Sat, May 18, 2024
Volume 9, Issue 3 (Summer 2023)
Caspian J Neurol Sci 2023, 9(3): 193-199 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Soltani M, Salimnia N, Nahayati M A, Payere M. Case Series of Cerebral Venous Sinus Thrombosis After COVID-19 Vaccination. Caspian J Neurol Sci 2023; 9 (3) :193-199
URL: http://cjns.gums.ac.ir/article-1-644-en.html
URL: http://cjns.gums.ac.ir/article-1-644-en.html
1- Department of Neurology, Faculty of Medicine, Qaem Hospital, Mashhad University of Medical Sciences, Mashhad, Iran.
Full-Text [PDF 1496 kb]
(203 Downloads)
| Abstract (HTML) (441 Views)
Full-Text: (255 Views)
Introduction
After vaccination with the AstraZeneca ChAdOx1-S against novel coronavirus disease 2019 (COVID-19), multiple cases of thrombosis with thrombocytopenia have been reported [1, 2, 3, 4, 5, 6]. The relationship between the thrombotic events and the COVID-19 vaccine was first reported on March 7, 2021. At that time, it was announced that using a batch of AstraZeneca's ChAdOx1 vaccine was suspended because of thromboembolic events. Medicines and Healthcare Products Regulatory Agency (MHRA) suggested a relationship between cerebral vein thrombosis and the COVID-19 vaccine, and as a result, a new guideline was put in effect. By May 18, 2021, two countries in the European :union:/European Economic Area have stopped using the vaccine, and 15 countries have only allowed its use in older ages [7]. Following the administration of AD26.COV2. S Johnson & Johnson (JJ) vaccine in the United States, cerebral venous thrombosis has also been reported. AD26.COV2. S Johnson & Johnson (JJ) vaccine, like AstraZeneca's ChAdOx1, uses recombinant adenoviral vectors that disrupt the severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) spike protein [2]. An underpinning pattern was denoted "vaccine-induced immune thrombotic thrombocytopenia" (VITT) [4, 5] or "thrombosis with thrombocytopenia syndrome" (TTS) [8]. VITT clinically resembles heparin-induced autoimmunity thrombocytopenia (HIT). HIT is caused by platelet-activating immunoglobulin G (IgG) antibodies against platelet factor 4 (PF4) complexed with heparin. This complex then binds to the platelet FcRγIIA receptors and causes platelet activation and formation of platelet microparticles [9]. These microparticles initiate the formation of blood clots and inducing a prothrombotic cascade, which consequently decreases platelet count and causes thrombocytopenia. Moreover, the reticuloendothelial system, particularly the spleen, removes the antibody-coated platelets and aggravates thrombocytopenia [9, 10, 11].
We report 3 cerebral venous thrombosis (CVT) cases due to severe thrombocytopenia after COVID-19 vaccination.
Case Presentation
Case 1
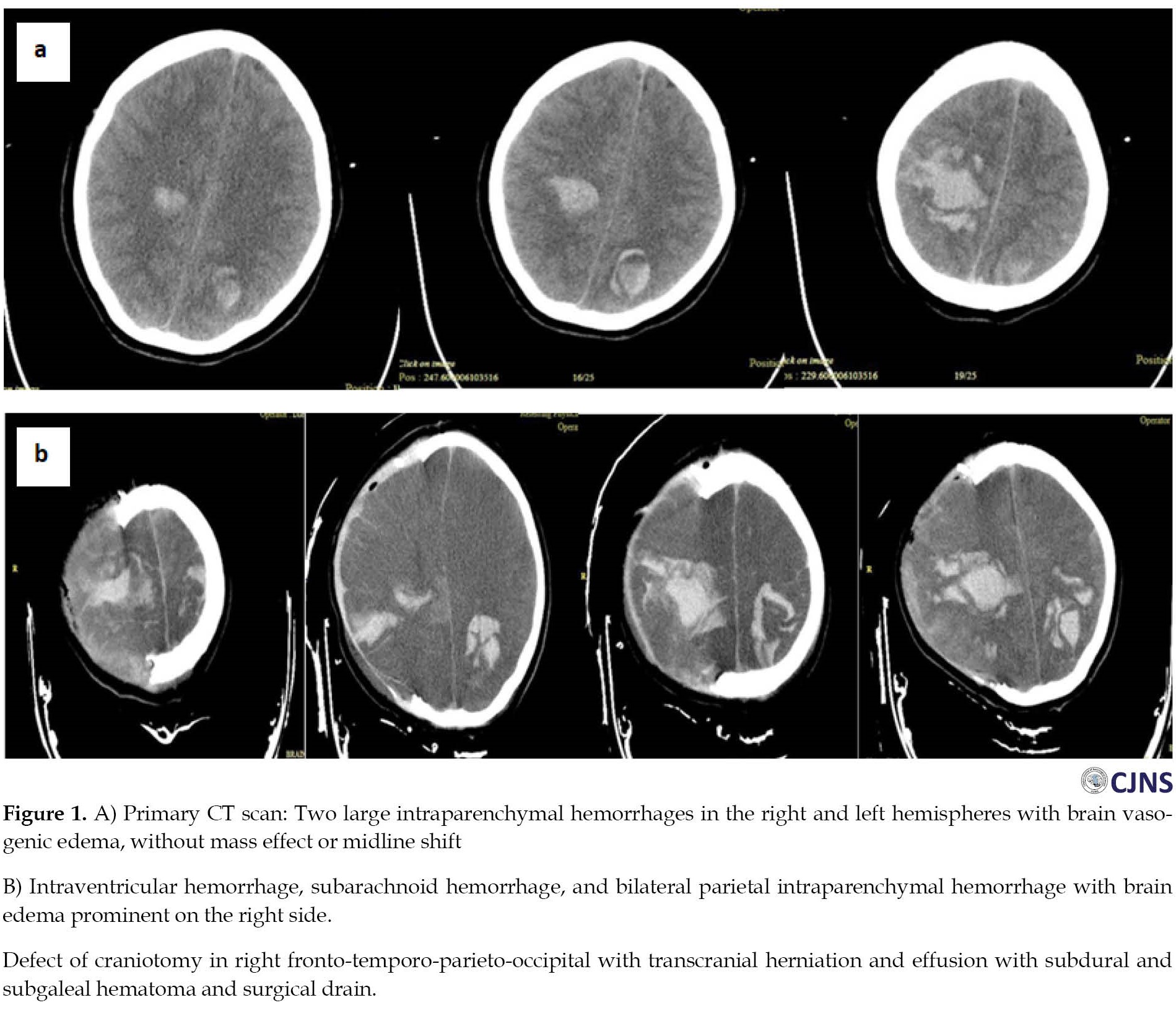
A 19-year-old lady was admitted to the emergency department with acute left-side hemiparesis, headache, nausea, and vomiting that started 2 hours before admission. The patient had no history of exposure to heparin or illness, especially prothrombotic medical conditions. She had no past clinical history or medication use. She received the first dose of AstraZeneca ChAdOx1-S COVID-19 vaccine 10 days before admission. Upon admission to the emergency department, an initial neurological examination revealed a Glasco coma scale (GCS) score of 15 with normal-sized and reactive pupils, bilateral Babinski signs, and left-sided hemiparesis. Primary computed tomography (CT) scan showed two expansive intraparenchymal hemorrhages within both hemispheres with vasogenic brain edema, causing no mass impact or midline shift (Figure 1A). Laboratory results showed marked thrombocytopenia, a very high D-dimer and international normalized ratio (INR), low fibrinogen, and imbalanced blood electrolytes (Table 1).

In magnetic resonance imaging (MRI) and magnetic resonance venography (MRV), superior sagittal veins were not seen; however, cerebral venous thrombosis (CVT) was suspected for her; she underwent emergency craniotomy with evacuation of intracerebral hemorrhage due to rapid neurological deterioration. Despite CVT suspicion because of thrombocytopenia and major bleeding, heparin therapy was not approved for her.
Upon arrival at the operating room, she had anisocoria with GCS=5. Although she had received 10 mg/kg platelet concentrates before craniotomy, she had a severe bleeding diathesis. As a result, hemostasis was difficult to achieve during craniotomy with persistently abnormal coagulation parameters.
We suspected a similar state of HIT caused by vaccination. Other tests for thrombotic thrombocytopenic purpura (TTP), vasculitis disorders such as antiphospholipid syndrome, and microangiopathic hemolytic anemia were negative.
Peripheral blood smear had normal morphology of platelets and showed a reduced count of platelets. Abdomen and pelvic sonography were normal. All vasculitis tests were negative.
Post-operatively, neurological examination showed a GCS=3, with mydriatic and reactive pupils. We treated the patient with 1 g/kg IV immunoglobulin and single donor platelet with fresh frozen plasma, but the platelets had no effective resolution.
One day after administration, gastrointestinal bleeding from the nasogastric tube and imbalanced blood electrolytes were seen (Table 1).
Post-surgery CT scan showed an intraventricular hemorrhage, subarachnoid hemorrhage, and bilateral parietal intraparenchymal hemorrhage with brain edema prominent on the right side, suspected of central vein thrombosis. Transcranial herniation and effusion with subdural and subgaleal hematoma were seen. No midline shift was seen (Figure 1B).
Three days after admission, unfortunately, a neurological examination showed abnormal brainstem reflexes, double fixed and mydriasis pupils, no spontaneous respiration, and no reaction to any stimulation; brain death was suspected considering this examination.
Case 2
A 24-year-old woman was admitted with a headache on the second day after the second AstraZeneca ChAdOx1-S COVID-19 vaccine shot. She received some calmative medication, but her headache increased. After 10 days, her level of conciseness decreased, so she was admitted to the hospital with GSC=10.
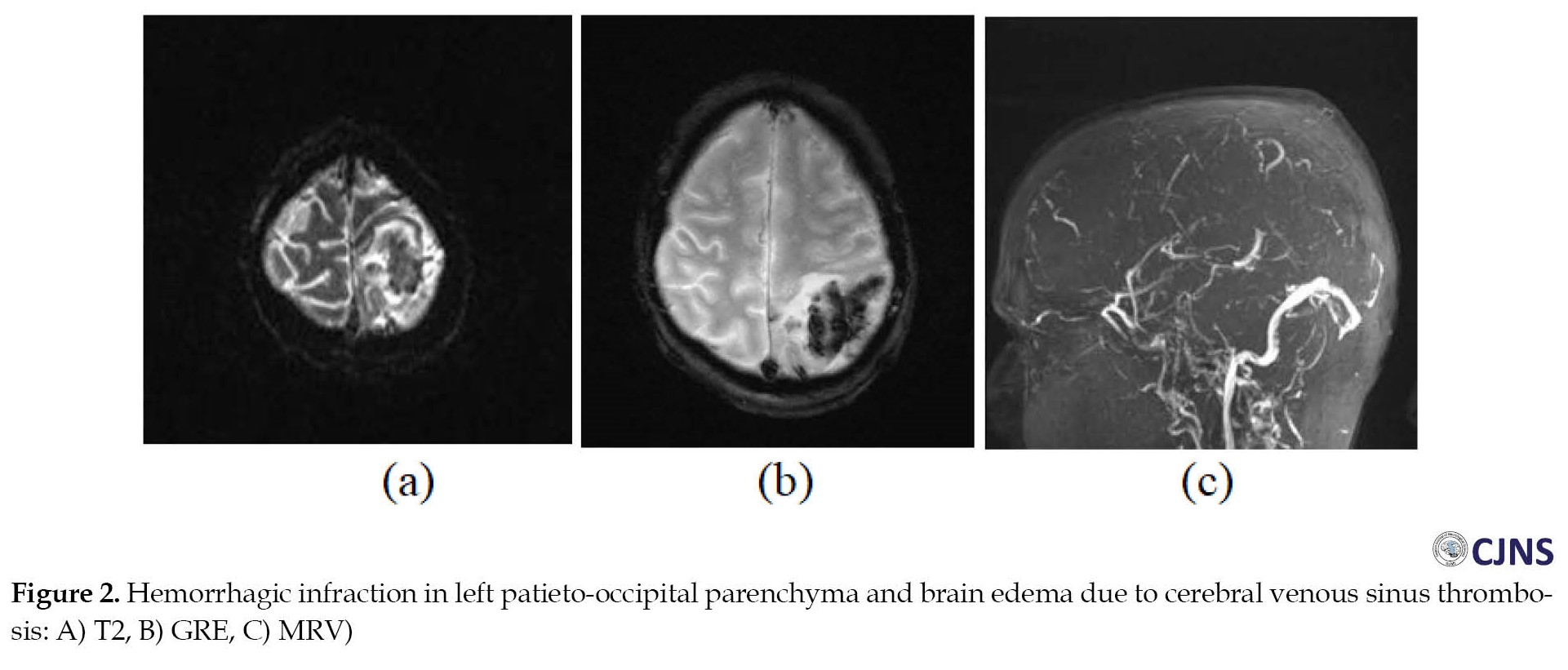
Her brain CT scan showed hemorrhagic infarction, and CVST was confirmed with brain MRI and MRV. The patient’s platelet count was 14000. The brain MRV with contrast showed a sagittal sinus and left transverse sinus clot (Figure 2). Afterward, vaccine-induced immune thrombotic thrombocytopenia (VITT) was suspected. She had no medical history of medication. Also, testing for TTP, vasculitis disorder, and microangiopathic hemolytic anemia was negative.
The peripheral blood smear had normal morphology of platelets but showed a reduced count of platelets. Abdomen and pelvic sonography were normal.
She underwent decompressive craniotomy and platelets transfusion, but unfortunately, her brain death was suspected one day later.
Case 3
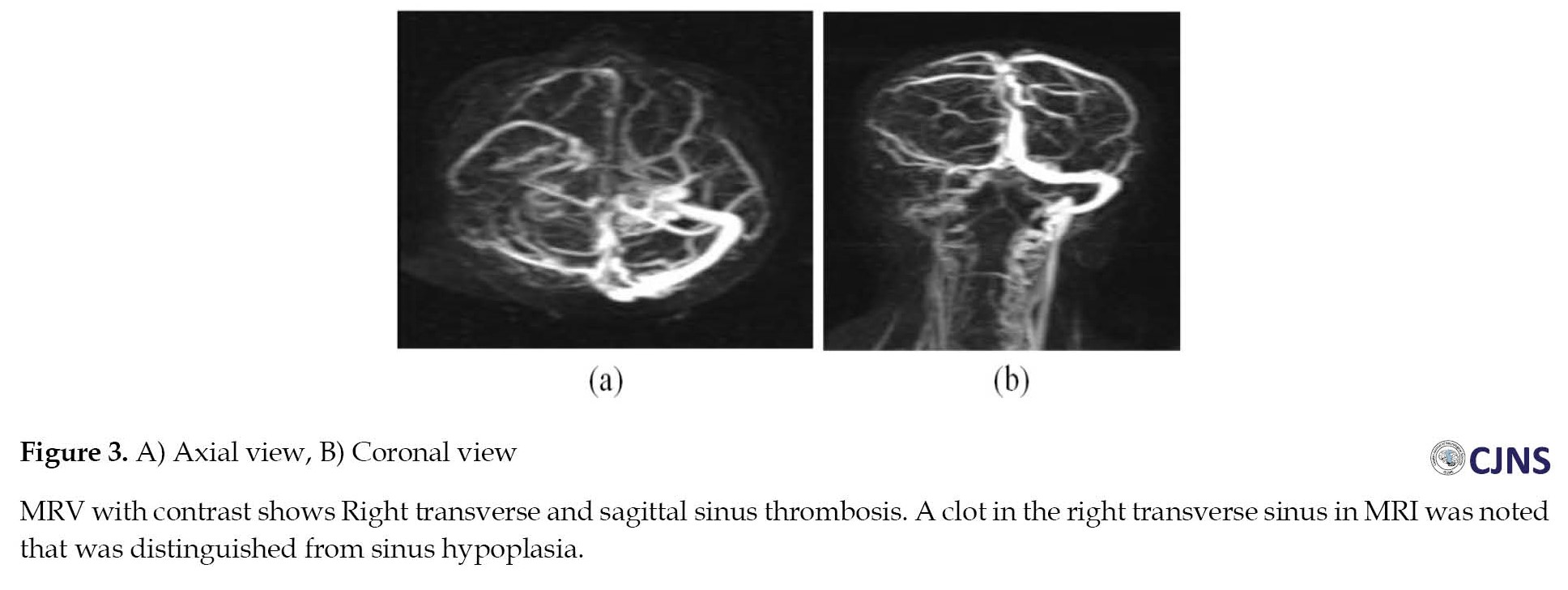
A 43-year-old woman was admitted with loss of consciousness 10 days after vaccination. She was admitted to the hospital with GCS=8. Her brain CT scan also showed intracranial hemorrhagic infarction, and CVST was confirmed with brain MRI and MRV. She had no past medical history and history of medication use (Figure 3). Also, testing for TTP, vasculitis disorder, and microangiopathic hemolytic anemia was negative. She underwent decompressive craniotomy and other medication, but unfortunately, brain death was suspected for her 20 days later.
Discussion
Several complications have been reported after vaccination against COVID-19. There are concerns about the possible association between some COVID-19 vaccines and hematological unfavorable events, such as cerebral venous sinus thrombosis (CVST) following ChAdOx1 nCoV-19 (Oxford/AstraZeneca; from now on ChAdOx1) administration. After the outbreak of the COVID-19 epidemic and widespread vaccination against it, several cases of CVST after vaccination with the adenovirus vector of COVID-19 have been reported [12, 13] several vaccines have been developed and millions of doses delivered. Our case series show evidence of VITT with an unusual course. In these cases, correct diagnosis and proper management were challenging. Their most common symptom after receiving the Ad26.COV2.S (Janssen) adenovirus-based SARS-CoV2 vaccine was a headache. The onset of the symptoms was 6 to 13 days after receiving the vaccine. Patients were between 18 and 48 years old. Five out of 6 patients had a headache, 1 had vomiting and a headache, and 1 had lethargy. Other symptoms of the patients included back pain (1 patient), hemiparesis (2 patients), aphasia (1 patient), neglect (1 patient), and loss of consciousness (1 patient). Portal vein thrombosis caused abdominal pain in two patients [5]. Other reports from Europe reported cases of venous thrombosis and thrombocytopenia after AstraZeneca ChAdOx1-S COVID-19 vaccine. People’s symptoms started 5 to 24 days after the first dose of 2-shot vaccination. All these patients had thrombocytopenia. In Germany, 11 patients (9 of whom were women) aged between 22 and 49 years, developed venous thrombosis. Nine patients had CVST, 3 had pulmonary embolism and 3 had splanchnic-vein thrombosis [14] potentially fatal neurological condition that can be frequently overlooked due to the vague nature of its clinical and radiological presentation. A literature search on PubMed using the keyword”cerebral sinus venous thrombosis” was performed. We searched for the epidemiology, risk factors, pathophysiology, clinical features, diagnosis, and treatment of CSVT. All full-text articles in the last 10 years, in adults (>18 years. In Norway, 5 patients aged 32-54 years presented with venous thrombosis and thrombocytopenia 7 to 10 days after receiving the first dose of the AstraZeneca ChAdOx1-S COVID-19 vaccine [7]. In the UK, 23 patients aged 21 to 77 years, 61% of whom were women, developed platelet factor 4 (PF4) antibodies after ChAdOx1 nCoV-19 vaccination. Out of 22 patients who presented with thrombosis, 13 patients were suspected of CVST, 4 had pulmonary embolism, 1 had deep venous thrombosis (DVT) and bilateral adrenal hemorrhage consistent with infarction, 2 people had middle cerebral artery territory ischemic stroke, and 2 persons had portal vein thrombosis [15]. VITT clinically resembles spontaneous autoimmune heparin-induced thrombocytopenia (HIT). HIT is caused by platelet-activating immunoglobulin G (IgG) antibodies against platelet factor 4 (PF4) complexed with heparin. This complex then binds to the platelet FcRγIIA receptors and causes platelet activation and formation of platelet microparticles. Interactions between the vaccine and platelets or PF4 could play a role in the pathogenesis of VITT.
Interactions between the vaccine (free DNA in the vaccines) and platelets or PF4 could trigger PF4-reactive autoantibodies and, subsequently, the pathogenesis of VITT [7].
The Oxford–AstraZeneca COVID-19 vaccine (AZD1222, ChAdOx1, and Vaxzevria) is a viral vector vaccine designed to prevent COVID-19, and its efficacy was about 66·7% beyond 14 days after the second dose [16].
Common side effects generally subside within a few days, and they include injection-site reaction, nausea, headache, and malaise [10] society and economics around the world. As a result, the development of vaccines to protect individuals from symptomatic COVID-19 infections has represented the only feasible health tool to combat the spread of the disease. However, at the same time the development and regulatory assessment of different vaccines has challenged pharmaceutical industries and regulatory agencies as this process has occurred in the shorter time ever though. So far, two mRNA and two adenovirus-vectored vaccines have received a conditional marketing authorisation in the EU and other countries. This review summarized and discusses the assessment reports of the European Medicine Agency (EMA) [6].
Reviewing all CVST cases during the COVID-19 pandemic provides a better incidence estimate and reduces case detection bias. Many neurological complications associated with COVID-19 are still unknown. Scientific data should not be published out of context and without considering the consequences of their publication in society and appropriate warnings [6]. For example, reports of vaccine-associated CVST may make people hesitant to vaccinate. Nevertheless, scientific studies showed that the risk of CVST due to COVID-19 infection significantly surpasses vaccination [3]. Specific diseases and conditions that may predispose individuals to CVST after vaccination are still unknown. Knowledge is scarce regarding the best therapy for CVST with VITT. However, the treatments are similar for heparin-induced thrombocytopenia (HIT) because the two conditions are similar [14]. Collaboration between vascular neurology and hematology is recommended for treating HIT with cerebral thrombosis.
IV immunoglobulin 1 g/kg daily for 2 days is recommended after laboratory testing for PF4 antibodies, based on evidence of response in HIT. However, there is no definitive information on efficacy in VITT [17]. Prescription of heparin products is not recommended. Sometimes specialists recommend the administration of steroids [18].
Anticoagulant administration in these patients should be properly managed and based on the latest scientific recommendations. The anticoagulant prescription should follow the latest guidelines on HIT with thrombosis, which recommend alternative anticoagulants to heparin/enoxaparin, including argatroban, bivalirudin, danaparoid, fondaparinux, or a novel oral anticoagulant (NOAC). A dose modification may be required in severe thrombocytopenia (i.e. less than 20000/mm3) or low fibrinogen. Even in the presence of secondary intracranial bleeding, anticoagulation should be used in CVST because it is necessary to control this bleeding to prevent progressive thrombosis.
Conclusion
Medical professionals must know about the symptoms, causes, and ways to diagnose and treat CVST in connection with VITT after receiving the COVID-19 vaccine. Even though this complication is highly uncommon, it should be taken seriously as it can result in death. Encouraging early identification and prompt treatment implementation can improve patients’ neurological prognosis [19].
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki 2013.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Investigation and original draft: Mohsen Soltani, Nasrin Salimnia, and Maryam Payere; Conceptualization, resources, supervision, review, and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We acknowledge the support of the Department of Neurology, Faculty of Medicine, Qaem Hospital.
References
After vaccination with the AstraZeneca ChAdOx1-S against novel coronavirus disease 2019 (COVID-19), multiple cases of thrombosis with thrombocytopenia have been reported [1, 2, 3, 4, 5, 6]. The relationship between the thrombotic events and the COVID-19 vaccine was first reported on March 7, 2021. At that time, it was announced that using a batch of AstraZeneca's ChAdOx1 vaccine was suspended because of thromboembolic events. Medicines and Healthcare Products Regulatory Agency (MHRA) suggested a relationship between cerebral vein thrombosis and the COVID-19 vaccine, and as a result, a new guideline was put in effect. By May 18, 2021, two countries in the European :union:/European Economic Area have stopped using the vaccine, and 15 countries have only allowed its use in older ages [7]. Following the administration of AD26.COV2. S Johnson & Johnson (JJ) vaccine in the United States, cerebral venous thrombosis has also been reported. AD26.COV2. S Johnson & Johnson (JJ) vaccine, like AstraZeneca's ChAdOx1, uses recombinant adenoviral vectors that disrupt the severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) spike protein [2]. An underpinning pattern was denoted "vaccine-induced immune thrombotic thrombocytopenia" (VITT) [4, 5] or "thrombosis with thrombocytopenia syndrome" (TTS) [8]. VITT clinically resembles heparin-induced autoimmunity thrombocytopenia (HIT). HIT is caused by platelet-activating immunoglobulin G (IgG) antibodies against platelet factor 4 (PF4) complexed with heparin. This complex then binds to the platelet FcRγIIA receptors and causes platelet activation and formation of platelet microparticles [9]. These microparticles initiate the formation of blood clots and inducing a prothrombotic cascade, which consequently decreases platelet count and causes thrombocytopenia. Moreover, the reticuloendothelial system, particularly the spleen, removes the antibody-coated platelets and aggravates thrombocytopenia [9, 10, 11].
We report 3 cerebral venous thrombosis (CVT) cases due to severe thrombocytopenia after COVID-19 vaccination.
Case Presentation
Case 1
A 19-year-old lady was admitted to the emergency department with acute left-side hemiparesis, headache, nausea, and vomiting that started 2 hours before admission. The patient had no history of exposure to heparin or illness, especially prothrombotic medical conditions. She had no past clinical history or medication use. She received the first dose of AstraZeneca ChAdOx1-S COVID-19 vaccine 10 days before admission. Upon admission to the emergency department, an initial neurological examination revealed a Glasco coma scale (GCS) score of 15 with normal-sized and reactive pupils, bilateral Babinski signs, and left-sided hemiparesis. Primary computed tomography (CT) scan showed two expansive intraparenchymal hemorrhages within both hemispheres with vasogenic brain edema, causing no mass impact or midline shift (Figure 1A). Laboratory results showed marked thrombocytopenia, a very high D-dimer and international normalized ratio (INR), low fibrinogen, and imbalanced blood electrolytes (Table 1).

In magnetic resonance imaging (MRI) and magnetic resonance venography (MRV), superior sagittal veins were not seen; however, cerebral venous thrombosis (CVT) was suspected for her; she underwent emergency craniotomy with evacuation of intracerebral hemorrhage due to rapid neurological deterioration. Despite CVT suspicion because of thrombocytopenia and major bleeding, heparin therapy was not approved for her.
Upon arrival at the operating room, she had anisocoria with GCS=5. Although she had received 10 mg/kg platelet concentrates before craniotomy, she had a severe bleeding diathesis. As a result, hemostasis was difficult to achieve during craniotomy with persistently abnormal coagulation parameters.
We suspected a similar state of HIT caused by vaccination. Other tests for thrombotic thrombocytopenic purpura (TTP), vasculitis disorders such as antiphospholipid syndrome, and microangiopathic hemolytic anemia were negative.
Peripheral blood smear had normal morphology of platelets and showed a reduced count of platelets. Abdomen and pelvic sonography were normal. All vasculitis tests were negative.
Post-operatively, neurological examination showed a GCS=3, with mydriatic and reactive pupils. We treated the patient with 1 g/kg IV immunoglobulin and single donor platelet with fresh frozen plasma, but the platelets had no effective resolution.
One day after administration, gastrointestinal bleeding from the nasogastric tube and imbalanced blood electrolytes were seen (Table 1).
Post-surgery CT scan showed an intraventricular hemorrhage, subarachnoid hemorrhage, and bilateral parietal intraparenchymal hemorrhage with brain edema prominent on the right side, suspected of central vein thrombosis. Transcranial herniation and effusion with subdural and subgaleal hematoma were seen. No midline shift was seen (Figure 1B).
Three days after admission, unfortunately, a neurological examination showed abnormal brainstem reflexes, double fixed and mydriasis pupils, no spontaneous respiration, and no reaction to any stimulation; brain death was suspected considering this examination.
Case 2
A 24-year-old woman was admitted with a headache on the second day after the second AstraZeneca ChAdOx1-S COVID-19 vaccine shot. She received some calmative medication, but her headache increased. After 10 days, her level of conciseness decreased, so she was admitted to the hospital with GSC=10.
Her brain CT scan showed hemorrhagic infarction, and CVST was confirmed with brain MRI and MRV. The patient’s platelet count was 14000. The brain MRV with contrast showed a sagittal sinus and left transverse sinus clot (Figure 2). Afterward, vaccine-induced immune thrombotic thrombocytopenia (VITT) was suspected. She had no medical history of medication. Also, testing for TTP, vasculitis disorder, and microangiopathic hemolytic anemia was negative.
The peripheral blood smear had normal morphology of platelets but showed a reduced count of platelets. Abdomen and pelvic sonography were normal.
She underwent decompressive craniotomy and platelets transfusion, but unfortunately, her brain death was suspected one day later.
Case 3
A 43-year-old woman was admitted with loss of consciousness 10 days after vaccination. She was admitted to the hospital with GCS=8. Her brain CT scan also showed intracranial hemorrhagic infarction, and CVST was confirmed with brain MRI and MRV. She had no past medical history and history of medication use (Figure 3). Also, testing for TTP, vasculitis disorder, and microangiopathic hemolytic anemia was negative. She underwent decompressive craniotomy and other medication, but unfortunately, brain death was suspected for her 20 days later.
Discussion
Several complications have been reported after vaccination against COVID-19. There are concerns about the possible association between some COVID-19 vaccines and hematological unfavorable events, such as cerebral venous sinus thrombosis (CVST) following ChAdOx1 nCoV-19 (Oxford/AstraZeneca; from now on ChAdOx1) administration. After the outbreak of the COVID-19 epidemic and widespread vaccination against it, several cases of CVST after vaccination with the adenovirus vector of COVID-19 have been reported [12, 13] several vaccines have been developed and millions of doses delivered. Our case series show evidence of VITT with an unusual course. In these cases, correct diagnosis and proper management were challenging. Their most common symptom after receiving the Ad26.COV2.S (Janssen) adenovirus-based SARS-CoV2 vaccine was a headache. The onset of the symptoms was 6 to 13 days after receiving the vaccine. Patients were between 18 and 48 years old. Five out of 6 patients had a headache, 1 had vomiting and a headache, and 1 had lethargy. Other symptoms of the patients included back pain (1 patient), hemiparesis (2 patients), aphasia (1 patient), neglect (1 patient), and loss of consciousness (1 patient). Portal vein thrombosis caused abdominal pain in two patients [5]. Other reports from Europe reported cases of venous thrombosis and thrombocytopenia after AstraZeneca ChAdOx1-S COVID-19 vaccine. People’s symptoms started 5 to 24 days after the first dose of 2-shot vaccination. All these patients had thrombocytopenia. In Germany, 11 patients (9 of whom were women) aged between 22 and 49 years, developed venous thrombosis. Nine patients had CVST, 3 had pulmonary embolism and 3 had splanchnic-vein thrombosis [14] potentially fatal neurological condition that can be frequently overlooked due to the vague nature of its clinical and radiological presentation. A literature search on PubMed using the keyword”cerebral sinus venous thrombosis” was performed. We searched for the epidemiology, risk factors, pathophysiology, clinical features, diagnosis, and treatment of CSVT. All full-text articles in the last 10 years, in adults (>18 years. In Norway, 5 patients aged 32-54 years presented with venous thrombosis and thrombocytopenia 7 to 10 days after receiving the first dose of the AstraZeneca ChAdOx1-S COVID-19 vaccine [7]. In the UK, 23 patients aged 21 to 77 years, 61% of whom were women, developed platelet factor 4 (PF4) antibodies after ChAdOx1 nCoV-19 vaccination. Out of 22 patients who presented with thrombosis, 13 patients were suspected of CVST, 4 had pulmonary embolism, 1 had deep venous thrombosis (DVT) and bilateral adrenal hemorrhage consistent with infarction, 2 people had middle cerebral artery territory ischemic stroke, and 2 persons had portal vein thrombosis [15]. VITT clinically resembles spontaneous autoimmune heparin-induced thrombocytopenia (HIT). HIT is caused by platelet-activating immunoglobulin G (IgG) antibodies against platelet factor 4 (PF4) complexed with heparin. This complex then binds to the platelet FcRγIIA receptors and causes platelet activation and formation of platelet microparticles. Interactions between the vaccine and platelets or PF4 could play a role in the pathogenesis of VITT.
Interactions between the vaccine (free DNA in the vaccines) and platelets or PF4 could trigger PF4-reactive autoantibodies and, subsequently, the pathogenesis of VITT [7].
The Oxford–AstraZeneca COVID-19 vaccine (AZD1222, ChAdOx1, and Vaxzevria) is a viral vector vaccine designed to prevent COVID-19, and its efficacy was about 66·7% beyond 14 days after the second dose [16].
Common side effects generally subside within a few days, and they include injection-site reaction, nausea, headache, and malaise [10] society and economics around the world. As a result, the development of vaccines to protect individuals from symptomatic COVID-19 infections has represented the only feasible health tool to combat the spread of the disease. However, at the same time the development and regulatory assessment of different vaccines has challenged pharmaceutical industries and regulatory agencies as this process has occurred in the shorter time ever though. So far, two mRNA and two adenovirus-vectored vaccines have received a conditional marketing authorisation in the EU and other countries. This review summarized and discusses the assessment reports of the European Medicine Agency (EMA) [6].
Reviewing all CVST cases during the COVID-19 pandemic provides a better incidence estimate and reduces case detection bias. Many neurological complications associated with COVID-19 are still unknown. Scientific data should not be published out of context and without considering the consequences of their publication in society and appropriate warnings [6]. For example, reports of vaccine-associated CVST may make people hesitant to vaccinate. Nevertheless, scientific studies showed that the risk of CVST due to COVID-19 infection significantly surpasses vaccination [3]. Specific diseases and conditions that may predispose individuals to CVST after vaccination are still unknown. Knowledge is scarce regarding the best therapy for CVST with VITT. However, the treatments are similar for heparin-induced thrombocytopenia (HIT) because the two conditions are similar [14]. Collaboration between vascular neurology and hematology is recommended for treating HIT with cerebral thrombosis.
IV immunoglobulin 1 g/kg daily for 2 days is recommended after laboratory testing for PF4 antibodies, based on evidence of response in HIT. However, there is no definitive information on efficacy in VITT [17]. Prescription of heparin products is not recommended. Sometimes specialists recommend the administration of steroids [18].
Anticoagulant administration in these patients should be properly managed and based on the latest scientific recommendations. The anticoagulant prescription should follow the latest guidelines on HIT with thrombosis, which recommend alternative anticoagulants to heparin/enoxaparin, including argatroban, bivalirudin, danaparoid, fondaparinux, or a novel oral anticoagulant (NOAC). A dose modification may be required in severe thrombocytopenia (i.e. less than 20000/mm3) or low fibrinogen. Even in the presence of secondary intracranial bleeding, anticoagulation should be used in CVST because it is necessary to control this bleeding to prevent progressive thrombosis.
Conclusion
Medical professionals must know about the symptoms, causes, and ways to diagnose and treat CVST in connection with VITT after receiving the COVID-19 vaccine. Even though this complication is highly uncommon, it should be taken seriously as it can result in death. Encouraging early identification and prompt treatment implementation can improve patients’ neurological prognosis [19].
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki 2013.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Investigation and original draft: Mohsen Soltani, Nasrin Salimnia, and Maryam Payere; Conceptualization, resources, supervision, review, and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We acknowledge the support of the Department of Neurology, Faculty of Medicine, Qaem Hospital.
References
- Mehta PR, Apap Mangion S, Benger M, Stanton BR, Czuprynska J, Arya R, et al. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination - A report of two UK cases. Brain Behav Immun. 2021; 95:514-7. [DOI:10.1016/j.bbi.2021.04.006] [PMID] [PMCID]
- Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021; 384(22):2124-30. [DOI:10.1056/NEJMoa2104882] [PMID] [PMCID]
- Silvis SM, de Sousa DA, Ferro JM, Coutinho JM. Cerebral venous thrombosis. Nat Rev Neurol. 2017; 13(9):555-65. [DOI:10.1038/nrneurol.2017.104] [PMID]
- Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after “COVID-19 Vaccine AstraZeneca” exposure. J Clin Med. 2021; 10(8):1599. [DOI:10.3390/jcm10081599] [PMID] [PMCID]
- Castelli GP, Pognani C, Sozzi C, Franchini M, Vivona L. Cerebral venous sinus thrombosis associated with thrombocytopenia post-vaccination for COVID-19. Crit Care. 2021; 25(1):137. [DOI:10.1186/s13054-021-03572-y] [PMID] [PMCID]
- Cumurciuc R, Crassard I, Sarov M, Valade D, Bousser MG. Headache as the only neurological sign of cerebral venous thrombosis: A series of 17 cases. J Neurol Neurosurg Psychiatry. 2005; 76(8):1084-7. [DOI:10.1136/jnnp.2004.056275] [PMID] [PMCID]
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021; 384(22):2092-101. [DOI:10.1056/NEJMoa2104840] [PMID] [PMCID]
- D'Agostino V, Caranci F, Negro A, Piscitelli V, Tuccillo B, Fasano F, et al. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration. J Pers Med. 2021; 11(4):285. [DOI:10.3390/jpm11040285] [PMID] [PMCID]
- Vayne C, Nguyen TH, Rollin J, Charuel N, Poupon A, Pouplard C, et al. Characterization of new monoclonal PF4-specific antibodies as useful tools for studies on typical and autoimmune heparin-induced thrombocytopenia. Thromb Haemost. 2021; 121(3):322-31. [DOI:10.1055/s-0040-1717078] [PMID]
- Hernández AF, Calina D, Poulas K, Docea AO, Tsatsakis AM. Safety of COVID-19 vaccines administered in the EU: Should we be concerned? Toxicol Rep. 2021; 8:871-9. [DOI:10.1016/j.toxrep.2021.04.003] [PMID] [PMCID]
- Cines DB, Bussel JB. SARS-CoV-2 Vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021; 384(23):2254-6. [DOI:10.1056/NEJMe2106315] [PMID] [PMCID]
- See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA. 2021; 325(24):2448-56. [DOI:10.1001/jama.2021.7517] [PMID] [PMCID]
- Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021; 384(23):2202-11. [DOI:10.1056/NEJMoa2105385] [PMID] [PMCID]
- Idiculla PS, Gurala D, Palanisamy M, Vijayakumar R, Dhandapani S, Nagarajan E. Cerebral venous thrombosis: A comprehensive review. Eur Neurol. 2020; 83(4):369-79. [DOI:10.1159/000509802] [PMID]
- Duman T, Uluduz D, Midi I, Bektas H, Kablan Y, Goksel BK, et al. A multicenter study of 1144 patients with cerebral venous thrombosis: The VENOST study. J Stroke Cerebrovasc Dis. 2017; 26(8):1848-57. [DOI:10.1016/j.jstrokecerebrovasdis.2017.04.020] [PMID]
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet (London, England). 2021; 397(10277):881-91. [DOI:10.1016/S0140-6736(21)00432-3] [PMID]
- Taquet M, Husain M, Geddes JR, Luciano S, Harrison PJ. Cerebral venous thrombosis and portal vein thrombosis: A retrospective cohort study of 537,913 COVID-19 cases. EClinicalMedicine. 2021; 39:101061. [DOI:10.1016/j.eclinm.2021.101061] [PMID] [PMCID]
- Chougar L, Mathon B, Weiss N, Degos V, Shor N. Atypical deep cerebral vein thrombosis with hemorrhagic venous infarction in a patient positive for COVID-19. AJNR Am J Neuroradiol. 2020; 41(8):1377-9. [DOI:10.3174/ajnr.A6642] [PMID] [PMCID]
- Bjørnstad-Tuveng TH, Rudjord A, Anker P. Fatal cerebral haemorrhage after COVID-19 vaccine. Tidsskr Nor Laegeforen. 2021; 141. [DOI:10.4045/tidsskr.21.0312] [PMID]
Type of Study: case report |
Subject:
Special
Received: 2023/07/5 | Accepted: 2023/07/28 | Published: 2023/07/28
Received: 2023/07/5 | Accepted: 2023/07/28 | Published: 2023/07/28
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |