Sat, May 18, 2024
Volume 10, Issue 1 (Winter 2024)
Caspian J Neurol Sci 2024, 10(1): 1-19 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hadipour E, Shahangian S S, Mashayekhi F. Monoterpenes and Diterpenes in Combating Alzheimer Disease, Mechanisms, and Clinical Insights: A Comprehensive Review. Caspian J Neurol Sci 2024; 10 (1) :1-19
URL: http://cjns.gums.ac.ir/article-1-688-en.html
URL: http://cjns.gums.ac.ir/article-1-688-en.html
1- Department of Biology, Faculty of Science, University of Guilan, Rasht, Iran.
Keywords: Alzheimer disease, Diterpenes, Monoterpenes, Neurodegeneration, Neuroinflammation, Oxidative stress
Full-Text [PDF 3377 kb]
(220 Downloads)
| Abstract (HTML) (219 Views)
Full-Text: (103 Views)
Introduction
Death, aging, and loss of mental abilities are natural processes that occur in all living organisms. However, in modern times, dementia and nervous breakdown have become increasingly prevalent. [1]. The most commonly used pharmacological treatments are acetylcholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists [2]. One of the latest trends in medical treatment is the exploration of medicinal plants and their active ingredients. Terpenes are among the most effective substances found in plants. These chemicals belong to the group of plant secondary metabolites with numerous biological activities. These activities include antioxidant properties, enzyme inhibition (acetylcholinesterase, amylase, and glucosidase), antifungal effects, liver protection, and sedation [1, 3-5]. Monoterpenes, responsible for plants’ aromatic properties, are a specific subgroup of terpenes derived from isoprene.
These secondary metabolites are produced in response to both biotic and abiotic stresses. They can be found in various plant families, such as Lamiaceae, including Citrus species [6]. Monoterpenes can be classified into three groups based on their chemical structure: Non-cyclic monoterpenes (e.g. citral, citronellal), monocyclic monoterpenes (e.g. menthol, carvone), and bicyclic and tricyclic terpenes (e.g. nepetalactone, santonin). Due to their low molecular weight, much research on monoterpenes has focused on the first two groups. One of the most intensively studied drug-based activities of monoterpenes is their neuroprotective effects against Alzheimer disease (AD) [7, 8]. Diterpenes, a diverse class of C20 natural compounds, are widely distributed in nature and are formed through the condensation of four isoprene units, utilizing either the mevalonate or deoxyxylulose phosphate pathways. Based on their skeletal core, diterpenes can be classified as linear, bi-, tri-, tetra-, penta-, and macrocyclic [9]. In their natural form, they often exhibit polyoxygenation with keto and hydroxyl groups, frequently esterified by small-sized aliphatic or aromatic acids. Derived from a common isoprene precursor, geranylgeranyl diphosphate, diterpenes undergo carbon skeleton formation and chemical modification, showing their structural versatility [10].
These natural products play a pivotal role in various biological activities, including antiviral, antibacterial, anti-inflammatory, antimalarial, and cytotoxic actions [10]. Notable examples of diterpene activity include the anticancer drug Taxol, used against ovarian, breast, and lung cancer. In addition, certain diterpenes exhibit potent and selective antagonistic activity toward platelet-activating factors, addressing conditions such as shock, burns, ulceration, and inflammatory skin diseases [9]. Moreover, there is emerging evidence suggesting anti-AD effects of specific diterpenes. This effect adds another layer to their diverse applications, making them promising candidates for medicinal contexts, including the ongoing exploration of therapeutic interventions for AD [11]. Another therapeutic diterpene, resiniferatoxin, is currently undergoing clinical trials to treat bladder hyperreflexia and diabetic neuropathy, highlighting diterpenes’ multifaceted and promising applications in various health-related domains [9]. In light of this information, the present study aims to investigate the mechanisms behind the therapeutic effects of these compounds against AD, as well as to provide an overview of the current studies regarding the role of monoterpenes and diterpenes in treating the disease mentioned above.
An overview of the Alzheimer disease pathology
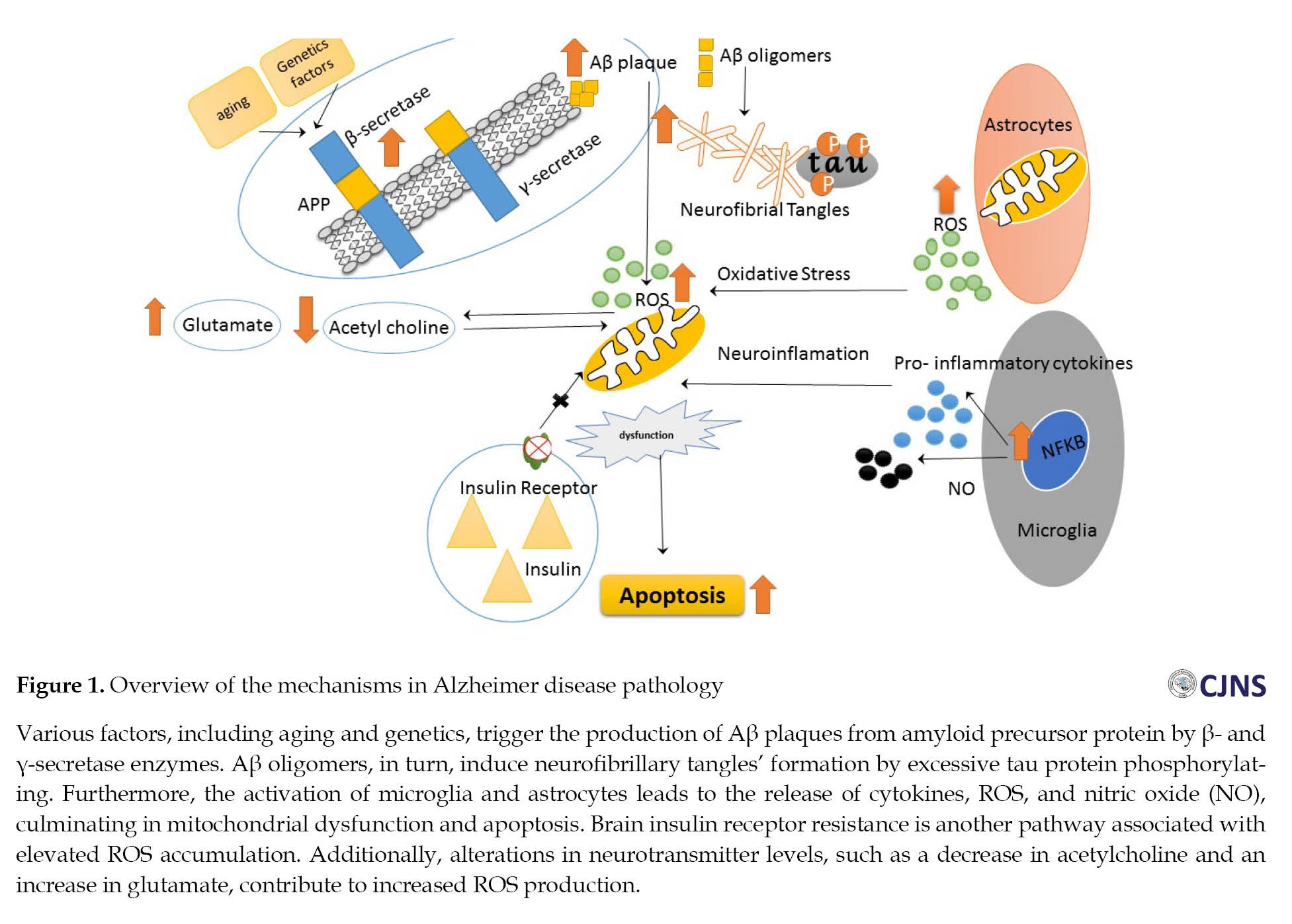
As previously mentioned, AD is a neurodegenerative disease characterized by two features of neural damage: Extracellular senile plaques and intracellular neurofibrillary tangles (NFT). The extracellular senile plaques are caused by the amyloid beta (Aβ) peptide deposition, while the NFT results from excessive phosphorylation of the tau protein [11, 12]. The accumulation of these factors in various parts of the nervous system, including the hippocampus, amygdala, entorhinal cortex, and basal parts of the forebrain, leads to memory, learning, and emotional behavior disorders [13, 14]. In addition to these features, AD is characterized by neuronal damage, synaptic loss, and, ultimately, neuronal death [11] (Figure 1).
Aβ protein
Amyloid precursor protein (APP) is a transmembrane protein with an extracellular amino-terminal region and a shorter intracellular carboxy-terminal region [15-17]. This protein is cleaved by α-, γ-, and β-secretase enzymes, ultimately producing non-amyloidogenic and amyloidogenic Aβ peptides. APP molecules on the cell surface are cleaved by α- and γ-secretase enzymes, and Aβ is not produced in this process [12]. However, APP precursors surrounded by clathrin vesicles enter endosomal compartments containing β- and γ-secretases, producing Aβ [18, 19]. Initially, Aβ, a part of the APP structure, has an α-helix structure and cannot be deposited or folded. However, once cleaved from its precursor into Aβ protein, it adopts a β-sheet conformation and folds [20, 21]. At the onset of the disease, the concentration of Aβ with 42 amino acids in the brain increases due to the reduction of its clearance from the brain to the cerebrospinal fluid, ultimately leading to accumulation and neurotoxicity. Initially, low molecular weight dimers and oligomers form primary fibrils, which expand into plaques and fibrillar strands [22, 23].
Tau protein
This protein, responsible for forming paired helical filaments (PHF), is encoded by a gene called microtubule-associated protein tau (MAPT). Tau protein is predominantly present in axons with smaller amounts in dendrites, the cell body of neurons, and in much lower amounts in astrocytes and oligodendrocytes. Its function is to maintain the stability of microtubules and enable axonal transmission. In AD, abnormal phosphorylation of this protein leads to its excessive accumulation and formation of insoluble PHF [24, 25]. As a result, the intracellular deposition of a tau protein leads to the formation of intracellular lesions known as NFTs. There is a bidirectional relationship between two protein molecules, Aβ and tau, in which oligomers obtained from Aβ accumulate in the lipid regions of the plasma membrane. When calcium enters the cell, it activates protein kinases that lead to the excessive phosphorylation of tau protein [26].
Oxidative stress and mitochondrial dysfunction
Chemical substances that have one or more unpaired electrons are considered free radicals, including hydroxyl radicals (•OH), superoxide radicals (O2•–), and nitric oxides (•NO). Due to their ability to alter cell oxidation, they are categorized as reactive oxygen species (ROS) [27]. Abnormal accumulations of these compounds result in oxidative stress, which refers to any disruption in the normal state of cellular oxidation and regeneration. Age-related, genetic, and environmental factors are all known factors contributing to ROS production. Therefore, disrupting the intracellular balance is one factor that contributes to AD [28, 29].
The increased free oxygen species has various effects, such as altered neurotransmitter levels, including increased glutamate and decreased acetylcholine. In addition, it causes an accumulation of proteins, damages glial cells, and activates genes associated with apoptosis and cell death, ultimately resulting in the loss of neurons [27]. Metal ions such as iron, copper, and aluminum, found in the brains of AD patients, can stimulate the production of free radicals, which serve as a major source of free oxygen species. Also, AD is associated with a reduction in the activity of cytochrome C oxidase and other enzymes involved in the electron transport chain in the mitochondria, which can lead to abnormal functioning of this organelle and the excessive production of free oxygen species [30].
Neuroinflammation
Neuroinflammation is another factor that can contribute to the development of AD. Specifically, the accumulation of Aβ fibrils can trigger the activation of microglia and astrocytes at the site of injury. This activation can release inflammatory cytokines, such as interleukin-1β, interleukin-6, interleukin-1-γ, and tumor necrosis factor-α, which in turn can activate the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway. This pathway can lead to the production of free oxygen species and activation of the oxidative stress pathway. Also, the activation of the β-secretase enzyme can result in the production of more Aβ protein [31, 32].
Apolipoprotein E
Apolipoprotein E is produced in astrocytes in three isoforms of apolipoprotein E2, E3, and E4 [33]. Apolipoprotein E contributes to the clearance of Aβ peptides by inducing their cleavage [34]. However, the apolipoprotein E4 allele, associated with an increased amyloidogenic process, is a genetic risk factor for AD. Individuals with more E4 alleles are three times more likely to develop AD than those with the E3 allele. The accumulation of Aβ and the increased phosphorylation of tau protein are associated with an increase in cholesterol concentration in the nerve cell membrane of these individuals [35].
Cholinergic system
Damage to the functioning of the acetylcholine neurotransmitter production system is associated with a decrease in the level of perception and various cognitive, physiological, and tissue disorders in the brain. Thus, the resulting dementia is associated with a reduction in the activity of acetylcholine [36]. This chemical is produced by the enzyme acetylcholine transferase in the synaptic terminal of nerve cells. Two types of receptors, nicotinic and muscarinic, are known for this neurotransmitter in the postsynaptic cell. At the end of the synaptic transmission, acetylcholine in the synaptic space is broken down into choline and acetate by the enzyme acetylcholinesterase. Chemical analyses of brain tissue from deceased Alzheimer patients indicate an extensive loss of the acetylcholine transferase enzyme. Acetylcholinesterase inhibitors are associated with improved cognition in these patients, confirming the cholinergic hypothesis in AD pathology. Also, nicotinic and muscarinic receptors for acetylcholine are low in AD patients [37].
Glutamatergic system
Excessive stimulation of nerve cells due to the over-activation of glutamate receptors and the resulting toxicity is also considered a contributing factor to nerve cell damage in AD. In these patients, NMDA receptors related to glutamate are overstimulated, which causes the influx of calcium ions into the cells and subsequently stimulates a series of biochemical reactions that can ultimately activate the cellular apoptosis cascade and result in cell death [38, 39].
Nerve cell damage
The destruction of cortical neurons, including the basal parts of the forebrain and subcortical areas such as the noradrenergic nucleus of the locus coeruleus, the serotonergic nucleus of the raphe, and the dopaminergic nucleus of the frontal tegmentum area, have been reported in AD patients. Also, cell damage has been confirmed in the areas of the limbic system in the temporal cortex, the middle-basal part of the dentate gyrus, and the superior parietal cortex [11].
Synapse loss
The loss of nerve synapses is considered a significant pathological factor in AD. This loss is due to the decrease in the number and density of dendritic spines, leading to memory deficits caused by reduced synaptic plasticity in the hippocampus [40]. Several factors contribute to this loss, including reduced sensory inputs and neuroinflammation by increasing the production of interleukin 1-β, which decreases the production of nerve-derived growth factor and alters the density of spines [41]. However, it should be noted that in AD, not only intercellular synapses are lost, but also communication with unaffected synapses is impaired. The loss of synapses is considered one of the earliest signs of nerve cell dysfunction before cell death in AD patients [42].
Cell death
In AD patients, a gradual loss of cells has been observed, which can be categorized into two types based on their appearance and biochemical characteristics: Apoptosis and necrosis. Apoptosis is a programmed cell death, where the cell undergoes a series of changes such as chromatin compaction, plasma membrane shrinkage, nuclear fragmentation, and formation of apoptotic bodies with a membrane [43]. On the other hand, in necrosis, depending on the type of damage inflicted on the cell membrane, the cell swells, and all the intracellular organelles are destroyed. The continuity of the plasma membrane is lost, and the cell contents spill into the intercellular space, initiating an intense inflammatory response [44].
Brain insulin resistance
An intriguing hypothesis suggests a direct link between diabetes and AD. This disease is sometimes called type 3 diabetes due to the shared molecular and cellular features of diabetes. Insulin is proposed to play a crucial role in forming amyloid plaques and tau phosphorylation, contributing to the development of NTFs. Just as insulin resistance in the body is associated with type 2 diabetes, insulin resistance in the brain is implicated in forming plaques and manifestations of AD [45].
Monoterpenes, diterpenes, and Alzheimer disease
As mentioned earlier, the common treatments used to control AD, such as acetylcholinesterase inhibitors, NMDA glutamate receptor antagonists, and other neuroprotective agents, can only alleviate the symptoms of the disease and slow down its progression. These treatments cannot directly interfere with the factors causing the disease. In contrast, monoterpenes and diterpenes represent an intriguing group of drug candidates that exhibit the potential to target multiple factors associated with AD pathology. Their unique properties make them promising candidates for therapeutic intervention [9, 46].
Outline of possible mechanism of effects of monoterpenes on Alzheimer disease
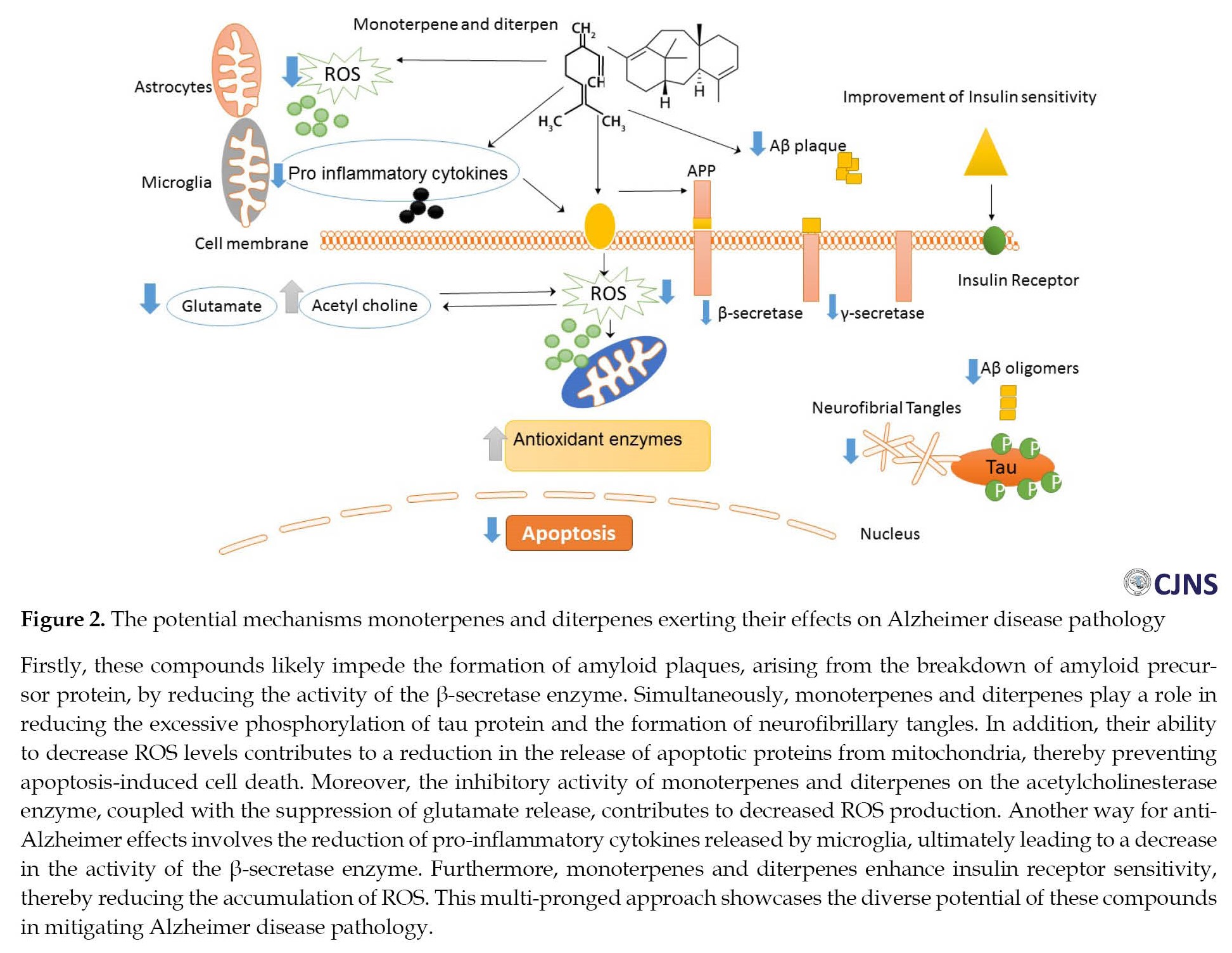
The therapeutic importance of monoterpenes appears to extend beyond their effect on the accumulation of Aβ protein in AD. Studies have revealed that monoterpenes only partially inhibit the β-secretase enzyme, and their impact on the activity of the α-secretase enzyme, responsible for initiating the non-amyloidogenic pathway in APP processing, is negligible. An alternative therapeutic approach for AD involves the up-regulation of Aβ-degrading enzymes such as Aβ proteases, which are associated with the low-density lipoprotein (LDL) receptor and apolipoprotein E systems [47, 48]. Notable protease enzymes include neprilysin, endothelin-converting enzymes, angiotensin-converting enzymes, and insulin-degrading enzymes. Moreover, monoterpenes have been found to prevent the accumulation of hyperphosphorylated tau proteins by down-regulating GSK-3β through the PI3K/Akt pathway. This reduction in GSK-3β activity contributes to a decrease in the production of free oxygen species from mitochondria, as evidenced by studies conducted by Sotolongo et al. [49]. Another significant mechanism of action for monoterpenes is their anti-inflammatory properties. These compounds effectively inhibit the production of key inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α), interleukin-1, cyclooxygenase (COX), and nitric oxide synthase (NOS). In addition, they regulate pro-inflammatory cytokines, including nuclear factor kappa light chain enhancer of activated B cells (NFκB), which plays a key role in the pathology of AD [50, 51]. Several monoterpene compounds, such as myrtenal, verbenone, carvacrol, 1,8-cineole (eucalyptol), and α-pinene, have been identified as potent inhibitors of the acetylcholinesterase enzyme. Also, monoterpenes have a moderate effect on the acetylcholinesterase enzyme. Furthermore, other monoterpene compounds, including β-pinene, α-terpinene, γ-terpinene, 3-carene, limonene, sabinene, trans-anethole, thymohydroquinone, carvacrol, thymoquinone, thymol, linalool, and pulegone [52] have shown moderate effects on acetylcholinesterase enzyme activity. By modeling the structure of these compounds, constructive studies can be made to improve the activity of anti-AD drugs. For instance, integrating the skeletal structure of the monoterpene camphene into the structure of galantamine results in a combined drug with inhibitory effects on the acetylcholinesterase enzyme that are one hundred times stronger than galantamine alone [53]. Linalool, another monoterpene, exhibits neuroprotective effects by inhibiting glutamate release and blocking NMDA receptors. Moreover, it prevents processes such as Aβ production and excessive phosphorylation of tau protein and reduces pro-inflammatory markers [54].
Furthermore, certain monoterpenes, including ecrodane ketone, geranyl acetone, and fenchone exhibit a moderate inhibitory effect on the β-secretase enzyme, leading to lesser production of Aβ protein. Also, specific monoterpene compounds like 1,8-cineole and genipin can mitigate the Aβ caused neurotoxicity [52, 55]. Moreover, linalool is pivotal in restoring antioxidant enzyme levels, such as superoxidase dismutase and glutathione peroxidase, to normal levels [56]. In addition, it boasts anti-inflammatory properties by inhibiting pro-inflammatory proteins such as p38, MAPK, cyclooxygenase-2 (COX-2), nitric oxide synthase-2 (NOS-2), and interleukin-1 [54, 57] (Table 1) (Figure 2).
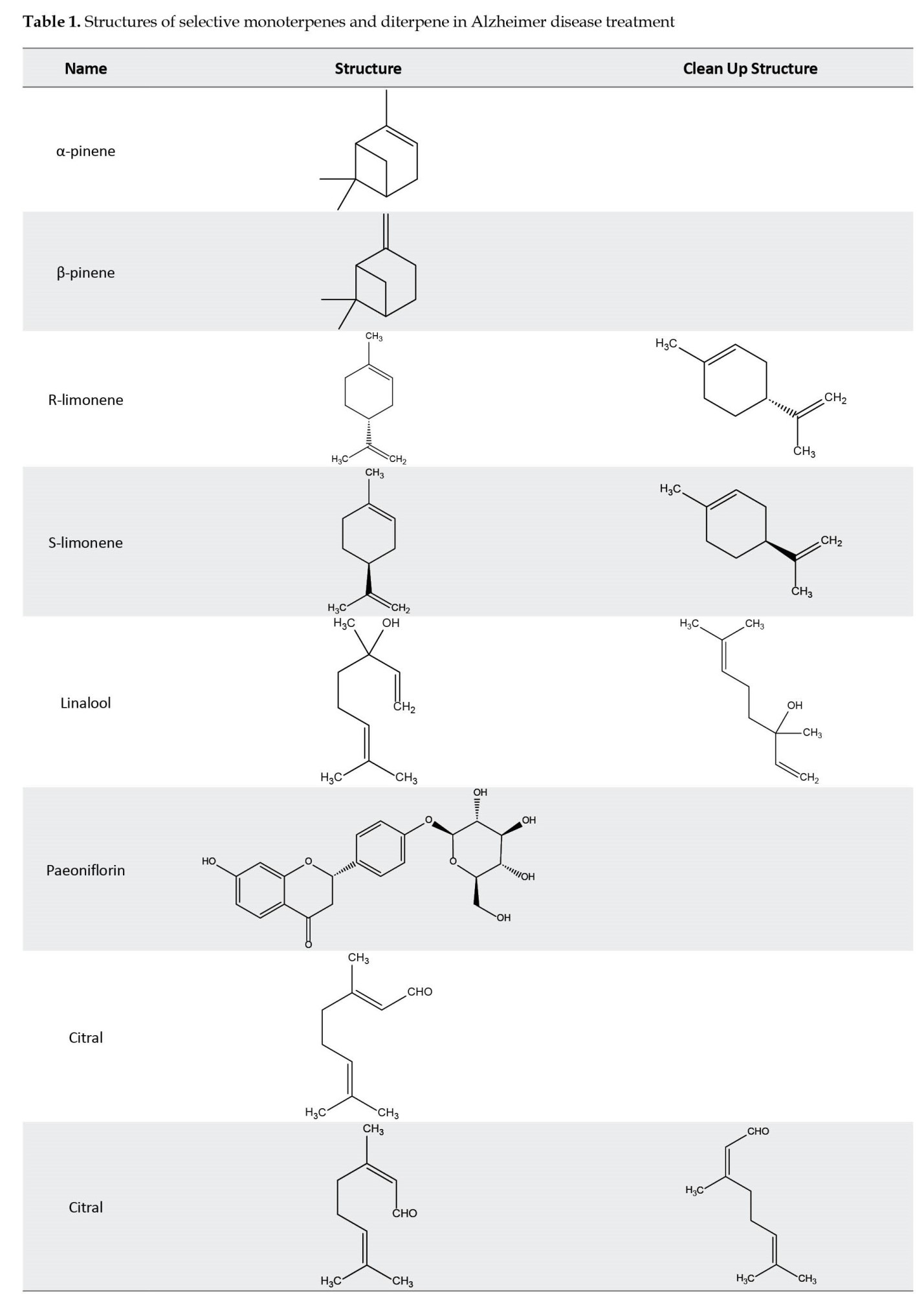
Outline of possible mechanism of effects of diterpenes on Alzheimer disease
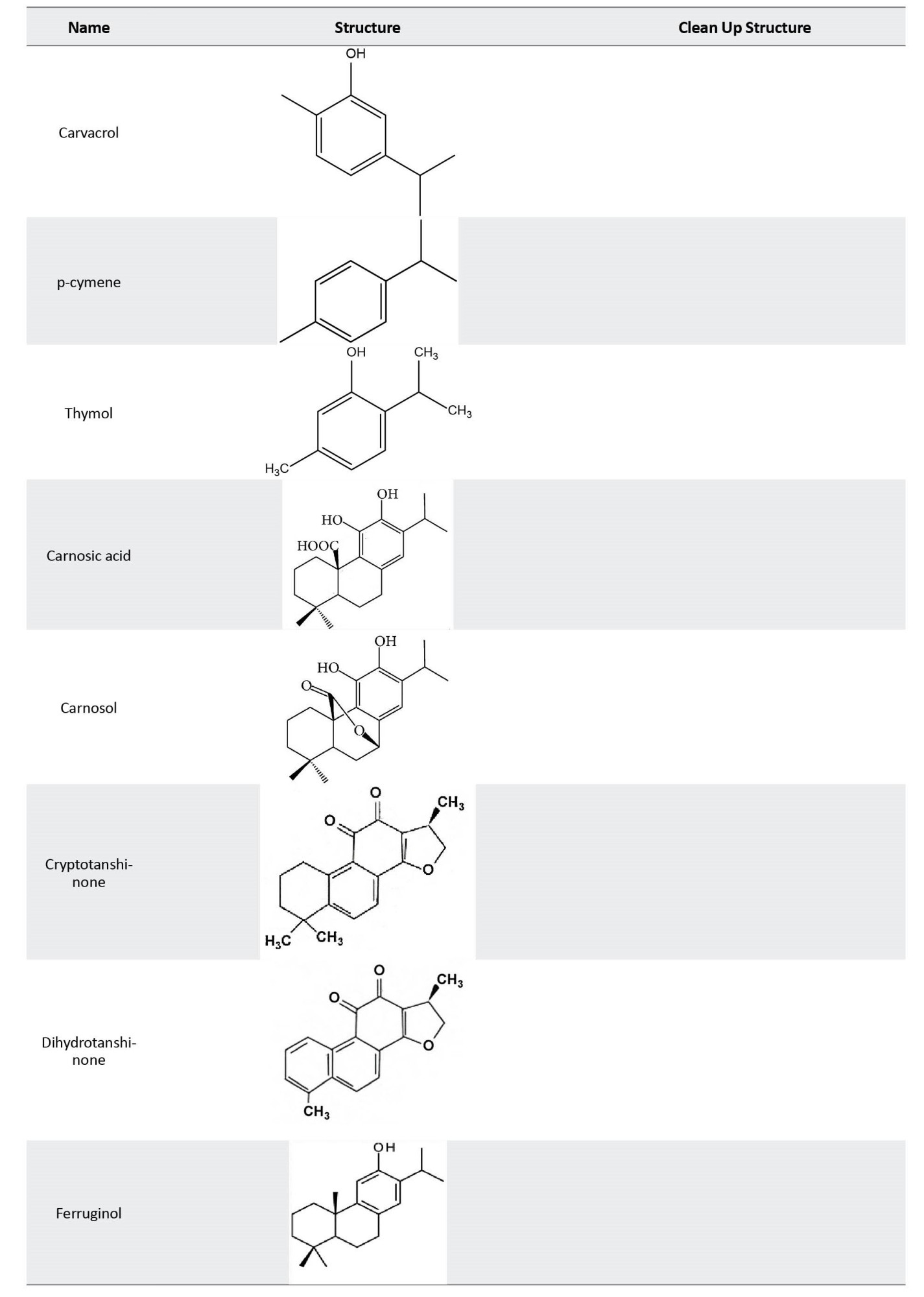
Diterpenes contribute to controlling AD via various mechanisms. The primary focus is on neuroprotective antioxidant mechanisms and cellular defense activation. Recent investigations into the impact of rosemary diterpenes on brain function have unveiled a spectrum of pharmacological effects. Carnosic acid is a prominent diterpene isolated from rosemary, constituting 1.5%–2.5% of dried leaves [58]. Carnosic acid is susceptible to oxidation, forming carnosol, rosmanol, and its epimeric form. Analyzing the relative concentrations in rosemary tissues reveals that carnosic acid and carnosol, two major rosemary antioxidants, provide antioxidant effects for the plant [59]. These compounds, particularly carnosic acid and carnosol, hold biological significance, reaching concentrations of approximately 5% of the dry weight in rosemary [11]. Rosemary diterpenes, particularly carnosic acid, and carnosol, demonstrate robust antioxidant properties in in vitro experiments, effectively preventing lipid peroxidation and providing protection against oxidative cell death [60]. The antioxidant mechanisms involve the removal of hydrogen from phenolic hydroxyl groups, leading to the formation of quinone derivatives. The antioxidant potential of rosemary extract and diterpenes has been extensively established, including applications in food preservation. Emphasizing relevance to neurodegenerative diseases, carnosic acid’s protective effects against oxidative damage in neuronal cells are prominent. The exceptional antioxidant mechanism and radical scavenging effects of polyphenolic natural products are attributed to the presence of the catechol functional group [11]. In addition, the formation of various diterpene derivatives is associated with carnosic acid’s ability to interact with ROS. Activating phase II detoxifying enzymes is a vital defense mechanism against internal and external toxins. Substantial evidence supports the involvement of nuclear factor erythroid-derived 2-related factor 2 (Nrf-2) in orchestrating the induction of antioxidant response elements (AREs), leading to the activation of genes responsible for various antioxidant enzymes, including phase II detoxifying enzymes [61]. Thiol-regulating enzymes like glutathione S-transferase (GST), glutamylcysteine synthetase, and thioredoxin reductase notably depend on Nrf-2 for their expression [62]. A specific mechanism identified for these antioxidant effects entails the direct S-alkylation of the cysteine thiol on Kelch-like ECH-associated protein 1 (Keap1) by the electrophilic quinone derivative of carnosic acid. Keap1, a regulatory protein associated with the Nrf2 transcription factor, binds to ARE. The interaction of electrophilic compounds with cysteine residues on Keap1 leads to the formation of S-alkyl adducts, facilitating the translocation of Nrf2 to the nucleus. Once in the nucleus, Nrf2 promotes gene expression by binding to AREs of phase II genes. This well-documented mechanism underscores the efficacy of electrophile compounds as antioxidants and neuroprotective agents. Carnosol exhibits high electrophilic activity, activating Nrf2 phase II detoxifying enzyme genes and antioxidant enzymes. It has also been demonstrated to directly interact with cysteine residues of the nuclear factor kappa B (NF-κB) [11]. The essential role of free carboxylic acid and catechol hydroxyl moieties in antioxidant effects has been highlighted. The evidence suggests that the primary constituents of rosemary, namely carnosic acid, and carnosol, safeguard neurons against oxidative stress by activating the Keap1/Nrf2 pathway. Carnosic acid and carnosol protect HT22 cells against oxidative glutamate toxicity by activating the transcriptional antioxidant-responsive element of phase II genes, including heme oxygenase-1 (HO-1), NADPH-dependent quinone oxidoreductase, and γ-glutamyl cysteine ligase [63]. These genes contribute to neuroprotection by regulating the cellular redox system. Also, carnosic acid protects against lipopolysaccharide-induced liver injury (LPS) by enhancing the body’s cellular antioxidant defense system through its antioxidant mechanism. This action is evidenced by restoring superoxide dismutase, glutathione peroxidase, and glutathione levels in serum and liver after the LPS challenge [11].
Now, we aim to elucidate the mechanisms underlying the inhibition of Aβ formation, polymerization, and metal chelation by diterpenes. In a study focusing on Aβ polymerization inhibition and metal chelation, carnosic acid exhibited a significant impact, partially reversing cellular changes induced by Aβ42 or Aβ43 monomers. This observed effect correlates with reduced levels of cellular oligomers, suggesting a potential mechanism involving the inhibition of oligomerization [11, 64]. The findings were consistent with in vivo observations, where carnosic acid demonstrated a beneficial effect in AD models, effectively reversing Aβ (1–40)-mediated neurodegeneration and cognitive impairment. Additionally, research further substantiated the therapeutic potential of carnosic acid for AD, exhibiting its ability to reverse Aβ (1–40)-mediated changes in neurodegeneration and cognitive impairment [11, 65]. The connection between Aβ formation/aggregation and metal ions, particularly copper, has been extensively reviewed. Metal chelators have proven effective in decreasing Alzheimer Aβ plaques. Aβ’s redox activity, involving the reduction of transition metals and the generation of ROS, establishes a link between polymerization and toxicity to metal ions and ROS. Rosemary diterpenes, known for their multifunctional nature in metal chelation and ROS scavenging, may contribute to their effectiveness against Aβ polymerization and toxicity in the context of AD [11, 66].
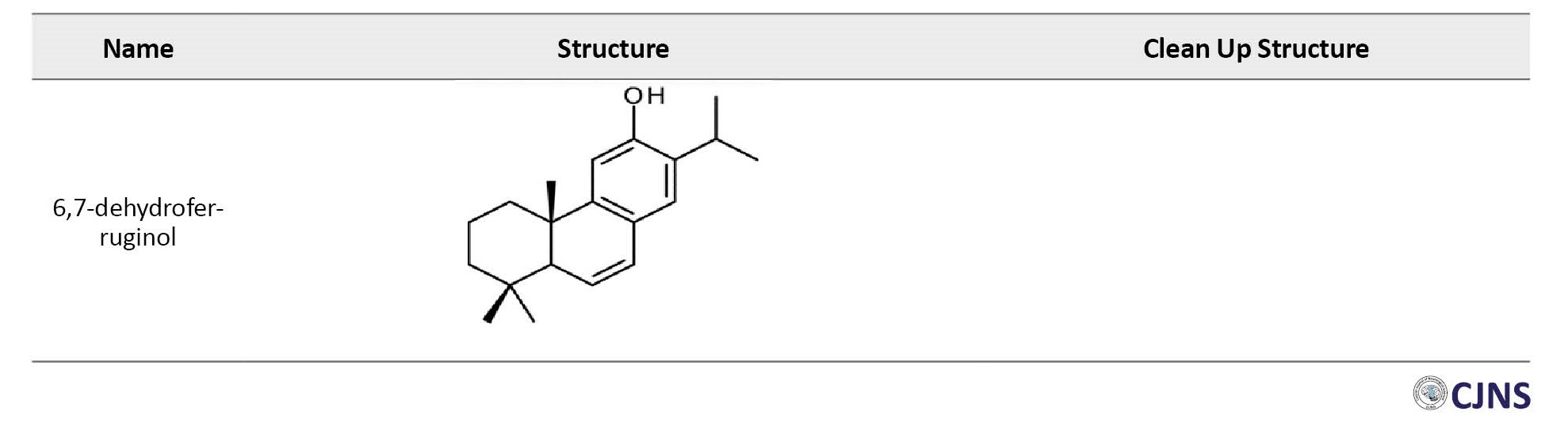
Among the 7 identified sugi wood diterpenes, ferruginol and 6,7-dehydroferruginol exhibited prominent reduction effects on Aβ toxicity. Their activity was comparable to known anti-AD active agents, including ginkgolide A, morin, rosmarinic acid, and carnosic acid. Ferruginol and 6,7-dehydroferruginol demonstrated antioxidative activity similar to other anti-AD agents in a DPPH radical scavenging activity test, suggesting a correlation between antioxidative activity and the Aβ toxicity reduction effect of sugi wood diterpenes [67]. Isolated from volatile compounds released during the sugi wood-drying process, ferruginol and 6,7-dehydroferruginol improved motor function disorder (paralysis) caused by Aβ toxicity in Caenorhabditis elegans CL4176. The Aβ toxicity reduction effect of ferruginol was comparable to known anti-AD active compounds. The antioxidative activity observed only in ferruginol and 6,7-dehydroferruginol, which demonstrated Aβ toxicity reduction effect, suggests the importance of antioxidative activity in alleviating Aβ toxicity. These active diterpenes are skin-permeable, volatile, and could be applied transdermally or nasally. This discovery opens avenues for their potential use as therapeutic or preventive drugs for AD based on their Aβ toxicity reduction effects [67].
In addition, this section delves into the mechanisms underlying the neuroprotective, cognitive-enhancing, and mood-modulating potential of diterpenes. Exploration of the influence of rosemary diterpenes on brain function has revealed a diverse range of pharmacological impacts. These effects include anxiolytic and antidepressant-like behaviors, as well as neuroprotective properties against cyanide-induced brain damage [68]. The underlying mechanisms involve the upregulation of transcriptional pathways associated with antioxidant and anti-inflammatory responses. Also, carnosol has demonstrated protective effects against rotenone-induced neurotoxicity, indicating its potential to modulate apoptotic mechanisms [69].
Furthermore, rosemary’s essential oil constituents, including carnosic acid and carnosol, exhibit cognitive-enhancing properties. The multifaceted pharmacological effects of rosemary diterpenes encompass anxiolytic and antidepressant-like behaviors, neuroprotective properties against brain damage, and cognitive enhancement. These findings highlight the potential therapeutic applications of rosemary in addressing various aspects of brain function and memory-related disorders [70, 11].
Another mechanism through which diterpenes modulate AD involves the inhibition of human cholinesterases. In the context of AD, characterized by a significant decline in acetylcholine neurotransmitter levels linked to memory loss, restoring central cholinergic function has been a focal point of exploration. A notable strategy involves using acetylcholinesterase inhibitors, given the pivotal role of acetylcholine hydrolysis [71]. Expanding on the investigation into rosemary diterpene mechanisms, this study scrutinizes the inhibitory properties of two compounds, cryptotanshinone (CT) and dihydrotanshinone (DT), derived from Salvia miltiorrhiza Bunge. Functioning as mixed non-competitive inhibitors for human acetylcholinesterase (AChE) and uncompetitive inhibitors for human butyrylcholinesterase, these diterpenes unveil a spectrum of pharmacological potential. Molecular docking analyses offer insights into their interactions within the active sites of both cholinesterases [72], unraveling the intricate mechanisms of rosemary diterpenes. In a separate study, kaurenoic acid (1), a natural product isolated from various plant species such as Sphagneticola trilobata (Asteraceae), Xylopia sericea, and X. frutescens, emerges as a potential candidate. Furthermore, gibberellic acids, diterpene plant hormones synthesized from geranylgeranyl diphosphate, have also been explored for their role in acetylcholinesterase inhibition. Both derivatives of kaurenoic acid and gibberellic acid underwent screening for their AChE inhibitory capacity, yielding promising results. Kaurenoic acid exhibited an inhibition percentage of 81.6%±1.8%, while gibberellic acid reached 85.2%±4.1%. These findings suggest that derivatives of kaurenoic and gibberellic acids hold promise as potential AChE inhibitors, opening new avenues for drug development in the treatment of AD [73].
Clinical studies of the effects of monoterpenes on Alzheimer disease
The beneficial effects of monoterpenes and their derivatives on AD have been extensively investigated in various models (Table 2) [52, 55, 74].

Among these, linalool stands out for its ability to reduce neurodegeneration and enhance cognitive function. Researchers have focused on understanding how linalool affects brain phospholipids, crucial in developing neurodegeneration and cognitive disorders [54]. In a study using mice genetically predisposed to AD, oral administration of the linalool (25 mg/kg) for three months resulted in improved behavioral functions. These positive effects were associated with reduced Aβ and tau phosphorylation levels and decreased pro-inflammatory markers such as p38, MAPK, COX-2, NOS-2, and interleukin-1β [75]. However, in another study using a different animal model, male rats, Aβ injected into their hippocampus treatment with linalool (intraperitoneally, 100 mg/kg) showed a different pattern. While there was an improvement in cognitive performance, no adverse effects on motor activities were observed. Notably, impairment was observed in long-term memory but not in short-term memory. The mechanisms behind these diverse effects seem to be related to linalool’s ability to reduce apoptosis and oxidative stress induced by Aβ through the Nrf2 transcription factor/HO-1 pathway [56]. These findings underscore the complex and context-dependent nature of monoterpenes’ effect in AD, warranting further research and exploration.
Citral, another monoterpene, has been investigated for its cognitive effects on spatial memory using the water maze test in an AD model of male rats. The results reveal an interesting dose-dependent relationship. Behavioral tests show that lower doses of citral (0.1 mg/kg) improve the learning capacity and memory of rats, while higher doses (1 mg/kg) lead to suppression of learning and improvement of spatial memory in rats. The possible mechanism behind these effects is citral’s influence on retinoic acid, subsequently affecting spatial memory function [76].
Limonene, another significant monoterpene, exhibits multiple biological effects, including antioxidant, anti-inflammatory, and neuroprotective properties [77]. Its impact on neurodegenerative symptoms such as memory impairment and hippocampal damage has been investigated using various behavioral tests. Oral consumption of limonene significantly prevents memory and learning deficits and reduces neuron loss due to stress in the hippocampus region of male rat brains. The underlying mechanism is attributed to its anti-inflammatory and antioxidant activities [78]. Furthermore, in another study, IP injection of limonene at 20 mg/kg in rats that suffered cerebral ischemia protected them against memory impairment. The possible mechanism involves the increase in the activity of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase, along with the reduction of malondialdehyde (MDA) levels and free oxygen species [79].
The effects of the monoterpene thymol were investigated in a study involving rats fed a high-fat diet, leading to memory impairment caused by Aβ accumulation. The results demonstrated a positive response to thymol treatment in this AD model. The therapeutic effects included improvements in spatial memory and learning in selective behavioral tests and a significant reduction in Aβ deposition [80]. In a similar study, mice on a high-fat diet treated with thymol exhibited enhanced cognitive function, reduced Aβ deposits, and decreased phosphorylation of tau protein in the hippocampus. These beneficial effects are likely attributed to thymol’s antioxidant and anti-inflammatory activities. Thymol was also found to down-regulate the level of P-Ser307IRS-1 and increase the expression of P-Ser473 AKT and P-Ser9 GSK3β, further contributing to its neuroprotective effects. Researchers also highlight thymol’s ability to protect cognitive functions by upregulating the HO-1/Nrf2 pathway [81, 82]. Furthermore, thymol showed promise in mitigating memory and cognitive impairments caused by Aβ injection into the hippocampus or IP injection of scopolamine in rats. Treatment with thymol (0.5, 1, or 2 mg/kg) improved cognitive functions and reversed the effects induced by Aβ or scopolamine. The mechanisms underlying these effects were associated with thymol’s antioxidant and anti-inflammatory activities [83].
Another noteworthy monoterpene, p-cymene, has demonstrated anti-AD effects by inhibiting the formation of Aβ deposits. In this study, the analysis was conducted in six separate groups of rats with AD induced through hippocampal injection. The results showed the positive effect of IP injection equivalent to 50 and 100 mg/kg of p-cymene on memory improvement, learning, and reducing Aβ plaque deposits [84]. On the other hand, paeoniflorin, another derivative of monoterpene, exhibits diverse biological activities. Chronic treatment with this monoterpene for 20 days enhanced cognitive performance in mice with memory impairment induced by Aβ injection into the hippocampus. The possible mechanism of action for both p-cymene and paeoniflorin involves calcium homeostasis, increasing reduced glutamate content, and reducing protein carbonyl and MDA levels by inhibiting NOS (nitic oxide synthesis) activity and NO (nitric oxide) levels [85]. Similarly, the positive effects of paeoniflorin were observed in rats transformed into an AD model by injecting Aβ into the dorsal part of the hippocampus. Treatment with this monoterpene for 20 days improved cognitive performance, particularly in spatial learning and memory abilities.
In addition, the activities of acetylcholinesterase and acetylcholine transferase enzymes in the hippocampus were evaluated, revealing that paeoniflorin enhances the activity of cholinergic neurons (by enhancing the sprouting of cholinergic neurons) in the hippocampus. This enhancement is achieved by regulating the signaling pathway of the nerve growth factor and simultaneously reducing the atrophy of cholinergic neurons, ultimately leading to increased survival of nerve cells [86-88]. In rats with memory damage and intra-neuronal fiber coil accumulation induced by Aβ injection into the lateral ventricle, treatment with 25 and 100 mg/kg of carvacrol (IP) improved memory and significantly reduced cognitive deficits. The possible mechanism behind these effects involves reducing inflammatory factors in the brain, including interleukin-1, TNF-α, COX-2, and expression of tropomyosin receptor kinase B protein, along with increased brain-derived neurotrophic factor expression [89].
Moreover, carvacrol has been found to significantly reduce brain edema induced by aquaporin-4 [90] in AD mice models. Similarly, in various studies, α-pinene, another monoterpene, has been investigated for its effects on AD [91, 92, 93]. In a study involving mice with scopolamine-induced memory deficits, IP injection of 10 mg/kg of α-pinene was observed to improve memory and learning. The possible mechanism behind these effects involves the regulation of proteins related to the production of acetylcholine (increased expression of acetylcholine transferase in the cortex) and the antioxidant defense system (increased expression of HO-1 and superoxide dismutase via the activation of tumor necrosis factor E2) in the hippocampus [94].
In addition, it has been shown that treatment with 0.1, 0.2, and 0.4 μg/kg of α-pinene in rats suffering from memory and learning impairments induced by capsaicin resulted in improved memory and learning by modulating GABA A receptors [95]. Moreover, α-pinene (IP, 0.4 μg/kg) reduces neurodegeneration in rats subjected to Aβ-injected toxicity in the CA1 region of the hippocampus [96]. Similarly, male rats that experienced oxidative toxicity and neuro-inflammation due to intra-hippocampal injection of Aβ treatment with 50 mg/kg of α-pinene (IP) for 14 days improved learning and spatial learning while reducing anxiety behaviors [97]. In PC12 cells exposed to hydrogen peroxide (H2O2), treatment with 10-400 µM of α-pinene and 1,8-cineole has been found to increase cell viability, reduce ROS levels, and raise the expression of antioxidant enzymes, such as catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase, and HO-1. Notably, these compounds can reduce apoptosis by decreasing caspase 3 activities. The possible mechanism underlying the antioxidant effects of these compounds involves scavenging ROS and inducing TNF-E2 [98]. Moreover, α-pinene administration via intraperitoneal (IP) injection at 25, 50, and 100 mg/kg doses to rats that experienced cerebral ischemia improved behavioral functions. The treatment also restored the function of antioxidant enzymes while reducing the concentrations of NO, MDA, and interleukin-6 in the hippocampus, cortex, and striated bodies [99].
Olibanum essential oil is abundant in various monoterpene compounds with different percentages, including limonene, α-pinene, and 4-tripeneol. It has been shown that treating mice with 150 to 600 mg/kg of the aforementioned olibanum essential oil for two weeks, following Aβ-induced toxicity, improved memory and learning. The possible mechanism behind these effects involves increasing acetylcholine levels, reducing Aβ plaques, protecting hippocampal neurons, and downregulating c-Fos in brain tissues [100]. Besides, treatment with Hypericum scabrum essential oil, containing approximately 51.3% of the monoterpene α-pinene, improved memory and learning in behavioral tests in rats suffering from dementia induced by scopolamine. The potential mechanism appears to be α-pinene acting as an inhibitor of the acetylcholinesterase enzyme, thereby prolonging the presence of acetylcholine in the synaptic space [101].
Clinical studies of the effects of diterpenes on Alzheimer disease
Recent investigations into the impact of rosemary diterpenes on brain function encompass a spectrum of pharmacological effects, extending beyond rodent models to potential clinical applications. Ferlemi et al. explored the anxiolytic and antidepressant-like behavior resulting from rosemary tea consumption in adult male mice [102]. Machado et al. demonstrated the antidepressant-like effects of rosemary extract in mice undergoing an olfactory bulbectomy procedure. The study suggests a potential role for rosemary diterpenes in alleviating depressive-like behaviors in a murine model, providing insights into their antidepressant properties [70]. The crude extract of rosemary has exhibited efficacy in improving memory impairment in an in vivo scopolamine-induced dementia model of AD [103, 11]. Moreover, cognitive enhancement is observed in clinical studies, with rosemary’s essential oil constituents, including 1,8-cineole, showcasing mental benefits [104].
This preclinical finding indicates a potential cognitive-enhancing effect of rosemary diterpenes, highlighting their neuroprotective and memory-improving properties. Carnosic acid’s neuroprotective prowess extends to cultured rodent and human-induced pluripotent stem cell-derived neurons in vitro and in vivo [68]. The multifaceted pharmacological effects of rosemary diterpenes, including anxiolytic and antidepressant-like behaviors, neuroprotective properties against brain damage, and cognitive enhancement, provide a foundation for considering their therapeutic potential in clinical settings. Further exploration in human subjects is essential to understand and validate these observed effects fully, emphasizing the need for clinical trials to establish the efficacy and safety of rosemary diterpenes in the context of AD and related conditions [11].
Carnosic acid has been shown to safeguard neuronal cells from ischemic injury by scavenging ROS both in vitro and in vivo. Carnosol and carnosic acid have been proven to protect neuronal HT22 cells by activating the antioxidant-responsive element [105]. The existing evidence strongly suggests that these primary constituents of rosemary safeguard HT22 cells against oxidative glutamate toxicity. Pretreatment of RAW264.7 macrophages with carnosic acid also significantly reduces H2O2 or LPS-induced ROS and nitric oxide generation, with concurrent time- and dose-dependent upregulation of HO-1 protein expression [63, 11]. Furthermore, carnosol has been shown to enhance GST and quinone reductase activity in vivo. In the exploration of promoting non-amyloidogenic pathways in APP processing, Meng et al. conducted an investigation using SH-SY5Y human neuroblastoma cells, where carnosic acid exhibited a significant 61% suppression of Aβ42 secretion at a concentration of 30 μM. This suppression was concurrent with increased mRNA expressions of α-secretase [64]. The findings suggest that the mechanism underlying the inhibition of APP processing by carnosic acid involves facilitating the normal non-amyloidogenic-dependent pathway. Yoshida et al. conducted similar observations, demonstrating that carnosic acid suppressed Aβ peptides (1–40, 1–42, and 1–43) production in U373MG human astrocytoma cells at a concentration of 50 μM. The study disclosed a substantial 55% to 71% inhibition of Aβ release and a notable impact on mRNA expressions of α-secretase [106]. In a study by Meng et al. investigating the Aβ polymerization inhibition and metal chelation, the impact of carnosic acid on cultured SH-SY5Y human neuroblastoma cells challenged by Aβ42 or Aβ43 was explored. The results demonstrated a partial reversal of cellular deletion induced by Aβ42 or Aβ43 monomers (10 μM each) with carnosic acid treatment (10 μM) [64]. This observed effect correlates with reduced levels of cellular oligomers, suggesting the inhibition of oligomerization as a potential mechanism. These findings align with in vivo observations, where carnosic acid exhibited a beneficial effect in AD models, effectively reversing Aβ (1–40)-mediated neurodegeneration and cognitive impairment. Rasoolijazi et al. further substantiated the therapeutic potential of carnosic acid for AD, demonstrating its ability to reverse Aβ (1–40)-mediated changes in neurodegeneration and cognitive impairment in rats [65].
In a distinct clinical investigation, diterpenes extracted from sugi wood (Cryptomeria japonica) were identified as ferruginol, 6,7-dehydroferruginol, sandaracopimarinal, sandaracopimarinol, abietadiene, abietatriene, and phyllocladenes; mixture of phyllocladene and isophyllocladene. In this study, focusing on the potential of sugi wood diterpenes as a preventive and therapeutic drug for AD, their β-amyloid toxicity reduction effect was scrutinized by administering each diterpenoid at a final concentration of 0.5 mg/mL to C. elegans [67]. Paralyzed rates were meticulously observed, and the subsequent analysis of non-paralysis rates revealed significantly positive effects in the experimental plots of two impactful diterpenes, namely ferruginol and 6,7-dehydroferruginol. This outcome emphasizes that the administration of these compounds effectively suppressed the expression of paralysis induced by Aβ toxicity in C. elegans CL4176. Conversely, no significant differences were observed in the experimental plots of other sugi wood diterpenes. This study concludes that the Aβ toxicity reduction effect is a distinctive property of ferruginol and related compounds. Furthermore, it unveils, for the first time, the anti-AD effects derived from extracts of sugi wood [67] (Table 2).
Conclusion
In conclusion, Alzheimer disease has remained a formidable global health challenge, impacting millions worldwide. While current pharmacological treatments, such as acetylcholinesterase inhibitors and NMDA receptor antagonists, have been employed, the quest for more effective therapeutic approaches continues. Recent years have witnessed a burgeoning interest in investigating natural compounds from medicinal plants as potential remedies for AD. Among these, monoterpenes and diterpenes, certain plant secondary metabolites, have emerged as promising candidates due to their diverse biological activities. These compounds show neuroprotective attributes, augmenting memory and cognitive functions while demonstrating potent antioxidant capabilities. Their capacity to influence non-amyloidogenic pathways in the processing of APP is complemented by their ability to hinder Aβ formation and accumulation. Moreover, they actively participate in metal chelation and exhibit notable cholinesterase inhibition. Encouragingly, preclinical studies conducted on animal models and cellular cultures have consistently affirmed the effectiveness of these compounds, fostering optimism for their future role in therapeutic interventions. However, to fully unlock the therapeutic potential of monoterpenes and diterpenes, additional research, particularly in clinical trials, is imperative to comprehend their safety and efficacy in human subjects. Continued exploration in neuropharmacology holds the promise of pioneering innovative treatments that could substantially enhance the quality of life for individuals grappling with AD and related memory disorders.
In summary, the investigation into monoterpenes as integral components in AD management offers an exciting prospect for the future of neurodegenerative disease research. As our understanding of these compounds grows, the development of novel and effective treatments is in sight, ushering a positive impact on the lives of those with cognitive impairments and neurodegenerative conditions.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in compliance with the ethi- cal guidelines of the Declaration of Helsinki 2013
Funding
The study was funded by the Research Council of the University of Guilan.
Authors contributions
All authors equally contribute to preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors are thankful to the Research Council of University of Guilan.
References
Death, aging, and loss of mental abilities are natural processes that occur in all living organisms. However, in modern times, dementia and nervous breakdown have become increasingly prevalent. [1]. The most commonly used pharmacological treatments are acetylcholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists [2]. One of the latest trends in medical treatment is the exploration of medicinal plants and their active ingredients. Terpenes are among the most effective substances found in plants. These chemicals belong to the group of plant secondary metabolites with numerous biological activities. These activities include antioxidant properties, enzyme inhibition (acetylcholinesterase, amylase, and glucosidase), antifungal effects, liver protection, and sedation [1, 3-5]. Monoterpenes, responsible for plants’ aromatic properties, are a specific subgroup of terpenes derived from isoprene.
These secondary metabolites are produced in response to both biotic and abiotic stresses. They can be found in various plant families, such as Lamiaceae, including Citrus species [6]. Monoterpenes can be classified into three groups based on their chemical structure: Non-cyclic monoterpenes (e.g. citral, citronellal), monocyclic monoterpenes (e.g. menthol, carvone), and bicyclic and tricyclic terpenes (e.g. nepetalactone, santonin). Due to their low molecular weight, much research on monoterpenes has focused on the first two groups. One of the most intensively studied drug-based activities of monoterpenes is their neuroprotective effects against Alzheimer disease (AD) [7, 8]. Diterpenes, a diverse class of C20 natural compounds, are widely distributed in nature and are formed through the condensation of four isoprene units, utilizing either the mevalonate or deoxyxylulose phosphate pathways. Based on their skeletal core, diterpenes can be classified as linear, bi-, tri-, tetra-, penta-, and macrocyclic [9]. In their natural form, they often exhibit polyoxygenation with keto and hydroxyl groups, frequently esterified by small-sized aliphatic or aromatic acids. Derived from a common isoprene precursor, geranylgeranyl diphosphate, diterpenes undergo carbon skeleton formation and chemical modification, showing their structural versatility [10].
These natural products play a pivotal role in various biological activities, including antiviral, antibacterial, anti-inflammatory, antimalarial, and cytotoxic actions [10]. Notable examples of diterpene activity include the anticancer drug Taxol, used against ovarian, breast, and lung cancer. In addition, certain diterpenes exhibit potent and selective antagonistic activity toward platelet-activating factors, addressing conditions such as shock, burns, ulceration, and inflammatory skin diseases [9]. Moreover, there is emerging evidence suggesting anti-AD effects of specific diterpenes. This effect adds another layer to their diverse applications, making them promising candidates for medicinal contexts, including the ongoing exploration of therapeutic interventions for AD [11]. Another therapeutic diterpene, resiniferatoxin, is currently undergoing clinical trials to treat bladder hyperreflexia and diabetic neuropathy, highlighting diterpenes’ multifaceted and promising applications in various health-related domains [9]. In light of this information, the present study aims to investigate the mechanisms behind the therapeutic effects of these compounds against AD, as well as to provide an overview of the current studies regarding the role of monoterpenes and diterpenes in treating the disease mentioned above.
An overview of the Alzheimer disease pathology
As previously mentioned, AD is a neurodegenerative disease characterized by two features of neural damage: Extracellular senile plaques and intracellular neurofibrillary tangles (NFT). The extracellular senile plaques are caused by the amyloid beta (Aβ) peptide deposition, while the NFT results from excessive phosphorylation of the tau protein [11, 12]. The accumulation of these factors in various parts of the nervous system, including the hippocampus, amygdala, entorhinal cortex, and basal parts of the forebrain, leads to memory, learning, and emotional behavior disorders [13, 14]. In addition to these features, AD is characterized by neuronal damage, synaptic loss, and, ultimately, neuronal death [11] (Figure 1).
Aβ protein
Amyloid precursor protein (APP) is a transmembrane protein with an extracellular amino-terminal region and a shorter intracellular carboxy-terminal region [15-17]. This protein is cleaved by α-, γ-, and β-secretase enzymes, ultimately producing non-amyloidogenic and amyloidogenic Aβ peptides. APP molecules on the cell surface are cleaved by α- and γ-secretase enzymes, and Aβ is not produced in this process [12]. However, APP precursors surrounded by clathrin vesicles enter endosomal compartments containing β- and γ-secretases, producing Aβ [18, 19]. Initially, Aβ, a part of the APP structure, has an α-helix structure and cannot be deposited or folded. However, once cleaved from its precursor into Aβ protein, it adopts a β-sheet conformation and folds [20, 21]. At the onset of the disease, the concentration of Aβ with 42 amino acids in the brain increases due to the reduction of its clearance from the brain to the cerebrospinal fluid, ultimately leading to accumulation and neurotoxicity. Initially, low molecular weight dimers and oligomers form primary fibrils, which expand into plaques and fibrillar strands [22, 23].
Tau protein
This protein, responsible for forming paired helical filaments (PHF), is encoded by a gene called microtubule-associated protein tau (MAPT). Tau protein is predominantly present in axons with smaller amounts in dendrites, the cell body of neurons, and in much lower amounts in astrocytes and oligodendrocytes. Its function is to maintain the stability of microtubules and enable axonal transmission. In AD, abnormal phosphorylation of this protein leads to its excessive accumulation and formation of insoluble PHF [24, 25]. As a result, the intracellular deposition of a tau protein leads to the formation of intracellular lesions known as NFTs. There is a bidirectional relationship between two protein molecules, Aβ and tau, in which oligomers obtained from Aβ accumulate in the lipid regions of the plasma membrane. When calcium enters the cell, it activates protein kinases that lead to the excessive phosphorylation of tau protein [26].
Oxidative stress and mitochondrial dysfunction
Chemical substances that have one or more unpaired electrons are considered free radicals, including hydroxyl radicals (•OH), superoxide radicals (O2•–), and nitric oxides (•NO). Due to their ability to alter cell oxidation, they are categorized as reactive oxygen species (ROS) [27]. Abnormal accumulations of these compounds result in oxidative stress, which refers to any disruption in the normal state of cellular oxidation and regeneration. Age-related, genetic, and environmental factors are all known factors contributing to ROS production. Therefore, disrupting the intracellular balance is one factor that contributes to AD [28, 29].
The increased free oxygen species has various effects, such as altered neurotransmitter levels, including increased glutamate and decreased acetylcholine. In addition, it causes an accumulation of proteins, damages glial cells, and activates genes associated with apoptosis and cell death, ultimately resulting in the loss of neurons [27]. Metal ions such as iron, copper, and aluminum, found in the brains of AD patients, can stimulate the production of free radicals, which serve as a major source of free oxygen species. Also, AD is associated with a reduction in the activity of cytochrome C oxidase and other enzymes involved in the electron transport chain in the mitochondria, which can lead to abnormal functioning of this organelle and the excessive production of free oxygen species [30].
Neuroinflammation
Neuroinflammation is another factor that can contribute to the development of AD. Specifically, the accumulation of Aβ fibrils can trigger the activation of microglia and astrocytes at the site of injury. This activation can release inflammatory cytokines, such as interleukin-1β, interleukin-6, interleukin-1-γ, and tumor necrosis factor-α, which in turn can activate the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway. This pathway can lead to the production of free oxygen species and activation of the oxidative stress pathway. Also, the activation of the β-secretase enzyme can result in the production of more Aβ protein [31, 32].
Apolipoprotein E
Apolipoprotein E is produced in astrocytes in three isoforms of apolipoprotein E2, E3, and E4 [33]. Apolipoprotein E contributes to the clearance of Aβ peptides by inducing their cleavage [34]. However, the apolipoprotein E4 allele, associated with an increased amyloidogenic process, is a genetic risk factor for AD. Individuals with more E4 alleles are three times more likely to develop AD than those with the E3 allele. The accumulation of Aβ and the increased phosphorylation of tau protein are associated with an increase in cholesterol concentration in the nerve cell membrane of these individuals [35].
Cholinergic system
Damage to the functioning of the acetylcholine neurotransmitter production system is associated with a decrease in the level of perception and various cognitive, physiological, and tissue disorders in the brain. Thus, the resulting dementia is associated with a reduction in the activity of acetylcholine [36]. This chemical is produced by the enzyme acetylcholine transferase in the synaptic terminal of nerve cells. Two types of receptors, nicotinic and muscarinic, are known for this neurotransmitter in the postsynaptic cell. At the end of the synaptic transmission, acetylcholine in the synaptic space is broken down into choline and acetate by the enzyme acetylcholinesterase. Chemical analyses of brain tissue from deceased Alzheimer patients indicate an extensive loss of the acetylcholine transferase enzyme. Acetylcholinesterase inhibitors are associated with improved cognition in these patients, confirming the cholinergic hypothesis in AD pathology. Also, nicotinic and muscarinic receptors for acetylcholine are low in AD patients [37].
Glutamatergic system
Excessive stimulation of nerve cells due to the over-activation of glutamate receptors and the resulting toxicity is also considered a contributing factor to nerve cell damage in AD. In these patients, NMDA receptors related to glutamate are overstimulated, which causes the influx of calcium ions into the cells and subsequently stimulates a series of biochemical reactions that can ultimately activate the cellular apoptosis cascade and result in cell death [38, 39].
Nerve cell damage
The destruction of cortical neurons, including the basal parts of the forebrain and subcortical areas such as the noradrenergic nucleus of the locus coeruleus, the serotonergic nucleus of the raphe, and the dopaminergic nucleus of the frontal tegmentum area, have been reported in AD patients. Also, cell damage has been confirmed in the areas of the limbic system in the temporal cortex, the middle-basal part of the dentate gyrus, and the superior parietal cortex [11].
Synapse loss
The loss of nerve synapses is considered a significant pathological factor in AD. This loss is due to the decrease in the number and density of dendritic spines, leading to memory deficits caused by reduced synaptic plasticity in the hippocampus [40]. Several factors contribute to this loss, including reduced sensory inputs and neuroinflammation by increasing the production of interleukin 1-β, which decreases the production of nerve-derived growth factor and alters the density of spines [41]. However, it should be noted that in AD, not only intercellular synapses are lost, but also communication with unaffected synapses is impaired. The loss of synapses is considered one of the earliest signs of nerve cell dysfunction before cell death in AD patients [42].
Cell death
In AD patients, a gradual loss of cells has been observed, which can be categorized into two types based on their appearance and biochemical characteristics: Apoptosis and necrosis. Apoptosis is a programmed cell death, where the cell undergoes a series of changes such as chromatin compaction, plasma membrane shrinkage, nuclear fragmentation, and formation of apoptotic bodies with a membrane [43]. On the other hand, in necrosis, depending on the type of damage inflicted on the cell membrane, the cell swells, and all the intracellular organelles are destroyed. The continuity of the plasma membrane is lost, and the cell contents spill into the intercellular space, initiating an intense inflammatory response [44].
Brain insulin resistance
An intriguing hypothesis suggests a direct link between diabetes and AD. This disease is sometimes called type 3 diabetes due to the shared molecular and cellular features of diabetes. Insulin is proposed to play a crucial role in forming amyloid plaques and tau phosphorylation, contributing to the development of NTFs. Just as insulin resistance in the body is associated with type 2 diabetes, insulin resistance in the brain is implicated in forming plaques and manifestations of AD [45].
Monoterpenes, diterpenes, and Alzheimer disease
As mentioned earlier, the common treatments used to control AD, such as acetylcholinesterase inhibitors, NMDA glutamate receptor antagonists, and other neuroprotective agents, can only alleviate the symptoms of the disease and slow down its progression. These treatments cannot directly interfere with the factors causing the disease. In contrast, monoterpenes and diterpenes represent an intriguing group of drug candidates that exhibit the potential to target multiple factors associated with AD pathology. Their unique properties make them promising candidates for therapeutic intervention [9, 46].
Outline of possible mechanism of effects of monoterpenes on Alzheimer disease
The therapeutic importance of monoterpenes appears to extend beyond their effect on the accumulation of Aβ protein in AD. Studies have revealed that monoterpenes only partially inhibit the β-secretase enzyme, and their impact on the activity of the α-secretase enzyme, responsible for initiating the non-amyloidogenic pathway in APP processing, is negligible. An alternative therapeutic approach for AD involves the up-regulation of Aβ-degrading enzymes such as Aβ proteases, which are associated with the low-density lipoprotein (LDL) receptor and apolipoprotein E systems [47, 48]. Notable protease enzymes include neprilysin, endothelin-converting enzymes, angiotensin-converting enzymes, and insulin-degrading enzymes. Moreover, monoterpenes have been found to prevent the accumulation of hyperphosphorylated tau proteins by down-regulating GSK-3β through the PI3K/Akt pathway. This reduction in GSK-3β activity contributes to a decrease in the production of free oxygen species from mitochondria, as evidenced by studies conducted by Sotolongo et al. [49]. Another significant mechanism of action for monoterpenes is their anti-inflammatory properties. These compounds effectively inhibit the production of key inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α), interleukin-1, cyclooxygenase (COX), and nitric oxide synthase (NOS). In addition, they regulate pro-inflammatory cytokines, including nuclear factor kappa light chain enhancer of activated B cells (NFκB), which plays a key role in the pathology of AD [50, 51]. Several monoterpene compounds, such as myrtenal, verbenone, carvacrol, 1,8-cineole (eucalyptol), and α-pinene, have been identified as potent inhibitors of the acetylcholinesterase enzyme. Also, monoterpenes have a moderate effect on the acetylcholinesterase enzyme. Furthermore, other monoterpene compounds, including β-pinene, α-terpinene, γ-terpinene, 3-carene, limonene, sabinene, trans-anethole, thymohydroquinone, carvacrol, thymoquinone, thymol, linalool, and pulegone [52] have shown moderate effects on acetylcholinesterase enzyme activity. By modeling the structure of these compounds, constructive studies can be made to improve the activity of anti-AD drugs. For instance, integrating the skeletal structure of the monoterpene camphene into the structure of galantamine results in a combined drug with inhibitory effects on the acetylcholinesterase enzyme that are one hundred times stronger than galantamine alone [53]. Linalool, another monoterpene, exhibits neuroprotective effects by inhibiting glutamate release and blocking NMDA receptors. Moreover, it prevents processes such as Aβ production and excessive phosphorylation of tau protein and reduces pro-inflammatory markers [54].
Furthermore, certain monoterpenes, including ecrodane ketone, geranyl acetone, and fenchone exhibit a moderate inhibitory effect on the β-secretase enzyme, leading to lesser production of Aβ protein. Also, specific monoterpene compounds like 1,8-cineole and genipin can mitigate the Aβ caused neurotoxicity [52, 55]. Moreover, linalool is pivotal in restoring antioxidant enzyme levels, such as superoxidase dismutase and glutathione peroxidase, to normal levels [56]. In addition, it boasts anti-inflammatory properties by inhibiting pro-inflammatory proteins such as p38, MAPK, cyclooxygenase-2 (COX-2), nitric oxide synthase-2 (NOS-2), and interleukin-1 [54, 57] (Table 1) (Figure 2).



Outline of possible mechanism of effects of diterpenes on Alzheimer disease
Diterpenes contribute to controlling AD via various mechanisms. The primary focus is on neuroprotective antioxidant mechanisms and cellular defense activation. Recent investigations into the impact of rosemary diterpenes on brain function have unveiled a spectrum of pharmacological effects. Carnosic acid is a prominent diterpene isolated from rosemary, constituting 1.5%–2.5% of dried leaves [58]. Carnosic acid is susceptible to oxidation, forming carnosol, rosmanol, and its epimeric form. Analyzing the relative concentrations in rosemary tissues reveals that carnosic acid and carnosol, two major rosemary antioxidants, provide antioxidant effects for the plant [59]. These compounds, particularly carnosic acid and carnosol, hold biological significance, reaching concentrations of approximately 5% of the dry weight in rosemary [11]. Rosemary diterpenes, particularly carnosic acid, and carnosol, demonstrate robust antioxidant properties in in vitro experiments, effectively preventing lipid peroxidation and providing protection against oxidative cell death [60]. The antioxidant mechanisms involve the removal of hydrogen from phenolic hydroxyl groups, leading to the formation of quinone derivatives. The antioxidant potential of rosemary extract and diterpenes has been extensively established, including applications in food preservation. Emphasizing relevance to neurodegenerative diseases, carnosic acid’s protective effects against oxidative damage in neuronal cells are prominent. The exceptional antioxidant mechanism and radical scavenging effects of polyphenolic natural products are attributed to the presence of the catechol functional group [11]. In addition, the formation of various diterpene derivatives is associated with carnosic acid’s ability to interact with ROS. Activating phase II detoxifying enzymes is a vital defense mechanism against internal and external toxins. Substantial evidence supports the involvement of nuclear factor erythroid-derived 2-related factor 2 (Nrf-2) in orchestrating the induction of antioxidant response elements (AREs), leading to the activation of genes responsible for various antioxidant enzymes, including phase II detoxifying enzymes [61]. Thiol-regulating enzymes like glutathione S-transferase (GST), glutamylcysteine synthetase, and thioredoxin reductase notably depend on Nrf-2 for their expression [62]. A specific mechanism identified for these antioxidant effects entails the direct S-alkylation of the cysteine thiol on Kelch-like ECH-associated protein 1 (Keap1) by the electrophilic quinone derivative of carnosic acid. Keap1, a regulatory protein associated with the Nrf2 transcription factor, binds to ARE. The interaction of electrophilic compounds with cysteine residues on Keap1 leads to the formation of S-alkyl adducts, facilitating the translocation of Nrf2 to the nucleus. Once in the nucleus, Nrf2 promotes gene expression by binding to AREs of phase II genes. This well-documented mechanism underscores the efficacy of electrophile compounds as antioxidants and neuroprotective agents. Carnosol exhibits high electrophilic activity, activating Nrf2 phase II detoxifying enzyme genes and antioxidant enzymes. It has also been demonstrated to directly interact with cysteine residues of the nuclear factor kappa B (NF-κB) [11]. The essential role of free carboxylic acid and catechol hydroxyl moieties in antioxidant effects has been highlighted. The evidence suggests that the primary constituents of rosemary, namely carnosic acid, and carnosol, safeguard neurons against oxidative stress by activating the Keap1/Nrf2 pathway. Carnosic acid and carnosol protect HT22 cells against oxidative glutamate toxicity by activating the transcriptional antioxidant-responsive element of phase II genes, including heme oxygenase-1 (HO-1), NADPH-dependent quinone oxidoreductase, and γ-glutamyl cysteine ligase [63]. These genes contribute to neuroprotection by regulating the cellular redox system. Also, carnosic acid protects against lipopolysaccharide-induced liver injury (LPS) by enhancing the body’s cellular antioxidant defense system through its antioxidant mechanism. This action is evidenced by restoring superoxide dismutase, glutathione peroxidase, and glutathione levels in serum and liver after the LPS challenge [11].
Now, we aim to elucidate the mechanisms underlying the inhibition of Aβ formation, polymerization, and metal chelation by diterpenes. In a study focusing on Aβ polymerization inhibition and metal chelation, carnosic acid exhibited a significant impact, partially reversing cellular changes induced by Aβ42 or Aβ43 monomers. This observed effect correlates with reduced levels of cellular oligomers, suggesting a potential mechanism involving the inhibition of oligomerization [11, 64]. The findings were consistent with in vivo observations, where carnosic acid demonstrated a beneficial effect in AD models, effectively reversing Aβ (1–40)-mediated neurodegeneration and cognitive impairment. Additionally, research further substantiated the therapeutic potential of carnosic acid for AD, exhibiting its ability to reverse Aβ (1–40)-mediated changes in neurodegeneration and cognitive impairment [11, 65]. The connection between Aβ formation/aggregation and metal ions, particularly copper, has been extensively reviewed. Metal chelators have proven effective in decreasing Alzheimer Aβ plaques. Aβ’s redox activity, involving the reduction of transition metals and the generation of ROS, establishes a link between polymerization and toxicity to metal ions and ROS. Rosemary diterpenes, known for their multifunctional nature in metal chelation and ROS scavenging, may contribute to their effectiveness against Aβ polymerization and toxicity in the context of AD [11, 66].
Among the 7 identified sugi wood diterpenes, ferruginol and 6,7-dehydroferruginol exhibited prominent reduction effects on Aβ toxicity. Their activity was comparable to known anti-AD active agents, including ginkgolide A, morin, rosmarinic acid, and carnosic acid. Ferruginol and 6,7-dehydroferruginol demonstrated antioxidative activity similar to other anti-AD agents in a DPPH radical scavenging activity test, suggesting a correlation between antioxidative activity and the Aβ toxicity reduction effect of sugi wood diterpenes [67]. Isolated from volatile compounds released during the sugi wood-drying process, ferruginol and 6,7-dehydroferruginol improved motor function disorder (paralysis) caused by Aβ toxicity in Caenorhabditis elegans CL4176. The Aβ toxicity reduction effect of ferruginol was comparable to known anti-AD active compounds. The antioxidative activity observed only in ferruginol and 6,7-dehydroferruginol, which demonstrated Aβ toxicity reduction effect, suggests the importance of antioxidative activity in alleviating Aβ toxicity. These active diterpenes are skin-permeable, volatile, and could be applied transdermally or nasally. This discovery opens avenues for their potential use as therapeutic or preventive drugs for AD based on their Aβ toxicity reduction effects [67].
In addition, this section delves into the mechanisms underlying the neuroprotective, cognitive-enhancing, and mood-modulating potential of diterpenes. Exploration of the influence of rosemary diterpenes on brain function has revealed a diverse range of pharmacological impacts. These effects include anxiolytic and antidepressant-like behaviors, as well as neuroprotective properties against cyanide-induced brain damage [68]. The underlying mechanisms involve the upregulation of transcriptional pathways associated with antioxidant and anti-inflammatory responses. Also, carnosol has demonstrated protective effects against rotenone-induced neurotoxicity, indicating its potential to modulate apoptotic mechanisms [69].
Furthermore, rosemary’s essential oil constituents, including carnosic acid and carnosol, exhibit cognitive-enhancing properties. The multifaceted pharmacological effects of rosemary diterpenes encompass anxiolytic and antidepressant-like behaviors, neuroprotective properties against brain damage, and cognitive enhancement. These findings highlight the potential therapeutic applications of rosemary in addressing various aspects of brain function and memory-related disorders [70, 11].
Another mechanism through which diterpenes modulate AD involves the inhibition of human cholinesterases. In the context of AD, characterized by a significant decline in acetylcholine neurotransmitter levels linked to memory loss, restoring central cholinergic function has been a focal point of exploration. A notable strategy involves using acetylcholinesterase inhibitors, given the pivotal role of acetylcholine hydrolysis [71]. Expanding on the investigation into rosemary diterpene mechanisms, this study scrutinizes the inhibitory properties of two compounds, cryptotanshinone (CT) and dihydrotanshinone (DT), derived from Salvia miltiorrhiza Bunge. Functioning as mixed non-competitive inhibitors for human acetylcholinesterase (AChE) and uncompetitive inhibitors for human butyrylcholinesterase, these diterpenes unveil a spectrum of pharmacological potential. Molecular docking analyses offer insights into their interactions within the active sites of both cholinesterases [72], unraveling the intricate mechanisms of rosemary diterpenes. In a separate study, kaurenoic acid (1), a natural product isolated from various plant species such as Sphagneticola trilobata (Asteraceae), Xylopia sericea, and X. frutescens, emerges as a potential candidate. Furthermore, gibberellic acids, diterpene plant hormones synthesized from geranylgeranyl diphosphate, have also been explored for their role in acetylcholinesterase inhibition. Both derivatives of kaurenoic acid and gibberellic acid underwent screening for their AChE inhibitory capacity, yielding promising results. Kaurenoic acid exhibited an inhibition percentage of 81.6%±1.8%, while gibberellic acid reached 85.2%±4.1%. These findings suggest that derivatives of kaurenoic and gibberellic acids hold promise as potential AChE inhibitors, opening new avenues for drug development in the treatment of AD [73].
Clinical studies of the effects of monoterpenes on Alzheimer disease
The beneficial effects of monoterpenes and their derivatives on AD have been extensively investigated in various models (Table 2) [52, 55, 74].

Among these, linalool stands out for its ability to reduce neurodegeneration and enhance cognitive function. Researchers have focused on understanding how linalool affects brain phospholipids, crucial in developing neurodegeneration and cognitive disorders [54]. In a study using mice genetically predisposed to AD, oral administration of the linalool (25 mg/kg) for three months resulted in improved behavioral functions. These positive effects were associated with reduced Aβ and tau phosphorylation levels and decreased pro-inflammatory markers such as p38, MAPK, COX-2, NOS-2, and interleukin-1β [75]. However, in another study using a different animal model, male rats, Aβ injected into their hippocampus treatment with linalool (intraperitoneally, 100 mg/kg) showed a different pattern. While there was an improvement in cognitive performance, no adverse effects on motor activities were observed. Notably, impairment was observed in long-term memory but not in short-term memory. The mechanisms behind these diverse effects seem to be related to linalool’s ability to reduce apoptosis and oxidative stress induced by Aβ through the Nrf2 transcription factor/HO-1 pathway [56]. These findings underscore the complex and context-dependent nature of monoterpenes’ effect in AD, warranting further research and exploration.
Citral, another monoterpene, has been investigated for its cognitive effects on spatial memory using the water maze test in an AD model of male rats. The results reveal an interesting dose-dependent relationship. Behavioral tests show that lower doses of citral (0.1 mg/kg) improve the learning capacity and memory of rats, while higher doses (1 mg/kg) lead to suppression of learning and improvement of spatial memory in rats. The possible mechanism behind these effects is citral’s influence on retinoic acid, subsequently affecting spatial memory function [76].
Limonene, another significant monoterpene, exhibits multiple biological effects, including antioxidant, anti-inflammatory, and neuroprotective properties [77]. Its impact on neurodegenerative symptoms such as memory impairment and hippocampal damage has been investigated using various behavioral tests. Oral consumption of limonene significantly prevents memory and learning deficits and reduces neuron loss due to stress in the hippocampus region of male rat brains. The underlying mechanism is attributed to its anti-inflammatory and antioxidant activities [78]. Furthermore, in another study, IP injection of limonene at 20 mg/kg in rats that suffered cerebral ischemia protected them against memory impairment. The possible mechanism involves the increase in the activity of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase, along with the reduction of malondialdehyde (MDA) levels and free oxygen species [79].
The effects of the monoterpene thymol were investigated in a study involving rats fed a high-fat diet, leading to memory impairment caused by Aβ accumulation. The results demonstrated a positive response to thymol treatment in this AD model. The therapeutic effects included improvements in spatial memory and learning in selective behavioral tests and a significant reduction in Aβ deposition [80]. In a similar study, mice on a high-fat diet treated with thymol exhibited enhanced cognitive function, reduced Aβ deposits, and decreased phosphorylation of tau protein in the hippocampus. These beneficial effects are likely attributed to thymol’s antioxidant and anti-inflammatory activities. Thymol was also found to down-regulate the level of P-Ser307IRS-1 and increase the expression of P-Ser473 AKT and P-Ser9 GSK3β, further contributing to its neuroprotective effects. Researchers also highlight thymol’s ability to protect cognitive functions by upregulating the HO-1/Nrf2 pathway [81, 82]. Furthermore, thymol showed promise in mitigating memory and cognitive impairments caused by Aβ injection into the hippocampus or IP injection of scopolamine in rats. Treatment with thymol (0.5, 1, or 2 mg/kg) improved cognitive functions and reversed the effects induced by Aβ or scopolamine. The mechanisms underlying these effects were associated with thymol’s antioxidant and anti-inflammatory activities [83].
Another noteworthy monoterpene, p-cymene, has demonstrated anti-AD effects by inhibiting the formation of Aβ deposits. In this study, the analysis was conducted in six separate groups of rats with AD induced through hippocampal injection. The results showed the positive effect of IP injection equivalent to 50 and 100 mg/kg of p-cymene on memory improvement, learning, and reducing Aβ plaque deposits [84]. On the other hand, paeoniflorin, another derivative of monoterpene, exhibits diverse biological activities. Chronic treatment with this monoterpene for 20 days enhanced cognitive performance in mice with memory impairment induced by Aβ injection into the hippocampus. The possible mechanism of action for both p-cymene and paeoniflorin involves calcium homeostasis, increasing reduced glutamate content, and reducing protein carbonyl and MDA levels by inhibiting NOS (nitic oxide synthesis) activity and NO (nitric oxide) levels [85]. Similarly, the positive effects of paeoniflorin were observed in rats transformed into an AD model by injecting Aβ into the dorsal part of the hippocampus. Treatment with this monoterpene for 20 days improved cognitive performance, particularly in spatial learning and memory abilities.
In addition, the activities of acetylcholinesterase and acetylcholine transferase enzymes in the hippocampus were evaluated, revealing that paeoniflorin enhances the activity of cholinergic neurons (by enhancing the sprouting of cholinergic neurons) in the hippocampus. This enhancement is achieved by regulating the signaling pathway of the nerve growth factor and simultaneously reducing the atrophy of cholinergic neurons, ultimately leading to increased survival of nerve cells [86-88]. In rats with memory damage and intra-neuronal fiber coil accumulation induced by Aβ injection into the lateral ventricle, treatment with 25 and 100 mg/kg of carvacrol (IP) improved memory and significantly reduced cognitive deficits. The possible mechanism behind these effects involves reducing inflammatory factors in the brain, including interleukin-1, TNF-α, COX-2, and expression of tropomyosin receptor kinase B protein, along with increased brain-derived neurotrophic factor expression [89].
Moreover, carvacrol has been found to significantly reduce brain edema induced by aquaporin-4 [90] in AD mice models. Similarly, in various studies, α-pinene, another monoterpene, has been investigated for its effects on AD [91, 92, 93]. In a study involving mice with scopolamine-induced memory deficits, IP injection of 10 mg/kg of α-pinene was observed to improve memory and learning. The possible mechanism behind these effects involves the regulation of proteins related to the production of acetylcholine (increased expression of acetylcholine transferase in the cortex) and the antioxidant defense system (increased expression of HO-1 and superoxide dismutase via the activation of tumor necrosis factor E2) in the hippocampus [94].
In addition, it has been shown that treatment with 0.1, 0.2, and 0.4 μg/kg of α-pinene in rats suffering from memory and learning impairments induced by capsaicin resulted in improved memory and learning by modulating GABA A receptors [95]. Moreover, α-pinene (IP, 0.4 μg/kg) reduces neurodegeneration in rats subjected to Aβ-injected toxicity in the CA1 region of the hippocampus [96]. Similarly, male rats that experienced oxidative toxicity and neuro-inflammation due to intra-hippocampal injection of Aβ treatment with 50 mg/kg of α-pinene (IP) for 14 days improved learning and spatial learning while reducing anxiety behaviors [97]. In PC12 cells exposed to hydrogen peroxide (H2O2), treatment with 10-400 µM of α-pinene and 1,8-cineole has been found to increase cell viability, reduce ROS levels, and raise the expression of antioxidant enzymes, such as catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase, and HO-1. Notably, these compounds can reduce apoptosis by decreasing caspase 3 activities. The possible mechanism underlying the antioxidant effects of these compounds involves scavenging ROS and inducing TNF-E2 [98]. Moreover, α-pinene administration via intraperitoneal (IP) injection at 25, 50, and 100 mg/kg doses to rats that experienced cerebral ischemia improved behavioral functions. The treatment also restored the function of antioxidant enzymes while reducing the concentrations of NO, MDA, and interleukin-6 in the hippocampus, cortex, and striated bodies [99].
Olibanum essential oil is abundant in various monoterpene compounds with different percentages, including limonene, α-pinene, and 4-tripeneol. It has been shown that treating mice with 150 to 600 mg/kg of the aforementioned olibanum essential oil for two weeks, following Aβ-induced toxicity, improved memory and learning. The possible mechanism behind these effects involves increasing acetylcholine levels, reducing Aβ plaques, protecting hippocampal neurons, and downregulating c-Fos in brain tissues [100]. Besides, treatment with Hypericum scabrum essential oil, containing approximately 51.3% of the monoterpene α-pinene, improved memory and learning in behavioral tests in rats suffering from dementia induced by scopolamine. The potential mechanism appears to be α-pinene acting as an inhibitor of the acetylcholinesterase enzyme, thereby prolonging the presence of acetylcholine in the synaptic space [101].
Clinical studies of the effects of diterpenes on Alzheimer disease
Recent investigations into the impact of rosemary diterpenes on brain function encompass a spectrum of pharmacological effects, extending beyond rodent models to potential clinical applications. Ferlemi et al. explored the anxiolytic and antidepressant-like behavior resulting from rosemary tea consumption in adult male mice [102]. Machado et al. demonstrated the antidepressant-like effects of rosemary extract in mice undergoing an olfactory bulbectomy procedure. The study suggests a potential role for rosemary diterpenes in alleviating depressive-like behaviors in a murine model, providing insights into their antidepressant properties [70]. The crude extract of rosemary has exhibited efficacy in improving memory impairment in an in vivo scopolamine-induced dementia model of AD [103, 11]. Moreover, cognitive enhancement is observed in clinical studies, with rosemary’s essential oil constituents, including 1,8-cineole, showcasing mental benefits [104].
This preclinical finding indicates a potential cognitive-enhancing effect of rosemary diterpenes, highlighting their neuroprotective and memory-improving properties. Carnosic acid’s neuroprotective prowess extends to cultured rodent and human-induced pluripotent stem cell-derived neurons in vitro and in vivo [68]. The multifaceted pharmacological effects of rosemary diterpenes, including anxiolytic and antidepressant-like behaviors, neuroprotective properties against brain damage, and cognitive enhancement, provide a foundation for considering their therapeutic potential in clinical settings. Further exploration in human subjects is essential to understand and validate these observed effects fully, emphasizing the need for clinical trials to establish the efficacy and safety of rosemary diterpenes in the context of AD and related conditions [11].
Carnosic acid has been shown to safeguard neuronal cells from ischemic injury by scavenging ROS both in vitro and in vivo. Carnosol and carnosic acid have been proven to protect neuronal HT22 cells by activating the antioxidant-responsive element [105]. The existing evidence strongly suggests that these primary constituents of rosemary safeguard HT22 cells against oxidative glutamate toxicity. Pretreatment of RAW264.7 macrophages with carnosic acid also significantly reduces H2O2 or LPS-induced ROS and nitric oxide generation, with concurrent time- and dose-dependent upregulation of HO-1 protein expression [63, 11]. Furthermore, carnosol has been shown to enhance GST and quinone reductase activity in vivo. In the exploration of promoting non-amyloidogenic pathways in APP processing, Meng et al. conducted an investigation using SH-SY5Y human neuroblastoma cells, where carnosic acid exhibited a significant 61% suppression of Aβ42 secretion at a concentration of 30 μM. This suppression was concurrent with increased mRNA expressions of α-secretase [64]. The findings suggest that the mechanism underlying the inhibition of APP processing by carnosic acid involves facilitating the normal non-amyloidogenic-dependent pathway. Yoshida et al. conducted similar observations, demonstrating that carnosic acid suppressed Aβ peptides (1–40, 1–42, and 1–43) production in U373MG human astrocytoma cells at a concentration of 50 μM. The study disclosed a substantial 55% to 71% inhibition of Aβ release and a notable impact on mRNA expressions of α-secretase [106]. In a study by Meng et al. investigating the Aβ polymerization inhibition and metal chelation, the impact of carnosic acid on cultured SH-SY5Y human neuroblastoma cells challenged by Aβ42 or Aβ43 was explored. The results demonstrated a partial reversal of cellular deletion induced by Aβ42 or Aβ43 monomers (10 μM each) with carnosic acid treatment (10 μM) [64]. This observed effect correlates with reduced levels of cellular oligomers, suggesting the inhibition of oligomerization as a potential mechanism. These findings align with in vivo observations, where carnosic acid exhibited a beneficial effect in AD models, effectively reversing Aβ (1–40)-mediated neurodegeneration and cognitive impairment. Rasoolijazi et al. further substantiated the therapeutic potential of carnosic acid for AD, demonstrating its ability to reverse Aβ (1–40)-mediated changes in neurodegeneration and cognitive impairment in rats [65].
In a distinct clinical investigation, diterpenes extracted from sugi wood (Cryptomeria japonica) were identified as ferruginol, 6,7-dehydroferruginol, sandaracopimarinal, sandaracopimarinol, abietadiene, abietatriene, and phyllocladenes; mixture of phyllocladene and isophyllocladene. In this study, focusing on the potential of sugi wood diterpenes as a preventive and therapeutic drug for AD, their β-amyloid toxicity reduction effect was scrutinized by administering each diterpenoid at a final concentration of 0.5 mg/mL to C. elegans [67]. Paralyzed rates were meticulously observed, and the subsequent analysis of non-paralysis rates revealed significantly positive effects in the experimental plots of two impactful diterpenes, namely ferruginol and 6,7-dehydroferruginol. This outcome emphasizes that the administration of these compounds effectively suppressed the expression of paralysis induced by Aβ toxicity in C. elegans CL4176. Conversely, no significant differences were observed in the experimental plots of other sugi wood diterpenes. This study concludes that the Aβ toxicity reduction effect is a distinctive property of ferruginol and related compounds. Furthermore, it unveils, for the first time, the anti-AD effects derived from extracts of sugi wood [67] (Table 2).
Conclusion
In conclusion, Alzheimer disease has remained a formidable global health challenge, impacting millions worldwide. While current pharmacological treatments, such as acetylcholinesterase inhibitors and NMDA receptor antagonists, have been employed, the quest for more effective therapeutic approaches continues. Recent years have witnessed a burgeoning interest in investigating natural compounds from medicinal plants as potential remedies for AD. Among these, monoterpenes and diterpenes, certain plant secondary metabolites, have emerged as promising candidates due to their diverse biological activities. These compounds show neuroprotective attributes, augmenting memory and cognitive functions while demonstrating potent antioxidant capabilities. Their capacity to influence non-amyloidogenic pathways in the processing of APP is complemented by their ability to hinder Aβ formation and accumulation. Moreover, they actively participate in metal chelation and exhibit notable cholinesterase inhibition. Encouragingly, preclinical studies conducted on animal models and cellular cultures have consistently affirmed the effectiveness of these compounds, fostering optimism for their future role in therapeutic interventions. However, to fully unlock the therapeutic potential of monoterpenes and diterpenes, additional research, particularly in clinical trials, is imperative to comprehend their safety and efficacy in human subjects. Continued exploration in neuropharmacology holds the promise of pioneering innovative treatments that could substantially enhance the quality of life for individuals grappling with AD and related memory disorders.
In summary, the investigation into monoterpenes as integral components in AD management offers an exciting prospect for the future of neurodegenerative disease research. As our understanding of these compounds grows, the development of novel and effective treatments is in sight, ushering a positive impact on the lives of those with cognitive impairments and neurodegenerative conditions.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in compliance with the ethi- cal guidelines of the Declaration of Helsinki 2013
Funding
The study was funded by the Research Council of the University of Guilan.
Authors contributions
All authors equally contribute to preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors are thankful to the Research Council of University of Guilan.
References
- Wojtunik-Kulesza K, Oniszczuk A, Waksmundzka-Hajnos M. An attempt to elucidate the role of iron and zinc ions in development of Alzheimer’s and Parkinson’s diseases. Biomed Pharmacother. 2019; 111:1277-89. [DOI:10.1016/j.biopha.2018.12.140] [PMID]
- Kulshreshtha A, Piplani P. Current pharmacotherapy and putative disease-modifying therapy for Alzheimer’s disease. Neurol Sci. 2016; 37(9):1403-35. [DOI:10.1007/s10072-016-2625-7] [PMID]
- Blackburn L, Achor S, Allen B, Bauchmire N, Dunnington D, Klisovic RB, et al. The effect of aromatherapy on insomnia and other common symptoms among patients with acute leukemia. Oncol Nurs Forum. 2017; 44(4):E185-93. [DOI:10.1188/17.ONF.E185-E193] [PMID]
- Popova A, Dalemska Z, Mihaylova D, Hristova I, Alexieva I. Melissa officinalis L.-GC profile and antioxidant activity. Int J Pharmacogn Phytochem Res. 2016; 8(4):634-8. [Link]
- Wojtunik‐Kulesza KA, Kasprzak K, Oniszczuk T, Oniszczuk A. Natural monoterpenes: Much more than only a scent. Chem Biodivers. 2019; 16(12):e1900434. [DOI:10.1002/cbdv.201900434] [PMID]
- Maggio A, Rosselli S, Bruno M. Essential oils and pure volatile compounds as potential drugs in Alzheimer’s disease therapy: An updated review of the literature. Curr Pharm Des. 2016; 22(26):4011-27. [DOI:10.2174/1381612822666160607065917] [PMID]
- Adorjan B, Buchbauer G. Biological properties of essential oils: An updated review. Flavour Fragr J. 2010; 25(6):407-26. [DOI:10.1002/ffj.2024]
- Chen J, Jiang QD, Chai YP, Zhang H, Peng P, Yang XX. Natural terpenes as penetration enhancers for transdermal drug delivery. Molecules. 2016; 21(12):1709. [DOI:10.3390/molecules21121709] [PMID]
- Islam MT, da Mata AM, de Aguiar RP, Paz MF, de Alencar MV, Ferreira PM, et al. Therapeutic potential of essential oils focusing on diterpenes. Phytother Res. 2016; 30(9):1420-44. [DOI:10.1002/ptr.5652] [PMID]
- Qiu P, Xia J, Zhang H, Lin D, Shao Z. A review of diterpenes from marine-derived fungi: 2009-2021. Molecules. 2022; 27(23):8303. [DOI:10.3390/molecules27238303] [PMID]
- Habtemariam S. The therapeutic potential of rosemary (Rosmarinus officinalis) diterpenes for Alzheimer’s disease. Evid Based Complement Alternat Med. 2016; 2016:2680409.[DOI:10.1155/2016/2680409] [PMID]
- Wippold FJ 2nd, Cairns N, Vo K, Holtzman DM, Morris JC. Neuropathology for the neuroradiologist: Plaques and tangles. AJNR Am J Neuroradiol. 2008; 29(1):18-22. [DOI:10.3174/ajnr.A0781] [PMID]
- Hadipour E, Tayarani-Najaran Z, Fereidoni M. Vitamin K2 protects PC12 cells against Aβ (1-42) and H2O2-induced apoptosis via p38 MAP kinase pathway. Nutr Neurosci. 2020; 23(5):343-52. [DOI:10.1080/1028415X.2018.1504428] [PMID]
- Yankner BA, Caceres A, Duffy LK. Nerve growth factor potentiates the neurotoxicity of beta amyloid. Proc Natl Acad Sci U S A. 1990; 87(22):9020-3. [DOI:10.1073/pnas.87.22.9020] [PMID]
- Evin G, Weidemann A. Biogenesis and metabolism of Alzheimer’s disease Aβ amyloid peptides. Peptides. 2002; 23(7):1285-97. [DOI:10.1016/S0196-9781(02)00063-3] [PMID]
- Glenner GG, Wong CW. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984; 120(3):885-90. [DOI:10.1016/S0006-291X(84)80190-4] [PMID]
- O’brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011; 34:185-204. [DOI:10.1146/annurev-neuro-061010-113613] [PMID]
- Chen M. The maze of APP processing in Alzheimer’s disease: where did we go wrong in reasoning? Front Cell Neurosci. 2015; 9:186. [DOI:10.3389/fncel.2015.00186]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron. 2008; 58(1):42-51. [DOI:10.1016/j.neuron.2008.02.003] [PMID]
- Thal DR, Walter J, Saido TC, Fändrich M. Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer’s disease. Acta Neuropathol. 2015; 129(2):167-82. [DOI:10.1007/s00401-014-1375-y] [PMID]
- Nisbet RM, Polanco JC, Ittner LM, Götz J. Tau aggregation and its interplay with amyloid-β. Acta Neuropathol. 2015; 129(2):207-20. [DOI:10.1007/s00401-014-1371-2] [PMID]
- Palop JJ, Mucke L. Amyloid-β-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010; 13(7):812-8. [DOI:10.1038/nn.2583] [PMID]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer’s disease. Nat Rev Neurosci. 2007; 8(7):499-509. [DOI:10.1038/nrn2168] [PMID]
- Derisbourg M, Leghay C, Chiappetta G, Fernandez-Gomez FJ, Laurent C, Demeyer D, Carrier S, et al. Role of the Tau N-terminal region in microtubule stabilization revealed by newendogenous truncated forms. Sci Rep. 2015; 5:9659. [DOI:10.1038/srep09659] [PMID]
- de Paula VJR, Guimarães FM, Diniz BS, Forlenza OV. Neurobiological pathways to Alzheimer's disease: Amyloid-beta, TAU protein or both? Dement Neuropsychol. 2009; 3(3):188-94. [DOI:10.1590/S1980-57642009DN30300003] [PMID]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer β-amyloid precursor protein depends on lipid rafts. J. Cell Biol. 2003; 160(1):113-23. [DOI:10.1083/jcb.200207113] [PMID]
- Gilgun-Sherki Y, Melamed E, Offen D. Antioxidant treatment in Alzheimer’s disease: Current state. J Mol Neurosci. 2003; 21(1):1-11. [DOI:10.1385/JMN:21:1:1] [PMID]
- Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer’s disease: Molecular mechanisms. Int J Dev Neurosci. 2006; 24(2-3):167-76. [DOI:10.1016/j.ijdevneu.2005.11.014] [PMID]
- Rafieipour F, Hadipour E, Emami SA, Asili J, Tayarani-Najaran Z. Safranal protects against beta-amyloid peptide-induced cell toxicity in PC12 cells via MAPK and PI3 K pathways. Metab. Brain Dis. 2019; 34(1):165-72. [DOI:10.1007/s11011-018-0329-9] [PMID]
- Small SA, Gandy S. Sorting through the cell biology of Alzheimer’s disease: Intracellular pathways to pathogenesis. Neuron. 2006; 52(1):15-31. [DOI:10.1016/j.neuron.2006.09.001] [PMID]
- Chami L, Checler F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and β-amyloid production in Alzheimer’s disease. Mol Neurodegener. 2012; 7:52. [DOI:10.1186/1750-1326-7-52] [PMID]
- Taheri R, Hadipour E, Tayarani-Najaran Z. Crocin protects against Beta-Amyloid peptide-induced apoptosis in PC12 Cells Via the PI3 K pathway. Curr Mol Pharmacol. 2021; 14(4):627-34. [DOI:10.2174/1874467213666201012160401] [PMID]
- Zhao N, Liu CC, Qiao W, Bu G. Apolipoprotein E, receptors, and modulation of Alzheimer’s disease. Biol Psychiatry. 2018; 83(4):347-57. [DOI:10.1016/j.biopsych.2017.03.003] [PMID]
- Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet. 2010; 19(R1): R12-20. [DOI:10.1093/hmg/ddq160] [PMID]
- Butterfield DA, Mattson MP. Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer’s disease. Neurobiol Dis. 2020; 138:104795. [DOI:10.1016/j.nbd.2020.104795] [PMID]
- Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: Targeting the cholinergic system. Curr Neuropharmacol. 2016; 14(1):101-15. [DOI:10.2174/1570159X13666150716165726] [PMID]
- Bekdash RA. The cholinergic system, the adrenergic system and the neuropathology of Alzheimer’s disease. Int J Mol Sci. 2021; 22(3):1273. [DOI:10.3390/ijms22031273] [PMID]
- Rupsingh R, Borrie M, Smith M, Wells JL, Bartha R. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging. 2011; 32(5):802-10. [DOI:10.1016/j.neurobiolaging.2009.05.002] [PMID]
- Bukke VN, Archana M, Villani R, Romano AD, Wawrzyniak A, Balawender K, et al. The dual role of glutamatergic neurotransmission in Alzheimer’s disease: From pathophysiology to pharmacotherapy. Int J Mol Sci. 2020; 21(20):7452. [DOI:10.3390/ijms21207452] [PMID]
- Kim DH, Yeo SH, Park JM, Choi JY, Lee TH, Park SY, et al. Genetic markers for diagnosis and pathogenesis of Alzheimer’s disease. Gene. 2014; 545(2):185-93. [DOI:10.1016/j.gene.2014.05.031] [PMID]
- Dorostkar MM, Zou C, Blazquez-Llorca L, Herms J. Analyzing dendritic spine pathology in Alzheimer’s disease: Problems and opportunities. Acta Neuropathol. 2015; 130(1):1-19. [DOI:10.1007/s00401-015-1449-5] [PMID]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol. Aging. 2003; 24(8):1063-70. [DOI:10.1016/j.neurobiolaging.2003.08.012] [PMID]
- Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005; 73(4):1907-16. [DOI:10.1128/IAI.73.4.1907-1916.2005] [PMID]
- Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: A specific form of programmed cell death? Exp Cell Res. 2003; 283(1):1-6. [DOI:10.1016/S0014-4827(02)00027-7] [PMID]
- Piccialli I, Tedeschi V, Caputo L, D’Errico S, Ciccone R, De Feo V, et al. Exploring the therapeutic potential of phytochemicals in Alzheimer’s disease: Focus on polyphenols and monoterpenes. Front Pharmacol. 2022; 13:876614. [DOI:10.3389/fphar.2022.876614] [PMID]
- Wojtunik-Kulesza K, Rudkowska M, Kasprzak-Drozd K, Oniszczuk A, Borowicz-Reutt K. Activity of selected group of monoterpenes in alzheimer’s disease symptoms in experimental model studies-A non-systematic review. Int J Mol Sci. 2021; 22(14):7366. [DOI:10.3390/ijms22147366] [PMID]
- Kothari S, Bala N, Patel AB, Donovan A, Narayanaswami V. The LDL receptor binding domain of apolipoprotein E directs the relative orientation of its C-terminal segment in reconstituted nascent HDL. Biochim Biophys Acta Biomembr. 2021; 1863(7):183618. [DOI:10.1016/j.bbamem.2021.183618] [PMID]
- Kim M, Bezprozvanny I. Differences in recycling of apolipoprotein e3 and e4-ldl receptor complexes-A mechanistic hypothesis. Int J Mol Sci. 2021; 22(9):5030. [DOI:10.3390/ijms22095030] [PMID]
- Sotolongo K, Ghiso J, Rostagno A. Nrf2 activation through the PI3K/GSK-3 axis protects neuronal cells from Aβ-mediated oxidative and metabolic damage. Alzheimers Res Ther. 2020; 12(1):13. [DOI:10.1186/s13195-019-0578-9] [PMID]
- Hamilton K, Harvey J. The neuronal actions of leptin and the implications for treating alzheimer’s disease. Pharmaceuticals. 2021; 14(1):52. [DOI:10.3390/ph14010052] [PMID]
- Arkali G, Aksakal M, Kaya ŞÖ. Protective effects of carvacrol against diabetes‐induced reproductive damage in male rats: Modulation of Nrf2/HO‐1 signalling pathway and inhibition of Nf‐kB‐mediated testicular apoptosis and inflammation. Andrologia. 2021; 53(2):e13899. [DOI:10.1111/and.13899] [PMID]
- Volcho KP, Laev SS, Ashraf GM, Aliev G, Salakhutdinov NF. Application of monoterpenoids and their derivatives for treatment of neurodegenerative disorders. Curr Med Chem. 2018; 25(39):5327-46. [DOI:10.2174/0929867324666170112101837] [PMID]
- Stavrakov G, Philipova I, Zheleva‐Dimitrova D, Valkova I, Salamanova E, Konstantinov S, et al. Docking‐based design and synthesis of galantamine-camphane hybrids as inhibitors of acetylcholinesterase. Chem Biol Drug Des. 2017; 90(5):709-18. [DOI:10.1111/cbdd.12991] [PMID]
- Sabogal-Guáqueta AM, Osorio E, Cardona-Gómez GP. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology. 2016; 102:111-20. [DOI:10.1016/j.neuropharm.2015.11.002] [PMID]
- Habtemariam S. Iridoids and other monoterpenes in the Alzheimer’s brain: Recent development and future prospects. Molecules. 2018; 23(1):117. [DOI:10.3390/molecules23010117] [PMID]
- Xu P, Wang K, Lu C, Dong L, Gao L, Yan M, et al. Protective effects of linalool against amyloid beta-induced cognitive deficits and damages in mice Life Sci. 2017; 174:21-7. [DOI:10.1016/j.lfs.2017.02.010] [PMID]
- Khan A, Vaibhav K, Javed H, Tabassum R, Ahmed ME, Khan MM, et al. 1, 8-cineole (eucalyptol) mitigates inflammation in amyloid Beta toxicated PC12 cells: Relevance to Alzheimer’s disease. Neurochem Res. 2014; 39(2):344-52.[DOI:10.1007/s11064-013-1231-9] [PMID]
- Petiwala SM, Puthenveetil AG, Johnson JJ. Polyphenols from the Mediterranean herb rosemary (Rosmarinus officinalis) for prostate cancer. Front Pharmacol. 2013; 25; 4:29. [DOI:10.3389/fphar.2013.00029] [PMID]
- Loussouarn M, Krieger-Liszkay A, Svilar L, Bily A, Birtić S, Havaux M. Carnosic acid and carnosol, two major antioxidants of rosemary, act through different mechanisms. Plant Physiol. 2017; 175(3):1381-94. [DOI:10.1104/pp.17.01183] [PMID]
- Wijeratne SS, Cuppett SL. Potential of rosemary (Rosemarinus officinalis L.) diterpenes in preventing lipid hydroperoxide-mediated oxidative stress in Caco-2 cells. J Agric Food Chem. 2007; 55(4):1193-9. [DOI:10.1021/jf063089m] [PMID]
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, et al. The Cap’ n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001; 61(8):3299-307. [PMID]
- Lian KC, Chuang JJ, Hsieh CW, Wung BS, Huang GD, Jian TY, et al. Dual mechanisms of NF-kappaB inhibition in carnosol-treated endothelial cells. Toxicol Appl Pharmacol. 2010; 245(1):21-35. [DOI:10.1016/j.taap.2010.01.003] [PMID]
- Xiang Q, Wang Y, Wu W, Meng X, Qiao Y, Xu L, et al. Carnosic acid protects against ROS/RNS-induced protein damage and upregulates HO-1 expression in RAW264. 7 macrophages. J Funct Foods. 2013; 5(1):362-9. [DOI:10.1016/j.jff.2012.11.007]
- Meng P, Yoshida H, Matsumiya T, Imaizumi T, Tanji K, Xing F, et al. Carnosic acid suppresses the production of amyloid-β 1-42 by inducing the metalloprotease gene TACE/ADAM17 in SH-SY5Y human neuroblastoma cells. Neurosci Res. 2013; 75(2):94-102. [DOI:10.1016/j.neures.2012.11.007] [PMID]
- Rasoolijazi H, Azad N, Joghataei MT, Kerdari M, Nikbakht F, Soleimani M. The protective role of carnosic acid against beta-amyloid toxicity in rats. Sci World J. 2013; 2013:917082.[DOI:10.1155/2013/917082] [PMID]
- Maynard CJ, Bush AI, Masters CL, Cappai R, Li QX. Metals and amyloid‐β in Alzheimer’s disease.Int J Exp Pathol. 2005; 86(3):147-59. [DOI:10.1111/j.0959-9673.2005.00434.x] [PMID]
- Yamaji Y, Wakabayashi T, Kofujita H. Potential of sugi wood diterpenes as an Alzheimer’s disease preventive and therapeutic drug by the β-amyloid toxicity reduction effect. J Wood Sci. 2020; 66(76):1-6. [DOI:10.1186/s10086-020-01923-x]
- Zhang D, Lee B, Nutter A, Song P, Dolatabadi N, Parker J, et al. Protection from cyanide‐induced brain injury by the Nrf2 transcriptional activator carnosic acid. J Neurochem. 2015; 133(6):898-908. [DOI:10.1111/jnc.13074] [PMID]
- Kim SJ, Kim JS, Cho HS, Lee HJ, Kim SY, Kim S, et al. Carnosol, a component of rosemary (Rosmarinus officinalis L.) protects nigral dopaminergic neuronal cells. Neuroreport. 2006; 17(16):1729-33. [DOI:10.1097/01.wnr.0000239951.14954.10] [PMID]
- Machado DG, Cunha MP, Neis VB, Balen GO, Colla AR, Grando J, et al. Rosmarinus officinalis L. hydroalcoholic extract, similar to fluoxetine, reverses depressive-like behavior without altering learning deficit in olfactory bulbectomized mice. J Ethnopharmacol. 2012 ; 143(1):158-69. [DOI:10.1016/j.jep.2012.06.017] [PMID]
- Merad M, Soufi W, Ghalem S, Boukli F, Baig MH, Ahmad K, et al. Molecular interaction of acetylcholinesterase with carnosic acid derivatives: A neuroinformatics study. CNS Neurol Disord Drug Targets. 2014; 13(3):440-6. [DOI:10.2174/18715273113126660157] [PMID]
- Wong KK, Ngo JC, Liu S, Lin HQ, Hu C, Shaw PC, et al. Interaction study of two diterpenes, cryptotanshinone and dihydrotanshinone, to human acetylcholinesterase and butyrylcholinesterase by molecular docking and kinetic analysis. Chem Biol Interact. 2010; 187(1-3):335-9. [DOI:10.1016/j.cbi.2010.03.026] [PMID]
- Santos GF, Pereira RG, Boaventura MA, Macias FA, Lima GD, Coelho A, et al. Structure-activity relationship study of diterpenes for treatment of Alzheimer’s Disease. Quím Nova. 2017; 40(9):1045-50. [DOI:10.21577/0100-4042.20170112]
- Dinda B, Debnath S, Harigaya Y. Naturally occurring secoiridoids and bioactivity of naturally occurring iridoids and secoiridoids. A review, part 2. Chem Pharm Bull (Tokyo). 2007; 55(5):689-728. [DOI:10.1248/cpb.55.689] [PMID]
- Coelho VR, Gianesini J, Von Borowski R, Mazzardo-Martins L, Martins DF, Picada JN, et al. (−)-Linalool, a naturally occurring monoterpene compound, impairs memory acquisition in the object recognition task, inhibitory avoidance test and habituation to a novel environment in rats. Phytomedicine. 2011; 18(10):896-901. [DOI:10.1016/j.phymed.2011.02.010] [PMID]
- Yang Z, Xi J, Li J, Qu W. Biphasic effect of citral, a flavoring and scenting agent, on spatial learning and memory in rats. Pharmacol Biochem Behav. 2009; 93(4):391-6. [DOI:10.1016/j.pbb.2009.05.016] [PMID]
- Murali R, Saravanan R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed Prev Nutr. 2012; 2(4):269-75. [DOI:10.1016/j.bionut.2012.08.008]
- Bigdeli Y, Asle-Rousta M, Rahnema M. Effects of limonene on chronic restraint stress-induced memory impairment and anxiety in male rats. Neurophysiology. 2019; 51(2):107-13. [DOI:10.1007/s11062-019-09800-0]
- Wang X, Li G, Shen W. Protective effects of D Limonene against transient cerebral ischemia in stroke prone spontaneously hypertensive rats. Exp Ther Med. 2018; 15(1):699-706. [DOI:10.3892/etm.2017.5509]
- Asadbegi M, Yaghmaei P, Salehi I, Komaki A, Ebrahim-Habibi A. Investigation of thymol effect on learning and memory impairment induced by intrahippocampal injection of amyloid beta peptide in high fat diet-fed rats. Metab Brain Dis. 2017; 32(3):827-39. [DOI:10.1007/s11011-017-9960-0] [PMID]
- FangFang, Li H, Qin T, Li M, Ma S. Thymol improves high-fat diet-induced cognitive deficits in mice via ameliorating brain insulin resistance and upregulating NRF2/HO-1 pathway. Metab Brain Dis. 2017; 32(2):385-93. [DOI:10.1007/s11011-016-9921-z] [PMID]
- Hong Y, An Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Arch Pharm Res. 2018; 41(6):655-63. [DOI:10.1007/s12272-015-0662-z] [PMID]
- Azizi Z, Ebrahimi S, Saadatfar E, Kamalinejad M, Majlessi N. Cognitive-enhancing activity of thymol and carvacrol in two rat models of dementia. Behav Pharmacol. 2012; 23(3):241-9. [DOI:10.1097/FBP.0b013e3283534301] [PMID]
- Seifi Nahavandi B, Yaghmaei P, Ahmadian S, Ebrahim-Habibi A, Ghobeh M. [Effects of terpinolene and physical activity on memory and learning in a model of Alzheimer’s disease among rats (Persan)]. Qom Univ Med Sci J. 2020; 14(10):25-33. [DOI:10.52547/qums.14.10.25]
- Zhong SZ, Ge QH, Li Q, Qu R, Ma SP. Peoniflorin attentuates Aβ (1-42)-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J Neurol Sci. 2009; 280(1-2):71-8. [DOI:10.1016/j.jns.2009.01.027] [PMID]
- Lan Z, Chen L, Fu Q, Ji W, Wang S, Liang Z, et al. Paeoniflorin attenuates amyloid-beta peptide-induced neurotoxicity by ameliorating oxidative stress and regulating the NGF-mediated signaling in rats. Brain Res. 2013; 1498:9-19. [DOI:10.1016/j.brainres.2012.12.040] [PMID]
- Niewiadomska G, Mietelska-Porowska A, Mazurkiewicz M. The cholinergic system, nerve growth factor and the cytoskeleton. Behav Brain Res. 2011; 221(2):515-26. [DOI:10.1016/j.bbr.2010.02.024] [PMID]
- Niewiadomska G, Komorowski S, Baksalerska-Pazera M. Amelioration of cholinergic neurons dysfunction in aged rats depends on the continuous supply of NGF. Neurobiol Aging. 2002; 23(4):601-13. [DOI:10.1016/S0197-4580(01)00345-1] [PMID]
- Lee B, Yeom M, Shim I, Lee H, Hahm DH. Protective effects of quercetin on anxiety-like symptoms and neuroinflammation induced by lipopolysaccharide in rats. Evid Based Complement Alternat Med. 2020; 2020:4892415.[DOI:10.1155/2020/4892415] [PMID]
- Zhong Z, Wang B, Dai M, Sun Y, Sun Q, Yang G, et al. Carvacrol alleviates cerebral edema by modulating AQP4 expression after intracerebral hemorrhage in mice. Neurosci Lett. 2013; 555:24-9. [DOI:10.1016/j.neulet.2013.09.023] [PMID]
- Weston-Green K, Clunas H, Jimenez Naranjo C. A review of the potential use of pinene and linalool as terpene-based medicines for brain health: Discovering novel therapeutics in the flavours and fragrances of cannabis. Front Psychiatry. 2021; 12:583211. [DOI:10.3389/fpsyt.2021.583211] [PMID]
- Aydin E, Türkez H, Geyikoğlu F. Antioxidative, anticancer and genotoxic properties of α-pinene on N2a neuroblastoma cells. Biologia. 2013; 68:1004-9. [DOI:10.2478/s11756-013-0230-2]
- Allenspach M, Steuer C. α-Pinene: A never-ending story. Phytochemistry. 2021; 190:112857. [DOI:10.1016/j.phytochem.2021.112857] [PMID]
- Lee GY, Lee C, Park GH, Jang JH. Amelioration of scopolamine-induced learning and memory impairment by -pinene in C57BL/6 mice. Evid Based Complementary Altern Med. 2018; 2017:1-9. [Link]
- Rafie F, Kooshki R, Abbasnejad M, Rahbar I, Raoof M, Nekouei AH. α-Pinene influence on pulpal pain-induced learning and memory impairment in rats via modulation of the GABAA receptor. Adv Biomed Res. 2022; 11:60. [DOI:10.4103/abr.abr_139_21] [PMID]
- Khan-Mohammadi-Khorrami M, Asle-Rousta M, Rahnema M, Amini R. [The effect of alpha-pinene on amyloid-beta-induced neuronal death and depression in male wistar rats (Persian)]. J Ardabil Univ Med Sci. 2020; 20(4):456-64. [DOI:10.52547/jarums.20.4.456]
- Khan‐Mohammadi‐Khorrami MK, Asle‐Rousta M, Rahnema M, Amini R. Neuroprotective effect of alpha‐pinene is mediated by suppression of the TNF‐α/NF‐κB pathway in Alzheimer’s disease rat model. J Biochem Mol Toxicol. 2022; 36(5):e23006. [DOI:10.1002/jbt.23006] [PMID]
- Porres-Martínez M, González-Burgos E, Carretero ME, Gómez-Serranillos MP. In vitro neuroprotective potential of the monoterpenes α-pinene and 1, 8-cineole against H2O2-induced oxidative stress in PC12 cells. Z Naturforsch C J Biosci. 2016; 71(7-8):191-9. [DOI:10.1515/znc-2014-4135] [PMID]
- Khoshnazar M, Bigdeli MR, Parvardeh S, Pouriran R. Attenuating effect of α-pinene on neurobehavioural deficit, oxidative damage and inflammatory response following focal ischaemic stroke in rat. J Pharm Pharmaco. 2019; 71(11):1725-33. [DOI:10.1111/jphp.13164] [PMID]
- Zhang Z, Chen W, Luan J, Chen D, Liu L, Feng X. Ameliorative effects of olibanum essential oil on learning and memory in Aβ1-42-induced Alzheimer’s disease mouse model. Trop J Pharm Res. 2020; 19(8):1643-51. [DOI:10.4314/tjpr.v19i8.12]
- Sur TM, Akbaba E, Bagci E. Memory-enhancing properties of hypericum scabrum essential oil in a rat model of dementia. Iran J Pharm Sci. 2019; 15(4):95-106. [Link]
- Ferlemi AV, Katsikoudi A, Kontogianni VG, Kellici TF, Iatrou G, Lamari FN, et al. Rosemary tea consumption results to anxiolytic-and anti-depressant-like behavior of adult male mice and inhibits all cerebral area and liver cholinesterase activity; phytochemical investigation and in silico studies. Chem Biol Interact. 2015; 237:47-57. [DOI:10.1016/j.cbi.2015.04.013] [PMID]
- Ozarowski M, Mikolajczak PL, Bogacz A, Gryszczynska A, Kujawska M, Jodynis-Liebert J, et al. Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia. 2013; 91:261-71. [DOI:10.1016/j.fitote.2013.09.012] [PMID]
- Touafek O, Nacer A, Kabouche A, Kabouche Z, Bruneau C. Chemical composition of the essential oil of Rosmarinus officinalis cultivated in the Algerian Sahara. Chem Nat Compd. 2004; 40:28-9. [DOI:10.1023/B:CONC.0000025460.78222.69]
- Satoh T, Izumi M, Inukai Y, Tsutsumi Y, Nakayama N, Kosaka K, et al. Carnosic acid protects neuronal HT22 cells through activation of the antioxidant-responsive element in free carboxylic acid-and catechol hydroxyl moieties-dependent manners. Neurosci Lett. 2008; 434(3):260-5. [DOI:10.1016/j.neulet.2008.01.079] [PMID]
- Yoshida H, Meng P, Matsumiya T, Tanji K, Hayakari R, Xing F, et al. Carnosic acid suppresses the production of amyloid-β 1-42 and 1-43 by inducing an α-secretase TACE/ADAM17 in U373MG human astrocytoma cells. Neurosci Res. 2014; 79:83-93. [DOI:10.1016/j.neures.2013.11.004] [PMID]
Type of Study: Review |
Subject:
Special
Received: 2024/01/27 | Accepted: 2024/01/20 | Published: 2024/01/20
Received: 2024/01/27 | Accepted: 2024/01/20 | Published: 2024/01/20
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |