Wed, Jan 7, 2026
Volume 10, Issue 3 (Summer 2024)
Caspian J Neurol Sci 2024, 10(3): 151-168 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kosari M, Asgari Taei A, Klegeris A, Soleimani S, Tavasol A, Jazi K, et al . The Role of Dysregulated Neuroinflammatory Molecular Pathways in Parkinson Disease: A Systematic Review. Caspian J Neurol Sci 2024; 10 (3) :151-168
URL: http://cjns.gums.ac.ir/article-1-712-en.html
URL: http://cjns.gums.ac.ir/article-1-712-en.html
Mohammadreza Kosari1 

 , Afsaneh Asgari Taei2
, Afsaneh Asgari Taei2 

 , Andis Klegeris3
, Andis Klegeris3 

 , Sevim Soleimani4
, Sevim Soleimani4 

 , Arian Tavasol4
, Arian Tavasol4 

 , Kimia Jazi5
, Kimia Jazi5 

 , Kimia Eyvani6
, Kimia Eyvani6 

 , Ashkan Bahrami7
, Ashkan Bahrami7 

 , Zahra Farrokhi8
, Zahra Farrokhi8 

 , Farnoosh Vosough9
, Farnoosh Vosough9 

 , Faraz Rahmani Khajeh10
, Faraz Rahmani Khajeh10 

 , Saleh Behzadi11
, Saleh Behzadi11 

 , Zohreh Zamani *12
, Zohreh Zamani *12 




 , Afsaneh Asgari Taei2
, Afsaneh Asgari Taei2 

 , Andis Klegeris3
, Andis Klegeris3 

 , Sevim Soleimani4
, Sevim Soleimani4 

 , Arian Tavasol4
, Arian Tavasol4 

 , Kimia Jazi5
, Kimia Jazi5 

 , Kimia Eyvani6
, Kimia Eyvani6 

 , Ashkan Bahrami7
, Ashkan Bahrami7 

 , Zahra Farrokhi8
, Zahra Farrokhi8 

 , Farnoosh Vosough9
, Farnoosh Vosough9 

 , Faraz Rahmani Khajeh10
, Faraz Rahmani Khajeh10 

 , Saleh Behzadi11
, Saleh Behzadi11 

 , Zohreh Zamani *12
, Zohreh Zamani *12 


1- Tongji Medical College, Wuhan Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China.
2- Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Biology, Faculty of Science, University of British Columbia Okanagan Campus, Kelowna, Canada.
4- School of Medicine, Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5- Student Research Committee, School of Medicine, Qom University of Medical Sciences, Qom, Iran.
6- Student Research Committee, Faculty of Medicine,Guilan University of Medical Sciences, Rasht, Iran.
7- Student Research Committee, Faculty of Medicine, Kashan University of Medical Science, Kashan, Iran.
8- Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
9- Student Research Committee, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
10- Student Research Committee, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
11- Student Research Committee, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
12- Department of Neurology, Firoozabadi Clinical Research Development Unit (FACRDU), Iran University of Medical Sciences, Tehran, Iran. ,zamani.zhrh@gmail.com
2- Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Biology, Faculty of Science, University of British Columbia Okanagan Campus, Kelowna, Canada.
4- School of Medicine, Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5- Student Research Committee, School of Medicine, Qom University of Medical Sciences, Qom, Iran.
6- Student Research Committee, Faculty of Medicine,Guilan University of Medical Sciences, Rasht, Iran.
7- Student Research Committee, Faculty of Medicine, Kashan University of Medical Science, Kashan, Iran.
8- Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
9- Student Research Committee, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
10- Student Research Committee, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
11- Student Research Committee, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
12- Department of Neurology, Firoozabadi Clinical Research Development Unit (FACRDU), Iran University of Medical Sciences, Tehran, Iran. ,
Keywords: C-reactive protein (CRP), Tumor necrosis factor (TNF)-α, α-Synuclein, Toll-like receptor (TLR)2, TLR9, Neuroinflammation, Parkinson disease (PD), Lipocalin-2 (LCN2)
Full-Text [PDF 2560 kb]
(661 Downloads)
| Abstract (HTML) (1555 Views)
Full-Text: (952 Views)
Introduction
Parkinson disease (PD) is a neurodegenerative disease characterized by the progressive degeneration of dopaminergic (DA) neurons in the substantia nigra (SN) and their projections to the striatum. Clinical hallmarks of PD consist of postural instability, bradykinesia, resting tremor, and rigidity. The major non-motor symptoms of PD are sensory deficits, sleep disorders, cognitive impairment, depression, anxiety, and autonomic dysfunctions [1-4].
Several pathological structures containing misfolded proteins are observed in PD brain tissue, including limbic and cortical Lewy bodies [5], senile plaques, and neurofibrillary tangles [6]. α-Synuclein (α-syn), the key factor of Lewy bodies, is the main protein involved in neurodegeneration and neuronal cell toxicity [7]. Monomeric α-syn and oligomeric aggregates of this protein are believed to be critical players in PD pathogenesis [8]. The deterioration of cognition in PD patients is caused by progressive neuronal dysfunction in various cortical regions affected by α-syn pathology [9], which is also associated with neuroinflammatory processes [10]. Neuroinflammation is crucial in different stages of PD progression, from early α-syn aggregation and degeneration of dopaminergic neurons to the onset of clinical symptoms [11-14]. Dysregulation of inflammatory pathways is one of the inflammatory components of PD, and it is likely caused by genetic predispositions, immunological changes brought on by aging, and the initial activation of glia as a result of neuronal injury [15, 16]. Also, the aggregation of misfolded proteins like α-syn and blood-brain barrier (BBB) disruption, are the initial changes in the pathogenesis of PD and can be induced by neuroinflammation [17, 18]. The selective injury of dopaminergic neurons in the nigrostriatal path can be due to the neurotoxic effects of inflammatory mediators mainly secreted by reactive microglia. The pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 have vital roles in the cellular death observed in PD and other chronic neuro-inflammatory conditions [19, 20]. Interferon-gamma (IFN-γ), which has an important role in autoimmune disease, also induces neurodegeneration in PD [21]. C-reactive protein (CRP) is another participant in neuro-inflammatory responses in PD. It is an acute-phase serum protein that becomes upregulated following an increase in IL-6 levels [22].
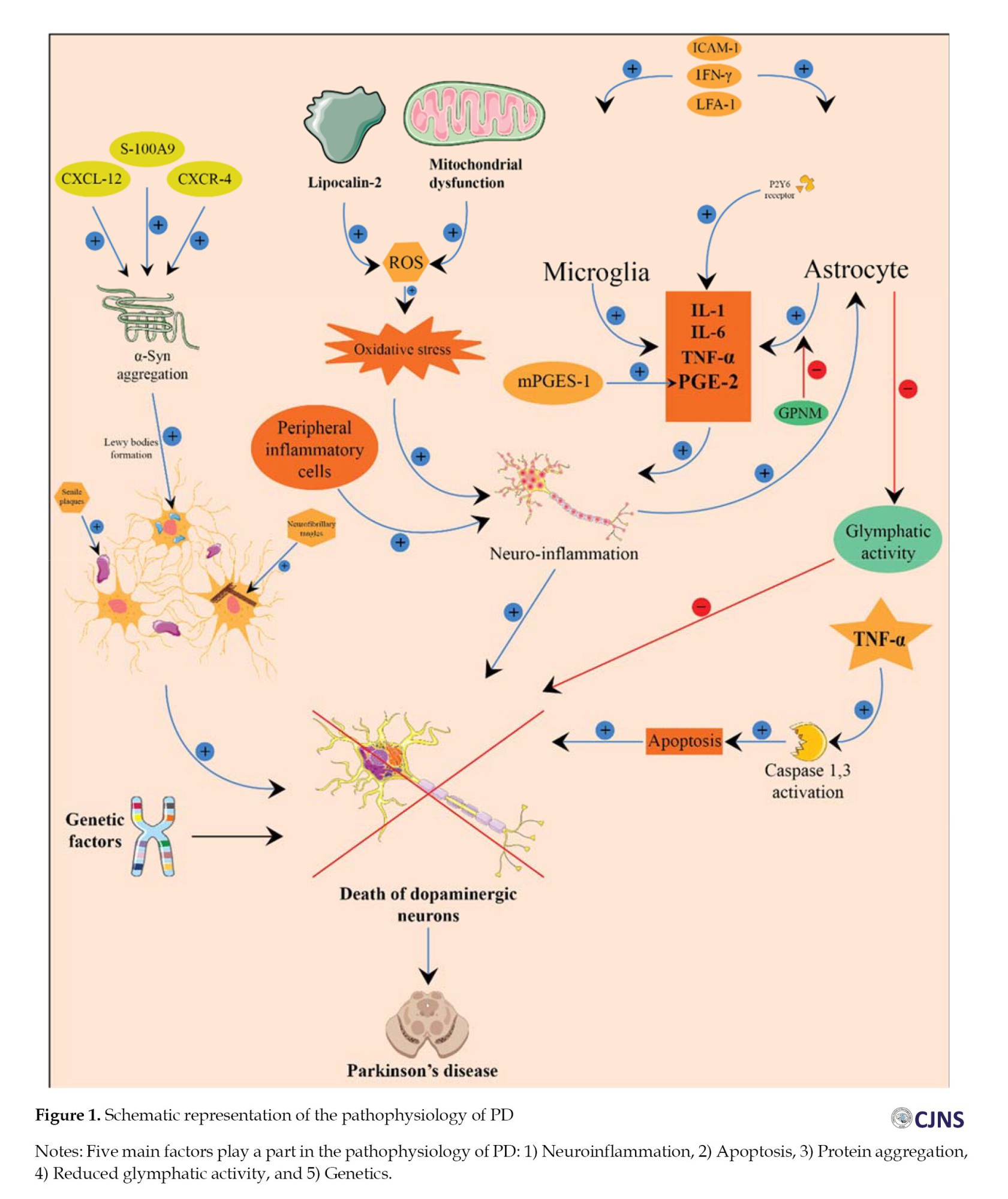
In PD, systemic oxidative stress and neuroinflammation may lead to astrogliosis, which damages the glymphatic system [23]. Notably, reduced glymphatic activity is associated with aging, the primary risk factor for PD [23]. Even though the etiology of PD is not fully established, the importance of genetic factors has become evident [24]. Thus, genetic mutations in 18 chromosomal regions have been identified in familial PD. For example, heterozygous mutations involving the GBA gene encoding lysosomal enzyme glucocerebrosidase are one of the greatest common risk factors for PD, increasing its risk 5-6 times [25, 26]. In addition, different single nucleotide polymorphisms (SNPs) which increased PD risk have been detected in the promoter of the TNF-α gene; especially 238 G/A (rs361525) and 308 G/A (rs1800629) SNPs are demonstrated as important functional polymorphism [27-29].
The current systematic review aims to determine the effects of each neuroinflammatory molecular pathway involved in PD pathogenesis.
Materials and Methods
Search strategy
The literature search was done till December 2023 by two authors independently in PubMed, Web of Science, Scopus, and Embase databases. The following terms, their abbreviations, and MeSH terms were used without any restriction on publication date: “Inflammation,” “neuroinflammation,” “neurodegenerative disease,” “Parkinson’s disease,” “brain,” “inflammatory markers,” “C-reactive protein,” “C–C chemokine receptor type 3,” and “prostaglandin.” The reference lists of all identified publications were checked to prevent any duplicate.
Inclusion and exclusion criteria
All observational studies written in English reporting qualitative or quantitative data on the connection between neuroinflammation and PD were included in this review. We excluded animal studies, case reports, conference papers, review studies, studies without randomized sampling, and low-quality studies identified by the quality assessment using the Newcastle-Ottawa scale (NOS). Two authors independently conducted the initial screening based on “titles” and “abstracts” and then selected qualified papers after considering the full text of the nominated articles. A third author resolved differences in opinions.
Quality assessment
NOS was used to evaluate the quality of selected articles. Based on this scale, each study can obtain a maximum of 9 points from the following parameters: Selection of participants (4 points), comparability (2 points), and the valuation of outcomes (3 points). The studies with a score above 6 were considered high-quality studies. Studies with modest quality had points of 4-6, and the quality of studies scored under 4 was low (Table 1).
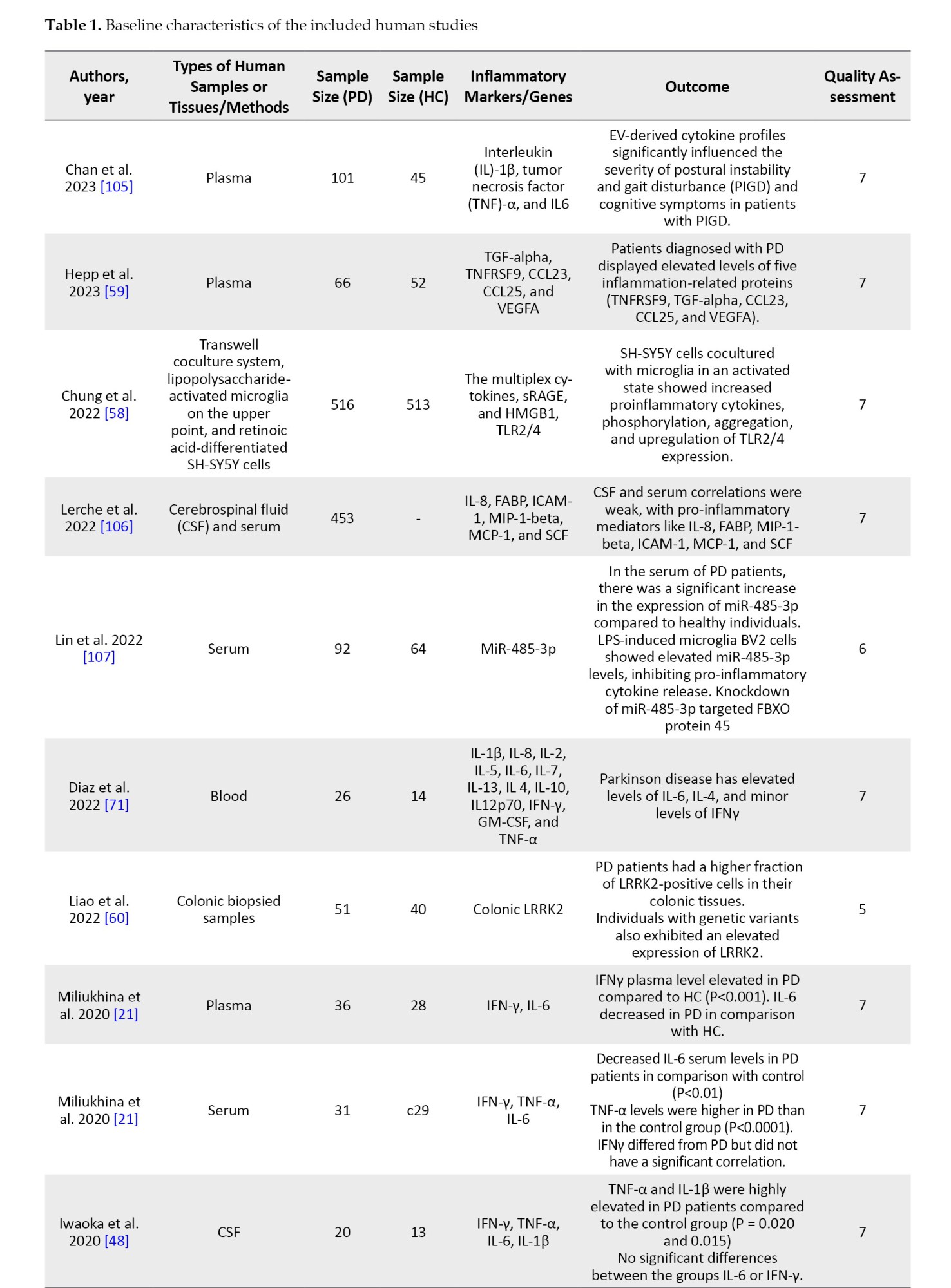
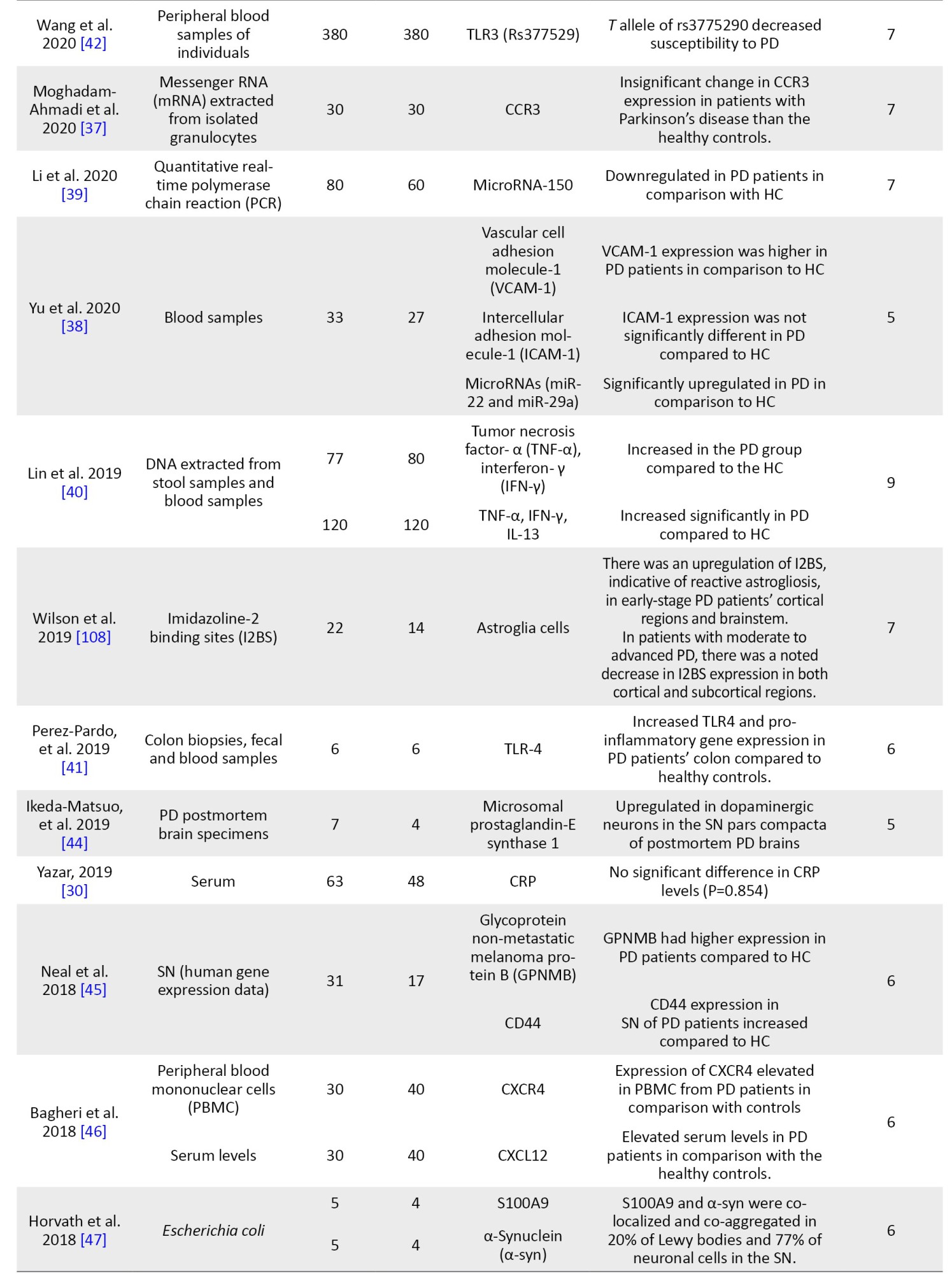
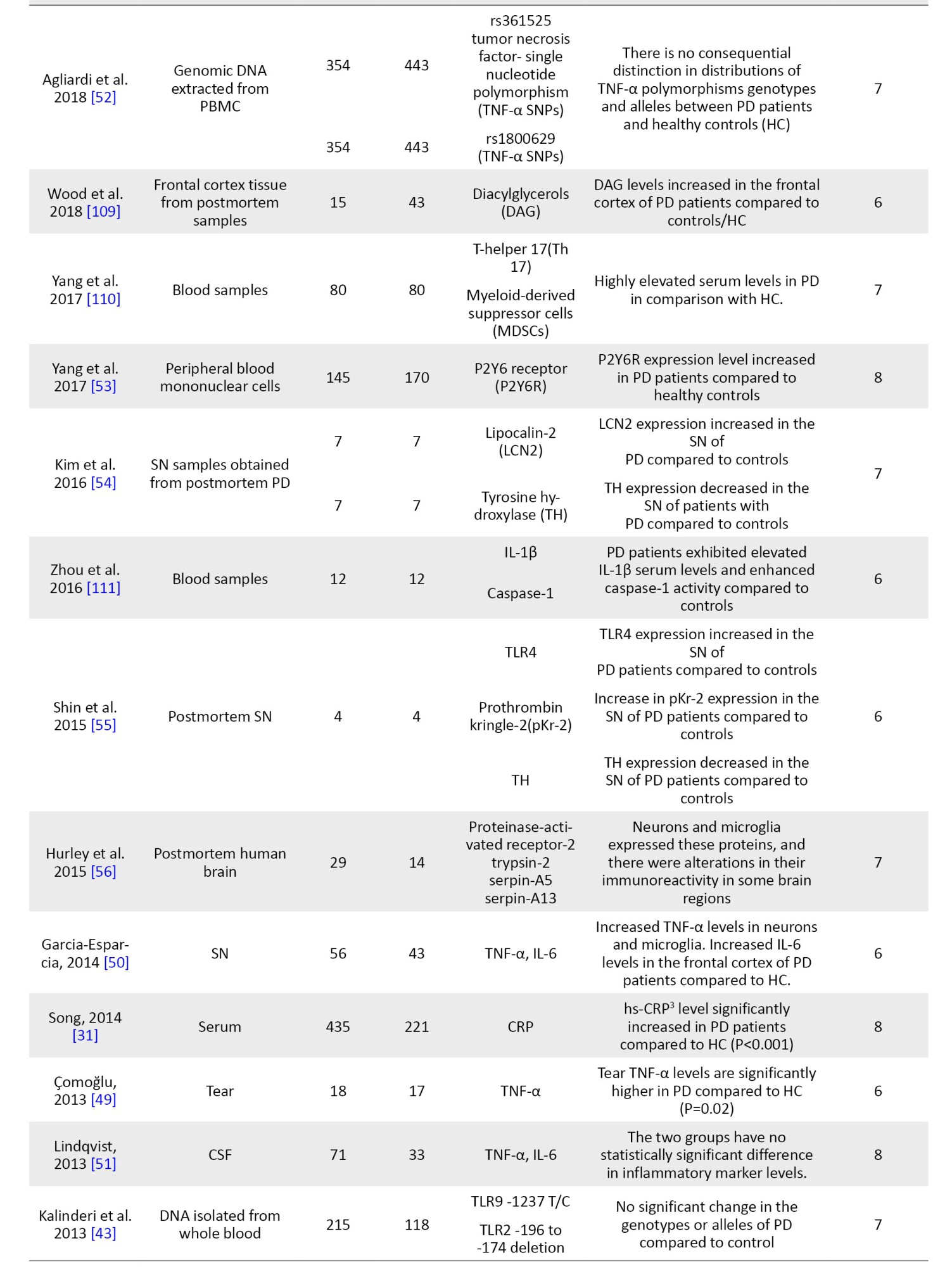
Data extraction
The following information was extracted from all qualified articles: The first author’s name, country of study, date of publication, sample size, and all characteristics of the patient and control groups, including studied inflammatory markers, factors, genes, and the clinical stage of the disease. Data extraction was conducted independently by two authors, and afterward, their results were compared to eliminate discrepancies.
Results
Study selection
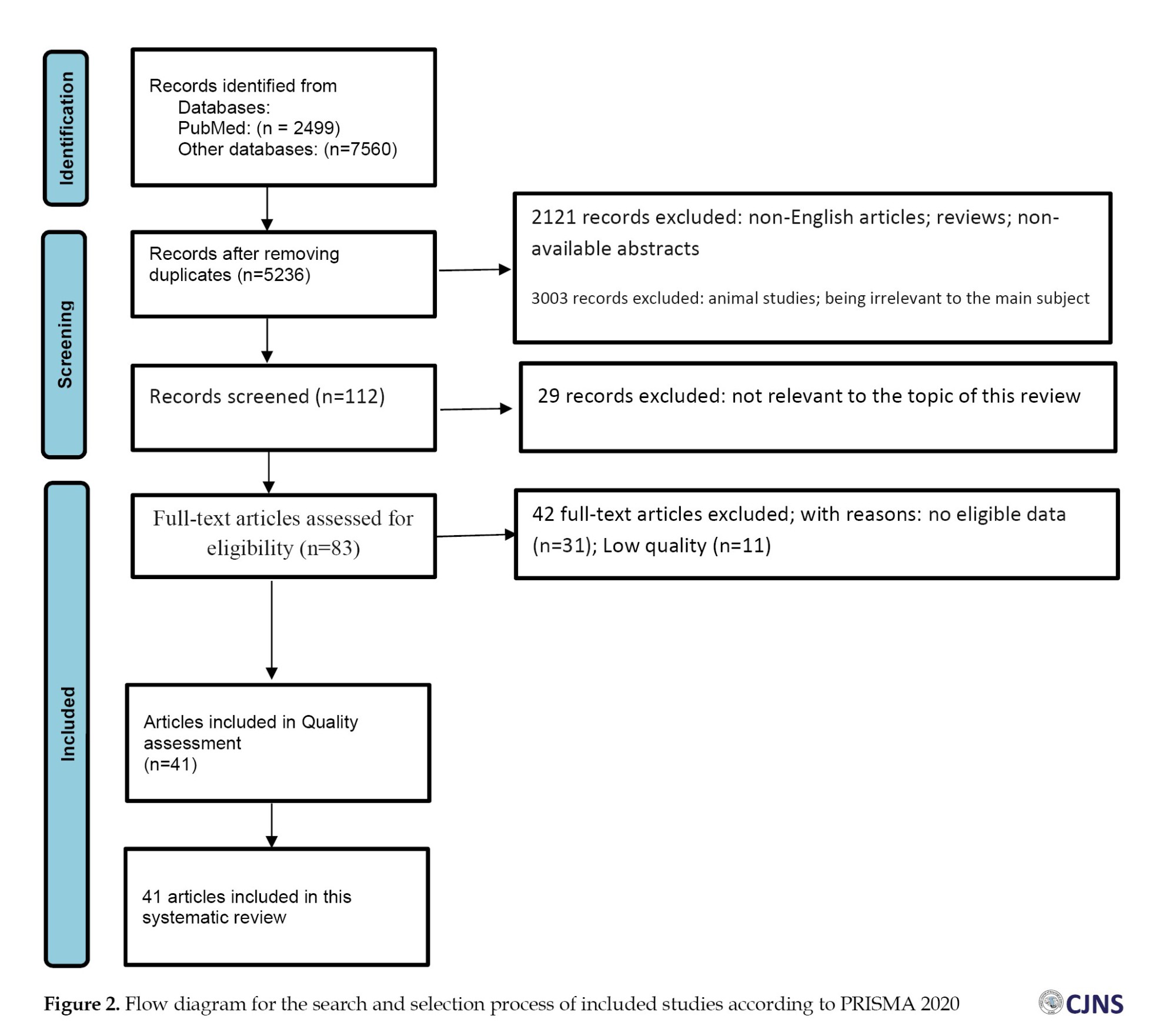
We used the PRISMA (preferred reporting items for systematic reviews and meta-analyses) checklist to conduct the present review [18]. A total of 2499 studies were extracted through the search on PubMed, and 7560 by searching other databases (Google Scholar, Scopus, Web of Science, and Embase). Next, 4823 papers were excluded because of repetition in various databases. After reviewing the “titles” and “abstracts” of these chosen articles, 2121 more studies were excluded because of the absence of validation in abstracts, being review studies or non-English. Furthermore, 3003 more papers were excluded because they were unrelated to the key subject or animal articles.
At last, from the 112 remaining studies, 29 records were excluded due to being irrelevant to the subject and the primary effect size of the study. The complete manuscripts of the 83 studies were utterly evaluated, and 42 papers were excluded because of unclear or insufficient data (n=31) and low quality (n=11). Lastly, 41 papers published before December 2023 were selected (Figure 2).
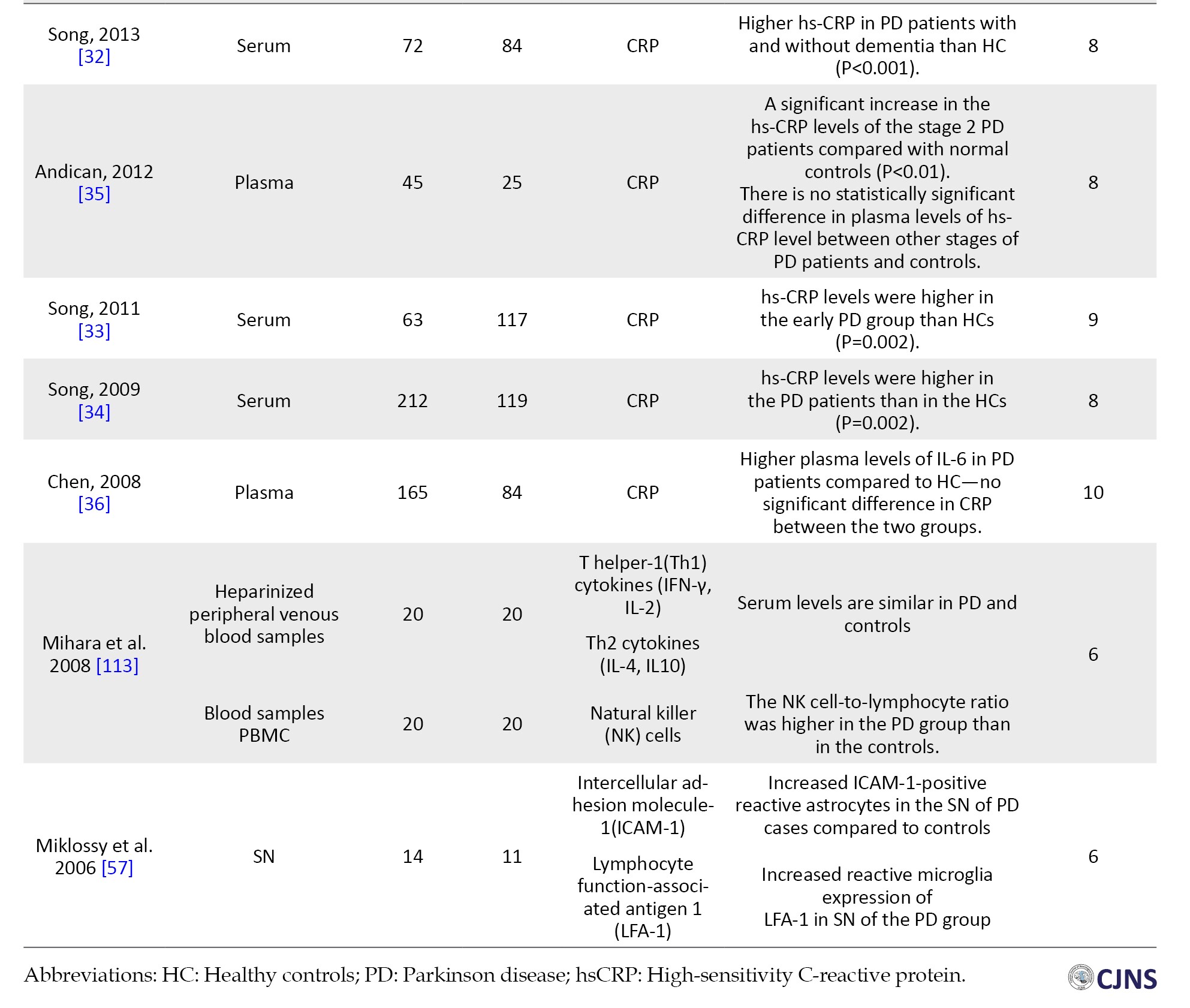
C-reactive protein (CRP)
Seven studies, including 1055 PD patients and 698 healthy controls, reported the participants’ CRP levels in the serum [30-34] and plasma [35, 36]. Among studies assessing the CRP in the serum samples of PD patients and their controls, there are four studies by Song et al. [31-34] stating significantly higher stages of high-sensitivity (hs)-CRP in the serum of early PD patients, PD patients with or without dementia, and a whole sample of PD patients in comparison with the control groups. However, Yazar et al. [30] reported that the difference between CRP stages in the serum sample of PD individuals and the control group was not significant (P=0.854).
C–C chemokine receptor type 3
The innate immune system mainly expresses c chemokine receptor type 3 (CCR3) and can bind with several chemokines to activate some inflammatory cells’ responses. CCR3 was extracted from isolated granulocytes of the cases, and no significant difference was detected in the CCR3 levels among the PD and health control (HC). Therefore, it is not eligible for diagnosing the disease [37].
Some microRNA subtypes role in the inflammatory pathogenesis of PD
MicroRNAs (miRNAs) are small single-stranded classes of RNAs that contribute to regulating gene expression. MiRNA-29a potentially regulates angiogenesis in endothelial cells, leading to BBB dysfunction and neuroinflammation. MiRNA-22 is also associated with angiogenesis-related alterations alongside miRNA-29a. Evaluation of the blood samples obtained from the subjects demonstrates that miRNA-29a and miRNA-22 are remarkably upregulated in the PD individuals compared to the HC group [38]. Quantitative real-time PCR has shown that the expression levels of microRNA-150 are reduced in the serum of PD patients. Thus, it can be considered a diagnostic biomarker for evaluating the disease. There was also a negative association between the pro-cytokines and miR‐150 in PD patients [39].
Gut microbiota and inflammatory cytokines responses
Examination of the stool and blood samples collected from PD and control subjects demonstrated an alteration in the fecal gut microbiota in the PD group. The alteration included an increment of Verrucomicrobia, Lactobacillus, Porphyromonas, Mucispirillum, and Parabacteroides in the PD samples. However, Prevotella was more abundant in HC. These alterations were also associated with the severity of some symptoms, such as tremors, in PD patients. The plasma levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin(IL)-1β, IL-4, IL-2, IL-18, IL-6, IL-13, TNFα, and IFNγ were analyzed. An increase in IFNγ, TNFα, and IL-13 was seen in the PD samples compared with the control group [40].
Toll-like receptors (TLRs) and their polymorphisms
Toll-like receptor-4 (TLR-4) is a member of the toll-like receptor family and produces an inflammatory response to lipopolysaccharide (LPS) of the gram-negative bacteria. Examining colon biopsies and fecal and blood samples from PD patients reveals that these patients are more susceptible to intestinal barrier rupture and display increased expression of TLR-4 and pro-inflammatory genes in colonic biopsy samples, including CD3+ T cells and cytokines. Also, examination of SN of the postmortem brain showed an increased level of TLR-4 in PD cases compared to controls [41]. TLR-3 is a pattern recognition receptor that is involved in innate immune responses. The T allele of TLR-3 gene SNP (rs3775290) was revealed to decrease the risk of developing PD [42].
Also, the evaluation of TLR-9 and TLR-2 polymorphisms showed no statistical difference in TLR-9 -1237 T/C nor TLR-2 -196 to -174 del polymorphism between PD and control cases [43].
Microsomal prostaglandin E synthase1
Microsomal prostaglandin E synthase1 (mPGES-1) is an inducible enzyme that, under inflammatory conditions, increases prostaglandin E2 production. Compared to control brains, assessing mPGES-1 protein levels demonstrated its up-regulation in dopaminergic SN pars compacta neurons from PD postmortem brain specimens [44].
Glycoprotein non-metastatic melanoma protein B (GPNMB) inflammatory response in interaction with CD44
CD44 is an astrocyte-expressed receptor that binds GPNMB, an endogenous glycoprotein. The binding of GPNMB to CD44 reduces nuclear factor kappa light chain enhancer of activated B cells (NFκB) activation and inflammatory responses in macrophages. Evaluation of GPNMB and CD44 gene expression showed their increased levels in the SN of PD patients compared to controls [45].
CXCL12/CXCR4 as potential biomarkers of inflammation in PD
CXCL12 is a chemokine responsible for physiological functions such as CNS development by cell migration, proliferation, and neuronal circuit formation. CXCL12/CXCR4 is expressed in astrocytes, microglia, and neuronal areas such as SN. The expression of CXCR4 was shown to be expressively increased in the peripheral blood mononuclear cells of PD patients. In addition, CXCL12 serum levels measured using enzyme-linked immunosorbent assay (ELISA) were significantly developed in PD patients [46].
Accumulation of S100A9 and α-synuclein in PD
S100A9, as a pro-inflammatory mediator, participates in neuro-inflammatory pathways in Alzheimer disease and is expressed by both intracellular and Lewy body amyloid deposits and a calcium-binding protein, which participates in a broad range of inflammatory and neurodegenerative diseases. Ex-vivo measurements of S100A9 and α-syn (a presynaptic neuronal protein) protein levels showed that they both were elevated in the same brain areas and structures, including the SN neurons, Lewy bodies of SN, and also the Lewy bodies of the frontal lobe [47].
TNF-α and its polymorphisms
TNF-α is an inflammatory cytokine that regulates neuroinflammation activates microglia, and astrocytes can produce it. In four studies, TNF-α was higher among PD patients than controls in plasma, SN, tear, and cerebrospinal fluid (CSF) samples [21, 48-50]. However, in one study on the CSF sample, there was no notable change in the TNF-α level between the two study groups [51]. Two of the TNF-α single-nucleotide polymorphisms (SNP), rs361525 and rs1800629, were identified in the TNF-α gene promoter extracted from peripheral blood mononuclear cells, and the evaluation of the genomic DNA demonstrated no significant difference in the TNF-α SNPs between PD and control cases [52].
Microglia P2Y6 receptor participation in PD neuroinflammatory processes
The purinoceptor P2Y6 receptor (P2Y6R) is mostly responsible for microglia activation and some inflammatory pathways like triggering pro-inflammatory responses in macrophages in the central nervous system. The evaluation of P2Y6R expression in the peripheral blood mononuclear cells demonstrated that its levels in PD were increased compared to healthy controls. P2Y6R is also activated in LPS-treated BV2 cells and is involved in releasing pro-inflammatory cytokines through an autocrine feedback loop based on LPS-triggered uridine diphosphate (UDP) secretion; therefore, blocking UDP/P2Y6R signaling can opposite pathological procedures [53].
Upregulation of lipocalin-2 expression in the SN of PD patients
Lipocalin-2 (LCN2) is a glycoprotein involved in innate immune system functions due to its antimicrobial activity. Meanwhile, tyrosine hydroxylase (TH) is the enzyme that changes tyrosine to dopamine. Evaluation of LCN2 and TH protein levels by western blotting of SN samples obtained from postmortem brains presented that LCN2 expression was increased significantly in the SN of PD patients compared with age-matched healthy controls. In contrast, TH expression was lower in PD’s SN than in healthy patients [54].
Microglia-mediated neuro-inflammation in the pathogenesis of PD
Evaluation of SN of the postmortem PD patients’ brains demonstrated a reduction in neuromelanin-positive neurons (DA neurons). There was also a decrease in tyrosine hydroxylase (TH) expression, which is a marker for DA neurons. Activation of TLR-4, a receptor in the neuro-inflammation pathway of microglial cells, generates inflammatory cytokines that lead to neurodegeneration of the brain, and the expression levels of TLR-4 increase in the SN of PD brains. Prothrombin kringle-2 (pKr-2) is produced from prothrombin through the activation of thrombin, and pKr-2 is related to inducing the death of DA neurons. The levels of pKr-2 increased significantly in SN of PD individuals compared to healthy patients [55].
Altered expression of proteinase-activated receptor-2 (PAR2) and associated proteins
PAR2 are G-protein-coupled receptors activated by proteases, which play a role in inflammation and pain pathways. PAR2 expression is increased in neurologic disorders, such as Alzheimer disease and multiple sclerosis. Levels of PAR2 activator trypsin-2 and its inhibitors, serpin-A5 and serpin-A13, are altered in different PD brain areas. With higher stages of the disease, a decrease in the number of neurons expressing the proteinase inhibitors was seen in brain regions, including the dorsal motor nucleus, locus coeruleus, SN, and primary motor cortex. Also, an increase in the expression of serpins in the microglia of the dorsal motor nucleus of the vagus and the SN, an increase of trypsin-2 expression in the microglia of the dorsal motor nucleus of the vagus and primary motor cortex, and PAR-2 expression increase in the locus coeruleus and cingulate cortex were seen. However, in the early stages of PD, there was an increase in serpin-A13 [56].
Roles of intracellular adhesion molecule-1, vascular cell adhesion molecule-1, and lymphocyte function-associated antigen-1 in neuroinflammation
Intracellular adhesion molecule-1 (ICAM-1), a transmembrane glycoprotein expressed chiefly on endothelial cells and some immune system cells, along with its counter receptor, lymphocyte function-associated antigen-1 (LFA-1), plays a crucial part in the inflammatory procedure and T-cell mediated defense. Increased ICAM-1-positive reactive astrocytes were detected in the SN of PD cases. ICAM-1-positive astrocytes are focused around residual neurons and areas containing extracellular melanin aggregates. LFA-1, expressed by activated microglia and activated T cells, is upregulated in reactive microglia from within the SN of the PD brain. Also, weak reactivity of LFA-1 in resting microglia is detected in the SN of control brains [57]. It is indicated that neurovascular changes can lead to BBB dysfunction through neo-angiogenesis; BBB dysfunction can cause neuro-inflammation in the CNS, and neutrophil infiltration can lead to neuronal injury in the SN. The presence of pro-inflammatory cytokines such as TNF-α and IL-1β in the CNS can cause BBB cells to upregulate ICAM-1 and VCAM-1. These factors can transfer leukocytes into the CNS and eventually cause neuro-inflammation. Blood samples from the cases demonstrated a higher vascular cell adhesion molecule-1 (VCAM-1) expression in PD subjects compared to HC. However, ICAM-1 expression wasn’t significantly different among the two groups [38].
Multiplex cytokines, soluble (s)RAGE, and high mobility group box-1 (HMGB1), TLR2/4 in PD neuroinflammation
Chung and colleagues’ study sheds light on how inflammation in the brain can trigger a cascade of events leading to the worsening of α-synuclein pathology and nerve cell degeneration. They found that certain receptors, TLR-2 and TLR-4, become more active in response to this inflammation, setting off a chain reaction involving a signaling pathway called p38/JNK. This pathway, in turn, affects the process of autophagy, which is like a cellular cleaning system. When this system gets disrupted, it cannot properly clear out harmful proteins like α-synuclein, leading to their buildup and damage to nerve cells. Their research also revealed that during inflammation, there is an increase in markers associated with autophagy, suggesting that the cell is trying to ramp up its cleaning efforts. However, they noticed a hitch in the process: The fusion of certain cellular compartments essential for proper cleaning seems to be impaired. This condition leads to a backlog of protein waste and further damage. Interestingly, when they blocked TLR-2 and TLR-4, they saw a reduction in one marker of protein buildup but not in others related to the cleanup process. This outcome suggests that while blocking these receptors might alleviate some aspects of the problem, it does not fully restore the cell’s ability to clean up. Their findings provide valuable insights into the complex interplay between inflammation, protein buildup, and cellular cleanup mechanisms in neurodegenerative diseases like PD [58].
TNFRSF9, CCL23, CCL25, VEGFA, and TGF-α neuroinflammatory role in PD
According to a panel of four proteins, in the research headed by Hepp et al., PD patients displayed a different immune response from controls (CXCL11, OPG, IL-12B, and CSF-1). Five inflammation-associated proteins (TNFRSF9, CCL23, CCL25, TGF-α, and VEGFA) had higher PD expression levels and were partially related to more severe motor and cognitive symptoms during follow-up. Decreased cognitive function and the APOE4 genotype were linked to elevated CCL23 levels [59].
Colonic LRRK2 neuroinflammatory role in PD
According to a study by Liao et al., colonic biopsied specimens from PD patients had higher levels of LRRK2 expression than did age- and gender-matched controls. Colonic LRRK2 levels in PD patients were found to be connected with disease severity in cognitive function and motor, and those with pathogenic mutations or those bearing LRRK2 risk had higher expression levels than the wild-type LRRK2. Furthermore, individuals with PD in the prodromal phase showed elevated levels of LRRK2 expression in the colon compared to those without the condition, and this expression increased further as the disease progressed. These results suggest that heightened expression of LRRK2 in the colon could serve as an indicative marker for PD with gut involvement [60].
Discussion
In addition to the hallmark degeneration of dopaminergic neurons in SN and accumulation of α-syn in neural cell bodies in PD [61, 62], several other biochemical and functional changes have been associated with this disease. They include mitochondrial dysfunction, oxidative stress, protein aggregation, impaired autophagy, and immune system overreaction, followed by neuroinflammation [63-65]. This systematic review studied 34 original studies involving PD patients to identify evidence supporting the contribution of neuro-inflammatory mechanisms to the pathogenesis of this disease.
The Mean±SD functional disability of included patients was 2.13±1.22 (Table 1). Hoehn and Yahr’s scale representing the disease severity has been significantly correlated with a cytokine blood level called RANTES. This finding highlights the importance of further investigations on cytokines as potential biomarkers for disease advancement [67].
IFN-γ has been proven to be associated with typical brain pathogenesis [68]. Studies on animal subjects have illustrated that a high serum level of INF-γ is responsible for microglia and astroglia activation, leading to dopaminergic neural death [69, 70]. Recent studies have consistently approved a correlation between IFN-γ expression network signatures and its part in the selective susceptibility of dopaminergic neurons in PD patients [71]. Comparing 26 PD patients with 14 healthy controls, Diaz and colleagues showed insignificantly higher levels of peripheral IFN-γ in PD patients [71]. However, although IFN-γ has been associated with microglia, astroglia activation, and dopaminergic neural degeneration [72], it has been reported that the lower levels of IFN-γ, the more severe the tremors [71, 73]. The involvement of cytokines in motor symptoms of PD and their independent, non-responsive innate to dopaminergic treatment [74, 75] suggests the importance of cytokine-mediated symptomatic therapies in PD along with dopaminergic interventions. A study on postpartum brain samples has also demonstrated the role of TNF-α in the apoptosis of neuronal and glial cells due to its interaction with TNF receptor (TNFR) 1 by activating caspase 1 and 3 in SN. In another study conducted in vitro, lipopolysaccharide (LPS) targeted glial cells containing IL1β and TNF-α caused the death of dopaminergic rat neurons [76, 77]. A recent study based on GWAS statistics implied that TNF-TNFR1 signaling inhibition could not be used to delay or prevent PD onset [78]. The multifactorial nature of PD pathogenesis could explain this phenomenon.
Similarly, according to our results of systematic review, IFN-γ, TNF-α, CRP, and IL-6 are the most important neuroinflammatory markers associated with PD pathogenesis (Table 1). Confirming our data, a recent meta-analysis on higher CRP levels in serum, plasma, and cerebrospinal fluid concluded that it could be a risk factor for PD, leading to inflammatory responses [79]. Interestingly, an ultrasensitive assay demonstrates that high serum CRP is highly associated with freezing of gait in PD patients [80]. IL-6 levels are higher in cerebrospinal fluid and nigrostriatal region of PD patients, and they positively correlated with disease severity and motor symptoms, similar to our results [81, 82]. A meta-analysis has also approved higher CSF levels of IL-6 (Hedges’g, 0.468; 95% CI, 0.049, 0.887; P=0.026) than controls [83].
Our results determined that LCN2 and mPGES-1 expression was upregulated in SN samples of postmortem PD patients, while TH expression showed a significant decrease. Upregulation of LCN2 and mPGES-1 indicates that they could be used as novel biomarkers of PD. In a study on both SN of postmortem brain tissue from neurotoxin 6-hydroxydopamine (6-OHDA)-induced PD mice and PD patients, it has been demonstrated that mPGES-1 contributes to the inflammatory process and PD pathogenesis, by increasing PGE2, which triggers dopaminergic neuronal loss. Ikeda-Matsuo et al. [44] showed elevated levels of mPGES-1 in the PD patients’ SN dopaminergic neurons of postmortem brain tissue. They suggested it could be a therapeutic target [84]. LCN2 is an iron-trafficking protein that upregulates oxidative stress and inflammation [84].
In contrast to the high LCN2 level in the SN samples of postpartum PD patients, Xiong et al. reported that the serum level of this protein is not considerably higher in PD patients than in healthy controls [85]. Thus, LCN2 may not be a biomarker for PD, but its suitability as a therapeutic target requires further investigation. TH, an iron-containing monooxygenase, plays a significant catalytic role in L-tyrosine to L-DOPA conversion [86]. It is considered a fundamental rate-limiting step in the biosynthesis of catecholamines [87]. Importantly, PD mainly affects TH-containing neurons. TH deficiency could be compensated by levodopa, DA agonists, inhibitors of DA metabolism, or grafts of brain cells expressing TH [88]. Our result showed an increase of CXCL12 and CXCR4, respectively, in peripheral blood mononuclear cells and serum of PD patients, revealing two new potential markers [46] (Table 1). A recent study also showed a positive correlation between CXCL12 and α-synuclein in the postmortem brain tissue of PD patients. In addition, they found upregulated levels of CXCR4 in SN microglia of transgenic expressing human A53T mutant α-synuclein (A53T) mice. The authors suggested that inhibition of CXCL12/CXCR4 is a potential preventive approach for α-synuclein-triggered microglial responses and, consequently, PD treatment [89].
As reported in the results, we have noticed that S100A9 and α-syn concentrations were elevated in the SN of PD patients [47] (Table 1). Recently, Toleikis et al. demonstrated the aggregation of α-syn depends on the presence of S100A9, which could promote an earlier onset or a novel pattern of α-syn aggregates, increasing their toxicity and the accompanying neurodegeneration [90]. Therefore, we suggest that by focusing on S100A9 and its effect on α-synuclein aggregation, further studies could create more effective therapeutic and preventive programs for diverse synucleinopathies like PD.
P2Y6R is expressed by immune cells, such as the microglia. It modulates the phagocytic activity of microglia and upregulates their inflammatory cytokine secretion, thus stimulating microglial-related inflammation in neurological disorders [91, 53] (Table 1).
MicroRNA-150 downregulation is a potential diagnostic biomarker in PD inflammation [92] (Table 1). The authors claimed that miR-150 is decreased in microglia by LPS treatment. A study by Yang et al. showed that micoRNA-105-5p was significantly overexpressed in PD patients compared with healthy control [93]. In addition, according to Caggiu et al., a considerable increase in miRNA-155-5p expression in PD patients showed its potential as a novel diagnostic biomarker by assessing peripheral blood samples [94]. Besides, the considerable decrease in microRNA-155-5p after levodopa treatment revealed its potential as a promising therapeutic target [94]. The authors also investigated the expression patterns of miR-26a-5p and miR132-5p, which did not show any difference between healthy controls and PD patients, while miRNA-146a-5p expression was decreased. We also found that blood levels of miRNA-29a and miRNA-22 were considerably upregulated in PD patients. Associated with apoptosis, neural survival, angiogenesis, and immune regulation, miRNA-29 has four different sequences called miRNA-29a, miRNA-29b-1, miRNA-29b-2, and miRNA-29c [95-98].
During PD progression, astrocytes produce TNF-α, IL-1β, NO, prostaglandin-E2, and other inflammatory mediators, which activate signal transduction pathways in dopaminergic neurons, leading to their degeneration [99]. Interestingly, GPNM prevents such inflammatory cytokine release by binding to CD44 receptors on astrocytes. Notably, the neuroprotective effects of GPNMB have already been reported in amyotrophic lateral sclerosis and ischemia [45]. CD44-dependent reduction of inflammatory mediators and oxidative stress by GPNMB has been reported in an astrocyte cell line and primary mouse astrocytes [45]. Recently demonstrated in the Chinese population that rs156429 single nucleotide polymorphism in the GPNMB gene could be related to pain symptoms and cognitive dysfunction in female PD patients [100].
PD patients had significantly higher levels of both CCL25 (C-C motif chemokine ligand 25, which has chemotactic action to inflammatory cells) and TNFRSF9 (a protein that induces T cell death) at follow-up, which is reliable with a prior finding for CCL25 [101]. At follow-up, a higher TNFRSF9 level was associated with worse cognitive function. TNFRSF9 may contribute to cell death in multiple sclerosis by inducing microglia activation [102]. Recently, modifications in the TNFRSF9 gene have been linked to PD; in individuals with a known DJ-1 mutation, the TNFRSF9 genotype may act as a disease modifier [103].
Upregulated production of miR-205 could offer a viable healing approach for PD patients since Goh et al. discovered that downregulated expression of miR-205 can cause the sporadic PD patients’ brains to show a pathogenic elevation of LRRK2 protein [95]. Additionally, some research has demonstrated that miR-19b, miR-24, and miR-195 serum levels may be highly accurately utilized to diagnose PD [104]. However, no research has been done on miR485-function 3p’s in PD diagnosis or treatment.
Conclusion
The current review aimed to summarize the outcomes of previous studies focused on mechanisms of neuro-inflammation involved in PD. We found that inflammatory pathways play key roles in the neuroinflammation and, subsequently, initiation and progression of PD. This study demonstrated that the neurodegeneration in PD could be initiated by neuron-derived α-syn protein, which leads to neuro-inflammation characterized by inflammatory responses in neurons. Also, inflammatory markers, astrocytes, and microglial cells can be involved in the progression of PD. Finally, chronic neuroinflammation could cause dopaminergic neuronal death in SN. By collecting and analyzing the latest outcomes and information, the impact of both single and all factors was assessed for planning possible further studies in a particular pathway to intercept the onset of inflammatory paths in favor of therapeutic purposes. Estimating the influence of each factor that has a part in the pathogenesis of PD gives us this opportunity to define new subsequent studies to dream up for solving the mystery of PD.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization: Mohammadreza Kosari, Supervision: Zohreh Zamani, Data collection: Sevim Soleimani; Writing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Parkinson disease (PD) is a neurodegenerative disease characterized by the progressive degeneration of dopaminergic (DA) neurons in the substantia nigra (SN) and their projections to the striatum. Clinical hallmarks of PD consist of postural instability, bradykinesia, resting tremor, and rigidity. The major non-motor symptoms of PD are sensory deficits, sleep disorders, cognitive impairment, depression, anxiety, and autonomic dysfunctions [1-4].
Several pathological structures containing misfolded proteins are observed in PD brain tissue, including limbic and cortical Lewy bodies [5], senile plaques, and neurofibrillary tangles [6]. α-Synuclein (α-syn), the key factor of Lewy bodies, is the main protein involved in neurodegeneration and neuronal cell toxicity [7]. Monomeric α-syn and oligomeric aggregates of this protein are believed to be critical players in PD pathogenesis [8]. The deterioration of cognition in PD patients is caused by progressive neuronal dysfunction in various cortical regions affected by α-syn pathology [9], which is also associated with neuroinflammatory processes [10]. Neuroinflammation is crucial in different stages of PD progression, from early α-syn aggregation and degeneration of dopaminergic neurons to the onset of clinical symptoms [11-14]. Dysregulation of inflammatory pathways is one of the inflammatory components of PD, and it is likely caused by genetic predispositions, immunological changes brought on by aging, and the initial activation of glia as a result of neuronal injury [15, 16]. Also, the aggregation of misfolded proteins like α-syn and blood-brain barrier (BBB) disruption, are the initial changes in the pathogenesis of PD and can be induced by neuroinflammation [17, 18]. The selective injury of dopaminergic neurons in the nigrostriatal path can be due to the neurotoxic effects of inflammatory mediators mainly secreted by reactive microglia. The pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 have vital roles in the cellular death observed in PD and other chronic neuro-inflammatory conditions [19, 20]. Interferon-gamma (IFN-γ), which has an important role in autoimmune disease, also induces neurodegeneration in PD [21]. C-reactive protein (CRP) is another participant in neuro-inflammatory responses in PD. It is an acute-phase serum protein that becomes upregulated following an increase in IL-6 levels [22].

In PD, systemic oxidative stress and neuroinflammation may lead to astrogliosis, which damages the glymphatic system [23]. Notably, reduced glymphatic activity is associated with aging, the primary risk factor for PD [23]. Even though the etiology of PD is not fully established, the importance of genetic factors has become evident [24]. Thus, genetic mutations in 18 chromosomal regions have been identified in familial PD. For example, heterozygous mutations involving the GBA gene encoding lysosomal enzyme glucocerebrosidase are one of the greatest common risk factors for PD, increasing its risk 5-6 times [25, 26]. In addition, different single nucleotide polymorphisms (SNPs) which increased PD risk have been detected in the promoter of the TNF-α gene; especially 238 G/A (rs361525) and 308 G/A (rs1800629) SNPs are demonstrated as important functional polymorphism [27-29].
The current systematic review aims to determine the effects of each neuroinflammatory molecular pathway involved in PD pathogenesis.
Materials and Methods
Search strategy
The literature search was done till December 2023 by two authors independently in PubMed, Web of Science, Scopus, and Embase databases. The following terms, their abbreviations, and MeSH terms were used without any restriction on publication date: “Inflammation,” “neuroinflammation,” “neurodegenerative disease,” “Parkinson’s disease,” “brain,” “inflammatory markers,” “C-reactive protein,” “C–C chemokine receptor type 3,” and “prostaglandin.” The reference lists of all identified publications were checked to prevent any duplicate.
Inclusion and exclusion criteria
All observational studies written in English reporting qualitative or quantitative data on the connection between neuroinflammation and PD were included in this review. We excluded animal studies, case reports, conference papers, review studies, studies without randomized sampling, and low-quality studies identified by the quality assessment using the Newcastle-Ottawa scale (NOS). Two authors independently conducted the initial screening based on “titles” and “abstracts” and then selected qualified papers after considering the full text of the nominated articles. A third author resolved differences in opinions.
Quality assessment
NOS was used to evaluate the quality of selected articles. Based on this scale, each study can obtain a maximum of 9 points from the following parameters: Selection of participants (4 points), comparability (2 points), and the valuation of outcomes (3 points). The studies with a score above 6 were considered high-quality studies. Studies with modest quality had points of 4-6, and the quality of studies scored under 4 was low (Table 1).




Data extraction
The following information was extracted from all qualified articles: The first author’s name, country of study, date of publication, sample size, and all characteristics of the patient and control groups, including studied inflammatory markers, factors, genes, and the clinical stage of the disease. Data extraction was conducted independently by two authors, and afterward, their results were compared to eliminate discrepancies.
Results
Study selection
We used the PRISMA (preferred reporting items for systematic reviews and meta-analyses) checklist to conduct the present review [18]. A total of 2499 studies were extracted through the search on PubMed, and 7560 by searching other databases (Google Scholar, Scopus, Web of Science, and Embase). Next, 4823 papers were excluded because of repetition in various databases. After reviewing the “titles” and “abstracts” of these chosen articles, 2121 more studies were excluded because of the absence of validation in abstracts, being review studies or non-English. Furthermore, 3003 more papers were excluded because they were unrelated to the key subject or animal articles.
At last, from the 112 remaining studies, 29 records were excluded due to being irrelevant to the subject and the primary effect size of the study. The complete manuscripts of the 83 studies were utterly evaluated, and 42 papers were excluded because of unclear or insufficient data (n=31) and low quality (n=11). Lastly, 41 papers published before December 2023 were selected (Figure 2).

C-reactive protein (CRP)
Seven studies, including 1055 PD patients and 698 healthy controls, reported the participants’ CRP levels in the serum [30-34] and plasma [35, 36]. Among studies assessing the CRP in the serum samples of PD patients and their controls, there are four studies by Song et al. [31-34] stating significantly higher stages of high-sensitivity (hs)-CRP in the serum of early PD patients, PD patients with or without dementia, and a whole sample of PD patients in comparison with the control groups. However, Yazar et al. [30] reported that the difference between CRP stages in the serum sample of PD individuals and the control group was not significant (P=0.854).
C–C chemokine receptor type 3
The innate immune system mainly expresses c chemokine receptor type 3 (CCR3) and can bind with several chemokines to activate some inflammatory cells’ responses. CCR3 was extracted from isolated granulocytes of the cases, and no significant difference was detected in the CCR3 levels among the PD and health control (HC). Therefore, it is not eligible for diagnosing the disease [37].
Some microRNA subtypes role in the inflammatory pathogenesis of PD
MicroRNAs (miRNAs) are small single-stranded classes of RNAs that contribute to regulating gene expression. MiRNA-29a potentially regulates angiogenesis in endothelial cells, leading to BBB dysfunction and neuroinflammation. MiRNA-22 is also associated with angiogenesis-related alterations alongside miRNA-29a. Evaluation of the blood samples obtained from the subjects demonstrates that miRNA-29a and miRNA-22 are remarkably upregulated in the PD individuals compared to the HC group [38]. Quantitative real-time PCR has shown that the expression levels of microRNA-150 are reduced in the serum of PD patients. Thus, it can be considered a diagnostic biomarker for evaluating the disease. There was also a negative association between the pro-cytokines and miR‐150 in PD patients [39].
Gut microbiota and inflammatory cytokines responses
Examination of the stool and blood samples collected from PD and control subjects demonstrated an alteration in the fecal gut microbiota in the PD group. The alteration included an increment of Verrucomicrobia, Lactobacillus, Porphyromonas, Mucispirillum, and Parabacteroides in the PD samples. However, Prevotella was more abundant in HC. These alterations were also associated with the severity of some symptoms, such as tremors, in PD patients. The plasma levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin(IL)-1β, IL-4, IL-2, IL-18, IL-6, IL-13, TNFα, and IFNγ were analyzed. An increase in IFNγ, TNFα, and IL-13 was seen in the PD samples compared with the control group [40].
Toll-like receptors (TLRs) and their polymorphisms
Toll-like receptor-4 (TLR-4) is a member of the toll-like receptor family and produces an inflammatory response to lipopolysaccharide (LPS) of the gram-negative bacteria. Examining colon biopsies and fecal and blood samples from PD patients reveals that these patients are more susceptible to intestinal barrier rupture and display increased expression of TLR-4 and pro-inflammatory genes in colonic biopsy samples, including CD3+ T cells and cytokines. Also, examination of SN of the postmortem brain showed an increased level of TLR-4 in PD cases compared to controls [41]. TLR-3 is a pattern recognition receptor that is involved in innate immune responses. The T allele of TLR-3 gene SNP (rs3775290) was revealed to decrease the risk of developing PD [42].
Also, the evaluation of TLR-9 and TLR-2 polymorphisms showed no statistical difference in TLR-9 -1237 T/C nor TLR-2 -196 to -174 del polymorphism between PD and control cases [43].
Microsomal prostaglandin E synthase1
Microsomal prostaglandin E synthase1 (mPGES-1) is an inducible enzyme that, under inflammatory conditions, increases prostaglandin E2 production. Compared to control brains, assessing mPGES-1 protein levels demonstrated its up-regulation in dopaminergic SN pars compacta neurons from PD postmortem brain specimens [44].
Glycoprotein non-metastatic melanoma protein B (GPNMB) inflammatory response in interaction with CD44
CD44 is an astrocyte-expressed receptor that binds GPNMB, an endogenous glycoprotein. The binding of GPNMB to CD44 reduces nuclear factor kappa light chain enhancer of activated B cells (NFκB) activation and inflammatory responses in macrophages. Evaluation of GPNMB and CD44 gene expression showed their increased levels in the SN of PD patients compared to controls [45].
CXCL12/CXCR4 as potential biomarkers of inflammation in PD
CXCL12 is a chemokine responsible for physiological functions such as CNS development by cell migration, proliferation, and neuronal circuit formation. CXCL12/CXCR4 is expressed in astrocytes, microglia, and neuronal areas such as SN. The expression of CXCR4 was shown to be expressively increased in the peripheral blood mononuclear cells of PD patients. In addition, CXCL12 serum levels measured using enzyme-linked immunosorbent assay (ELISA) were significantly developed in PD patients [46].
Accumulation of S100A9 and α-synuclein in PD
S100A9, as a pro-inflammatory mediator, participates in neuro-inflammatory pathways in Alzheimer disease and is expressed by both intracellular and Lewy body amyloid deposits and a calcium-binding protein, which participates in a broad range of inflammatory and neurodegenerative diseases. Ex-vivo measurements of S100A9 and α-syn (a presynaptic neuronal protein) protein levels showed that they both were elevated in the same brain areas and structures, including the SN neurons, Lewy bodies of SN, and also the Lewy bodies of the frontal lobe [47].
TNF-α and its polymorphisms
TNF-α is an inflammatory cytokine that regulates neuroinflammation activates microglia, and astrocytes can produce it. In four studies, TNF-α was higher among PD patients than controls in plasma, SN, tear, and cerebrospinal fluid (CSF) samples [21, 48-50]. However, in one study on the CSF sample, there was no notable change in the TNF-α level between the two study groups [51]. Two of the TNF-α single-nucleotide polymorphisms (SNP), rs361525 and rs1800629, were identified in the TNF-α gene promoter extracted from peripheral blood mononuclear cells, and the evaluation of the genomic DNA demonstrated no significant difference in the TNF-α SNPs between PD and control cases [52].
Microglia P2Y6 receptor participation in PD neuroinflammatory processes
The purinoceptor P2Y6 receptor (P2Y6R) is mostly responsible for microglia activation and some inflammatory pathways like triggering pro-inflammatory responses in macrophages in the central nervous system. The evaluation of P2Y6R expression in the peripheral blood mononuclear cells demonstrated that its levels in PD were increased compared to healthy controls. P2Y6R is also activated in LPS-treated BV2 cells and is involved in releasing pro-inflammatory cytokines through an autocrine feedback loop based on LPS-triggered uridine diphosphate (UDP) secretion; therefore, blocking UDP/P2Y6R signaling can opposite pathological procedures [53].
Upregulation of lipocalin-2 expression in the SN of PD patients
Lipocalin-2 (LCN2) is a glycoprotein involved in innate immune system functions due to its antimicrobial activity. Meanwhile, tyrosine hydroxylase (TH) is the enzyme that changes tyrosine to dopamine. Evaluation of LCN2 and TH protein levels by western blotting of SN samples obtained from postmortem brains presented that LCN2 expression was increased significantly in the SN of PD patients compared with age-matched healthy controls. In contrast, TH expression was lower in PD’s SN than in healthy patients [54].
Microglia-mediated neuro-inflammation in the pathogenesis of PD
Evaluation of SN of the postmortem PD patients’ brains demonstrated a reduction in neuromelanin-positive neurons (DA neurons). There was also a decrease in tyrosine hydroxylase (TH) expression, which is a marker for DA neurons. Activation of TLR-4, a receptor in the neuro-inflammation pathway of microglial cells, generates inflammatory cytokines that lead to neurodegeneration of the brain, and the expression levels of TLR-4 increase in the SN of PD brains. Prothrombin kringle-2 (pKr-2) is produced from prothrombin through the activation of thrombin, and pKr-2 is related to inducing the death of DA neurons. The levels of pKr-2 increased significantly in SN of PD individuals compared to healthy patients [55].
Altered expression of proteinase-activated receptor-2 (PAR2) and associated proteins
PAR2 are G-protein-coupled receptors activated by proteases, which play a role in inflammation and pain pathways. PAR2 expression is increased in neurologic disorders, such as Alzheimer disease and multiple sclerosis. Levels of PAR2 activator trypsin-2 and its inhibitors, serpin-A5 and serpin-A13, are altered in different PD brain areas. With higher stages of the disease, a decrease in the number of neurons expressing the proteinase inhibitors was seen in brain regions, including the dorsal motor nucleus, locus coeruleus, SN, and primary motor cortex. Also, an increase in the expression of serpins in the microglia of the dorsal motor nucleus of the vagus and the SN, an increase of trypsin-2 expression in the microglia of the dorsal motor nucleus of the vagus and primary motor cortex, and PAR-2 expression increase in the locus coeruleus and cingulate cortex were seen. However, in the early stages of PD, there was an increase in serpin-A13 [56].
Roles of intracellular adhesion molecule-1, vascular cell adhesion molecule-1, and lymphocyte function-associated antigen-1 in neuroinflammation
Intracellular adhesion molecule-1 (ICAM-1), a transmembrane glycoprotein expressed chiefly on endothelial cells and some immune system cells, along with its counter receptor, lymphocyte function-associated antigen-1 (LFA-1), plays a crucial part in the inflammatory procedure and T-cell mediated defense. Increased ICAM-1-positive reactive astrocytes were detected in the SN of PD cases. ICAM-1-positive astrocytes are focused around residual neurons and areas containing extracellular melanin aggregates. LFA-1, expressed by activated microglia and activated T cells, is upregulated in reactive microglia from within the SN of the PD brain. Also, weak reactivity of LFA-1 in resting microglia is detected in the SN of control brains [57]. It is indicated that neurovascular changes can lead to BBB dysfunction through neo-angiogenesis; BBB dysfunction can cause neuro-inflammation in the CNS, and neutrophil infiltration can lead to neuronal injury in the SN. The presence of pro-inflammatory cytokines such as TNF-α and IL-1β in the CNS can cause BBB cells to upregulate ICAM-1 and VCAM-1. These factors can transfer leukocytes into the CNS and eventually cause neuro-inflammation. Blood samples from the cases demonstrated a higher vascular cell adhesion molecule-1 (VCAM-1) expression in PD subjects compared to HC. However, ICAM-1 expression wasn’t significantly different among the two groups [38].
Multiplex cytokines, soluble (s)RAGE, and high mobility group box-1 (HMGB1), TLR2/4 in PD neuroinflammation
Chung and colleagues’ study sheds light on how inflammation in the brain can trigger a cascade of events leading to the worsening of α-synuclein pathology and nerve cell degeneration. They found that certain receptors, TLR-2 and TLR-4, become more active in response to this inflammation, setting off a chain reaction involving a signaling pathway called p38/JNK. This pathway, in turn, affects the process of autophagy, which is like a cellular cleaning system. When this system gets disrupted, it cannot properly clear out harmful proteins like α-synuclein, leading to their buildup and damage to nerve cells. Their research also revealed that during inflammation, there is an increase in markers associated with autophagy, suggesting that the cell is trying to ramp up its cleaning efforts. However, they noticed a hitch in the process: The fusion of certain cellular compartments essential for proper cleaning seems to be impaired. This condition leads to a backlog of protein waste and further damage. Interestingly, when they blocked TLR-2 and TLR-4, they saw a reduction in one marker of protein buildup but not in others related to the cleanup process. This outcome suggests that while blocking these receptors might alleviate some aspects of the problem, it does not fully restore the cell’s ability to clean up. Their findings provide valuable insights into the complex interplay between inflammation, protein buildup, and cellular cleanup mechanisms in neurodegenerative diseases like PD [58].
TNFRSF9, CCL23, CCL25, VEGFA, and TGF-α neuroinflammatory role in PD
According to a panel of four proteins, in the research headed by Hepp et al., PD patients displayed a different immune response from controls (CXCL11, OPG, IL-12B, and CSF-1). Five inflammation-associated proteins (TNFRSF9, CCL23, CCL25, TGF-α, and VEGFA) had higher PD expression levels and were partially related to more severe motor and cognitive symptoms during follow-up. Decreased cognitive function and the APOE4 genotype were linked to elevated CCL23 levels [59].
Colonic LRRK2 neuroinflammatory role in PD
According to a study by Liao et al., colonic biopsied specimens from PD patients had higher levels of LRRK2 expression than did age- and gender-matched controls. Colonic LRRK2 levels in PD patients were found to be connected with disease severity in cognitive function and motor, and those with pathogenic mutations or those bearing LRRK2 risk had higher expression levels than the wild-type LRRK2. Furthermore, individuals with PD in the prodromal phase showed elevated levels of LRRK2 expression in the colon compared to those without the condition, and this expression increased further as the disease progressed. These results suggest that heightened expression of LRRK2 in the colon could serve as an indicative marker for PD with gut involvement [60].
Discussion
In addition to the hallmark degeneration of dopaminergic neurons in SN and accumulation of α-syn in neural cell bodies in PD [61, 62], several other biochemical and functional changes have been associated with this disease. They include mitochondrial dysfunction, oxidative stress, protein aggregation, impaired autophagy, and immune system overreaction, followed by neuroinflammation [63-65]. This systematic review studied 34 original studies involving PD patients to identify evidence supporting the contribution of neuro-inflammatory mechanisms to the pathogenesis of this disease.
The Mean±SD functional disability of included patients was 2.13±1.22 (Table 1). Hoehn and Yahr’s scale representing the disease severity has been significantly correlated with a cytokine blood level called RANTES. This finding highlights the importance of further investigations on cytokines as potential biomarkers for disease advancement [67].
IFN-γ has been proven to be associated with typical brain pathogenesis [68]. Studies on animal subjects have illustrated that a high serum level of INF-γ is responsible for microglia and astroglia activation, leading to dopaminergic neural death [69, 70]. Recent studies have consistently approved a correlation between IFN-γ expression network signatures and its part in the selective susceptibility of dopaminergic neurons in PD patients [71]. Comparing 26 PD patients with 14 healthy controls, Diaz and colleagues showed insignificantly higher levels of peripheral IFN-γ in PD patients [71]. However, although IFN-γ has been associated with microglia, astroglia activation, and dopaminergic neural degeneration [72], it has been reported that the lower levels of IFN-γ, the more severe the tremors [71, 73]. The involvement of cytokines in motor symptoms of PD and their independent, non-responsive innate to dopaminergic treatment [74, 75] suggests the importance of cytokine-mediated symptomatic therapies in PD along with dopaminergic interventions. A study on postpartum brain samples has also demonstrated the role of TNF-α in the apoptosis of neuronal and glial cells due to its interaction with TNF receptor (TNFR) 1 by activating caspase 1 and 3 in SN. In another study conducted in vitro, lipopolysaccharide (LPS) targeted glial cells containing IL1β and TNF-α caused the death of dopaminergic rat neurons [76, 77]. A recent study based on GWAS statistics implied that TNF-TNFR1 signaling inhibition could not be used to delay or prevent PD onset [78]. The multifactorial nature of PD pathogenesis could explain this phenomenon.
Similarly, according to our results of systematic review, IFN-γ, TNF-α, CRP, and IL-6 are the most important neuroinflammatory markers associated with PD pathogenesis (Table 1). Confirming our data, a recent meta-analysis on higher CRP levels in serum, plasma, and cerebrospinal fluid concluded that it could be a risk factor for PD, leading to inflammatory responses [79]. Interestingly, an ultrasensitive assay demonstrates that high serum CRP is highly associated with freezing of gait in PD patients [80]. IL-6 levels are higher in cerebrospinal fluid and nigrostriatal region of PD patients, and they positively correlated with disease severity and motor symptoms, similar to our results [81, 82]. A meta-analysis has also approved higher CSF levels of IL-6 (Hedges’g, 0.468; 95% CI, 0.049, 0.887; P=0.026) than controls [83].
Our results determined that LCN2 and mPGES-1 expression was upregulated in SN samples of postmortem PD patients, while TH expression showed a significant decrease. Upregulation of LCN2 and mPGES-1 indicates that they could be used as novel biomarkers of PD. In a study on both SN of postmortem brain tissue from neurotoxin 6-hydroxydopamine (6-OHDA)-induced PD mice and PD patients, it has been demonstrated that mPGES-1 contributes to the inflammatory process and PD pathogenesis, by increasing PGE2, which triggers dopaminergic neuronal loss. Ikeda-Matsuo et al. [44] showed elevated levels of mPGES-1 in the PD patients’ SN dopaminergic neurons of postmortem brain tissue. They suggested it could be a therapeutic target [84]. LCN2 is an iron-trafficking protein that upregulates oxidative stress and inflammation [84].
In contrast to the high LCN2 level in the SN samples of postpartum PD patients, Xiong et al. reported that the serum level of this protein is not considerably higher in PD patients than in healthy controls [85]. Thus, LCN2 may not be a biomarker for PD, but its suitability as a therapeutic target requires further investigation. TH, an iron-containing monooxygenase, plays a significant catalytic role in L-tyrosine to L-DOPA conversion [86]. It is considered a fundamental rate-limiting step in the biosynthesis of catecholamines [87]. Importantly, PD mainly affects TH-containing neurons. TH deficiency could be compensated by levodopa, DA agonists, inhibitors of DA metabolism, or grafts of brain cells expressing TH [88]. Our result showed an increase of CXCL12 and CXCR4, respectively, in peripheral blood mononuclear cells and serum of PD patients, revealing two new potential markers [46] (Table 1). A recent study also showed a positive correlation between CXCL12 and α-synuclein in the postmortem brain tissue of PD patients. In addition, they found upregulated levels of CXCR4 in SN microglia of transgenic expressing human A53T mutant α-synuclein (A53T) mice. The authors suggested that inhibition of CXCL12/CXCR4 is a potential preventive approach for α-synuclein-triggered microglial responses and, consequently, PD treatment [89].
As reported in the results, we have noticed that S100A9 and α-syn concentrations were elevated in the SN of PD patients [47] (Table 1). Recently, Toleikis et al. demonstrated the aggregation of α-syn depends on the presence of S100A9, which could promote an earlier onset or a novel pattern of α-syn aggregates, increasing their toxicity and the accompanying neurodegeneration [90]. Therefore, we suggest that by focusing on S100A9 and its effect on α-synuclein aggregation, further studies could create more effective therapeutic and preventive programs for diverse synucleinopathies like PD.
P2Y6R is expressed by immune cells, such as the microglia. It modulates the phagocytic activity of microglia and upregulates their inflammatory cytokine secretion, thus stimulating microglial-related inflammation in neurological disorders [91, 53] (Table 1).
MicroRNA-150 downregulation is a potential diagnostic biomarker in PD inflammation [92] (Table 1). The authors claimed that miR-150 is decreased in microglia by LPS treatment. A study by Yang et al. showed that micoRNA-105-5p was significantly overexpressed in PD patients compared with healthy control [93]. In addition, according to Caggiu et al., a considerable increase in miRNA-155-5p expression in PD patients showed its potential as a novel diagnostic biomarker by assessing peripheral blood samples [94]. Besides, the considerable decrease in microRNA-155-5p after levodopa treatment revealed its potential as a promising therapeutic target [94]. The authors also investigated the expression patterns of miR-26a-5p and miR132-5p, which did not show any difference between healthy controls and PD patients, while miRNA-146a-5p expression was decreased. We also found that blood levels of miRNA-29a and miRNA-22 were considerably upregulated in PD patients. Associated with apoptosis, neural survival, angiogenesis, and immune regulation, miRNA-29 has four different sequences called miRNA-29a, miRNA-29b-1, miRNA-29b-2, and miRNA-29c [95-98].
During PD progression, astrocytes produce TNF-α, IL-1β, NO, prostaglandin-E2, and other inflammatory mediators, which activate signal transduction pathways in dopaminergic neurons, leading to their degeneration [99]. Interestingly, GPNM prevents such inflammatory cytokine release by binding to CD44 receptors on astrocytes. Notably, the neuroprotective effects of GPNMB have already been reported in amyotrophic lateral sclerosis and ischemia [45]. CD44-dependent reduction of inflammatory mediators and oxidative stress by GPNMB has been reported in an astrocyte cell line and primary mouse astrocytes [45]. Recently demonstrated in the Chinese population that rs156429 single nucleotide polymorphism in the GPNMB gene could be related to pain symptoms and cognitive dysfunction in female PD patients [100].
PD patients had significantly higher levels of both CCL25 (C-C motif chemokine ligand 25, which has chemotactic action to inflammatory cells) and TNFRSF9 (a protein that induces T cell death) at follow-up, which is reliable with a prior finding for CCL25 [101]. At follow-up, a higher TNFRSF9 level was associated with worse cognitive function. TNFRSF9 may contribute to cell death in multiple sclerosis by inducing microglia activation [102]. Recently, modifications in the TNFRSF9 gene have been linked to PD; in individuals with a known DJ-1 mutation, the TNFRSF9 genotype may act as a disease modifier [103].
Upregulated production of miR-205 could offer a viable healing approach for PD patients since Goh et al. discovered that downregulated expression of miR-205 can cause the sporadic PD patients’ brains to show a pathogenic elevation of LRRK2 protein [95]. Additionally, some research has demonstrated that miR-19b, miR-24, and miR-195 serum levels may be highly accurately utilized to diagnose PD [104]. However, no research has been done on miR485-function 3p’s in PD diagnosis or treatment.
Conclusion
The current review aimed to summarize the outcomes of previous studies focused on mechanisms of neuro-inflammation involved in PD. We found that inflammatory pathways play key roles in the neuroinflammation and, subsequently, initiation and progression of PD. This study demonstrated that the neurodegeneration in PD could be initiated by neuron-derived α-syn protein, which leads to neuro-inflammation characterized by inflammatory responses in neurons. Also, inflammatory markers, astrocytes, and microglial cells can be involved in the progression of PD. Finally, chronic neuroinflammation could cause dopaminergic neuronal death in SN. By collecting and analyzing the latest outcomes and information, the impact of both single and all factors was assessed for planning possible further studies in a particular pathway to intercept the onset of inflammatory paths in favor of therapeutic purposes. Estimating the influence of each factor that has a part in the pathogenesis of PD gives us this opportunity to define new subsequent studies to dream up for solving the mystery of PD.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization: Mohammadreza Kosari, Supervision: Zohreh Zamani, Data collection: Sevim Soleimani; Writing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Kim BW, Koppula S, Kumar H, Park JY, Kim IW, More SV, et al. alpha-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology. 2015; 97:46-57. [DOI:10.1016/j.neuropharm.2015.04.037] [PMID]
- Kim SR, Chen X, Oo TF, Kareva T, Yarygina O, Wang C, et al. Dopaminergic pathway reconst ion by Akt/Rheb-induced axon regeneration. Ann Neurol. 2011; 70(1):110-20. [DOI:10.1002/ana.22383] [PMID] [PMCID]
- Braak H, Del Tredici K. Neuropathological Staging of brain pathology in sporadic parkinson’s disease: Separating the wheat from the chaff. J Parkinsons Dis. 2017; 7(s1):S71-85. [DOI:10.3233/JPD-179001] [PMID] [PMCID]
- Magrinelli F, Picelli A, Tocco P, Federico A, Roncari L, Smania N, et al. Pathophysiology of motor dysfunction in Parkinson’s Disease as the rationale for drug treatment and rehabilitation. Parkinsons Dis. 2016; 2016:9832839. [DOI:10.1155/2016/9832839] [PMID] [PMCID]
- Braak H, Rüb U, Jansen Steur EN, Del Tredici K, de Vos RA. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. 2005; 64(8):1404-10. [DOI:10.1212/01.WNL.0000158422.41380.82] [PMID]
- Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van Deerlin V, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012; 72(4):587-98. [DOI:10.1002/ana.23659] [PMID] [PMCID]
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011; 108(10):4194-9. [DOI:10.1073/pnas.1100976108] [PMID] [PMCID]
- Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013; 14(1):38-48. [DOI:10.1038/nrn3406] [PMID] [PMCID]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003; 106(6):518-26. [DOI:10.1007/s00401-003-0766-2] [PMID]
- Petrou M, Dwamena BA, Foerster BR, MacEachern MP, Bohnen NI, Müller ML, et al. Amyloid deposition in Parkinson’s disease and cognitive impairment: A systematic review. Mov Disord. 2015; 30(7):928-35. [DOI:10.1002/mds.26191] [PMID] [PMCID]
- Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson’s disease. Ann Neurol. 2003; 53 (Suppl 3):S49-58; discussion S-60. [DOI:10.1002/ana.10481] [PMID]
- Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: Role for cytokines. Curr Pharm Des. 2005; 11(8):999-1016. [DOI:10.2174/1381612053381620] [PMID]
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010; 37(3):510-8. [DOI:10.1016/j.nbd.2009.11.004] [PMID] [PMCID]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007; 150(8):963-76. [DOI:10.1038/sj.bjp.0707167] [PMID] [PMCID]
- Tiwari PC, Pal R. The potential role of neuroinflammation and transcription factors in Parkinson disease. Dialogues Clin Neurosci. 2017; 19(1):71-80. [PMID]
- Saberi A, Pourshafie SH, Kazemnejad-Leili E, Nemati S, Sutohian S, Sayad-Fathi S. Ondansetron or promethazine: Which one is better for the treatment of acute peripheral vertigo? Am J Otolaryngol. 2019; 40(1):10-5. [DOI:10.1016/j.amjoto.2018.09.010] [PMID]
- Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016; 353(6301):777-83. [DOI:10.1126/science.aag2590] [PMID]
- Ezzati K, Ravarian B, Saberi A, Salari A, Reyhanian Z, Khakpour M, et al. Prevalence of cervical myofascial pain syndrome and its correlation with the severity of pain and disability in patients with chronic non-specific neck pain. Arch Bone Jt Surg. 2021; 9(2):230-4. [PMID]
- Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol. 2012; 7(1):42-59. [DOI:10.1007/s11481-011-9287-2] [PMID]
- Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011; 10(3):391-403. [DOI:10.2174/187152711794653751] [PMID] [PMCID]
- Miliukhina IV, Usenko TS, Senkevich KA, Nikolaev MA, Timofeeva AA, Agapova EA, et al. Plasma cytokines profile in patients with parkinson’s disease associated with mutations in GBA Gene. Bull Exp Biol Med. 2020; 168(4):423-6. [DOI:10.1007/s10517-020-04723-x] [PMID]
- Jin H, Gu HY, Mao CJ, Chen J, Liu CF. Association of inflammatory factors and aging in Parkinson’s disease. Neurosci Lett. 2020; 736:135259. [DOI:10.1016/j.neulet.2020.135259] [PMID]
- Chiang PL, Chen HL, Lu CH, Chen YS, Chou KH, Hsu TW, et al. Interaction of systemic oxidative stress and mesial temporal network degeneration in Parkinson’s disease with and without cognitive impairment. J Neuroinflammation. 2018; 15(1):281. [DOI:10.1186/s12974-018-1317-z] [PMID] [PMCID]
- Nemati S, Gerami H, Soltanipour S, Saberi A, Moghadam SK, Setva F. The effects of oropharyngeal-lingual exercises in patients with primary snoring. Eur Arch Otorhinolaryngol. 2015; 272(4):1027-31. [DOI:10.1007/s00405-014-3382-y] [PMID]
- Emelyanov AK, Usenko TS, Tesson C, Senkevich KA, Nikolaev MA, Miliukhina IV, et al. Mutation analysis of Parkinson’s disease genes in a Russian data set. Neurobiol Aging. 2018; 71:267.e7-.e10. [DOI:10.1016/j.neurobiolaging.2018.06.027] [PMID]
- Sidransky E, Lopez G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012; 11(11):986-98. [DOI:10.1016/S1474-4422(12)70190-4] [PMID]
- Kroeger KM, Carville KS, Abraham LJ. The −308 tumor necrosis factor-α promoter polymorphism effects transcription. Mol Immunol. 1997; 34(5):391-9. [DOI:10.1016/S0161-5890(97)00052-7] [PMID]
- Muñoz-Valle JF, Oregón-Romero E, Rangel-Villalobos H, Martínez-Bonilla GE, Castañeda-Saucedo E, Salgado-Goytia L, et al. High expression of TNF alpha is associated with -308 and -238 TNF alpha polymorphisms in knee osteoarthritis. Clin Exp Med. 2014; 14(1):61-7. [DOI:10.1007/s10238-012-0216-3] [PMID]
- Kaluza W, Reuss E, Grossmann S, Hug R, Schopf RE, Galle PR, et al. Different transcriptional activity and in vitro TNF-alpha production in psoriasis patients carrying the TNF-alpha 238A promoter polymorphism. J Invest Dermatol. 2000; 114(6):1180-3. [DOI:10.1046/j.1523-1747.2000.00001.x] [PMID]
- Yazar HO, Yazar T, Cihan M. A preliminary data: Evaluation of serum Galectin-3 levels in patients with Idiopathic Parkinson’s Disease. J Clin Neurosci. 2019; 70:164-8. [DOI:10.1016/j.jocn.2019.08.032] [PMID]
- Song IU, Cho HJ, Kim JS, Park IS, Lee KS. Serum hs-CRP levels are increased in de Novo Parkinson’s disease independently from age of onset. Eur Neurol. 2014; 72(5-6):285-9. [DOI:10.1159/000363570] [PMID]
- Song IU, Kim YD, Cho HJ, Chung SW. Is neuroinflammation involved in the development of dementia in patients with Parkinson’s disease? Intern Med. 2013; 52(16):1787-92.[DOI:10.2169/internalmedicine.52.0474] [PMID]
- Song IU, Chung SW, Kim JS, Lee KS. Association between high-sensitivity C-reactive protein and risk of early idiopathic Parkinson’s disease. Neurol Sci. 2011; 32(1):31-4. [DOI:10.1007/s10072-010-0335-0] [PMID]
- Song IU, Kim JS, Chung SW, Lee KS. Is there an association between the level of high-sensitivity C-reactive protein and idiopathic Parkinson’s disease? A comparison of Parkinson’s disease patients, disease controls and healthy individuals. Eur Neurol. 2009; 62(2):99-104. [DOI:10.1159/000222780] [PMID]
- Andican G, Konukoglu D, Bozluolcay M, Bayülkem K, Firtiına S, Burcak G. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson’s disease. Acta Neurol Belg. 2012; 112(2):155-9. [DOI:10.1007/s13760-012-0015-3] [PMID]
- Chen H, O’Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am J Epidemiol. 2008; 167(1):90-5. [DOI:10.1093/aje/kwm260] [PMID]
- Moghadam-Ahmadi A, Khorramdelazad H, Hassanshahi G, Shahsavari S, Moadab A, Vakilian A. Eotaxins and C-C chemokine receptor type 3 in Parkinson’s disease. Acta Neurol Belg. 2020; 120(3):589-94. [DOI:10.1007/s13760-018-01061-8] [PMID]
- Yu CC, Chen HL, Chen MH, Lu CH, Tsai NW, Huang CC, et al. Vascular inflammation is a risk factor associated with brain atrophy and disease severity in parkinson’s disease: A case-control study. Oxid Med Cell Longev. 2020; 2020:2591248. [DOI:10.1155/2020/2591248] [PMID] [PMCID]
- Li H, Yu L, Li M, Chen X, Tian Q, Jiang Y, et al. MicroRNA-150 serves as a diagnostic biomarker and is involved in the inflammatory pathogenesis of Parkinson’s disease. Mol Genet Genomic Med. 2020; 8(4):e1189. [DOI:10.1002/mgg3.1189] [PMID] [PMCID]
- Lin CH, Chen CC, Chiang HL, Liou JM, Chang CM, Lu TP, et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflammation. 2019; 16(1):129. [DOI:10.1186/s12974-019-1528-y] [PMID] [PMCID]
- Perez-Pardo P, Dodiya HB, Engen PA, Forsyth CB, Huschens AM, Shaikh M, et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: A translational study from men to mice. Gut. 2019; 68(5):829-43. [DOI:10.1136/gutjnl-2018-316844] [PMID]
- Wang J, Liu Y, Liu Y, Zhu K, Xie A. The association between TLR3 rs3775290 polymorphism and sporadic Parkinson’s disease in Chinese Han population. Neurosci Lett. 2020; 728:135005. [DOI:10.1016/j.neulet.2020.135005] [PMID]
- Kalinderi K, Bostantjopoulou S, Katsarou Z, Fidani L. TLR9 -1237 T/C and TLR2 -194 to -174 del polymorphisms and the risk of Parkinson’s disease in the Greek population: A pilot study. Neurol Sci. 2013; 34(5):679-82. [DOI:10.1007/s10072-012-1106-x] [PMID]
- Ikeda-Matsuo Y, Miyata H, Mizoguchi T, Ohama E, Naito Y, Uematsu S, et al. Microsomal prostaglandin E synthase-1 is a critical factor in dopaminergic neurodegeneration in Parkinson’s disease. Neurobiol Dis. 2019; 124:81-92. [DOI:10.1016/j.nbd.2018.11.004] [PMID]
- Neal ML, Boyle AM, Budge KM, Safadi FF, Richardson JR. The glycoprotein GPNMB attenuates astrocyte inflammatory responses through the CD44 receptor. J Neuroinflammation. 2018; 15(1):73. [DOI:10.1186/s12974-018-1100-1] [PMID] [PMCID]
- Bagheri V, Khorramdelazad H, Hassanshahi G, Moghadam-Ahmadi A, Vakilian A. CXCL12 and CXCR4 in the peripheral blood of patients with parkinson’s disease. Neuroimmunomodulation. 2018; 25(4):201-5. [DOI:10.1159/000494435] [PMID]
- Horvath I, Iashchishyn IA, Moskalenko RA, Wang C, Wärmländer SKTS, Wallin C, et al. Co-aggregation of pro-inflammatory S100A9 with α-synuclein in Parkinson’s disease: Ex vivo and in vitro studies. J Neuroinflammation. 2018; 15(1):172. [DOI:10.1186/s12974-018-1210-9] [PMID] [PMCID]
- Iwaoka K, Otsuka C, Maeda T, Yamahara K, Kato K, Takahashi K, et al. Impaired metabolism of kynurenine and its metabolites in CSF of parkinson’s disease. Neurosci Lett. 2020; 714:134576. [DOI:10.1016/j.neulet.2019.134576] [PMID]
- Çomoğlu SS, Güven H, Acar M, Öztürk G, Koçer B. Tear levels of tumor necrosis factor-alpha in patients with Parkinson’s disease. Neurosci Lett. 2013; 553:63-7. [DOI:10.1016/j.neulet.2013.08.019] [PMID]
- Garcia-Esparcia P, Llorens F, Carmona M, Ferrer I. Complex deregulation and expression of cytokines and mediators of the immune response in Parkinson’s disease brain is region dependent. Brain Pathol. 2014; 24(6):584-98. [DOI:10.1111/bpa.12137] [PMID] [PMCID]
- Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, et al. Cerebrospinal fluid inflammatory markers in Parkinson’s disease--associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. 2013; 33:183-9. [DOI:10.1016/j.bbi.2013.07.007] [PMID]
- Agliardi C, Guerini FR, Zanzottera M, Riboldazzi G, Zangaglia R, Bono G, et al. TNF-α -308 G/A and -238 G/A promoter polymorphisms and sporadic Parkinson’s disease in an Italian cohort. J Neurol Sci. 2018; 385:45-8. [DOI:10.1016/j.jns.2017.12.011] [PMID]
- Yang X, Lou Y, Liu G, Wang X, Qian Y, Ding J, et al. Microglia P2Y6 receptor is related to Parkinson’s disease through neuroinflammatory process. J Neuroinflammation. 2017; 14(1):38. [DOI:10.1186/s12974-017-0795-8] [PMID] [PMCID]
- Kim BW, Jeong KH, Kim JH, Jin M, Kim JH, Lee MG, et al. Pathogenic Upregulation of Glial Lipocalin-2 in the Parkinsonian dopaminergic system. J Neurosci. 2016; 36(20):5608-22. [DOI:10.1523/JNEUROSCI.4261-15.2016] [PMID] [PMCID]
- Shin WH, Jeon MT, Leem E, Won SY, Jeong KH, Park SJ, et al. Induction of microglial toll-like receptor 4 by prothrombin kringle-2: A potential pathogenic mechanism in Parkinson’s disease. Sci Rep. 2015; 5:14764. [DOI:10.1038/srep14764] [PMID] [PMCID]
- Hurley MJ, Durrenberger PF, Gentleman SM, Walls AF, Dexter DT. Altered expression of brain proteinase-activated receptor-2, trypsin-2 and serpin proteinase inhibitors in parkinson’s disease. J Mol Neurosci. 2015; 57(1):48-62. [DOI:10.1007/s12031-015-0576-8] [PMID]
- Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol. 2006; 197(2):275-83. [DOI:10.1016/j.expneurol.2005.10.034] [PMID]
- Chung LY, Lin YT, Liu C, Tai YC, Lin HY, Lin CH, et al. Neuroinflammation upregulated neuronal toll-like receptors 2 and 4 to drive synucleinopathy in neurodegeneration. Front Pharmacol. 2022; 13:845930. [DOI:10.3389/fphar.2022.845930] [PMID] [PMCID]
- Hepp DH, van Wageningen TA, Kuiper KL, van Dijk KD, Oosterveld LP, Berendse HW, et al. Inflammatory blood biomarkers are associated with long-term clinical disease severity in Parkinson’s Disease. Int J Mol Sci. 2023; 24(19):14915.[DOI:10.3390/ijms241914915] [PMID] [PMCID]
- Liao PH, Chiang HL, Shun CT, Hang JF, Chiu HM, Wu MS, et al. Colonic leucine-rich repeat kinase 2 expression is increased and associated with disease severity in patients with Parkinson’s Disease. Front Aging Neurosci. 2022; 13:819373. [DOI:10.3389/fnagi.2021.819373] [PMID] [PMCID]
- Zafar S, Yaddanapudi SS. Parkinson Disease. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021. [Link]
- Guo X, Namekata K, Kimura A, Harada C, Harada T. ASK1 in neurodegeneration.Adv Biol Regul. 2017; 66:63-71. [DOI:10.1016/j.jbior.2017.08.003] [PMID]
- Simon DK, Tanner CM, Brundin P. Parkinson Disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020; 36(1):1-12. [DOI:10.1016/j.cger.2019.08.002] [PMID] [PMCID]
- Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012; 18 (Suppl 1):S210-2. [DOI:10.1016/S1353-8020(11)70065-7] [PMID]
- Naderinabi B, Soltanipour S, Nemati S, Saberi A, Parastesh S. Acupuncture for chronic nonpulsatile tinnitus: A randomized clinical trial. Caspian J Intern Med. 2018; 9(1):38-45. [PMID]
- Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, Hansson O. Non-motor symptoms in patients with Parkinson’s disease-correlations with inflammatory cytokines in serum. PLoS One. 2012; 7(10):e47387. [DOI:10.1371/journal.pone.0047387] [PMID] [PMCID]
- Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson Disease: A systematic review and meta-analysis. JAMA Neurol. 2016; 73(11):1316-24. [DOI:10.1001/jamaneurol.2016.2742] [PMID]
- Deczkowska A, Baruch K, Schwartz M. Type I/II interferon balance in the regulation of brain physiology and pathology. Trends Immunol. 2016; 37(3):181-92. [DOI:10.1016/j.it.2016.01.006] [PMID]
- Abdeldayem M, Anani M, Hassan H, Taha S. Association of serum interferon gamma level with Parkinson’s Disease in Egyptian Patients. Egypt J Med Microbiol. 2014; 23(1):1-5. [Link]
- Keo A, Mahfouz A, Ingrassia AMT, Meneboo JP, Villenet C, Mutez E, et al. Transcriptomic signatures of brain regional vulnerability to Parkinson’s disease. Commun Biol. 2020; 3(1):101. [DOI:10.1038/s42003-020-0804-9] [PMID] [PMCID]
- Diaz K, Kohut ML, Russell DW, Stegemöller EL. Peripheral inflammatory cytokines and motor symptoms in persons with Parkinson’s disease. Brain Behav Immun Health. 2022; 21:100442. [DOI:10.1016/j.bbih.2022.100442] [PMID] [PMCID]
- Wang J, Yang QX, Sun X, Vesek J, Mosher Z, Vasavada M, et al. MRI evaluation of asymmetry of nigrostriatal damage in the early stage of early-onset Parkinson’s disease. Parkinsonism Relat Disord. 2015; 21(6):590-6. [DOI:10.1016/j.parkreldis.2015.03.012] [PMID]
- Lian TH, Guo P, Zuo LJ, Hu Y, Yu SY, Yu QJ, et al. Tremor-dominant in Parkinson disease: The relevance to iron metabolism and inflammation. Front Neurosci. 2019; 13:255. [DOI:10.3389/fnins.2019.00255] [PMID] [PMCID]
- Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain. 2012; 135(11):3206-26. [DOI:10.1093/brain/aws023] [PMID] [PMCID]
- Fishman PS. Paradoxical aspects of parkinsonian tremor. Mov Disord. 2008; 23(2):168-73. [DOI:10.1002/mds.21736] [PMID]
- Long-Smith CM, Collins L, Toulouse A, Sullivan AM, Nolan YM. Interleukin-1β contributes to dopaminergic neuronal death induced by lipopolysaccharide-stimulated rat glia in vitro. J Neuroimmunol. 2010; 226(1-2):20-6. [DOI:10.1016/j.jneuroim.2010.05.030] [PMID]
- Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson’s disease. Ann N Y Acad Sci. 2003; 991:214-28. [DOI:10.1111/j.1749-6632.2003.tb07478.x] [PMID]
- Kang X, Ploner A, Pedersen NL, Bandres-Ciga S, Noyce AJ, Wirdefeldt K, et al. Tumor necrosis factor inhibition and Parkinson Disease: A Mendelian Randomization Study. Neurology. 2021; 96(12):e1672-9. [DOI:10.1212/WNL.0000000000011630]
- Qiu X, Xiao Y, Wu J, Gan L, Huang Y, Wang J. C-Reactive protein and risk of Parkinson’s Disease: a systematic review and meta-analysis. Front Neurol. 2019; 10:384. [DOI:10.3389/fneur.2019.00384] [PMID] [PMCID]
- Santos-García D, de Deus Fonticoba T, Suárez Castro E, Aneiros Díaz A, Paz González JM, Feal Panceiras MJ, et al. High ultrasensitive serum C-reactive protein may be related to freezing of gait in Parkinson’s disease patients. J Neural Transm (Vienna). 2019; 126(12):1599-608. [DOI:10.1007/s00702-019-02096-8] [PMID]
- Green HF, Khosousi S, Svenningsson P. Plasma IL-6 and IL-17A correlate with severity of motor and non-motor symptoms in Parkinson’s Disease. J Parkinsons Dis. 2019; 9(4):705-9. [DOI:10.3233/JPD-191699] [PMID] [PMCID]
- Hofmann KW, Schuh AF, Saute J, Townsend R, Fricke D, Leke R, et al. Interleukin-6 serum levels in patients with Parkinson’s Disease. Neurochem Res. 2009; 34(8):1401-4. [DOI:10.1007/s11064-009-9921-z] [PMID]
- Chen X, Hu Y, Cao Z, Liu Q, Cheng Y. Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s Disease, Parkinson’s Disease and amyotrophic lateral sclerosis: A systematic review and meta-analysis. Front Immunol. 2018; 9:2122. [DOI:10.3389/fimmu.2018.02122] [PMID] [PMCID]
- Shin HJ, Jeong EA, Lee JY, An HS, Jang HM, Ahn YJ, et al. Lipocalin-2 deficiency reduces oxidative stress and neuroinflammation and results in attenuation of kainic acid-induced hippocampal cell death. Antioxidants. 2021; 10(1):100. [DOI:10.3390/antiox10010100] [PMID] [PMCID]
- Xiong M, Qian Q, Liang X, Wei YD. Serum levels of lipocalin-2 in patients with Parkinson’s disease. Neurol Sci. 2022; 43(3):1755-9. [DOI:10.1007/s10072-021-05579-3] [PMID]
- Nagatsu T, Nakashima A, Ichinose H, Kobayashi K. Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. J Neural Transm (Vienna). 2019; 126(4):397-409. [DOI:10.1007/s00702-018-1903-3] [PMID]
- Alam G, Richardson JR. Chapter 4 - Regulation of tyrosine hydroxylase: Relevance to Parkinson’s disease. In: Martin CR, Preedy VR, editors. Genetics, Neurology, Behavior, and Diet in Parkinson’s Disease. Massachusetts: Academic Press; 2020. [Link]
- Haavik J, Toska K. Tyrosine hydroxylase and Parkinson’s disease. Mol Neurobiol. 1998; 16(3):285-309. [DOI:10.1007/BF02741387] [PMID]
- Li Y, Niu M, Zhao A, Kang W, Chen Z, Luo N, et al. CXCL12 is involved in α-synuclein-triggered neuroinflammation of Parkinson’s disease. J Neuroinflammation. 2019; 16(1):263. [DOI:10.1186/s12974-019-1646-6] [PMID]
- Toleikis Z, Ziaunys M, Baranauskiene L, Petrauskas V, Jaudzems K, Smirnovas V. S100A9 Alters the Pathway of Alpha-synuclein amyloid aggregation. Int J Mol Sci. 2021; 22(15):7972. [DOI:10.3390/ijms22157972] [PMID] [PMCID]
- Anwar S, Pons V, Rivest S. Microglia Purinoceptor P2Y6: An emerging therapeutic target in CNS Diseases. Cells. 2020; 9(7):1595. [DOI:10.3390/cells9071595] [PMID] [PMCID]
- Li H, Yu L, Li M, Chen X, Tian Q, Jiang Y, et al. MicroRNA-150 serves as a diagnostic biomarker and is involved in the inflammatory pathogenesis of Parkinson’s disease. Mol Genet Genomic Med. 2020; 8(4):e1189. [DOI:10.1002/mgg3.1189] [PMID] [PMCID]
- Yang Z, Li T, Cui Y, Li S, Cheng C, Shen B, et al. Elevated Plasma microRNA-105-5p level in patients with idiopathic Parkinson’s Disease: A potential disease biomarker. Front Neurosci. 2019; 13:218. [DOI:10.3389/fnins.2019.00218] [PMID] [PMCID]
- Caggiu E, Paulus K, Mameli G, Arru G, Sechi GP, Sechi LA. Differential expression of miRNA 155 and miRNA 146a in Parkinson’s disease patients. eNeurologicalSci. 2018; 13:1-4. [DOI:10.1016/j.ensci.2018.09.002] [PMID] [PMCID]
- Goh SY, Chao YX, Dheen ST, Tan EK, Tay SS. Role of MicroRNAs in Parkinson’s Disease. Int J Mol Sci. 2019; 20(22):5649. [DOI:10.3390/ijms20225649] [PMID] [PMCID]
- Kole AJ, Swahari V, Hammond SM, Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011; 25(2):125-30. [DOI:10.1101/gad.1975411] [PMID] [PMCID]
- Roshan R, Shridhar S, Sarangdhar MA, Banik A, Chawla M, Garg M, et al. Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in mice. RNA. 2014; 20(8):1287-97. [DOI:10.1261/rna.044008.113] [PMID] [PMCID]
- Cao L, Zhang Y, Zhang S, Jiang TP, Chen L, Liu J, et al. MicroRNA-29b alleviates oxygen and glucose deprivation/reperfusion-induced injury via inhibition of the p53dependent apoptosis pathway in N2a neuroblastoma cells. Exp Ther Med. 2018; 15(1):67-74. [PMID]
- Bai Y, Su X, Piao L, Jin Z, Jin R. Involvement of Astrocytes and microRNA dysregulation in neurodegenerative diseases: From pathogenesis to therapeutic potential. Front Mol Neurosci. 2021; 14:556215. [DOI:10.3389/fnmol.2021.556215] [PMID] [PMCID]
- Liu J, Li G, He Y, He G, Zhang P, Shen X, et al. The association analysis of GPNMB rs156429 with clinical manifestations in Chinese population with Parkinson’s Disease. Front Genet. 2020; 11:952. [DOI:10.3389/fgene.2020.00952] [PMID] [PMCID]
- Umeh CC, Mahajan A, Mihailovic A, Pontone GM. APOE4 allele, sex, and dementia Risk in Parkinson’s Disease: Lessons from a longitudinal cohort. J Geriatr Psychiatry Neurol. 2022; 35(6):810-5. [DOI:10.1177/08919887211060019] [PMID] [PMCID]
- Wong HY, Prasad A, Gan SU, Chua JJE, Schwarz H. Identification of CD137-Expressing B Cells in multiple sclerosis which secrete IL-6 upon engagement by CD137 ligand. Front Immunol. 2020; 11:571964. [DOI:10.3389/fimmu.2020.571964] [PMID] [PMCID]
- Güler S, Gül T, Güler Ş, Haerle MC, Başak AN. Early-onset Parkinson’s Disease: A novel deletion comprising the DJ-1 and TNFRSF9 Genes. Mov Disord. 2021; 36(12):2973-6. [DOI:10.1002/mds.28812] [PMID]
- Cho HJ, Liu G, Jin SM, Parisiadou L, Xie C, Yu J, et al. MicroRNA-205 regulates the expression of Parkinson’s disease-related leucine-rich repeat kinase 2 protein. Hum Mol Genet. 2013; 22(3):608-20. [DOI:10.1093/hmg/dds470] [PMID] [PMCID]
- Chan L, Chung CC, Yu RC, Hong CT. Cytokine profiles of plasma extracellular vesicles as progression biomarkers in Parkinson’s disease. Aging (Albany NY). 2023; 15(5):1603-14. [DOI:10.18632/aging.204575] [PMID] [PMCID]
- Lerche S, Zimmermann M, Wurster I, Roeben B, Fries FL, Deuschle C, et al. CSF and serum levels of inflammatory markers in PD: Sparse correlation, sex differences and association with neurodegenerative biomarkers. Front Neurol. 2022; 13:834580. [DOI:10.3389/fneur.2022.834580] [PMID] [PMCID]
- Lin X, Wang R, Li R, Tao T, Zhang D, Qi Y. Diagnostic performance of miR-485-3p in Patients with Parkinson’s Disease and its relationship with neuroinflammation. Neuromolecular Med. 2022; 24(2):195-201. [DOI:10.1007/s12017-021-08676-w] [PMID]
- Wilson H, Dervenoulas G, Pagano G, Tyacke RJ, Polychronis S, Myers J, et al. Imidazoline 2 binding sites reflecting astroglia pathology in Parkinson’s disease: An in vivo11C-BU99008 PET study. Brain. 2019; 142(10):3116-28. [DOI:10.1093/brain/awz260] [PMID]
- Wood PL, Tippireddy S, Feriante J, Woltjer RL. Augmented frontal cortex diacylglycerol levels in Parkinson’s disease and Lewy Body Disease. Plos One. 2018; 13(3):e0191815. [DOI:10.1371/journal.pone.0191815] [PMID] [PMCID]
- Yang L, Guo C, Zhu J, Feng Y, Chen W, Feng Z, et al. Increased levels of pro-inflammatory and anti-inflammatory cellular responses in Parkinson’s Disease Patients: Search for a disease indicator. Med Sci Monit. 2017; 23:2972-8. [DOI:10.12659/MSM.904240] [PMID] [PMCID]
- Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, et al. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol Neurodegener. 2016; 11:28. [DOI:10.1186/s13024-016-0094-3] [PMID] [PMCID]
- Mihara T, Nakashima M, Kuroiwa A, Akitake Y, Ono K, Hosokawa M, et al. Natural killer cells of Parkinson’s disease patients are set up for activation: a possible role for innate immunity in the pathogenesis of this disease. Parkinsonism Relat Disord. 2008; 14(1):46-51. [DOI:10.1016/j.parkreldis.2007.05.013] [PMID]
Type of Study: Review |
Subject:
Special
Received: 2024/04/23 | Accepted: 2024/04/30 | Published: 2024/07/17
Received: 2024/04/23 | Accepted: 2024/04/30 | Published: 2024/07/17
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



