Sat, May 18, 2024
Volume 10, Issue 1 (Winter 2024)
Caspian J Neurol Sci 2024, 10(1): 38-45 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sopuluchukwu Udodi P, Vivian Aneke O, Udodi R E. Upregulation of Metabolic Defense System After Short-term Exposure to Formulated Insecticide. Caspian J Neurol Sci 2024; 10 (1) :38-45
URL: http://cjns.gums.ac.ir/article-1-689-en.html
URL: http://cjns.gums.ac.ir/article-1-689-en.html
1- Department of Anatomy, Faculty of Basic Medical Sciences, College of Health Sciences, Nnamdi Azikiwe University, Nnewi Campus, Nnewi, Nigeria.
2- Department of Anatomy, Faculty of Basic Medical Sciences, College of Health Sciences, Delta State University, Abraka, Nigeria
2- Department of Anatomy, Faculty of Basic Medical Sciences, College of Health Sciences, Delta State University, Abraka, Nigeria
Full-Text [PDF 2750 kb]
(86 Downloads)
| Abstract (HTML) (185 Views)
Full-Text: (63 Views)
Introduction
The recent increase in malaria vector resistance to insecticide has led to several efforts to control the vectors in areas prone to mosquito activity [1]. Due to this resistance, the only choice left to the residents of the affected areas is to use multiple insecticides to stop the spread of malaria.
The substantia nigra, which is significantly affected in Parkinson disease, is one of the motor areas of the brain impacted by this insecticide mixture [2]. The substantia nigra is a midbrain dopaminergic nucleus with nigrostriatal fibers that interacts with astrocytes (metabolic cells) to maintain its function in regulating movement and reward behavior. Astrocytes generally play the vital roles of structural support, neuroprotection, metabolic regulation, growth, and homeostatic maintenance of the central nervous system components [3, 4]. Thus, ischemia and other brain injuries that affect neuron survival may make astrocytes vulnerable. During times of high demand, astrocytes produce glycogen, provide neuronal cells with glycolytic components, and clear the brain tissues of free radicals [5]. Astrocytes carry out these actions through functional gap junctions, ion channels, and several projections, which link them to neurons and their vascular supply within the central nervous system (CNS). Because of this structural link, the astrocytes can monitor and communicate with the extracellular environment and the neuronal connections. Inter-astrocyte communication is made possible by gap junctions created between astrocytes and glial transmission. They function through calcium signaling and contain connexins 30 and 43 [5]. When cells are first exposed to toxicity, astrocytes use their structural and functional advantages to shield the cells, build resistance, and boost their metabolic activity to store more energy for cellular survival [5].
Several studies have established the neurodegenerative impact of formulated pyrethroids [6]. However, there is a paucity of research on the defense system of substantia nigra to unveil its role in the increasing cases of motor diseases among residents of mosquito-prone environments and possible therapeutic windows. This study aims to investigate the defense mechanisms of astrocytes in the substantia nigra against formulated pyrethroids and explore potential therapeutic strategies for motor diseases prevalent in insecticide-heavy environments.
Materials and Methods
Chemical procurement
Analytical standards for cypermethrin and dichlorvos were bought from the Oblong Science Store in Onitsha, Anambra State, Nigeria. The Industrial Chemistry Department at Nnamdi Azikiwe University in Awka (NAU) verified the chemicals using the authentication code AU131.
Experimental animals
A total of 32 male adult Wistar rats weighing around 180–200 g were placed in a controlled environment at a temperature of 25°C–28°C, relative humidity of 60%–80%, and a photoperiodicity of 12 hours light and 12 hours dark for 14 days to acclimate them. Agro Feed Mill Nigeria Ltd. supplied the animals with guinea feed pellets and unrestricted access to water. The animals were handled with the approval of the Ethics Committee of the Faculty of Basic Medical Sciences, College of Health Sciences, Nnamdi Azikiwe University, Nnewi Campus. The committee followed the guidelines for the care and use of laboratory animals based on the publication of “guide for the care and use of laboratory animals” [7].
Acute toxicity test (LC50) of cypermethrin and dichlorvos insecticide
The results of the insecticides’ acute toxicity tests at the Department of Biochemistry at Nnamdi Azikiwe University were 5 mm-1 (4.4 ppm) for dichlorvos and 10 mm-1 (8.7 ppm) for cypermethrin. These findings aligned with the Organization for Economic Cooperation and Development’s (OCED) methodology [8].
Animal grouping and exposure
The animals were randomly assigned to 4 groups: A, B, C, and D, each containing 8 animals. In group A, rats were exposed to fresh air, and over 4 weeks, rats in groups B, C, and D were exposed to a pyrethroid insecticide mixture prepared with dichlorvos and cypermethrin for 2, 3, and 4 h/d, respectively.
The exposure procedure
The animals’ whole bodies could spend time inside a controlled chamber thanks to using a dynamic inhalation exposure system as a whole-body inhalation chamber. The animal’s trunk was covered in local skin coverings (a local nonharmful gelatinous substance used to cover treated wounds to prevent absorbents) to avoid the little amount of pyrethroid known to be absorbed via the skin. Two gas heater pumps for the two chemicals were used to create the insecticides’ gaseous form. Depending on the group exposed, the gaseous levels of cypermethrin and dichlorvos in the air were maintained for a specific amount of time while the animals in each group were immersed in the test atmosphere that was regulated with the amount of gas necessary as determined by the LC50. This measure was required to guarantee the insecticide’s precise concentration during the study period, as stated by Cheng et al. [9].
Animal sacrifice
There were no fatalities in the study. The animals were killed 24 hours after their final insecticide exposure, and three brain samples from each group were preserved in 10% neutral-buffered formalin for 48 hours to be used for astrocyte immunohistochemistry research, while 5 brain samples from each group were homogenized immediately for glucose assessment.
Behavioral function test
Wire suspension test was utilized for motor assessment, according to Moran et al. [10]. The animal’s forepaws were hung 30 cm above a soft, flat surface on a 2-mm diameter metal rod. In three successive trials, the pups’ time to lose their hold and fall to the soft surface was recorded and used to gauge the animals’ motor skills. The reflex was deemed fully acquired when the animal could hang on to the bar for 30 s [10].
Glucose assessment
A key substrate for energy metabolism in brain tissue was determined to be glucose. Five animal brain tissues weighing about 1 g each were homogenized at room temperature after being dipped in 10 mL of 0.9% normal saline. The supernatant was extracted and centrifuged for 20 minutes at ambient temperature at 3000 rpm. Using reagents from the Trace Company, glucose was tested by the glucose oxidase-peroxidase (GOD-PAP) technique [11]. At 500 nm, the samples’ absorbance was measured and compared to a blank and a standard.
Tissue processing
Forty-eight hours after fixing, the tissues were rinsed under running water to remove any remaining fixatives. The brain tissues were dehydrated using alcohol in a graded sequence to remove water and other impurities. After effectively dehydrating the tissues, they were washed for 2 h in xylene. Then, for 4 h, they were infiltrated with melted paraffin wax at a temperature of 60°C. After the paraffin wax set, the tissues were molded onto a tissue block and sectioned to obtain 3-µm tissue sections [12].
Glial fibrillary acidic protein (GFAP) staining method
Sections measuring approximately 3-µm thick were cut, deparaffinized, and rehydrated on coated slides. In a pH-6.0 citrate-based antigen retrieval solution, antigen retrieval was conducted for 30 minutes. After that, the sections were processed using the mouse and rabbit HRP/DAB IHC detection kits. For 10 minutes, endogenous peroxidase was blocked. After that, the primary antibodies were added, specifically mouse anti-GFAP (1:5000 dilutions; 2 h, 30 min). The sections were treated with the HRP (horseradish peroxidase) micro-polymer goat anti-rabbit HRP (Abcam USA) for 30 minutes. The reaction was produced using DAB chromogen (Abcam, USA). After rinsing the sections with water, Mayer’s hematoxylin was used as a counterstain. The procedure was dehydration, cleaning, and mounting with Dako dibutyl phthalate xylene.
Photomicrograph and image quantification
The treated tissues were examined using a digital light microscope, and OMAX software, version 32.0, Patch #7 was used to take digital photomicrographs of the samples at x400 magnifications. Using the cell counter plugin, NIH-sponsored ImageJ software, version 1.5.3 was utilized to analyze photomicrographs [13] digitally.
Data analysis
The data analysis program utilized was SPSS software, version 27.0.1. One-way analysis of variance (ANOVA) was used to get the Mean±SE of the mean when comparing values between groups. P<0.05 were used for determining statistical significance, and the data were given as Mean±SEM.
Result
Wire suspension test for the assessment of motor function
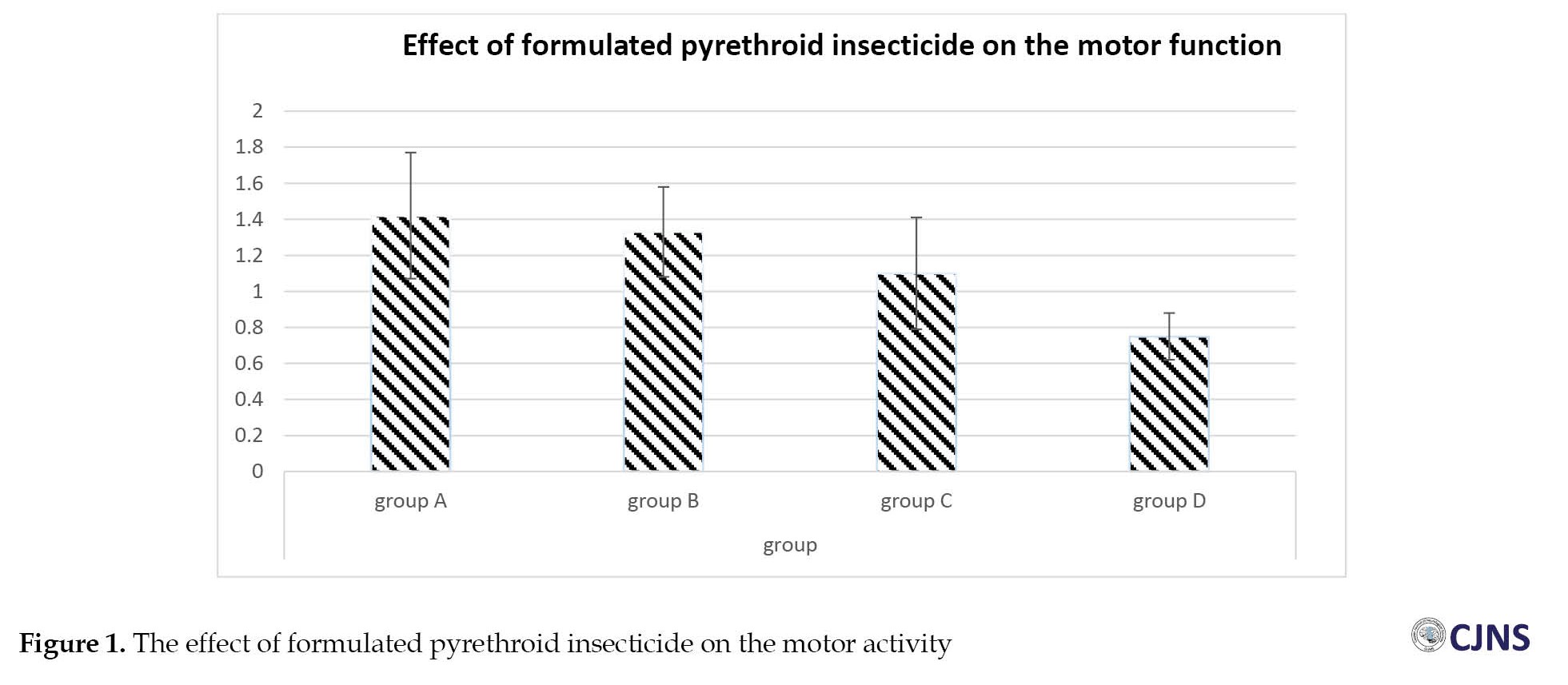
The neurobehavioral function test results are shown in Table 1.

The mean time spent by the experimental and the control groups while hanging on a wire is contrasted in the table. There is no significant decrease (P=0.05) in the time the animals spent hanging on the wire among the experimental groups.
The outcome of the neurobehavioral function test, performed on the final day of the exposure, is shown in the bar chart in Figure 1. The mean period spent by the experimental and control groups is shown in the above bar chart. Compared to group A, groups B, C, and D showed a decrease in the time the animals spent hanging on the wire; however, none of the differences were statistically significant.
Substrate for energy metabolism in the brain
The data about brain glucose levels in each group is shown in Table 2.

Compared to the control, all three test groups (B, C, and D) showed elevated glucose levels, all statistically significant.
The effect of a pyrethroid insecticide mixture consisting of dichlorvos and cypermethrin on glucose levels in the brain is shown in the bar chart in Figure 2. This outcome was assessed after the animals were exposed. The above bar chart describes the mean value of the animals in the control group (A) and the mean values of the experimental groups (B, C, and D). Compared to the control group, there is a statistically significant increase in the experimental groups.
The expression of astrocyte in substantia nigra
The expression of astrocytes in the substantia nigra of animals across different groups is shown in Table 3.

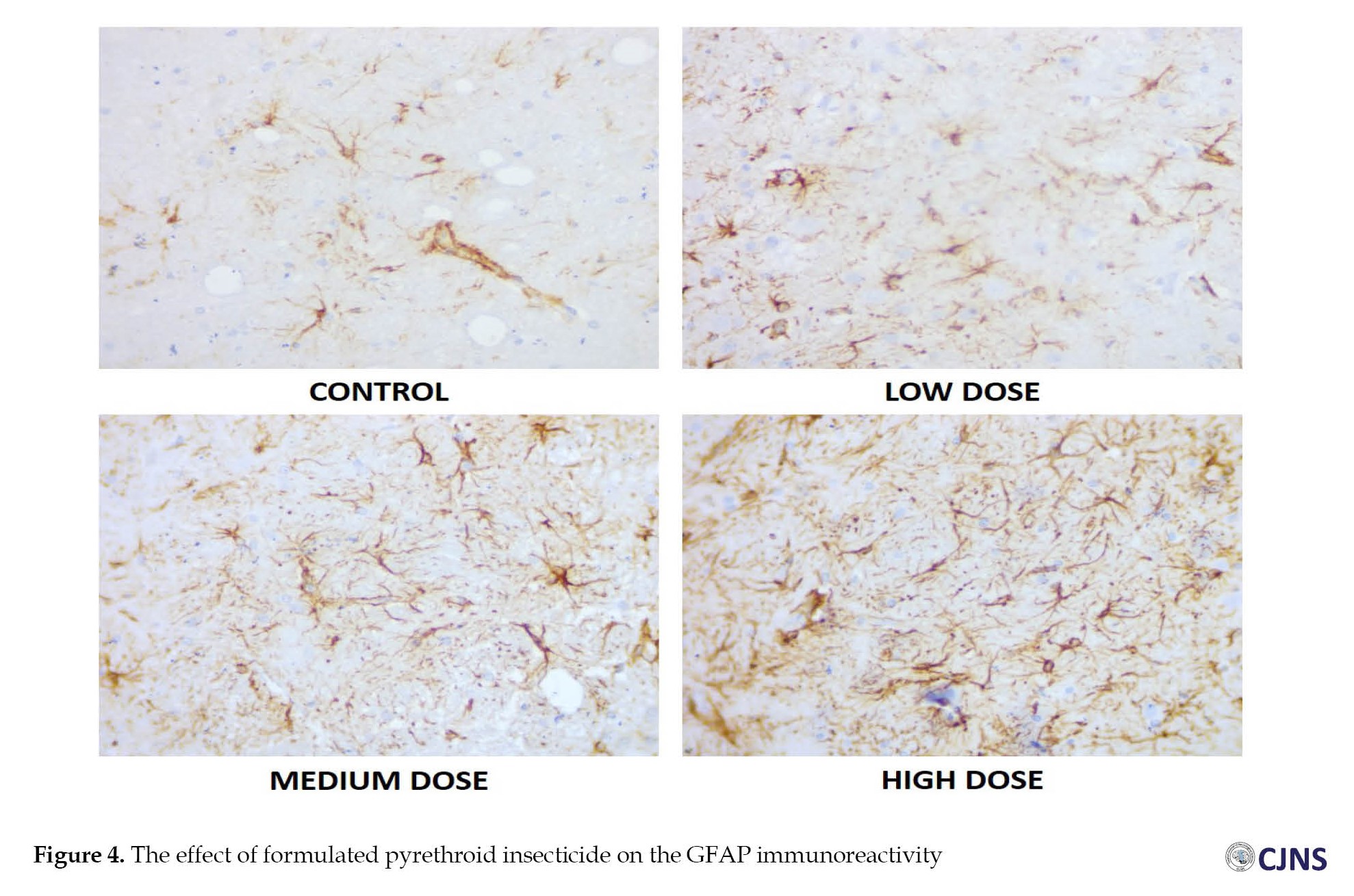
Compared to the control, the astrocyte expression in group B (short-term exposed animals) shows a non-significant increase. In contrast, group C (medium-term exposed animals) and group D (long-term exposed animals) present a statistically significant increase (Figure 3). The effect of the synthetic pyrethroid insecticide on astrocyte expression is shown in the bar chart in Figure 4. A comparison was made between the experimental groups’ (B, C, and D) and the control group’s (A) mean values. Although there was an increase in the experimental groups relative to the control, only groups C and D showed a statistically significant increase (Figure 4).
Immunohistochemical demonstration of astrocyte using GFAP
The expression of astrocytes in the substantia nigra of an adult Wistar rat is shown in the micrograph above across 4 groups. The short-term, medium-term, and long-term groups exhibit increased astrocytic expression compared to the control group following a four-week exposure to a formulated pyrethroid insecticide mixture. The animals in the high-dose group show the highest expression of astrocytes in the test groups, whereas the animals in the low-dose group show the lowest expression.
Discussion
Numerous studies have identified pyrethroid insecticide mixtures as neurotoxic substances. Still, it is unknown what the activities of the brain’s defensive structure are in such xenobiotic inversions to establish a viable therapeutic window. The substantia nigra is one of the motor centers impacted by this toxin [6]. Due to increased astrocyte homeostatic function, the exposed group shows a non-significant motor deficit in the neurobehavioral test [14]. Less time was spent hanging on the wire by the animals in the exposed groups during the neurobehavioral test. This result reveals how pyrethroids impact nigrostriatal dopaminergic neurodegeneration and behavioral abnormalities [15]. According to Tennekes [16] and Verma et al., time dependency is a characteristic feature of toxicity [17]. They proposed that the longer a toxic substance is exposed, the greater the effects. So, the period of suspension decreases with high exposure time. This finding suggests that the animals exposed to high doses of the substance spent the shortest time on the suspension wire, followed by those exposed to medium and low doses. Extended (more than one month) exposure to these insecticides may result in more severe manifestations of adverse health consequences [18, 19], including the inability of an astrocyte to sustain its homeostatic function in the brain.
Pyrethroid insecticides are frequently used and can have a toxic effect on the nervous system, presenting symptoms like limb weakness, memory loss, vision loss, cognitive and behavioral problems, and metabolic diseases like metabolic syndrome and diabetes mellitus, which involve improper glucose regulation and are conspicuously linked to environmental toxins [20]. The astrocytic defense system was activated in this scientific study, which required the proliferating astrocyte to absorb more circulatory glucose and reduce its glycolytic capability [21]. This condition of high glucose level was demonstrated in this study to satisfy the high energy need for cellular survival in xenobiotic penetrance conditions. Like other organ systems, glucose metabolism in the nervous system uses the tricarboxylic acid cycle (TCA) activity, the pentose phosphate route, glycolysis, and glycogenesis, unique to astrocytes. These processes also produce numerous metabolic intermediates, including lactate and glutamate, which are energy storage. Interestingly, aspartate and glutathione, crucial for many cellular functions, are also made from CNS glucose. Gamma-aminobutyric acid and glutamate are just two neurotransmitters produced from CNS glucose [22]. Also, glutamate, the main excitatory neurotransmitter released from neurons and causes the induction of glycolysis in nearby astrocytes, further proves the metabolic connection between astrocytic glucose uptake and neuronal activity [23].
Astrocytes normally provide structural support for the nervous system, regulate metabolism, protect neurons from harm, and promote the growth and homeostasis of neurons [3]. Astrocytes in the brain are responsible for tissue repair following trauma and for regulating water, ions, and glutamate in all brains, regardless of age or health [24]. By fostering neuronal health and function in certain ways, astrocytes regulate the microenvironment and maintain homeostasis [25]. In this study, we found that the expression of astrocytes considerably increased when the experimental mice were subjected to a pyrethroid insecticide mixture of dichlorvos and cypermethrin. The downregulation of normal astrocyte function can be a major neurodegeneration factor because astrocytes are necessary for neuronal survival [26, 27]. An increase in glucose was observed here to ensure neuronal activation along the nigrostriatal pathway [22]. Since disruption of astrocytic function has far-reaching effects, understanding how astrocytes react to toxins is crucial for internalizing the impact on the entire brain. This outcome is partially explained by the fact that astrocytes are more abundant than other synapses [28] and that they are connected to the brain’s blood vessels [29], where they act as the first line of defense to prevent foreign objects from entering the central nervous system [30]. The differences observed between the glucose concentration in the parenchyma (0.5–1 mM) and the blood glucose concentration in CNS capillaries (roughly 3–6 mM) [31] indicate that astrocytic projections wrapping around the vasculature [32] are necessary for glucose uptake and subsequent entry into the parenchyma, specifically through the glucose transporter-1 (GLUT1). The idea that the cytoarchitectural proximity of astrocytes to neurons is essential for neuronal support is corroborated by the fact that, despite requiring much less glucose than neurons do to maintain their physiological function, astrocytes are positioned to be the first recipients of this energetic substrate [22]. The GLUT1 transporter at the astrocyte-to-neuron interface provides a viable method by which astrocytes react to metabolic stress and meet glutamate clearance requirements [32]. Astrocytes and glucose increased simultaneously in response to pyrethroid toxicity, demonstrates how important these cells are for maintaining the overall health of the central nervous system, including the brain. This scientific study expands our understanding of how astrocytes behave when exposed to harmful chemicals like a mixture of pyrethroid insecticides. This study recommends boosting astrocytes and glucose as a therapeutic strategy for mitigating motor impairments in long-term exposure to such toxicity as pyrethroid insecticides, which will continue to be used.
Conclusion
According to the findings of this study, astrocytes and glucose are both elevated at the outset of xenobiotic toxicity to stabilize the activity of the substantia nigra. This study recommends boosting astrocytes and glucose as a therapeutic intervention for mitigating motor deficits caused by long-term exposure to such toxicity because pyrethroid insecticides will continue to be used.
Ethical Considerations
Compliance with ethical guidelines
The study was authorized by the Ethics Committee of the Faculty of Basic Medical Sciences, College of Health Sciences, Nnamdi Azikiwe University (Code: EA/2022/108).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Study design and writing: Princewill Sopuluchukwu Udodi; Acquiration of the animals; training the animals, exposing the animals to toxic agents, anesthetization, harvesting and the tissue of interest and fixation: Oluoma Vivian Aneke; Processing the tissues, biochemical and histological investigation: Roseline Ebube Udodi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors appreciate Damian N Ezejindu and Izuchukwu J Abugu for their help during this research work.
References
The recent increase in malaria vector resistance to insecticide has led to several efforts to control the vectors in areas prone to mosquito activity [1]. Due to this resistance, the only choice left to the residents of the affected areas is to use multiple insecticides to stop the spread of malaria.
The substantia nigra, which is significantly affected in Parkinson disease, is one of the motor areas of the brain impacted by this insecticide mixture [2]. The substantia nigra is a midbrain dopaminergic nucleus with nigrostriatal fibers that interacts with astrocytes (metabolic cells) to maintain its function in regulating movement and reward behavior. Astrocytes generally play the vital roles of structural support, neuroprotection, metabolic regulation, growth, and homeostatic maintenance of the central nervous system components [3, 4]. Thus, ischemia and other brain injuries that affect neuron survival may make astrocytes vulnerable. During times of high demand, astrocytes produce glycogen, provide neuronal cells with glycolytic components, and clear the brain tissues of free radicals [5]. Astrocytes carry out these actions through functional gap junctions, ion channels, and several projections, which link them to neurons and their vascular supply within the central nervous system (CNS). Because of this structural link, the astrocytes can monitor and communicate with the extracellular environment and the neuronal connections. Inter-astrocyte communication is made possible by gap junctions created between astrocytes and glial transmission. They function through calcium signaling and contain connexins 30 and 43 [5]. When cells are first exposed to toxicity, astrocytes use their structural and functional advantages to shield the cells, build resistance, and boost their metabolic activity to store more energy for cellular survival [5].
Several studies have established the neurodegenerative impact of formulated pyrethroids [6]. However, there is a paucity of research on the defense system of substantia nigra to unveil its role in the increasing cases of motor diseases among residents of mosquito-prone environments and possible therapeutic windows. This study aims to investigate the defense mechanisms of astrocytes in the substantia nigra against formulated pyrethroids and explore potential therapeutic strategies for motor diseases prevalent in insecticide-heavy environments.
Materials and Methods
Chemical procurement
Analytical standards for cypermethrin and dichlorvos were bought from the Oblong Science Store in Onitsha, Anambra State, Nigeria. The Industrial Chemistry Department at Nnamdi Azikiwe University in Awka (NAU) verified the chemicals using the authentication code AU131.
Experimental animals
A total of 32 male adult Wistar rats weighing around 180–200 g were placed in a controlled environment at a temperature of 25°C–28°C, relative humidity of 60%–80%, and a photoperiodicity of 12 hours light and 12 hours dark for 14 days to acclimate them. Agro Feed Mill Nigeria Ltd. supplied the animals with guinea feed pellets and unrestricted access to water. The animals were handled with the approval of the Ethics Committee of the Faculty of Basic Medical Sciences, College of Health Sciences, Nnamdi Azikiwe University, Nnewi Campus. The committee followed the guidelines for the care and use of laboratory animals based on the publication of “guide for the care and use of laboratory animals” [7].
Acute toxicity test (LC50) of cypermethrin and dichlorvos insecticide
The results of the insecticides’ acute toxicity tests at the Department of Biochemistry at Nnamdi Azikiwe University were 5 mm-1 (4.4 ppm) for dichlorvos and 10 mm-1 (8.7 ppm) for cypermethrin. These findings aligned with the Organization for Economic Cooperation and Development’s (OCED) methodology [8].
Animal grouping and exposure
The animals were randomly assigned to 4 groups: A, B, C, and D, each containing 8 animals. In group A, rats were exposed to fresh air, and over 4 weeks, rats in groups B, C, and D were exposed to a pyrethroid insecticide mixture prepared with dichlorvos and cypermethrin for 2, 3, and 4 h/d, respectively.
The exposure procedure
The animals’ whole bodies could spend time inside a controlled chamber thanks to using a dynamic inhalation exposure system as a whole-body inhalation chamber. The animal’s trunk was covered in local skin coverings (a local nonharmful gelatinous substance used to cover treated wounds to prevent absorbents) to avoid the little amount of pyrethroid known to be absorbed via the skin. Two gas heater pumps for the two chemicals were used to create the insecticides’ gaseous form. Depending on the group exposed, the gaseous levels of cypermethrin and dichlorvos in the air were maintained for a specific amount of time while the animals in each group were immersed in the test atmosphere that was regulated with the amount of gas necessary as determined by the LC50. This measure was required to guarantee the insecticide’s precise concentration during the study period, as stated by Cheng et al. [9].
Animal sacrifice
There were no fatalities in the study. The animals were killed 24 hours after their final insecticide exposure, and three brain samples from each group were preserved in 10% neutral-buffered formalin for 48 hours to be used for astrocyte immunohistochemistry research, while 5 brain samples from each group were homogenized immediately for glucose assessment.
Behavioral function test
Wire suspension test was utilized for motor assessment, according to Moran et al. [10]. The animal’s forepaws were hung 30 cm above a soft, flat surface on a 2-mm diameter metal rod. In three successive trials, the pups’ time to lose their hold and fall to the soft surface was recorded and used to gauge the animals’ motor skills. The reflex was deemed fully acquired when the animal could hang on to the bar for 30 s [10].
Glucose assessment
A key substrate for energy metabolism in brain tissue was determined to be glucose. Five animal brain tissues weighing about 1 g each were homogenized at room temperature after being dipped in 10 mL of 0.9% normal saline. The supernatant was extracted and centrifuged for 20 minutes at ambient temperature at 3000 rpm. Using reagents from the Trace Company, glucose was tested by the glucose oxidase-peroxidase (GOD-PAP) technique [11]. At 500 nm, the samples’ absorbance was measured and compared to a blank and a standard.
Tissue processing
Forty-eight hours after fixing, the tissues were rinsed under running water to remove any remaining fixatives. The brain tissues were dehydrated using alcohol in a graded sequence to remove water and other impurities. After effectively dehydrating the tissues, they were washed for 2 h in xylene. Then, for 4 h, they were infiltrated with melted paraffin wax at a temperature of 60°C. After the paraffin wax set, the tissues were molded onto a tissue block and sectioned to obtain 3-µm tissue sections [12].
Glial fibrillary acidic protein (GFAP) staining method
Sections measuring approximately 3-µm thick were cut, deparaffinized, and rehydrated on coated slides. In a pH-6.0 citrate-based antigen retrieval solution, antigen retrieval was conducted for 30 minutes. After that, the sections were processed using the mouse and rabbit HRP/DAB IHC detection kits. For 10 minutes, endogenous peroxidase was blocked. After that, the primary antibodies were added, specifically mouse anti-GFAP (1:5000 dilutions; 2 h, 30 min). The sections were treated with the HRP (horseradish peroxidase) micro-polymer goat anti-rabbit HRP (Abcam USA) for 30 minutes. The reaction was produced using DAB chromogen (Abcam, USA). After rinsing the sections with water, Mayer’s hematoxylin was used as a counterstain. The procedure was dehydration, cleaning, and mounting with Dako dibutyl phthalate xylene.
Photomicrograph and image quantification
The treated tissues were examined using a digital light microscope, and OMAX software, version 32.0, Patch #7 was used to take digital photomicrographs of the samples at x400 magnifications. Using the cell counter plugin, NIH-sponsored ImageJ software, version 1.5.3 was utilized to analyze photomicrographs [13] digitally.
Data analysis
The data analysis program utilized was SPSS software, version 27.0.1. One-way analysis of variance (ANOVA) was used to get the Mean±SE of the mean when comparing values between groups. P<0.05 were used for determining statistical significance, and the data were given as Mean±SEM.
Result
Wire suspension test for the assessment of motor function
The neurobehavioral function test results are shown in Table 1.

The mean time spent by the experimental and the control groups while hanging on a wire is contrasted in the table. There is no significant decrease (P=0.05) in the time the animals spent hanging on the wire among the experimental groups.
The outcome of the neurobehavioral function test, performed on the final day of the exposure, is shown in the bar chart in Figure 1. The mean period spent by the experimental and control groups is shown in the above bar chart. Compared to group A, groups B, C, and D showed a decrease in the time the animals spent hanging on the wire; however, none of the differences were statistically significant.
Substrate for energy metabolism in the brain
The data about brain glucose levels in each group is shown in Table 2.

Compared to the control, all three test groups (B, C, and D) showed elevated glucose levels, all statistically significant.
The effect of a pyrethroid insecticide mixture consisting of dichlorvos and cypermethrin on glucose levels in the brain is shown in the bar chart in Figure 2. This outcome was assessed after the animals were exposed. The above bar chart describes the mean value of the animals in the control group (A) and the mean values of the experimental groups (B, C, and D). Compared to the control group, there is a statistically significant increase in the experimental groups.
The expression of astrocyte in substantia nigra
The expression of astrocytes in the substantia nigra of animals across different groups is shown in Table 3.

Compared to the control, the astrocyte expression in group B (short-term exposed animals) shows a non-significant increase. In contrast, group C (medium-term exposed animals) and group D (long-term exposed animals) present a statistically significant increase (Figure 3). The effect of the synthetic pyrethroid insecticide on astrocyte expression is shown in the bar chart in Figure 4. A comparison was made between the experimental groups’ (B, C, and D) and the control group’s (A) mean values. Although there was an increase in the experimental groups relative to the control, only groups C and D showed a statistically significant increase (Figure 4).
Immunohistochemical demonstration of astrocyte using GFAP
The expression of astrocytes in the substantia nigra of an adult Wistar rat is shown in the micrograph above across 4 groups. The short-term, medium-term, and long-term groups exhibit increased astrocytic expression compared to the control group following a four-week exposure to a formulated pyrethroid insecticide mixture. The animals in the high-dose group show the highest expression of astrocytes in the test groups, whereas the animals in the low-dose group show the lowest expression.
Discussion
Numerous studies have identified pyrethroid insecticide mixtures as neurotoxic substances. Still, it is unknown what the activities of the brain’s defensive structure are in such xenobiotic inversions to establish a viable therapeutic window. The substantia nigra is one of the motor centers impacted by this toxin [6]. Due to increased astrocyte homeostatic function, the exposed group shows a non-significant motor deficit in the neurobehavioral test [14]. Less time was spent hanging on the wire by the animals in the exposed groups during the neurobehavioral test. This result reveals how pyrethroids impact nigrostriatal dopaminergic neurodegeneration and behavioral abnormalities [15]. According to Tennekes [16] and Verma et al., time dependency is a characteristic feature of toxicity [17]. They proposed that the longer a toxic substance is exposed, the greater the effects. So, the period of suspension decreases with high exposure time. This finding suggests that the animals exposed to high doses of the substance spent the shortest time on the suspension wire, followed by those exposed to medium and low doses. Extended (more than one month) exposure to these insecticides may result in more severe manifestations of adverse health consequences [18, 19], including the inability of an astrocyte to sustain its homeostatic function in the brain.
Pyrethroid insecticides are frequently used and can have a toxic effect on the nervous system, presenting symptoms like limb weakness, memory loss, vision loss, cognitive and behavioral problems, and metabolic diseases like metabolic syndrome and diabetes mellitus, which involve improper glucose regulation and are conspicuously linked to environmental toxins [20]. The astrocytic defense system was activated in this scientific study, which required the proliferating astrocyte to absorb more circulatory glucose and reduce its glycolytic capability [21]. This condition of high glucose level was demonstrated in this study to satisfy the high energy need for cellular survival in xenobiotic penetrance conditions. Like other organ systems, glucose metabolism in the nervous system uses the tricarboxylic acid cycle (TCA) activity, the pentose phosphate route, glycolysis, and glycogenesis, unique to astrocytes. These processes also produce numerous metabolic intermediates, including lactate and glutamate, which are energy storage. Interestingly, aspartate and glutathione, crucial for many cellular functions, are also made from CNS glucose. Gamma-aminobutyric acid and glutamate are just two neurotransmitters produced from CNS glucose [22]. Also, glutamate, the main excitatory neurotransmitter released from neurons and causes the induction of glycolysis in nearby astrocytes, further proves the metabolic connection between astrocytic glucose uptake and neuronal activity [23].
Astrocytes normally provide structural support for the nervous system, regulate metabolism, protect neurons from harm, and promote the growth and homeostasis of neurons [3]. Astrocytes in the brain are responsible for tissue repair following trauma and for regulating water, ions, and glutamate in all brains, regardless of age or health [24]. By fostering neuronal health and function in certain ways, astrocytes regulate the microenvironment and maintain homeostasis [25]. In this study, we found that the expression of astrocytes considerably increased when the experimental mice were subjected to a pyrethroid insecticide mixture of dichlorvos and cypermethrin. The downregulation of normal astrocyte function can be a major neurodegeneration factor because astrocytes are necessary for neuronal survival [26, 27]. An increase in glucose was observed here to ensure neuronal activation along the nigrostriatal pathway [22]. Since disruption of astrocytic function has far-reaching effects, understanding how astrocytes react to toxins is crucial for internalizing the impact on the entire brain. This outcome is partially explained by the fact that astrocytes are more abundant than other synapses [28] and that they are connected to the brain’s blood vessels [29], where they act as the first line of defense to prevent foreign objects from entering the central nervous system [30]. The differences observed between the glucose concentration in the parenchyma (0.5–1 mM) and the blood glucose concentration in CNS capillaries (roughly 3–6 mM) [31] indicate that astrocytic projections wrapping around the vasculature [32] are necessary for glucose uptake and subsequent entry into the parenchyma, specifically through the glucose transporter-1 (GLUT1). The idea that the cytoarchitectural proximity of astrocytes to neurons is essential for neuronal support is corroborated by the fact that, despite requiring much less glucose than neurons do to maintain their physiological function, astrocytes are positioned to be the first recipients of this energetic substrate [22]. The GLUT1 transporter at the astrocyte-to-neuron interface provides a viable method by which astrocytes react to metabolic stress and meet glutamate clearance requirements [32]. Astrocytes and glucose increased simultaneously in response to pyrethroid toxicity, demonstrates how important these cells are for maintaining the overall health of the central nervous system, including the brain. This scientific study expands our understanding of how astrocytes behave when exposed to harmful chemicals like a mixture of pyrethroid insecticides. This study recommends boosting astrocytes and glucose as a therapeutic strategy for mitigating motor impairments in long-term exposure to such toxicity as pyrethroid insecticides, which will continue to be used.
Conclusion
According to the findings of this study, astrocytes and glucose are both elevated at the outset of xenobiotic toxicity to stabilize the activity of the substantia nigra. This study recommends boosting astrocytes and glucose as a therapeutic intervention for mitigating motor deficits caused by long-term exposure to such toxicity because pyrethroid insecticides will continue to be used.
Ethical Considerations
Compliance with ethical guidelines
The study was authorized by the Ethics Committee of the Faculty of Basic Medical Sciences, College of Health Sciences, Nnamdi Azikiwe University (Code: EA/2022/108).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Study design and writing: Princewill Sopuluchukwu Udodi; Acquiration of the animals; training the animals, exposing the animals to toxic agents, anesthetization, harvesting and the tissue of interest and fixation: Oluoma Vivian Aneke; Processing the tissues, biochemical and histological investigation: Roseline Ebube Udodi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors appreciate Damian N Ezejindu and Izuchukwu J Abugu for their help during this research work.
References
- Wondji CS, Coleman M, Kleinschmidt I, Mzilahowa T, Irving H, Ndula M, et al. Impact of pyrethroid resistance on operational malaria control in Malawi. Proc Natl Acad Sci U S A. 2012; 109(47):19063-70. [DOI:10.1073/pnas.1217229109] [PMID]
- Udodi PS, Nnadi EI, Ezejindu DN, Okafor EC, Obiesie IJ, Oyinbo CA, et al. The neurotoxic impact of formulated pyrethroid insecticide on the substantia Nigra of adult wistar rat. J Chem Health Risks. 2022; 12(2):323-34. [DOI:10.22034/JCHR.2022.1938288.1392]
- Ransom BR, Ransom CB. Astrocytes: Multitalented stars of the central nervous system. Methods Mol Biol. 2012; 814:3-7. [DOI:10.1007/978-1-61779-452-0_1] [PMID]
- Penky M, Pekna M, Messing A, Steinhauseer C, Lee JM, Parpura V, et al. Astrocytes: A central element in neurological diseases. Acta Neuropathol. 2016; 131(3):323-45. [DOI:10.1007/s00401-015-1513-1] [PMID]
- Benarroch EE. Neuron-astrocyte interactions: Partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005; 80(10):1326-38. [OI:10.4065/80.10.1326] [PMID]
- Udodi PS, Anonye TC, Ezejindu DN, Abugu JI, Omile CI, Obiesie IJ. et al. Exposure to insecticide mixture of cypermethrin and dichlorvos induced neurodegeneration by reducing antioxidant capacity in striatum. J Chem Health Risks. 2023; 13(3):423-39. [DOI:10.22034/JCHR.2022.1961632.1577]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. “Guide for the Care and Use of Laboratory Animals”, eighth ed. Washington, DC:The National Academies Press; 2011. [Link]
- Organisation for Economic Cooperation and Development (OECD). Test No. 433: Acute inhalation toxicity: Fixed concentration procedure. Paris: OECD Publishing; 2018. [Link]
- Cheng YS, Bowen L, Rando RJ, Postlethwait EM, Squadrito GL, Matalon S. Exposing animals to oxidant gases: Nose only vs. Whole body. Proc Am Thorac Soc. 2010; 7(4):264-8. [DOI:10.1513/pats.201001-001SM] [PMID]
- Moran PM, Higgins LS, Cordell B, Moser PC. Age-related learning deficits in transgenic mice expressing the 751-amino acid isoform of human beta-amyloid precursor protein. Proc Natl Acad Sci U S A. 1995; 92(12):5341-5. [DOI:10.1073/pnas.92.12.5341] [PMID]
- Mihara M, Uchiyama M. Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1997; 86(1):271-8. [DOI:10.1016/0003-2697(78)90342-1] [PMID]
- Feldman AT, Wolfe D. Tissue processing and hematoxylin and eosin staining. Methods Mol Biol. 2014; 1180:31-43. [DOI:10.1007/978-1-4939-1050-2_3] [PMID]
- Erukainure OL, Oyebode OA, Ibeji CU, Koorbanally NA, Islam M. Vernonia Amygdalina Del. stimulated glucose uptake in brain tissues enhances Antioxidative activities; and modulates functional chemistry and dysregulated metabolic pathways. Metab Brain Disease. 2019; 34(3):721-32. [DOI:10.1007/s11011-018-0363-7] [PMID]
- Singh S, Nag SK, Kundu SS, Maity SB. Relative intake, eating pattern, nutrient digestibility, nitrogen metabolism, fermentation pattern and growth performance of lambs fed organically and inorganically produced cowpea hay-barley grain diets. Trop Grassl. 2010; 44:55-61. [Link]
- Tiwari AK, Viswanadh V, Gowri PM, Ali AZ, Radhakrishnan SV. Oleanolic acid, an α-glucosidase inhibitory and antihyperglycemic active compound from the fruits of Sonneratia caseolaris. J Med Aromatic Plants. 2010; 1(1):19-23. [Link]
- Tennekes HA. The significance of the Druckrey-Küpfmüller equation for risk assessment-the toxicity of Neonicotinoid insecticides to arthropods is reinforced by exposure time. Toxicology. 2010; 276(1):1-4. [DOI:10.1016/j.tox.2010.07.005] [PMID]
- Verma P, Yadav AN, Kazy SK, Saxena AK, Suman A. Evaluating the diversity and phylogeny of plant growth promoting bacteria associated with wheat (Triticum aestivum) growing in central zone of India. Int J Curr Microbiol Appl Sci. 2014; 3(5):432-47. [Link]
- Casida JE, Quistad GB. Pyrethrum flowers: Production, chemistry, toxicology, and uses. Oxford: Oxford University Press; 1995. [Link]
- Meurer-Grimes B. Pyrethrum flowers: Production, chemistry, toxicology and uses. Brittonia. 1996; 48:613–4. [Link]
- Sargis RM. The hijacking of cellular signaling and the diabetes epidemic: Mechanisms of environmental disruption of insulin action and glucose homeostasis. Diabetes Metab J. 2014; 38(1):13-24. [DOI:10.4093/dmj.2014.38.1.13] [PMID]
- Pavlou S, Lindsay J, Ingram R, Xu H, Chen M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunology. 2018; 19(1):24. [DOI:10.1186/s12865-018-0261-0] [PMID]
- Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011; 14(6):724-38. [DOI:10.1016/j.cmet.2011.08.016] [PMID]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994; 91(22):10625-9. [DOI:10.1073/pnas.91.22.10625] [PMID]
- Bélanger M, Magistretti PJ. The role of astroglia in neuroprotection. Dialogues Clin Neurosci. 2009; 11(3):281-95. [DOI:10.31887/DCNS.2009.11.3/mbelanger] [PMID]
- Verkhratsky A, Parpura V, Vardjan N, Zorec R. Physiology of astroglia. Adv Exp Med Biol. 2019; 175:45-91.[DOI:10.1007/978-981-13-9913-8_3] [PMID]
- Wagner B, Natarajan A, Grünaug S, Kroismayr R, Wagner EF, Sibilia M. Neuronal survival depends on EGFR signaling in cortical but not midbrain astrocytes. EMBO J. 2006; 25(4):752-62. [DOI:10.1038/sj.emboj.7600988] [PMID]
- Brenner M, Johnson AB, Boespflug-Tanguy O, Rodriguez D, Goldman JE, Messing A. Mutations in GFAP, en-coding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet. 2001; 27(1):117-20. [DOI:10.1038/83679] [PMID]
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, et al. What is the role of astrocyte calcium in neurophysiology. Neuron. 2008; 59(6):932-46. [DOI:10.1016/j.neuron.2008.09.004] [PMID]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: A confocal microscopy study. Glia. 1998; 23(1):1-10. [PMID]
- Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003; 384(4):505-16. [DOI:10.1515/BC.2003.059] [PMID]
- Dienel GA. Brain glucose metabolism: Integration of energetics with function. Physiol Rev. 2019; 99(1):949-1045.[DOI:10.1152/physrev.00062.2017] [PMID]
- Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003; 23(19):7337-42. [DOI:10.1523/JNEUROSCI.23-19-07337.2003] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2024/01/28 | Accepted: 2024/01/20 | Published: 2024/01/20
Received: 2024/01/28 | Accepted: 2024/01/20 | Published: 2024/01/20
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |