Wed, May 8, 2024
Volume 10, Issue 2 (Spring 2024)
Caspian J Neurol Sci 2024, 10(2): 102-110 |
Back to browse issues page
Ethics code: IR.DU.REC.1401.006
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rahimi M, Goudarzi I, Rohampour K. Effect of Fluvoxamine on Electrophysiological and Behavioral Alterations of Rats With Depression. Caspian J Neurol Sci 2024; 10 (2) :102-110
URL: http://cjns.gums.ac.ir/article-1-684-en.html
URL: http://cjns.gums.ac.ir/article-1-684-en.html
1- School of Biology, Damghan University, Damghan, Iran.
2- Department of Physiology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran. , rohampour@gums.ac.ir
2- Department of Physiology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran. , rohampour@gums.ac.ir
Keywords: Depression, Hippocampus, Posterior cingulate cortex, Fluvoxamine, Local field potential recording
Full-Text [PDF 1705 kb]
(73 Downloads)
| Abstract (HTML) (132 Views)
Full-Text: (58 Views)
Introduction
Depression, a debilitating mental health condition, can affect individuals’ emotional well-being, cognitive functioning, and overall behavioral patterns. In 2020, 280 million people worldwide had depression [1]. The prevalence of depression varies across different regions and age groups. In the US, 17.3 million adults experienced an episode of major depressive disorder (MDD) in 2020 [2]. Depression is the result of a complex interplay of genetic, biological, psychological, and environmental factors. The imbalance of neurotransmitters, such as serotonin, norepinephrine, and dopamine, can develop depression. These neurotransmitters play crucial roles in emotional regulation, and their dysregulation contributes to the hallmark symptoms of depression, including sadness, anhedonia (inability to feel pleasure), and fatigue [3]. Changes in brain activity, such as reduced hippocampal volume, are also related to depression [4]. The brain regions involved in memory and learning have a role in the pathogenesis of depression [5]. The hypothalamus-pituitary-adrenal (HPA) axis is also related to the development of depression. Chronic stress, a common trigger for depression, leads to sustained activation of the HPA axis, resulting in elevated cortisol levels. This prolonged cortisol exposure can disrupt neurotransmitter signaling and contribute to neuroplastic changes in the mood-regulating brain regions [6].
Functional magnetic resonance imaging (fMRI) studies have revealed that the altered connectivity in brain networks is involved in the pathogenesis of depression [7]. These studies have identified disruptions in several key brain networks, such as the salience network (SN), the default mode network (DMN) [8], and the central executive network (CEN) [9]. The DMN is a brain network that is usually active during resting and when the individual is engaged in self-referential thoughts and memories. In depression, the DMN exhibits increased connectivity, suggesting a tendency for individuals to ruminate on negative thoughts and emotions. Alterations in the DMN have been reported in individuals with depression [10]. DMN connectivity may contribute to anhedonia by interfering with the ability to engage in joyful activities [11]. Moreover, the depression is believed to be exacerbated by reduced connectivity between DMN and SN. Individuals with depression often experience cognitive impairments, such as impaired memory, attention, and decision-making. Reduced connectivity between DMN and CEN may contribute to these cognitive deficits by interfering with the ability to focus and plan [12]. The hippocampus and posterior cingulate cortex (PCC) are the core areas of the DMN which are involved in self-referential processing, episodic memory, attention, and information integration [13]. The hippocampus mediate memory formation and the PCC has a role in integrating self-referential information. Alterations in the activity and connectivity of these brain regions are associated with depression, potentially contributing to cognitive and emotional disorders [14].
Fluvoxamine (FLX), a selective serotonin reuptake inhibitor (SSRI), is considered as an effective drug for treatment of depression. Several clinical trials and meta-analyses have reported its efficacy in reducing depressive symptoms and improving overall functioning in individuals with MDD [15]. In this study, we aim to investigate whether FLX can affect electrophysiological characteristics and depressive-like behavior of rats with depression.
Materials and Methods
Study design and animals
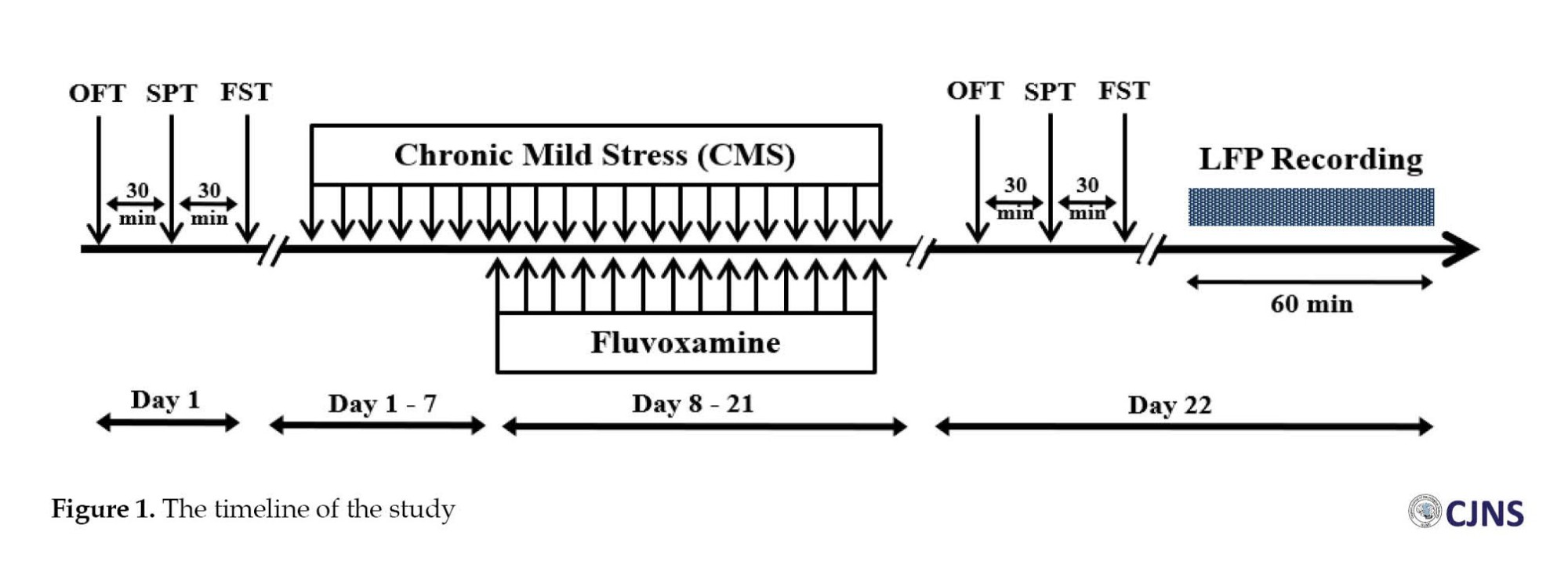
Thirty-two male albino rats (weighing 250-300 g) were transferred to the animal house and kept in a 12: 12 light-dark cycle at 20±2°C with free access to water and food. They were randomly assigned to four groups: Control (sham), chronic mild stress (CMS), FLX, and CMS+FLX. Rats participated in an open field test (OFT), a sucrose preference test (SPT), and a forced swim test (FST) as part of baseline behavioral assessments conducted one week after electrode implantation. The rats were then subjected to CMS for three weeks. The groups receiving FLX had daily intraperitoneal injection of FLX (25 mg/Kg) for 2 weeks (day 8-21). A second round of behavioral testing was conducted on Day 22. The local field potential (LFP) recordings were completed one hour after the assessment of depressive-like behaviors (Figure 1).
CMS induction
For the CMS induction, the rats were housed separately for one week to acclimatize to their new environment. The four-week CMS regimen was then applied to the stressed groups. The animal was subjected to the stimuli for two hours by tilting of the cage, wet cage, food deprivation (24 h), water deprivation (18 h), continuous lighting (36 h), white noise (85 dB), and cold water. The white noise had intensity similar to at many random frequencies across the audible spectrum. Though they were applied according to a planned and unexpected manner [16].
Surgery
After an intraperitoneal injection of xylazine and ketamine (20 and 100 mg/kg, respectively) the stimulating and recording electrodes were inserted into the brain of the rats and were put into the stereotaxic apparatus. The electrodes were placed on the hippocampus (anterior-posterior: -5.28; mediolateral: ±2.5; dorsoventral: -4.5 mm) and PCC (anterior-posterior: -1.32; mediolateral: +0.5 mm, dorsoventral: -2.8 mm) based on the coordinates of the Paxinos Watson rat brain atlas [17]. An eyeglass screw was linked to the telephone jack and fastened to the skull’s surface to create a reference electrode.
Measures
Open field test
The rats were put in a 70×70×35 cm plexiglas box with 16 squares on its floor (4 in the middle and 12 in the corners). For five minutes, the rats investigated the box, and a camera recorded their actions. Their time spent in the central area of the box and the frequency of crossing the lines were recorded [18].
Sucrose preference test
The SPT was employed to examine behaviors associated with anhedonia. In this regard, rats received 2% sucrose for 48 hours to facilitate their adaption process. The rats were fasted for 12 hours before each test, and they were denied food and drink for 12 hours in the following day. On the test day, two bottles were provided for three hours, one containing water and the other with 2% sucrose solution. The difference in preference of each bottle was measured and recorded before and after the experiment. Sucrose preference percentage was calculated as follows (Equation 1) [18]:
1. SP (%)=Water intake+Sucrose intake/100
Forced swim test
For habituation, rats were allowed to swim in a water-filled (23–25°C) tube for 15 minutes, before testing. The rats then remained in the tube for an additional six minutes. A camera was used to record the animals’ movements. Being immobile or floating was defined as immobility [16].
Local field potential recording
On the 23rd day of the experiment, 40 minutes of LFP recording from the hippocampus and PCC in the resting state was done. An analogue-to-digital converter (BioDAQ) was used to digitize the brain signal at 10 kHz. The signal was amplified (1000×) by a homemade differential amplifier. After pre-processing phase (noise and artifact removal), the fast Fourier transform (FFT) method was used in MATLAB software, version 2016b) to examine the recorded signals. Each group’s power in different frequency bands was calculated under different conditions. After analysis, the power was presented as the various frequency bands’ average absolute power. The delta band ranged 0.5-4 Hz, theta ranged 4-8 Hz, alpha ranged 8-12 Hz, and beta ranged 13-30 Hz. Low-gamma and high-gamma band ranges were 30-60 Hz and 60-100 Hz, respectively.
Statistical analysis
The normality of behavioral data distribution was assessed using the Shapiro-Wilk test. One-way analysis of variance (ANOVA) followed by Tukey’s post-test (if data had a normal distribution) or Kruskal-Wallis test (if data had an abnormal distribution). For the LFP analysis, two-way ANOVA with pairwise comparison was used. The GraphPad PRISM software, version 9 was utilized for analysis. The Mean±SEM was used to describe the data.
Results
Effect of FLX on depressive symptoms
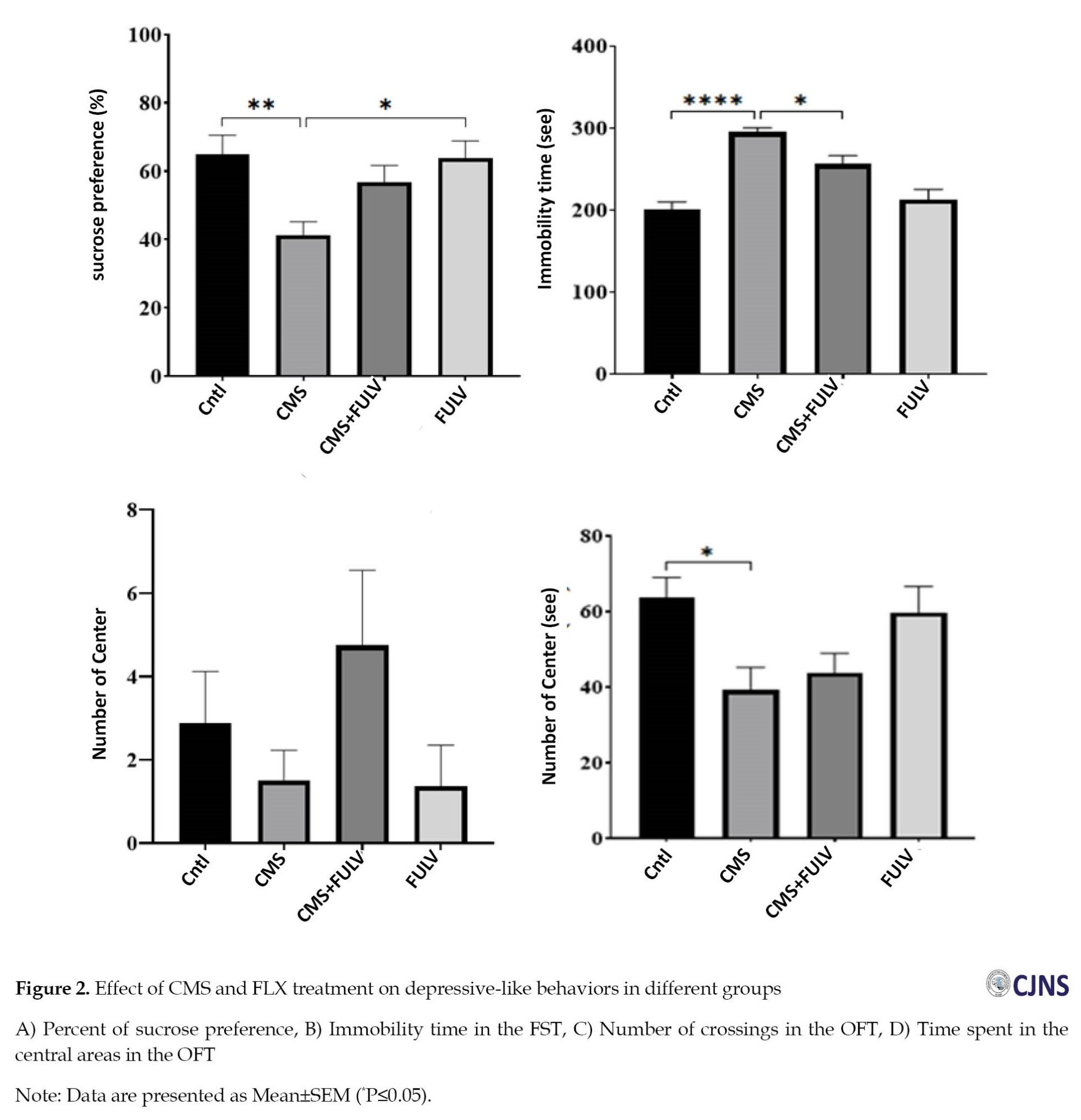
The CMS induction reduced the sucrose preference significantly (P≤0.01). Administration of FLX showed a significant increase in both FLX and CMS+FLX groups (P≤0.05) compared to the CMS group (Figure 2A). The immobility time in the CMS group showed a significant increase (P≤0.0001). A significantly shorter immobility time was observed in the CMS+FLX group (P≤0.05) compared to the CMS group (Figure 2B). The FLX injection caused that the control and CMS+FLX groups to have more crossings than the CMS ones, but this increase was not significant (Figure 2C). The rats in the CMS+FLX group spent shorter time in the central zone compared to control group (Figure 2D).
Effect of FLX on hippocampal low-frequency oscillations
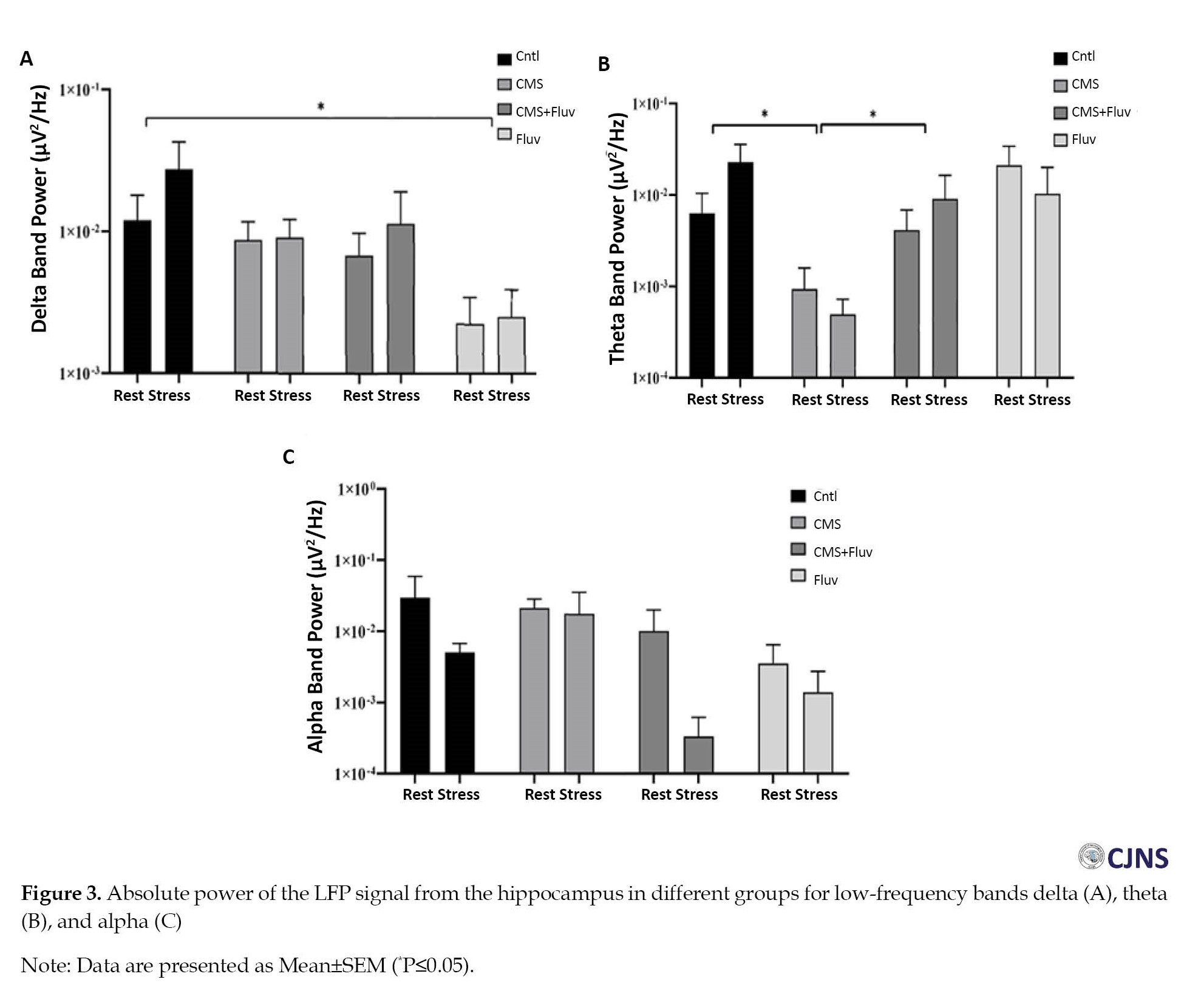
Figure 3 shows the absolute power alterations in the low-frequency bands (delta, theta, and alpha) in the hippocampus for different groups. The two-way ANOVA results showed a significant difference between groups but not between the conditions (resting or stressed). The multiple comparison indicated that the delta band power reduced significantly in the hippocampus of rats in the FLX group (P≤0.05). In the theta band, the CMS reduced the band power, but FLX injection for the CMS rats reversed the theta band power significantly (P≤0.05; Figure 3B).
Effect of FLX on hippocampal high-frequency oscillations
Figure 4 shows the changes in beta and gamma absolute powers for different groups and conditions, in the hippocampus. The multiple comparison of the two-way ANOVA revealed that FLX injection reduced the absolute beta band power compared to the CMS group (P≤0.05). The low-gamma absolute band power increased in the CMS group compared to the control group, but FLX injection was able to significantly reverse this band power to the level of controls (P≤0.05; Figure 4C). The FLX group showed lower low-gamma band power compared to CMS group (P≤0.05).
Effect of FLX on low-frequency oscillations in the PCC
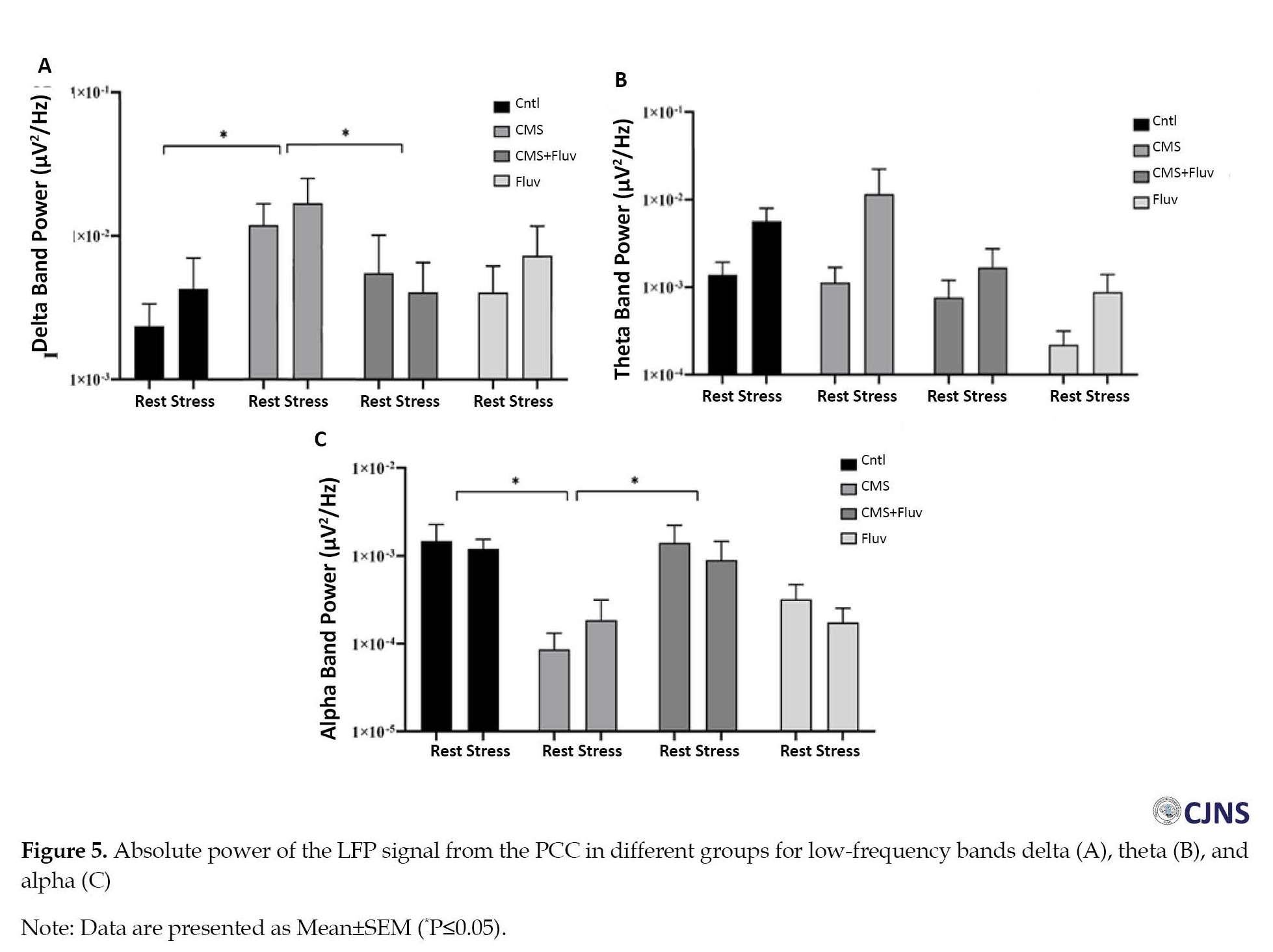
The results of FFT analysis showed that the delta band power in the PCC was significantly higher in the CMS group compared to control group (P≤0.05; Figure 5A), but FLX injection reduced the delta band power in both resting and stressed conditions (P≤0.05). Conversely, the absolute power of alpha band was lower in the CMS group (P≤0.05), while the CMS+FLX group showed higher alpha band power compared to the CMS group (P≤0.05; Figure 5C).
Effect of FLX on high-frequency oscillations in the PCC
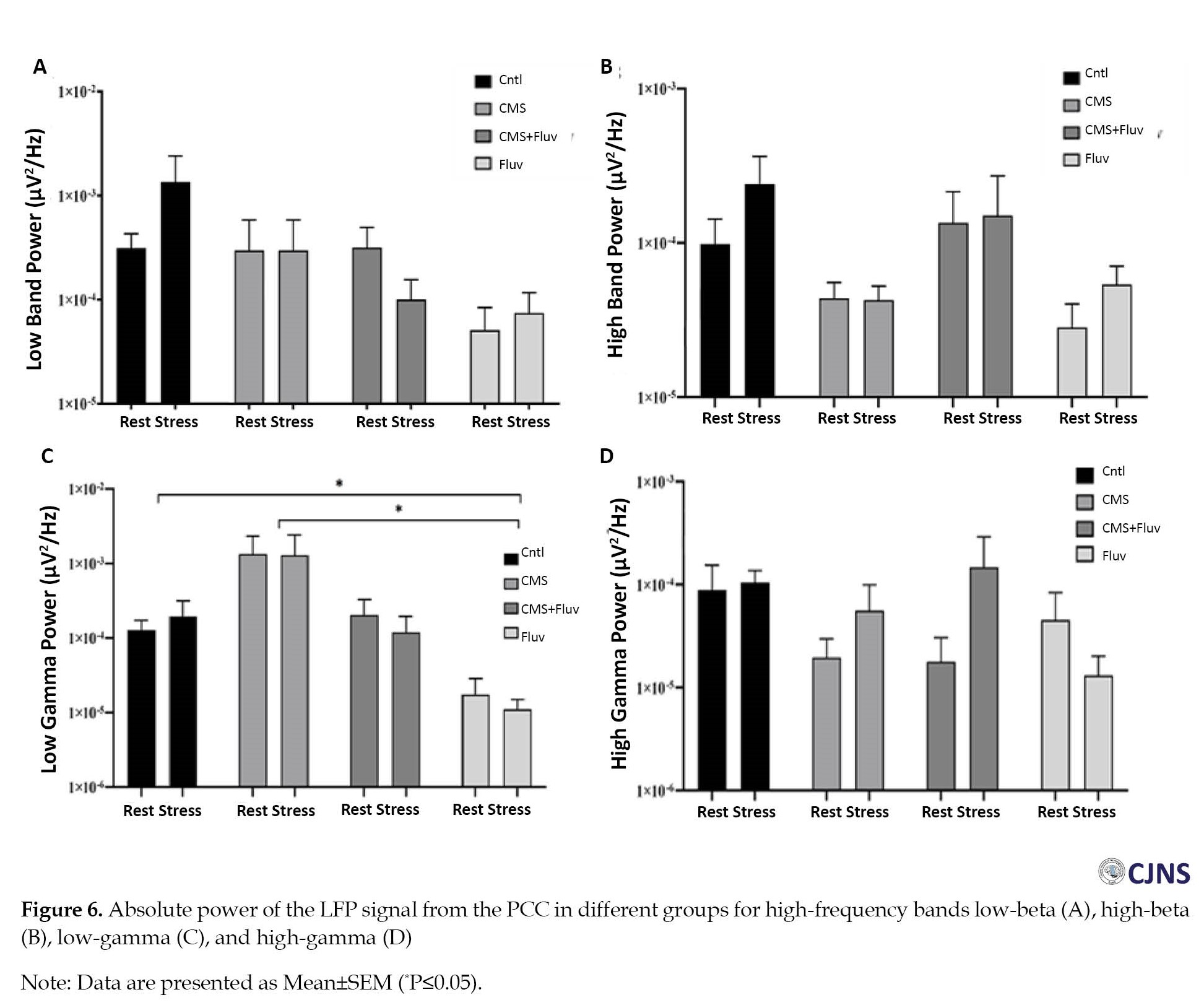
Figure 6 depicts the absolute power of high-frequency bands in the PCC of different groups. The only significant difference was observed in the low-gamma band (Figure 6C). According to the two-way ANOVA results, the injection of chronic FLX reduced the low-gamma band power compared to control and CMS groups (P≤0.05).
Discussion
The results showed that two weeks of FLX treatment reduced the immobility time in rats with CMS, which indicates an improvement in symptoms like hopelessness and lack of interest, but it did not affect anhedonia in depressed rats, since the sucrose preference did not change significantly. In a systematic review study, Omori et al. found no evidence to suggest that FLX performed better or worse than other antidepressants in terms of response and tolerability [19]. FLX acts by preventing serotonin reuptake, leading to higher levels of serotonin in the brain. This boosts serotonin’s mood-regulating and overall effect on well-being. FLX also affects other neurotransmitters such as norepinephrine, dopamine, and GABA, contributing to its antidepressant effect [20].
The electrophysiological study revealed that CMS-induced depression reduced the absolute power of delta and theta bands in the hippocampus of rats. Mormann et al. also suggested that the interaction between theta and delta bands in the hippocampus can affect many cognitive functions [21]. Delta band oscillations in the hippocampus are involved in emotional processing. Studies have shown that delta band power can be modulated by emotional stimuli via increasing delta band activity during emotional experiences [22]. The interaction between the delta band frequency and the hippocampus is thought to affect the encoding and retrieval of emotional memories as well as the regulation of emotional responses [23]. On the other hand, evidence suggests that disruption of the dynamics of hippocampal theta wave and its connectivity with other emotion-related brain regions such as the amygdala, may contribute to the dysregulation of emotional responses observed in depression [24]. Theta oscillations are particularly involved in behaviors such as active exploration and memory encoding processing. The theta oscillations are crucial for memory and cognitive control [25].
The present study demonstrated that depression resulted in an overall reduction in alpha band power and an increased power in delta and gamma band range in the PCC. Consistent with our results, Foti et al. found that the delta band power increased following monetary loss in the anterior cingulate cortex [26]. Additionally, our research revealed that depression increases the low-gamma band power in the ACC, which contrasts with the results of Vannest et al., who reported that the power of this band increased in the cingulate cortex during distress such as tinnitus [27].
Conclusion
FLX administration can significantly reduce depressive-like behaviors in depressed rats with CMS. Electrophysiological recordings revealed depression-induced changes in LFP signals recorded from the hippocampus and PCC. In the hippocampus, depression can reduce the power of theta, delta, and low-gamma bands and increase the power of low gamma band. In the PCC, depression can increase the power of low-gamma and delta bands and reduce the power of alpha band. Overall, these findings implicate disturbed LFP signals in CMS-induced depression and suggest that FLX’s antidepressant effects, at least partly, can reduce these disrupted LFP signals.
Ethical Considerations
Compliance with ethical guidelines
The study procedures are in accordance to the guidance for the care and use of laboratory animals of the National Institute of Health and approved by the Research Ethics Committee of Damghan University (Code: IR.DU.REC.1401.006).
Funding
This study was extracted from the Master’s thesis of Mahsa Rahimi, approved by School of Biology, Damghan University, and was funded by Damghan University.
Authors contributions
Conceptualization, study design and data curation: Kambiz Rohampour and Iran Goudarzi; Investigation: Mahsa Rahimi; Writing the original draft: Kambiz Rohampour; Review & editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank Damghan University for the support.
References
Depression, a debilitating mental health condition, can affect individuals’ emotional well-being, cognitive functioning, and overall behavioral patterns. In 2020, 280 million people worldwide had depression [1]. The prevalence of depression varies across different regions and age groups. In the US, 17.3 million adults experienced an episode of major depressive disorder (MDD) in 2020 [2]. Depression is the result of a complex interplay of genetic, biological, psychological, and environmental factors. The imbalance of neurotransmitters, such as serotonin, norepinephrine, and dopamine, can develop depression. These neurotransmitters play crucial roles in emotional regulation, and their dysregulation contributes to the hallmark symptoms of depression, including sadness, anhedonia (inability to feel pleasure), and fatigue [3]. Changes in brain activity, such as reduced hippocampal volume, are also related to depression [4]. The brain regions involved in memory and learning have a role in the pathogenesis of depression [5]. The hypothalamus-pituitary-adrenal (HPA) axis is also related to the development of depression. Chronic stress, a common trigger for depression, leads to sustained activation of the HPA axis, resulting in elevated cortisol levels. This prolonged cortisol exposure can disrupt neurotransmitter signaling and contribute to neuroplastic changes in the mood-regulating brain regions [6].
Functional magnetic resonance imaging (fMRI) studies have revealed that the altered connectivity in brain networks is involved in the pathogenesis of depression [7]. These studies have identified disruptions in several key brain networks, such as the salience network (SN), the default mode network (DMN) [8], and the central executive network (CEN) [9]. The DMN is a brain network that is usually active during resting and when the individual is engaged in self-referential thoughts and memories. In depression, the DMN exhibits increased connectivity, suggesting a tendency for individuals to ruminate on negative thoughts and emotions. Alterations in the DMN have been reported in individuals with depression [10]. DMN connectivity may contribute to anhedonia by interfering with the ability to engage in joyful activities [11]. Moreover, the depression is believed to be exacerbated by reduced connectivity between DMN and SN. Individuals with depression often experience cognitive impairments, such as impaired memory, attention, and decision-making. Reduced connectivity between DMN and CEN may contribute to these cognitive deficits by interfering with the ability to focus and plan [12]. The hippocampus and posterior cingulate cortex (PCC) are the core areas of the DMN which are involved in self-referential processing, episodic memory, attention, and information integration [13]. The hippocampus mediate memory formation and the PCC has a role in integrating self-referential information. Alterations in the activity and connectivity of these brain regions are associated with depression, potentially contributing to cognitive and emotional disorders [14].
Fluvoxamine (FLX), a selective serotonin reuptake inhibitor (SSRI), is considered as an effective drug for treatment of depression. Several clinical trials and meta-analyses have reported its efficacy in reducing depressive symptoms and improving overall functioning in individuals with MDD [15]. In this study, we aim to investigate whether FLX can affect electrophysiological characteristics and depressive-like behavior of rats with depression.
Materials and Methods
Study design and animals
Thirty-two male albino rats (weighing 250-300 g) were transferred to the animal house and kept in a 12: 12 light-dark cycle at 20±2°C with free access to water and food. They were randomly assigned to four groups: Control (sham), chronic mild stress (CMS), FLX, and CMS+FLX. Rats participated in an open field test (OFT), a sucrose preference test (SPT), and a forced swim test (FST) as part of baseline behavioral assessments conducted one week after electrode implantation. The rats were then subjected to CMS for three weeks. The groups receiving FLX had daily intraperitoneal injection of FLX (25 mg/Kg) for 2 weeks (day 8-21). A second round of behavioral testing was conducted on Day 22. The local field potential (LFP) recordings were completed one hour after the assessment of depressive-like behaviors (Figure 1).
CMS induction
For the CMS induction, the rats were housed separately for one week to acclimatize to their new environment. The four-week CMS regimen was then applied to the stressed groups. The animal was subjected to the stimuli for two hours by tilting of the cage, wet cage, food deprivation (24 h), water deprivation (18 h), continuous lighting (36 h), white noise (85 dB), and cold water. The white noise had intensity similar to at many random frequencies across the audible spectrum. Though they were applied according to a planned and unexpected manner [16].
Surgery
After an intraperitoneal injection of xylazine and ketamine (20 and 100 mg/kg, respectively) the stimulating and recording electrodes were inserted into the brain of the rats and were put into the stereotaxic apparatus. The electrodes were placed on the hippocampus (anterior-posterior: -5.28; mediolateral: ±2.5; dorsoventral: -4.5 mm) and PCC (anterior-posterior: -1.32; mediolateral: +0.5 mm, dorsoventral: -2.8 mm) based on the coordinates of the Paxinos Watson rat brain atlas [17]. An eyeglass screw was linked to the telephone jack and fastened to the skull’s surface to create a reference electrode.
Measures
Open field test
The rats were put in a 70×70×35 cm plexiglas box with 16 squares on its floor (4 in the middle and 12 in the corners). For five minutes, the rats investigated the box, and a camera recorded their actions. Their time spent in the central area of the box and the frequency of crossing the lines were recorded [18].
Sucrose preference test
The SPT was employed to examine behaviors associated with anhedonia. In this regard, rats received 2% sucrose for 48 hours to facilitate their adaption process. The rats were fasted for 12 hours before each test, and they were denied food and drink for 12 hours in the following day. On the test day, two bottles were provided for three hours, one containing water and the other with 2% sucrose solution. The difference in preference of each bottle was measured and recorded before and after the experiment. Sucrose preference percentage was calculated as follows (Equation 1) [18]:
1. SP (%)=Water intake+Sucrose intake/100
Forced swim test
For habituation, rats were allowed to swim in a water-filled (23–25°C) tube for 15 minutes, before testing. The rats then remained in the tube for an additional six minutes. A camera was used to record the animals’ movements. Being immobile or floating was defined as immobility [16].
Local field potential recording
On the 23rd day of the experiment, 40 minutes of LFP recording from the hippocampus and PCC in the resting state was done. An analogue-to-digital converter (BioDAQ) was used to digitize the brain signal at 10 kHz. The signal was amplified (1000×) by a homemade differential amplifier. After pre-processing phase (noise and artifact removal), the fast Fourier transform (FFT) method was used in MATLAB software, version 2016b) to examine the recorded signals. Each group’s power in different frequency bands was calculated under different conditions. After analysis, the power was presented as the various frequency bands’ average absolute power. The delta band ranged 0.5-4 Hz, theta ranged 4-8 Hz, alpha ranged 8-12 Hz, and beta ranged 13-30 Hz. Low-gamma and high-gamma band ranges were 30-60 Hz and 60-100 Hz, respectively.
Statistical analysis
The normality of behavioral data distribution was assessed using the Shapiro-Wilk test. One-way analysis of variance (ANOVA) followed by Tukey’s post-test (if data had a normal distribution) or Kruskal-Wallis test (if data had an abnormal distribution). For the LFP analysis, two-way ANOVA with pairwise comparison was used. The GraphPad PRISM software, version 9 was utilized for analysis. The Mean±SEM was used to describe the data.
Results
Effect of FLX on depressive symptoms
The CMS induction reduced the sucrose preference significantly (P≤0.01). Administration of FLX showed a significant increase in both FLX and CMS+FLX groups (P≤0.05) compared to the CMS group (Figure 2A). The immobility time in the CMS group showed a significant increase (P≤0.0001). A significantly shorter immobility time was observed in the CMS+FLX group (P≤0.05) compared to the CMS group (Figure 2B). The FLX injection caused that the control and CMS+FLX groups to have more crossings than the CMS ones, but this increase was not significant (Figure 2C). The rats in the CMS+FLX group spent shorter time in the central zone compared to control group (Figure 2D).
Effect of FLX on hippocampal low-frequency oscillations
Figure 3 shows the absolute power alterations in the low-frequency bands (delta, theta, and alpha) in the hippocampus for different groups. The two-way ANOVA results showed a significant difference between groups but not between the conditions (resting or stressed). The multiple comparison indicated that the delta band power reduced significantly in the hippocampus of rats in the FLX group (P≤0.05). In the theta band, the CMS reduced the band power, but FLX injection for the CMS rats reversed the theta band power significantly (P≤0.05; Figure 3B).
Effect of FLX on hippocampal high-frequency oscillations
Figure 4 shows the changes in beta and gamma absolute powers for different groups and conditions, in the hippocampus. The multiple comparison of the two-way ANOVA revealed that FLX injection reduced the absolute beta band power compared to the CMS group (P≤0.05). The low-gamma absolute band power increased in the CMS group compared to the control group, but FLX injection was able to significantly reverse this band power to the level of controls (P≤0.05; Figure 4C). The FLX group showed lower low-gamma band power compared to CMS group (P≤0.05).
Effect of FLX on low-frequency oscillations in the PCC
The results of FFT analysis showed that the delta band power in the PCC was significantly higher in the CMS group compared to control group (P≤0.05; Figure 5A), but FLX injection reduced the delta band power in both resting and stressed conditions (P≤0.05). Conversely, the absolute power of alpha band was lower in the CMS group (P≤0.05), while the CMS+FLX group showed higher alpha band power compared to the CMS group (P≤0.05; Figure 5C).
Effect of FLX on high-frequency oscillations in the PCC
Figure 6 depicts the absolute power of high-frequency bands in the PCC of different groups. The only significant difference was observed in the low-gamma band (Figure 6C). According to the two-way ANOVA results, the injection of chronic FLX reduced the low-gamma band power compared to control and CMS groups (P≤0.05).
Discussion
The results showed that two weeks of FLX treatment reduced the immobility time in rats with CMS, which indicates an improvement in symptoms like hopelessness and lack of interest, but it did not affect anhedonia in depressed rats, since the sucrose preference did not change significantly. In a systematic review study, Omori et al. found no evidence to suggest that FLX performed better or worse than other antidepressants in terms of response and tolerability [19]. FLX acts by preventing serotonin reuptake, leading to higher levels of serotonin in the brain. This boosts serotonin’s mood-regulating and overall effect on well-being. FLX also affects other neurotransmitters such as norepinephrine, dopamine, and GABA, contributing to its antidepressant effect [20].
The electrophysiological study revealed that CMS-induced depression reduced the absolute power of delta and theta bands in the hippocampus of rats. Mormann et al. also suggested that the interaction between theta and delta bands in the hippocampus can affect many cognitive functions [21]. Delta band oscillations in the hippocampus are involved in emotional processing. Studies have shown that delta band power can be modulated by emotional stimuli via increasing delta band activity during emotional experiences [22]. The interaction between the delta band frequency and the hippocampus is thought to affect the encoding and retrieval of emotional memories as well as the regulation of emotional responses [23]. On the other hand, evidence suggests that disruption of the dynamics of hippocampal theta wave and its connectivity with other emotion-related brain regions such as the amygdala, may contribute to the dysregulation of emotional responses observed in depression [24]. Theta oscillations are particularly involved in behaviors such as active exploration and memory encoding processing. The theta oscillations are crucial for memory and cognitive control [25].
The present study demonstrated that depression resulted in an overall reduction in alpha band power and an increased power in delta and gamma band range in the PCC. Consistent with our results, Foti et al. found that the delta band power increased following monetary loss in the anterior cingulate cortex [26]. Additionally, our research revealed that depression increases the low-gamma band power in the ACC, which contrasts with the results of Vannest et al., who reported that the power of this band increased in the cingulate cortex during distress such as tinnitus [27].
Conclusion
FLX administration can significantly reduce depressive-like behaviors in depressed rats with CMS. Electrophysiological recordings revealed depression-induced changes in LFP signals recorded from the hippocampus and PCC. In the hippocampus, depression can reduce the power of theta, delta, and low-gamma bands and increase the power of low gamma band. In the PCC, depression can increase the power of low-gamma and delta bands and reduce the power of alpha band. Overall, these findings implicate disturbed LFP signals in CMS-induced depression and suggest that FLX’s antidepressant effects, at least partly, can reduce these disrupted LFP signals.
Ethical Considerations
Compliance with ethical guidelines
The study procedures are in accordance to the guidance for the care and use of laboratory animals of the National Institute of Health and approved by the Research Ethics Committee of Damghan University (Code: IR.DU.REC.1401.006).
Funding
This study was extracted from the Master’s thesis of Mahsa Rahimi, approved by School of Biology, Damghan University, and was funded by Damghan University.
Authors contributions
Conceptualization, study design and data curation: Kambiz Rohampour and Iran Goudarzi; Investigation: Mahsa Rahimi; Writing the original draft: Kambiz Rohampour; Review & editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank Damghan University for the support.
References
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022; 9(2):137-50. [DOI:10.1016/S2215-0366(21)00395-3] [PMID]
- Birk SL, Olino TM, Klein DN, Seeley JR. Validity of retrospectively-reported depressive episodes. J Affect Disord. 2020; 277:908-13. [DOI:10.1016/j.jad.2020.08.067] [PMID]
- Li Z, Ruan M, Chen J, Fang Y. Major depressive disorder: Advances in neuroscience research and translational applications. Neurosci Bull. 2021; 37(6):863-80. [DOI:10.1007/s12264-021-00638-3]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: Neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009; 33(5):699-771. [DOI:10.1016/j.neubiorev.2009.01.004] [PMID]
- Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019; 102(1):75-90. [DOI:10.1016/j.neuron.2019.03.013] [PMID]
- Pariante CM, Lightman SL. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008; 31(9):464-8. [DOI:10.1016/j.tins.2008.06.006] [PMID]
- Tura A, Goya-Maldonado R. Brain connectivity in major depressive disorder: A precision component of treatment modalities? Transl Psychiatry. 2023; 13(1):196. [DOI:10.1038/s41398-023-02499-y]
- Ju Y, Wang M, Liu J, Liu B, Yan D, Lu X, et al. Modulation of resting-state functional connectivity in default mode network is associated with the long-term treatment outcome in major depressive disorder. Psychol Med. 2023; 53(13):5963-75. [DOI:10.1017/S0033291722002628] [PMID]
- Zhang T, He K, Bai T, Lv H, Xie X, Nie J, et al. Altered neural activity in the reward-related circuit and executive control network associated with amelioration of anhedonia in major depressive disorder by electroconvulsive therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2021; 109:110193. [DOI:10.1016/j.pnpbp.2020.110193] [PMID]
- Posner J, Cha J, Wang Z, Talati A, Warner V, Gerber A, et al. Increased default mode network connectivity in individuals at high familial risk for depression. Neuropsychopharmacology. 2016; 41(7):1759-67. [DOI:10.1038/npp.2015.342] [PMID]
- Zhong X, Pu W, Yao S. Functional alterations of fronto-limbic circuit and default mode network systems in first-episode, drug-naïve patients with major depressive disorder: A meta-analysis of resting-state fMRI data. J Affect Disord. 2016; 206:280-6. [DOI:10.1016/j.jad.2016.09.005] [PMID]
- Vidal-Piñeiro D, Valls-Pedret C, Fernández-Cabello S, Arenaza-Urquijo EM, Sala-Llonch R, Solana E, et al. Decreased Default Mode Network connectivity correlates with age-associated structural and cognitive changes. Front Aging Neurosci. 2014; 6:256. [DOI:10.3389/fnagi.2014.00256] [PMID]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008; 1124:1-38. [DOI:10.1196/annals.1440.011] [PMID]
- Finlayson-Short L, Davey CG, Harrison BJ. Neural correlates of integrated self and social processing. Soc Cogn Affect Neurosci. 2020; 15(9):941-9. [DOI:10.1093/scan/nsaa121] [PMID]
- Westenberg HG, Sandner C. Tolerability and safety of fluvoxamine and other antidepressants. Int J Clin Pract. 2006; 60(4):482-91. [DOI:10.1111/j.1368-5031.2006.00865.x] [PMID]
- Akhoondian M, Rashtiani S, Khakpour-Taleghani B, Rostampour M, Jafari A, Rohampour K. Lateral habenula deep brain stimulation alleviates depression-like behaviors and reverses the oscillatory pattern in the nucleus accumbens in an animal model of depression. Brain Res Bull. 2023; 202:110745. [DOI:10.1016/j.brainresbull.2023.110745] [PMID]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Massachusetts: Academic Press; 2007. [Link]
- Mirbolouk B, Rohampour K, Rostampour M, Jafari A, Khakpour-Taleghani B. Chronic orexin-1 receptor blockage attenuates depressive behaviors and provokes PSD-95 expression in a rat model of depression. Behav Brain Res. 2023; 437:114123. [DOI:10.1016/j.bbr.2022.114123] [PMID]
- Omori IM, Watanabe N, Nakagawa A, Cipriani A, Barbui C, McGuire H, et al. Fluvoxamine versus other anti-depressive agents for depression. Cochrane Database Syst Rev. 2010; 3. [DOI:10.1002/14651858.CD006114.pub2]
- Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme VV. Fluvoxamine: A review of its mechanism of action and its role in covid-19. Front Pharmacol. 2021; 12:652688. [DOI:10.3389/fphar.2021.652688] [PMID]
- Mormann F, Osterhage H, Andrzejak RG, Weber B, Fernández G, Fell J, et al. Independent delta/theta rhythms in the human hippocampus and entorhinal cortex. Front Hum Neurosci. 2008; 2:3. [DOI:10.3389/neuro.09.003.2008] [PMID]
- Schönfeld LM, Wojtecki L. Beyond emotions: Oscillations of the amygdala and their implications for electrical neuromodulation. Front Neurosci. 2019; 13:366. [DOI:10.3389/fnins.2019.00366] [PMID]
- Lv X, Zhang X, Zhao Q, Li C, Zhang T, Yang X. Acute stress promotes brain oscillations and hippocampal-cortical dialog in emotional processing. Biochem Biophys Res Commun. 2022; 598:55-61. [DOI:10.1016/j.bbrc.2022.01.116] [PMID]
- Helm K, Viol K, Weiger TM, Tass PA, Grefkes C, Del Monte D, et al. Neuronal connectivity in major depressive disorder: A systematic review. Neuropsychiatr Dis Treat. 2018; 14:2715-37. [DOI:10.2147/NDT.S170989] [PMID]
- Sadaghiani S, Kleinschmidt A. Brain networks and α-Oscillations: Structural and functional foundations of cognitive control. Trends Cogn Sci. 2016; 20(11):805-17. [DOI:10.1016/j.tics.2016.09.004] [PMID]
- Foti D, Weinberg A, Bernat EM, Proudfit GH. Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clin Neurophysiol. 2015; 126(7):1338-47. [DOI:10.1016/j.clinph.2014.08.025] [PMID]
- Vanneste S, De Ridder D. Stress-related functional connectivity changes between auditory cortex and cingulate in tinnitus. Brain Connect. 2015; 5(6):371-83. [DOI:10.1089/brain.2014.0255] [PMID]
Type of Study: Research |
Subject:
General
Received: 2023/05/8 | Accepted: 2023/06/26 | Published: 2024/04/1
Received: 2023/05/8 | Accepted: 2023/06/26 | Published: 2024/04/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |