Fri, Jan 2, 2026
Volume 10, Issue 3 (Summer 2024)
Caspian J Neurol Sci 2024, 10(3): 235-242 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Niryana I W, Mardhika P E, Awyono S, Wismayasa A, Rahayu N M M. Malignant Peripheral Nerve Sheath Tumor of the Scalp: A Case Report. Caspian J Neurol Sci 2024; 10 (3) :235-242
URL: http://cjns.gums.ac.ir/article-1-685-en.html
URL: http://cjns.gums.ac.ir/article-1-685-en.html
I Wayan Niryana *1 

 , Putu Eka Mardhika2
, Putu Eka Mardhika2 

 , Steven Awyono2
, Steven Awyono2 

 , Adi Wismayasa2
, Adi Wismayasa2 

 , Ni Made Maharini Rahayu3
, Ni Made Maharini Rahayu3 




 , Putu Eka Mardhika2
, Putu Eka Mardhika2 

 , Steven Awyono2
, Steven Awyono2 

 , Adi Wismayasa2
, Adi Wismayasa2 

 , Ni Made Maharini Rahayu3
, Ni Made Maharini Rahayu3 


1- Department of Surgery, Faculty of Medicine, Prof. Dr. IGNG Ngoerah General Hospital, Udayana University, Denpasar, Indonesia. , niryanawayan@gmail.com
2- Department of Surgery, Faculty of Medicine, Prof. Dr. IGNG Ngoerah General Hospital, Udayana University, Denpasar, Indonesia.
3- Department of Pathology, Prima Medika Hospital, Denpasar, Indonesia.
2- Department of Surgery, Faculty of Medicine, Prof. Dr. IGNG Ngoerah General Hospital, Udayana University, Denpasar, Indonesia.
3- Department of Pathology, Prima Medika Hospital, Denpasar, Indonesia.
Full-Text [PDF 4613 kb]
(811 Downloads)
| Abstract (HTML) (1027 Views)
Full-Text: (436 Views)
Introduction
Malignant peripheral nerve sheath tumors (MPNSTs) are rare tumors commonly arising from somatic soft tissues or cells within the peripheral nerve sheath [1-6]. Classified as a soft tissue sarcoma, these tumors stem from peripheral nerve sheath cells and exhibit diverse differentiation potential [2-4, 7]. MPNSTs with an estimated incidence of approximately 0.001% are uncommon, with infrequent occurrences on the scalp [1, 8, 9]. The propensity of this tumor is mainly toward the proximal extremities and the trunk [10]. MPNSTs are known to be aggressive neoplasms and thus have a high incidence of recurrence and metastasis [6, 8, 11-13]. The tumor prognosis is poor, with a survival rate of only 30% for patients who survive beyond 5 years [1, 4].
The management of MPNSTs includes surgical excision, radiation, and systemic chemotherapy. The gold standard management of MPNSTs is surgical excision, for which the benefits of radiation and systemic chemotherapy are still undetermined [14]. MPNSTs often coincide with neurofibromatosis type 1 (NF-1), affecting 20%–50% of patients with NF-1 [15]. Apart from originating as primary growths, MPNSTs can also emerge through the malignant evolution of plexiform neurofibromas [16]. In this article, we aimed to report a single case of a de novo MPNSTs of the scalp due to its rarity in line with the surgical case report (SCARE) criteria with the literature review to summarize the clinical presentation and management for patients with MPNSTs.
Case Presentation
The patient was a 40-year-old man of Balinese ethnicity and an employee at a private company. He was right-handed with a normal body mass index. He was referred to the neurosurgery clinic with a complaint of rapid mass growth on the left side of the head. A small mass was not present before the mass grew larger without any pain or tenderness. The patient lacked a history of chronic headache, nausea, or vomiting. He had not previously taken any medication nor undergone any intervention for this mass. There was no history of family or relatives having a similar condition. There was no history of inherited disease in the family. She had no history of smoking, alcohol abuse, or recreational drug use.
Clinical findings
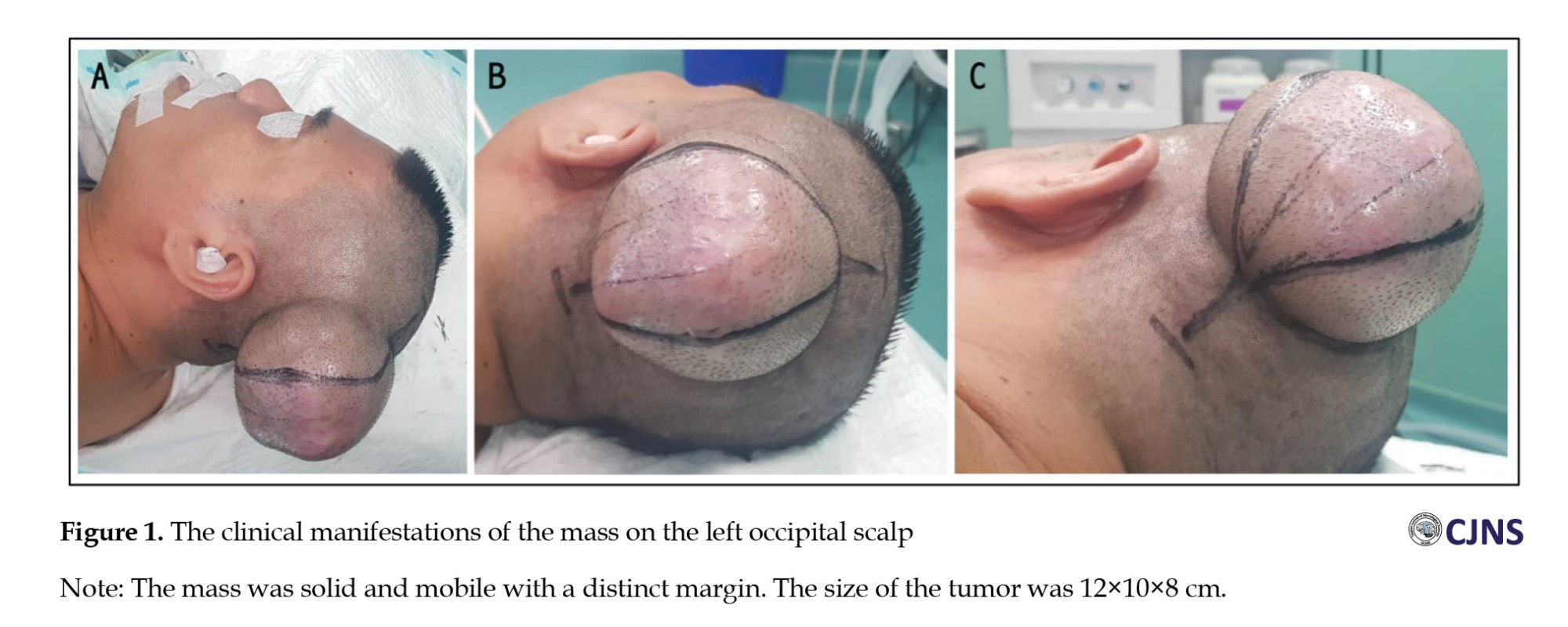
The patient was fully conscious and had normal vital signs. On physical examination, NF-1 signs, such as café au lait spots, tumors (neurofibroma) and a cluster of freckles, were not found. On neurological examination, cranial nerve function was normal, without any sign of focal neurological deficit. The mass was on the left occipital side. It was 12×10×8 cm. Based on the physical examination findings, the mass was solid and mobile with a distinct margin (Figure 1), suggesting a benign tumor.
Diagnostic assessment and interpretation
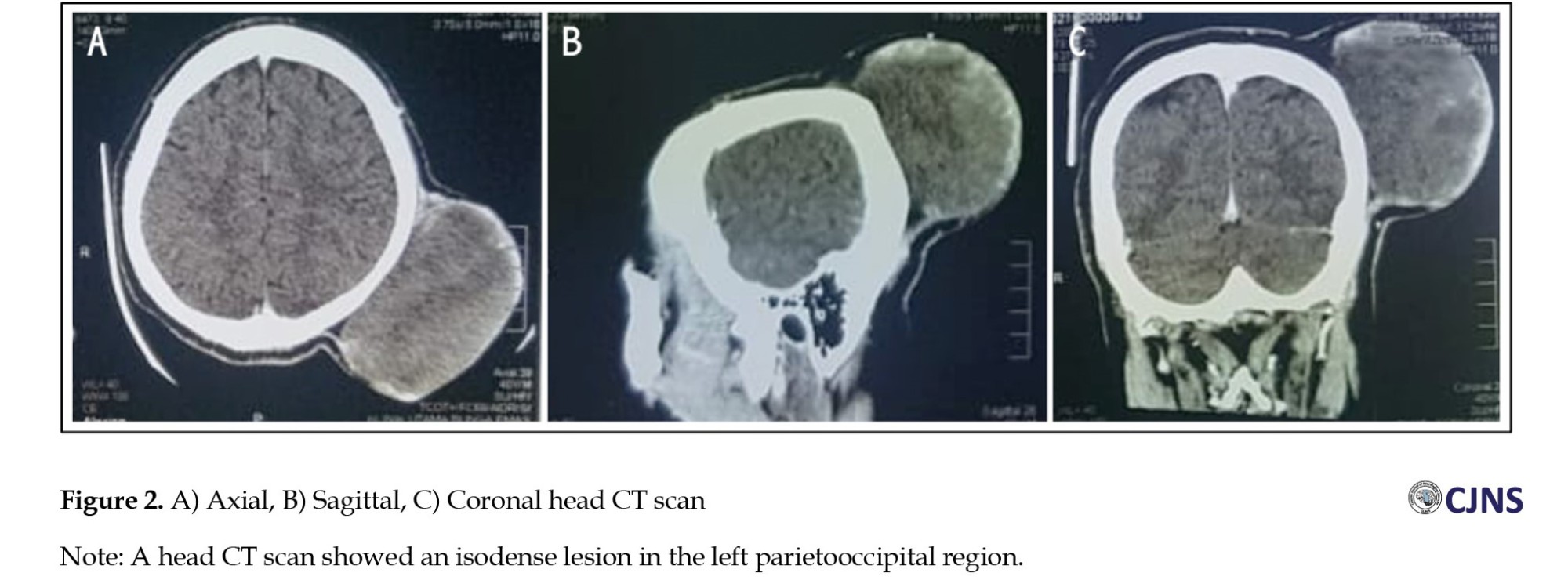
A computed tomography (CT) scan of the head was conducted to assess the mass. The results showed an isodense, non-enhancing mass outside the skull on the left occipital, measuring 12×10×8 cm in size (Figure 2). The tumor had a distinct margin in the subgaleal region with no bone involvement. The head CT scan results were in favor of a benign soft tissue tumor.
Intervention
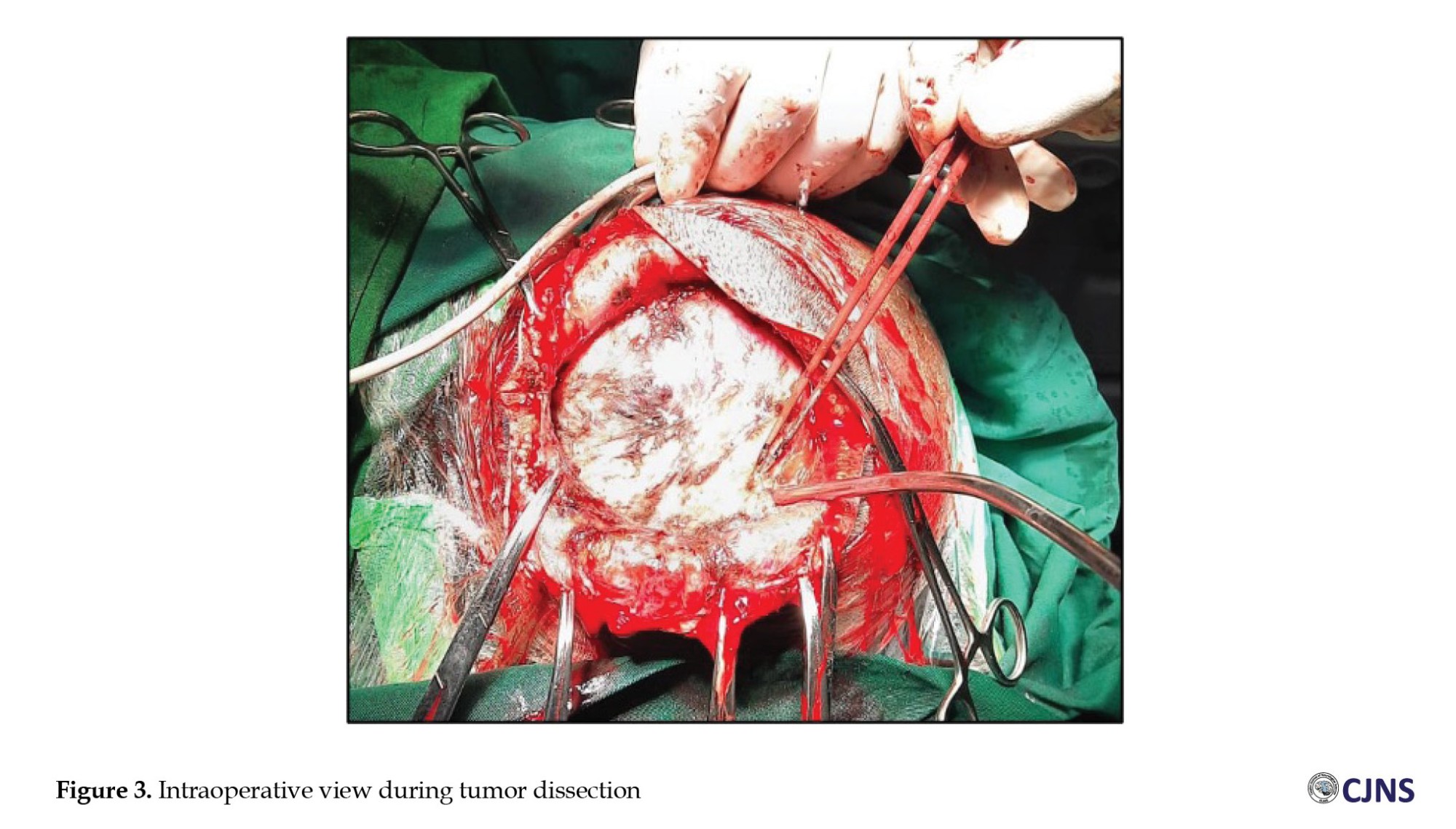
The patient was in optimal condition preoperatively, and we performed the surgery under general anesthesia. He was positioned supine and tilted to the right, placing the mass in the uppermost position for easier access during the procedure. There was no specific preparation other than a blood product. The tumor was completely removed during surgery (Figure 3). The tumor was solid and had a distinct margin; therefore, tumor dissection was performed without problem (Figure 4). No bone involvement was observed intraoperatively. The patient was discharged 3 days after surgery without any postoperative complications.
Follow-up and outcomes
The patient visited the neurosurgery clinic every 3 months and reported no obvious problems related to the disease. The patient could also sleep normally because the tumor had been excised. No adverse events or complications were found during follow-up.
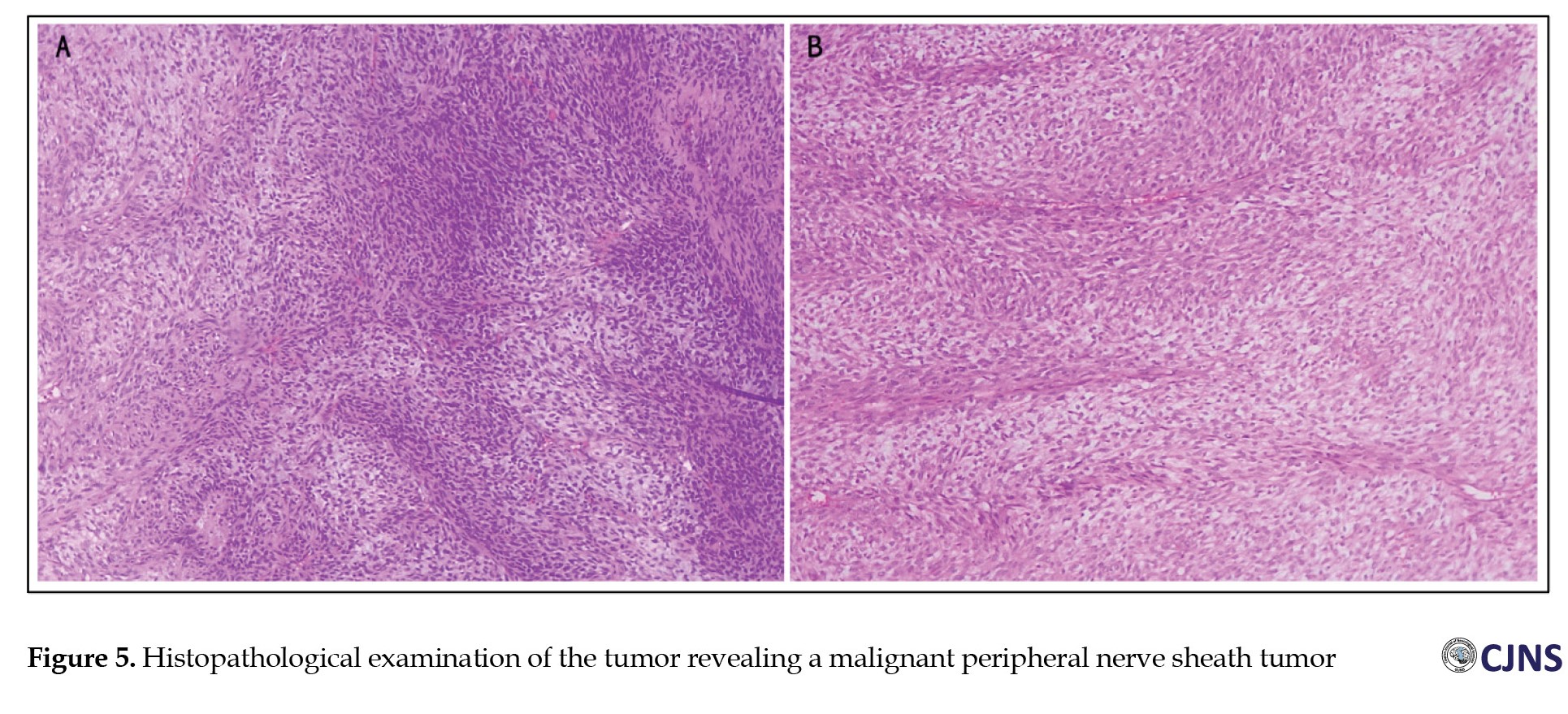
The histopathology results showed a tumor mass, with alternating hypercellular and hypocellular patterns, consisting of predominantly atypical spindle-shaped proliferating cells forming a short fascicle structure and irregular fasciculus and partly in the form of a vaguely storiform pattern and whorled-like focus. It also contains vascular components and hemangiopericytoma on several blood vessels; some cases occur in a “peculiar fashion,” and some tumor cells appear to be located between the stroma, which is loosely arranged (myxoid-like). Necrosis was present. Some tumor cells in other foci had focal cytoplasmic vacuoles. There were also foci of neoplastic cells with vaguely palisading arranged nuclei. Mitosis was found in 27 per 10 high-power fields (Figure 5).
Discussion
MPNSTs are classified as nervous system neoplasms originating from peripheral nerve sheath structures, such as Schwann cells, and exhibit malignant behavior [17, 18]. MPNSTs are rare and classified as soft tissue sarcoma, accounting for 5%-10% of this sarcoma [3, 6, 7]. There is a higher incidence of this tumor in males, with an average onset of around 50 [1].
MPNSTs can develop throughout the human body in the presence of any peripheral nerve. However, the predilection is on the major nerve in the proximal lower extremities and the trunk [1, 9]. The occurrence of MPNSTs in the scalp is rare[1, 9, 10] and we found only 22 published cases (Table 1).
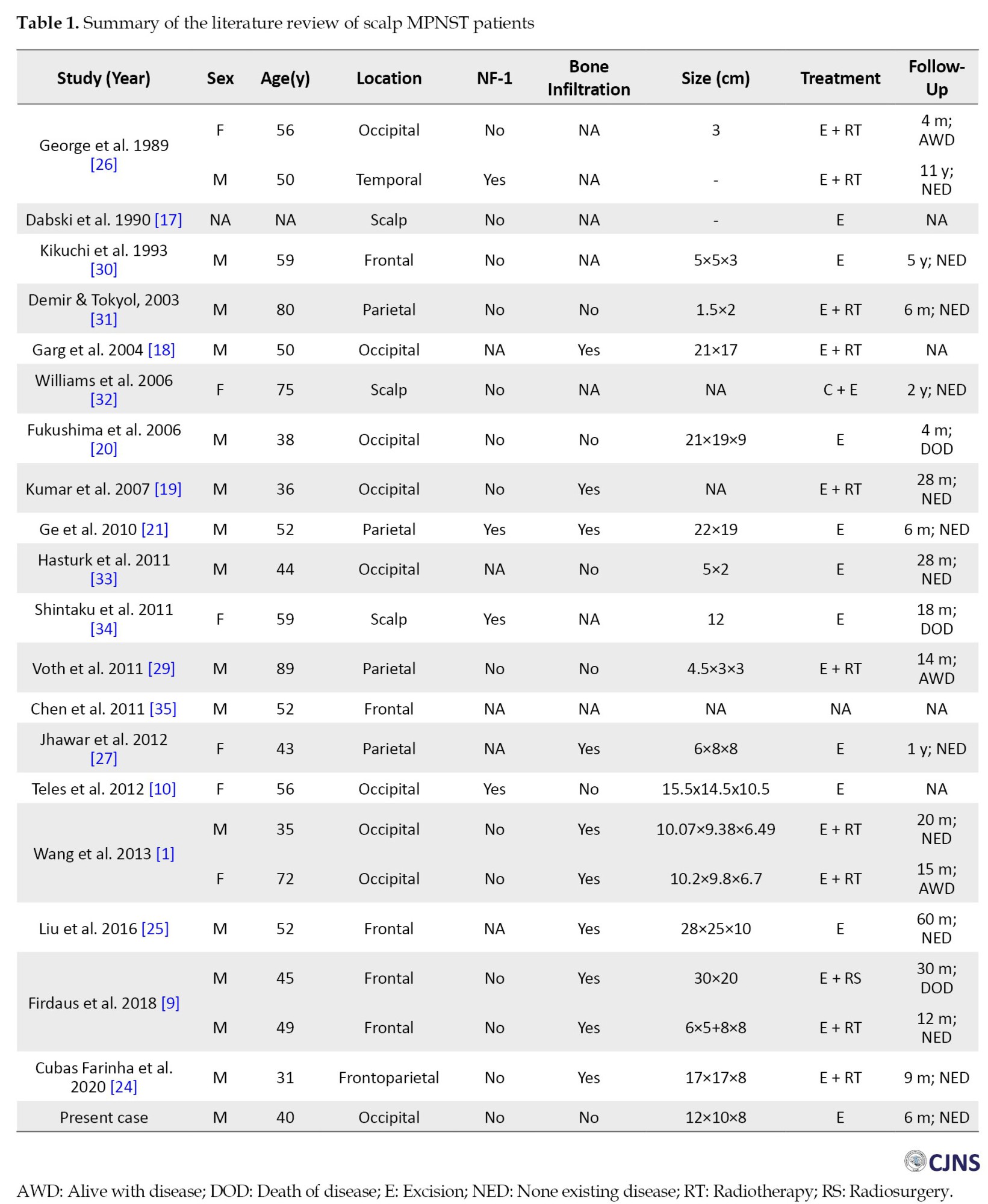
A scalp MPNST can arise from any scalp site with the highest incidence on the occipital (9 patients), followed by frontal, parietal, and temporal, as shown in Table 1. Regarding sex, MPNSTs have a preference for men, with 16 cases reported. The mean age was 52 years, ranging from 31 to 89 years. Four patients had NF-1 and 14 patients reported the absence of NF-1. The presence of bone infiltration was reported in 10 patients. In our patient, there was no bone infiltration or NF-1 present.
However, the etiology of MPNSTs is still unknown. Still, its association with the absence of the NF-1 protein product [19], as well as the p16 (INK4A) gene reordering, has been reported [19, 20, 21] NF-1 is a hereditary condition characterized by the presence of NF-1 tumor suppressor gene on chromosome [17], which follows an autosomal dominant inheritance pattern [15]. It was also reported that up to thirty percent of MPNSTs arise de novo and that 60% are a transformation of preexisting benign neurofibroma [8, 19, 22]. Furthermore, a history of radiation exposure is also associated with MPNSTs, which account for 10% of cases [1, 23].
The clinical manifestations of scalp MPNSTs are atypical [1]. Mostly, it manifests as the initially firm, globular, non-tender, no pulsatile, and noncompressible mass [24]. The mass gradually increases in size on the scalp, whether cutaneous or subcutaneous and can reach around 10 cm in diameter [24]. MPNSTs arising from the transformation of benign neurofibroma exhibit slow growth during the initial stages and accelerate growth during the later stages of the disease. On the other hand, MPNSTs arising de novo proliferate rapidly from the beginning [8, 19]. This neoplasm can invade the scalp, cranial bone, and dura mater due to its aggressiveness and rapid cell growth [1]. Involvement of the skin causes skin erosion and pain, where skin erosion can lead to bleeding and infection [25]. Invasion of the cranial bone and dura mater leads to destruction and further invasion of the intracranial compartment. Intracranial invasion manifests as symptoms of mass effects that cause raised intracranial pressure [1, 9, 26].
Imaging has a role in defining the tumor margin and resectability, assessing bone infiltration, and evaluating the anatomic relationship with important muscle and neurovascular structures adjacent to the tumor. CT scan is used to determine bone infiltration, and magnetic resonance imaging (MRI) can be performed to assess adjacent important structures [1, 6, 9, 21]. In MRI scans, the tumor appears hypointense to isointense in T1- and hyperintense in T2-weighted and fluid attenuated inversion recovery (FLAIR) sequences [22]. Additional venography can be performed for scalp MPNSTs that originate adjacent to the dural venous sinus system [1].
An MPNST often presents a swirling organization of intermixed myxoid and compact areas in a marbleized pattern on histopathology examination. During histopathological examination, an MPNST commonly displays a marbleized pattern characterized by swirling myxoid and compact areas. This tumor is densely cellular, with spindle-shaped cells and irregular contours, featuring fascicular regions interspersed with myxoid areas [27]. The presence of nuclear pleomorphism, necrosis, increased mitotic activity, and invasion of vascular and surrounding tissue are features of malignancy [9].
MPNSTs are treated like other high-grade sarcomas due to their aggressive behavior. However, no established guidelines exist for treating MPNSTs occurring on the scalp. The gold standard of treatment is total surgical excision to achieve gross total removal. A wide excision should be performed for a resectable tumor with a margin of more than 2 cm [1, 9]. In a condition where the excision width is limited due to an anatomic barrier, a marginal or positive margin microscopic excision should be performed to preserve physical function [28]. To achieve this goal, the presence of a giant skull defect is highly possible. Therefore, collaboration with plastic and reconstructive surgery is necessary to reconstruct defects by large cutaneous, myocutaneous pediculated, or free flaps [24]. We were using an elliptical surgical incision, identifying the tumor around 15 mm from the skin and meticulously dissecting the tumor from the surrounding tissue. The main feeder artery was from the left occipital artery, which was identified early in the surgery and then coagulated. This early identification assists during the surgery, which helps minimize blood loss. We were using blunt dissection to separate the tumor from the surrounding structure. The base of the tumor is located just above the periosteum (Figure 3). The tumor was firm in consistency with a distinct margin that facilitated our dissection of the tumor with the surrounding tissue without significant problems. No bone involvement was seen on the intraoperative evaluation. Furthermore, radiation treatment was also proposed following tumor excision with a safe margin. Adjuvant radiotherapy was suggested to enhance local tumor control [1, 22, 27, 29]. However, no clear evidence supports this measure. As shown in Table 1, 11 patients underwent adjuvant radiation therapy. Upon final follow-up, 6 patients showed no signs of disease, 3 patients were alive with signs of disease, 1 had already passed away, and there was no information regarding the last patient.
Conclusion
Even though MPNSTs are rare, a pathological diagnosis should be made for rapidly growing tumors on the scalp. Wide excision with a two cm free margin should be performed first. Adjuvant radiotherapy for local tumor control should be considered individually for each patient, as well as the benefits and risks.
Ethical Considerations
Compliance with ethical guidelines
All procedures conducted in this study were in accordance with the ethical principles outlined in the 2013 Declaration of Helsinki.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, resources and supervision: I Wayan Niryana; Visualization and writing the original draft: I Wayan Niryana, Putu Eka Mardhika and Steven Awyono; Investigation, review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Malignant peripheral nerve sheath tumors (MPNSTs) are rare tumors commonly arising from somatic soft tissues or cells within the peripheral nerve sheath [1-6]. Classified as a soft tissue sarcoma, these tumors stem from peripheral nerve sheath cells and exhibit diverse differentiation potential [2-4, 7]. MPNSTs with an estimated incidence of approximately 0.001% are uncommon, with infrequent occurrences on the scalp [1, 8, 9]. The propensity of this tumor is mainly toward the proximal extremities and the trunk [10]. MPNSTs are known to be aggressive neoplasms and thus have a high incidence of recurrence and metastasis [6, 8, 11-13]. The tumor prognosis is poor, with a survival rate of only 30% for patients who survive beyond 5 years [1, 4].
The management of MPNSTs includes surgical excision, radiation, and systemic chemotherapy. The gold standard management of MPNSTs is surgical excision, for which the benefits of radiation and systemic chemotherapy are still undetermined [14]. MPNSTs often coincide with neurofibromatosis type 1 (NF-1), affecting 20%–50% of patients with NF-1 [15]. Apart from originating as primary growths, MPNSTs can also emerge through the malignant evolution of plexiform neurofibromas [16]. In this article, we aimed to report a single case of a de novo MPNSTs of the scalp due to its rarity in line with the surgical case report (SCARE) criteria with the literature review to summarize the clinical presentation and management for patients with MPNSTs.
Case Presentation
The patient was a 40-year-old man of Balinese ethnicity and an employee at a private company. He was right-handed with a normal body mass index. He was referred to the neurosurgery clinic with a complaint of rapid mass growth on the left side of the head. A small mass was not present before the mass grew larger without any pain or tenderness. The patient lacked a history of chronic headache, nausea, or vomiting. He had not previously taken any medication nor undergone any intervention for this mass. There was no history of family or relatives having a similar condition. There was no history of inherited disease in the family. She had no history of smoking, alcohol abuse, or recreational drug use.
Clinical findings
The patient was fully conscious and had normal vital signs. On physical examination, NF-1 signs, such as café au lait spots, tumors (neurofibroma) and a cluster of freckles, were not found. On neurological examination, cranial nerve function was normal, without any sign of focal neurological deficit. The mass was on the left occipital side. It was 12×10×8 cm. Based on the physical examination findings, the mass was solid and mobile with a distinct margin (Figure 1), suggesting a benign tumor.
Diagnostic assessment and interpretation
A computed tomography (CT) scan of the head was conducted to assess the mass. The results showed an isodense, non-enhancing mass outside the skull on the left occipital, measuring 12×10×8 cm in size (Figure 2). The tumor had a distinct margin in the subgaleal region with no bone involvement. The head CT scan results were in favor of a benign soft tissue tumor.
Intervention
The patient was in optimal condition preoperatively, and we performed the surgery under general anesthesia. He was positioned supine and tilted to the right, placing the mass in the uppermost position for easier access during the procedure. There was no specific preparation other than a blood product. The tumor was completely removed during surgery (Figure 3). The tumor was solid and had a distinct margin; therefore, tumor dissection was performed without problem (Figure 4). No bone involvement was observed intraoperatively. The patient was discharged 3 days after surgery without any postoperative complications.
Follow-up and outcomes
The patient visited the neurosurgery clinic every 3 months and reported no obvious problems related to the disease. The patient could also sleep normally because the tumor had been excised. No adverse events or complications were found during follow-up.
The histopathology results showed a tumor mass, with alternating hypercellular and hypocellular patterns, consisting of predominantly atypical spindle-shaped proliferating cells forming a short fascicle structure and irregular fasciculus and partly in the form of a vaguely storiform pattern and whorled-like focus. It also contains vascular components and hemangiopericytoma on several blood vessels; some cases occur in a “peculiar fashion,” and some tumor cells appear to be located between the stroma, which is loosely arranged (myxoid-like). Necrosis was present. Some tumor cells in other foci had focal cytoplasmic vacuoles. There were also foci of neoplastic cells with vaguely palisading arranged nuclei. Mitosis was found in 27 per 10 high-power fields (Figure 5).
Discussion
MPNSTs are classified as nervous system neoplasms originating from peripheral nerve sheath structures, such as Schwann cells, and exhibit malignant behavior [17, 18]. MPNSTs are rare and classified as soft tissue sarcoma, accounting for 5%-10% of this sarcoma [3, 6, 7]. There is a higher incidence of this tumor in males, with an average onset of around 50 [1].
MPNSTs can develop throughout the human body in the presence of any peripheral nerve. However, the predilection is on the major nerve in the proximal lower extremities and the trunk [1, 9]. The occurrence of MPNSTs in the scalp is rare[1, 9, 10] and we found only 22 published cases (Table 1).

A scalp MPNST can arise from any scalp site with the highest incidence on the occipital (9 patients), followed by frontal, parietal, and temporal, as shown in Table 1. Regarding sex, MPNSTs have a preference for men, with 16 cases reported. The mean age was 52 years, ranging from 31 to 89 years. Four patients had NF-1 and 14 patients reported the absence of NF-1. The presence of bone infiltration was reported in 10 patients. In our patient, there was no bone infiltration or NF-1 present.
However, the etiology of MPNSTs is still unknown. Still, its association with the absence of the NF-1 protein product [19], as well as the p16 (INK4A) gene reordering, has been reported [19, 20, 21] NF-1 is a hereditary condition characterized by the presence of NF-1 tumor suppressor gene on chromosome [17], which follows an autosomal dominant inheritance pattern [15]. It was also reported that up to thirty percent of MPNSTs arise de novo and that 60% are a transformation of preexisting benign neurofibroma [8, 19, 22]. Furthermore, a history of radiation exposure is also associated with MPNSTs, which account for 10% of cases [1, 23].
The clinical manifestations of scalp MPNSTs are atypical [1]. Mostly, it manifests as the initially firm, globular, non-tender, no pulsatile, and noncompressible mass [24]. The mass gradually increases in size on the scalp, whether cutaneous or subcutaneous and can reach around 10 cm in diameter [24]. MPNSTs arising from the transformation of benign neurofibroma exhibit slow growth during the initial stages and accelerate growth during the later stages of the disease. On the other hand, MPNSTs arising de novo proliferate rapidly from the beginning [8, 19]. This neoplasm can invade the scalp, cranial bone, and dura mater due to its aggressiveness and rapid cell growth [1]. Involvement of the skin causes skin erosion and pain, where skin erosion can lead to bleeding and infection [25]. Invasion of the cranial bone and dura mater leads to destruction and further invasion of the intracranial compartment. Intracranial invasion manifests as symptoms of mass effects that cause raised intracranial pressure [1, 9, 26].
Imaging has a role in defining the tumor margin and resectability, assessing bone infiltration, and evaluating the anatomic relationship with important muscle and neurovascular structures adjacent to the tumor. CT scan is used to determine bone infiltration, and magnetic resonance imaging (MRI) can be performed to assess adjacent important structures [1, 6, 9, 21]. In MRI scans, the tumor appears hypointense to isointense in T1- and hyperintense in T2-weighted and fluid attenuated inversion recovery (FLAIR) sequences [22]. Additional venography can be performed for scalp MPNSTs that originate adjacent to the dural venous sinus system [1].
An MPNST often presents a swirling organization of intermixed myxoid and compact areas in a marbleized pattern on histopathology examination. During histopathological examination, an MPNST commonly displays a marbleized pattern characterized by swirling myxoid and compact areas. This tumor is densely cellular, with spindle-shaped cells and irregular contours, featuring fascicular regions interspersed with myxoid areas [27]. The presence of nuclear pleomorphism, necrosis, increased mitotic activity, and invasion of vascular and surrounding tissue are features of malignancy [9].
MPNSTs are treated like other high-grade sarcomas due to their aggressive behavior. However, no established guidelines exist for treating MPNSTs occurring on the scalp. The gold standard of treatment is total surgical excision to achieve gross total removal. A wide excision should be performed for a resectable tumor with a margin of more than 2 cm [1, 9]. In a condition where the excision width is limited due to an anatomic barrier, a marginal or positive margin microscopic excision should be performed to preserve physical function [28]. To achieve this goal, the presence of a giant skull defect is highly possible. Therefore, collaboration with plastic and reconstructive surgery is necessary to reconstruct defects by large cutaneous, myocutaneous pediculated, or free flaps [24]. We were using an elliptical surgical incision, identifying the tumor around 15 mm from the skin and meticulously dissecting the tumor from the surrounding tissue. The main feeder artery was from the left occipital artery, which was identified early in the surgery and then coagulated. This early identification assists during the surgery, which helps minimize blood loss. We were using blunt dissection to separate the tumor from the surrounding structure. The base of the tumor is located just above the periosteum (Figure 3). The tumor was firm in consistency with a distinct margin that facilitated our dissection of the tumor with the surrounding tissue without significant problems. No bone involvement was seen on the intraoperative evaluation. Furthermore, radiation treatment was also proposed following tumor excision with a safe margin. Adjuvant radiotherapy was suggested to enhance local tumor control [1, 22, 27, 29]. However, no clear evidence supports this measure. As shown in Table 1, 11 patients underwent adjuvant radiation therapy. Upon final follow-up, 6 patients showed no signs of disease, 3 patients were alive with signs of disease, 1 had already passed away, and there was no information regarding the last patient.
Conclusion
Even though MPNSTs are rare, a pathological diagnosis should be made for rapidly growing tumors on the scalp. Wide excision with a two cm free margin should be performed first. Adjuvant radiotherapy for local tumor control should be considered individually for each patient, as well as the benefits and risks.
Ethical Considerations
Compliance with ethical guidelines
All procedures conducted in this study were in accordance with the ethical principles outlined in the 2013 Declaration of Helsinki.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, resources and supervision: I Wayan Niryana; Visualization and writing the original draft: I Wayan Niryana, Putu Eka Mardhika and Steven Awyono; Investigation, review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Wang J, Ou S, Guo Z, Wang Y, Xing D. Microsurgical management of giant malignant peripheral nerve sheath tumor of the scalp: Two case reports and a literature review. World J Surg Oncol. 2013; 11:269. [DOI:10.1186/1477-7819-11-269]
- Hajdu SI. Peripheral nerve sheath tumors. Histogenesis, classification, and prognosis. Cancer. 1993; 72(12):3549-52.[DOI:10.1002/1097-0142(19931215)72:123.0.CO;2-Y] [PMID]
- Wanebo JE, Malik JM, VandenBerg SR, Wanebo HJ, Driesen N, Persing JA. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 28 cases. Cancer. 1993; 71(4):1247-53. [DOI:10.1002/1097-0142(19930215)71:43.0.CO;2-S] [PMID]
- Baehring JM, Betensky RA, Batchelor TT. Malignant peripheral nerve sheath tumor: The clinical spectrum and outcome of treatment. Neurology. 2003; 61:696 -8. [DOI:10.1212/01.WNL.0000078813.05925.2C] [PMID]
- Jo VY, Fletcher CD. WHO classification of soft tissue tumors: An update based on the 2013 (4th) edition. Pathology. 2014; 46(2):95-104. [DOI:10.1097/PAT.0000000000000050] [PMID]
- Owosho AA, Estilo CL, Huryn JM, Chi P, Antonescu CR. A clinicopathologic study of head and neck malignant peripheral nerve sheath tumors. Head Neck Pathol. 2018; 12(2):151-9. [DOI:10.1007/s12105-017-0841-y] [PMID]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016. 131(6):803-20.[DOI:10.1007/s00401-016-1545-1] [PMID]
- Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986; 57(10):2006-21. [DOI:10.1002/1097-0142(19860515)57:103.0.CO;2-6] [PMID]
- Firdaus M, Gill AS, Mukarramah DA, Andriani R, Sari L, Cahyanti D, et al. Malignant peripheral nerve sheath tumor of the scalp: Two rare case reports. Surg Neurol Int. 2018; 9:102.[DOI:10.4103/sni.sni_196_17] [PMID]
- Teles F, Ataíde AMM, De Lima BA, Costa TCC, Lins RC, Barbosa GHTS, et al. Giant malignant peripheral nerve sheath tumor of the scalp. Acta Neurol Taiwan. 2012; 21(3):133-5. [PMID]
- Das Gupta TK, Brasfield RD. Solitary malignant schwannoma. Ann Surg. 1970; 171(3):419-28. [DOI:10.1097/00000658-197003000-00016] [PMID]
- Vege DS, Chinov RF, Ganesh B, Parikh DM. Malignant peripheral nerve sheath tumors of the head and neck: A clinicopathologic study. J Surg Oncol. 1994; 55(2):100-3. [DOI:10.1002/jso.2930550208] [PMID]
- LaFemina J, Qin LX, Moraco NH, Antonescu CR, Fields RC, Crago AM, et al. Oncologic outcomes of sporadic, neurofibromatosis-associated, and radiation-induced malignant peripheral nerve sheath tumors. Ann Surg Oncol. 2013; 20(1):66 - 72. [DOI:10.1245/s10434-012-2573-2] [PMID]
- Yuan ZN, Xu LB, Zhao ZG, Xu SF, Zhang XX, Liu T, et al. Clinicopathological features and prognosis of malignant peripheral nerve sheath tumor: A retrospective study of 140 cases. Zhonghua Zhong Liu Za Zhi. 2017; 39(6):439-44. [PMID]
- Evans DG, Huson SM, Birch JM. Malignant peripheral nerve sheath tumors in inherited disease. Clin Sarcoma Res. 2012; 2(1):17. [DOI:10.1186/2045-3329-2-17] [PMID]
- Farid M, Demicco EG, Garcia R, Ahn L, Merola PR, Cioffi A, et al. Malignant peripheral nerve sheath tumors. Oncologist. 2014; 19(2):193-201. [DOI:10.1634/theoncologist.2013-0328] [PMID]
- Dabski C, Reiman HM Jr, Muller SA. Neurofibrosarcoma of skin and subcutaneous tissues. Mayo Clin Proc. 1990; 65(2):164-72. [DOI:10.1016/S0025-6196(12)65011-3] [PMID]
- Garg A, Gupta V, Gaikwad SB, Mishra NK, Ojha BK, Chugh M, et al. Scalp malignant peripheral nerve sheath tumor (MPNST) with bony involvement and new bone formation: Case report. Clin Neurol Neurosurg. 2004; 106(4):340-4. [DOI:10.1016/j.clineuro.2004.01.003] [PMID]
- Kumar P, Jaiswal S, Agrawal T, Verma A, Datta NR. Malignant peripheral nerve sheath tumor of the occipital region: Case report. Neurosurgery. 2007; 61(6):E1334-5; discussion E1335. [DOI:10.1227/01.neu.0000306115.85668.ec] [PMID]
- Fukushima S, Kageshita T, Wakasugi S, Matsushita S, Kaguchi A, Ishihara T, et al. Giant malignant peripheral nerve sheath tumor of the scalp. J Dermatol. 2006; 33(12):865-8. [DOI:10.1111/j.1346-8138.2006.00197.x] [PMID]
- Ge P, Fu S, Lu L, Zhong Y, Qi B, Luo Y. Diffuse scalp malignant peripheral nerve sheath tumor with intracranial extension in a patient with neurofibromatosis type 1. J Clin Neurosci. 2010; 17(11):1443-4. [DOI:10.1016/j.jocn.2010.01.055] [PMID]
- Yamada K, Harada M, Kunitoku N, Goto S, Kochi M, Ushio Y. MR imaging features of a scalp plexiform schwannoma. AJNR Am J Neuroradiol. 2004; 25(2):291-4. [PMID]
- Woodruff JM, Selig AM, Crowley K, Allen PW. Schwannoma (neurilemoma) with malignant transformation. A rare, distinctive peripheral nerve tumor. Am J Surg Pathol. 1994; 18(9):882-95. [DOI:10.1097/00000478-199409000-00003] [PMID]
- Cubas Farinha N, Belo D, Freitas H, Coiteiro D. Giant malignant peripheral nerve sheath tumor of the scalp: Case report and review of the literature. World Neurosurg. 2020; 138:246-52. [DOI:10.1016/j.wneu.2020.03.039] [PMID]
- Liu X, Li W, Yuan H, Gu W, Chen D. Surgical treatment of rare giant malignant tumors of the scalp: A report of 3 cases with different tumor types. Oncol Lett. 2016; 12(5):3411-6.[DOI:10.3892/ol.2016.5113] [PMID]
- George E, Swanson PE, Wick MR. Malignant peripheral nerve sheath tumors of the skin. Am J Dermatopathol. 1989; 11(3):213-21 [DOI:10.1097/00000372-198906000-00004] [PMID]
- Jhawar SS, Mahore A, Goel N, Goel A. Malignant peripheral nerve sheath tumor of scalp with extradural extension: Case report. Turk Neurosurg. 2012; 22(2):254-6. [PMID]
- Dangoor A, Seddon B, Gerrand C, Grimer R, Whelan J, Judson I. UK guidelines for the management of soft tissue sarcomas. Clin Sarcoma Res. 2016; 6:20. [DOI:10.1186/s13569-016-0060-4] [PMID]
- Voth H, Nakai N, Wardelmann E, Wenzel J, Bieber T, Wendtner CM, et al. Malignant peripheral nerve sheath tumor of the scalp: Case report and review of the literature. Dermatol Surg. 2011; 37(11):1684-8. [DOI:10.1111/j.1524-4725.2011.02113.x] [PMID]
- Kikuchi A, Akiyama M, Han-Yaku H, Shimizu H, Naka W, Nishikawa T. Solitary cutaneous malignant schwannoma. Immunohistochemical and ultrastructural studies. Am J Dermatopathol. 1993; 15(1):15-9. [DOI:10.1097/00000372-199302000-00003] [PMID]
- Demir Y, Tokyol C. Superficial malignant schwannoma of the scalp. Dermatol Surg. 2003; 29(8):879-81. [PMID]
- Williams SB, Szlyk GR, Manyak MJ. Malignant peripheral nerve sheath tumor of the kidney. Int J Urol. 2006; 13(1):74-5.[DOI:10.1111/j.1442-2042.2006.01238.x] [PMID]
- Hasturk AE, Basmaci M, Bayram C, Bozdogan N. Surgical management of recurrent malignant schwannoma of the scalp. J Craniofac Surg. 2011; 22(3):1120-2. [DOI:10.1097/SCS.0b013e3182108f69] [PMID]
- Shintaku M, Wada K, Wakasa T, Ueda M. Malignant peripheral nerve sheath tumor with fibroblastic differentiation in a patient with neurofibromatosis type 1: Imprint cytological findings. Acta Cytol. 2011; 55(5):467-72.[DOI:10.1159/000330676] [PMID]
- Chen DW, Gu WH, Fu SL. [Giant scalp malignant peripheral nerve sheath tumor: one case report (Chinese)]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011; 46(12):1047-8. [PMID]
Type of Study: case report |
Subject:
General
Received: 2024/01/21 | Accepted: 2024/04/23 | Published: 2024/07/7
Received: 2024/01/21 | Accepted: 2024/04/23 | Published: 2024/07/7
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |