Sat, May 18, 2024
Volume 9, Issue 4 (Autumn 2023)
Caspian J Neurol Sci 2023, 9(4): 244-251 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Razazian N, Sahraian M, Eskandarieh S, Jafari N, Rezaei M, Fakhri N. Follow-up of Patients With Multiple Sclerosis After COVID-19 Vaccination With Sinopharm Vaccine. Caspian J Neurol Sci 2023; 9 (4) :244-251
URL: http://cjns.gums.ac.ir/article-1-666-en.html
URL: http://cjns.gums.ac.ir/article-1-666-en.html
Nazanin Razazian * 

 1, Mohammad-Ali Sahraian2
1, Mohammad-Ali Sahraian2 

 , Sharareh Eskandarieh2
, Sharareh Eskandarieh2 

 , Nooshin Jafari1
, Nooshin Jafari1 

 , Mansour Rezaei3
, Mansour Rezaei3 

 , Negin Fakhri4
, Negin Fakhri4 




 1, Mohammad-Ali Sahraian2
1, Mohammad-Ali Sahraian2 

 , Sharareh Eskandarieh2
, Sharareh Eskandarieh2 

 , Nooshin Jafari1
, Nooshin Jafari1 

 , Mansour Rezaei3
, Mansour Rezaei3 

 , Negin Fakhri4
, Negin Fakhri4 


1- Department of Neurology, Neuroscience Research Center, Imam Reza Hospital, Kermanshah University of Medical Sciences, Kermanshah, Iran
2- Multiple Sclerosis Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran
3- Social Development and Health Promotion Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
4- Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran
2- Multiple Sclerosis Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran
3- Social Development and Health Promotion Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
4- Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran
Full-Text [PDF 1136 kb]
(123 Downloads)
| Abstract (HTML) (294 Views)
Full-Text: (70 Views)
Introduction
Multiple sclerosis (MS) is the most common non-traumatic debilitating disease [1] that affects young adults [2]. MS is a chronic inflammatory disorder of the central nervous system in which neuroaxonal damage contributes to clinical events and prognosis [3]. In late 2019, the highly transmissible RNA virus SARS-CoV-2 (coronavirus 2019 [COVID-19]) spread in China [4]. The coronavirus, the causative agent of human COVID-19, has been identified by the World Health Organization (WHO) as a pandemic that has spread rapidly in many parts of the world [5]. Among people with COVID-19, people with chronic diseases need special attention [6]. Ghayeghran et al. reported the frequency of COVID-19 infection in MS patients as 12.6% [7]. Immunosuppressive therapies for MS are associated with an increased risk of infection [8], and infectious diseases contribute significantly to the complications of MS [4]. Sinopharm is a COVID-19-inactivated vaccine developed by Sinopharm’s Beijing Institute of Biological Products and approved by the United Arab Emirates [9]. Although people with MS (PWMS) should be vaccinated against COVID-19 [10], the risks and benefits must be considered. There is an urgent need for further research on vaccination in PWMS to help with evidence-based decision-making [11]. Considering the increasing prevalence of MS in Kermanshah Province, Iran during the last decade [2], we aimed to follow up PWMS and evaluate the relapse of MS and COVID-19 infection within 3 months after both doses of the Sinopharm vaccine in Kermanshah, Iran.
Materials and Methods
Study design and research community
This study was a case series and analytical study conducted between August and November 2021. The study coincided with the fifth peak of COVID-19 in Iran. Sampling was conducted using the Nationwide MS Registry of Iran [12]. The study population consisted of all PWMS living in Kermanshah who received two doses of the Sinopharm vaccine. The inclusion criteria were as follows: Definitive diagnosis of MS based on the latest McDonald criteria by a neurologist, being over 18 years of age, and injection of both the Sinopharm vaccine doses. Refusing to answer the phone and dissatisfaction with participating in the study were the exclusion criteria. Before injecting the first dose of the vaccine, the information of all PWMS in Kermanshah Province from the nationwide MS registry of Iran was prepared and provided to the deputy director of treatment and the MS Clinic for advice on whether they should receive the vaccine. After vaccination, a list of PWMS who received the Sinopharm vaccine was sent to researchers through the MS Clinic. After the second dose of the Sinopharm vaccine, PWMS was followed for 3 months.
Data collection tools and methods
The data collection tool was a researcher-made form, consisting of 3 parts. In the first part, the patients’ demographic information and clinical characteristics like gender, age, marital status, age of diagnosis, type of MS, and MS medicine were collected. Participants were asked about COVID-19 infection within 3 months after the second vaccination dose. If there was an infection, the time interval between the infection and the injection of the vaccine, hospitalization, the severity of symptoms, and the type of MS medication used at the time of vaccination was recorded. Infection with COVID-19 was confirmed if the person has symptoms of COVID-19 and the result of the COVID-19 test of the person himself or his family members who lived in the same house was positive. Participants were asked about MS relapses within 3 months after the second vaccination dose in the third part of the survey. If there was a relapse, the interval between relapse with vaccine injections, the need for corticosteroids, and the need for hospitalization were asked, as well as the name of the MS medication used at the time of vaccination. In addition, information about hospitalizations and thrombosis was also collected within 3 months after the second dose. The information was obtained by telephone [13].
Data analysis
The collected data were entered into SPSS software, version 25 and analyzed according to the project objectives. Descriptive statistics such as mean, median, interquartile range, frequency, and percentage were used. Using the McNemar test, the frequency before and after the vaccination was compared after checking the data’s normality by the Kolmogorov-Smirnov test. Mann-Whitney, Fisher Exact, and chi-square tests were used to compare means and frequencies.
Results
The study evaluated 197 PWMS who had been vaccinated with the Sinopharm. The Mean±SD age of PWMS was 41.84±11.0 years (age range: 21-79 years). A total of 155 PWMS (78.7%) were female. More than 42% of the PWMS had a college education, and 74.4% were married. The Mean±SD number of years since people were diagnosed with MS was 9.57±7.0, and 142 people (73.6%) had RR-type MS. Before vaccination, 49 people (25.1%) had been infected with COVID-19 (Table 1).

Both vaccine doses were injected for all people. Out of 197 PWMS, for 10 people (5.1%), two doses were injected 28 days apart, but in others, the doses were more than 28 days apart. The Mean±SD interval between injections of both vaccine doses was 47.91±20.6, ranging from 28 to 152 days.
The results showed that 15 people (7.6%) had a relapse of MS within 3 months before the first dose of the vaccine, and 15 patients (7.6%) had a relapse of MS within 3 months after the second dose. There was no difference in MS relapse rates between these two time points (P=1.000). Among 15 PWMS who experienced a relapse after the second dose, most were treated with oral and anti-CD20 therapy and had motor and sensory symptoms during relapses. Relapses of MS that occurred up to 3 months after the second dose, required corticosteroids in 8 patients (53.3%) and led to hospitalization in 5(33.3%). There were no significant differences in age, disease duration, gender, and type of MS between those who had a relapse between the 3 months after the second dose and those who did not (P>0.05). Consumption of oral and anti-CD20 treatments (rituximab, ocrelizumab, fingolimod, giomide) was significantly (P=0.046) higher among those with a recurrence after the second dose compared to those without (Table 2).

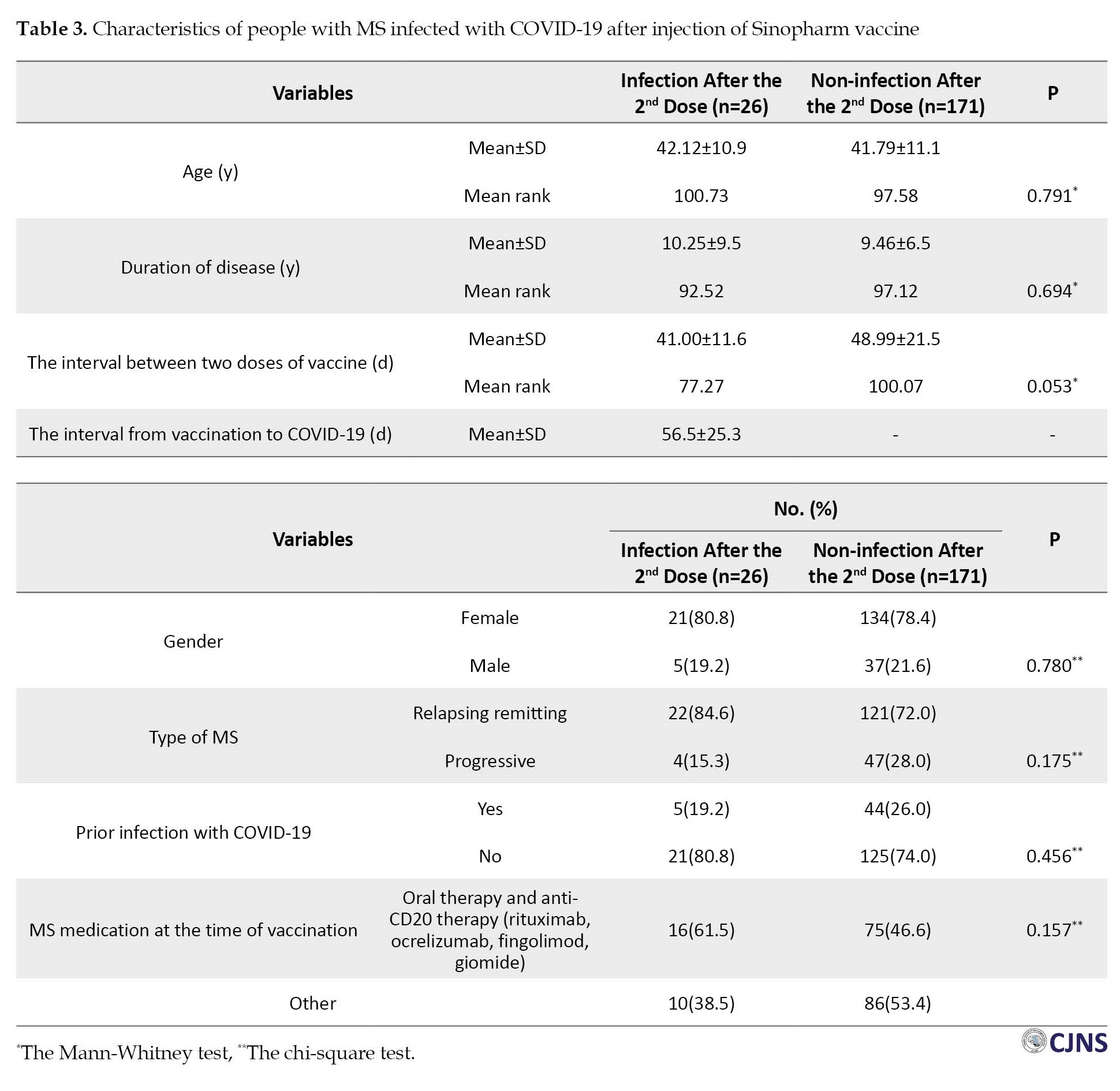
Within 3 months before the first dose of the vaccine, 12 PWMS (6.1%) had been infected with COVID-19, and within 3 months after the second dose, 26 PWMS (13.2%) became infected with COVID-19. The frequency of COVID-19 during the 3 months after the second dose of the vaccine was significantly higher than in the three months before the first dose (P=0.017). There were no significant differences (P>0.05) between those who had a recurrence within 3 months after the second dose and those who did not regarding age, disease duration, interval between two doses of vaccine, gender, type of MS, or prior infection with COVID-19. Although not significant (P=0.157), those infected with COVID-19 after the second dose consumed more oral and anti-CD20 treatments than those without (Table 3).
Within 3 months after the second dose of the vaccine, only 3 PWMS (11.5%) developed severe symptoms of COVID-19 and were hospitalized. The remaining 23 people (88.5%) had mild to moderate symptoms and did not need hospitalization (severe cases; people with severe symptoms such as poor breathing and requiring immediate medical assistance).
Out of the 26 PWMS who became infected after the second dose, 16(61.5%) were treated with oral and anti-CD20 therapy (rituximab, ocrelizumab, fingolimod, giomide), and only 3(11.5%) had severe COVID-19 symptoms requiring hospitalization.
In those who became infected within 3 months after receiving the second dose, 5 people (19.2%) already had COVID-19 before vaccination; their symptoms were mild and did not require hospitalization. The drugs used by these 5 were as follows: 2 people Copamer, 1 Rituximab, and 2 without medicine.
Among 197 PWMS who received two doses of the vaccine, no cases of thrombosis were observed among PWMS within 3 months after the second dose.
Discussion
In the present study, after receiving the Sinopharm vaccine, PWMS were followed up for 3 months. MS relapses and COVID-19 infections in these people were compared within 3 months before the first dose and 3 months after the second dose. The relapse of MS in PWMS showed no difference between these two time points (15 patients at each time point).
Kavosh et al. reported MS relapse after COVID-19 vaccination in 23 patients, one had two relapses, one after each vaccine dose [14]. Although a systematic review showed increased MS relapse after vaccination, the review advised continuing vaccination in PWMS regarding the benefits of vaccination [15]. In contrast to these studies, in another study on 583 PWMS after vaccination, only 0.9% experienced a relapse [16], or the risk of relapse is low either with infection or vaccination [17].
Razazian et al.’s study showed no significant difference in MS relapse rate within the 3 months before and after the vaccination with the AstraZeneca vaccine [18]. Similarly, Filippo et al. [19] found that the number of relapses before and after vaccination (6 and 7 cases, respectively) was not significantly different. We found that motor and sensory symptoms were the most common in MS relapses after the second dose. Lotan et al. [20] also found that most people who reported new or worsening neurological symptoms after vaccination had sensory and motor symptoms. Our study found that out of 15 PWMS who relapsed after the second dose, 7(46.7%) took oral medicine for MS, including fingolimod, dimethyl fumarate, and teriflunomide. Fragoso et al. [21] reported that among patients with MS who had relapsed after vaccination with the AZD1222 vaccine, 4 out of 8 patients (50%) took oral medicines for MS (among them two people took fingolimod, 1 person took dimethyl fumarate and one person took teriflunomide), which is consistent with our findings.
The present study revealed that out of 197 PWMS, 26(13.2%) became infected with COVID-19 after receiving the Sinopharm vaccine. The incidence of COVID-19 infection within 3 months after the second dose was significantly higher than 3 months before the first dose. In contrast to our study, the results of Sedighi et al.’s study [22] showed that the incidence of COVID-19 was much higher before vaccination (24.5% before vaccination versus 10.1% after vaccination). There are some noteworthy points. First of all, in our study, 3 months following the injection of the second dose coincided with the time when the spread of COVID-19 in Kermanshah and other cities of Iran was very high again. During a period of about 3 months immediately before the vaccination, i.e. from March 15, 2021, to June 7, 2021, the incidence of COVID-19 infections in Iran was 1281162, as well as in the 3 months immediately following the second dose, i.e. from July 19, 2021, to October 11, 2021, when the incidence of COVID-19 infections in Iran was 2272340 [23]. Secondly, within 3 months after the second dose, people may have followed health protocols less strictly. We can say that the higher incidence of COVID-19 in our study could also be attributed to non-compliance with health protocols. Thirdly, the interval between two vaccine doses in our study was longer than the standard interval for most people. This latency can lead to a reduction in the effectiveness of the vaccine within three months after the second dose.
In our study, most individuals infected with COVID-19 after vaccination reported mild symptoms and did not require hospitalization. Also, most people with a family history of COVID-19 reported milder symptoms 3 months after immunization compared to their family members who had not been vaccinated. It can be concluded that the Sinopharm vaccine prevents severe symptoms, as Mehrotra et al. [24] suggested that a shift toward asymptomatic COVID-19 may accompany vaccination against COVID-19 infections.
Our study showed that 16 of the 26 PWMS who developed COVID-19 infection after the second dose were treated with oral and anti-CD20 therapy. This finding is consistent with the results of a study by Garjani et al. [25], who reported that PWMS receiving fingolimod and ocrelizumab were less resistant to COVID-19 infection. In addition, Tallantyre et al. [26] reported that some disease-modifying therapies are associated with the risk of attenuated serological response to COVID-19 vaccination in PWMS.
Conclusion
According to this study, there was no difference in MS relapse frequency before and after vaccination with the Sinopharm vaccine. Most PWMS who relapsed after vaccination received oral therapy and anti-CD20 therapy. The Sinopharm vaccine also prevented the severe symptoms of COVID-19. Even though the number of people with COVID-19 after vaccination was high, their diseases were not as severe. Most PWMS who developed COVID-19 infection after vaccination received oral therapy and anti-CD20 medication.
Ethical Considerations
Compliance with ethical guidelines
The study process was in compliance with the ethical guidelines of the Declaration of Helsinki 2013. The Ethics Committee of Kermanshah University of Medical Sciences approved this study (Code: IR.KUMS.MED.REC.1400.103). Consent to participate in the study was obtained with clear explanations of the study’s objectives and the preservation of their information. In addition, they were assured that the obtained data would be used for research purposes. Every participant was assigned a code or number on all forms and questions.
Funding
This article is part of residency thesis of Nooshin Jafari, approved by Department of Neurology, Kermanshah University of Medical Sciences (Code: 50000693) and was sponsored by the Deputy for Research and Technology of Kermanshah University of Medical Sciences.
Authors contributions
Conceptualization and project administration: Mohammad Ali Sahraeian, Sharareh Eskandarieh and Nazanin Razazian; Methodology: Mansour Rezaei; Visiting patients: Nazanin Razazian and Nooshin Jafari; Questionnaire completion, writing and review: Nooshin Jafari; Data collection, software and writing original draft: Negin Fakhri; Formal analysis: Mansour Rezaei, Negin Fakhri and Mansour Rezaei; Editing: Mansour Rezaei, Mohammad Ali Sahraeian, Sharareh Eskandarieh and Nazanin Razazian.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We acknowledge the support of the Deputy for Research and Technology of Kermanshah University of Medical Sciences and the Clinical Research Development Center of Imam Reza Hospital.
References
Multiple sclerosis (MS) is the most common non-traumatic debilitating disease [1] that affects young adults [2]. MS is a chronic inflammatory disorder of the central nervous system in which neuroaxonal damage contributes to clinical events and prognosis [3]. In late 2019, the highly transmissible RNA virus SARS-CoV-2 (coronavirus 2019 [COVID-19]) spread in China [4]. The coronavirus, the causative agent of human COVID-19, has been identified by the World Health Organization (WHO) as a pandemic that has spread rapidly in many parts of the world [5]. Among people with COVID-19, people with chronic diseases need special attention [6]. Ghayeghran et al. reported the frequency of COVID-19 infection in MS patients as 12.6% [7]. Immunosuppressive therapies for MS are associated with an increased risk of infection [8], and infectious diseases contribute significantly to the complications of MS [4]. Sinopharm is a COVID-19-inactivated vaccine developed by Sinopharm’s Beijing Institute of Biological Products and approved by the United Arab Emirates [9]. Although people with MS (PWMS) should be vaccinated against COVID-19 [10], the risks and benefits must be considered. There is an urgent need for further research on vaccination in PWMS to help with evidence-based decision-making [11]. Considering the increasing prevalence of MS in Kermanshah Province, Iran during the last decade [2], we aimed to follow up PWMS and evaluate the relapse of MS and COVID-19 infection within 3 months after both doses of the Sinopharm vaccine in Kermanshah, Iran.
Materials and Methods
Study design and research community
This study was a case series and analytical study conducted between August and November 2021. The study coincided with the fifth peak of COVID-19 in Iran. Sampling was conducted using the Nationwide MS Registry of Iran [12]. The study population consisted of all PWMS living in Kermanshah who received two doses of the Sinopharm vaccine. The inclusion criteria were as follows: Definitive diagnosis of MS based on the latest McDonald criteria by a neurologist, being over 18 years of age, and injection of both the Sinopharm vaccine doses. Refusing to answer the phone and dissatisfaction with participating in the study were the exclusion criteria. Before injecting the first dose of the vaccine, the information of all PWMS in Kermanshah Province from the nationwide MS registry of Iran was prepared and provided to the deputy director of treatment and the MS Clinic for advice on whether they should receive the vaccine. After vaccination, a list of PWMS who received the Sinopharm vaccine was sent to researchers through the MS Clinic. After the second dose of the Sinopharm vaccine, PWMS was followed for 3 months.
Data collection tools and methods
The data collection tool was a researcher-made form, consisting of 3 parts. In the first part, the patients’ demographic information and clinical characteristics like gender, age, marital status, age of diagnosis, type of MS, and MS medicine were collected. Participants were asked about COVID-19 infection within 3 months after the second vaccination dose. If there was an infection, the time interval between the infection and the injection of the vaccine, hospitalization, the severity of symptoms, and the type of MS medication used at the time of vaccination was recorded. Infection with COVID-19 was confirmed if the person has symptoms of COVID-19 and the result of the COVID-19 test of the person himself or his family members who lived in the same house was positive. Participants were asked about MS relapses within 3 months after the second vaccination dose in the third part of the survey. If there was a relapse, the interval between relapse with vaccine injections, the need for corticosteroids, and the need for hospitalization were asked, as well as the name of the MS medication used at the time of vaccination. In addition, information about hospitalizations and thrombosis was also collected within 3 months after the second dose. The information was obtained by telephone [13].
Data analysis
The collected data were entered into SPSS software, version 25 and analyzed according to the project objectives. Descriptive statistics such as mean, median, interquartile range, frequency, and percentage were used. Using the McNemar test, the frequency before and after the vaccination was compared after checking the data’s normality by the Kolmogorov-Smirnov test. Mann-Whitney, Fisher Exact, and chi-square tests were used to compare means and frequencies.
Results
The study evaluated 197 PWMS who had been vaccinated with the Sinopharm. The Mean±SD age of PWMS was 41.84±11.0 years (age range: 21-79 years). A total of 155 PWMS (78.7%) were female. More than 42% of the PWMS had a college education, and 74.4% were married. The Mean±SD number of years since people were diagnosed with MS was 9.57±7.0, and 142 people (73.6%) had RR-type MS. Before vaccination, 49 people (25.1%) had been infected with COVID-19 (Table 1).

Both vaccine doses were injected for all people. Out of 197 PWMS, for 10 people (5.1%), two doses were injected 28 days apart, but in others, the doses were more than 28 days apart. The Mean±SD interval between injections of both vaccine doses was 47.91±20.6, ranging from 28 to 152 days.
The results showed that 15 people (7.6%) had a relapse of MS within 3 months before the first dose of the vaccine, and 15 patients (7.6%) had a relapse of MS within 3 months after the second dose. There was no difference in MS relapse rates between these two time points (P=1.000). Among 15 PWMS who experienced a relapse after the second dose, most were treated with oral and anti-CD20 therapy and had motor and sensory symptoms during relapses. Relapses of MS that occurred up to 3 months after the second dose, required corticosteroids in 8 patients (53.3%) and led to hospitalization in 5(33.3%). There were no significant differences in age, disease duration, gender, and type of MS between those who had a relapse between the 3 months after the second dose and those who did not (P>0.05). Consumption of oral and anti-CD20 treatments (rituximab, ocrelizumab, fingolimod, giomide) was significantly (P=0.046) higher among those with a recurrence after the second dose compared to those without (Table 2).

Within 3 months before the first dose of the vaccine, 12 PWMS (6.1%) had been infected with COVID-19, and within 3 months after the second dose, 26 PWMS (13.2%) became infected with COVID-19. The frequency of COVID-19 during the 3 months after the second dose of the vaccine was significantly higher than in the three months before the first dose (P=0.017). There were no significant differences (P>0.05) between those who had a recurrence within 3 months after the second dose and those who did not regarding age, disease duration, interval between two doses of vaccine, gender, type of MS, or prior infection with COVID-19. Although not significant (P=0.157), those infected with COVID-19 after the second dose consumed more oral and anti-CD20 treatments than those without (Table 3).

Within 3 months after the second dose of the vaccine, only 3 PWMS (11.5%) developed severe symptoms of COVID-19 and were hospitalized. The remaining 23 people (88.5%) had mild to moderate symptoms and did not need hospitalization (severe cases; people with severe symptoms such as poor breathing and requiring immediate medical assistance).
Out of the 26 PWMS who became infected after the second dose, 16(61.5%) were treated with oral and anti-CD20 therapy (rituximab, ocrelizumab, fingolimod, giomide), and only 3(11.5%) had severe COVID-19 symptoms requiring hospitalization.
In those who became infected within 3 months after receiving the second dose, 5 people (19.2%) already had COVID-19 before vaccination; their symptoms were mild and did not require hospitalization. The drugs used by these 5 were as follows: 2 people Copamer, 1 Rituximab, and 2 without medicine.
Among 197 PWMS who received two doses of the vaccine, no cases of thrombosis were observed among PWMS within 3 months after the second dose.
Discussion
In the present study, after receiving the Sinopharm vaccine, PWMS were followed up for 3 months. MS relapses and COVID-19 infections in these people were compared within 3 months before the first dose and 3 months after the second dose. The relapse of MS in PWMS showed no difference between these two time points (15 patients at each time point).
Kavosh et al. reported MS relapse after COVID-19 vaccination in 23 patients, one had two relapses, one after each vaccine dose [14]. Although a systematic review showed increased MS relapse after vaccination, the review advised continuing vaccination in PWMS regarding the benefits of vaccination [15]. In contrast to these studies, in another study on 583 PWMS after vaccination, only 0.9% experienced a relapse [16], or the risk of relapse is low either with infection or vaccination [17].
Razazian et al.’s study showed no significant difference in MS relapse rate within the 3 months before and after the vaccination with the AstraZeneca vaccine [18]. Similarly, Filippo et al. [19] found that the number of relapses before and after vaccination (6 and 7 cases, respectively) was not significantly different. We found that motor and sensory symptoms were the most common in MS relapses after the second dose. Lotan et al. [20] also found that most people who reported new or worsening neurological symptoms after vaccination had sensory and motor symptoms. Our study found that out of 15 PWMS who relapsed after the second dose, 7(46.7%) took oral medicine for MS, including fingolimod, dimethyl fumarate, and teriflunomide. Fragoso et al. [21] reported that among patients with MS who had relapsed after vaccination with the AZD1222 vaccine, 4 out of 8 patients (50%) took oral medicines for MS (among them two people took fingolimod, 1 person took dimethyl fumarate and one person took teriflunomide), which is consistent with our findings.
The present study revealed that out of 197 PWMS, 26(13.2%) became infected with COVID-19 after receiving the Sinopharm vaccine. The incidence of COVID-19 infection within 3 months after the second dose was significantly higher than 3 months before the first dose. In contrast to our study, the results of Sedighi et al.’s study [22] showed that the incidence of COVID-19 was much higher before vaccination (24.5% before vaccination versus 10.1% after vaccination). There are some noteworthy points. First of all, in our study, 3 months following the injection of the second dose coincided with the time when the spread of COVID-19 in Kermanshah and other cities of Iran was very high again. During a period of about 3 months immediately before the vaccination, i.e. from March 15, 2021, to June 7, 2021, the incidence of COVID-19 infections in Iran was 1281162, as well as in the 3 months immediately following the second dose, i.e. from July 19, 2021, to October 11, 2021, when the incidence of COVID-19 infections in Iran was 2272340 [23]. Secondly, within 3 months after the second dose, people may have followed health protocols less strictly. We can say that the higher incidence of COVID-19 in our study could also be attributed to non-compliance with health protocols. Thirdly, the interval between two vaccine doses in our study was longer than the standard interval for most people. This latency can lead to a reduction in the effectiveness of the vaccine within three months after the second dose.
In our study, most individuals infected with COVID-19 after vaccination reported mild symptoms and did not require hospitalization. Also, most people with a family history of COVID-19 reported milder symptoms 3 months after immunization compared to their family members who had not been vaccinated. It can be concluded that the Sinopharm vaccine prevents severe symptoms, as Mehrotra et al. [24] suggested that a shift toward asymptomatic COVID-19 may accompany vaccination against COVID-19 infections.
Our study showed that 16 of the 26 PWMS who developed COVID-19 infection after the second dose were treated with oral and anti-CD20 therapy. This finding is consistent with the results of a study by Garjani et al. [25], who reported that PWMS receiving fingolimod and ocrelizumab were less resistant to COVID-19 infection. In addition, Tallantyre et al. [26] reported that some disease-modifying therapies are associated with the risk of attenuated serological response to COVID-19 vaccination in PWMS.
Conclusion
According to this study, there was no difference in MS relapse frequency before and after vaccination with the Sinopharm vaccine. Most PWMS who relapsed after vaccination received oral therapy and anti-CD20 therapy. The Sinopharm vaccine also prevented the severe symptoms of COVID-19. Even though the number of people with COVID-19 after vaccination was high, their diseases were not as severe. Most PWMS who developed COVID-19 infection after vaccination received oral therapy and anti-CD20 medication.
Ethical Considerations
Compliance with ethical guidelines
The study process was in compliance with the ethical guidelines of the Declaration of Helsinki 2013. The Ethics Committee of Kermanshah University of Medical Sciences approved this study (Code: IR.KUMS.MED.REC.1400.103). Consent to participate in the study was obtained with clear explanations of the study’s objectives and the preservation of their information. In addition, they were assured that the obtained data would be used for research purposes. Every participant was assigned a code or number on all forms and questions.
Funding
This article is part of residency thesis of Nooshin Jafari, approved by Department of Neurology, Kermanshah University of Medical Sciences (Code: 50000693) and was sponsored by the Deputy for Research and Technology of Kermanshah University of Medical Sciences.
Authors contributions
Conceptualization and project administration: Mohammad Ali Sahraeian, Sharareh Eskandarieh and Nazanin Razazian; Methodology: Mansour Rezaei; Visiting patients: Nazanin Razazian and Nooshin Jafari; Questionnaire completion, writing and review: Nooshin Jafari; Data collection, software and writing original draft: Negin Fakhri; Formal analysis: Mansour Rezaei, Negin Fakhri and Mansour Rezaei; Editing: Mansour Rezaei, Mohammad Ali Sahraeian, Sharareh Eskandarieh and Nazanin Razazian.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We acknowledge the support of the Deputy for Research and Technology of Kermanshah University of Medical Sciences and the Clinical Research Development Center of Imam Reza Hospital.
References
- Dobson R, Giovannoni G. Multiple sclerosis-a review. Eur J Neurol. 2019; 26(1):27-40. [DOI:10.1111/ene.13819] [PMID]
- Razazian N, Eskandarieh S, Siabani S, Afshari D, Sahraian MA, Khezri O, et al. Prevalence of multiple sclerosis and its clinical and demographic characteristics in Kurdish populations in western Iran (2020). Mult Scler Relat Disord. 2022; 57:103441. [DOI:10.1016/j.msard.2021.103441] [PMID]
- Bittner S, Oh J, Havrdová EK, Tintoré M, Zipp F. The potential of serum neurofilament as biomarker for multiple sclerosis. Brain. 2021; 144(10):2954-63. [DOI:10.1093/brain/awab241] [PMID] [PMCID]
- Monschein T, Hartung HP, Zrzavy T, Barnett M, Boxberger N, Berger T, et al. Vaccination and multiple sclerosis in the era of the COVID-19 pandemic. J Neurol Neurosurg Psychiatry. 2021; 92(10):1033-43. [DOI:10.1136/jnnp-2021-326839] [PMID] [PMCID]
- Mansoor S, Kelly S, Murphy K, Waters A, Siddiqui NS. COVID-19 pandemic and the risk of infection in multiple sclerosis patients on disease modifying therapies: “What the bleep do we know?”. Egypt J Neurol Psychiatr Neurosurg. 2020; 56(1):44. [DOI:10.1186/s41983-020-00177-0] [PMID] [PMCID]
- Laroni A, Schiavetti I, Sormani MP, Uccelli A. COVID-19 in patients with multiple sclerosis undergoing disease-modifying treatments. Mult Scler. 2021; 27(14):2126-36. [DOI:10.1177/1352458520971817] [PMID]
- Ghayeghran A, Ghahramani E, Saberi A, Hatamian H, Homaie Rad E, Ghorbani Shirkouhi S, et al. COVID-19 infection and seropositivity in multiple sclerosis patients in Guilan in 2021. Caspian J Neurol Sci. 2023; 9(1):39-49. [DOI:10.32598/CJNS.9.32.8.29]
- Winkelmann A, Loebermann M, Reisinger EC, Hartung HP, Zettl UK. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol. 2016; 12(4):217-33. [DOI:10.1038/nrneurol.2016.21] [PMID]
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021; 21(2):181-92. [DOI:10.1016/S1473-3099(20)30843-4] [PMID]
- Drulovic J, Ivanovic J, Martinovic V, Tamas O, Veselinovic N, Cujic D, et al. Humoral response to SARS-CoV-2 COVID-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Mult Scler Relat Disord. 2021; 54:103150. [DOI:10.1016/j.msard.2021.103150] [PMID] [PMCID]
- Riva A, Barcella V, Benatti SV, Capobianco M, Capra R, Cinque P, et al. Vaccinations in patients with multiple sclerosis: A Delphi consensus statement. Mult Scler. 2021; 27(3):347-59. [DOI:10.1177/1352458520952310] [PMID]
- Shahin S, Eskandarieh S, Moghadasi AN, Razazian N, Baghbanian SM, Ashtari F, et al. Multiple sclerosis national registry system in Iran: Validity and reliability of a minimum data set. Mult Scler Relat Disord. 2019; 33:158-61. [DOI:10.1016/j.msard.2019.06.009] [PMID]
- Eskandarieh S, Sahraian MA, Moghadasi AN. Implementing coronavirus disease 2019 scale-up registry protocol in national multiple sclerosis registry system of Iran. Curr J Neurol. 2021; 20(4):241–5. [DOI:10.18502/cjn.v20i4.8351] [PMCID]
- Kavosh A, Ashtari F, Naghavi S, Adibi I, Shaygannejad V, Karimi Z, et al. Safety of Sinopharm vaccine for people with Multiple Sclerosis: Study of adverse reactions and disease activity. Mult Scler Relat Disord. 2022; 61:103708. [DOI:10.1016/j.msard.2022.103708] [PMID]
- Nabizadeh F, Balabandian M, Rostami MR, Owji M, Sahraian MA, Bidadian M, et al. Association of cognitive impairment and quality of life in patients with multiple sclerosis: A cross-sectional study. Curr J Neurol. 2022; 21(3):144-50. [DOI:10.18502/cjn.v21i3.11106] [PMCID]
- Ali Sahraian M, Ghadiri F, Azimi A, Naser Moghadasi A. Adverse events reported by Iranian patients with multiple sclerosis after the first dose of Sinopharm BBIBP-CorV. Vaccine. 2021; 39(43):6347-50. [DOI:10.1016/j.vaccine.2021.09.030] [PMID] [PMCID]
- Gad AHE, Ahmed SM, Garadah MYA, Dahshan A. Multiple sclerosis patients’ response to COVID-19 pandemic and vaccination in Egypt. Egypt J Neurol Psychiatr Neurosurg. 2022; 58(1):131. [DOI:10.1186/s41983-022-00573-8] [PMID] [PMCID]
- Razazian N, Sahraian MA, Eskandarieh S, Jafari N, Rezaei M, Fakhri N. Outcomes of AstraZeneca vaccine for relapse and Covid-19 infection in people with multiple sclerosis. Tehran Univ Med J. 2022; 80(6):462-9. [Link]
- Di Filippo M, Cordioli C, Malucchi S, Annovazzi P, Cavalla P, Clerici VT, et al. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2022; 93(4):448-50. . [DOI:10.1136/jnnp-2021-327200] [PMID]
- Lotan I, Wilf-Yarkoni A, Friedman Y, Stiebel-Kalish H, Steiner I, Hellmann MA. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): Early experience from a tertiary MS center in Israel. Eur J Neurol. 2021; 28(11):3742-8. [DOI:10.1111/ene.15028] [PMID] [PMCID]
- Fragoso YD, Gomes S, Gonçalves MVM, Mendes Junior E, Oliveira BES, Rocha CF, et al. New relapse of multiple sclerosis and neuromyelitis optica as a potential adverse event of AstraZeneca AZD1222 vaccination for COVID-19. Mult Scler Relat Disord. 2022; 57:103321. [DOI:10.1016/j.msard.2021.103321] [PMID] [PMCID]
- Sedighi B, Haghdoost A, Jangipour Afshar P, Abna Z, Bahmani S, Jafari S. Multiple sclerosis and COVID-19: A retrospective study in Iran. Plos One. 2023; 18(3):e0283538. [DOI:10.1371/journal.pone.0283538] [PMID] [PMCID]
- World Health Organization. COVID-19 In Iran (Islamic Republic of). Geneva: World Health Organization; 2021. [Link]
- Mehrotra DV, Janes HE, Fleming TR, Annunziato PW, Neuzil KM, Carpp LN, et al. Clinical endpoints for evaluating efficacy in COVID-19 vaccine trials. Ann Intern Med. 2021; 174(2):221-8. [DOI:10.7326/M20-6169] [PMID] [PMCID]
- Garjani A, Patel S, Bharkhada D, Rashid W, Coles A, Law GR, et al. Impact of mass vaccination on SARS-CoV-2 infections among multiple sclerosis patients taking immunomodulatory disease-modifying therapies in England. Mult Scler Relat Disord. 2022; 57:103458. [DOI:10.1016/j.msard.2021.103458] [PMID] [PMCID]
- Tallantyre EC, Vickaryous N, Anderson V, Asardag AN, Baker D, Bestwick J, et al. COVID-19 vaccine response in people with multiple sclerosis. Ann Neurol. 2022; 91(1):89-100. [DOI:10.1002/ana.26251] [PMID] [PMCID]
Type of Study: Research |
Subject:
Special
Received: 2023/10/11 | Accepted: 2023/10/17 | Published: 2023/10/17
Received: 2023/10/11 | Accepted: 2023/10/17 | Published: 2023/10/17
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



