Tue, Dec 30, 2025
Volume 10, Issue 3 (Summer 2024)
Caspian J Neurol Sci 2024, 10(3): 225-234 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Saeedi N, Hatamian H, Erfanian M, Shafiei F, Homam S M. Mental Health and Seizure Patterns in Epileptic Patients During COVID-19 Pandemic: A Case Study in Northeast Iran. Caspian J Neurol Sci 2024; 10 (3) :225-234
URL: http://cjns.gums.ac.ir/article-1-699-en.html
URL: http://cjns.gums.ac.ir/article-1-699-en.html
1- Faculty of Medicine, Student Research Committee, Mashhad Medical Sciences Branch, Islamic Azad University, Mashhad, Iran.
2- Department of Neurology, Faculty of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
3- British Columbia Institute of Technology, Burnaby, Canada.
4- Neuroscience Research Center, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
5- Department of Neurology, Faculty of Medicine, Mashhad Medical Sciences Branch, Islamic Azad University, Mashhad, Iran. ,mehrhomam@mshdiau.ac.ir
2- Department of Neurology, Faculty of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
3- British Columbia Institute of Technology, Burnaby, Canada.
4- Neuroscience Research Center, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
5- Department of Neurology, Faculty of Medicine, Mashhad Medical Sciences Branch, Islamic Azad University, Mashhad, Iran. ,
Full-Text [PDF 1408 kb]
(578 Downloads)
| Abstract (HTML) (1204 Views)
Full-Text: (472 Views)
Introduction
The novel coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2. The disease was accompanied by unprecedented changes worldwide, leading to significant consequences in physical and psychopathological health [1]. Following its rapid spread, it became a pandemic and severely affected all countries’ health systems [2]. Therefore, the governments have implemented several preventive strategies, including quarantines, travel bans, and lockdowns, to decrease the spread of the virus. Although inevitable, the strict isolation is accompanied by the feeling of loneliness, which is followed by the onset or deterioration of depression, anxiety, and sleep disorders [3]. In this situation, people with chronic diseases such as epilepsy receive inadequate level of medical care. It is reported that patients with epilepsy (PWE) are more prone to mental illnesses, and approximately 25% of them have psychopathological comorbidities [4, 5]. Although public health incidents can bring vast amounts of emotional problems to the general population, PWE experience more severe physical and mental consequences. For example, during the outbreak of SARS in 2003, PWE were banned from referring patients to hospitals for their epilepsy medications because of safety concerns. This restriction raised the number and severity of seizures [6] and also caused psychopathological problems such as anxiety and depression in these patients [7]. Stress has an essential role in the recurrence of epileptic seizures. Also, it is a commonly reported cause of seizure recurrence [8]. Limited access to health care centers and changes in lifestyle regarding remote work can potentially develop stress in PWE. In addition, the COVID-19 outbreak led to extended delays in outpatient visits and cancellation of epilepsy paraclinical tests, such as electroencephalogram test and magnetic resonance imaging, and even problems in accessing emergency [9]. Not only can the social and behavioral influences of COVID-19 lockdown increase seizure frequency in PWE, but COVID-19 infection also causes a febrile status, reducing the seizure threshold [10]. The lockdown of pharmaceutical industries hampers antiepileptic drug supply. Meanwhile, the care services were limited to emergencies, and PWE could not visit their neurologists regularly. Also, the SARS virus family has neurotropic potencies explaining the neurological manifestations of COVID-19, such as headache, seizure, Guillan-Bare syndrome, paresthesia, changes in consciousness level and so on [11, 12].
In light of limited research conducted in Iran, especially in the northeastern region, we undertook this study to assess the levels of psychopathological distress, depression, and sleep quality among PWE. Additionally, our investigation examined the variations in seizure frequency before and after COVID-19 infection among affected patients. Furthermore, a comprehensive analysis was conducted to elucidate the potential risk factors associated with the development of depression, psychopathological distress, sleep disturbances, and exacerbations of seizures in this population.
Materials and Methods
This study was a single-center, case-control study. The survey was conducted during the post-COVID-19 period from January 28 to March 7, 2023. The inclusion criteria of the epilepsy group were as follows: Having the diagnosis of epilepsy for at least one year based on a neurologist’s diagnosis and being 18 years or older. The exclusion criteria were as follows: Developing hemorrhage, infarction, tumor, and infection in the central nervous system; having a history of substance abuse, psychosis, or any psychiatric diseases; and having a change in the type or dosage of the AEDs. Before patient enrollment, a comprehensive review of electronic health records (EHRs) was conducted to assess eligibility criteria. Each patient’s EHR contains essential information, including medical history, physical examination findings, imaging results, and medication details. During each visit, physicians document all physical examination findings and any alterations to the medication regimen. Subsequently, eligible patients underwent a structured interview conducted by a neurologist to evaluate their psychiatric and mental status.
Additionally, in this interview, the patients were asked if they have experienced any changes in the number, type, or duration of their seizures experienced during the COVID-19 pandemic. A total of 12 patients (13.6%) were excluded from the study. For the patients with no remarkable psychiatric disease and normal mental state, the study’s objective and process were clearly explained, and the patients who were willing to participate in the study signed the informed consent. Eight patients (9%) were excluded because of changes in the type or dosage of their drugs within the previous month, and 4 patients (4%) because they refused to participate. After that, the patients completely understood the objective and process of the study and signed the consent form, three printed questionnaires, including Beck depression inventory (BDI), 6-item Kessler (K-6), and Pittsburgh sleep quality index (PSQI) questionnaires, were given to them to complete. While answering the questions, if a patient was illiterate, researchers facilitated the process by reading the questions aloud verbally and providing clear explanations in a simplified manner. Moreover, if patients encountered difficulty understanding any aspect of the questionnaires, researchers provided additional explanations until the questions were entirely understandable.
Also, healthy individuals with the same age and gender distribution of patients were enrolled as the normal group. The normal group was recruited from the patients’ companions without any remarkable past medical history, who were referred to the 22 Bahman Hospital, Mashhad, Khorasan Razavi. The inclusion criteria for the normal group were age 18 or older and signing the consent form. The exclusion criteria of the normal group were as follows: Having a history of seizures or any neurologic disease and having depressive or anxiety disorders. The normal group was completely matched to the patients in terms of age and gender. The sample size was calculated according to previous similar studies [13] and by utilizing the Equation 1 [14]:

Their psychiatric and mental status were assessed through standard psychological questionnaires and also the epilepsy-related information was checked. The study’s goal and process were clearly explained to the patients. The patients willing to participate in the study signed the informed consent form.
A questionnaire was designed and customized for this study to collect demographic information, such as age, gender, education level and marital status. Also, epilepsy information was asked from the PWE as follows: Type of epilepsy (according to the classification approved by the International League Against Epilepsy (ILAE) [15], duration of disease, the number of antiepileptic drugs (AEDs), drug resistance, and the number of seizures before and after COVID-19 infection.
BDI is a 21-item self-report measure of typical depressive symptoms [16]. Each item possesses four possible responses; higher total scores indicate a greater number and severity of depressive symptoms. Scores ranging from 0 to 13 indicate no symptoms, 14–19 marks mild depression, 20–28 denotes moderate depression, and 29–63 points to severe depression. The validity and reliability of the Persian version of this questionnaire have been confirmed by a study as 0.87 and 0.74, respectively [17].
PSQI was first developed in 1988 by Buysse et al. [18]. This questionnaire is a self-report instrument used to assess the quality and patterns of sleep in adults over the last month. This questionnaire evaluates seven aspects: Subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. A total score of “5” or higher indicates poor sleep. The validity and reliability of the Persian version of the PSQI questionnaire were reported as 0.94 and 0.72, respectively [19].
The K-6 psychopathological distress scale is a short screening tool developed to identify persons with a high probability of having diagnosable psychopathological distress, which is severe enough to induce moderate to serious impairment in functioning. Total scores range from 0 to 30, with higher scores indicating more severe psychopathological distress. Dadfar and colleagues reported the sensitivity and specificity of the Persian version of this questionnaire as 0.93 and 0.91, respectively [20].
Statistical analysis was conducted by using SPSS software, version 20. We presented categorical variables as numbers and percentages and continuous variables as Mean±SD. Intergroup differences were compared using the independent t-test or Mann-Whitney U test. Multivariate logistic regression was employed to investigate factors independently associated with severe mental health problems among PWE. The multivariate model included variables that exhibited a significant association (P<0.05) with the outcome in univariate analysis.
Results
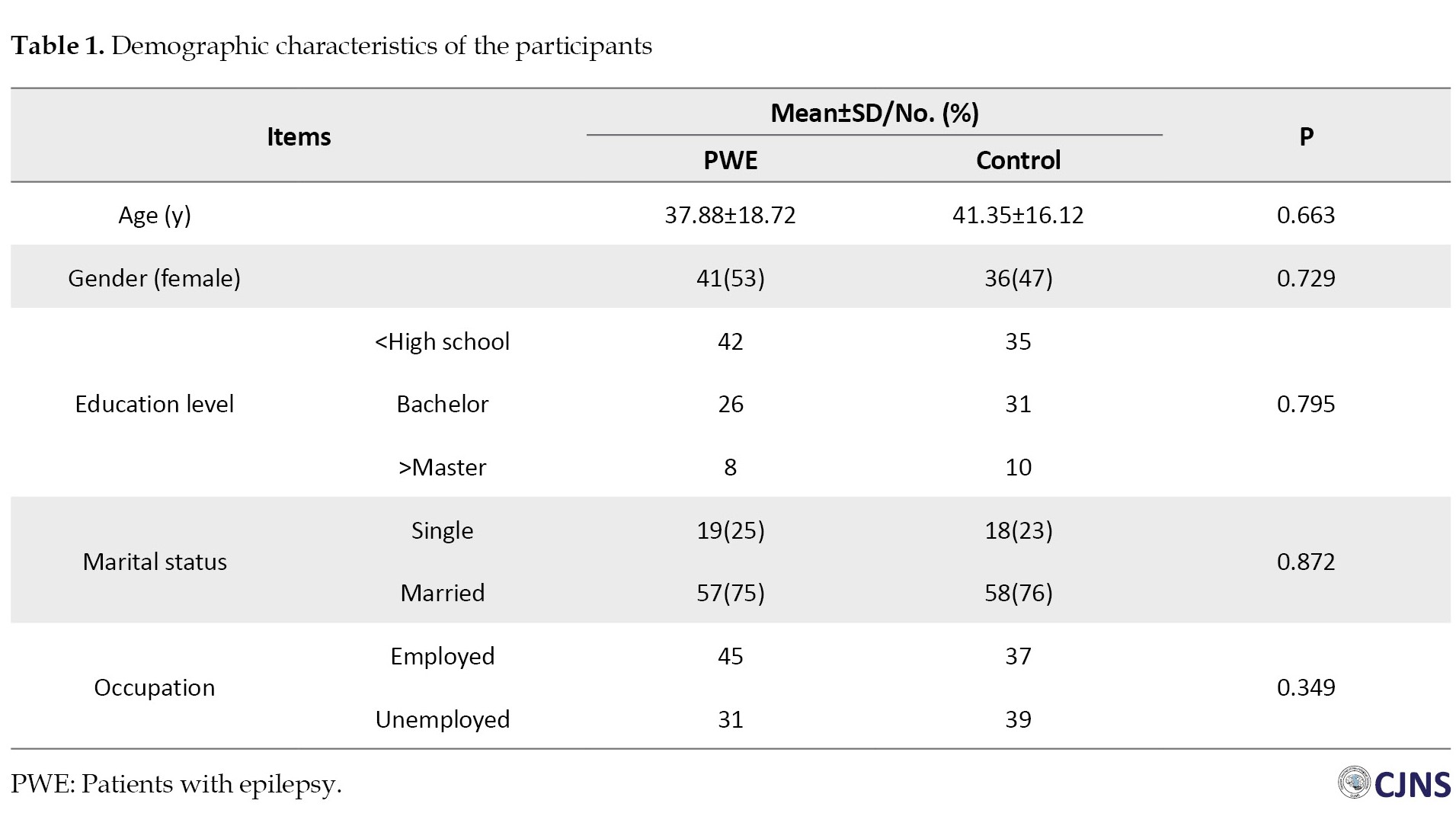
A total of 88 PWE were eligible to participate in our study. Of them, 8 patients (9%) were excluded because of changes in the type or dosage of their drugs within the previous month, and 4 patients (4%) because they refused to participate. Also, 76 healthy individuals were enrolled as control participants. These individuals were matched regarding their age and gender with the patients (Table 1).
Demographic data
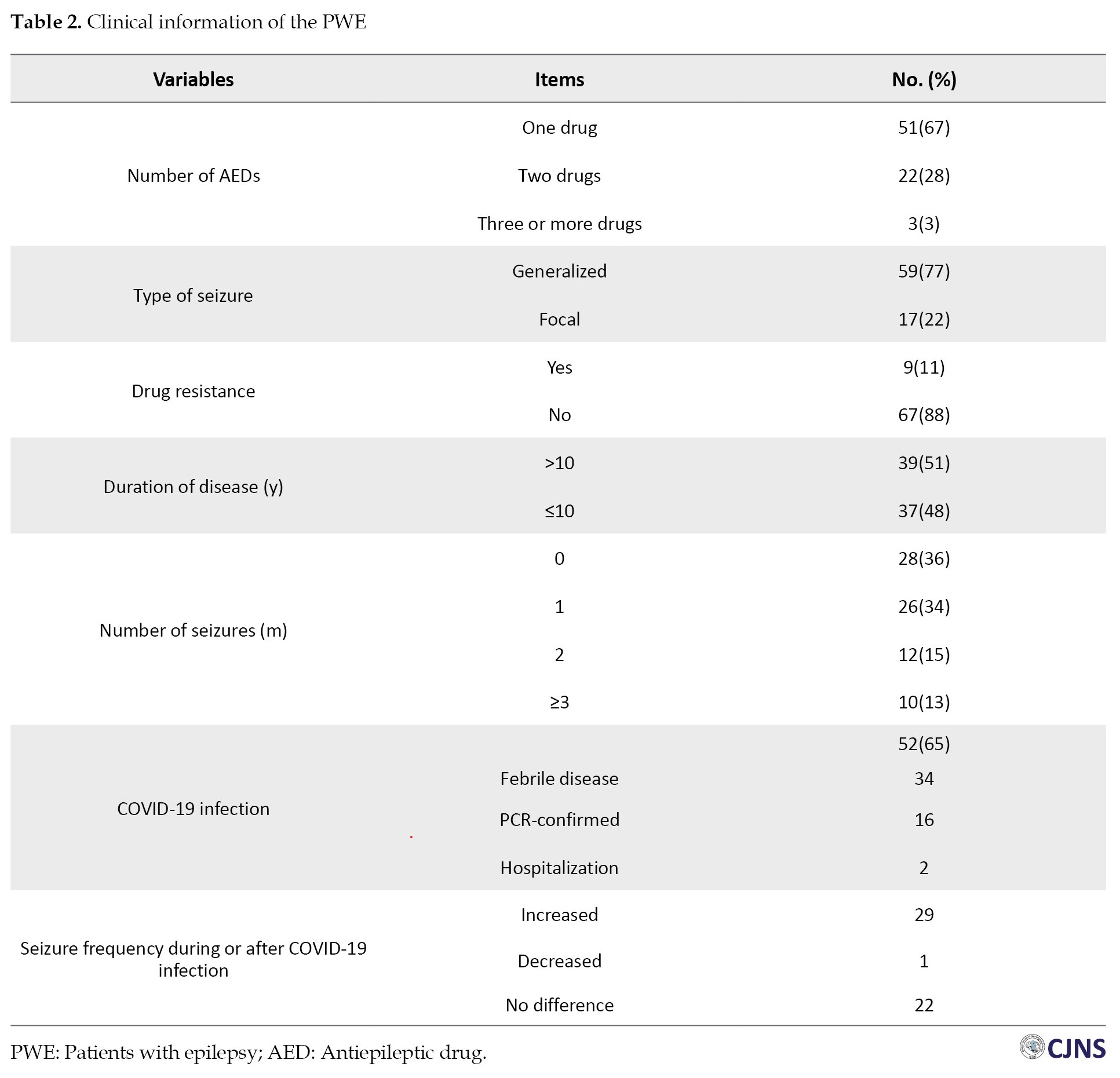
Ultimately, 76 PWE were included, and the same number of normal individuals were enrolled as the control group. The mean age of the patients was 37.88±18.72 years. Females constituted 50.6% of the participants. No significant difference regarding age and gender was found between the groups (P>0.05). Three-fourths of the patients were married. Forty-five patients were employed. Most of the patients were using one antiepileptic drug, had generalized seizures and lacked drug resistance. Thirty-nine patients were suffering from epilepsy for more than 10 years. Although 28 patients did not have any seizures, 26 had one, 12 had two, and 10 patients had more than three seizures per month. Fifty-two patients reported infection by COVID-19, but PCR confirmed the definitive diagnosis of COVID-19 in only 16 patients. Moreover, 2 patients had severe COVID-19 infection, which led to their hospitalization. Our results demonstrated that 29 PWE (38%) experienced exacerbated epileptic seizures, while 22(28%) did not report any changes in the number of their seizures. Only one of the patients stated decreased frequency of their seizures after COVID-19 infection. Table 2 demonstrates the detailed clinical information of the patients.
Depression
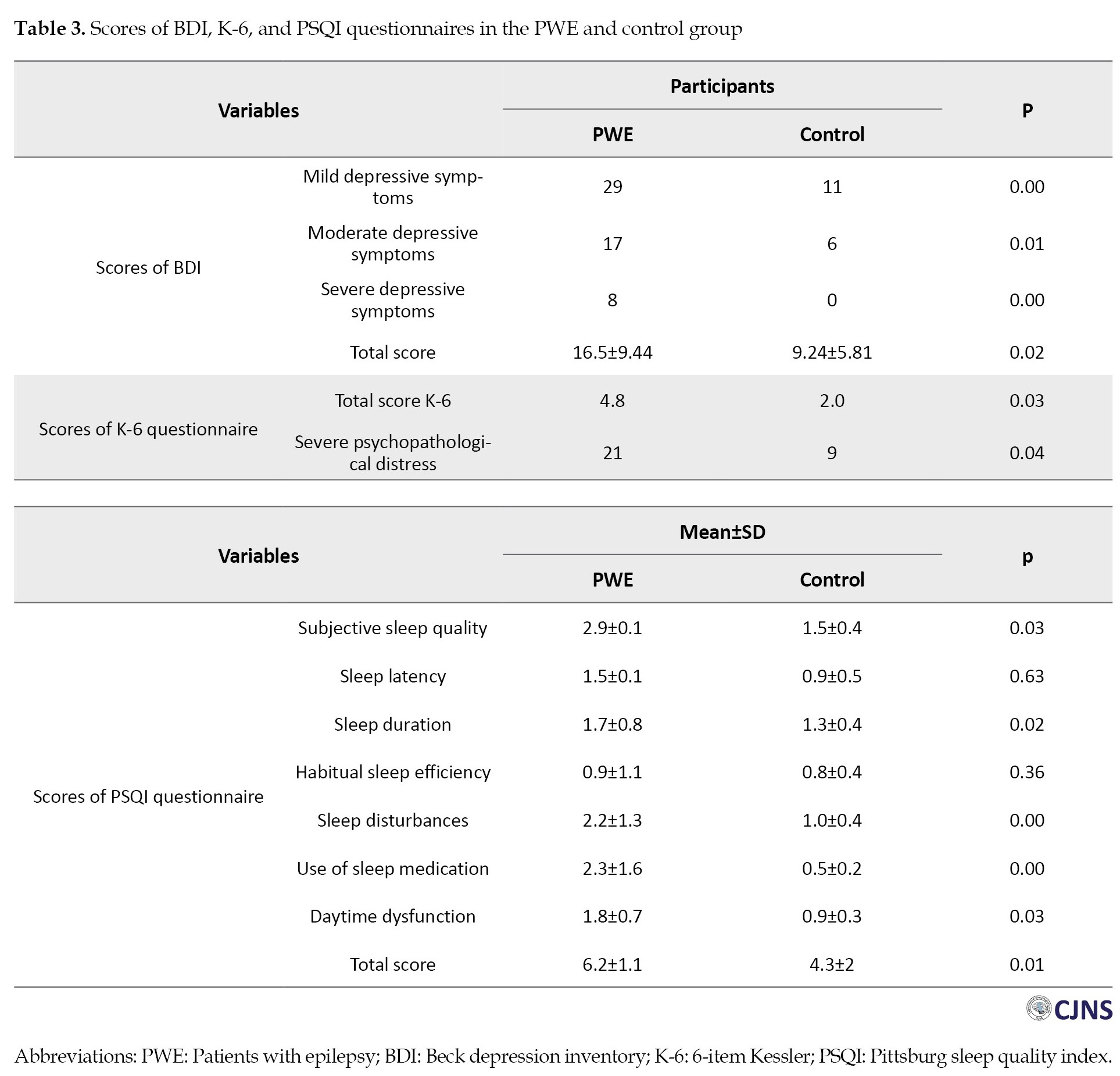
PWE scored higher in the BDI questionnaire (16.5±9.44 vs 9.24±5.81, P=0.02). Among PWE, 22 patients had normal BDI scores, 29 had mild, 17 had moderate, and 8 had severe depressive symptoms. Though, in the control group, 59 participants had normal values of BDI, 11 had mild symptoms of depression, and 6 had moderate symptoms. None of the participants in the control group showed severe symptoms of depression (Table 3).
Psychopathological distress
Our findings revealed that PWE exhibited significantly elevated K-6 scores compared to the normal individual group (P=0.03). K-6 score ≥12 was considered severe psychopathological distress, and we observed that the proportion of patients with K-6 score >12 was notably greater in PWE (21 vs 9, P=0.04) (Table 3).
Sleep
The PSQI questionnaire scores represented a significantly worse sleep quality in PWE than the normal individual group (6.2±1.1 vs 4.3±2, P=0.01). Different subscales of the PSQI questionnaire are also presented in Table 3. The patients had remarkably poor subjective sleep quality, longer sleep duration, and more sleep disturbances. Moreover, daytime dysfunction was more prevalent in patients than in the normal individual group (Table 3).
Table 4 shows correlations between depression (BDI scores), psychopathological distress (K-6 scores), and sleep quality (PSQI scores) with disease duration in PWE.
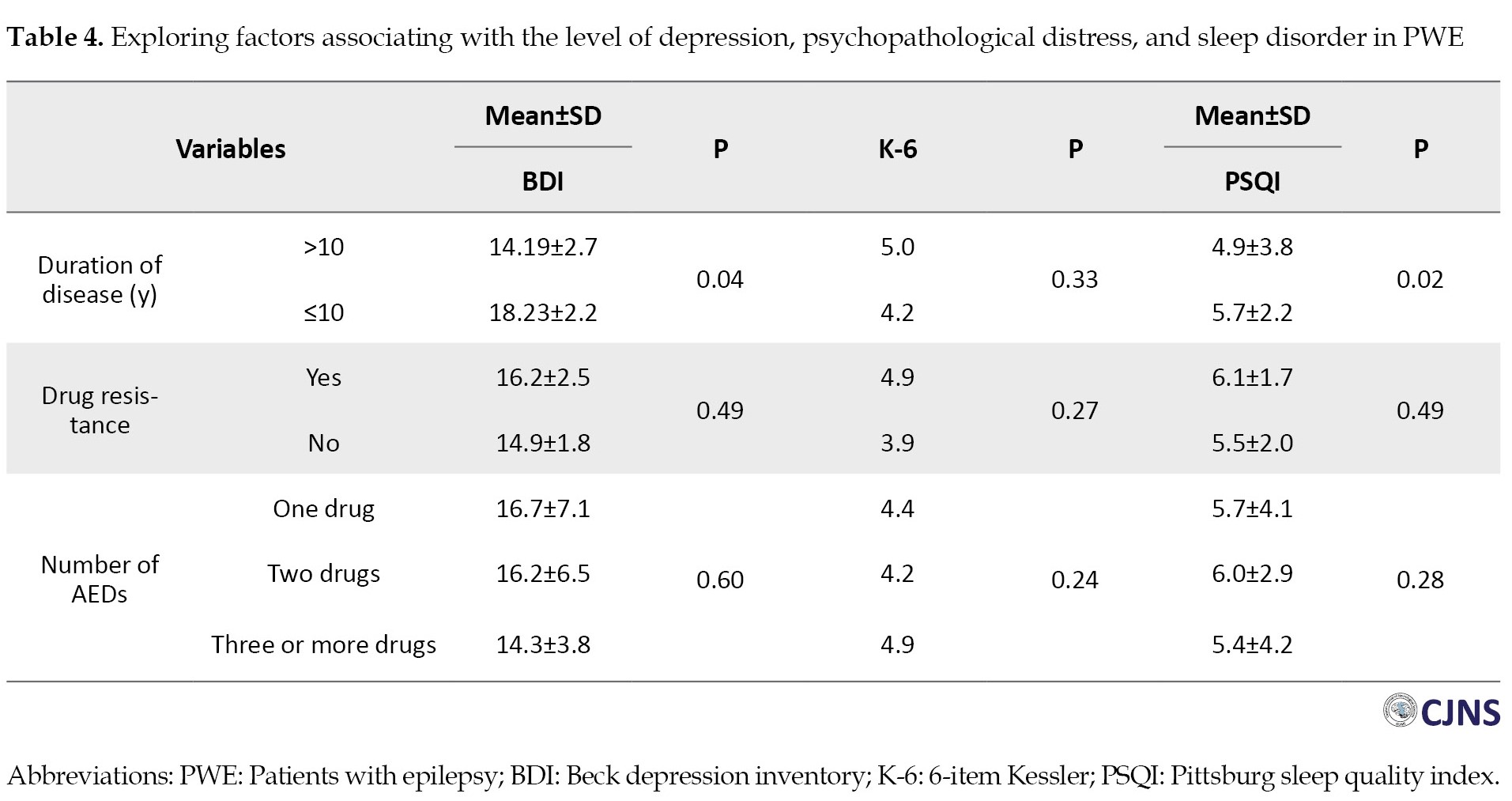
We found that both depressive symptoms (P=0.04) and poor sleep quality (P=0.02) were significantly associated with longer disease duration. However, correlations do not necessarily imply that one variable directly causes another. To understand if disease duration independently predicts depression and sleep problems, we performed a multivariate logistic regression analysis. This analysis reveals that even after accounting for other factors, disease duration remains a significant predictor for both depression (OR=1.128; 95% CI, 1.110%, 1.729%; P=0.002) and sleep disturbances (OR=1.860; 95% CI, 1.363%, 1.922%; P=0.01). In other words, for every unit increase in disease duration, the odds of experiencing depression increase by 12.8% and the odds of having sleep problems increase by 86.0% while controlling for other variables.
Discussion
In the present study, we explored the mental health status of PWE in northeast Iran during the COVID-19 pandemic. We compared our results with the mental health status of healthy individuals from the same region in addition to evaluating risk factors for poor mental status. Our findings revealed that PWE had significantly higher levels of psychopathological distress, sleep disturbances, and depression than the normal group, which was closely related to the duration of the disease.
It is reported that the COVID-19 pandemic resulted in increased mental health problems due to high numbers of mortality, high levels of infection, and quick distribution. Moreover, stress is more frequent during the pandemic [21]. Besides this, recent studies on the general population have shown that following the pandemic, anxiety and depression increase, which is accompanied by sleep disorders. Accordingly, PWEs are at considerable risk to the psychosocial influence of the pandemic [22]. These data on the frequency of depression in COVID-19 patients can be compared to that of the pre-pandemic prevalence of depressive disorders in hospital inpatients and outpatients, which are estimated to be between 5% to 34% [23] and 27.0% [24], respectively. In this study, we found the prevalence of depression for COVID-19 outpatients to be 71%, which is a substantial increase compared with pre-pandemic depression levels. Furthermore, these estimated prevalence rates of depression and anxiety display a remarkable increase compared to the general population during the COVID-19 pandemic, which are estimated to be 33.7% and 31.9%, respectively [25, 26]. The discrepancy between our results and those of other studies can be explained by the various screening tools, which significantly impact the resultant frequency. However, it should be mentioned that substantial variations in the sample size of studies may contribute to this issue.
On the other side, it seems that restrictions during the COVID-19 pandemic negatively correlate with sleep quality in the general population because of the increasing rate of psychological disorders, decreasing exercise activity, social isolation, and inadequate access to sunlight [27]. In this regard, results showed that 7.1%–71.2% of PWE had sleep problems, and the prevalence of insomnia was also 28.2% among these patients [9, 28]. However, one study reported no significant difference in the sleep quality between PWE and healthy persons [29]. Sleep disturbance in PWE is a vital activator that can increase motor excitability, which develops the frequency of seizures in these patients [30]. Generally, disturbed sleep-wake cycles during the COVID-19 restriction can influence PWE and healthy persons. However, sleep problems may increase EEG epileptiform abnormalities that result in a worsening of seizures in PWE [31]. Previous data show that sleep disturbances have been reported in 20.1% of the PWE during the COVID-19 pandemic, significantly higher than in the pre-pandemic period [26]. Our results showed that PWE have significantly higher sleep problems than the general population. We also noticed that sleep problems were significantly associated with longer disease duration.
Various factors, including social separation and loneliness, affect the high prevalence of psychopathological distress in PWE during the COVID-19 pandemic [32]. The development of social restrictions to control the pandemic leads to a number of behavioral and social consequences that may also trigger epileptic seizures and decrease the threshold level of seizures in PWE [33]. Also, stress has been shown to exacerbate epilepsy. There is a significant correlation between stress and the number of epileptic seizures among PWE [34]. Results of studies focusing on the impact of the COVID-19 pandemic on stress levels among PWE represented a higher stress and anxiety level among PWE. In addition, the studies reported that PWE experienced worse mental health conditions during the COVID-19 restrictions [13]. The elevated stress associated with the pandemic has a direct correlation with the worsening of seizures in some PWE [29]. Our data support notable psychopathological distress among PWE compared to the normal individual group. Also, the number of people with severe psychopathological distress was remarkably higher than the healthy individuals. We did not find any significant relationship between drug resistance and psychopathological distress, which is in line with the results of 3 meta-analyses [5, 35, 36]. In contrast, the study by Hao et al. found a significant association [13].
Also, it is estimated that one-third of PWE are affected by mental issues. Furthermore, psychopathological distress frequently happens in their daily life [37]. Therefore, it is expected that in stressful conditions like COVID-19 restrictions, the occurrence of depression and anxiety increases. Among psychopathological distress, anxiety, and depression are the most frequent epilepsy-relate disorders which is observed among PWE during the COVID-19 pandemic. Results from studies on COVID-19 in Italy demonstrated that PWE (19%) and the general population (17%) reported psychiatric disorders, including depression. However, PWE had more severe depressive symptoms than the control group [33]. In addition, Van Hees et al. found remarkable gender differences among PWE, and it was reported that women are at increased risk of more depressive problems during the COVID-19 pandemic [38]. Excessive exposure to stressor factors not only increases seizures in PWE but also triggers seizure development even in people without any history of epilepsy [39]. As regards, the study by Huang et al. documented that depression among PWE and the control group increased the number of seizures (22.58% vs 12.08%, respectively) [29]. In line with this, several studies performed during the COVID-19 pandemic indicated gender and age differences in relation to the prevalence of stress and anxiety symptoms among PWE. In this respect, female and older subjects had higher anxiety levels [40]. Other relevant studies also showed that both PWE and the general population experienced anxiety symptoms during the pandemic, though PWEs experienced a significantly higher prevalence of anxiety [40].
Although our study provided significant evidence of high levels of psychopathological distress, depression, and sleep disorders among PWE, it still has several limitations. First, we utilized online questionnaires, which possessed several biases and had low scientific evidence strength. Second, most of our responders were young adults who use the Internet the most, so our sample could not properly represent the general population. The lower age of patients could bias the number of reported seizures, the rate of drug resistance, and antiepileptic medications. Third, we only measured the depression and anxiety of patients during the pandemic, and our study lacked a pre-pandemic baseline of their mental condition.
Conclusion
In conclusion, the significant disparities in psychopathological distress, depression, and sleep disorders between PWE and healthy individuals highlight the vulnerability of PWE to mental health challenges during crises. Importantly, our results indicate that longer disease duration is associated with increased susceptibility to depression and sleep disturbances among PWE, emphasizing the need for targeted interventions to address their mental health needs, especially during public health emergencies like the COVID-19 pandemic. Moreover, these findings underscore the importance of integrating mental health support into the comprehensive care of PWE, with tailored interventions aimed at enhancing psychopathological well-being and sleep quality. By acknowledging and addressing the unique mental health challenges faced by epilepsy patients, healthcare providers can effectively promote quality of life in this vulnerable population. Further future research should continue to explore the dynamic interplay between epilepsy, mental health, and external stressors.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in compliance with the ethical guidelines of the 2013 Declaration of Helsinki. This study was approved by the Ethics Committee of Islamic Azad University, Mashhad Medical Sciences Branch (Code: IR.IAU.MSHD.REC.1401.163).
Funding
This article was extracted from the Doctor of Medicine thesis of Nikoo Saeedi, approved by Islamic Azad University, Mashhad Medical Sciences Branch (Code: 162467234).
Authors contributions
Conceptualization: Hamidreza Hatamian and Mehrdad Erfanian; Methodology: Fatemeh Shafiei; Investigation and writing the original draft: Nikoo Saeedi; Review, editing and supervision: Seyed Mehran Homam.
Conflict of interest
The authors declared no conflict of interest.
References
The novel coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2. The disease was accompanied by unprecedented changes worldwide, leading to significant consequences in physical and psychopathological health [1]. Following its rapid spread, it became a pandemic and severely affected all countries’ health systems [2]. Therefore, the governments have implemented several preventive strategies, including quarantines, travel bans, and lockdowns, to decrease the spread of the virus. Although inevitable, the strict isolation is accompanied by the feeling of loneliness, which is followed by the onset or deterioration of depression, anxiety, and sleep disorders [3]. In this situation, people with chronic diseases such as epilepsy receive inadequate level of medical care. It is reported that patients with epilepsy (PWE) are more prone to mental illnesses, and approximately 25% of them have psychopathological comorbidities [4, 5]. Although public health incidents can bring vast amounts of emotional problems to the general population, PWE experience more severe physical and mental consequences. For example, during the outbreak of SARS in 2003, PWE were banned from referring patients to hospitals for their epilepsy medications because of safety concerns. This restriction raised the number and severity of seizures [6] and also caused psychopathological problems such as anxiety and depression in these patients [7]. Stress has an essential role in the recurrence of epileptic seizures. Also, it is a commonly reported cause of seizure recurrence [8]. Limited access to health care centers and changes in lifestyle regarding remote work can potentially develop stress in PWE. In addition, the COVID-19 outbreak led to extended delays in outpatient visits and cancellation of epilepsy paraclinical tests, such as electroencephalogram test and magnetic resonance imaging, and even problems in accessing emergency [9]. Not only can the social and behavioral influences of COVID-19 lockdown increase seizure frequency in PWE, but COVID-19 infection also causes a febrile status, reducing the seizure threshold [10]. The lockdown of pharmaceutical industries hampers antiepileptic drug supply. Meanwhile, the care services were limited to emergencies, and PWE could not visit their neurologists regularly. Also, the SARS virus family has neurotropic potencies explaining the neurological manifestations of COVID-19, such as headache, seizure, Guillan-Bare syndrome, paresthesia, changes in consciousness level and so on [11, 12].
In light of limited research conducted in Iran, especially in the northeastern region, we undertook this study to assess the levels of psychopathological distress, depression, and sleep quality among PWE. Additionally, our investigation examined the variations in seizure frequency before and after COVID-19 infection among affected patients. Furthermore, a comprehensive analysis was conducted to elucidate the potential risk factors associated with the development of depression, psychopathological distress, sleep disturbances, and exacerbations of seizures in this population.
Materials and Methods
This study was a single-center, case-control study. The survey was conducted during the post-COVID-19 period from January 28 to March 7, 2023. The inclusion criteria of the epilepsy group were as follows: Having the diagnosis of epilepsy for at least one year based on a neurologist’s diagnosis and being 18 years or older. The exclusion criteria were as follows: Developing hemorrhage, infarction, tumor, and infection in the central nervous system; having a history of substance abuse, psychosis, or any psychiatric diseases; and having a change in the type or dosage of the AEDs. Before patient enrollment, a comprehensive review of electronic health records (EHRs) was conducted to assess eligibility criteria. Each patient’s EHR contains essential information, including medical history, physical examination findings, imaging results, and medication details. During each visit, physicians document all physical examination findings and any alterations to the medication regimen. Subsequently, eligible patients underwent a structured interview conducted by a neurologist to evaluate their psychiatric and mental status.
Additionally, in this interview, the patients were asked if they have experienced any changes in the number, type, or duration of their seizures experienced during the COVID-19 pandemic. A total of 12 patients (13.6%) were excluded from the study. For the patients with no remarkable psychiatric disease and normal mental state, the study’s objective and process were clearly explained, and the patients who were willing to participate in the study signed the informed consent. Eight patients (9%) were excluded because of changes in the type or dosage of their drugs within the previous month, and 4 patients (4%) because they refused to participate. After that, the patients completely understood the objective and process of the study and signed the consent form, three printed questionnaires, including Beck depression inventory (BDI), 6-item Kessler (K-6), and Pittsburgh sleep quality index (PSQI) questionnaires, were given to them to complete. While answering the questions, if a patient was illiterate, researchers facilitated the process by reading the questions aloud verbally and providing clear explanations in a simplified manner. Moreover, if patients encountered difficulty understanding any aspect of the questionnaires, researchers provided additional explanations until the questions were entirely understandable.
Also, healthy individuals with the same age and gender distribution of patients were enrolled as the normal group. The normal group was recruited from the patients’ companions without any remarkable past medical history, who were referred to the 22 Bahman Hospital, Mashhad, Khorasan Razavi. The inclusion criteria for the normal group were age 18 or older and signing the consent form. The exclusion criteria of the normal group were as follows: Having a history of seizures or any neurologic disease and having depressive or anxiety disorders. The normal group was completely matched to the patients in terms of age and gender. The sample size was calculated according to previous similar studies [13] and by utilizing the Equation 1 [14]:

Their psychiatric and mental status were assessed through standard psychological questionnaires and also the epilepsy-related information was checked. The study’s goal and process were clearly explained to the patients. The patients willing to participate in the study signed the informed consent form.
A questionnaire was designed and customized for this study to collect demographic information, such as age, gender, education level and marital status. Also, epilepsy information was asked from the PWE as follows: Type of epilepsy (according to the classification approved by the International League Against Epilepsy (ILAE) [15], duration of disease, the number of antiepileptic drugs (AEDs), drug resistance, and the number of seizures before and after COVID-19 infection.
BDI is a 21-item self-report measure of typical depressive symptoms [16]. Each item possesses four possible responses; higher total scores indicate a greater number and severity of depressive symptoms. Scores ranging from 0 to 13 indicate no symptoms, 14–19 marks mild depression, 20–28 denotes moderate depression, and 29–63 points to severe depression. The validity and reliability of the Persian version of this questionnaire have been confirmed by a study as 0.87 and 0.74, respectively [17].
PSQI was first developed in 1988 by Buysse et al. [18]. This questionnaire is a self-report instrument used to assess the quality and patterns of sleep in adults over the last month. This questionnaire evaluates seven aspects: Subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. A total score of “5” or higher indicates poor sleep. The validity and reliability of the Persian version of the PSQI questionnaire were reported as 0.94 and 0.72, respectively [19].
The K-6 psychopathological distress scale is a short screening tool developed to identify persons with a high probability of having diagnosable psychopathological distress, which is severe enough to induce moderate to serious impairment in functioning. Total scores range from 0 to 30, with higher scores indicating more severe psychopathological distress. Dadfar and colleagues reported the sensitivity and specificity of the Persian version of this questionnaire as 0.93 and 0.91, respectively [20].
Statistical analysis was conducted by using SPSS software, version 20. We presented categorical variables as numbers and percentages and continuous variables as Mean±SD. Intergroup differences were compared using the independent t-test or Mann-Whitney U test. Multivariate logistic regression was employed to investigate factors independently associated with severe mental health problems among PWE. The multivariate model included variables that exhibited a significant association (P<0.05) with the outcome in univariate analysis.
Results
A total of 88 PWE were eligible to participate in our study. Of them, 8 patients (9%) were excluded because of changes in the type or dosage of their drugs within the previous month, and 4 patients (4%) because they refused to participate. Also, 76 healthy individuals were enrolled as control participants. These individuals were matched regarding their age and gender with the patients (Table 1).

Demographic data
Ultimately, 76 PWE were included, and the same number of normal individuals were enrolled as the control group. The mean age of the patients was 37.88±18.72 years. Females constituted 50.6% of the participants. No significant difference regarding age and gender was found between the groups (P>0.05). Three-fourths of the patients were married. Forty-five patients were employed. Most of the patients were using one antiepileptic drug, had generalized seizures and lacked drug resistance. Thirty-nine patients were suffering from epilepsy for more than 10 years. Although 28 patients did not have any seizures, 26 had one, 12 had two, and 10 patients had more than three seizures per month. Fifty-two patients reported infection by COVID-19, but PCR confirmed the definitive diagnosis of COVID-19 in only 16 patients. Moreover, 2 patients had severe COVID-19 infection, which led to their hospitalization. Our results demonstrated that 29 PWE (38%) experienced exacerbated epileptic seizures, while 22(28%) did not report any changes in the number of their seizures. Only one of the patients stated decreased frequency of their seizures after COVID-19 infection. Table 2 demonstrates the detailed clinical information of the patients.

Depression
PWE scored higher in the BDI questionnaire (16.5±9.44 vs 9.24±5.81, P=0.02). Among PWE, 22 patients had normal BDI scores, 29 had mild, 17 had moderate, and 8 had severe depressive symptoms. Though, in the control group, 59 participants had normal values of BDI, 11 had mild symptoms of depression, and 6 had moderate symptoms. None of the participants in the control group showed severe symptoms of depression (Table 3).

Psychopathological distress
Our findings revealed that PWE exhibited significantly elevated K-6 scores compared to the normal individual group (P=0.03). K-6 score ≥12 was considered severe psychopathological distress, and we observed that the proportion of patients with K-6 score >12 was notably greater in PWE (21 vs 9, P=0.04) (Table 3).
Sleep
The PSQI questionnaire scores represented a significantly worse sleep quality in PWE than the normal individual group (6.2±1.1 vs 4.3±2, P=0.01). Different subscales of the PSQI questionnaire are also presented in Table 3. The patients had remarkably poor subjective sleep quality, longer sleep duration, and more sleep disturbances. Moreover, daytime dysfunction was more prevalent in patients than in the normal individual group (Table 3).
Table 4 shows correlations between depression (BDI scores), psychopathological distress (K-6 scores), and sleep quality (PSQI scores) with disease duration in PWE.

We found that both depressive symptoms (P=0.04) and poor sleep quality (P=0.02) were significantly associated with longer disease duration. However, correlations do not necessarily imply that one variable directly causes another. To understand if disease duration independently predicts depression and sleep problems, we performed a multivariate logistic regression analysis. This analysis reveals that even after accounting for other factors, disease duration remains a significant predictor for both depression (OR=1.128; 95% CI, 1.110%, 1.729%; P=0.002) and sleep disturbances (OR=1.860; 95% CI, 1.363%, 1.922%; P=0.01). In other words, for every unit increase in disease duration, the odds of experiencing depression increase by 12.8% and the odds of having sleep problems increase by 86.0% while controlling for other variables.
Discussion
In the present study, we explored the mental health status of PWE in northeast Iran during the COVID-19 pandemic. We compared our results with the mental health status of healthy individuals from the same region in addition to evaluating risk factors for poor mental status. Our findings revealed that PWE had significantly higher levels of psychopathological distress, sleep disturbances, and depression than the normal group, which was closely related to the duration of the disease.
It is reported that the COVID-19 pandemic resulted in increased mental health problems due to high numbers of mortality, high levels of infection, and quick distribution. Moreover, stress is more frequent during the pandemic [21]. Besides this, recent studies on the general population have shown that following the pandemic, anxiety and depression increase, which is accompanied by sleep disorders. Accordingly, PWEs are at considerable risk to the psychosocial influence of the pandemic [22]. These data on the frequency of depression in COVID-19 patients can be compared to that of the pre-pandemic prevalence of depressive disorders in hospital inpatients and outpatients, which are estimated to be between 5% to 34% [23] and 27.0% [24], respectively. In this study, we found the prevalence of depression for COVID-19 outpatients to be 71%, which is a substantial increase compared with pre-pandemic depression levels. Furthermore, these estimated prevalence rates of depression and anxiety display a remarkable increase compared to the general population during the COVID-19 pandemic, which are estimated to be 33.7% and 31.9%, respectively [25, 26]. The discrepancy between our results and those of other studies can be explained by the various screening tools, which significantly impact the resultant frequency. However, it should be mentioned that substantial variations in the sample size of studies may contribute to this issue.
On the other side, it seems that restrictions during the COVID-19 pandemic negatively correlate with sleep quality in the general population because of the increasing rate of psychological disorders, decreasing exercise activity, social isolation, and inadequate access to sunlight [27]. In this regard, results showed that 7.1%–71.2% of PWE had sleep problems, and the prevalence of insomnia was also 28.2% among these patients [9, 28]. However, one study reported no significant difference in the sleep quality between PWE and healthy persons [29]. Sleep disturbance in PWE is a vital activator that can increase motor excitability, which develops the frequency of seizures in these patients [30]. Generally, disturbed sleep-wake cycles during the COVID-19 restriction can influence PWE and healthy persons. However, sleep problems may increase EEG epileptiform abnormalities that result in a worsening of seizures in PWE [31]. Previous data show that sleep disturbances have been reported in 20.1% of the PWE during the COVID-19 pandemic, significantly higher than in the pre-pandemic period [26]. Our results showed that PWE have significantly higher sleep problems than the general population. We also noticed that sleep problems were significantly associated with longer disease duration.
Various factors, including social separation and loneliness, affect the high prevalence of psychopathological distress in PWE during the COVID-19 pandemic [32]. The development of social restrictions to control the pandemic leads to a number of behavioral and social consequences that may also trigger epileptic seizures and decrease the threshold level of seizures in PWE [33]. Also, stress has been shown to exacerbate epilepsy. There is a significant correlation between stress and the number of epileptic seizures among PWE [34]. Results of studies focusing on the impact of the COVID-19 pandemic on stress levels among PWE represented a higher stress and anxiety level among PWE. In addition, the studies reported that PWE experienced worse mental health conditions during the COVID-19 restrictions [13]. The elevated stress associated with the pandemic has a direct correlation with the worsening of seizures in some PWE [29]. Our data support notable psychopathological distress among PWE compared to the normal individual group. Also, the number of people with severe psychopathological distress was remarkably higher than the healthy individuals. We did not find any significant relationship between drug resistance and psychopathological distress, which is in line with the results of 3 meta-analyses [5, 35, 36]. In contrast, the study by Hao et al. found a significant association [13].
Also, it is estimated that one-third of PWE are affected by mental issues. Furthermore, psychopathological distress frequently happens in their daily life [37]. Therefore, it is expected that in stressful conditions like COVID-19 restrictions, the occurrence of depression and anxiety increases. Among psychopathological distress, anxiety, and depression are the most frequent epilepsy-relate disorders which is observed among PWE during the COVID-19 pandemic. Results from studies on COVID-19 in Italy demonstrated that PWE (19%) and the general population (17%) reported psychiatric disorders, including depression. However, PWE had more severe depressive symptoms than the control group [33]. In addition, Van Hees et al. found remarkable gender differences among PWE, and it was reported that women are at increased risk of more depressive problems during the COVID-19 pandemic [38]. Excessive exposure to stressor factors not only increases seizures in PWE but also triggers seizure development even in people without any history of epilepsy [39]. As regards, the study by Huang et al. documented that depression among PWE and the control group increased the number of seizures (22.58% vs 12.08%, respectively) [29]. In line with this, several studies performed during the COVID-19 pandemic indicated gender and age differences in relation to the prevalence of stress and anxiety symptoms among PWE. In this respect, female and older subjects had higher anxiety levels [40]. Other relevant studies also showed that both PWE and the general population experienced anxiety symptoms during the pandemic, though PWEs experienced a significantly higher prevalence of anxiety [40].
Although our study provided significant evidence of high levels of psychopathological distress, depression, and sleep disorders among PWE, it still has several limitations. First, we utilized online questionnaires, which possessed several biases and had low scientific evidence strength. Second, most of our responders were young adults who use the Internet the most, so our sample could not properly represent the general population. The lower age of patients could bias the number of reported seizures, the rate of drug resistance, and antiepileptic medications. Third, we only measured the depression and anxiety of patients during the pandemic, and our study lacked a pre-pandemic baseline of their mental condition.
Conclusion
In conclusion, the significant disparities in psychopathological distress, depression, and sleep disorders between PWE and healthy individuals highlight the vulnerability of PWE to mental health challenges during crises. Importantly, our results indicate that longer disease duration is associated with increased susceptibility to depression and sleep disturbances among PWE, emphasizing the need for targeted interventions to address their mental health needs, especially during public health emergencies like the COVID-19 pandemic. Moreover, these findings underscore the importance of integrating mental health support into the comprehensive care of PWE, with tailored interventions aimed at enhancing psychopathological well-being and sleep quality. By acknowledging and addressing the unique mental health challenges faced by epilepsy patients, healthcare providers can effectively promote quality of life in this vulnerable population. Further future research should continue to explore the dynamic interplay between epilepsy, mental health, and external stressors.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in compliance with the ethical guidelines of the 2013 Declaration of Helsinki. This study was approved by the Ethics Committee of Islamic Azad University, Mashhad Medical Sciences Branch (Code: IR.IAU.MSHD.REC.1401.163).
Funding
This article was extracted from the Doctor of Medicine thesis of Nikoo Saeedi, approved by Islamic Azad University, Mashhad Medical Sciences Branch (Code: 162467234).
Authors contributions
Conceptualization: Hamidreza Hatamian and Mehrdad Erfanian; Methodology: Fatemeh Shafiei; Investigation and writing the original draft: Nikoo Saeedi; Review, editing and supervision: Seyed Mehran Homam.
Conflict of interest
The authors declared no conflict of interest.
References
- Varma P, Junge M, Meaklim H, Jackson ML.Younger people are more vulnerable to stress, anxiety and depression during COVID-19 pandemic: A global cross-sectional survey. Prog Neuropsychopharmacol Biol Psychiatry. 2021; 109:110236. [DOI:10.1016/j.pnpbp.2020.110236] [PMID] [PMCID]
- Mostacci B, Licchetta L, Cacciavillani C, Di Vito L, Ferri L, Menghi V, et al. The impact of the COVID-19 pandemic on people with epilepsy. An Italian survey and a global perspective. Front Neurol. 2020; 11:613719. [DOI:10.3389/fneur.2020.613719] [PMID] [PMCID]
- Chou KL, Liang K, Sareen J. The association between social isolation and DSM-IV mood, anxiety, and substance use disorders: wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2011; 72(11):1468-76. [DOI:10.4088/JCP.10m06019gry] [PMID]
- Fiest KM, Dykeman J, Patten SB, Wiebe S, Kaplan GG, Maxwell CJ, et al. Depression in epilepsy: A systematic review and meta-analysis. Neurology. 2013; 80(6):590-9. [DOI:10.1212/WNL.0b013e31827b1ae0] [PMID] [PMCID]
- Scott AJ, Sharpe L, Hunt C, Gandy M. Anxiety and depressive disorders in people with epilepsy: A meta-analysis. Epilepsia. 2017; 58(6):973-82. [DOI:10.1111/epi.13769] [PMID]
- Lai SL, Hsu MT, Chen SS. The impact of SARS on epilepsy: The experience of drug withdrawal in epileptic patients. Seizure. 2005; 14(8):557-61. [DOI:10.1016/j.seizure.2005.08.010] [PMID] [PMCID]
- Shehata GA, Bateh Ael-A. Cognitive function, mood, behavioral aspects, and personality traits of adult males with idiopathic epilepsy. Epilepsy Behav. 2009; 14(1):121-4.[DOI:10.1016/j.yebeh.2008.08.014] [PMID]
- Ou S, Xia L, Li R, Wang L, Xia L, Zhou Q, Pan S. Long-term outcome of seizure-free patients and risk factors of relapse following antiepileptic drug withdrawal. Epilepsy Behav. 2018; 88:295-300. [DOI:10.1016/j.yebeh.2018.09.028] [PMID]
- Fonseca E, Quintana M, Lallana S, Luis Restrepo J, Abraira L, Santamarina E, et al. Epilepsy in time of COVID-19: A survey-based study. Acta Neurol Scand. 2020; 142(6):545-54.[DOI:10.1111/ane.13335] [PMID] [PMCID]
- Frucht MM, Quigg M, Schwaner C, Fountain NB. Distribution of seizure precipitants among epilepsy syndromes. Epilepsia. 2000; 41(12):1534-9. [DOI:10.1111/j.1499-1654.2000.001534.x] [PMID]
- Nordvig AS, Fong KT, Willey JZ, Thakur KT, Boehme AK, Vargas WS, et al. Potential neurologic manifestations of COVID-19. Neurol Clin Pract. 2021; 11(2):e135-46. [DOI:10.1212/CPJ.0000000000000897] [PMID] [PMCID]
- Misra S, Kolappa K, Prasad M, Radhakrishnan D, Thakur KT, Solomon T, et al. Frequency of neurologic manifestations in COVID-19: A systematic review and meta-analysis. Neurology. 2021; 97(23):e2269-81. [DOI:10.1212/WNL.0000000000012930] [PMID] [PMCID]
- Hao X, Zhou D, Li Z, Zeng G, Hao N, Li E, et al. Severe psychological distress among patients with epilepsy during the COVID-19 outbreak in southwest China. Epilepsia. 2020; 61(6):1166-73. [DOI:10.1111/epi.16544] [PMID] [PMCID]
- Sepandi M, Taghdir M. [Useful Research Methods in Medical Sciences. Tehran: Asare Sobhan; 2023. [Link]
- Riney K, Bogacz A, Somerville E, Hirsch E, Nabbout R, Scheffer IE, et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset at a variable age: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022; 63(6):1443-74. [DOI:10.1111/epi.17240] [PMID]
- Jackson-Koku G. Beck depression inventory. Occup Med (Lond). 2016; 66(2):174-5. [DOI:10.1093/occmed/kqv087] [PMID]
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N. Psychometric properties of a Persian-language version of the Beck Depression Inventory-Second edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21(4):185-92. [DOI:10.1002/da.20070] [PMID]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2):193-213. [DOI:10.1016/0165-1781(89)90047-4] [PMID]
- Farrahi Moghaddam J, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep Breath. 2012; 16(1):79-82. [DOI:10.1007/s11325-010-0478-5] [PMID]
- Dadfar M, Atef Vahid MK, Lester D, Bahrami F. Kessler psychological distress scale (K6): Psychometric testing of the Farsi form in psychiatric outpatients. Adv Biores. 2016; 7(2):105-8. [Link]
- Yuan K, Gong YM, Liu L, Sun YK, Tian SS, Wang YJ, et al. Prevalence of posttraumatic stress disorder after infectious disease pandemics in the twenty-first century, including COVID-19: A meta-analysis and systematic review. Mol Psychiatry. 2021; 26(9):4982-98. [DOI:10.1038/s41380-021-01036-x] [PMID] [PMCID]
- French JA, Brodie MJ, Caraballo R, Devinsky O, Ding D, Jehi L, et al. Keeping people with epilepsy safe during the COVID-19 pandemic. Neurology. 2020; 94(23):1032-7. [DOI:10.1212/WNL.0000000000009632] [PMID] [PMCID]
- Walker J, Burke K, Wanat M, Fisher R, Fielding J, Mulick A, et al. The prevalence of depression in general hospital inpatients: A systematic review and meta-analysis of interview-based studies. Psychol Med. 2018; 48(14):2285-98.[DOI:10.1017/S0033291718000624] [PMID]
- Dong M, Wang SB, Li Y, Xu DD, Ungvari GS, Ng CH, et al. Prevalence of suicidal behaviors in patients with major depressive disorder in China: A comprehensive meta-analysis. J Affect Disord. 2018; 225:32-39. [DOI:10.1016/j.jad.2017.07.043] [PMID]
- Salari N, Khazaie H, Hosseinian-Far A, Ghasemi H, Mohammadi M, Shohaimi S, et al. The prevalence of sleep disturbances among physicians and nurses facing the COVID-19 patients: A systematic review and meta-analysis. Global Health. 2020; 16(1):92. [PMID]
- Lin LY, Wang J, Ou-Yang XY, Miao Q, Chen R, Liang FX, et al. The immediate impact of the 2019 novel coronavirus (COVID-19) outbreak on subjective sleep status. Sleep Med. 2021; 77:348-54. [DOI:10.1016/j.sleep.2020.05.018] [PMID] [PMCID]
- Abokalawa F, Ahmad SF, Al-Hashel J, Hassan AM, Arabi M. The effects of coronavirus disease 2019 (COVID-19) pandemic on people with epilepsy (PwE): An online survey-based study. Acta Neurol Belg. 2022; 122(1):59-66. [DOI:10.1007/s13760-021-01609-1] [PMID] [PMCID]
- Alkhotani A, Siddiqui MI, Almuntashri F, Baothman R. The effect of COVID-19 pandemic on seizure control and self-reported stress on patient with epilepsy. Epilepsy Behav. 2020; 112:107323. [DOI:10.1016/j.yebeh.2020.107323] [PMID] [PMCID]
- Huang S, Wu C, Jia Y, Li G, Zhu Z, Lu K, et al. COVID-19 outbreak: The impact of stress on seizures in patients with epilepsy. Epilepsia. 2020; 61(9):1884-93. [DOI:10.1111/epi.16635] [PMID] [PMCID]
- Manganotti P, Bongiovanni LG, Fuggetta G, Zanette G, Fiaschi A. Effects of sleep deprivation on cortical excitability in patients affected by juvenile myoclonic epilepsy: A combined transcranial magnetic stimulation and EEG study. J Neurol Neurosurg Psychiatry. 2006; 77(1):56-60. [DOI:10.1136/jnnp.2004.041137] [PMID] [PMCID]
- Pratt KL, Mattson RH, Weikers NJ, Williams R. EEG activation of epileptics following sleep deprivation: A prospective study of 114 cases. Electroencephalogr Clin Neurophysiol. 1968; 24(1):11-5. [DOI:10.1016/0013-4694(68)90061-8] [PMID]
- Cénat JM, Blais-Rochette C, Kokou-Kpolou CK, Noorishad PG, Mukunzi JN, McIntee SE, et al. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Res. 2021; 295:113599. [DOI:10.1016/j.psychres.2020.113599] [PMID] [PMCID]
- Assenza G, Lanzone J, Brigo F, Coppola A, Di Gennaro G, Di Lazzaro V, et al. Epilepsy care in the time of COVID-19 pandemic in Italy: Risk factors for seizure worsening. Front Neurol. 2020; 11:737. [DOI:10.3389/fneur.2020.00737] [PMID] [PMCID]
- McKee HR, Privitera MD. Stress as a seizure precipitant: Identification, associated factors, and treatment options. Seizure. 2017; 44:21-6. [DOI:10.1016/j.seizure.2016.12.009] [PMID]
- Kanner AM, Barry JJ, Gilliam F, Hermann B, Meador KJ. Anxiety disorders, subsyndromic depressive episodes, and major depressive episodes: Do they differ on their impact on the quality of life of patients with epilepsy? Epilepsia. 2010; 51(7):1152-8. [DOI:10.1111/j.1528-1167.2010.02582.x] [PMID]
- Bragatti JA, Torres CM, Londero RG, Martin KC, Souza AC, Hidalgo MP, et al. Prevalence of psychiatric comorbidities in temporal lobe epilepsy in a Southern Brazilian population. Arq Neuropsiquiatr. 2011; 69(2A):159-65. [DOI:10.1590/S0004-282X2011000200003] [PMID]
- Ribot R, Kanner AM. Neurobiologic properties of mood disorders may have an impact on epilepsy: Should this motivate neurologists to screen for this psychiatric comorbidity in these patients? Epilepsy Behav. 2019; 98(Pt B):298-301.[DOI:10.1016/j.yebeh.2019.01.026] [PMID]
- Van Hees S, Siewe Fodjo JN, Wijtvliet V, Van den Bergh R, Faria de Moura Villela E, da Silva CF, et al. Access to healthcare and prevalence of anxiety and depression in persons with epilepsy during the COVID-19 pandemic: A multicountry online survey. Epilepsy Behav. 2020; 112:107350. [DOI:10.1016/j.yebeh.2020.107350] [PMID] [PMCID]
- Christensen J, Li J, Vestergaard M, Olsen J. Stress and epilepsy: A population-based cohort study of epilepsy in parents who lost a child. Epilepsy Behav. 2007; 11(3):324-8. [DOI:10.1016/j.yebeh.2007.06.003] [PMID]
- Giordano A, Siciliano M, De Micco R, Sant'Elia V, Russo A, Tedeschi G, et al. Correlates of psychological distress in epileptic patients during the COVID-19 outbreak. Epilepsy Behav. 2021; 115:107632. [DOI:10.1016/j.yebeh.2020.107632] [PMID] [PMCID]
Type of Study: Research |
Subject:
General
Received: 2024/02/26 | Accepted: 2024/05/12 | Published: 2024/07/7
Received: 2024/02/26 | Accepted: 2024/05/12 | Published: 2024/07/7
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






