Sat, May 18, 2024
Volume 9, Issue 3 (Summer 2023)
Caspian J Neurol Sci 2023, 9(3): 162-168 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghasemi M, Alizadeh M, Basiri K, Ansari B, Tayebi Khorami R. Abductor Pollicis Brevis/Abductor Digiti Minimi Compound Muscle Action Potential Ratio as a Diagnostic Marker for Amyotrophic Lateral Sclerosis. Caspian J Neurol Sci 2023; 9 (3) :162-168
URL: http://cjns.gums.ac.ir/article-1-640-en.html
URL: http://cjns.gums.ac.ir/article-1-640-en.html
1- Department of Neurology, Isfahan Neuroscience Research Center, Isfahan University of Medical Science, Isfahan, Iran.
2- Department of Neurology, Isfahan Neuroscience Research Center, Alzahra Research Institute, Isfahan University of Medical Science, Isfahan, Iran.
3- Department of Mathematics , Ahvaz Branch , Islamic Azad University , Ahvaz , Iran.
2- Department of Neurology, Isfahan Neuroscience Research Center, Alzahra Research Institute, Isfahan University of Medical Science, Isfahan, Iran.
3- Department of Mathematics , Ahvaz Branch , Islamic Azad University , Ahvaz , Iran.
Full-Text [PDF 1253 kb]
(211 Downloads)
| Abstract (HTML) (394 Views)
Full-Text: (185 Views)
Introduction
Amyotrophic lateral sclerosis (ALS), a neurodegenerative disease, manifests with progressive paralysis in skeletal muscles [1] reports on its incidence range from 0.6 to 3.8 per 100000 person-years. The ALS incidence has continuously risen in recent years [2]. The time between the onset of disease to dependency on respiratory support or death is reported to be around 24 to 50 months [3, 4].
Several diagnostic criteria and markers have been suggested in the literature [5], but more consistent markers are required due to difficulties and delays in diagnosing ALS.
Split hand phenomenon, first introduced by Wilbourn and Sweeney [6], is a specific pattern characterized by atrophy of thenar muscles (abductor pollicis brevis [APB] and first dorsal interosseous muscles [FDI]) with relative preservation of hypothenar muscles (abductor digiti minimi [ADM]) [7]. Since its introduction in the literature, the split hand has been considered a specific and characteristic sign that helps diagnose ALS [8]. Markers of the split hand, including split hand index (SI) and APB/ADM ratio measured by compound muscle action potentials (CMAP), have been evaluated for their diagnostic accuracy for ALS. Still, no agreement has been reached on the best choice [9, 10]. Normal adult values of APB and ADM CMAP amplitude are ≥4.0 and ≥6.0, respectively, and their normal ratio is 0.6 [11].
In nerve conduction studies (NCSs), a decreased APB/ADM CMAP amplitude ratio (<0.6) reflects the split hand phenomenon, which in ALS indicates cortical motor neuron compromise, particularly because APB and FDI are muscles with extensive corticospinal connections affected by glutamate excitotoxicity [7]. The split hand phenomenon is observed in 55% of ALS patients. The dissociated hand muscle atrophy, particularly the combination of APB/ADM ratio <0.6 and FDI/ADM ratio <0.9, is rarely found in pure LMN disease, cervical spondylotic amyotrophy, and polyneuropathies [12].
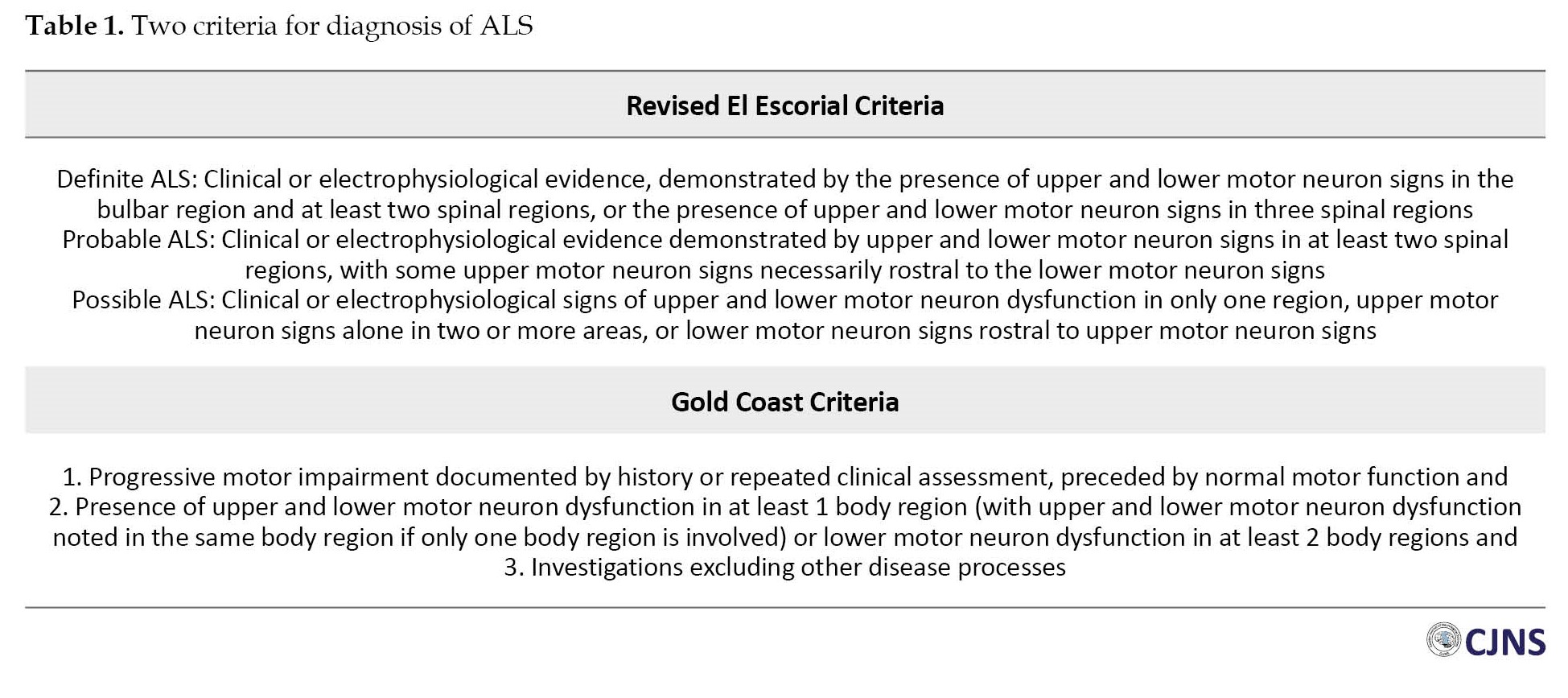
Diagnosing ALS is based on the coexistence of upper and lower motor neuron involvements and progressive symptoms [13]. Numerous ALS phenotypes and the lack of a definitive diagnostic test have led to frequent diagnostic delays [14]. Subsequently, initiation of neuroprotective treatment strategies is deferred, and the disease progresses. Clinical diagnostic criteria such as El Escorial criteria are not sensitive enough to identify earlier stages of ALS [14, 15]. To enhance diagnostic sensitivity, the gold coast criteria for diagnosis of ALS has recently been proposed (Table 1) [16].
Reports have designated split hand as an early sign of ALS which can assist in the prompt diagnosis of ALS patients [7]. Thus, in this study, we evaluated the utility of the APB/ADM CMAP ratio as a diagnostic marker in evaluating ALS and its differentiation from healthy controls.
Materials and Methods
This cross-sectional retrospective study was conducted at Alzahra and Kashani hospitals of Isfahan, Iran, in 2021. The study population consisted of all patients with a definite or probable diagnosis of ALS based on the revised El Escorial criteria who had undergone electrophysiological studies within the previous 5 years. Patients with clinical suspicion of other diseases mimicking ALS and compression neuropathies, such as C8 radiculopathy and carpal tunnel syndrome, were excluded from the study. Individuals with non-specific complaints who had undergone electrophysiological studies but were not diagnosed with ALS or any other neuromuscular abnormality were enrolled in the study as healthy controls. A total of 200 ALS patients and 200 healthy controls were evaluated in our study by consecutive convenient sampling.
Demographic data, including age and gender, were collected from medical records. Electrophysiological studies of patients were retrieved to collect CMAP amplitudes of APB and ADM muscles in both study groups. Then, APB/ADM CMAP ratio was calculated in both groups based on right/left hand and below/over 50 years of age. The statistical analysis also evaluated the diagnostic accuracy of the APB/ADM amplitude ratio.
Statistical analysis was performed with IBM SPSS software, version 25 (IBM Corp., Armonk, N.Y., USA). Descriptive analysis is presented as frequency and percentage or Mean±SD. The independent t-test and chi-square test were used to compare continuous and categorical parameters between the two groups. The diagnostic yield of the APB/ADM amplitude ratio for the differentiation of ALS from healthy controls was assessed by the ROC curve to determine cut-off values and their corresponding sensitivity, specificity, positive predictive value, negative predictive value, and area under the curve. P≤0.05 was considered statistically significant.
Results
A total of 200 patients with ALS and 200 healthy controls were enrolled in this study. Overall, 225 participants (56.3%) were males, and 185(46.25%) were females. The mean age of participants was 53.29±12.40 years (16-80 years). To be more specific, 247 participants (61.75%) were in the >50 years age group, while 153 participants (38.3%) were below 50 years. Also, 63 participants were patients, and 90 participants were healthy.
The mean age of the patients and controls was 54.44±13.25 years (16-77) and 52.15±11.41 years (25-80), respectively (P=0.065). The patients’ group consisted of 122 males (61%) and 78 females (39%), while 105 subjects (52.5%) and 95 (47.5%) in the control group were males and females, respectively (P=0.086).
APB/ADM amplitude ratio for diagnosis of ALS
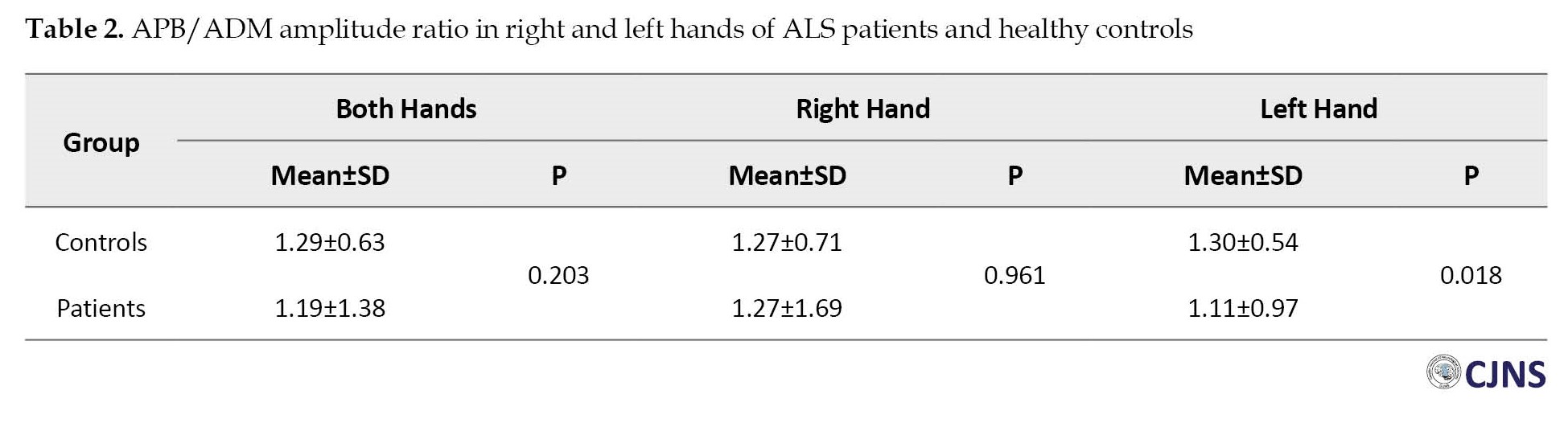
APB/ADM amplitude ratio showed no significant difference in the right hand and both hands together between patients and controls. However, this ratio in the left hand was significantly higher in healthy controls than in patients (P=0.018). The results showed that in patients below 50 years of age, a significant difference in APB/ADM amplitude ratio between patients and controls was only detected in the left hand (P=0.008), with higher levels in healthy controls compared to patients. In the age group over 50, no significant difference in APB/ADM amplitude ratio between healthy controls and patients was found in any of the hands and neither in both hands together (P>0.05). The details of APB/ADM amplitude ratio measurements are presented in Table 2 and Table 3.

Diagnostic accuracy parameters of APB/ADM amplitude ratio for diagnosis of ALS
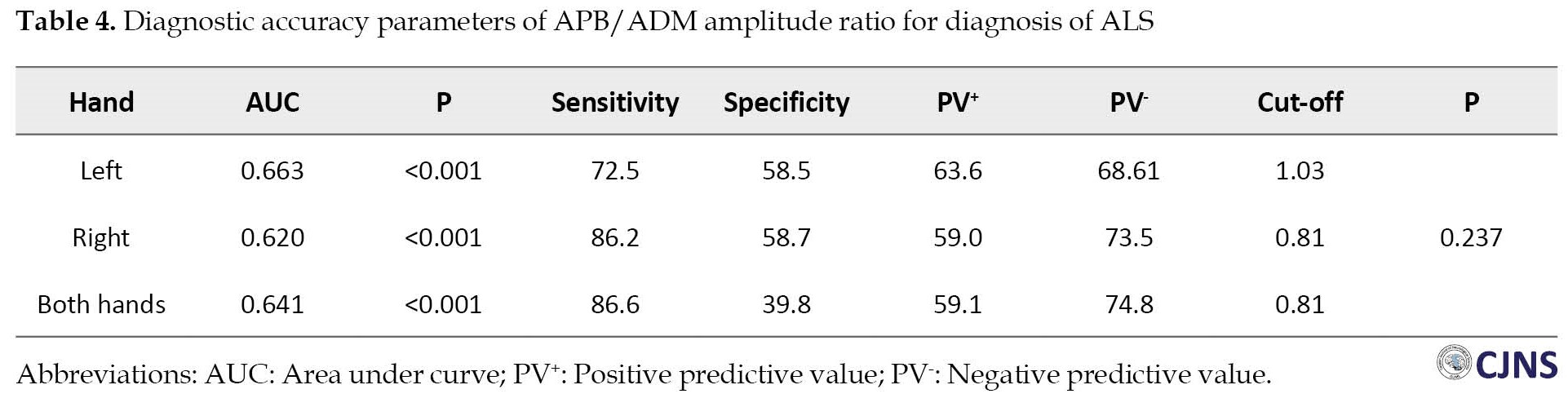
ROC curve (receiver operating characteristic curve) analysis was performed to assess the sensitivity and specificity of the APB/ADM amplitude ratio of right and left hands and both hands to discriminate patients from healthy subjects to select the best cut-off points and their respective sensitivity and specificity. The APB/ADM amplitude ratio cut-off in both hands was 0.81, with a sensitivity of 86.6% and specificity of 39.8%. A cut-off ratio of 0.81 in the right hand revealed a sensitivity of 86.2% and specificity of 58.7%. The APB/ADM amplitude ratio cut-off in the left hand was 1.03, which resulted in a sensitivity of specificity of 72.5% and 58.5%. The area under curve values for both hands was 0.641, and for left and right hands were 0.663 and 0.620 (P=0.237). Table 4 and Figure 1 present more details on APB/ADM amplitude ratio diagnostic accuracy parameters for diagnosing ALS.
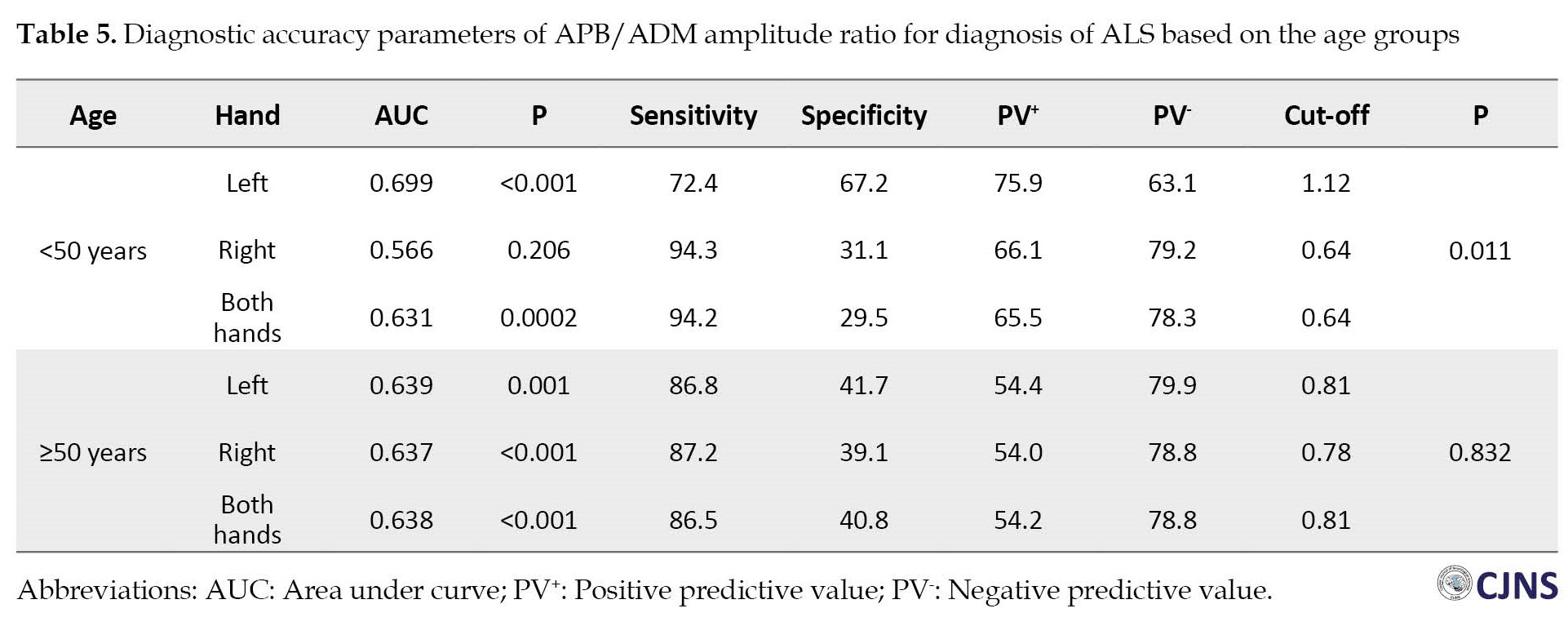
To be more specific, data were categorized into two classes below and over 50 years of age. In patients below 50 years of age, APB/ADM amplitude ratio cut-off point was calculated as 1.12 (sensitivity=72.4%, specificity=67.2%) and 0.64 (sensitivity=94.3% and specificity=31.1%) and 0.64 (sensitivity=94.2% and specificity=29.5%) for left, right, and both hands together, respectively. In participants over 50 years of age, these figures were 0.81 (sensitivity=86.8% and specificity=41.7%) and 0.78 (sensitivity=87.2% and specificity=39.1%), and 0.81 (sensitivity=86.5% and specificity=40.8%), respectively. In the group below 50 years of age, the area under the curve of APB/ADM amplitude ratio in the left hand was significantly higher than the right hand (0.699 vs 0.566, P=0.011), while in the over 50 years of age, no significant difference was found between right and left hands (0.637 vs 0.639, P=0.832). The area under the curve of APB/ADM amplitude ratio in both hands together in participants below and over 50 years of age were 0.631 and 0.638, respectively. More details are presented in Table 5.
Discussion
The current study assessed APB/ADM CMAP ratio as one of the key indicators of the split hand phenomenon in patients with ALS. In this study, we found that APB/ADM CMAP ratio is significantly higher in the left hands of controls compared to patients, while no significant difference was observed in the right hands or both hands together. Analysis based on age groups also showed that in the <50 years of age group, APB/ADM CMAP ratio was significantly higher for the right hand in controls compared to patients, while no remarkable difference was found in the age group of over 50 years. Our results indicated that APB/ADM CMAP ratio could yield cut-off values for discriminating ALS patients from healthy controls with satisfactory sensitivities.
The split hand is reported to be present in around 50% of ALS cases, meaning that not every patient with ALS has a split hand. On the other hand, not all patients with split hands have ALS. Thus, the differentiation of ALS from other conditions in the case of split hand is a field of interest. In our study, APB/ADM amplitude ratio was equal between patients and controls in the right hand (1.27), while in the left hand, patients showed significantly lower values compared to controls (1.11 vs. 1.30, P=0.018) and in both hands together, patients showed higher values compared to controls (1.38 vs. 0.63, P=0.203). Consistent with our findings, Hu et al. reported in a systematic review that APB/ADM CMAP in ALS patients was significantly lower than in healthy controls [17]. Also, De Carvalho et al. reported that CMAP amplitudes of APB and FDI showed significant decrements over 6 months. In contrast, ADM muscle amplitude decrement was much lower than APB and FDI muscles, leading to a low APB/ADM amplitude ratio in ALS patients [18]. Kim et al. also showed that APB/ADM CMAP ratio was significantly lower in ALS patients (0.64) compared to healthy controls (1.01) [19]. Although it is well-established that APB/ADM amplitude ratio is reduced in ALS patients, its diagnostic yield is still debatable. We measured this ratio in two age groups below and over 50 for more elucidation. In the over 50 years group, no significant difference in APB/ADM amplitude ratio was noted between controls and patients, while in the below 50 years group, ALS patients showed significantly lower values compared to controls in the left hand. No previous report has found and discussed differences between right and left hands as in our study. To justify our findings, we may attribute this discrepancy between these two hands to the fact that most of the population is right-handed. Right-handedness can put nerves and muscles under the compressive burden and subsequent degenerative changes. Thus, left hands can be more reliably judged in these clinical settings and assessments.
Disease progression usually involves thenar muscles (APB and FDI) at first and spares the hypothenar muscles (ADM). More advanced stages of the disease are associated with the involvement of hypothenar muscles. Simultaneous involvement of ADM and APB muscles in the progressive stages of ALS raises the APB/ADM ratio from the lower values usually found in ALS patients. Higher ages are usually associated with higher stages of the disease. As explained, we expect the APB/ADM ratio to increase to around normal values in the healthy population. Thus, our study found no significant difference between ALS patients and controls regarding the APB/ADM amplitude ratio in the age group of over 50 years. Consistent with our findings, Fang et al. have stated that aging was associated with the decline of APB and ADM amplitudes, but the extent of their change is equivalent, which makes the use of the APM/ADM amplitude ratio limited in aged patients. Thus, APB/ADM ratio is more beneficial in assessing individuals below the age of 50 years [20].
The diagnostic accuracy of the APB/ADM amplitude ratio has been evaluated in several studies to figure out the clinical utility of this marker in the differentiation of ALS patients from healthy controls. In our research, the cut-off points of 0.81 (sensitivity=86.2% and specificity=58.7%) and 1.03 (sensitivity=72.5% and specificity=58.5%), and 0.81 (sensitivity=76.6% and specificity=39.8%) were calculated for right, left, and both hands together, respectively. Jiang et al. reported that a cut-off value of 0.93 for APB/ADM yielded a sensitivity of 75.8% and specificity of 83.1% for diagnosing ALS. Another study by Kim et al. with 0.58 as the cut-off value of the APB/ADM ratio resulted in a sensitivity of 67% and specificity of 92% for discrimination of ALS from other mimicking disorders and controls [19]. Kalita et al. also reported a sensitivity of 61% and specificity of 91% for the differentiation of ALS from other similar clinical conditions mimicking ALS with a cut-off value of 1.2 [21]. The mutual finding of the mentioned studies is that APB/ADM amplitude ratio is a highly specific marker for ALS with moderate sensitivity, but our results are in contrast with their findings indicating high sensitivity and moderate specificity for APB/ADM ratio. The reasons for this finding should be investigated in future studies, but selection bias, sample population, and study design can play roles in achieving these results. For instance, most of these studies have reported the sensitivity and specificity of the APB/ADM ratio in differentiating ALS from other neurologic/mimicking disorders.
In contrast, in our study, we tried to discriminate ALS from healthy controls. In addition, we provided cut-off points of APB/ADM amplitude ratio in two age groups below and over 50 years which were not previously reported in the literature. These figures must be evaluated and confirmed in future studies with larger sample sizes.
Our study had several limitations. First, we did not evaluate the split hand index (SI) as another key indicator for comparing its clinical utility and diagnostic accuracy with APB/ADM amplitude ratio. Second, we did not assess study subjects throughout the time to evaluate disease progression and its corresponding changes in measured ratios. Third, our study did not include disorders mimicking ALS and was limited only to healthy controls. Evaluation of ALS mimicking disorders in addition to ALS can provide more valuable information on the clinical utility of APB/ADM amplitude ratio in daily practice.
Conclusions
APB/ADM CMAP ratio is a relatively highly sensitive but moderately specific diagnostic marker for differentiating ALS patients from healthy controls with higher diagnostic utility in patients <50.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki (2013). The study protocol was approved by the Ethics Committee of Alzahra Research Centers (Code: IR.ARI.MUI.REC.1400.072).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, methodology, and investigation: Majid Ghasemi, Maryam Alizadeh, Keyvan Basiri, and Behnaz Ansari; Data collection: Maryam Alizadeh and Reza Tayebi Khorami; Writing draft, review, and editing: Maryam Alizadeh; Funding acquisition: Majid Ghasemi; Resources and supervision: Majid Ghasemi and Maryam Alizadeh.
Conflict of interest
The authors declared no competing interests.
References
Amyotrophic lateral sclerosis (ALS), a neurodegenerative disease, manifests with progressive paralysis in skeletal muscles [1] reports on its incidence range from 0.6 to 3.8 per 100000 person-years. The ALS incidence has continuously risen in recent years [2]. The time between the onset of disease to dependency on respiratory support or death is reported to be around 24 to 50 months [3, 4].
Several diagnostic criteria and markers have been suggested in the literature [5], but more consistent markers are required due to difficulties and delays in diagnosing ALS.
Split hand phenomenon, first introduced by Wilbourn and Sweeney [6], is a specific pattern characterized by atrophy of thenar muscles (abductor pollicis brevis [APB] and first dorsal interosseous muscles [FDI]) with relative preservation of hypothenar muscles (abductor digiti minimi [ADM]) [7]. Since its introduction in the literature, the split hand has been considered a specific and characteristic sign that helps diagnose ALS [8]. Markers of the split hand, including split hand index (SI) and APB/ADM ratio measured by compound muscle action potentials (CMAP), have been evaluated for their diagnostic accuracy for ALS. Still, no agreement has been reached on the best choice [9, 10]. Normal adult values of APB and ADM CMAP amplitude are ≥4.0 and ≥6.0, respectively, and their normal ratio is 0.6 [11].
In nerve conduction studies (NCSs), a decreased APB/ADM CMAP amplitude ratio (<0.6) reflects the split hand phenomenon, which in ALS indicates cortical motor neuron compromise, particularly because APB and FDI are muscles with extensive corticospinal connections affected by glutamate excitotoxicity [7]. The split hand phenomenon is observed in 55% of ALS patients. The dissociated hand muscle atrophy, particularly the combination of APB/ADM ratio <0.6 and FDI/ADM ratio <0.9, is rarely found in pure LMN disease, cervical spondylotic amyotrophy, and polyneuropathies [12].
Diagnosing ALS is based on the coexistence of upper and lower motor neuron involvements and progressive symptoms [13]. Numerous ALS phenotypes and the lack of a definitive diagnostic test have led to frequent diagnostic delays [14]. Subsequently, initiation of neuroprotective treatment strategies is deferred, and the disease progresses. Clinical diagnostic criteria such as El Escorial criteria are not sensitive enough to identify earlier stages of ALS [14, 15]. To enhance diagnostic sensitivity, the gold coast criteria for diagnosis of ALS has recently been proposed (Table 1) [16].

Reports have designated split hand as an early sign of ALS which can assist in the prompt diagnosis of ALS patients [7]. Thus, in this study, we evaluated the utility of the APB/ADM CMAP ratio as a diagnostic marker in evaluating ALS and its differentiation from healthy controls.
Materials and Methods
This cross-sectional retrospective study was conducted at Alzahra and Kashani hospitals of Isfahan, Iran, in 2021. The study population consisted of all patients with a definite or probable diagnosis of ALS based on the revised El Escorial criteria who had undergone electrophysiological studies within the previous 5 years. Patients with clinical suspicion of other diseases mimicking ALS and compression neuropathies, such as C8 radiculopathy and carpal tunnel syndrome, were excluded from the study. Individuals with non-specific complaints who had undergone electrophysiological studies but were not diagnosed with ALS or any other neuromuscular abnormality were enrolled in the study as healthy controls. A total of 200 ALS patients and 200 healthy controls were evaluated in our study by consecutive convenient sampling.
Demographic data, including age and gender, were collected from medical records. Electrophysiological studies of patients were retrieved to collect CMAP amplitudes of APB and ADM muscles in both study groups. Then, APB/ADM CMAP ratio was calculated in both groups based on right/left hand and below/over 50 years of age. The statistical analysis also evaluated the diagnostic accuracy of the APB/ADM amplitude ratio.
Statistical analysis was performed with IBM SPSS software, version 25 (IBM Corp., Armonk, N.Y., USA). Descriptive analysis is presented as frequency and percentage or Mean±SD. The independent t-test and chi-square test were used to compare continuous and categorical parameters between the two groups. The diagnostic yield of the APB/ADM amplitude ratio for the differentiation of ALS from healthy controls was assessed by the ROC curve to determine cut-off values and their corresponding sensitivity, specificity, positive predictive value, negative predictive value, and area under the curve. P≤0.05 was considered statistically significant.
Results
A total of 200 patients with ALS and 200 healthy controls were enrolled in this study. Overall, 225 participants (56.3%) were males, and 185(46.25%) were females. The mean age of participants was 53.29±12.40 years (16-80 years). To be more specific, 247 participants (61.75%) were in the >50 years age group, while 153 participants (38.3%) were below 50 years. Also, 63 participants were patients, and 90 participants were healthy.
The mean age of the patients and controls was 54.44±13.25 years (16-77) and 52.15±11.41 years (25-80), respectively (P=0.065). The patients’ group consisted of 122 males (61%) and 78 females (39%), while 105 subjects (52.5%) and 95 (47.5%) in the control group were males and females, respectively (P=0.086).
APB/ADM amplitude ratio for diagnosis of ALS
APB/ADM amplitude ratio showed no significant difference in the right hand and both hands together between patients and controls. However, this ratio in the left hand was significantly higher in healthy controls than in patients (P=0.018). The results showed that in patients below 50 years of age, a significant difference in APB/ADM amplitude ratio between patients and controls was only detected in the left hand (P=0.008), with higher levels in healthy controls compared to patients. In the age group over 50, no significant difference in APB/ADM amplitude ratio between healthy controls and patients was found in any of the hands and neither in both hands together (P>0.05). The details of APB/ADM amplitude ratio measurements are presented in Table 2 and Table 3.


Diagnostic accuracy parameters of APB/ADM amplitude ratio for diagnosis of ALS
ROC curve (receiver operating characteristic curve) analysis was performed to assess the sensitivity and specificity of the APB/ADM amplitude ratio of right and left hands and both hands to discriminate patients from healthy subjects to select the best cut-off points and their respective sensitivity and specificity. The APB/ADM amplitude ratio cut-off in both hands was 0.81, with a sensitivity of 86.6% and specificity of 39.8%. A cut-off ratio of 0.81 in the right hand revealed a sensitivity of 86.2% and specificity of 58.7%. The APB/ADM amplitude ratio cut-off in the left hand was 1.03, which resulted in a sensitivity of specificity of 72.5% and 58.5%. The area under curve values for both hands was 0.641, and for left and right hands were 0.663 and 0.620 (P=0.237). Table 4 and Figure 1 present more details on APB/ADM amplitude ratio diagnostic accuracy parameters for diagnosing ALS.

To be more specific, data were categorized into two classes below and over 50 years of age. In patients below 50 years of age, APB/ADM amplitude ratio cut-off point was calculated as 1.12 (sensitivity=72.4%, specificity=67.2%) and 0.64 (sensitivity=94.3% and specificity=31.1%) and 0.64 (sensitivity=94.2% and specificity=29.5%) for left, right, and both hands together, respectively. In participants over 50 years of age, these figures were 0.81 (sensitivity=86.8% and specificity=41.7%) and 0.78 (sensitivity=87.2% and specificity=39.1%), and 0.81 (sensitivity=86.5% and specificity=40.8%), respectively. In the group below 50 years of age, the area under the curve of APB/ADM amplitude ratio in the left hand was significantly higher than the right hand (0.699 vs 0.566, P=0.011), while in the over 50 years of age, no significant difference was found between right and left hands (0.637 vs 0.639, P=0.832). The area under the curve of APB/ADM amplitude ratio in both hands together in participants below and over 50 years of age were 0.631 and 0.638, respectively. More details are presented in Table 5.

Discussion
The current study assessed APB/ADM CMAP ratio as one of the key indicators of the split hand phenomenon in patients with ALS. In this study, we found that APB/ADM CMAP ratio is significantly higher in the left hands of controls compared to patients, while no significant difference was observed in the right hands or both hands together. Analysis based on age groups also showed that in the <50 years of age group, APB/ADM CMAP ratio was significantly higher for the right hand in controls compared to patients, while no remarkable difference was found in the age group of over 50 years. Our results indicated that APB/ADM CMAP ratio could yield cut-off values for discriminating ALS patients from healthy controls with satisfactory sensitivities.
The split hand is reported to be present in around 50% of ALS cases, meaning that not every patient with ALS has a split hand. On the other hand, not all patients with split hands have ALS. Thus, the differentiation of ALS from other conditions in the case of split hand is a field of interest. In our study, APB/ADM amplitude ratio was equal between patients and controls in the right hand (1.27), while in the left hand, patients showed significantly lower values compared to controls (1.11 vs. 1.30, P=0.018) and in both hands together, patients showed higher values compared to controls (1.38 vs. 0.63, P=0.203). Consistent with our findings, Hu et al. reported in a systematic review that APB/ADM CMAP in ALS patients was significantly lower than in healthy controls [17]. Also, De Carvalho et al. reported that CMAP amplitudes of APB and FDI showed significant decrements over 6 months. In contrast, ADM muscle amplitude decrement was much lower than APB and FDI muscles, leading to a low APB/ADM amplitude ratio in ALS patients [18]. Kim et al. also showed that APB/ADM CMAP ratio was significantly lower in ALS patients (0.64) compared to healthy controls (1.01) [19]. Although it is well-established that APB/ADM amplitude ratio is reduced in ALS patients, its diagnostic yield is still debatable. We measured this ratio in two age groups below and over 50 for more elucidation. In the over 50 years group, no significant difference in APB/ADM amplitude ratio was noted between controls and patients, while in the below 50 years group, ALS patients showed significantly lower values compared to controls in the left hand. No previous report has found and discussed differences between right and left hands as in our study. To justify our findings, we may attribute this discrepancy between these two hands to the fact that most of the population is right-handed. Right-handedness can put nerves and muscles under the compressive burden and subsequent degenerative changes. Thus, left hands can be more reliably judged in these clinical settings and assessments.
Disease progression usually involves thenar muscles (APB and FDI) at first and spares the hypothenar muscles (ADM). More advanced stages of the disease are associated with the involvement of hypothenar muscles. Simultaneous involvement of ADM and APB muscles in the progressive stages of ALS raises the APB/ADM ratio from the lower values usually found in ALS patients. Higher ages are usually associated with higher stages of the disease. As explained, we expect the APB/ADM ratio to increase to around normal values in the healthy population. Thus, our study found no significant difference between ALS patients and controls regarding the APB/ADM amplitude ratio in the age group of over 50 years. Consistent with our findings, Fang et al. have stated that aging was associated with the decline of APB and ADM amplitudes, but the extent of their change is equivalent, which makes the use of the APM/ADM amplitude ratio limited in aged patients. Thus, APB/ADM ratio is more beneficial in assessing individuals below the age of 50 years [20].
The diagnostic accuracy of the APB/ADM amplitude ratio has been evaluated in several studies to figure out the clinical utility of this marker in the differentiation of ALS patients from healthy controls. In our research, the cut-off points of 0.81 (sensitivity=86.2% and specificity=58.7%) and 1.03 (sensitivity=72.5% and specificity=58.5%), and 0.81 (sensitivity=76.6% and specificity=39.8%) were calculated for right, left, and both hands together, respectively. Jiang et al. reported that a cut-off value of 0.93 for APB/ADM yielded a sensitivity of 75.8% and specificity of 83.1% for diagnosing ALS. Another study by Kim et al. with 0.58 as the cut-off value of the APB/ADM ratio resulted in a sensitivity of 67% and specificity of 92% for discrimination of ALS from other mimicking disorders and controls [19]. Kalita et al. also reported a sensitivity of 61% and specificity of 91% for the differentiation of ALS from other similar clinical conditions mimicking ALS with a cut-off value of 1.2 [21]. The mutual finding of the mentioned studies is that APB/ADM amplitude ratio is a highly specific marker for ALS with moderate sensitivity, but our results are in contrast with their findings indicating high sensitivity and moderate specificity for APB/ADM ratio. The reasons for this finding should be investigated in future studies, but selection bias, sample population, and study design can play roles in achieving these results. For instance, most of these studies have reported the sensitivity and specificity of the APB/ADM ratio in differentiating ALS from other neurologic/mimicking disorders.
In contrast, in our study, we tried to discriminate ALS from healthy controls. In addition, we provided cut-off points of APB/ADM amplitude ratio in two age groups below and over 50 years which were not previously reported in the literature. These figures must be evaluated and confirmed in future studies with larger sample sizes.
Our study had several limitations. First, we did not evaluate the split hand index (SI) as another key indicator for comparing its clinical utility and diagnostic accuracy with APB/ADM amplitude ratio. Second, we did not assess study subjects throughout the time to evaluate disease progression and its corresponding changes in measured ratios. Third, our study did not include disorders mimicking ALS and was limited only to healthy controls. Evaluation of ALS mimicking disorders in addition to ALS can provide more valuable information on the clinical utility of APB/ADM amplitude ratio in daily practice.
Conclusions
APB/ADM CMAP ratio is a relatively highly sensitive but moderately specific diagnostic marker for differentiating ALS patients from healthy controls with higher diagnostic utility in patients <50.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki (2013). The study protocol was approved by the Ethics Committee of Alzahra Research Centers (Code: IR.ARI.MUI.REC.1400.072).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, methodology, and investigation: Majid Ghasemi, Maryam Alizadeh, Keyvan Basiri, and Behnaz Ansari; Data collection: Maryam Alizadeh and Reza Tayebi Khorami; Writing draft, review, and editing: Maryam Alizadeh; Funding acquisition: Majid Ghasemi; Resources and supervision: Majid Ghasemi and Maryam Alizadeh.
Conflict of interest
The authors declared no competing interests.
References
- Al-Chalabi A, Hardiman O. The epidemiology of ALS: A conspiracy of genes, environment and time. Nat Rev Neurol. 2013; 9(11):617-28. [DOI:10.1038/nrneurol.2013.203] [PMID]
- Longinetti E, Fang F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr Opin Neurol. 2019; 32(5):771-6. [DOI:10.1097/WCO.0000000000000730] [PMID] [PMCID]
- Benjaminsen E, Alstadhaug KB, Gulsvik M, Baloch FK, Odeh F. Amyotrophic lateral sclerosis in Nordland county, Norway, 2000-2015: Prevalence, incidence, and clinical features. Amyotroph Lateral Scler Frontotemporal Degener. 2018; 19(7-8):522-7. [DOI:10.1080/21678421.2018.1513534] [PMID]
- Jun KY, Park J, Oh KW, Kim EM, Bae JS, Kim I, et al. Epidemiology of ALS in Korea using nationwide big data. J Neurol Neurosurg Psychiatry. 2019; 90(4):395-403. [DOI:10.1136/jnnp-2018-318974] [PMID] [PMCID]
- Xu RS, Yuan M. Considerations on the concept, definition, and diagnosis of amyotrophic lateral sclerosis. Neural Regen Res. 2021; 16(9):1723-9. [DOI:10.4103/1673-5374.306065] [PMID] [PMCID]
- Wilbourn AJ. Plexopathies. Neurol Clin. 2007; 25(1):139-71. [DOI:10.1016/j.ncl.2006.11.005] [PMID]
- Kuwabara S, Sonoo M, Komori T, Shimizu T, Hirashima F, Inaba A, et al. Dissociated small hand muscle atrophy in amyotrophic lateral sclerosis: Frequency, extent, and specificity. Muscle Nerve. 2008; 37(4):426-30. [DOI:10.1002/mus.20949] [PMID]
- Abraham A, Fainmesser Y, Drory VE, Bril V. Split-hand phenomenon in motor neuron diseases: Sonographic assesment of muscle thickness. Clin Neurophysiol. 2020; 131(8):1721-5.[DOI:10.1016/j.clinph.2020.04.163] [PMID]
- Bae JS, Menon P, Mioshi E, Kiernan MC, Vucic S. Cortical hyperexcitability and the split-hand plus phenomenon: pathophysiological insights in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014; 15(3-4):250-6. [DOI:10.3109/21678421.2013.872150] [PMID]
- Menon P, Bae JS, Mioshi E, Kiernan MC, Vucic S. Split-hand plus sign in ALS: Differential involvement of the flexor pollicis longus and intrinsic hand muscles. Amyotroph Lateral Scler Frontotemporal Degener. 2013; 14(4):315-8. [DOI:10.3109/21678421.2012.734521] [PMID]
- Preston DC, Shapiro B. Electromyography and neuromuscular disorders: clinical-electrophysiologic-ultrasound correlations. Amsterdam: Elsevier; 2020. [Link]
- Wang ZL, Cui L, Liu M, Zhang K, Liu S, Ding Q. Split-hand syndrome in amyotrophic lateral sclerosis: Differences in dysfunction of the FDI and ADM spinal motoneurons. Front Neurosci. 2019; 13:371. [DOI:10.3389/fnins.2019.00371] [PMID] [PMCID]
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. 2011; 377(9769):942-55. [DOI:10.1016/S0140-6736(10)61156-7] [PMID]
- Chiò A, Cucatto A, Calvo A, Terreni AA, Magnani C, Schiffer D. Amyotrophic lateral sclerosis among the migrant population to Piemonte, northwestern Italy. J Neurol. 1999; 246(3):175-80. [DOI:10.1007/s004150050330] [PMID]
- Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 2009; 8(1):94-109. [DOI:10.1016/S1474-4422(08)70293-X] [PMID]
- Shefner JM, Al-Chalabi A, Baker MR, Cui LY, de Carvalho M, Eisen A, et al. A proposal for new diagnostic criteria for ALS. Clin Neurophysiol. 2020; 131(8):1975-8. [DOI:10.1016/j.clinph.2020.04.005] [PMID]
- Hu N, Wang J, Liu M. Split hand in amyotrophic lateral sclerosis: A systematic review and meta-analysis. J Clin Neurosci. 2021; 90:293-301. [DOI:10.1016/j.jocn.2021.06.015] [PMID]
- de Carvalho M, Swash M. The split hand in amyotrophic lateral sclerosis: A possible role for the neuromuscular junction. Amyotroph Lateral Scler Frontotemporal Degener. 2019; 20(5-6):368-75. [DOI:10.1080/21678421.2019.1606245] [PMID]
- Kim JE, Hong YH, Lee JH, Ahn SW, Kim SM, Park KS, et al. Pattern difference of dissociated hand muscle atrophy in amyotrophic lateral sclerosis and variants. Muscle Nerve. 2015; 51(3):333-7. [DOI:10.1002/mus.24323] [PMID]
- Fang J, Liu MS, Guan YZ, Du H, Li BH, Cui B, et al. Pattern differences of small hand muscle atrophy in amyotrophic lateral sclerosis and mimic disorders. Chin Med J. 2016; 129(7):792-8. [DOI:10.4103/0366-6999.178953] [PMID] [PMCID]
- Kalita J, Kumar S, Misra UK, Neyaz Z. Split hand index and ulnar to median ratio in Hirayama disease and amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017; 18(7-8):598-603. [DOI:10.1080/21678421.2017.1336561] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2023/07/5 | Accepted: 2023/07/28 | Published: 2023/07/28
Received: 2023/07/5 | Accepted: 2023/07/28 | Published: 2023/07/28
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







