Sat, May 18, 2024
Volume 9, Issue 4 (Autumn 2023)
Caspian J Neurol Sci 2023, 9(4): 259-267 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
R Prabhu R. Phosphorylation Status of GluN2B-Ser1303 Under Glutamate-induced Excitotoxicity in Primary Cortical Neurons: An In Vitro Study. Caspian J Neurol Sci 2023; 9 (4) :259-267
URL: http://cjns.gums.ac.ir/article-1-668-en.html
URL: http://cjns.gums.ac.ir/article-1-668-en.html
Department of Biotechnology, Faculty of Applied Science, Government Arts College, Trivandrum, India
Full-Text [PDF 3546 kb]
(154 Downloads)
| Abstract (HTML) (381 Views)
Full-Text: (92 Views)
Introduction
The excitatory neurotransmission in the mammalian brain is crucial for neuronal development, differentiation, migration, and survival [1]. The major neurotransmitter involved in excitatory neurotransmission is glutamate. It is also the major neurotransmitter in the mammalian brain. Glutamate plays a critical role in synaptic plasticity, a foundation for learning and memory processes. However, excessively elevated levels of glutamate are fatal, acting as a potential neurotoxin and causing excitotoxicity with damage to neurons, injury, and cell death [2]. Ionotropic receptors represent the main receptors of glutamate, functioning as ligand-gated channels and opening upon binding to the major glutamate ligand. The major ionotropic receptors are N-methyl-D-aspartate receptors (NMDARs), which play vital roles in the brain’s excitatory synaptic transmission, like plasticity and excitotoxicity [3]. NMDARs exhibit a unique characteristic known as Mg2+ block at the opening of the channel. Two events are required for the opening of NMDARs: The binding of glutamate and strong membrane depolarization due to excitation, leading to the removal of the Mg2+ block [4]. NMDAR is a heterotetramer comprising a combination of any of the major seven subunits: One GluN1, four GluN2(A-D), and two GluN3(A, B) [5], with GluN1 being obligatory. The subunits GluN2A and GluN2B are enriched in the forebrain [6]. In the adult rat brain, the GluN2B subunit is expressed at the highest levels in the brain regions like the hippocampus, olfactory tubercle and bulb, and cerebral cortex [7]. This elevated expression is attributed to its primary role in mediating plasticity and excitotoxicity. Additionally, GluN2B expression level is predominant in prenatal rodents, peaking around 14 days after birth and continuously decreasing after that.
GluN2B undergoes phosphorylation at Ser1303 by kinases like CaMKII and DAPK1. These kinases bind to the phosphorylation site and affect synaptic plasticity [8]. GluN2B is also involved in the progress and pathophysiology of neurodegenerative disorders like Alzheimer disease, Parkinson disease, ischemic stroke, etc [9]. GluN2B-Ser1303 phosphorylation is also associated with the modulation of chronic neuropathic pain [10]. The functions of NMDAR, including its channel properties, binding of kinases like CaMKII or DAPK1, and its trafficking in the synaptic and extrasynaptic sites, can be regulated by phosphorylation [11]. Although the significance of phosphorylation of Ser1303 on GluN2B has been explored in some conditions, little is known about its regulation or dephosphorylation by protein phosphatases [12]. Preliminary studies have shown that phosphatases like protein phosphatase 1 (PP1) in postsynaptic density (PSD) preparations could act on phospho-GluN2B-Ser1303 and dephosphorylate the same [13]. Thus, this site could be a potential target for phosphatase action to regulate phosphorylation.
We further wanted to investigate the regulation of phosphorylation at GluN2B-Ser1303 under excitotoxic conditions. To investigate that, we utilized primary cortical neuronal preparation from embryonic rat brains and exposed them to glutamate treatment in the presence and absence of various phosphatase inhibitors.
Materials and Methods
All cell culture media and chemicals were procured from GIBCO-Invitrogen, USA. The antibodies for Western blot, protein A agarose beads, Poly-D-lysine, and laminin were procured from Sigma-Aldrich, USA. Phosphatase inhibitors were purchased from Calbiochem, USA, whereas cyclosporin and MK-801 were from Sigma-Aldrich, USA. The antibody for GluN2B- Ser1303 was purchased from Millipore, USA.
Primary cortical neurons preparation from E18 rat embryos
The cortical neurons’ primary culture was prepared from embryos aged 18 days (E18) from pregnant rats. The animal experiments were carried out according to guidelines recommended by the institutional animal ethics committee. The mother rat was euthanized, her abdomen was cut open, and E18 embryos were procured. After decapitating embryos, the meninges were removed, and the cortex was isolated. The cortical tissues were minced gently in Hanks balanced salt solution. Tissue dissociation was carried out by adding 0.25% Trypsin-EDTA and 20 μL DNase I (4000 U/mL) for 10 minutes at 37oC with intermittent tapping. Trypsin was inactivated by adding 500 µL of fetal bovine serum (FBS) and 5 mL Dulbecco’s modified Eagle’s medium (DMEM). The pelleted tissue was centrifuged at 1800 rpm for 5 minutes, and the supernatant was removed by aspiration. The tissue was again washed with 5 mL DMEM. Next, the tissue was subjected to trituration by pipetting about 50 times by adding 100 µL of DNase in 1 mL DMEM to make a single-cell suspension. The cells were pelleted by centrifugation at 1800 rpm for 5 minutes. The supernatant was removed, and the cells were resuspended in 1-2 mL of reconstituted neurobasal medium NBM supplemented with 1X antibiotic/antimycotic solution, and B27 supplement). The cells were counted using a hemocytometer and were plated in either 24- or 6-well plates precoated with poly-D-lysine (100 μg/mL) and laminin (100 μg/mL).
Treatment of primary cortical neurons with glutamate for induction of excitotoxicity
The primary cortical neuronal preparation from E18 rat embryos was seeded in 6-well plates. On the ninth day in vitro (DIV 9), these were treated with glutamate for excitotoxicity induction as described in the Remya et al. (2021) [14] study with slight modifications. The cells were washed with solution I (Hanks’ balanced salt solution [HBSS], 10 mM N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid [HEPES], and 0.2 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid [EGTA]) for 10 minutes, followed by washing with solution II (HBSS, 10 mM HEPES). Subsequently, the cells were treated with 100 μM glutamate and 10 μM glycine for about 60 minutes, supplemented with 1.2 mM CaCl2 (solution III). The various phosphatase inhibitors were also preincubated for 10 minutes along with solution II and given along with glutamate treatment in solution III. The phosphatase inhibitors used were I-2 at 10 nM and okadaic acid (OA) at 1 µM for inhibiting PP1, as well as OA at a concentration of 1 nM, I1PP2A at 2 nM for inhibiting PP2A, and cyclosporin (CsA) at a concentration of 1 µM for inhibiting PP2B. MK-801 is the open channel blocker of NMDAR and was used at a concentration of 20 µM. After the treatment, the cells were harvested in 1 mL of 1X phosphate-buffered saline (PBS). The cells were lysed in radioimmunoprecipitation assay buffer (RIPA buffer - 50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS), and proteins were identified using BCA method. The cortical cell lysate was subjected to Western blotting to detect phospho-GluN2B-Ser1303 and GluN2B levels [14].
Hoechst staining of glutamate treated primary cortical cells to check for excitotoxic neuronal death
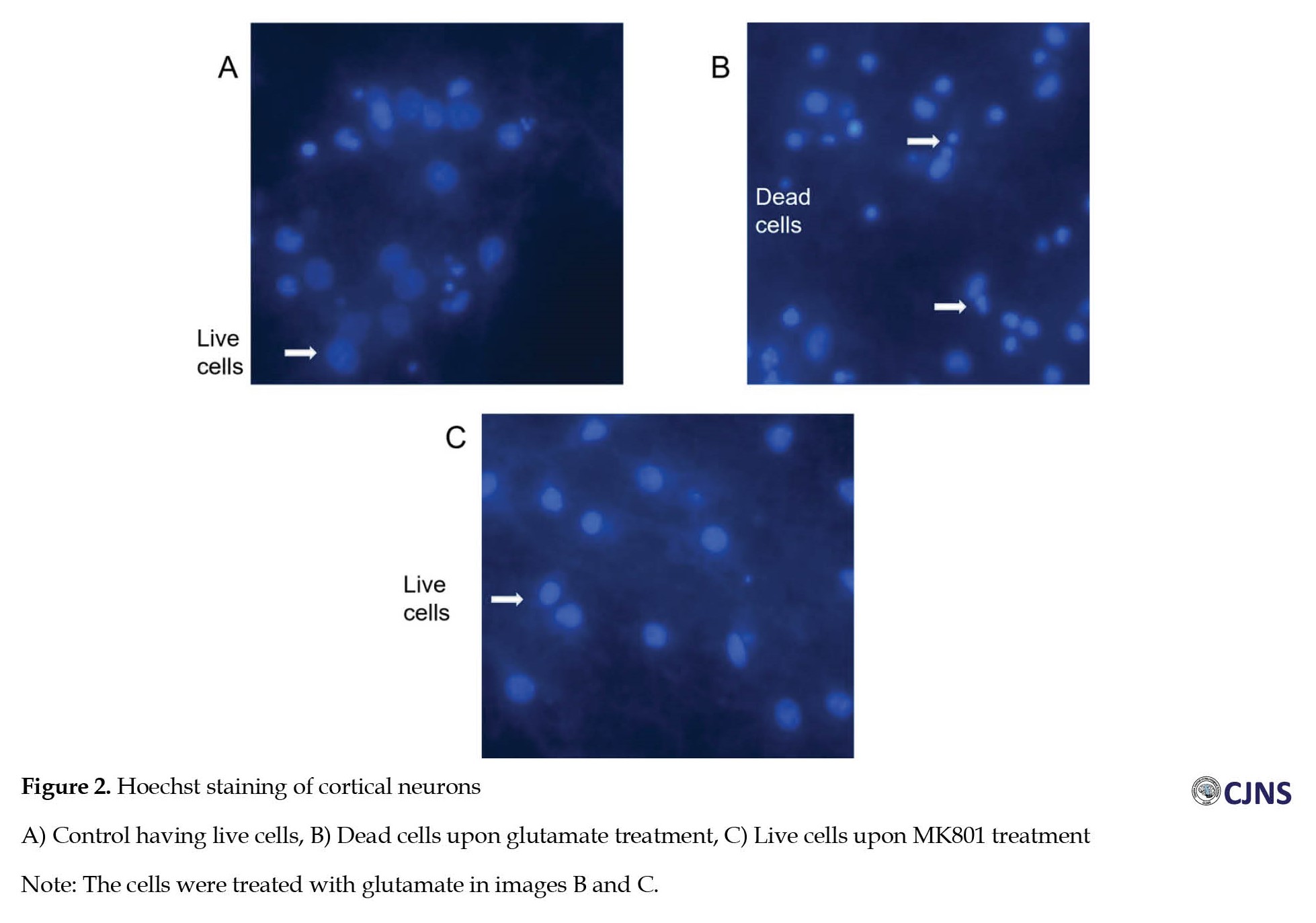
Primary cortical neurons were treated with glutamate, as mentioned earlier. After the treatment, glutamate was removed, and normal NBM was added to the wells, maintained at 37oC for 24 hours. After 24 hours, the medium was removed, and the cells were fixed with 4% paraformaldehyde. The cells were washed with 1X PBS, stained with 5 µg/mL Hoechst for 10 minutes, then the Hoechst stain was removed, and 1X PBS was added, and proceeded for imaging at 280 nm. The cells were examined for condensed nuclei, indicating dead cells, whereas healthy cells exhibited viable nuclei without morphological condensation. This experiment was done to check if the glutamate treatment at the given concentration can induce excitotoxicity and cell death in primary neurons.
Characterization of primary cortical neurons and immunofluorescence to check for the co-localization of PP1α with GluN2B in primary neurons
Primary neuronal cells isolated from E18 embryos were seeded on coverslips in a 24-well plate. The experiments to check the co-localization of GluN2B with PP1α were done on DIV 7. For immunocytochemistry, the cells were fixed with 250 μL of 4% paraformaldehyde at room temperature for 20 minutes after removing the medium, followed by a wash with 1X PBS. The cells were then washed thrice with 1X PBS. Next, 200 μL of blocking solution (0.3% Triton-X-100 in 5% normal goat serum (NGS) in 1X PBS) was added to cells, and the mixture was incubated at room temperature for 30 minutes. Following this, the primary antibody diluted in the corresponding blocking solution was added at a concentration of 1:100. This solution was incubated overnight at 4oC. After incubation, the cells were washed with 1X PBS thrice, and the secondary antibody was added.
For the GluN2B primary antibody, the corresponding secondary antibody used for immunofluorescence was a sheep anti-rabbit antibody conjugated with Cy3. Following removing the secondary antibody, the cells were washed thoroughly with 1X PBS, and then the mouse anti-PP1α antibody was added and incubated overnight. The secondary antibody used for immunofluorescence for the mouse anti-PP1α primary antibody was a goat anti-mouse antibody conjugated with Alexa 488. The images were taken with a confocal microscope.
Preparation of rat forebrain cortex homogenate for co-immunoprecipitation
The homogenate used for co-immunoprecipitation experiments was prepared from the rat forebrain cortex. The forebrains were homogenized in an ice-cold solution containing 320 mM sucrose and 10 mM Tris-HCl (pH-7.4), 1 mM EDTA, 1 mM EGTA, and 1X PIC with 12 strokes at 800 rpm. The large cell fragments were removed by centrifuging at 5000 rpm for about 2-3 minutes. Then, the membrane fraction was sedimented at 50000 rpm at 4oC for about 30 minutes. The obtained membrane fractions were solubilized with 1% deoxycholate in 50 mM Tris-HCl (pH-7.4), 10 mM EDTA, and 10 mM EGTA for about 20 minutes on ice at the rate of 10 mL solution per gram of rat brain. The solubilized membrane fractions were obtained in the supernatant and were used directly for immunoprecipitation or stored at -80oC as aliquots.
Co-immunoprecipitation
50 μL of protein A agarose beads were washed thrice in 1 mL 1X ice-cold PBS. These beads were subjected to blocking in 1% BSA for about 1 h at 4oC with constant agitation at 8 rpm. After removing the blocking solution, about 6 µg of anti-rabbit polyclonal antibody in 1 mL 1 X PBS was added to the beads. Correspondingly, anti-rabbit non-immune IgG as a negative control was also subjected to the same treatment. The antibody bead complex was allowed to form at about 7 h in agitation at 4oC. The previously prepared homogenate was subjected to preclearance before proceeding to immunoprecipitation. About 500 µL of homogenate was mixed with 50 µL of protein A agarose beads with agitation at 4oC for about an hour. This is done to remove the non-specific binding of homogenate to beads, if any. After 1 h, the mixture was centrifugated at 2000 rpm for 5 minutes, and the supernatant collected was subjected to immunoprecipitation. The antibody bead complex was subjected to washes with 500 µL 1X PBS, thrice by centrifuging at 2000 rpm for 4 minutes each, to remove the unbound antibody, if any. About 500 µL of precleared homogenate is added, followed by incubation for about 12-15 hours at 4oC with constant agitation. After this, the supernatant consisting of unbound proteins and the immunoprecipitated complex bound to the beads was subjected to washes with 500 µL 1X PBS with 0.1% Triton X 100 thrice, and the last wash was given without any detergent. The immunoprecipitants were denatured by boiling an equal amount of 2X SDS sample buffer and proceeded for Western blotting.
Statistical analysis
The significance of immunoblots was quantitated by one-way ANOVA followed by Scheffe’s post hoc analysis.
Results
Phosphorylation status of GluN2B-Ser1303 in cortical neurons upon glutamate treatment
The primary cortical neurons exposed to 100 µM glutamate for 60 minutes exhibited a reduction in GluN2B-Ser1303 phosphorylation, observed at about 50%, as revealed by Western blots. PP1 seems to be the main mediator of this effect, as evidenced by the effective prevention of dephosphorylation at GluN2B-Ser1303 when PP1 was inhibited by I-2 or OA at high concentration (1 µM) (Figure 1a and 1b). Treatment with MK801, an NMDAR channel blocker, also prevented the dephosphorylation, showing that the effect is mediated through NMDARs. Thus, glutamate treatment significantly changed the dephosphorylation of GluN2B-Ser1303, as evidenced by both PP1 inhibitor and MK801. When other phosphatase inhibitors were used, there was no significant change in phosphorylation levels of GluN2B at Ser1303. Notably, OA at 1 µM concentration reverses the dephosphorylation by blocking PP1. In contrast, other inhibitors like CsA, OA at 1 nM concentration, I-1, and others did not affect the dephosphorylation, indicating that the principal phosphatase involved in dephosphorylation at Ser1303 is PP1. There was no change in the level of GluN2B, indicating that the glutamate treatment results only in the shift of phosphorylation status at Ser1303 and does not affect the total protein at the receptor level. Staining with Hoechst after 24-h post-treatment of glutamate 100 µM for 60 minutes revealed the presence of dead neurons in conditions where glutamate was given to cells, whereas the control and MK 801-treated cells showed no significant death (Figure 2). This outcome indicates that glutamate treatment at the given concentration was excitotoxic.
Co-localization of PP1 and PP2A with GluN2B in primary cortical neurons
The cortical neurons were stained for the proteins GluN2B and PP1. Immunocytochemical staining showed co-localization of PP1 with GluN2B in primary cortical neurons (Figure 3), indicating that PP1 could access the phosphorylation site. Immunoprecipitation of PP1 But Not PP2A using GluN2B antibody
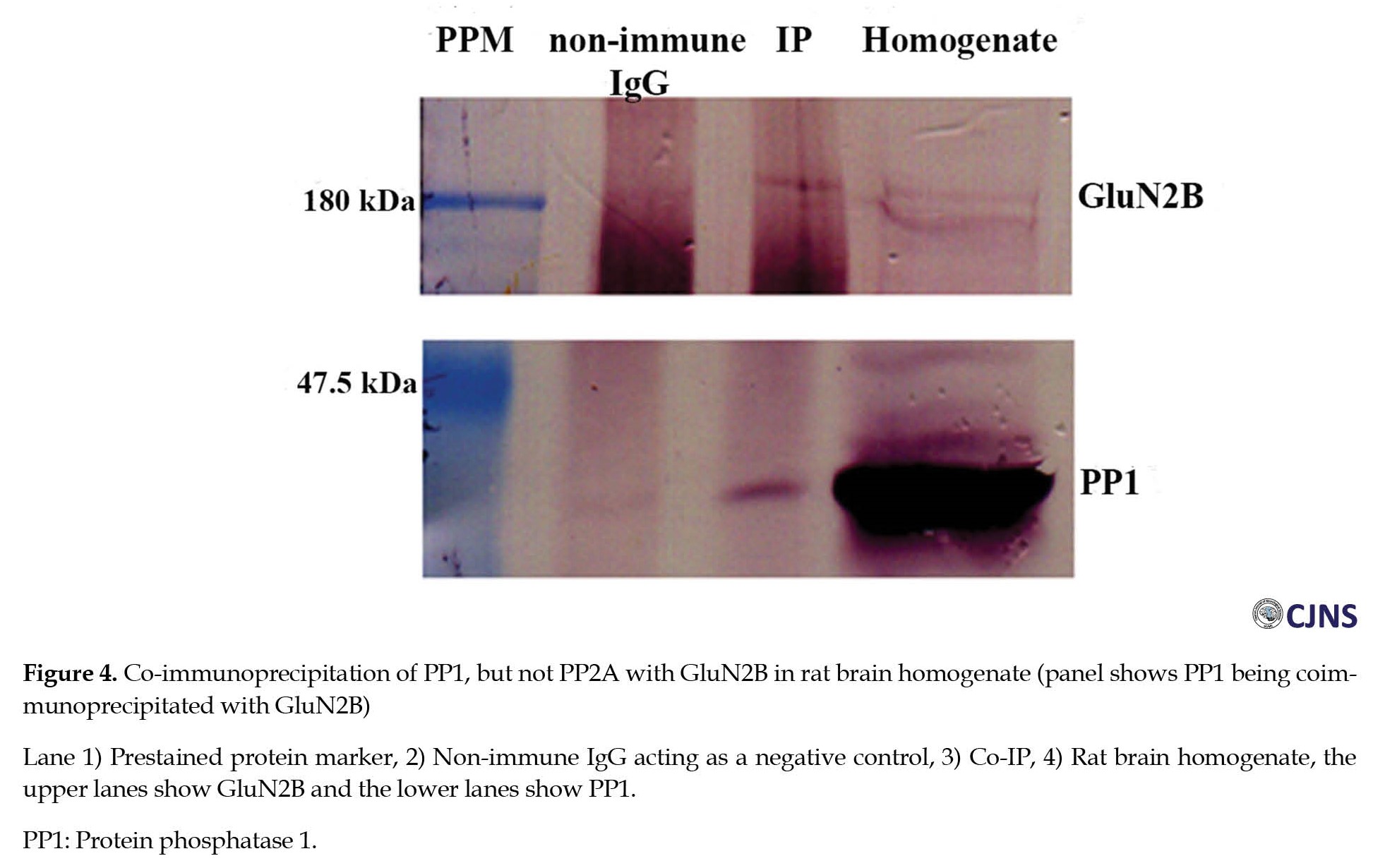
Immunoprecipitation studies were conducted to study whether PP1 and PP2A would interact with GluN2B in rat brain homogenate. Deoxycholate-solubilized homogenate was immunoprecipitated with rabbit polyclonal GluN2B antibody, previously bound to protein-A-agarose beads. Immunoprecipitation with GluN2B antibody resulted in the co-precipitation of only PP1, not PP2A (Figure 4). As a control, a non-immune IgG was used in the immunoprecipitation. GluN2B, PP1, and PP2A were detected by Western blotting using respective antibodies. The immunoprecipitation with GluN2B antibody showed reactivity with PP1 but not PP2A. The immunoreactivity band for all the proteins is absent in the control lane.
Discussion
Phosphorylation, as a post translational mechanism, induces covalent changes in the properties of a protein [15], thereby regulating the cellular processes such as activity, localization, and mobility of proteins, especially receptors. Regarding GluN2B, calcium influx through either NMDAR or voltage gate calcium channels activates the phosphorylation at Ser1303 [16]. Still, the mechanism of dephosphorylation has remained unclear in vitro or in vivo except for a study suggesting that overexpression of PP1 in hippocampal neurons may inhibit NMDAR activation, a possible pathway for neuroprotection [17].
Our previous studies have shown that phosphorylation of GluN2B-Ser1303 is regulated by phosphatases in PSD, especially by PP1 [13]. The current research shows that PP1 is the principal phosphatase responsible for the regulation of phosphorylation at GluN2B-Ser1303 in primary cortical neurons under excitotoxic conditions, consistent with our observations in the case of PSD. Inhibition of calcium entry via NMDAR results in the survival of neurons, indicating that the cell death is mediated by glutamate treatment at a concentration of 100 µM. Therefore, reduction in phosphorylation at GluN2B-Ser1303 could be an outcome of the excitotoxic event, resembling observations of GluN2B-Ser1303 phosphorylation reduction in patients with depression-like disorders showing a modulation of glutamatergic transmission [18].
Importantly, no change is observed in the total receptor levels, GluN2B, during glutamate treatment; only changes in phosphorylation levels at the Ser1303 site, as indicated by Western blots. This alteration in phosphorylation is inhibited only when inhibitors of PP1, such as okadaic acid at high concentration and I-2, are used, indicating that the principal phosphatase involved in phosphorylation of Ser1303 is PP1, but not other phosphatases like PP2A or PP2B. Compared to other Ser-Thr phosphatases, PP1 might have access to phosphorylation at GluN2B-Ser1303 as it is colocalized with the latter, as seen in in vitro staining and co-immunoprecipitation studies. Okadaic acid at 1 nM, used to inhibit PP2A, showed a slightly enhanced inhibition of phosphorylation at GluN2B- Ser1303, possibly inhibiting PP1 to some extent. However, the specific inhibitor of PP2A, I1PP2A, failed to reverse dephosphorylation, indicating that PP2A is not involved in the dephosphorylation of GluN2B- Ser1303. Further, in vivo, studies need to be carried out to validate the observations found in these studies and previous studies, which show that PP1 is responsible for regulating the phosphorylation of GluN2B-Ser1303 in both PSD and primary neurons in vitro.
Conclusion
Our study focussed on the effect of glutamate treatment on cortical neurons isolated from embryonic rat brains, primarily excitatory. Activating these primary neurons in vitro with 100 µM glutamate for 60 minutes resulted in excitotoxicity as visualized by cell death by Hoechst staining and a significant reduction in GluN2B-Ser1303 phosphorylation. Using phosphatase inhibitors like I-2, we showed that PP1 regulated the reduction of GluN2B-Ser1303 phosphorylation in primary cortical neurons upon glutamate treatment, indicating its major phosphatase involved in the regulation of the latter under excitotoxic conditions, with no discernible role for other phosphatases. This study lays the groundwork for future in vivo experiments to explore the regulation of phosphorylation at the GluN2B receptor, especially at the Ser1303 site, which PP1 regulates. The identified site could be a possible drug target for inhibiting excitotoxicity. Further studies need to be conducted in search of novel inhibitors of this target and their potential roles in mitigating the excitotoxicity induced by glutamate.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were done in compliance with the ethical guidelines of the Declaration of Helsinki 2013. This study was also approved by the Rajiv Gandhi Centre for Biotechnology (RGCB) (Code: IAEC/112/OMK/2010).
Funding
This study is financially supported by Rajiv Gandhi Centre for Biotechnology and the fellowship received from Council of scientific and industrial research, Government of India (CSIR).
Conflict of interest
The author declared no conflict of interest.
Acknowledgements
The author thanks the PhD supervisor, director, faculty, staff, and students at both RGCB and Government Arts College for the support extended in designing and carrying out the work.
References
The excitatory neurotransmission in the mammalian brain is crucial for neuronal development, differentiation, migration, and survival [1]. The major neurotransmitter involved in excitatory neurotransmission is glutamate. It is also the major neurotransmitter in the mammalian brain. Glutamate plays a critical role in synaptic plasticity, a foundation for learning and memory processes. However, excessively elevated levels of glutamate are fatal, acting as a potential neurotoxin and causing excitotoxicity with damage to neurons, injury, and cell death [2]. Ionotropic receptors represent the main receptors of glutamate, functioning as ligand-gated channels and opening upon binding to the major glutamate ligand. The major ionotropic receptors are N-methyl-D-aspartate receptors (NMDARs), which play vital roles in the brain’s excitatory synaptic transmission, like plasticity and excitotoxicity [3]. NMDARs exhibit a unique characteristic known as Mg2+ block at the opening of the channel. Two events are required for the opening of NMDARs: The binding of glutamate and strong membrane depolarization due to excitation, leading to the removal of the Mg2+ block [4]. NMDAR is a heterotetramer comprising a combination of any of the major seven subunits: One GluN1, four GluN2(A-D), and two GluN3(A, B) [5], with GluN1 being obligatory. The subunits GluN2A and GluN2B are enriched in the forebrain [6]. In the adult rat brain, the GluN2B subunit is expressed at the highest levels in the brain regions like the hippocampus, olfactory tubercle and bulb, and cerebral cortex [7]. This elevated expression is attributed to its primary role in mediating plasticity and excitotoxicity. Additionally, GluN2B expression level is predominant in prenatal rodents, peaking around 14 days after birth and continuously decreasing after that.
GluN2B undergoes phosphorylation at Ser1303 by kinases like CaMKII and DAPK1. These kinases bind to the phosphorylation site and affect synaptic plasticity [8]. GluN2B is also involved in the progress and pathophysiology of neurodegenerative disorders like Alzheimer disease, Parkinson disease, ischemic stroke, etc [9]. GluN2B-Ser1303 phosphorylation is also associated with the modulation of chronic neuropathic pain [10]. The functions of NMDAR, including its channel properties, binding of kinases like CaMKII or DAPK1, and its trafficking in the synaptic and extrasynaptic sites, can be regulated by phosphorylation [11]. Although the significance of phosphorylation of Ser1303 on GluN2B has been explored in some conditions, little is known about its regulation or dephosphorylation by protein phosphatases [12]. Preliminary studies have shown that phosphatases like protein phosphatase 1 (PP1) in postsynaptic density (PSD) preparations could act on phospho-GluN2B-Ser1303 and dephosphorylate the same [13]. Thus, this site could be a potential target for phosphatase action to regulate phosphorylation.
We further wanted to investigate the regulation of phosphorylation at GluN2B-Ser1303 under excitotoxic conditions. To investigate that, we utilized primary cortical neuronal preparation from embryonic rat brains and exposed them to glutamate treatment in the presence and absence of various phosphatase inhibitors.
Materials and Methods
All cell culture media and chemicals were procured from GIBCO-Invitrogen, USA. The antibodies for Western blot, protein A agarose beads, Poly-D-lysine, and laminin were procured from Sigma-Aldrich, USA. Phosphatase inhibitors were purchased from Calbiochem, USA, whereas cyclosporin and MK-801 were from Sigma-Aldrich, USA. The antibody for GluN2B- Ser1303 was purchased from Millipore, USA.
Primary cortical neurons preparation from E18 rat embryos
The cortical neurons’ primary culture was prepared from embryos aged 18 days (E18) from pregnant rats. The animal experiments were carried out according to guidelines recommended by the institutional animal ethics committee. The mother rat was euthanized, her abdomen was cut open, and E18 embryos were procured. After decapitating embryos, the meninges were removed, and the cortex was isolated. The cortical tissues were minced gently in Hanks balanced salt solution. Tissue dissociation was carried out by adding 0.25% Trypsin-EDTA and 20 μL DNase I (4000 U/mL) for 10 minutes at 37oC with intermittent tapping. Trypsin was inactivated by adding 500 µL of fetal bovine serum (FBS) and 5 mL Dulbecco’s modified Eagle’s medium (DMEM). The pelleted tissue was centrifuged at 1800 rpm for 5 minutes, and the supernatant was removed by aspiration. The tissue was again washed with 5 mL DMEM. Next, the tissue was subjected to trituration by pipetting about 50 times by adding 100 µL of DNase in 1 mL DMEM to make a single-cell suspension. The cells were pelleted by centrifugation at 1800 rpm for 5 minutes. The supernatant was removed, and the cells were resuspended in 1-2 mL of reconstituted neurobasal medium NBM supplemented with 1X antibiotic/antimycotic solution, and B27 supplement). The cells were counted using a hemocytometer and were plated in either 24- or 6-well plates precoated with poly-D-lysine (100 μg/mL) and laminin (100 μg/mL).
Treatment of primary cortical neurons with glutamate for induction of excitotoxicity
The primary cortical neuronal preparation from E18 rat embryos was seeded in 6-well plates. On the ninth day in vitro (DIV 9), these were treated with glutamate for excitotoxicity induction as described in the Remya et al. (2021) [14] study with slight modifications. The cells were washed with solution I (Hanks’ balanced salt solution [HBSS], 10 mM N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid [HEPES], and 0.2 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid [EGTA]) for 10 minutes, followed by washing with solution II (HBSS, 10 mM HEPES). Subsequently, the cells were treated with 100 μM glutamate and 10 μM glycine for about 60 minutes, supplemented with 1.2 mM CaCl2 (solution III). The various phosphatase inhibitors were also preincubated for 10 minutes along with solution II and given along with glutamate treatment in solution III. The phosphatase inhibitors used were I-2 at 10 nM and okadaic acid (OA) at 1 µM for inhibiting PP1, as well as OA at a concentration of 1 nM, I1PP2A at 2 nM for inhibiting PP2A, and cyclosporin (CsA) at a concentration of 1 µM for inhibiting PP2B. MK-801 is the open channel blocker of NMDAR and was used at a concentration of 20 µM. After the treatment, the cells were harvested in 1 mL of 1X phosphate-buffered saline (PBS). The cells were lysed in radioimmunoprecipitation assay buffer (RIPA buffer - 50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS), and proteins were identified using BCA method. The cortical cell lysate was subjected to Western blotting to detect phospho-GluN2B-Ser1303 and GluN2B levels [14].
Hoechst staining of glutamate treated primary cortical cells to check for excitotoxic neuronal death
Primary cortical neurons were treated with glutamate, as mentioned earlier. After the treatment, glutamate was removed, and normal NBM was added to the wells, maintained at 37oC for 24 hours. After 24 hours, the medium was removed, and the cells were fixed with 4% paraformaldehyde. The cells were washed with 1X PBS, stained with 5 µg/mL Hoechst for 10 minutes, then the Hoechst stain was removed, and 1X PBS was added, and proceeded for imaging at 280 nm. The cells were examined for condensed nuclei, indicating dead cells, whereas healthy cells exhibited viable nuclei without morphological condensation. This experiment was done to check if the glutamate treatment at the given concentration can induce excitotoxicity and cell death in primary neurons.
Characterization of primary cortical neurons and immunofluorescence to check for the co-localization of PP1α with GluN2B in primary neurons
Primary neuronal cells isolated from E18 embryos were seeded on coverslips in a 24-well plate. The experiments to check the co-localization of GluN2B with PP1α were done on DIV 7. For immunocytochemistry, the cells were fixed with 250 μL of 4% paraformaldehyde at room temperature for 20 minutes after removing the medium, followed by a wash with 1X PBS. The cells were then washed thrice with 1X PBS. Next, 200 μL of blocking solution (0.3% Triton-X-100 in 5% normal goat serum (NGS) in 1X PBS) was added to cells, and the mixture was incubated at room temperature for 30 minutes. Following this, the primary antibody diluted in the corresponding blocking solution was added at a concentration of 1:100. This solution was incubated overnight at 4oC. After incubation, the cells were washed with 1X PBS thrice, and the secondary antibody was added.
For the GluN2B primary antibody, the corresponding secondary antibody used for immunofluorescence was a sheep anti-rabbit antibody conjugated with Cy3. Following removing the secondary antibody, the cells were washed thoroughly with 1X PBS, and then the mouse anti-PP1α antibody was added and incubated overnight. The secondary antibody used for immunofluorescence for the mouse anti-PP1α primary antibody was a goat anti-mouse antibody conjugated with Alexa 488. The images were taken with a confocal microscope.
Preparation of rat forebrain cortex homogenate for co-immunoprecipitation
The homogenate used for co-immunoprecipitation experiments was prepared from the rat forebrain cortex. The forebrains were homogenized in an ice-cold solution containing 320 mM sucrose and 10 mM Tris-HCl (pH-7.4), 1 mM EDTA, 1 mM EGTA, and 1X PIC with 12 strokes at 800 rpm. The large cell fragments were removed by centrifuging at 5000 rpm for about 2-3 minutes. Then, the membrane fraction was sedimented at 50000 rpm at 4oC for about 30 minutes. The obtained membrane fractions were solubilized with 1% deoxycholate in 50 mM Tris-HCl (pH-7.4), 10 mM EDTA, and 10 mM EGTA for about 20 minutes on ice at the rate of 10 mL solution per gram of rat brain. The solubilized membrane fractions were obtained in the supernatant and were used directly for immunoprecipitation or stored at -80oC as aliquots.
Co-immunoprecipitation
50 μL of protein A agarose beads were washed thrice in 1 mL 1X ice-cold PBS. These beads were subjected to blocking in 1% BSA for about 1 h at 4oC with constant agitation at 8 rpm. After removing the blocking solution, about 6 µg of anti-rabbit polyclonal antibody in 1 mL 1 X PBS was added to the beads. Correspondingly, anti-rabbit non-immune IgG as a negative control was also subjected to the same treatment. The antibody bead complex was allowed to form at about 7 h in agitation at 4oC. The previously prepared homogenate was subjected to preclearance before proceeding to immunoprecipitation. About 500 µL of homogenate was mixed with 50 µL of protein A agarose beads with agitation at 4oC for about an hour. This is done to remove the non-specific binding of homogenate to beads, if any. After 1 h, the mixture was centrifugated at 2000 rpm for 5 minutes, and the supernatant collected was subjected to immunoprecipitation. The antibody bead complex was subjected to washes with 500 µL 1X PBS, thrice by centrifuging at 2000 rpm for 4 minutes each, to remove the unbound antibody, if any. About 500 µL of precleared homogenate is added, followed by incubation for about 12-15 hours at 4oC with constant agitation. After this, the supernatant consisting of unbound proteins and the immunoprecipitated complex bound to the beads was subjected to washes with 500 µL 1X PBS with 0.1% Triton X 100 thrice, and the last wash was given without any detergent. The immunoprecipitants were denatured by boiling an equal amount of 2X SDS sample buffer and proceeded for Western blotting.
Statistical analysis
The significance of immunoblots was quantitated by one-way ANOVA followed by Scheffe’s post hoc analysis.
Results
Phosphorylation status of GluN2B-Ser1303 in cortical neurons upon glutamate treatment
The primary cortical neurons exposed to 100 µM glutamate for 60 minutes exhibited a reduction in GluN2B-Ser1303 phosphorylation, observed at about 50%, as revealed by Western blots. PP1 seems to be the main mediator of this effect, as evidenced by the effective prevention of dephosphorylation at GluN2B-Ser1303 when PP1 was inhibited by I-2 or OA at high concentration (1 µM) (Figure 1a and 1b). Treatment with MK801, an NMDAR channel blocker, also prevented the dephosphorylation, showing that the effect is mediated through NMDARs. Thus, glutamate treatment significantly changed the dephosphorylation of GluN2B-Ser1303, as evidenced by both PP1 inhibitor and MK801. When other phosphatase inhibitors were used, there was no significant change in phosphorylation levels of GluN2B at Ser1303. Notably, OA at 1 µM concentration reverses the dephosphorylation by blocking PP1. In contrast, other inhibitors like CsA, OA at 1 nM concentration, I-1, and others did not affect the dephosphorylation, indicating that the principal phosphatase involved in dephosphorylation at Ser1303 is PP1. There was no change in the level of GluN2B, indicating that the glutamate treatment results only in the shift of phosphorylation status at Ser1303 and does not affect the total protein at the receptor level. Staining with Hoechst after 24-h post-treatment of glutamate 100 µM for 60 minutes revealed the presence of dead neurons in conditions where glutamate was given to cells, whereas the control and MK 801-treated cells showed no significant death (Figure 2). This outcome indicates that glutamate treatment at the given concentration was excitotoxic.
Co-localization of PP1 and PP2A with GluN2B in primary cortical neurons
The cortical neurons were stained for the proteins GluN2B and PP1. Immunocytochemical staining showed co-localization of PP1 with GluN2B in primary cortical neurons (Figure 3), indicating that PP1 could access the phosphorylation site. Immunoprecipitation of PP1 But Not PP2A using GluN2B antibody
Immunoprecipitation studies were conducted to study whether PP1 and PP2A would interact with GluN2B in rat brain homogenate. Deoxycholate-solubilized homogenate was immunoprecipitated with rabbit polyclonal GluN2B antibody, previously bound to protein-A-agarose beads. Immunoprecipitation with GluN2B antibody resulted in the co-precipitation of only PP1, not PP2A (Figure 4). As a control, a non-immune IgG was used in the immunoprecipitation. GluN2B, PP1, and PP2A were detected by Western blotting using respective antibodies. The immunoprecipitation with GluN2B antibody showed reactivity with PP1 but not PP2A. The immunoreactivity band for all the proteins is absent in the control lane.
Discussion
Phosphorylation, as a post translational mechanism, induces covalent changes in the properties of a protein [15], thereby regulating the cellular processes such as activity, localization, and mobility of proteins, especially receptors. Regarding GluN2B, calcium influx through either NMDAR or voltage gate calcium channels activates the phosphorylation at Ser1303 [16]. Still, the mechanism of dephosphorylation has remained unclear in vitro or in vivo except for a study suggesting that overexpression of PP1 in hippocampal neurons may inhibit NMDAR activation, a possible pathway for neuroprotection [17].
Our previous studies have shown that phosphorylation of GluN2B-Ser1303 is regulated by phosphatases in PSD, especially by PP1 [13]. The current research shows that PP1 is the principal phosphatase responsible for the regulation of phosphorylation at GluN2B-Ser1303 in primary cortical neurons under excitotoxic conditions, consistent with our observations in the case of PSD. Inhibition of calcium entry via NMDAR results in the survival of neurons, indicating that the cell death is mediated by glutamate treatment at a concentration of 100 µM. Therefore, reduction in phosphorylation at GluN2B-Ser1303 could be an outcome of the excitotoxic event, resembling observations of GluN2B-Ser1303 phosphorylation reduction in patients with depression-like disorders showing a modulation of glutamatergic transmission [18].
Importantly, no change is observed in the total receptor levels, GluN2B, during glutamate treatment; only changes in phosphorylation levels at the Ser1303 site, as indicated by Western blots. This alteration in phosphorylation is inhibited only when inhibitors of PP1, such as okadaic acid at high concentration and I-2, are used, indicating that the principal phosphatase involved in phosphorylation of Ser1303 is PP1, but not other phosphatases like PP2A or PP2B. Compared to other Ser-Thr phosphatases, PP1 might have access to phosphorylation at GluN2B-Ser1303 as it is colocalized with the latter, as seen in in vitro staining and co-immunoprecipitation studies. Okadaic acid at 1 nM, used to inhibit PP2A, showed a slightly enhanced inhibition of phosphorylation at GluN2B- Ser1303, possibly inhibiting PP1 to some extent. However, the specific inhibitor of PP2A, I1PP2A, failed to reverse dephosphorylation, indicating that PP2A is not involved in the dephosphorylation of GluN2B- Ser1303. Further, in vivo, studies need to be carried out to validate the observations found in these studies and previous studies, which show that PP1 is responsible for regulating the phosphorylation of GluN2B-Ser1303 in both PSD and primary neurons in vitro.
Conclusion
Our study focussed on the effect of glutamate treatment on cortical neurons isolated from embryonic rat brains, primarily excitatory. Activating these primary neurons in vitro with 100 µM glutamate for 60 minutes resulted in excitotoxicity as visualized by cell death by Hoechst staining and a significant reduction in GluN2B-Ser1303 phosphorylation. Using phosphatase inhibitors like I-2, we showed that PP1 regulated the reduction of GluN2B-Ser1303 phosphorylation in primary cortical neurons upon glutamate treatment, indicating its major phosphatase involved in the regulation of the latter under excitotoxic conditions, with no discernible role for other phosphatases. This study lays the groundwork for future in vivo experiments to explore the regulation of phosphorylation at the GluN2B receptor, especially at the Ser1303 site, which PP1 regulates. The identified site could be a possible drug target for inhibiting excitotoxicity. Further studies need to be conducted in search of novel inhibitors of this target and their potential roles in mitigating the excitotoxicity induced by glutamate.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were done in compliance with the ethical guidelines of the Declaration of Helsinki 2013. This study was also approved by the Rajiv Gandhi Centre for Biotechnology (RGCB) (Code: IAEC/112/OMK/2010).
Funding
This study is financially supported by Rajiv Gandhi Centre for Biotechnology and the fellowship received from Council of scientific and industrial research, Government of India (CSIR).
Conflict of interest
The author declared no conflict of interest.
Acknowledgements
The author thanks the PhD supervisor, director, faculty, staff, and students at both RGCB and Government Arts College for the support extended in designing and carrying out the work.
References
- Jansson LC, Åkerman KE. The role of glutamate and its receptors in the proliferation, migration, differentiation and survival of neural progenitor cells. J Neural Transm (Vienna). 2014; 121(8):819-36. [DOI:10.1007/s00702-014-1174-6] [PMID]
- Zhou Y, Danbolt NC. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm (Vienna). 2014; 121(8):799-817. [DOI:10.1007/s00702-014-1180-8] [PMID]
- Blanke ML, VanDongen AM. Chapter 13 Activation Mechanisms of the NMDA Receptor. In: Van Dongen AM, editor. Biology of the NMDA Receptor. Boca Raton: CRC Press/Taylor & Francis; 2009. [PMID]
- Kampa BM, Clements J, Jonas P, Stuart GJ. Kinetics of Mg2+ unblock of NMDA receptors: Implications for spike-timing dependent synaptic plasticity. J Physiol. 2004; 556(Pt 2):337-45. [DOI:10.1113/jphysiol.2003.058842] [PMID]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013; 14(6):383-400. [DOI:10.1038/nrn3504] [PMID]
- Shipton OA, Paulsen O. GluN2A and GluN2B subunit-containing NMDA receptors in hippocampal plasticity. Philos Trans R Soc Lond B Biol Sci. 2013; 369(1633):20130163. [DOI:10.1098/rstb.2013.0163] [PMID]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003; 97(1):55-85. [DOI:10.1016/S0163-7258(02)00302-9] [PMID]
- Tullis JE, Buonarati OR, Coultrap SJ, Bourke AM, Tiemeier EL, Kennedy MJ, et al. GluN2B S1303 phosphorylation by CaMKII or DAPK1: No indication for involvement in ischemia or LTP. iScience. 2021; 24(10): 103214. [DOI:10.1016/j.isci.2021.103214] [PMID]
- Kim N, Chen D, Zhou XZ, Lee TH. Death-associated protein kinase 1 phosphorylation in neuronal cell death and neurodegenerative disease. Int J Mol Sci. 2019; 20(13):3131. [DOI:10.3390/ijms20133131] [PMID]
- Zhou XL, Zhang CJ, Peng YN, Wang Y, Xu HJ, Liu CM. ROR2 modulates neuropathic pain via phosphorylation of NMDA receptor subunit GluN2B in rats.. Br J Anaesth. 2019; 123(2):e239-48. [DOI:10.1016/j.bja.2018.08.025] [PMID]
- Sanz-Clemente A, Nicoll RA, Roche KW. Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist. 2013; 19(1):62-75. [DOI:10.1177/1073858411435129] [PMID]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007; 53(3):362-8. [DOI:10.1016/j.neuropharm.2007.05.018] [PMID]
- Prabhu Ramya R, Suma Priya S, Mayadevi M, Omkumar RV. Regulation of phosphorylation at Ser(1303) of GluN2B receptor in the postsynaptic density. Neurochem Int. 2012; 61(7):981-5. [DOI:10.1016/j.neuint.2012.08.016] [PMID]
- Remya C, Dileep KV, Koti Reddy E, Mantosh K, Lakshmi K, Sarah Jacob R, et al. Neuroprotective derivatives of tacrine that target NMDA receptor and acetyl cholinesterase-design, synthesis and biological evaluation. Comput Struct Biotechnol J. 2021; 19:4517-37. [DOI:10.1016/j.csbj.2021.07.041] [PMID]
- Ramazi S, Zahiri J. Posttranslational modifications in proteins: Resources, tools and prediction methods. Database (Oxford). 2021; 2021:baab012. [DOI:10.1093/database/baab012] [PMID]
- Kumar M, John M, Madhavan M, James J, Omkumar RV. Alteration in the phosphorylation status of NMDA receptor GluN2B subunit by activation of both NMDA receptor and L-type voltage gated calcium channel. Neurosci Lett. 2019; 709:134343. [DOI:10.1016/j.neulet.2019.134343] [PMID]
- Farinelli M, Heitz FD, Grewe BF, Tyagarajan SK, Helmchen F, Mansuy IM. Selective regulation of NR2B by protein phosphatase-1 for the control of the NMDA receptor in neuroprotection. PloS One. 2012; 7(3):e34047. [DOI:10.1371/journal.pone.0034047] [PMID]
- Fumagalli F, Pasini M, Sartorius A, Scherer R, Racagni G, Riva MA, et al. Repeated electroconvulsive shock (ECS) alters the phosphorylation of glutamate receptor subunits in the rat hippocampus. Int J Neuropsychopharmacol. 2010; 13(9):1255-60. [DOI:10.1017/S1461145710000544] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2023/10/11 | Accepted: 2023/10/17 | Published: 2023/10/17
Received: 2023/10/11 | Accepted: 2023/10/17 | Published: 2023/10/17
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |