Sat, May 18, 2024
Volume 10, Issue 1 (Winter 2024)
Caspian J Neurol Sci 2024, 10(1): 47-56 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yahak H, Farjah G H, Pourheydar B, Karimipour M. Protective Effect of Gum Arabic on Spinal Cord Ischemia-reperfusion Injury in Rats. Caspian J Neurol Sci 2024; 10 (1) :47-56
URL: http://cjns.gums.ac.ir/article-1-690-en.html
URL: http://cjns.gums.ac.ir/article-1-690-en.html
1- Neurophysiology Research Center, Cellular and Molecular Medicine Research Institute, Urmia University of Medical Sciences, Urmia, Iran.
2- Department of Anatomical Sciences, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran.
2- Department of Anatomical Sciences, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran.
Full-Text [PDF 2577 kb]
(208 Downloads)
| Abstract (HTML) (193 Views)
Full-Text: (62 Views)
Introduction
The aorta artery should be clamped during abdominal and thoracic surgeries [1]. Ischemia is caused by clamping the artery, and its release and subsequent tissue reperfusion damage these tissues [2]. Paraplegia is one of the most serious complications of aortic ischemia-reperfusion (I-R) injury [3]. Recently, many studies have been conducted to reduce the complications of I-R, such as saffron extract [4], lutein [5], erythropoietin [6], and thymoquinone [7].
Gum arabic (GA) comprises several polysaccharides secreted from Senegal Acacia trees [8]. GA also shows anti-oxidant and anti-inflammatory properties [9, 10] and is involved in the apoptotic pathway [11].
A review of the literature shows that GA has a protective effect on several diseases, including lung tumors [12], heart disease [10], and diabetic nephropathy [13]. Our previous study showed that GA and propolis are effective in sciatic nerve regeneration [14]. A previous study reported that the administration of GA protects I-R heart damage through anti-inflammatory and anti-oxidant pathways [10]. To our knowledge, there is no study on the protective effect of GA on I-R injury of the spinal cord. So, the present study aimed to investigate the GA’s protective effect on the spinal cord’s motor neurons after ischemia-reperfusion (I-R) injury using neurological, biochemical, and histological assessments.
Materials and Methods
Animals
Thirty-five Sprague-Dawley male rats (250~280 g weight, 8~10 weeks old) were divided into 5 groups: Intact, sham surgery, control, GA (1 g/kg), and GA (4 g/kg). The GA (Merck, Germany) solution was prepared with distilled water in suitable concentrations (1 and 4 g/kg). In GA groups, oral gavages’ treatment was performed for 21 days before surgery. In the control group, the animals received gavage administration of distilled water (1 mL) for 21 days before surgery [15].
Surgery procedure
The rats were anesthetized by ketamine (90 mg/kg) and xylazine (10 mg/kg) through intraperitoneal injection, followed by heparin (400 IU) [16]. Then, under sterile conditions, the abdominal cavity was opened through a midline longitudinal incision (linea alba). The abdominal viscera were gently removed (from the right side of the peritoneal cavity) and placed on sterile gauze moistened with normal saline. To establish ischemia, the abdominal aortic artery was clamped at two points: Below the left kidney vein and before the bifurcation of the abdominal aortic artery. During the ischemia, the bilateral femoral artery was absent. After 60 minutes, the clamps were gently removed to restore blood flow (reperfusion). At the end of the surgery, the abdominal viscera were returned to the abdomen, and the animal’s muscles and skin were closed with 4-0 nylon threads [5]. During the surgery, the animal’s body temperature was maintained constant around 37ºC. After recovery, the animals were kept in their cages.
Neurological evaluation
The motor deficit index (MDI) test was used to determine the possible paralysis of the hind limbs (before spinal cord I-R and 24, 48, and 72 hours after the start of reperfusion). The score of MDI ranges between 0 (intact) and 6 (complete defect). The animals with an MDI score higher than, were marked as paraplegic [17].
Withdrawal reflex latency (WRL)
The withdrawal reflex latency (WRL) test was used to evaluate the sensation of the hind limbs at 24, 48, and 72 hours after surgery. The plantar surface was placed on a hot plate (56°C). Then, the contact time was recorded. To prevent possible damage, the maximum time allowed to contact the animal’s hind limbs with the hot plate was considered 12 seconds [18].
Blood sampling
The blood was obtained directly from the heart after anesthetizing the rats with ketamine (90 mg/kg). Then, the plasma was separated by centrifuge (1500 g; 15 min; 4°C). The collected plasmas were stored at -80°C until the biochemical evaluations [16].
Biochemical study
The hydroxylamine method was used to measure the level of superoxide dismutase (SOD) in plasma. This method converted hydroxylamine to nitrite and then measured at a wavelength of 550 nm by a spectrophotometer. The catalase (CAT) level in plasma was measured spectrophotometrically after observing the yellow complex at 450 nm wavelength. The plasma’s total anti-oxidant capacity (TAC) level was evaluated using an appropriate kit (LDN, GmbH & Co KG, Germany). The TAC is determined by the production of tetramethylbenzidine, which is detected by color produced at a wavelength of 450 nm by a spectrophotometer. The plasma malondialdehyde (MDA) level was measured using the thiobarbituric acid (TBA) method. MDA is a colorless liquid that reacts with TBA reagent to produce a pink color at a wavelength of 532 nm by spectrophotometer [19].
Histologic evaluation
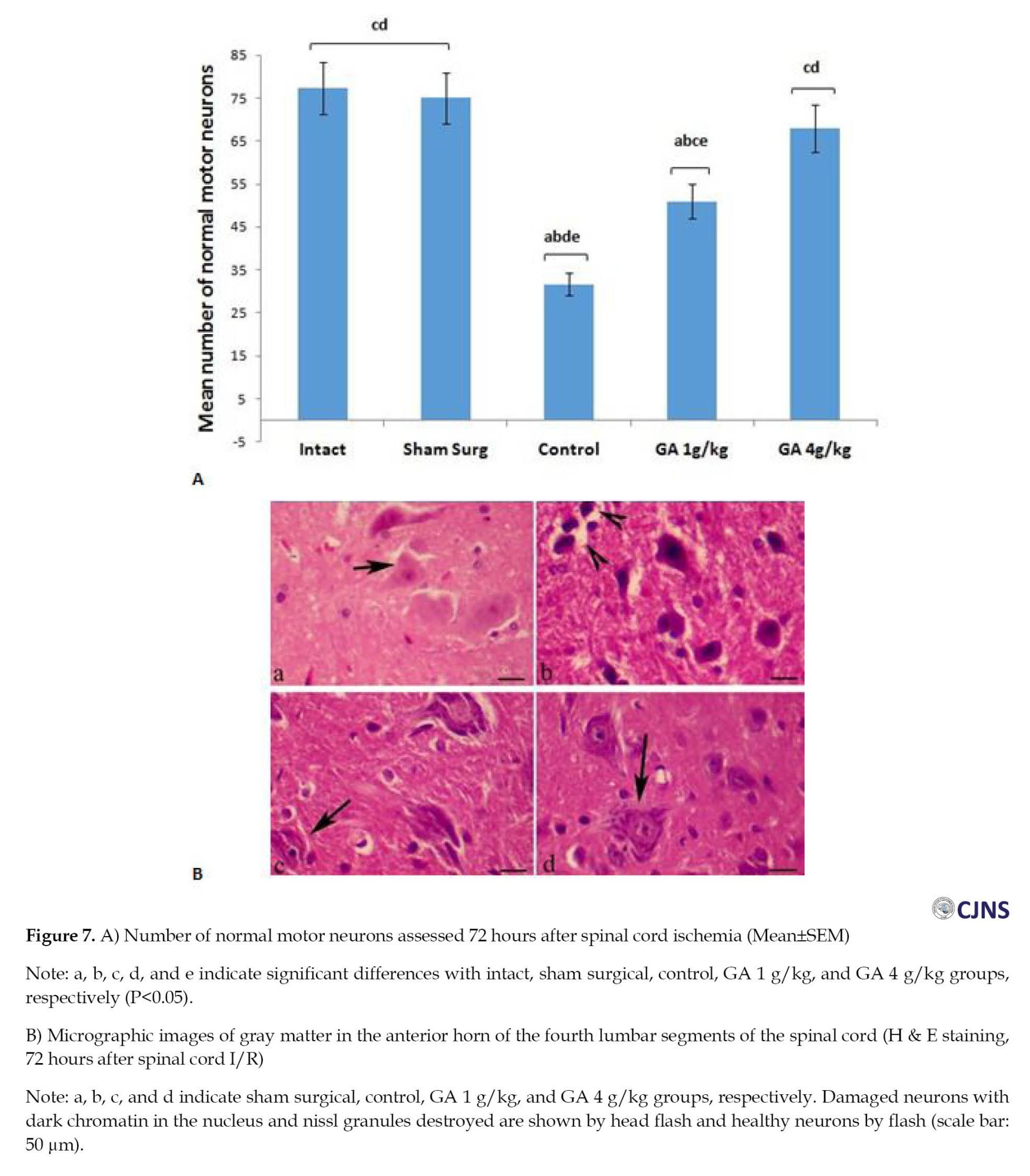
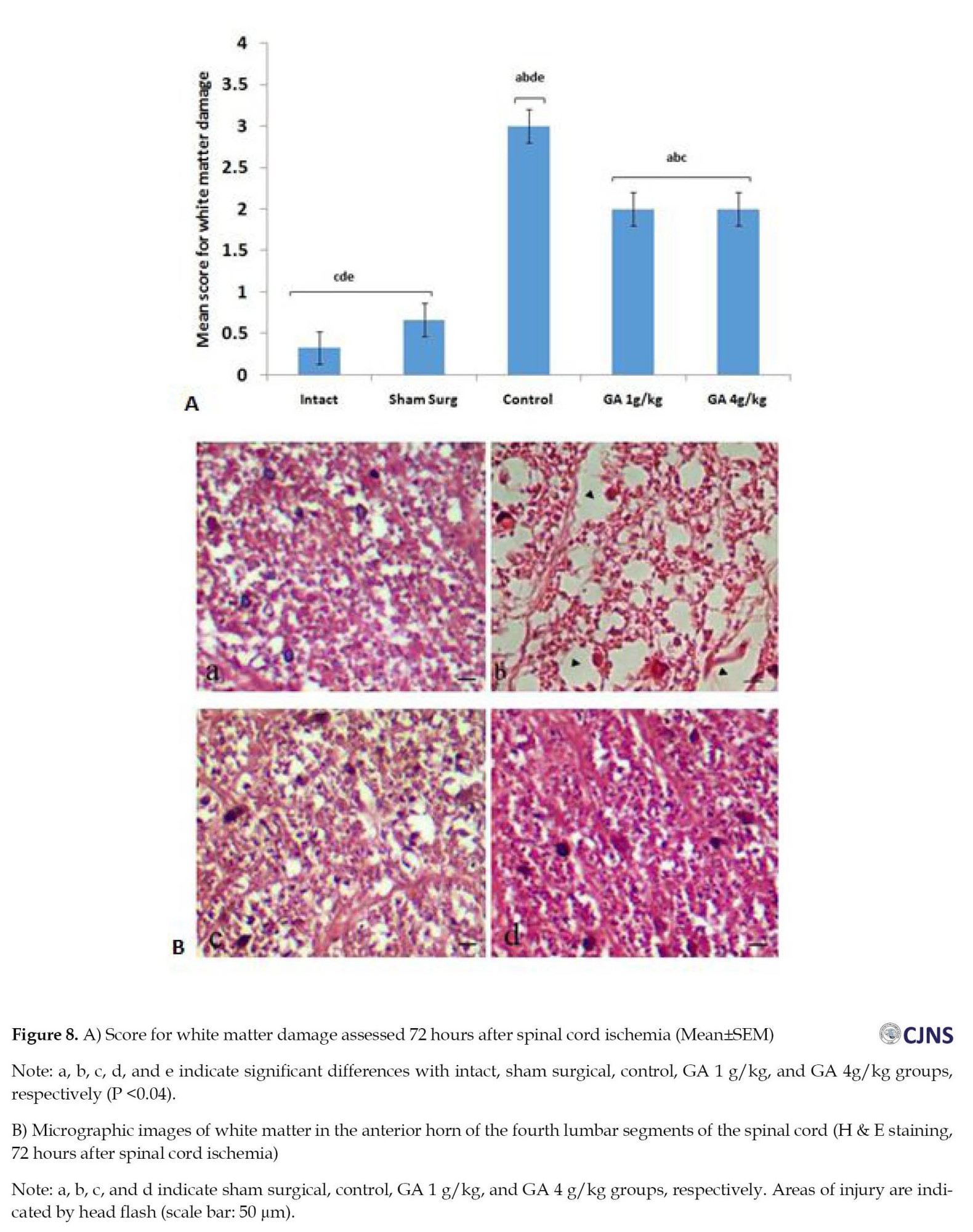
The animals were fixed through cardiac perfusion, and then the fourth segment of the lumbar spinal cord was isolated. After performing the tissue preparation procedures, 5-μm sections were prepared using H&E staining. Then, slices with an interval of 100 μm were prepared from the fourth lumbar spinal cord segment and observed by a digital light microscope. The number of healthy motor neurons was counted using OLYSIA Autobioreport Software (Olympus Optical, Co. LTD, Tokyo, Japan). Afterward, in the anterior horns of the spinal cord, the neurons with prominent nuclei with delicate chromatin bodies and Nissl bodies found within their cytoplasm were considered healthy motor neurons, and neurons with eosinophilic, non-nucleated cytoplasm were considered damaged cells. An area of 0.04-2 mm was evaluated in the anterior and lateral funiculi. In the samples that did not have cavitation, the tissue was considered normal, and the number 0 was reported; in the tissues with low cavitation (less than 10%), number 1; moderate (10% to 50%), number 2; and the tissues with severe cavitation (more than 50%), the number 3 was reported [20].
Statistical analysis
The data of the present study were displayed as Mean±SEM. Statistical analysis was performed using one-way ANOVA and Tukey’s test in SPSS software, version 16 (Chicago, IL, USA). The Kruskal-Wallis and Mann-Whitney tests were used to find the differences in MDI and cavitations between groups. P<0.05 was statistically significant.
Results
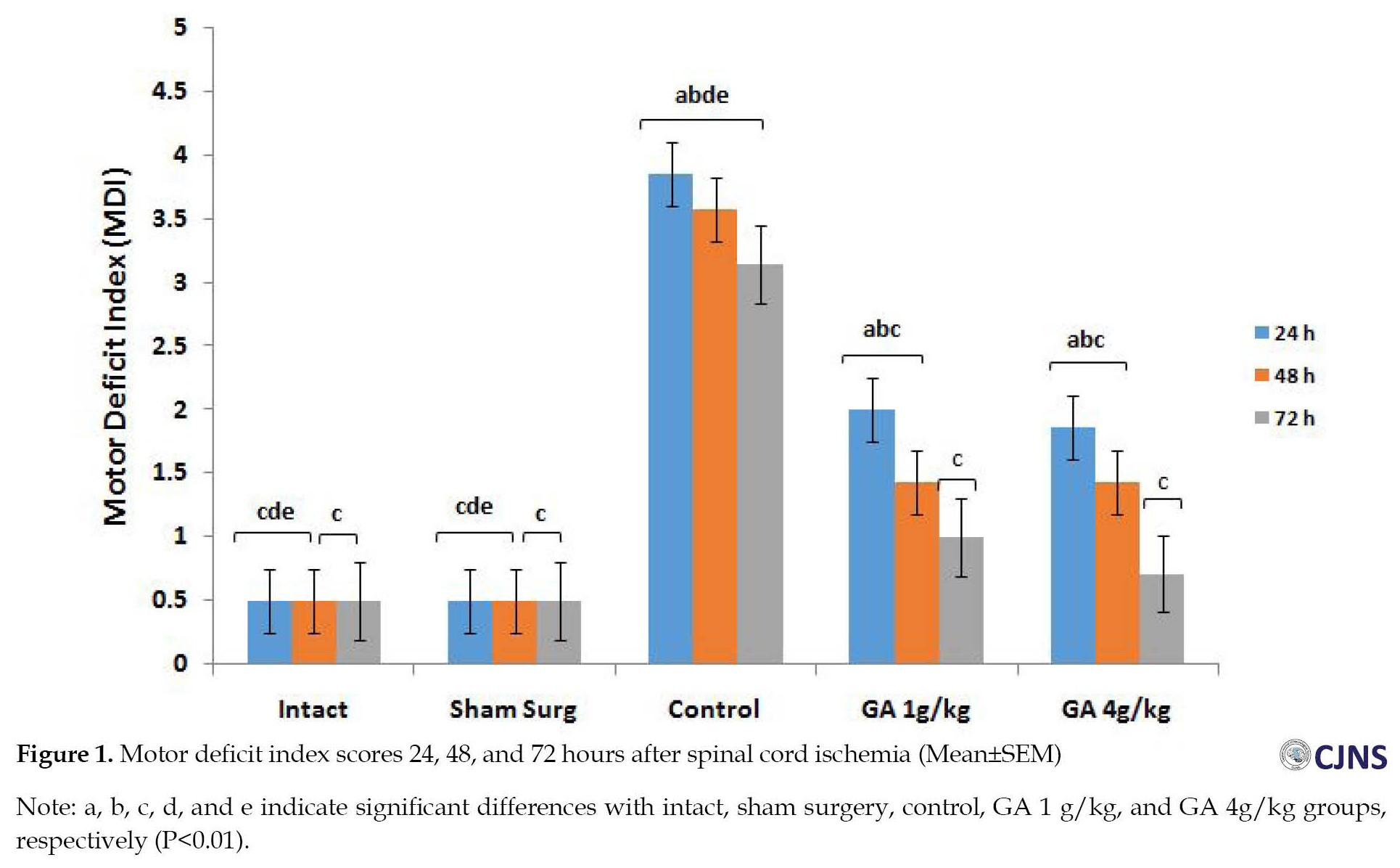
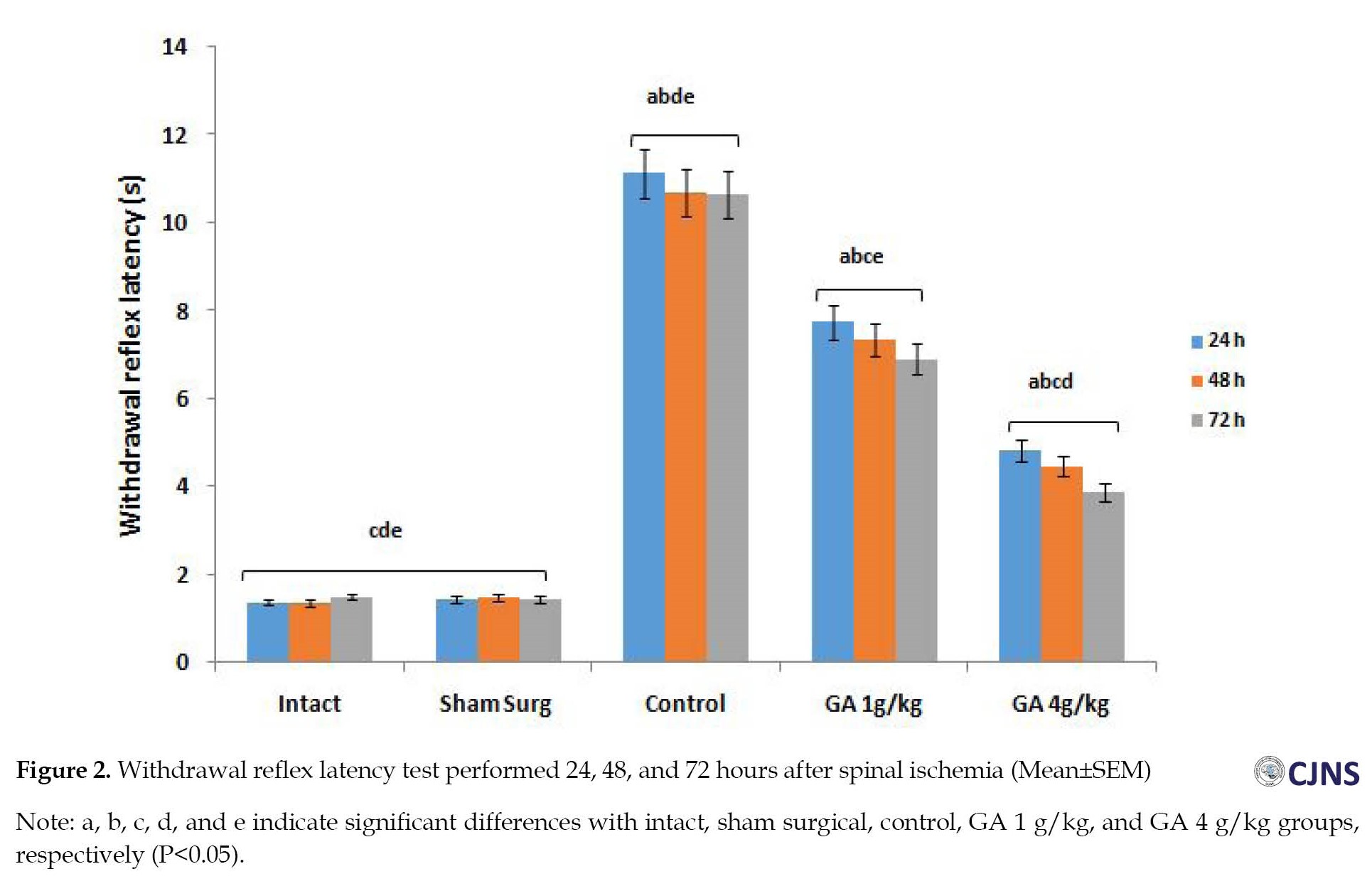
The mean scores of MDI in the control group versus other groups were significantly different up to 72 hours after I-R (P<0.01). The difference between the GA groups of ischemia (after 72 hours) and the intact and surgical sham groups was not significant (P>0.01) (Figure 1). The results of the WRL test show that intact, surgical sham, and GA 4 g/kg groups are significantly different from all other groups up to 72 hours after I-R (P<0.05) (Figure 2). At the mean value of SOD, 72 hours after spinal cord I-R, there was a significant (P<0.05) decrease in the control group versus the intact, surgical sham, and GA 4 g/kg groups (Figure 3). The mean value of CAT, 72 hours after spinal cord I-R, was significantly (P<0.04) reduced in the control group versus the intact and GA 5 g/kg group. CAT level is higher in the GA 1 g/kg group versus the control group (Figure 4). However, it was not significant (P˃0.02).
Mean TAC, 72 hours after spinal cord I-R, was remarkably reduced in the control group versus the intact and surgical sham groups (P=0.00). So, 72 hours after I-R, the TAC level in the GA 4 g/kg group compared to the control group showed a significant (P<0.02) increase (Figure 5). The mean plasma MDA level was higher in the control group versus the intact and surgical sham groups 72 hours after spinal cord I/R (P<0.01). This index decreased in the GA 4 g/kg group versus the control group (P<0.01) (Figure 6). The mean number of healthy spinal motor neurons in the L4 lumbar segment in GA groups considerably increased compared to the control group (P<0.01). In addition, the mean number of healthy motor neurons in the GA 4 g/kg group was significantly higher than the GA 1 g/kg group (P<0.05). The number of healthy motor neurons in the GA 4 g/kg group did not show a significant difference (P>0.05) compared to the intact and surgical sham groups (Figure 7). The mean injury of spinal cord white matter 72 hours after ischemia in the control group increased considerably versus the intact and surgical sham groups (P<0.001). It also significantly decreased in GA groups versus the control group (P<0.04). In addition, GA groups showed a significant (P<0.01) difference compared to intact and surgical sham groups (Figure 8).
Discussion
According to the results of this study, after 60 minutes of ischemia and then up to 72 hours of reperfusion, less damage was observed in the spinal cords, white and gray matter in animals receiving GA, and the hind limbs also showed better performance.
Ischemia and reperfusion may eventually lead to paralysis of the lower limbs and a decrease in the quality of nerve function in the affected areas, which can be attributed to factors such as disharmony in the regulation of neurotransmitters [21].
A previous study showed that GA combined with propolis as a nerve guide channel improves motor reflexes [14]. The present study is the first one to investigate the protective effect of GA on ischemia-reperfusion injury in the spinal cord.
MDI can be used in neurological assessment, and lower scores are desirable. Our results showed that the MDI in the GA groups was significantly less than in the control group, and the results of the WRL test showed an improvement in the sensory function of the hind limbs of the animals. After I-R, oxygen free radicals are formed, resulting in the cell membrane losing its proper function due to the disruption of cytokine secretion, resulting in leakage of the cell internals, which produces more reactive oxygen species [22]. These species and free radicals affect lipids by making lipid peroxides, causing direct damage to nerve cells since lipids are involved in the structure of neurons [23]. GA can be beneficial in neutralizing oxidative stress and inflammation caused by oxygen free radicals [22] and prevents lipid peroxide formation [24]. A study showed the use of GA decreased the oxidants and inflammatory factors (pro-inflammatory cytokines, tumor necrosis factor-alpha, interleukin-1β, interleukin-6) and increased SOD and anti-inflammatory factors (cytokine IL-10) [10]. SOD and CAT are among the anti-oxidant enzymes that neutralize oxygen free radicals, so their measurement can help understand the effectiveness of the substance used [25]. In addition, TAC is a suitable biochemical parameter for measuring the overall status of anti-oxidants [26]. In addition, MDA is a reliable indicator for evaluating oxygen free radicals and lipid peroxidation [25] and is used as an indicator of oxidative stress in cells [27]. The previous study shows that the levels of CAT and TAC have an inverse relationship with oxidative stress. In addition, a higher level of SOD has a direct effect on reducing oxidative stress. High levels of MDA were observed in acute oxidative stress, which indicates a direct relationship between the MDA index and levels of oxidative stress [28]. The results of the present study show that the levels of SOD, CAT, and TAC, as well as the number of healthy motor neurons in the GA 4 g/kg group, were significantly more than the control group, which may be due to the protective effect of GA on the motor neurons of the spinal cord. The role of GA in reducing MDA production [24] is another reason for increasing the percentage of healthy motor neurons and improving the function of the lower limbs. GA is a natural substance that can be taken orally without side effects [8], and at the same time, it can appear as a drug to strengthen and improve motor reflexes [29].
In this study, the white matter in the GA groups was healthier than the control group. Due to the protective effect of GA on neurons, more myelinated axons remained intact, and fewer cavities were formed in the white matter compared to the control group.
In the present study, GA was administered two doses of 1 and 4 g/kg through gavage. The previous study showed that the results obtained from the administration of GA in doses between 3 and 6 g do not show a significant difference in the coagulation system [30]. In another study, GA was prescribed with doses of 2, 3, and 4 g/kg body weight, and they concluded that the dose of 4 g/kg has the most beneficial effect [31]. GA shows different effects depending on the conditions and context of use, which is an interesting feature. Still, in general, it can be concluded that it can show more favorable effects at a dose of about 4 g/kg [21]. Similar mechanisms may effectively explain the role of the GA in protecting the spinal cord. Thus, GA increased the level of the anti-oxidant enzyme SOD, which showed that it plays an essential role in protecting the spinal cord against I-R injury [10]. The protection effect observed in this study is related to the anti-oxidant and anti-inflammatory activities of GA [9]. In addition, many mechanisms are unknown so far, and more experimental evidence is needed to understand them.
Conclusion
This study shows that GA has anti-oxidant and anti-inflammatory properties and, therefore, may be effective in reducing oxidative stress, preventing damage to spinal cord motor neurons, and protecting spinal cord white matter.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Urmia University of Medical Sciences approved this study (Code:IR.UMSU.REC.1399.124, date: July 22, 2020).
Funding
The present research is part of master's thesis of Hesam Yahak, approved by Anatomical Sciences, School of Medicine, Urmia University of Medical Sciences and was financially supported by the Urmia University of Medical Sciences (Grant No.:1398-94).
Authors contributions
Conceptualization, methodology, investigation, writing, and funding: All authors; Supervision: Gholam Hossein Farjah.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the Urmia University of Medical Sciences for their financial support.
References
The aorta artery should be clamped during abdominal and thoracic surgeries [1]. Ischemia is caused by clamping the artery, and its release and subsequent tissue reperfusion damage these tissues [2]. Paraplegia is one of the most serious complications of aortic ischemia-reperfusion (I-R) injury [3]. Recently, many studies have been conducted to reduce the complications of I-R, such as saffron extract [4], lutein [5], erythropoietin [6], and thymoquinone [7].
Gum arabic (GA) comprises several polysaccharides secreted from Senegal Acacia trees [8]. GA also shows anti-oxidant and anti-inflammatory properties [9, 10] and is involved in the apoptotic pathway [11].
A review of the literature shows that GA has a protective effect on several diseases, including lung tumors [12], heart disease [10], and diabetic nephropathy [13]. Our previous study showed that GA and propolis are effective in sciatic nerve regeneration [14]. A previous study reported that the administration of GA protects I-R heart damage through anti-inflammatory and anti-oxidant pathways [10]. To our knowledge, there is no study on the protective effect of GA on I-R injury of the spinal cord. So, the present study aimed to investigate the GA’s protective effect on the spinal cord’s motor neurons after ischemia-reperfusion (I-R) injury using neurological, biochemical, and histological assessments.
Materials and Methods
Animals
Thirty-five Sprague-Dawley male rats (250~280 g weight, 8~10 weeks old) were divided into 5 groups: Intact, sham surgery, control, GA (1 g/kg), and GA (4 g/kg). The GA (Merck, Germany) solution was prepared with distilled water in suitable concentrations (1 and 4 g/kg). In GA groups, oral gavages’ treatment was performed for 21 days before surgery. In the control group, the animals received gavage administration of distilled water (1 mL) for 21 days before surgery [15].
Surgery procedure
The rats were anesthetized by ketamine (90 mg/kg) and xylazine (10 mg/kg) through intraperitoneal injection, followed by heparin (400 IU) [16]. Then, under sterile conditions, the abdominal cavity was opened through a midline longitudinal incision (linea alba). The abdominal viscera were gently removed (from the right side of the peritoneal cavity) and placed on sterile gauze moistened with normal saline. To establish ischemia, the abdominal aortic artery was clamped at two points: Below the left kidney vein and before the bifurcation of the abdominal aortic artery. During the ischemia, the bilateral femoral artery was absent. After 60 minutes, the clamps were gently removed to restore blood flow (reperfusion). At the end of the surgery, the abdominal viscera were returned to the abdomen, and the animal’s muscles and skin were closed with 4-0 nylon threads [5]. During the surgery, the animal’s body temperature was maintained constant around 37ºC. After recovery, the animals were kept in their cages.
Neurological evaluation
The motor deficit index (MDI) test was used to determine the possible paralysis of the hind limbs (before spinal cord I-R and 24, 48, and 72 hours after the start of reperfusion). The score of MDI ranges between 0 (intact) and 6 (complete defect). The animals with an MDI score higher than, were marked as paraplegic [17].
Withdrawal reflex latency (WRL)
The withdrawal reflex latency (WRL) test was used to evaluate the sensation of the hind limbs at 24, 48, and 72 hours after surgery. The plantar surface was placed on a hot plate (56°C). Then, the contact time was recorded. To prevent possible damage, the maximum time allowed to contact the animal’s hind limbs with the hot plate was considered 12 seconds [18].
Blood sampling
The blood was obtained directly from the heart after anesthetizing the rats with ketamine (90 mg/kg). Then, the plasma was separated by centrifuge (1500 g; 15 min; 4°C). The collected plasmas were stored at -80°C until the biochemical evaluations [16].
Biochemical study
The hydroxylamine method was used to measure the level of superoxide dismutase (SOD) in plasma. This method converted hydroxylamine to nitrite and then measured at a wavelength of 550 nm by a spectrophotometer. The catalase (CAT) level in plasma was measured spectrophotometrically after observing the yellow complex at 450 nm wavelength. The plasma’s total anti-oxidant capacity (TAC) level was evaluated using an appropriate kit (LDN, GmbH & Co KG, Germany). The TAC is determined by the production of tetramethylbenzidine, which is detected by color produced at a wavelength of 450 nm by a spectrophotometer. The plasma malondialdehyde (MDA) level was measured using the thiobarbituric acid (TBA) method. MDA is a colorless liquid that reacts with TBA reagent to produce a pink color at a wavelength of 532 nm by spectrophotometer [19].
Histologic evaluation
The animals were fixed through cardiac perfusion, and then the fourth segment of the lumbar spinal cord was isolated. After performing the tissue preparation procedures, 5-μm sections were prepared using H&E staining. Then, slices with an interval of 100 μm were prepared from the fourth lumbar spinal cord segment and observed by a digital light microscope. The number of healthy motor neurons was counted using OLYSIA Autobioreport Software (Olympus Optical, Co. LTD, Tokyo, Japan). Afterward, in the anterior horns of the spinal cord, the neurons with prominent nuclei with delicate chromatin bodies and Nissl bodies found within their cytoplasm were considered healthy motor neurons, and neurons with eosinophilic, non-nucleated cytoplasm were considered damaged cells. An area of 0.04-2 mm was evaluated in the anterior and lateral funiculi. In the samples that did not have cavitation, the tissue was considered normal, and the number 0 was reported; in the tissues with low cavitation (less than 10%), number 1; moderate (10% to 50%), number 2; and the tissues with severe cavitation (more than 50%), the number 3 was reported [20].
Statistical analysis
The data of the present study were displayed as Mean±SEM. Statistical analysis was performed using one-way ANOVA and Tukey’s test in SPSS software, version 16 (Chicago, IL, USA). The Kruskal-Wallis and Mann-Whitney tests were used to find the differences in MDI and cavitations between groups. P<0.05 was statistically significant.
Results
The mean scores of MDI in the control group versus other groups were significantly different up to 72 hours after I-R (P<0.01). The difference between the GA groups of ischemia (after 72 hours) and the intact and surgical sham groups was not significant (P>0.01) (Figure 1). The results of the WRL test show that intact, surgical sham, and GA 4 g/kg groups are significantly different from all other groups up to 72 hours after I-R (P<0.05) (Figure 2). At the mean value of SOD, 72 hours after spinal cord I-R, there was a significant (P<0.05) decrease in the control group versus the intact, surgical sham, and GA 4 g/kg groups (Figure 3). The mean value of CAT, 72 hours after spinal cord I-R, was significantly (P<0.04) reduced in the control group versus the intact and GA 5 g/kg group. CAT level is higher in the GA 1 g/kg group versus the control group (Figure 4). However, it was not significant (P˃0.02).
Mean TAC, 72 hours after spinal cord I-R, was remarkably reduced in the control group versus the intact and surgical sham groups (P=0.00). So, 72 hours after I-R, the TAC level in the GA 4 g/kg group compared to the control group showed a significant (P<0.02) increase (Figure 5). The mean plasma MDA level was higher in the control group versus the intact and surgical sham groups 72 hours after spinal cord I/R (P<0.01). This index decreased in the GA 4 g/kg group versus the control group (P<0.01) (Figure 6). The mean number of healthy spinal motor neurons in the L4 lumbar segment in GA groups considerably increased compared to the control group (P<0.01). In addition, the mean number of healthy motor neurons in the GA 4 g/kg group was significantly higher than the GA 1 g/kg group (P<0.05). The number of healthy motor neurons in the GA 4 g/kg group did not show a significant difference (P>0.05) compared to the intact and surgical sham groups (Figure 7). The mean injury of spinal cord white matter 72 hours after ischemia in the control group increased considerably versus the intact and surgical sham groups (P<0.001). It also significantly decreased in GA groups versus the control group (P<0.04). In addition, GA groups showed a significant (P<0.01) difference compared to intact and surgical sham groups (Figure 8).
Discussion
According to the results of this study, after 60 minutes of ischemia and then up to 72 hours of reperfusion, less damage was observed in the spinal cords, white and gray matter in animals receiving GA, and the hind limbs also showed better performance.
Ischemia and reperfusion may eventually lead to paralysis of the lower limbs and a decrease in the quality of nerve function in the affected areas, which can be attributed to factors such as disharmony in the regulation of neurotransmitters [21].
A previous study showed that GA combined with propolis as a nerve guide channel improves motor reflexes [14]. The present study is the first one to investigate the protective effect of GA on ischemia-reperfusion injury in the spinal cord.
MDI can be used in neurological assessment, and lower scores are desirable. Our results showed that the MDI in the GA groups was significantly less than in the control group, and the results of the WRL test showed an improvement in the sensory function of the hind limbs of the animals. After I-R, oxygen free radicals are formed, resulting in the cell membrane losing its proper function due to the disruption of cytokine secretion, resulting in leakage of the cell internals, which produces more reactive oxygen species [22]. These species and free radicals affect lipids by making lipid peroxides, causing direct damage to nerve cells since lipids are involved in the structure of neurons [23]. GA can be beneficial in neutralizing oxidative stress and inflammation caused by oxygen free radicals [22] and prevents lipid peroxide formation [24]. A study showed the use of GA decreased the oxidants and inflammatory factors (pro-inflammatory cytokines, tumor necrosis factor-alpha, interleukin-1β, interleukin-6) and increased SOD and anti-inflammatory factors (cytokine IL-10) [10]. SOD and CAT are among the anti-oxidant enzymes that neutralize oxygen free radicals, so their measurement can help understand the effectiveness of the substance used [25]. In addition, TAC is a suitable biochemical parameter for measuring the overall status of anti-oxidants [26]. In addition, MDA is a reliable indicator for evaluating oxygen free radicals and lipid peroxidation [25] and is used as an indicator of oxidative stress in cells [27]. The previous study shows that the levels of CAT and TAC have an inverse relationship with oxidative stress. In addition, a higher level of SOD has a direct effect on reducing oxidative stress. High levels of MDA were observed in acute oxidative stress, which indicates a direct relationship between the MDA index and levels of oxidative stress [28]. The results of the present study show that the levels of SOD, CAT, and TAC, as well as the number of healthy motor neurons in the GA 4 g/kg group, were significantly more than the control group, which may be due to the protective effect of GA on the motor neurons of the spinal cord. The role of GA in reducing MDA production [24] is another reason for increasing the percentage of healthy motor neurons and improving the function of the lower limbs. GA is a natural substance that can be taken orally without side effects [8], and at the same time, it can appear as a drug to strengthen and improve motor reflexes [29].
In this study, the white matter in the GA groups was healthier than the control group. Due to the protective effect of GA on neurons, more myelinated axons remained intact, and fewer cavities were formed in the white matter compared to the control group.
In the present study, GA was administered two doses of 1 and 4 g/kg through gavage. The previous study showed that the results obtained from the administration of GA in doses between 3 and 6 g do not show a significant difference in the coagulation system [30]. In another study, GA was prescribed with doses of 2, 3, and 4 g/kg body weight, and they concluded that the dose of 4 g/kg has the most beneficial effect [31]. GA shows different effects depending on the conditions and context of use, which is an interesting feature. Still, in general, it can be concluded that it can show more favorable effects at a dose of about 4 g/kg [21]. Similar mechanisms may effectively explain the role of the GA in protecting the spinal cord. Thus, GA increased the level of the anti-oxidant enzyme SOD, which showed that it plays an essential role in protecting the spinal cord against I-R injury [10]. The protection effect observed in this study is related to the anti-oxidant and anti-inflammatory activities of GA [9]. In addition, many mechanisms are unknown so far, and more experimental evidence is needed to understand them.
Conclusion
This study shows that GA has anti-oxidant and anti-inflammatory properties and, therefore, may be effective in reducing oxidative stress, preventing damage to spinal cord motor neurons, and protecting spinal cord white matter.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Urmia University of Medical Sciences approved this study (Code:IR.UMSU.REC.1399.124, date: July 22, 2020).
Funding
The present research is part of master's thesis of Hesam Yahak, approved by Anatomical Sciences, School of Medicine, Urmia University of Medical Sciences and was financially supported by the Urmia University of Medical Sciences (Grant No.:1398-94).
Authors contributions
Conceptualization, methodology, investigation, writing, and funding: All authors; Supervision: Gholam Hossein Farjah.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the Urmia University of Medical Sciences for their financial support.
References
- Behzadi F, Simon JE, Zielke TJ, Cook JT, Costa RA, Bechara CF, et al. Risk factors associated with spinal cord ischemia during aortic aneurysm repair. Ann Vasc Surg. 2023; 91:36-49. [DOI:10.1016/j.avsg.2022.12.079] [PMID]
- Sánchez-Hernández CD, Torres-Alarcón LA, González-Cortés A, Peón AN. Ischemia/reperfusion injury: Pathophysiology, current clinical management, and potential preventive approaches. Mediators Inflamm. 2020; 2020:8405370. [DOI:10.1155/2020/8405370] [PMID]
- Khachatryan Z, Haunschild J, von Aspern K, Borger MA, Etz CD. Ischemic spinal cord injury-experimental evidence and evolution of protective measures. Ann Thorac Surg. 2022; 113(5):1692-702. [DOI:10.1016/j.athoracsur.2020.12.028] [PMID]
- Farjah GH, Salehi S, Ansari MH, Pourheidar B. Protective effect of Crocus sativus L. (Saffron) extract on spinal cord ischemia-reperfusion injury in rats. Iran J Basic Med Sci. 2017; 20(3):334-7. [PMID]
- Mohammad Pour M, Farjah GH, Karimipour M, Pourheidar B, Khadem Ansari MH. Protective effect of lutein on spinal cord ischemia-reperfusion injury in rats. Iran J Basic Med Sci. 2019; 22(4):412-7. [PMID]
- Golmohammadi MG, Banaei S, Nejati K, Chinifroush-Asl MM. Vitamin D3 and erythropoietin protect against renal ischemia-reperfusion injury via heat shock protein 70 and microRNA-21 expression. Sci Rep. 2020; 10(1):20906. [DOI:10.1038/s41598-020-78045-3] [PMID]
- Parlar A, Arslan SO. Thymoquinone reduces ischemia and reperfusion-induced intestinal injury in rats, through anti-oxidative and anti-inflammatory effects. Turk J Surg. 2020; 36(1):96-104. [DOI:10.5578/turkjsurg.4583] [PMID]
- Al-Jubori Y, Ahmed NTB, Albusaidi R, Madden J, Das S, Sirasanagandla SR. The efficacy of gum arabic in managing diseases: A Systematic review of evidence-based clinical trials. Biomolecules. 2023; 13(1):138. [DOI:10.3390/biom13010138] [PMID]
- García-Martínez E, Andújar I, Yuste Del Carmen A, Prohens J, Martínez-Navarrete N. Anti-oxidant and anti-inflammatory activities of freeze-dried grapefruit phenolics as affected by gum arabic and bamboo fibre addition and microwave pretreatment. J Sci Food Agric. 2018; 98(8):3076-83. [DOI:10.1002/jsfa.8807] [PMID]
- Gouda E, Babiker F. Gum Arabic protects the rat heart from ischemia/reperfusion injury through anti-inflammatory and anti-oxidant pathways. Sci Rep. 2022; 12(1):17235.[DOI:10.1038/s41598-022-22097-0] [PMID]
- Ahmed N, El-Rayes SM, Khalil WF, Abdeen A, Abdelkader A, Youssef M, et al. Arabic gum could alleviate the aflatoxin B1-provoked hepatic injury in rat: The involvement of oxidative stress, inflammatory, and apoptotic pathways. Toxins (Basel). 2022; 14(9):605. [DOI:10.3390/toxins14090605] [PMID]
- Gamal-Eldeen AM, Moustafa D, El-Daly SM, Abo-Zeid MAM, Saleh S, Khoobchandani M, et al. Gum Arabic-encapsulated gold nanoparticles for a non-invasive photothermal ablation of lung tumor in mice. Biomed Pharmacother. 2017; 89:1045-54. [DOI:10.1016/j.biopha.2017.03.006] [PMID]
- Mohammed ME, Abbas AM, Badi RM, Bashir SO, Osman OM, Morsy MD, et al. Effect of Acacia senegal on TGF-β1 and vascular mediators in a rat model of diabetic nephropathy. Arch Physiol Biochem. 2022; 128(6):1548-58. [DOI:10.1080/13813455.2020.1781901] [PMID]
- Nosratiyan M, Farjah GH, Karimipour M, Pourheidar B. The effect of propolis-gum Arabic as a novel nerve guidance channel on regeneration of sciatic nerve in male rats. Turk Neurosurg. 2021; 31(3):361-7. [DOI:10.5137/1019-5149.JTN.29813-20.2] [PMID]
- Jarrar AH, Stojanovska L, Apostolopoulos V, Feehan J, Bataineh MF, Ismail LC, et al. The effect of gum Arabic (Acacia Senegal) on cardiovascular risk factors and gastrointestinal symptoms in adults at risk of metabolic syndrome: A randomized clinical trial. Nutrients. 2021; 13(1):194. [DOI:10.3390/nu13010194] [PMID]
- Karimipour M, Farjah GH, Molazadeh F, Ansari M, Pourheidar B. Protective effect of contralateral, ipsilateral, and bilateral remote ischemic preconditioning on spinal cord ischemia reperfusion injury in rats. Turk Neurosurg. 2019; 29(6):933-9. [DOI:10.5137/1019-5149.JTN.26237-19.3] [PMID]
- Taira Y, Marsala M. Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke. 1996; 27(10):1850-8. [DOI:10.1161/01.STR.27.10.1850] [PMID]
- Farjah GH, Fazli F, Karimipour M, Pourheidar B, Heshmatiyan B, Pourheidar M. The effect of bone marrow mesenchymal stem cells on recovery of skeletal muscle after neurotization surgery in rat. Iran J Basic Med Sci. 2018; 21(3):236-43. [PMID]
- Hajizadeh N, Farjah GH, Karimipour M, Pourheidar B. Protective Effect of catechin hydrate on spinal cord ischemia-reperfusion injury in rats. Caspian J Neurol Sci. 2021; 7 (4):193-201. [DOI:10.32598/CJNS.7.27.4]
- Kanellopoulos GK, Xu XM, Hsu CY, Lu X, Sundt TM, Kouchoukos NT. White matter injury in spinal cord ischemia: Protection by AMPA/kainate glutamate receptor antagonism. Stroke. 2000; 31(8):1945-52. [DOI:10.1161/01.STR.31.8.1945] [PMID]
- Wang L, Li S, Liu Y, Feng DL, Jiang L, Long ZY, Wu YM. Motor neuron degeneration following glycine-mediated excitotoxicity induces spastic paralysis after spinal cord ischemia/reperfusion injury in rabbit. Am J Transl Res. 2017; 9(7):3411-21. [PMID]
- Ali BH, Al Za’abi M, Al Suleimani Y, Manoj P, Ali H, Ribeiro DA, et al. Gum arabic reduces inflammation, oxidative, and nitrosative stress in the gastrointestinal tract of mice with chronic kidney disease.Naunyn Schmiedebergs Arch Pharmacol. 2020; 393(8):1427-36. [DOI:10.1007/s00210-020-01844-y] [PMID]
- Hall ED. Anti-oxidant therapies for acute spinal cord injury. Neurotherapeutics. 2011; 8(2):152-67. [DOI:10.1007/s13311-011-0026-4] [PMID]
- Ahmed AA, Fedail JS, Musa HH, Musa TH, Sifaldin AZ. Gum Arabic supplementation improved anti-oxidant status and alters expression of oxidative stress gene in ovary of mice fed high fat diet. Middle East Fertil Soc J. 2016; 21(2): 101-8. [DOI:10.1016/j.mefs.2015.10.001]
- Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019; 2019:5080843. [DOI:10.1155/2019/5080843] [PMID]
- Mahjoub S, Ghasempour M, Gharage A, Bijani A, Masrourroudsari J. Comparison of total anti-oxidant capacity in saliva of children with severe early childhood caries and caries-free children. Caries Res. 2014; 48(4):271-5. [DOI:10.1159/000355581] [PMID]
- Makri OE, Ferlemi AV, Lamari FN, Georgakopoulos CD. Saffron administration prevents selenite-induced cataractogenesis. Mol Vis. 2013; 19:1188-97. [PMID]
- Goyal T, Mitra P, Singh P, Sharma P, Sharma S. Evaluation of oxidative stress and pro-inflammatory cytokines in occupationally cadmium exposed workers. Work. 2021; 69(1):67-73. [DOI:10.3233/WOR-203302] [PMID]
- Binjumah M, Ajarem J, Ahmad M. Effects of the perinatal exposure of Gum Arabic on the development, behavior and biochemical parameters of mice offspring. Saudi J Biol Sci. 2018; 25(7):1332-38. [DOI:10.1016/j.sjbs.2016.04.008] [PMID]
- Hadi AA, Elderbi MA, Mohamed AH. Effect of gum Arabic on coagulation system of albino rats. Int J Pharm Tech Res. 2010; 2(3):1762-6. [Link]
- Wadood A, Wadood N, Shah SA. Effects of Acacia arabica and Caralluma edulis on blood glucose levels of normal and alloxan diabetic rabbits. J Pak Med Assoc. 1989; 39(8):208-12. [PMID]
Type of Study: Research |
Subject:
Special
Received: 2024/01/28 | Accepted: 2024/01/20 | Published: 2024/01/20
Received: 2024/01/28 | Accepted: 2024/01/20 | Published: 2024/01/20
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |