Thu, May 9, 2024
Volume 10, Issue 2 (Spring 2024)
Caspian J Neurol Sci 2024, 10(2): 139-145 |
Back to browse issues page
Ethics code: N/A
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Golden N, Kristian Putra K, Awyono S, Lauren C. Suprasellar Immature Teratoma in A 20-year-old Male: A Case Report. Caspian J Neurol Sci 2024; 10 (2) :139-145
URL: http://cjns.gums.ac.ir/article-1-678-en.html
URL: http://cjns.gums.ac.ir/article-1-678-en.html
1- Neurosurgery Division, Department of Surgery, Faculty of Medicine, Prof. Dr. I.G.N.G. Ngoerah General Hospital, Universitas Udayana, Bali, Indonesia. , nyoman_golden@yahoo.co.id
2- Neurosurgery Division, Department of Surgery, Faculty of Medicine, Prof. Dr. I.G.N.G. Ngoerah General Hospital, Universitas Udayana, Bali, Indonesia.
2- Neurosurgery Division, Department of Surgery, Faculty of Medicine, Prof. Dr. I.G.N.G. Ngoerah General Hospital, Universitas Udayana, Bali, Indonesia.
Full-Text [PDF 1944 kb]
(70 Downloads)
| Abstract (HTML) (138 Views)
Full-Text: (30 Views)
Introduction
Primary brain tumors are uncommon compared to brain metastases. Histomorphologically diagnosed primary brain tumors include glioblastoma, astrocytoma, oligodendroglioma, ependymoma, medulloblastoma, meningioma, germ cell tumor, and lymphoma [1]. Germ cell tumors (GCTs) are sporadic, constituting 0.3-0.6% of all primary central nervous system (CNS) tumors. They are categorized into germinomatous, non-germinomatous, and subtypes. Teratomas, a subtype of non-embryonal GCTs, make up only 18-20% of all GCTs and, consequently, only 0.1-1.5% of all intracranial tumors. Teratomas can coexist with other germline elements such as germinomas, yolk sac tumors, and embryonal carcinomas [2, 3]. Teratomas have a higher incidence in children, with a frequency of 1.8-5% in adults and over 15% in children in East Asian countries like Japan, Korea, and Taiwan. The composition of teratomas is distinctive as they originate from all germ cell layers endoderm, ectoderm, and mesoderm [4, 5]. Based on histologic findings, teratomas are classified as mature, immature, and malignant, with incidences of 60%, 25%, and 15%, respectively [3-5]. Immature teratomas consist of components resembling fetal tissue, often mixed with elements of mature tissue. Teratomas and other GCTs primarily occur in midline structures of the brain, including the third ventricle, the suprasellar region, and the pineal region. These tumors carry a poor prognosis and are considered malignant [4-6]. Therefore, appropriate treatment strategies can significantly affect the patient outcomes. Despite the rarity and limited reports of teratomas cases, in this study we present a case of immature teratomas in the suprasellar region of a young male.
Case Presentation
A 20-year-old male presented to the emergency department with a chief complaint of generalized weakness for the past seven days. He was also experiencing progressively worsening headaches in the past three months, with the severity escalating in the past month. The pain was described as if being tied up and affecting the entire head. Initially, he could tolerate the pain, but in the past week, the headaches became unbearable and did not respond to oral analgesics, suggesting an increase in intracranial pressure. The patient also reported blurred vision in the past month. No other relevant history was associated with the current complaint.
Clinical findings
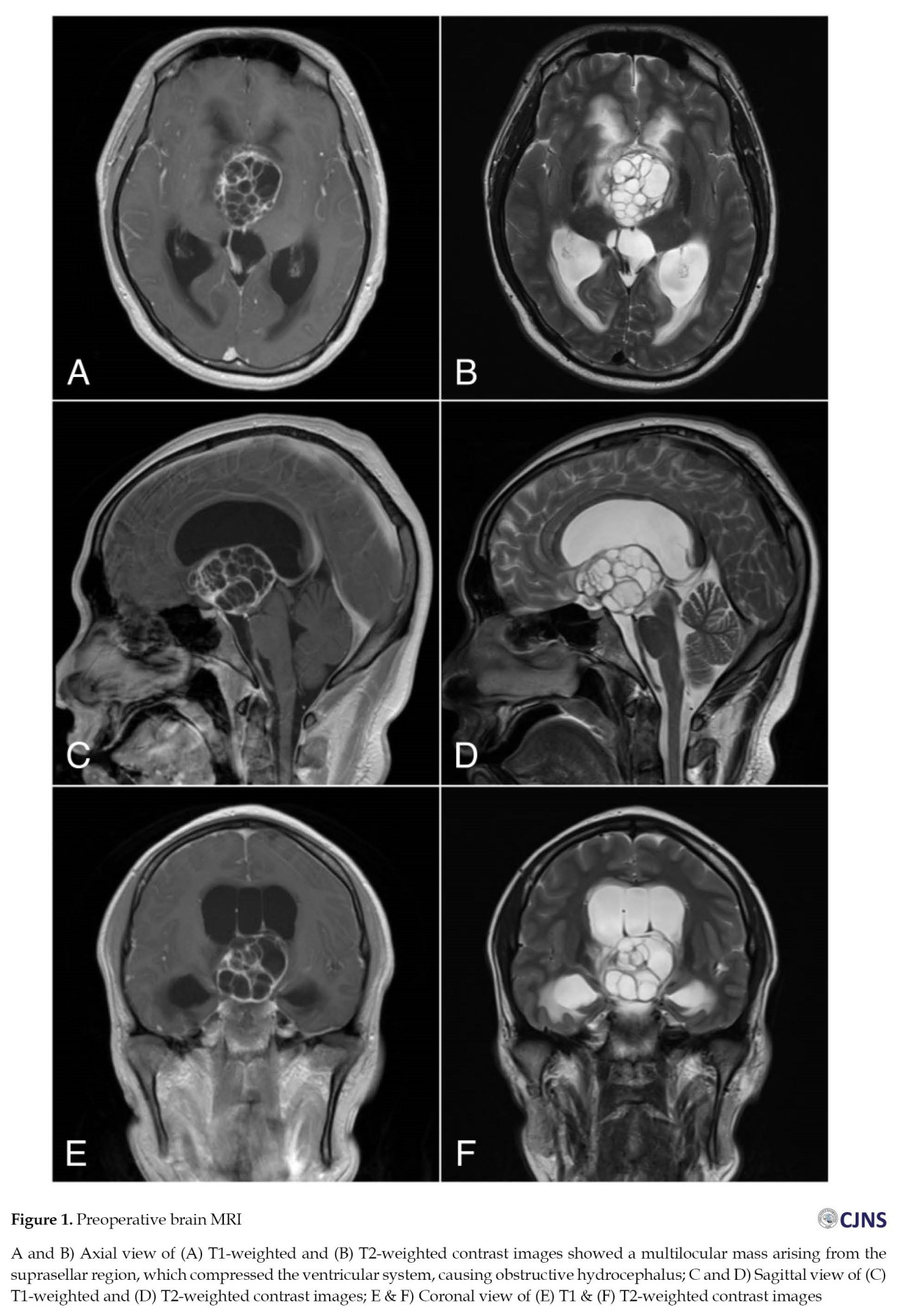
Magnetic resonance imaging (MRI) of the brain with a gadolinium contrast agent was performed (Figure 1) due to progressive headache, blurred vision, and general weakness. The results reveal a multilocular cystic mass in the suprasellar region surrounded by vasogenic edema. The cystic mass had compressed the anterior cavernous sinus and the posterior medulla oblongata, and had occluded the third ventricle, leading to hydrocephalus due to the blockage of the third ventricle. Tumor serum markers, such as beta-human chorionic gonadotropin (beta-hCG) and alpha-fetoprotein (AFP), were assayed at 2.3 mIU/mL and 2.34 ng/mL, respectively, all of which were within normal limits.
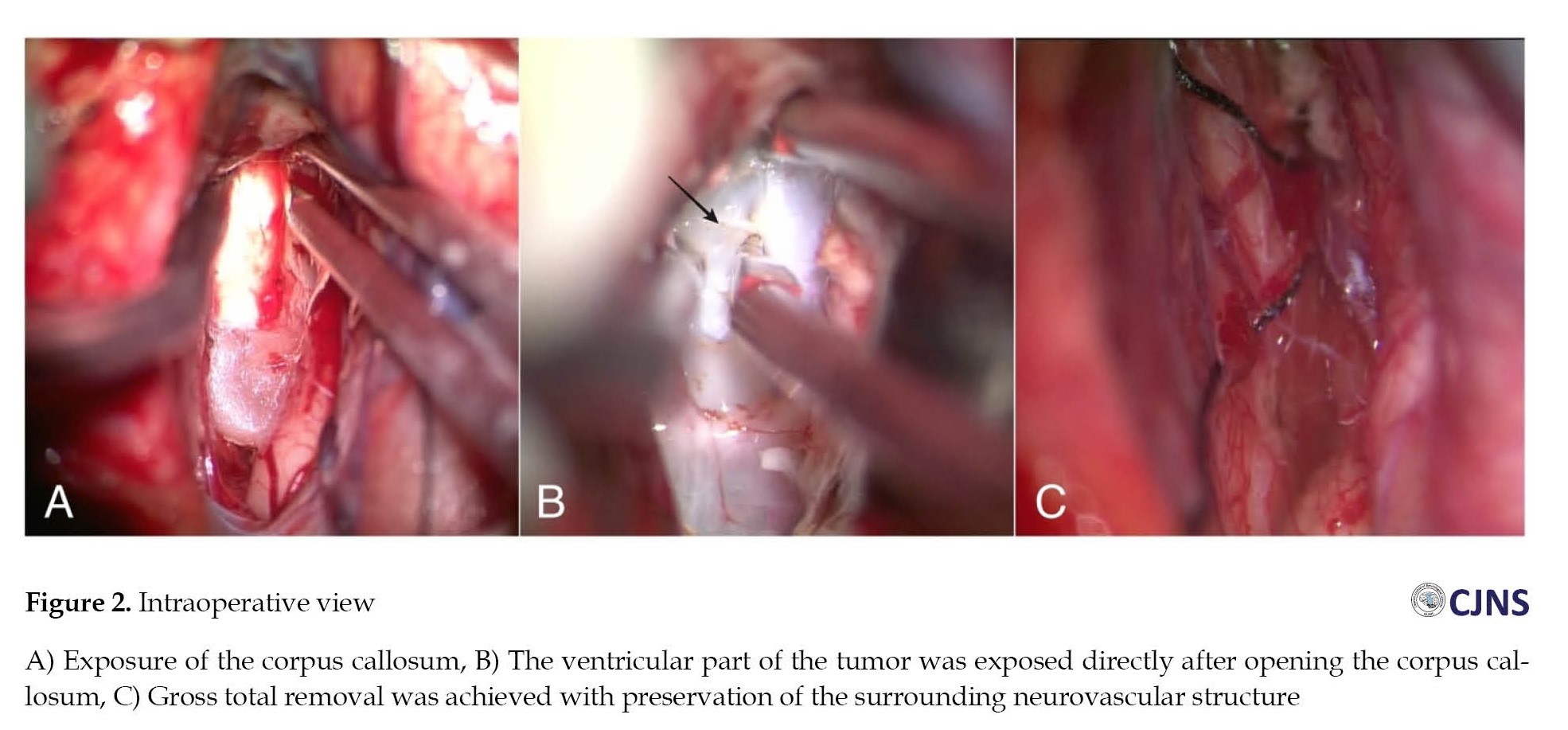
Initially, a medium-pressure ventriculoperitoneal (VP) shunting was performed due to hydrocephalus. Following the treatment, there was a significant improvement in the headache. Gross tumor resection was carried out utilizing an interhemispheric anterior transcallosal approach. A horizontal midline incision was made, followed by a 4×4 cm craniotomy. Identification of midline structures was achieved by exposing the corpus callosum and ventricular system (Figure 2A). An approximately 1.5-cm incision was made to the genu of the corpus callosum to directly visualize the mass (Figure 2B). Meticulous intraventricular tumor dissection of the lamina terminalis cause the exposure of the inferior border of the tumor. Then, the tumor resection was conducted using an ultrasonic aspirator to debulk the tumor. Macroscopically, a gross and complete resection was achieved with good preservation of the surrounding neurovascular structures (Figure 2C).
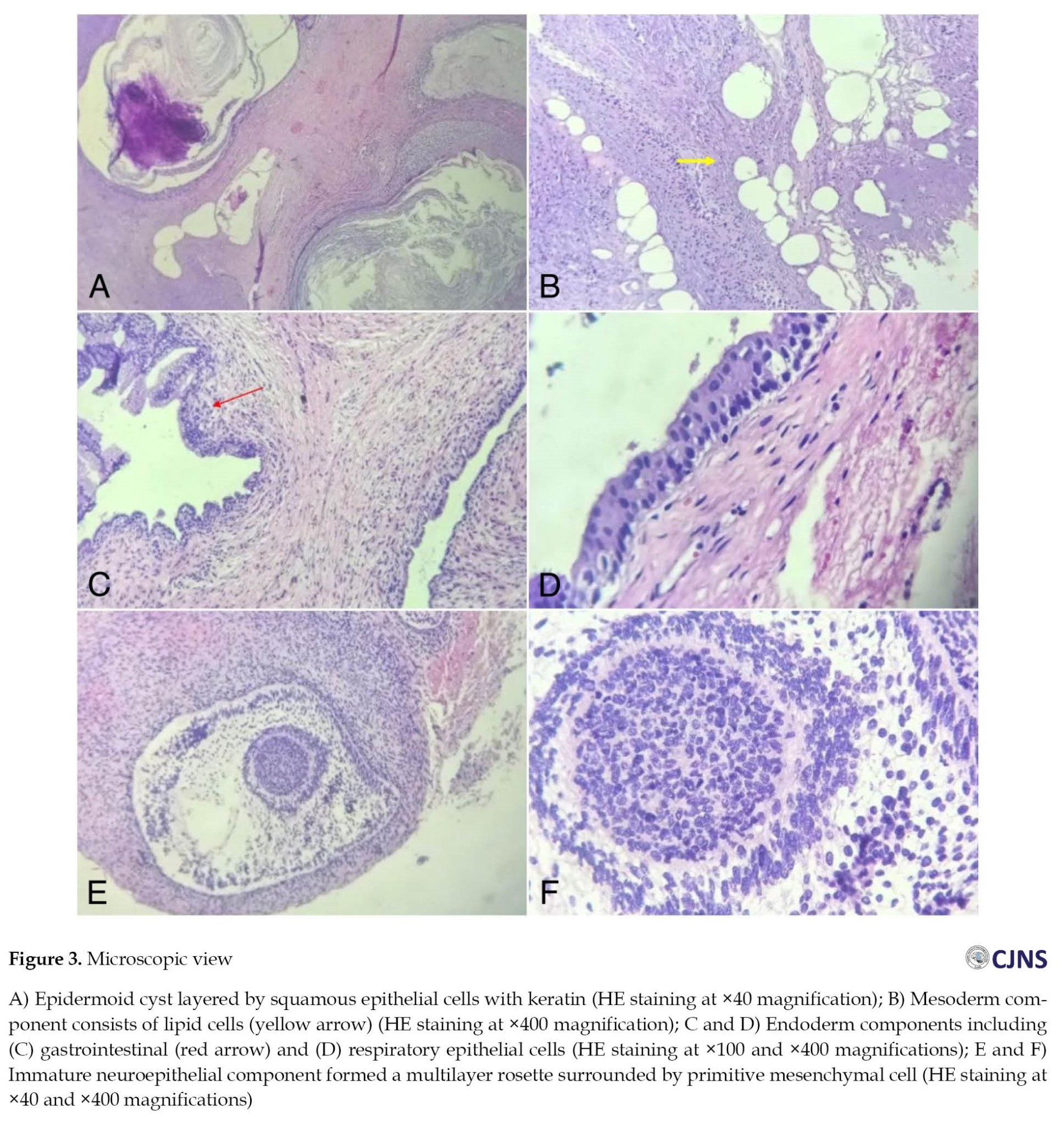
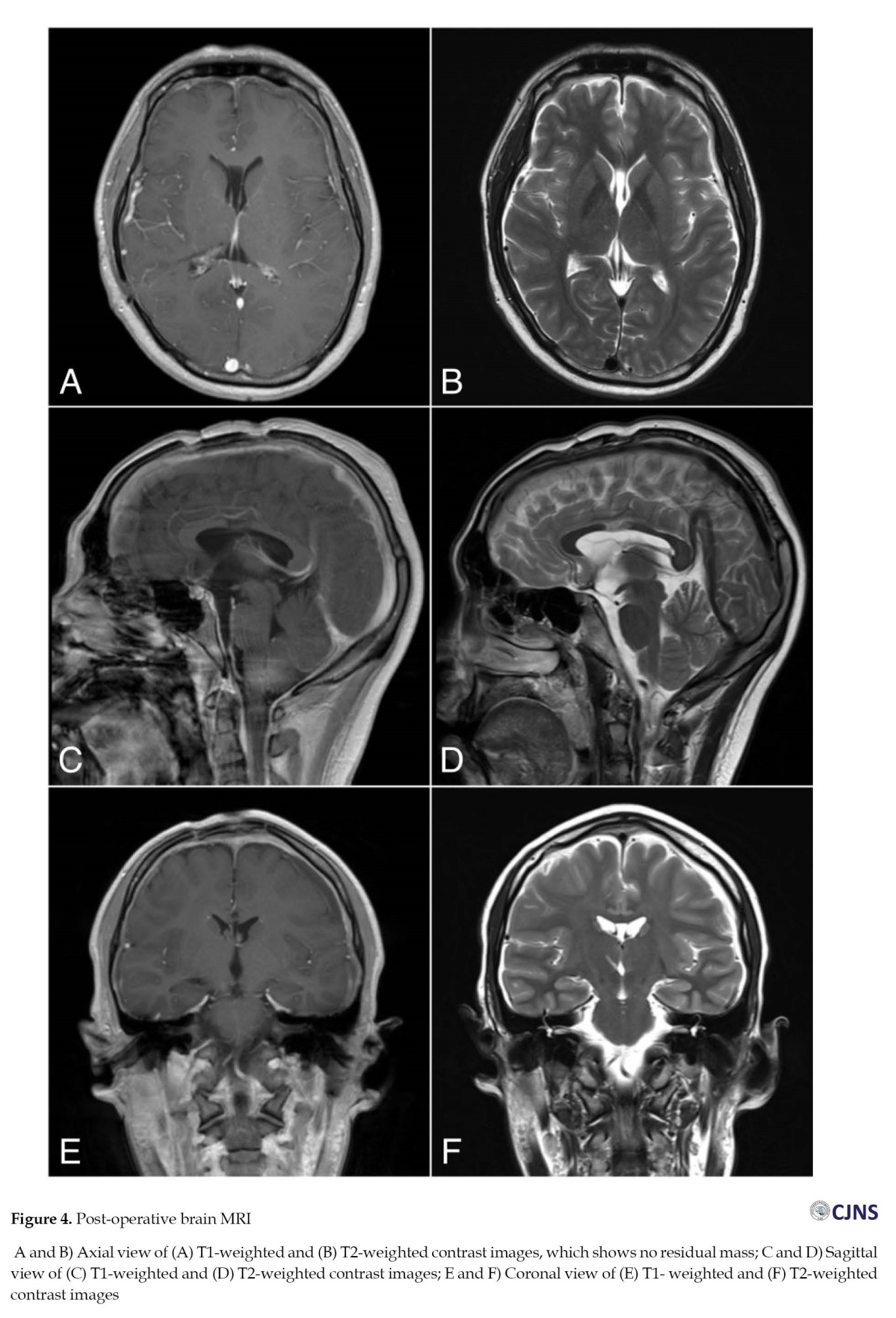
The histopathological finding was the immature teratomas (Figure 3). The components of the stratum corneum included ectoderm, adipocytes as mesoderm components, and endoderm components such as gastrointestinal and respiratory epithelia. Multi-layered rosettes surrounding mesenchymal progenitor cells were also identified. After surgery, the patient developed diabetes insipidus, a common complication of suprasellar tumor resection. Vasopressin and steroids were administered, and he recovered after 16 days of post-operative treatment. The patient was periodically followed up for up to 6 months, during which no signs of infection, seizures, or neurological deficits were observed. Post-operative imaging evaluation using MRI showed complete tumor resection (Figure 4).
Discussion
The GCTs originate from primordial germ cells found in neural tissues during early embryonic development, leading to histological and immunostaining features and some genetic alterations similar to the off-axis neural network components. Teratoma is one of the GCTs. Mixed and malignant subtypes of the CNS teratomas account for approximately 0.5-1% of adult primary intracranial tumors. The most of the CNS teratomas is found in the midline area, especially in the pineal region. The CNS GCTs in the pineal region are more common in males than in females [7, 8]. However, we encountered a conflicting case where a male patient in his early twenties had a teratoma in the suprasellar region. Tumors in the suprasellar region exhibit a wide range of clinical manifestations. From an anatomical perspective, visual impairment can occur due to the compression of the optic chiasm. However, non-communicating hydrocephalus may be developed, due to the compression of the ventricular system, by the posterior displacement, which is rare. The common signs of increased intracranial pressure, resulting from masses or hydrocephalus, include headache, nausea, vomiting, and seizures [7, 8]. Tumor serum markers such as beta-hCG and AFP, secreted by the GCTs, can be measured to help diagnose [9]. Patients report general weakness and progressive headaches as their predominant symptoms.
In our case, there was an evidence of increased intracranial pressure but it did not specifically indicate the possible location of the mass. Tumor serum markers were at a normal level and, thus, no specific diagnosis was possible. A head MRI with gadolinium enhancement revealed a lobular cystic mass in the suprasellar region compressing the ventricular system, and causing obstructive hydrocephalus. An emergency shunting procedure was performed to divert cerebrospinal fluid and reduce intracranial pressure. Complete resection of the tumor was then carried out seven days later. Tumors were removed using an interhemispheric transcallosal approach. After the incision of the ventricle wall, the tumor was exposed. Meticulous gross resection was then performed to minimize injury while accessing the suprasellar region where the base of the tumor was located. Although several complications, such as post-operative seizures, hemiparesis, and transient short-term memory deficit, have been reported, they did not occur in our patient. The subfrontal approach may be recommended for the patient, but there was a risk of injury in the frontal lobe [10].
The transcallosal approach is a surgical technique involving an incision in the corpus callosum to gain direct access to the lateral ventricle and lamina terminalis. This approach is commonly employed for intraventricular lesions, beginning with the dissection of the interhemispheric fissure to expose the corpus callosum [11]. The incision at the genu of the corpus callosum was made between the pericallosal arteries, entering the frontal horn and identifying the foramen of monro to expose the rostrum of the corpus callosum and the pre-commissural cistern. The arachnoid was dissected to expose the lamina terminalis, hypothalamic region, and precallosal branches at the bottom of the fissure. Complications of this procedure have been reported, including hydrocephalus (33.8%), transient hemiparesis (30.8%), seizures (18.5%), and short-term memory deficits (9.2%) [10-12]. Based on our experience, hydrocephalus might occur, typically requiring only temporary cerebrospinal fluid diversion. We employed external ventricular drainage for evaluating and monitoring impending hydrocephalus.
The histopathological findings of our study supported the existence of three germ cell layers: The stratum corneum representing ectoderm, lipid cells as mesoderm components, and endoderm components such as gastrointestinal and respiratory epithelium. These findings are characteristic of an immature teratoma. Mature teratomas exhibit a favorable 10-year survival rates of 90-93% with radical surgical removal. In contrast, survival rates for immature teratomas range from 50 to 70 years. Immature teratoma is a malignant tumor requiring multimodal therapy for the best patient outcome [7, 8, 9, 12] we present a 31-year-old female who presented with intermittent headache and oligomenorrhea of over 10 years’ duration. Imaging revealed a large suprasellar mass with sellar extension. The patient underwent an endoscopic endonasal trans-sphenoidal surgery to resection of the mass. Clinical, radiological, and operative findings from this patient were initially considered to be Rathke’s cleft cyst (RCC). However, since this is a rare case, more research is needed to understand the outcome and determine the best treatment for patients.
Conclusion
Immature teratomas are rare in adults, particularly in the suprasellar region. Therefore, the consideration of immature teratomas or GCTs is essential for the differential diagnosis, especially when dealing with tumors in the midline area. It is classified as a malignant brain tumor. Due to the rarity of this case and the limited number of reported cases, further research is needed to determine the optimal treatment.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in accordance with the ethical guidelines of the Declaration of Helsinki 2013.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors contributions
Conceptualization, investigation, and writing: All authors; Resources and supervision: Nyoman Golden.
Conflict of interest
The authors declared no conflict of interest.
References
Primary brain tumors are uncommon compared to brain metastases. Histomorphologically diagnosed primary brain tumors include glioblastoma, astrocytoma, oligodendroglioma, ependymoma, medulloblastoma, meningioma, germ cell tumor, and lymphoma [1]. Germ cell tumors (GCTs) are sporadic, constituting 0.3-0.6% of all primary central nervous system (CNS) tumors. They are categorized into germinomatous, non-germinomatous, and subtypes. Teratomas, a subtype of non-embryonal GCTs, make up only 18-20% of all GCTs and, consequently, only 0.1-1.5% of all intracranial tumors. Teratomas can coexist with other germline elements such as germinomas, yolk sac tumors, and embryonal carcinomas [2, 3]. Teratomas have a higher incidence in children, with a frequency of 1.8-5% in adults and over 15% in children in East Asian countries like Japan, Korea, and Taiwan. The composition of teratomas is distinctive as they originate from all germ cell layers endoderm, ectoderm, and mesoderm [4, 5]. Based on histologic findings, teratomas are classified as mature, immature, and malignant, with incidences of 60%, 25%, and 15%, respectively [3-5]. Immature teratomas consist of components resembling fetal tissue, often mixed with elements of mature tissue. Teratomas and other GCTs primarily occur in midline structures of the brain, including the third ventricle, the suprasellar region, and the pineal region. These tumors carry a poor prognosis and are considered malignant [4-6]. Therefore, appropriate treatment strategies can significantly affect the patient outcomes. Despite the rarity and limited reports of teratomas cases, in this study we present a case of immature teratomas in the suprasellar region of a young male.
Case Presentation
A 20-year-old male presented to the emergency department with a chief complaint of generalized weakness for the past seven days. He was also experiencing progressively worsening headaches in the past three months, with the severity escalating in the past month. The pain was described as if being tied up and affecting the entire head. Initially, he could tolerate the pain, but in the past week, the headaches became unbearable and did not respond to oral analgesics, suggesting an increase in intracranial pressure. The patient also reported blurred vision in the past month. No other relevant history was associated with the current complaint.
Clinical findings
Magnetic resonance imaging (MRI) of the brain with a gadolinium contrast agent was performed (Figure 1) due to progressive headache, blurred vision, and general weakness. The results reveal a multilocular cystic mass in the suprasellar region surrounded by vasogenic edema. The cystic mass had compressed the anterior cavernous sinus and the posterior medulla oblongata, and had occluded the third ventricle, leading to hydrocephalus due to the blockage of the third ventricle. Tumor serum markers, such as beta-human chorionic gonadotropin (beta-hCG) and alpha-fetoprotein (AFP), were assayed at 2.3 mIU/mL and 2.34 ng/mL, respectively, all of which were within normal limits.
Initially, a medium-pressure ventriculoperitoneal (VP) shunting was performed due to hydrocephalus. Following the treatment, there was a significant improvement in the headache. Gross tumor resection was carried out utilizing an interhemispheric anterior transcallosal approach. A horizontal midline incision was made, followed by a 4×4 cm craniotomy. Identification of midline structures was achieved by exposing the corpus callosum and ventricular system (Figure 2A). An approximately 1.5-cm incision was made to the genu of the corpus callosum to directly visualize the mass (Figure 2B). Meticulous intraventricular tumor dissection of the lamina terminalis cause the exposure of the inferior border of the tumor. Then, the tumor resection was conducted using an ultrasonic aspirator to debulk the tumor. Macroscopically, a gross and complete resection was achieved with good preservation of the surrounding neurovascular structures (Figure 2C).
The histopathological finding was the immature teratomas (Figure 3). The components of the stratum corneum included ectoderm, adipocytes as mesoderm components, and endoderm components such as gastrointestinal and respiratory epithelia. Multi-layered rosettes surrounding mesenchymal progenitor cells were also identified. After surgery, the patient developed diabetes insipidus, a common complication of suprasellar tumor resection. Vasopressin and steroids were administered, and he recovered after 16 days of post-operative treatment. The patient was periodically followed up for up to 6 months, during which no signs of infection, seizures, or neurological deficits were observed. Post-operative imaging evaluation using MRI showed complete tumor resection (Figure 4).
Discussion
The GCTs originate from primordial germ cells found in neural tissues during early embryonic development, leading to histological and immunostaining features and some genetic alterations similar to the off-axis neural network components. Teratoma is one of the GCTs. Mixed and malignant subtypes of the CNS teratomas account for approximately 0.5-1% of adult primary intracranial tumors. The most of the CNS teratomas is found in the midline area, especially in the pineal region. The CNS GCTs in the pineal region are more common in males than in females [7, 8]. However, we encountered a conflicting case where a male patient in his early twenties had a teratoma in the suprasellar region. Tumors in the suprasellar region exhibit a wide range of clinical manifestations. From an anatomical perspective, visual impairment can occur due to the compression of the optic chiasm. However, non-communicating hydrocephalus may be developed, due to the compression of the ventricular system, by the posterior displacement, which is rare. The common signs of increased intracranial pressure, resulting from masses or hydrocephalus, include headache, nausea, vomiting, and seizures [7, 8]. Tumor serum markers such as beta-hCG and AFP, secreted by the GCTs, can be measured to help diagnose [9]. Patients report general weakness and progressive headaches as their predominant symptoms.
In our case, there was an evidence of increased intracranial pressure but it did not specifically indicate the possible location of the mass. Tumor serum markers were at a normal level and, thus, no specific diagnosis was possible. A head MRI with gadolinium enhancement revealed a lobular cystic mass in the suprasellar region compressing the ventricular system, and causing obstructive hydrocephalus. An emergency shunting procedure was performed to divert cerebrospinal fluid and reduce intracranial pressure. Complete resection of the tumor was then carried out seven days later. Tumors were removed using an interhemispheric transcallosal approach. After the incision of the ventricle wall, the tumor was exposed. Meticulous gross resection was then performed to minimize injury while accessing the suprasellar region where the base of the tumor was located. Although several complications, such as post-operative seizures, hemiparesis, and transient short-term memory deficit, have been reported, they did not occur in our patient. The subfrontal approach may be recommended for the patient, but there was a risk of injury in the frontal lobe [10].
The transcallosal approach is a surgical technique involving an incision in the corpus callosum to gain direct access to the lateral ventricle and lamina terminalis. This approach is commonly employed for intraventricular lesions, beginning with the dissection of the interhemispheric fissure to expose the corpus callosum [11]. The incision at the genu of the corpus callosum was made between the pericallosal arteries, entering the frontal horn and identifying the foramen of monro to expose the rostrum of the corpus callosum and the pre-commissural cistern. The arachnoid was dissected to expose the lamina terminalis, hypothalamic region, and precallosal branches at the bottom of the fissure. Complications of this procedure have been reported, including hydrocephalus (33.8%), transient hemiparesis (30.8%), seizures (18.5%), and short-term memory deficits (9.2%) [10-12]. Based on our experience, hydrocephalus might occur, typically requiring only temporary cerebrospinal fluid diversion. We employed external ventricular drainage for evaluating and monitoring impending hydrocephalus.
The histopathological findings of our study supported the existence of three germ cell layers: The stratum corneum representing ectoderm, lipid cells as mesoderm components, and endoderm components such as gastrointestinal and respiratory epithelium. These findings are characteristic of an immature teratoma. Mature teratomas exhibit a favorable 10-year survival rates of 90-93% with radical surgical removal. In contrast, survival rates for immature teratomas range from 50 to 70 years. Immature teratoma is a malignant tumor requiring multimodal therapy for the best patient outcome [7, 8, 9, 12] we present a 31-year-old female who presented with intermittent headache and oligomenorrhea of over 10 years’ duration. Imaging revealed a large suprasellar mass with sellar extension. The patient underwent an endoscopic endonasal trans-sphenoidal surgery to resection of the mass. Clinical, radiological, and operative findings from this patient were initially considered to be Rathke’s cleft cyst (RCC). However, since this is a rare case, more research is needed to understand the outcome and determine the best treatment for patients.
Conclusion
Immature teratomas are rare in adults, particularly in the suprasellar region. Therefore, the consideration of immature teratomas or GCTs is essential for the differential diagnosis, especially when dealing with tumors in the midline area. It is classified as a malignant brain tumor. Due to the rarity of this case and the limited number of reported cases, further research is needed to determine the optimal treatment.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in accordance with the ethical guidelines of the Declaration of Helsinki 2013.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors contributions
Conceptualization, investigation, and writing: All authors; Resources and supervision: Nyoman Golden.
Conflict of interest
The authors declared no conflict of interest.
References
- Mukherjee T, Dutta R, Ghosh J, Sharma M. Brain tumors with review of literature: Immunohistochemistry or biomarkers versus histomorphology. J Neuro Oncol Neurosci. 2016; 1(1):14. [Link]
- Zygourakis CC, Davis JL, Kaur G, Ames CP, Gupta N, Auguste KI, et al. Management of central nervous system teratoma. J Clin Neurosci. 2015; 22(1):98-104. [DOI:10.1016/j.jocn.2014.03.039] [PMID]
- Lacruz CR, de Santamaría JS, Bardales RH. Central nervous system intraoperative cytopathology. New York: Springer; 2014. [DOI:10.1007/978-1-4614-8429-5]
- Takami H, Graffeo CS, Perry A, Giannini C, Nakazato Y, Saito N, et al. Roles of tumor markers in central nervous system germ cell tumors revisited with histopathology-proven cases in a large international cohort. Cancers (Basel). 2022; 14(4):979. [DOI:10.3390/cancers14040979] [PMID]
- Romić D, Raguž M, Marčinković P, Sesar P, Špero M, Čolak Romić Z, et al. Intracranial mature teratoma in an adult patient: A case report. J Neurol Surg Rep. 2019; 80(1):e14-7. [DOI:10.1055/s-0039-1685213] [PMID]
- Zhang RP, Chen J, Hu XL, Fang YL, Cai CQ. Immature teratoma of the posterior fossa in an infant: Case report. Turk Pediatri Ars. 2019 ; 54(2):125-8. [PMID]
- Jiang S, Wang Z, You Y, Wang R, Bao X. Suprasellar Mature cystic teratoma mimicking rathke’s cleft cyst: A case report and systematic review of the literature. Front Endocrinol (Lausanne). 2021; 12:731088. [DOI:10.3389/fendo.2021.731088] [PMID]
- Guk MO, Danevych OO, Chukov AA, Zemskova O V, Chuvashova OY, Iegorova KS, et al. Suprasellar mature teratoma: case report. Ukr Neurosurg J. 2021; 27(1):44-50. [DOI:10.25305/unj.211642]
- Zain SM, Mirchia K, Galbraith K, Galgano MA, Lee M, Richardson TE, et al. Mediastinal metastases from a primary immature teratoma of the CNS. Radiol Case Rep. 2022; 17(9):3339-44. [DOI:10.1016/j.radcr.2022.06.059] [PMID]
- Aldave G. Enhancing access to the suprasellar region: The transcallosal translamina terminalis approach. J Neurosurg Pediatr. 2020; 26(5):572-7. [DOI:10.3171/2020.5.PEDS20369] [PMID]
- Patel PG, Cohen-Gadol AA, Mercier P, Boop FA, Klimo P Jr. The posterior transcallosal approach to the pineal region and posterior third ventricle: Intervenous and paravenous variants. Oper Neurosurg (Hagerstown). 2017; 13(1):77-88. [DOI:10.1227/NEU.0000000000001268] [PMID]
- Aryan HE, Ozgur BM, Jandial R, Levy ML. Complications of interhemispheric transcallosal approach in children: Review of 15 years experience. Clin Neurol Neurosurg. 2006; 108(8):790-3. [DOI:10.1016/j.clineuro.2005.10.009] [PMID]
Type of Study: case report |
Subject:
General
Received: 2023/07/13 | Accepted: 2023/09/3 | Published: 2024/04/1
Received: 2023/07/13 | Accepted: 2023/09/3 | Published: 2024/04/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |