].
Survival time from the first immunization dose
The survival time of patients after injecting the first dose of AN1792 varies from 4 up to 184 months across different studies [29]. The longest and shortest survival times were observed in patients receiving AN1792 (225 µg and 50 µg, respectively) [30].
The time between the first dose of vaccine and booster
The time between the vaccine and the booster dose in the studies varied from 15 to 45 days. The most common choice was 30 days, as it was used in 7 out of 29 studies.
Adverse effects of vaccination
Cerebral microhemorrhage and vasogenic edema can be induced by passive and active Aβ immunization. Clinical trials have been suspended due to unfavorable outcomes such as meningoencephalitis [5], microhemorrhage in the brain, and cerebral amyloid angiopathy (CAA) [5, 31]. Thus, humoral antibody responses to AN1792 are restricted to the brain Aβ deposits, reducing the likelihood of post-vaccination aseptic meningoencephalitis in these patients.
In a study by Boche et al. [32], none of the patients receiving AN1792 experienced significant intracerebral hemorrhages caused by CAA. Still, they displayed a greater density of cortical microhemorrhages and microvascular deficits than the control group. Vaccination with AN1792 dissolves plaque deposits of Aβ42, which departs the brain through the perivascular route, temporarily worsening CAA. Gilman et al. [33] permanently stopped the administration of AN1792 after the initial reports of meningoencephalitis and monitored patients closely for the next 9 months. The inflammatory response was speculated to be due to polysorbate 80 in the vaccine, which may have exposed more amino acids of the Aβ42 peptide, escalating inflammatory T-cell responses. Orgogozo et al. [18] reported that 18 out of 298 AN1792 recipients experienced post-vaccination aseptic meningoencephalitis. Sakai et al. [31] evaluated the impact of ApoE, an Aβ transporter, on CAA and the formation of CAA-associated vasculopathy following Aβ immunotherapy with AN1792. They concluded that ApoE contributed to plaque clearance and Aβ delivery to the cerebral vasculature, with concentric splitting of the vessel wall being about three times more common in leptomeningeal arteries of immunized compared to non-immunized ones.
Unlike AN1792, no meningoencephalitis or vasogenic edema cases have been reported in patients receiving the AADvac1 vaccine. Among the studies using AADvac1, only one ApoE4 homozygote showed signs of new microhemorrhages [34]. Similar to AADvac1, no signs of central nervous system inflammation or clinical or subclinical cases of meningoencephalitis were observed in CAD106 studies [7, 28]. However, Vandenberghe et al. [7] reported amyloid-related imaging abnormalities (ARIAs) in 6 out of 120 patients, all of whom had a robust serological response to CAD106. None of those ARIA patients displayed neurological symptoms. However, this study recorded several severe adverse events (SAEs) reported in a single patient, including allergic dermatitis (immediately following the fourth injection), atrial fibrillation (6 weeks following the first injection), and acute psychosis (14 weeks following the seventh injection) were 3 of the 26 SAEs in the entire study group of 58 patients. The researchers thought that these SAEs might be linked to CAD106 injection. Nasopharyngitis and injection site erythema were the most common adverse events associated with the CAD106 vaccine [28].
ApoE4
ApoE4 was the most significant genetic risk factor for sporadic AD and severe CAA development in Aβ-immunized AD patients [35, 36]. AD patients carrying the ε4 allele are up to 15 times more likely to develop AD than ε3. Moreover, they experience more severe and frequent CAA comorbidity compared to carriers of other ApoE alleles or ApoE-negative AD patients [37]. The ApoE4 has been linked to tonic-clonic seizures independent of AD and Aβ. The complex mechanisms underlying the association between ApoE4 and AD are believed to involve tau phosphorylation, Aβ peptide clearance, and neuronal death [38]. Several mechanisms have been proposed for Aβ removal from the brain parenchyma without immunotherapy. First, ApoE influences Aβ uptake by cells since the Aβ and ApoE complex is endocytosed and digested enzymatically [39]. Second, ApoE facilitates the Aβ transport across the blood-brain barrier (BBB) [40] via the very low-density lipoprotein receptor and low-density lipoprotein receptor-related protein 1 [41]. Moreover, a perivascular drainage channel has been suggested as an essential Aβ elimination mechanism. Failure of this channel due to age-related mechanisms plays a significant role in the development of CAA [42, 43, 44]. Sakai et al. [31] reported that ApoE mediates the amyloid plaque clearance. Aβ delivery to the brain microvasculature is consistent with ApoE’s significant role in removing Aβ from the brain after Aβ immunotherapy. Thus, ARIAs associated with Aβ immunotherapy may reflect a severe age-related vascular alteration in some AD patients, particularly in ApoE4 carriers, who display rapid plaque disaggregation. According to preclinical data, CAD106 can decrease the buildup of Aβ in the brain by triggering antibodies that prevent amyloid deposition. Notably, CAD106 was shown to be safe and effective in a patient population with a higher proportion of ApoE4 carriers (>60%) than the general population (>23%), supporting this association [2].
Clinical outcomes and immunological findings
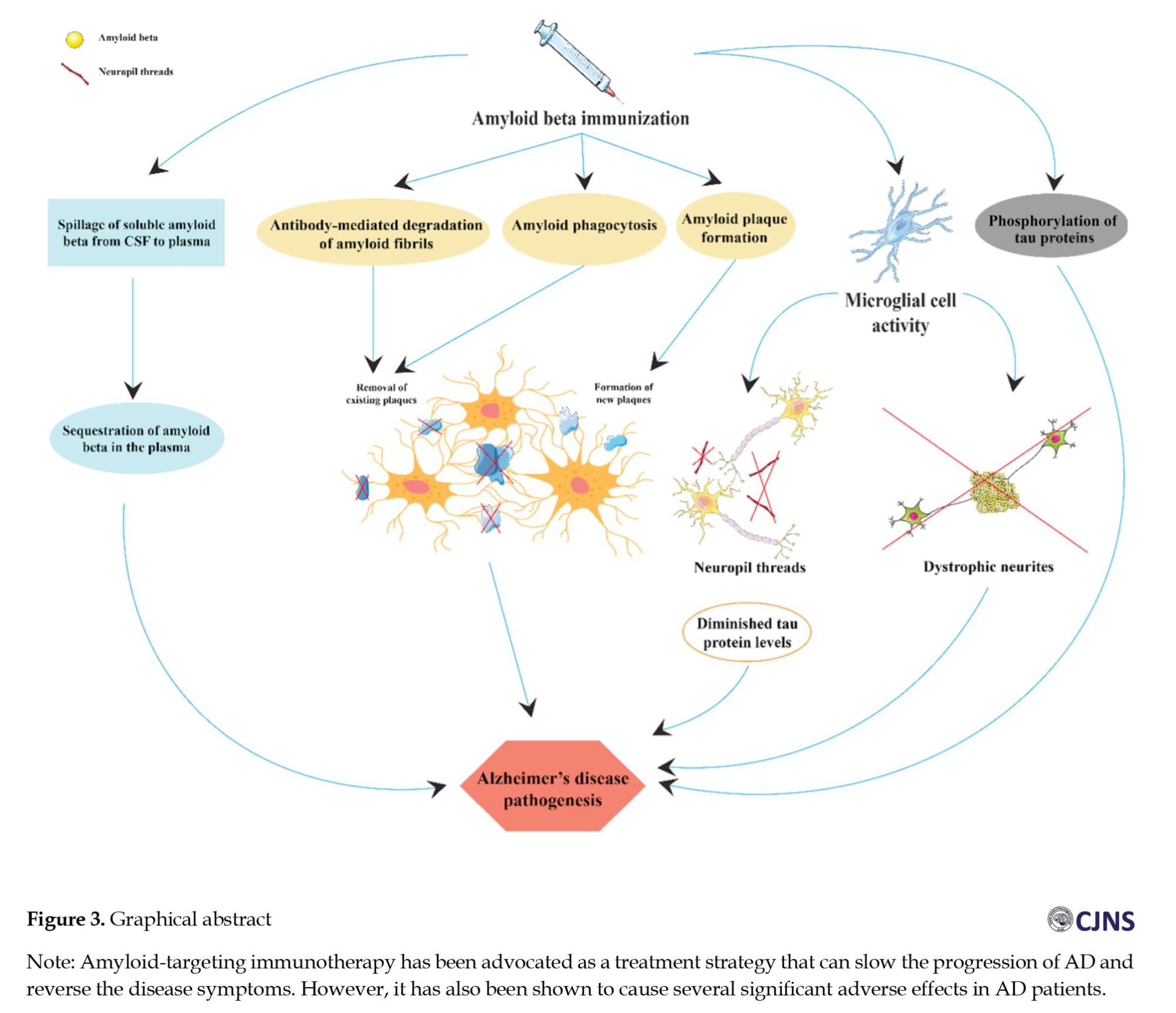
Although the precise biological mechanisms underlying vaccination-induced clearance of Aβ from brain tissue are still unknown, they possibly involve a combination of phagocytosis by microglial Fc-receptors, sequestration of Aβ in plasma that leads to the release of soluble Aβ from CSF into plasma, and the breakdown of Aβ fibrils by antibodies (Figure 2). Most AD patients immunized with aggregated Aβ42 (AN1792) show a favorable immune response by generating antibodies with a high selectivity towards Aβ plaques, which identify amyloid plaques, diffuse Aβ deposits, and vascular amyloid in subarachnoid and perforating cerebral blood vessels. However, while Aβ immunization leads to the removal of Aβ from the brain, the cognitive decline persists, probably because of the continued presence of phospho-tau in the brain. For instance, Boche et al. [16] showed that the reduction of Aβ in neuronal processes such as neuropil threads and dystrophic neurites was observed in AD patients actively immunized with AN1792. In contrast, the development of neurofibrillary tangles due to phospho-tau buildup in the neuronal cell bodies remained unaffected. In another study, Holmes et al. (Long-term effects of Aβ42) [45] performed a post-mortem assessment of AD patients immunized with AN1792 (50 μg or 225 μg). They demonstrated that 7 out of 8 patients with complete plaque removal experienced severe end-stage dementia before death. This finding indicates that plaque clearance did not correlate with the AN1792 dosage or improved clinical outcomes. The study found no significant difference in survival rates (hazard ratio 0.93; 95% CI, 0.43-3.11; P=0.86) or delay in dementia progression (1.18; 95% CI, 0.45-3.11; P=0.73) between the AN1792 and placebo group.
Elevation in the levels of neurodegeneration-associated protein, eukaryotic initiation factor 2α and neurotic curvature indicate neuronal health. Despite the apparent improvement in the health of remaining neurons, there was a more significant loss of neurons, suggesting that only damaged neurons may be removed following vaccination. This discrepancy between the decrease in brain volume and improved cognitive performance was also noted in other studies [4, 21]. Zotova et al. reported that levels of the activated macrophage and microglia marker CD68 in both white and gray matter were higher in the immunized Alzheimer’s disease (iAD) group compared to non-immunized control Alzheimer’s disease (cAD) cases. Moreover, the Aβ42 load was lower (P=0.036) in the brains of iAD compared to cAD patients.
Additionally, in the iAD group, the CD68 levels are inversely associated with Aβ42 load, suggesting that Aβ immunization enhances microglial reactivity and phagocytic activity in the presence of plaques. Interestingly, in long-surviving iAD patients, CD68 expression is lower despite complete plaque clearance, indicating a reduction in phagocytic activity post-plaque removal. The assessment of major histocompatibility complex (MHC) class II cell surface receptor HLA-DR, another microglia activation marker, on the cells that clustered around amyloid plaques reveals no significant differences between iAD and cAD groups, indicating that this microglia activation marker may have been exclusively connected to their phagocytic activity specifically when plaques are present.
The similarity of CD68 and HLA-DR loading in the pons of both groups raises the possibility that AD-associated pathology caused the observed variations in microglial activation in the cortex. It has been proposed that phagocytic activity initially increases after immunization with Aβ42 microglial. Still, it may decrease once the plaques are cleared, returning to levels similar to those observed in unimmunized AD patients at the same disease stage [46]. Aβ vaccination may also minimize tau phosphorylation and improve tau pathology in the hippocampus. According to some review studies, AN1792 immunization significantly reduces plaque load and increases amyloid phagocytosis in the frontal cortex and, to a lesser extent, in the temporal lobe [1, 47]. AN1792 vaccination reduced amyloid plaques, attenuated hippocampal neurite abnormalities, and potentially minimized tau phosphorylation. Some studies noted that despite these reductions, there are fewer and smaller remaining dense-core amyloid plaques; however, they might still be harmful [22]. This issue raises concerns that plaque clearance alone might not be sufficient to prevent disease progression.
The long-term outcome of AN1792 immunization was further examined by Vellas et al. [30], who showed low but detectable and persistent anti-Aβ antibody titers in responders from a phase II vaccine trial. Moreover, the same study also reported a significantly slower functional decline in immunized compared to placebo-treated patients over approximately 4.6 years following vaccination. Despite no significant difference in brain volume reduction between the two groups after 3.6 years, no new encephalitis was reported in immunized individuals. These results highlight the potential of Aβ immunotherapy in improving long-term functional outcomes in AD [30]. Notably, as reported, AD patients who were actively immunized with Aβ (AN1792) could remain virtually plaque-free for up to 14 years [29].
CAD106 is the first active immunotherapy shown to reduce amyloid depositions in AD patients. Winblad et al. [28] reported robust anti-Aβ antibody responses in CAD106-treated patients, with 67% and 82% in the first and second cohorts, respectively, meeting the expected criteria for antibody response [48]. These findings imply that CAD106 induces a better antibody response over 7 injections, consistent with the conclusions of an earlier phase I study. Notably, CAD106 also had a favorable safety profile, with no serious adverse effects reported in AD patients. The good safety profile, combined with the immunogenicity of CAD106, supports its potential as a viable therapeutic option for managing AD. The neuropsychological test battery (NTB) appears to be a valid and sensitive assessment of changes in cognitive functions in individuals with mild and moderate AD. The psychometric characteristics of the NTB denote its potential value in assessing treatment efficacy in clinical trials that include participants with moderate AD [28, 49]. In the clinical trials by Novak et al. [34, 50],
AADvac1 shows an acceptable safety profile, with booster doses leading to sustained IgG titers throughout immunization-free intervals. According to MRI evaluations, patients with higher anti-Aβ antibody titers exhibit slower deterioration in cognitive function and reduced brain atrophy, suggesting the vaccine’s efficacy in slowing down the disease progression. Although AADvac1 is still relatively new compared to AN1792, the evidence thus far indicates promising results, particularly regarding safety and cognitive benefits. The long-term impact of AADvac1 is still under investigation, but the early data suggest that it could be a valuable addition to the arsenal of AD treatments.
Discussion
Our review demonstrates sufficient published data to conclude that the Aβ vaccination significantly impacts the pathogenesis of AD. Placebo-controlled randomized trials discussed in this review show that Aβ vaccination can slow cognitive decline in AD patients [51]. Several other studies have demonstrated that Aβ immunotherapy reduces amyloid plaque burden and promotes amyloid phagocytosis in the frontal cortex and, to a lesser extent, in the temporal lobe. AD patients who experience a significant reduction in amyloid load can remain plaque-free for nearly 14 years [29]. This efficacy period prevents repeated accumulations of neurodegeneration-associated proteins, including Aβ. Notably, immunization of AD patients with Aβ42 induces the production of antibodies with a great degree of selectivity for the pathogenic amyloid structures. For example, they do not cross-react with denatured or native full-length amyloid precursor protein or its physiological derivatives, such as soluble Aβ42 monomers, dimers, and trimers. However, one of the most concerning side effects of Aβ immunotherapy is CAA-associated vasculopathy, which can develop after Aβ plaque removal. It has been proposed that ApoE may induce structural abnormalities in the blood vessel walls after transporting Aβ out of the brain [31]. In addition to amyloid clearance, Aβ immunotherapy has significantly reduced in AD brains. This reduction likely reflects the clearance of the tau-containing dystrophic neurite component of plaques. Immunization effectively reduces tau aggregation and decreases tau hyperphosphorylation, critical factors in AD progression. Furthermore, active immunization could effectively improve or reverse memory deficits [22]. Interestingly, while Aβ immunotherapy has been shown to improve microglial phagocytic activity, after the clearance of Aβ plaques, microglia phagocytic activity decreases to levels similar to those in unimmunized AD cases [29, 52].
However, while Aβ immunization is pivotal in preventing plaque formation and removing existing plaques, it does not necessarily stop the progression of neurodegeneration. Nearly all immunized individuals who died because of the AD neurodegenerative processes during the follow-up phase of the Aβ immunization trial had the highest anti-Aβ antibody titers, and their brains were free from amyloid plaques. A longer time interval does not lower the effect of the immunization, and there was no significant correlation between the amount of plaque removal and survival time since the first immunization dose. These findings suggest substantial plaque removal does not benefit survival [29]. This finding implies that despite the clearance of plaques, progressive neurodegeneration can continue in AD brains. An in vivo imaging demonstrates an increased rate of cerebral atrophy in Aβ-immunized AD patients, possibly indicating accelerated loss of damaged and degenerating neurons [21]. One of the most significant adverse effects of Aβ vaccination was observed by Orgogozo et al. [18]. They found that some AD patients developed signs and symptoms related to aseptic meningoencephalitis during the Aβ immunization process. The potential mechanism responsible for the induction of mild-to-severe meningoencephalitis includes T-cell and microglial activation, which must be considered carefully when developing safer Aβ immunotherapy for AD patients. However, the exact relationship between post-vaccination meningoencephalitis and serum anti-Aβ42 antibody titer has remained unclear. Many studies have documented the dramatic adverse effects of Aβ immunotherapy, such as neuroinflammatory reactions, meningoencephalitis, and leukoencephalopathy [47]. Other phenomena, including CAA, T-cell encephalitis, and macrophage infiltration in the white matter, were observed in the preclinical studies by Masliah et al. [47]. The MRI imaging of AD patients immunized with AN1792/QS-21 demonstrates reduced brain volume in antibody responders. The decrease in brain volume did not worsen cognitive performance. Although the reason for this decline is still unclear, it may be due to amyloid removal and the associated CSF shifts [4]. This review identifies cerebral micro hemorrhage, vasogenic edema, meningoencephalitis, and CAA as the most common adverse effects that have led to the postponement of AN1792 immunization trials.
In contrast, second-generation active immunotherapies, including CAD106, have offered a significant safety profile. They have avoided the T-cell response by using fragmented Aβ peptides or peptide mimetics of the Aβ N-terminus, which are recognized by B-cells only. Recent studies show that CAD106 is the first active Aβ immunotherapy that slows amyloid deposition in AD patients. Using CAD106 with an alum adjuvant leads to one of the best balances between antibody response and tolerability recorded thus far. The acceptable antibody response and the favorable safety profile of CAD106 indicate its potential as a valuable therapeutic option for treating AD patients [28]. Additionally, AD patients who respond to AADvac1 vaccination with high titers of anti-tau antibodies exhibit slower brain atrophy in MRI evaluation and a slower decline in cognitive assessment scores than those with low antibody titers. Notably, AN1792, AADvac1, and CAD106 vaccinations did not cause microhemorrhage inflammation in the central nervous system. However, there are a few reports of minor side effects, such as ARIAs, in patients receiving CAD106 injections. Other minor adverse effects associated with CAD106 vaccination include allergic dermatitis, atrial fibrillation, acute psychosis, nasopharyngitis, and erythema at the injection site [21, 53] (Figure 3).
Conclusion
This systematic review evaluated the effects of Aβ immunotherapy on AD pathogenesis and the progression of the disease processes. Aβ immunotherapy has been shown to prevent the formation of new Aβ plaques and even reduce the pre-existing plaques. The inhibitory effect of Aβ vaccination on amyloid plaque formation appears relatively specific and is not associated with general disturbances of the physiological processes. Most studies report improved cognitive performance and memory in vaccinated AD patients compared to unvaccinated ones. Despite the positive overall results observed with three different types of vaccines, there are remaining uncertainties about the exact mechanisms of their action, particularly concerning the effects of immunization on the progression of neurodegeneration and brain atrophy in AD patients. This review also highlighted the main adverse effects associated with the use of different Aβ vaccines in AD. Given the development of these side effects has led to the suspension of some clinical trials, more immunization studies need to be performed to document the adverse effects caused by vaccinations more comprehensively and decipher the causes of these complications. Moreover, future studies should also focus on optimizing vaccine strategies, combining these immunotherapies with other treatments, and extending follow-up durations to thoroughly understand the long-term impacts on AD progression.
Ethical Considerations
Compliance with ethical guidelines
The current study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (Code: IR.SBMU.RETECH.REC.1399.960).
Funding
This research was supported by the research project (No. 1399/61318), Funded by Shahid Beheshti University of Medical Science.
Authors contributions
Conceptualization and study design: Haideh Mosleh and Hani Ghayyem; Supervising: Mohammadreza Kosari; Writing–original draf, review & editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors appreciate the "Student Research Committee" and "Research & Technology Chancellor" at Shahid Beheshti University of Medical Sciences, as well as the Jack Brown and Family Alzheimer’s Disease Research Foundation, for their financial support of this study.
References
Alzheimer disease (AD) is the most common form of dementia [1], with nearly 10 million new cases every year [2]. Cholinesterase inhibitors, N-methyl-D-aspartate receptor partial antagonists, and other medications used to treat secondary behavioral symptoms associated with AD are currently used to manage this disease [2]. However, no drug or other intervention can currently prevent, slow down, or stop AD progression [3]. Therefore, developing a clinically validated method to slow down the progression of AD is challenging but essential [4].
Although the exact mechanism of Aβ toxicity is still debated, interventions that prevent Aβ aggregation and deposition are among promising therapeutic strategies. In transgenic AD animal models, passive immunization with anti-Aβ antibodies and vaccination with Aβ42 or other Aβ fragments have led to the elimination of Aβ deposits and improved behavioral deficits. These preclinical observations prompted clinical trials, one of which was abruptly stopped when several patients developed adverse neuroinflammatory reactions. Although the exact biological mechanisms of vaccination-induced Aβ clearance from brain tissue have remained unclear, they may involve a combination of microglial Fc-receptor-mediated phagocytosis, the sequestration of Aβ in plasma with subsequent release of soluble Aβ from cerebrospinal fluid (CSF) into plasma, and antibody-mediated breakdown of Aβ fibrils [5]. In an AD animal model, antibody-induced Aβ clearance from brain parenchyma occurred within three days [6]. As a result, a multicenter trial was set up to test active immunization with a pre-aggregated synthetic Aβ42 preparation (AN1792) in mild-to-moderate AD [5]. Due to the continuous production and maturation of therapeutic antibodies and the need for less frequent vaccine administration, active immunotherapy offers long-term advantages over passive immunotherapy. Furthermore, in phase III clinical trials in patients with mild-to-moderate AD, passive immunotherapies with monoclonal antibodies, including bapineuzumab and solanezumab, failed to meet their primary endpoint criteria, such as an improvement in cognition and activities of daily living index [7].
Active immunization with pre-aggregated human Aβ42 effectively reduced the number of Aβ plaques in the transgenic mice [8, 9, 10, 11, 12] that expressed mutations associated with the dominantly inherited AD. Intriguingly, there was an inverse correlation between the levels of remaining amyloid load in the brain and serum anti-Aβ antibody titers. The amyloid plaques that remain after vaccination are poorly identifiable, even though collapsed “moth-eaten” plaques have been documented.
The link between Aβ clearance and the status of neurofibrillary tangles, tau phosphorylation, and aggregation has not been fully described in AN1792-immunized patients. Nevertheless, post-mortem studies [1, 13] and in vivo amyloid imaging [14, 15] show the removal of Aβ following immunotherapy in patients living with AD [16, 17]. Although side effects of Aβ immunization have been observed in clinical trials [13, 17-19], the removal of Aβ is associated with potentially beneficial effects on the disease pathology, such as reduced tau phosphorylation in the brain [16] and diminished tau protein levels in the CSF [20]. Aβ immunization also alleviated disease-related deterioration of neuronal processes [21]. Notably, however, patients with mild-to-moderate cognitive impairment participating in the study by Serrano-Pozo et al. [22] developed persistent, progressive dementia and subsequently died of AD despite immunization.
Since the Aβ immunotherapy was successful in transgenic AD mice, a clinical trial was conducted in AD patients using aggregated Aβ42 (AN1792) in an adjuvant formulated with QS-21. Aseptic meningoencephalitis occurred in a subset of patients (6%) in this study and the clinical trial was stopped. Instead of the six injections initially scheduled in this phase II study protocol, patients received only one to three injections of AN1792 [23]. Despite the interruption of this trial, modest but significant positive effects of immunization on select cognitive and functional outcome measures were documented. Further evaluation of the entire study cohort revealed limited cognitive benefits and “less worsening” of clinical outcome measures [23]. Similarly, the recently reported complications associated with bapineuzumab passive immunotherapy are attributed to the development of brain vasogenic edema in some participants carrying the apolipoprotein E ε4 allele (ApoE4), which resulted in their exclusion from the study. Despite these adverse events, the pathology of abnormal neurites and tau levels/phosphorylation are reduced in AN-1792-immunized AD patients [24].
This review summarizes the effects of Aβ immunotherapy on AD pathological features and clinical outcomes. The evidence indicates that immunization with Aβ42 has protective effects at the early stages of AD and significantly affects the pathogenesis of established AD. By clearing Aβ deposits in the brain, particularly in the frontal cortex, Aβ immunotherapy slows the decline of cognitive and functional capacities of AD patients.
Materials and Methods
This systematic review was conducted according to the 2009 Preferred reporting items for systematic reviews and meta-analyses (PRISMA) guideline [25].
Search strategy
PubMed, Scopus, Web of Science, Google Scholar, and Embase were systematically searched using relevant search terms to identify pertinent articles published before 2023. Only articles written in English were considered in this review. Preclinical studies using animal models were excluded. The specific search terms used to identify AD immunotherapy studies are listed in Table 1.







This systematic review includes original, investigational, and follow-up studies undertaken to assess the potential effects of Aβ vaccination in AD patients. Only articles focusing on adult human populations were considered for this review. We included original research articles published from 2002 until 2023 written in English involving AD patients. Finally, we only included studies investigating the outcomes of vaccination trials in AD.
Inclusion and exclusion criteria
All relevant articles considering AD vaccines, including AADvac1, AN-1792 and CAD106, were screened before including them in the final analysis. Non-human studies, systematic reviews, meta-analyses, non-English language articles and duplicate publications were excluded.
Study selection methodology
The studies included in this review were chosen following a three-step process illustrated in Figure 1. All duplicate publications were eliminated first. Then, based on the inclusion and exclusion criteria stated above, the titles and abstracts of articles were independently assessed by two reviewers to create a shortlist of eligible publications. Subsequently, the same reviewers evaluated the full text of the publications. Finally, a third reviewer evaluated the quality and accuracy of the selected data. Disagreements among the three reviewers were resolved through discussions until a consensus was reached. The Newcastle-Ottawa scale (NOS) was used to assess the quality of each included study. The eight evaluation criteria of the Ottawa checklist were utilized to assess the features of the studies, including selection, comparability and outcome. All studies were categorized into three groups based on their NOS scores: High quality (scores 7-9), moderate quality (scores 5-6) and low quality (scores 0-4).
Results
Demographic characteristics of the subjects
The review included 2725 AD patients in this review. The mean age of the patients was 75.69 years and 51% were female. The mean mini-mental state examination score (MMSE) score of AD patients was 21.24, while the control group had a mean MMSE score of 21.21.
Anti-Aβ immunization in AD
Most immunotherapies and vaccinations target Aβ to trigger a suitable immune response (anti-Aβ antibodies) that removes the Aβ deposits and enhances cognitive and functional capacities. AN1792 was the first vaccine used for active immunotherapy in AD, and it was used in 26 out of 29 studies we reviewed. AN-1792 includes aggregated human Aβ42 combined with a saponin-based adjuvant QS-21. The safety and tolerability of this vaccine were first demonstrated in a phase I study. Anti-Aβ42 antibodies from AN1792 recipients could distinguish between Aβ plaques located extracellularly and those in brain blood vessels [5, 26].
AADvac1 is another vaccine comprising synthetic peptides resembling the misfolded tau protein, with aluminum hydroxide and keyhole limpet hemocyanin as adjuvants. AADvac1 attenuated neurofibrillary pathology and insoluble tau deposits in the brains of immunized AD patients. IgG antibodies against tau were produced in 29 out of 30 treated older individuals with mild-to-moderate AD, demonstrating its strong immunogenicity in humans [27].
CAD106 is a second-generation vaccine for active Aβ immunotherapy that includes several copies of the Aβ1-6 peptide attached to a carrier that consists of 180 copies of the bacteriophage Q coat protein. It successfully induces the production of anti-Aβ antibodies without causing an Aβ-specific T-cell response [28]. The first human study of CAD106 also shows a favorable safety profile and an encouraging antibody response when administered with adjuvants alum (50 µg and 150 µg) and MF59 (squalene-based oil-in-water emulsion, 125 µL and 250 µL) [28]. CAD106 administeration without adjuvants at doses up to 150 µg generates consistent anti-Aβ antibody responses in otherwise healthy AD patients without significant safety concerns. The combination of alum adjuvant and CAD106 (450 μg) shows the optimum balance between tolerability and antibody response [7].
Survival time from the first immunization dose
The survival time of patients after injecting the first dose of AN1792 varies from 4 up to 184 months across different studies [29]. The longest and shortest survival times were observed in patients receiving AN1792 (225 µg and 50 µg, respectively) [30].
The time between the first dose of vaccine and booster
The time between the vaccine and the booster dose in the studies varied from 15 to 45 days. The most common choice was 30 days, as it was used in 7 out of 29 studies.
Adverse effects of vaccination
Cerebral microhemorrhage and vasogenic edema can be induced by passive and active Aβ immunization. Clinical trials have been suspended due to unfavorable outcomes such as meningoencephalitis [5], microhemorrhage in the brain, and cerebral amyloid angiopathy (CAA) [5, 31]. Thus, humoral antibody responses to AN1792 are restricted to the brain Aβ deposits, reducing the likelihood of post-vaccination aseptic meningoencephalitis in these patients.
In a study by Boche et al. [32], none of the patients receiving AN1792 experienced significant intracerebral hemorrhages caused by CAA. Still, they displayed a greater density of cortical microhemorrhages and microvascular deficits than the control group. Vaccination with AN1792 dissolves plaque deposits of Aβ42, which departs the brain through the perivascular route, temporarily worsening CAA. Gilman et al. [33] permanently stopped the administration of AN1792 after the initial reports of meningoencephalitis and monitored patients closely for the next 9 months. The inflammatory response was speculated to be due to polysorbate 80 in the vaccine, which may have exposed more amino acids of the Aβ42 peptide, escalating inflammatory T-cell responses. Orgogozo et al. [18] reported that 18 out of 298 AN1792 recipients experienced post-vaccination aseptic meningoencephalitis. Sakai et al. [31] evaluated the impact of ApoE, an Aβ transporter, on CAA and the formation of CAA-associated vasculopathy following Aβ immunotherapy with AN1792. They concluded that ApoE contributed to plaque clearance and Aβ delivery to the cerebral vasculature, with concentric splitting of the vessel wall being about three times more common in leptomeningeal arteries of immunized compared to non-immunized ones.
Unlike AN1792, no meningoencephalitis or vasogenic edema cases have been reported in patients receiving the AADvac1 vaccine. Among the studies using AADvac1, only one ApoE4 homozygote showed signs of new microhemorrhages [34]. Similar to AADvac1, no signs of central nervous system inflammation or clinical or subclinical cases of meningoencephalitis were observed in CAD106 studies [7, 28]. However, Vandenberghe et al. [7] reported amyloid-related imaging abnormalities (ARIAs) in 6 out of 120 patients, all of whom had a robust serological response to CAD106. None of those ARIA patients displayed neurological symptoms. However, this study recorded several severe adverse events (SAEs) reported in a single patient, including allergic dermatitis (immediately following the fourth injection), atrial fibrillation (6 weeks following the first injection), and acute psychosis (14 weeks following the seventh injection) were 3 of the 26 SAEs in the entire study group of 58 patients. The researchers thought that these SAEs might be linked to CAD106 injection. Nasopharyngitis and injection site erythema were the most common adverse events associated with the CAD106 vaccine [28].
ApoE4
ApoE4 was the most significant genetic risk factor for sporadic AD and severe CAA development in Aβ-immunized AD patients [35, 36]. AD patients carrying the ε4 allele are up to 15 times more likely to develop AD than ε3. Moreover, they experience more severe and frequent CAA comorbidity compared to carriers of other ApoE alleles or ApoE-negative AD patients [37]. The ApoE4 has been linked to tonic-clonic seizures independent of AD and Aβ. The complex mechanisms underlying the association between ApoE4 and AD are believed to involve tau phosphorylation, Aβ peptide clearance, and neuronal death [38]. Several mechanisms have been proposed for Aβ removal from the brain parenchyma without immunotherapy. First, ApoE influences Aβ uptake by cells since the Aβ and ApoE complex is endocytosed and digested enzymatically [39]. Second, ApoE facilitates the Aβ transport across the blood-brain barrier (BBB) [40] via the very low-density lipoprotein receptor and low-density lipoprotein receptor-related protein 1 [41]. Moreover, a perivascular drainage channel has been suggested as an essential Aβ elimination mechanism. Failure of this channel due to age-related mechanisms plays a significant role in the development of CAA [42, 43, 44]. Sakai et al. [31] reported that ApoE mediates the amyloid plaque clearance. Aβ delivery to the brain microvasculature is consistent with ApoE’s significant role in removing Aβ from the brain after Aβ immunotherapy. Thus, ARIAs associated with Aβ immunotherapy may reflect a severe age-related vascular alteration in some AD patients, particularly in ApoE4 carriers, who display rapid plaque disaggregation. According to preclinical data, CAD106 can decrease the buildup of Aβ in the brain by triggering antibodies that prevent amyloid deposition. Notably, CAD106 was shown to be safe and effective in a patient population with a higher proportion of ApoE4 carriers (>60%) than the general population (>23%), supporting this association [2].
Clinical outcomes and immunological findings
Although the precise biological mechanisms underlying vaccination-induced clearance of Aβ from brain tissue are still unknown, they possibly involve a combination of phagocytosis by microglial Fc-receptors, sequestration of Aβ in plasma that leads to the release of soluble Aβ from CSF into plasma, and the breakdown of Aβ fibrils by antibodies (Figure 2). Most AD patients immunized with aggregated Aβ42 (AN1792) show a favorable immune response by generating antibodies with a high selectivity towards Aβ plaques, which identify amyloid plaques, diffuse Aβ deposits, and vascular amyloid in subarachnoid and perforating cerebral blood vessels. However, while Aβ immunization leads to the removal of Aβ from the brain, the cognitive decline persists, probably because of the continued presence of phospho-tau in the brain. For instance, Boche et al. [16] showed that the reduction of Aβ in neuronal processes such as neuropil threads and dystrophic neurites was observed in AD patients actively immunized with AN1792. In contrast, the development of neurofibrillary tangles due to phospho-tau buildup in the neuronal cell bodies remained unaffected. In another study, Holmes et al. (Long-term effects of Aβ42) [45] performed a post-mortem assessment of AD patients immunized with AN1792 (50 μg or 225 μg). They demonstrated that 7 out of 8 patients with complete plaque removal experienced severe end-stage dementia before death. This finding indicates that plaque clearance did not correlate with the AN1792 dosage or improved clinical outcomes. The study found no significant difference in survival rates (hazard ratio 0.93; 95% CI, 0.43-3.11; P=0.86) or delay in dementia progression (1.18; 95% CI, 0.45-3.11; P=0.73) between the AN1792 and placebo group.
Elevation in the levels of neurodegeneration-associated protein, eukaryotic initiation factor 2α and neurotic curvature indicate neuronal health. Despite the apparent improvement in the health of remaining neurons, there was a more significant loss of neurons, suggesting that only damaged neurons may be removed following vaccination. This discrepancy between the decrease in brain volume and improved cognitive performance was also noted in other studies [4, 21]. Zotova et al. reported that levels of the activated macrophage and microglia marker CD68 in both white and gray matter were higher in the immunized Alzheimer’s disease (iAD) group compared to non-immunized control Alzheimer’s disease (cAD) cases. Moreover, the Aβ42 load was lower (P=0.036) in the brains of iAD compared to cAD patients.
Additionally, in the iAD group, the CD68 levels are inversely associated with Aβ42 load, suggesting that Aβ immunization enhances microglial reactivity and phagocytic activity in the presence of plaques. Interestingly, in long-surviving iAD patients, CD68 expression is lower despite complete plaque clearance, indicating a reduction in phagocytic activity post-plaque removal. The assessment of major histocompatibility complex (MHC) class II cell surface receptor HLA-DR, another microglia activation marker, on the cells that clustered around amyloid plaques reveals no significant differences between iAD and cAD groups, indicating that this microglia activation marker may have been exclusively connected to their phagocytic activity specifically when plaques are present.
The similarity of CD68 and HLA-DR loading in the pons of both groups raises the possibility that AD-associated pathology caused the observed variations in microglial activation in the cortex. It has been proposed that phagocytic activity initially increases after immunization with Aβ42 microglial. Still, it may decrease once the plaques are cleared, returning to levels similar to those observed in unimmunized AD patients at the same disease stage [46]. Aβ vaccination may also minimize tau phosphorylation and improve tau pathology in the hippocampus. According to some review studies, AN1792 immunization significantly reduces plaque load and increases amyloid phagocytosis in the frontal cortex and, to a lesser extent, in the temporal lobe [1, 47]. AN1792 vaccination reduced amyloid plaques, attenuated hippocampal neurite abnormalities, and potentially minimized tau phosphorylation. Some studies noted that despite these reductions, there are fewer and smaller remaining dense-core amyloid plaques; however, they might still be harmful [22]. This issue raises concerns that plaque clearance alone might not be sufficient to prevent disease progression.
The long-term outcome of AN1792 immunization was further examined by Vellas et al. [30], who showed low but detectable and persistent anti-Aβ antibody titers in responders from a phase II vaccine trial. Moreover, the same study also reported a significantly slower functional decline in immunized compared to placebo-treated patients over approximately 4.6 years following vaccination. Despite no significant difference in brain volume reduction between the two groups after 3.6 years, no new encephalitis was reported in immunized individuals. These results highlight the potential of Aβ immunotherapy in improving long-term functional outcomes in AD [30]. Notably, as reported, AD patients who were actively immunized with Aβ (AN1792) could remain virtually plaque-free for up to 14 years [29].
CAD106 is the first active immunotherapy shown to reduce amyloid depositions in AD patients. Winblad et al. [28] reported robust anti-Aβ antibody responses in CAD106-treated patients, with 67% and 82% in the first and second cohorts, respectively, meeting the expected criteria for antibody response [48]. These findings imply that CAD106 induces a better antibody response over 7 injections, consistent with the conclusions of an earlier phase I study. Notably, CAD106 also had a favorable safety profile, with no serious adverse effects reported in AD patients. The good safety profile, combined with the immunogenicity of CAD106, supports its potential as a viable therapeutic option for managing AD. The neuropsychological test battery (NTB) appears to be a valid and sensitive assessment of changes in cognitive functions in individuals with mild and moderate AD. The psychometric characteristics of the NTB denote its potential value in assessing treatment efficacy in clinical trials that include participants with moderate AD [28, 49]. In the clinical trials by Novak et al. [34, 50],
AADvac1 shows an acceptable safety profile, with booster doses leading to sustained IgG titers throughout immunization-free intervals. According to MRI evaluations, patients with higher anti-Aβ antibody titers exhibit slower deterioration in cognitive function and reduced brain atrophy, suggesting the vaccine’s efficacy in slowing down the disease progression. Although AADvac1 is still relatively new compared to AN1792, the evidence thus far indicates promising results, particularly regarding safety and cognitive benefits. The long-term impact of AADvac1 is still under investigation, but the early data suggest that it could be a valuable addition to the arsenal of AD treatments.
Discussion
Our review demonstrates sufficient published data to conclude that the Aβ vaccination significantly impacts the pathogenesis of AD. Placebo-controlled randomized trials discussed in this review show that Aβ vaccination can slow cognitive decline in AD patients [51]. Several other studies have demonstrated that Aβ immunotherapy reduces amyloid plaque burden and promotes amyloid phagocytosis in the frontal cortex and, to a lesser extent, in the temporal lobe. AD patients who experience a significant reduction in amyloid load can remain plaque-free for nearly 14 years [29]. This efficacy period prevents repeated accumulations of neurodegeneration-associated proteins, including Aβ. Notably, immunization of AD patients with Aβ42 induces the production of antibodies with a great degree of selectivity for the pathogenic amyloid structures. For example, they do not cross-react with denatured or native full-length amyloid precursor protein or its physiological derivatives, such as soluble Aβ42 monomers, dimers, and trimers. However, one of the most concerning side effects of Aβ immunotherapy is CAA-associated vasculopathy, which can develop after Aβ plaque removal. It has been proposed that ApoE may induce structural abnormalities in the blood vessel walls after transporting Aβ out of the brain [31]. In addition to amyloid clearance, Aβ immunotherapy has significantly reduced in AD brains. This reduction likely reflects the clearance of the tau-containing dystrophic neurite component of plaques. Immunization effectively reduces tau aggregation and decreases tau hyperphosphorylation, critical factors in AD progression. Furthermore, active immunization could effectively improve or reverse memory deficits [22]. Interestingly, while Aβ immunotherapy has been shown to improve microglial phagocytic activity, after the clearance of Aβ plaques, microglia phagocytic activity decreases to levels similar to those in unimmunized AD cases [29, 52].
However, while Aβ immunization is pivotal in preventing plaque formation and removing existing plaques, it does not necessarily stop the progression of neurodegeneration. Nearly all immunized individuals who died because of the AD neurodegenerative processes during the follow-up phase of the Aβ immunization trial had the highest anti-Aβ antibody titers, and their brains were free from amyloid plaques. A longer time interval does not lower the effect of the immunization, and there was no significant correlation between the amount of plaque removal and survival time since the first immunization dose. These findings suggest substantial plaque removal does not benefit survival [29]. This finding implies that despite the clearance of plaques, progressive neurodegeneration can continue in AD brains. An in vivo imaging demonstrates an increased rate of cerebral atrophy in Aβ-immunized AD patients, possibly indicating accelerated loss of damaged and degenerating neurons [21]. One of the most significant adverse effects of Aβ vaccination was observed by Orgogozo et al. [18]. They found that some AD patients developed signs and symptoms related to aseptic meningoencephalitis during the Aβ immunization process. The potential mechanism responsible for the induction of mild-to-severe meningoencephalitis includes T-cell and microglial activation, which must be considered carefully when developing safer Aβ immunotherapy for AD patients. However, the exact relationship between post-vaccination meningoencephalitis and serum anti-Aβ42 antibody titer has remained unclear. Many studies have documented the dramatic adverse effects of Aβ immunotherapy, such as neuroinflammatory reactions, meningoencephalitis, and leukoencephalopathy [47]. Other phenomena, including CAA, T-cell encephalitis, and macrophage infiltration in the white matter, were observed in the preclinical studies by Masliah et al. [47]. The MRI imaging of AD patients immunized with AN1792/QS-21 demonstrates reduced brain volume in antibody responders. The decrease in brain volume did not worsen cognitive performance. Although the reason for this decline is still unclear, it may be due to amyloid removal and the associated CSF shifts [4]. This review identifies cerebral micro hemorrhage, vasogenic edema, meningoencephalitis, and CAA as the most common adverse effects that have led to the postponement of AN1792 immunization trials.
In contrast, second-generation active immunotherapies, including CAD106, have offered a significant safety profile. They have avoided the T-cell response by using fragmented Aβ peptides or peptide mimetics of the Aβ N-terminus, which are recognized by B-cells only. Recent studies show that CAD106 is the first active Aβ immunotherapy that slows amyloid deposition in AD patients. Using CAD106 with an alum adjuvant leads to one of the best balances between antibody response and tolerability recorded thus far. The acceptable antibody response and the favorable safety profile of CAD106 indicate its potential as a valuable therapeutic option for treating AD patients [28]. Additionally, AD patients who respond to AADvac1 vaccination with high titers of anti-tau antibodies exhibit slower brain atrophy in MRI evaluation and a slower decline in cognitive assessment scores than those with low antibody titers. Notably, AN1792, AADvac1, and CAD106 vaccinations did not cause microhemorrhage inflammation in the central nervous system. However, there are a few reports of minor side effects, such as ARIAs, in patients receiving CAD106 injections. Other minor adverse effects associated with CAD106 vaccination include allergic dermatitis, atrial fibrillation, acute psychosis, nasopharyngitis, and erythema at the injection site [21, 53] (Figure 3).
Conclusion
This systematic review evaluated the effects of Aβ immunotherapy on AD pathogenesis and the progression of the disease processes. Aβ immunotherapy has been shown to prevent the formation of new Aβ plaques and even reduce the pre-existing plaques. The inhibitory effect of Aβ vaccination on amyloid plaque formation appears relatively specific and is not associated with general disturbances of the physiological processes. Most studies report improved cognitive performance and memory in vaccinated AD patients compared to unvaccinated ones. Despite the positive overall results observed with three different types of vaccines, there are remaining uncertainties about the exact mechanisms of their action, particularly concerning the effects of immunization on the progression of neurodegeneration and brain atrophy in AD patients. This review also highlighted the main adverse effects associated with the use of different Aβ vaccines in AD. Given the development of these side effects has led to the suspension of some clinical trials, more immunization studies need to be performed to document the adverse effects caused by vaccinations more comprehensively and decipher the causes of these complications. Moreover, future studies should also focus on optimizing vaccine strategies, combining these immunotherapies with other treatments, and extending follow-up durations to thoroughly understand the long-term impacts on AD progression.
Ethical Considerations
Compliance with ethical guidelines
The current study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (Code: IR.SBMU.RETECH.REC.1399.960).
Funding
This research was supported by the research project (No. 1399/61318), Funded by Shahid Beheshti University of Medical Science.
Authors contributions
Conceptualization and study design: Haideh Mosleh and Hani Ghayyem; Supervising: Mohammadreza Kosari; Writing–original draf, review & editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors appreciate the "Student Research Committee" and "Research & Technology Chancellor" at Shahid Beheshti University of Medical Sciences, as well as the Jack Brown and Family Alzheimer’s Disease Research Foundation, for their financial support of this study.
References
- Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, et al. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006; 65(11):1040-8. [DOI:10.1097/01.jnen.0000240466.10758.ce]
- Farlow MR, Andreasen N, Riviere ME, Vostiar I, Vitaliti A, Sovago J, et al. Long-term treatment with active Aβ immunotherapy with CAD106 in mild Alzheimer’s disease. Alzheimers Res Ther. 2015; 7(1):23. [DOI:10.1186/s13195-015-0108-3]
- Lambracht-Washington D, Rosenberg RN. Active DNA Aβ42 vaccination as immunotherapy for Alzheimer disease. Transl Neurosci. 2012; 3(4):307-13. [DOI:10.2478/s13380-012-0037-6]
- Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005; 64(9):1563-72. [DOI:10.1212/01.WNL.0000159743.08996.99]
- Hock C, Konietzko U, Papassotiropoulos A, Wollmer A, Streffer J, von Rotz RC, et al. Generation of antibodies specific for beta-amyloid by vaccination of patients with Alzheimer disease. Nat Med. 2002; 8(11):1270-5. [DOI:10.1038/nm783]
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, et al. Imaging of amyloid-β deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001; 7(3):369-72. [DOI:10.1038/85525]
- Vandenberghe R, Riviere ME, Caputo A, Sovago J, Maguire RP, Farlow M, et al. Active Aβ immunotherapy CAD106 in Alzheimer’s disease: A phase 2b study. Alzheimers Dement. 2017; 3(1):10-22. [DOI:10.1016/j.trci.2016.12.003]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000; 408(6815):979-82. [DOI:10.1038/35050110]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, et al. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000; 408(6815):982-5. [DOI:10.1038/35050116]
- Petrushina I, Ghochikyan A, Mktrichyan M, Mamikonyan G, Movsesyan N, Davtyan H, et al. Alzheimer’s disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric Abeta species in amyloid precursor protein transgenic mice. J Neurosci. 2007; 27(46):12721-31. [DOI:10.1523/JNEUROSCI.3201-07.2007]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999; 400(6740):173-7. [DOI:10.1038/22124]
- Seabrook TJ, Thomas K, Jiang L, Bloom J, Spooner E, Maier M, et al. Dendrimeric Abeta1-15 is an effective immunogen in wildtype and APP-tg mice. Neurobiol Aging. 2007; 28(6):813-23. [DOI:10.1016/j.neurobiolaging.2006.04.007]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: A case report. Nat Med. 2003; 9(4):448-52. [DOI:10.1038/nm840]
- Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012; 69(2):198-207. [DOI:10.1001/archneurol.2011.1538]
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: A phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010; 9(4):363-72. [DOI:10.1016/S1474-4422(10)70043-0]
- Boche D, Donald J, Love S, Harris S, Neal JW, Holmes C, et al. Reduction of aggregated Tau in neuronal processes but not in the cell bodies after Abeta42 immunisation in Alzheimer’s disease. Acta Neuropathol. 2010; 120(1):13-20. [DOI:10.1007/s00401-010-0705-y]
- Roher AE, Maarouf CL, Daugs ID, Kokjohn TA, Hunter JM, Sabbagh MN, et al. Neuropathology and amyloid-β spectrum in a bapineuzumab immunotherapy recipient. J Alzheimer Dis. 2011; 24(2):315-25. [DOI:10.3233/JAD-2011-101809]
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology. 2003; 61(1):46-54. [DOI:10.1212/01.WNL.0000073623.84147.A8]
- Sperling R, Salloway S, Brooks DJ, Tampieri D, Barakos J, Fox NC, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012; 11(3):241-9. [DOI:10.1016/S1474-4422(12)70015-7]
- Blennow K, Zetterberg H, Rinne JO, Salloway S, Wei J, Black R, et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol. 2012; 69(8):1002-10. [DOI:10.1001/archneurol.2012.90]
- Paquet C, Amin J, Mouton-Liger F, Nasser M, Love S, Gray F, et al. Effect of active Aβ immunotherapy on neurons in human Alzheimer’s disease. J Pathol. 2015; 235(5):721-30. [DOI:10.1002/path.4491]
- Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, et al. Beneficial effect of human anti-amyloid-β active immunization on neurite morphology and tau pathology. Brain. 2010; 133(5):1312-27. [DOI:10.1093/brain/awq056]
- Vasilevko V, Pop V, Kim HJ, Saing T, Glabe CC, Milton S, et al. Linear and conformation specific antibodies in aged beagles after prolonged vaccination with aggregated Abeta. Neurobiol Dis. 2010; 39(3):301-10. [DOI:10.1016/j.nbd.2010.04.014]
- Maarouf CL, Daugs ID, Kokjohn TA, Kalback WM, Patton RL, Luehrs DC, et al. The biochemical aftermath of anti-amyloid immunotherapy. Mol Neurodegener. 2010; 5(1):39. [DOI:10.1186/1750-1326-5-39]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 2009; 6(7):e1000097. [DOI:10.1371/journal.pmed.1000097]
- Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, et al. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005; 64(1):94-101. [DOI:10.1212/01.WNL.0000148604.77591.67]
- Novak P, Zilka N, Zilkova M, Kovacech B, Skrabana R, Ondrus M, et al. AADvac1, an active immunotherapy for alzheimer’s disease and non alzheimer tauopathies: An overview of preclinical and clinical development. J Prev Alzheimers Dis. 2019; 6(1):63-9. [DOI:10.14283/jpad.2018.45]
- Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, et al. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: Randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012; 11(7):597-604. [DOI:10.1016/S1474-4422(12)70140-0]
- Nicoll JAR, Buckland GR, Harrison CH, Page A, Harris S, Love S, et al. Persistent neuropathological effects 14 years following amyloid-beta immunization in Alzheimer’s disease. Brain. 2019; 142(7):2113-26. [DOI:10.1093/brain/awz142]
- Vellas B, Black R, Thal LJ, Fox NC, Daniels M, McLennan G, et al. Long-term follow-up of patients immunized with AN1792: Reduced functional decline in antibody responders. Curr Alzheimer Res. 2009; 6(2):144-51. [DOI:10.2174/156720509787602852]
- Sakai K, Boche D, Carare R, Johnston D, Holmes C, Love S, et al. Aβ immunotherapy for Alzheimer’s disease: Effects on apoE and cerebral vasculopathy. Acta Neuropathol. 2014; 128(6):777-89. [DOI:10.1007/s00401-014-1340-9]
- Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, et al. Consequence of Aβ immunization on the vasculature of human Alzheimer’s disease brain. Brain. 2008; 131(Pt 12):3299-310. [DOI:10.1093/brain/awn261]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005; 64(9):1553-62. [DOI:10.1212/01.WNL.0000159740.16984.3C]
- Novak P, Schmidt R, Kontsekova E, Kovacech B, Smolek T, Katina S, et al. Fundamant: An interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimers Res Ther. 2018; 10(1):108. [DOI:10.1186/s13195-018-0436-1]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993; 261(5123):921-3. [DOI:10.1126/science.8346443]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009; 41(10):1094-9. [DOI:10.1038/ng.439]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. Jama. 1997; 278(16):1349-56. [DOI:10.1001/jama.1997.03550160069041]
- Gharbi-Meliani A, Dugravot A, Sabia S, Regy M, Fayosse A, Schnitzler A, et al. The association of APOE ε4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimer Res Ther. 2021;13(1):1-11. [DOI:10.1186/s13195-020-00740-0]
- Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Symposium: Clearance of Aβ from the brain in Alzheimer’s disease: Aβ-degrading enzymes in Alzheimer’s disease. Brain Pathology. 2008; 18(2):240-52. [DOI:10.1111/j.1750-3639.2008.00132.x]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, et al. Clearance of Alzheimer’s amyloid-β1-40 peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000; 106(12):1489-99. [DOI:10.1172/JCI10498]
- Zlokovic BV. Cerebrovascular Effects of apolipoprotein E: Implications for Alzheimer disease. JAMA Neurol. 2013; 70(4):440. [DOI:10.1001/jamaneurol.2013.2152]
- Hawkes CA, Härtig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 2011; 121(4):431-43. [DOI:10.1007/s00401-011-0801-7]
- Hawkes CA, Gatherer M, Sharp MM, Dorr A, Yuen HM, Kalaria R, et al. Regional differences in the morphological and functional effects of aging on cerebral basement membranes and perivascular drainage of amyloid-β from the mouse brain. Aging Cell. 2013; 12(2):224-36. [DOI:10.1111/acel.12045]
- Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathologica. 2009; 117(1):1-14. [DOI:10.1007/s00401-008-0457-0]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008; 372(9634):216-23. [DOI:10.1016/S0140-6736(08)61075-2]
- Zotova E, Bharambe V, Cheaveau M, Morgan W, Holmes C, Harris S, et al. Inflammatory components in human Alzheimer’s disease and after active amyloid-β42 immunization. Brain. 2013; 136(Pt 9):2677-96. [DOI:10.1093/brain/awt210]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, et al. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005; 64(1):129-31. [DOI:10.1212/01.WNL.0000148590.39911.DF]
- Reiman EM, Tariot PN, Borowsky B, Liu F, Riviere ME, Rouzade-Dominguez ML, et al. The API generation program: Umibecestat treatment and discontinuation effects on hippocampal and whole brain volumes in the overall population and amyloid-negative APOE4 homozygotes. Alzheimer Dement. 2020; 16(S9):e041142. [DOI:10.1002/alz.041142]
- Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. 2007; 64(9):1323-9. [DOI:10.1001/archneur.64.9.1323]
- Novak P, Schmidt R, Kontsekova E, Zilka N, Kovacech B, Skrabana R, et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 2017; 16(2):123-34. [DOI:10.1016/S1474-4422(16)30331-3]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Müller-Tillmanns B, et al. Antibodies against β-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003; 38(4):547-54. [DOI:10.1016/S0896-6273(03)00294-0]
- Zotova E, Holmes C, Johnston D, Neal JW, Nicoll JA, Boche D. Microglial alterations in human Alzheimer’s disease following Abeta42 immunization. Neuropathol Appl Neurobiol. 2011; 37(5):513-24. [DOI:10.1111/j.1365-2990.2010.01156.x]
- Amin J, Paquet C, Baker A, Asuni AA, Love S, Holmes C, et al. Effect of amyloid-beta (Abeta) immunization on hyperphosphorylated tau: A potential role for glycogen synthase kinase (GSK)-3beta. Neuropathol Appl Neurobiol. 2015; 41(4):445-57. [DOI:10.1111/nan.12205]
- Paquet C, Nicoll JA, Love S, Mouton-Liger F, Holmes C, Hugon J, et al. Downregulated apoptosis and autophagy after anti-Abeta immunotherapy in Alzheimer's disease. Brain Pathol. 2018; 28(5):603-10. [DOI:10.1111/bpa.12567] [PMID]
- Riviere ME, Langbaum JB, Turner RS, Rinne JO, Sui Y, Cazorla P, et al. Effects of the active amyloid beta immunotherapy CAD106 on PET measurements of amyloid plaque deposition in cognitively unimpaired APOE ε4 homozygotes. Alzheimers Dement. 2024; 20(3):1839-50. [PMID]
Type of Study: Review |
Subject:
Special
Received: 2024/04/23 | Accepted: 2024/09/11 | Published: 2024/10/26
Received: 2024/04/23 | Accepted: 2024/09/11 | Published: 2024/10/26
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |