Sat, May 18, 2024
Volume 9, Issue 3 (Summer 2023)
Caspian J Neurol Sci 2023, 9(3): 154-161 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bakhshayesh Eghbali B, Saberi A, Jalali Emam S Z, Fallah-Arzpayma S, Zare R, Ramezani S et al . Changes in Seizure Frequency and Characteristics During COVID-19 Pandemic: A Hospital-based Study. Caspian J Neurol Sci 2023; 9 (3) :154-161
URL: http://cjns.gums.ac.ir/article-1-639-en.html
URL: http://cjns.gums.ac.ir/article-1-639-en.html
Babak Bakhshayesh Eghbali * 

 1, Alia Saberi2
1, Alia Saberi2 

 , Seyede Zahra Jalali Emam3
, Seyede Zahra Jalali Emam3 

 , Sima Fallah-Arzpayma4
, Sima Fallah-Arzpayma4 

 , Roghaye Zare5
, Roghaye Zare5 

 , Sara Ramezani6
, Sara Ramezani6 

 , Mohammad Ali Yazdanipour5
, Mohammad Ali Yazdanipour5 




 1, Alia Saberi2
1, Alia Saberi2 

 , Seyede Zahra Jalali Emam3
, Seyede Zahra Jalali Emam3 

 , Sima Fallah-Arzpayma4
, Sima Fallah-Arzpayma4 

 , Roghaye Zare5
, Roghaye Zare5 

 , Sara Ramezani6
, Sara Ramezani6 

 , Mohammad Ali Yazdanipour5
, Mohammad Ali Yazdanipour5 


1- Department of Neurology, Neuroscience Research Center, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Neurology, Neuroscience Research Center, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
3- Neuroscience Research Center, Student Research Committee, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Radiology, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
5- Neuroscience Research Center, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
6- Guilan Road Trauma Research Center, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Neurology, Neuroscience Research Center, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
3- Neuroscience Research Center, Student Research Committee, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Radiology, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
5- Neuroscience Research Center, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
6- Guilan Road Trauma Research Center, Guilan University of Medical Sciences, Rasht, Iran.
Full-Text [PDF 1148 kb]
(258 Downloads)
| Abstract (HTML) (483 Views)
Full-Text: (111 Views)
Introduction
The novel coronavirus disease 2019 (COVID-19) pandemic has been since December 2019 [1]. Based on May 18, 2022, World Health Organization (WHO) situation report, there have been 520372000 confirmed cases of COVID-19, including 6270000 deaths worldwide [2]. Given the COVID-19 pandemic continues, a better understanding of the neurological manifestations and accompanying symptoms of COVID-19 is a serious priority [3]. Different neurological manifestations have been reported that are caused or provoked by COVID-19, including stroke, cerebral vein thrombosis, myelitis, and peripheral neuropathy [4, 5, 6, 7, 8, 9, 10, 11].
Evidence has signified the incidence of acute seizure as one of the well-known complications in critically-ill patients with sepsis, presumably affected by COVID-19 [12]. Likewise, some consequences of COVID-19, such as multi-organ failure and severe metabolic and electrolyte disturbance, may induce a subclinical and clinical acute symptomatic seizure in some patients [13, 14]. It has been reported that the first wave of the COVID-19 pandemic led to worsening seizure control in patients with epilepsy [15]. Also, there are some reports of COVID-19 presenting with a seizure [16].
In assessing 2 cohort studies in the UK, the incidence of new seizures or epilepsy diagnoses in the 6 months after COVID-19 was low but higher than in matched patients with influenza. This difference was more obvious among patients not hospitalized, indicating the risk of epilepsy and seizures in COVID-19 with different severity [17].
On the other hand, based on the evidence, epilepsy may bring about high hospital burdens such as increased costs and length of hospitalization [18].
However, the impact of the COVID-19 period on seizure burden changes in hospitalized patients is still unclear. Hence, this study aimed to investigate the burden and characteristics of seizures in hospitalized patients during the COVID-19 pandemic compared to last year.
Materials and Methods
In this observational cross-sectional comparative study, patients hospitalized in the neurology unit (NU) of an academic hospital in the north of Iran from February to August 2019 and 2020 were included in the emergency unit (EU). The emergency physician admitted those with recurrent or prolonged convulsive seizures, abnormal neurologic exam, ongoing seizure, or within <90 minutes of seizure termination. Otherwise, patients are usually discharged to the rapid-access outpatient neurology clinic. The admitted patients in the EU were then stabilized, treated as necessary, and then visited by the neurology consultant to decide early discharge versus admission to NU. We also included patients with seizures during hospitalization due to other neurologic conditions.
Probable coronavirus infection was defined when the clinical symptoms were accompanied by either a positive result of SARS-CoV-2 RT-PCR (reverse transcription-polymerase chain reaction) assay [19] or COVID-19 manifestations on chest computerized tomography (CT) findings.
Data including gender, age, seizure history, type, and etiology during hospitalization were recorded. A neurological examination was done on each patient to diagnose the seizure. In addition, routine neuro-radiological techniques consisting of CT scan or structural magnetic resonance imaging (MRI) were carried out to determine structural abnormality of the brain. Furthermore, conventional EEG was accomplished for all patients according to the international 10-20 system. The patients suspected of having neuro infection and subarachnoid hemorrhage were tested using a lumbar puncture. Routine laboratory experiments such as blood gases and cells, electrolytes, liver and renal function, C-reactive protein, and coagulant profile were performed for all patients. Demographic, clinical, and paraclinical data were collected and entered into the statistical software.
Statistical analysis
All analyses and calculations were performed in SPSS software, version 16 (SPSS Inc., Chicago, IL). Continuous variables are presented as Mean±SD, whereas categorical variables are expressed as numbers and percentages. The independent sample t-test, chi-square test, and Fisher exact test were applied to analyze the quantitative and categorical data differences between variables. The significant level was considered at P<0.05.
Results
A total of 1525 patients were hospitalized in the NU of Poursina Hospital in Rasht City, Iran, before COVID-19 and 985 patients during COVID-19. In particular, 149 patients with seizures (15.1%) were admitted between February and August 2020 during COVID-19, and 179 patients with seizures (11.7%) were admitted in the same period the year before (Figure 1). This increase in seizure frequency during COVID-19 versus pre-pandemic was statistically significant (P=0.014).
According to the findings in Table 1, there was no significant difference in the mean age between seizure patients hospitalized in NU during COVID-19 (51.3±18.9 years) and the same period last year (52.3±19.3 years).
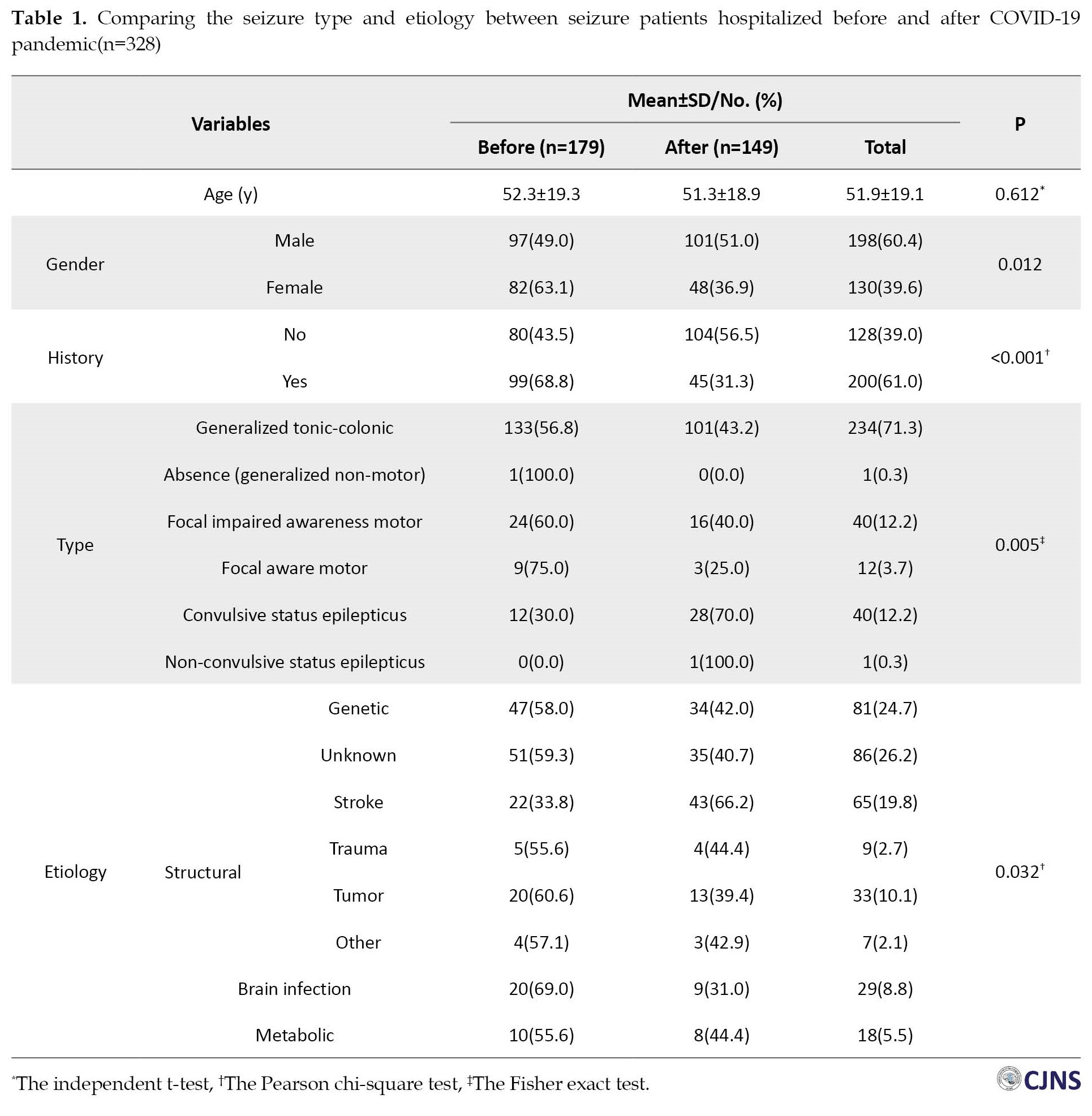
However, a considerable difference was observed between the two study periods regarding gender (P=0.012). Notably, a drastic decrease in the frequency of female patients with seizures was reported during the COVID-19 pandemic (36.9%) versus the pre-pandemic period (63.1%). Also, the previous history of seizure was significantly related to the study period, and it was more frequent before the COVID-19 pandemic (68.8% vs 31.3%, P<0.001). The results showed that the distribution of seizure types was significantly different between the COVID-19 pandemic and the same period the year before (P=0.005). The percentage of convulsive status epilepticus (CSE) seizures during the COVID-19 pandemic (70%) was substantially elevated relative to pre-pandemic (40%). Furthermore, the frequency of some types of seizure, such as focal impaired awareness motor (FIAM), focal aware motor (FAM), and generalized tonic-clonic (GTC) before the COVID-19 pandemic were 60%, 75%, and 56.8%, respectively that hugely declined during the COVID-19 period. Based on the results of this study, a significant difference was disclosed between the two periods of the study in terms of seizure etiology (P=0.032). A remarkable increase was seen in the frequency of seizure patients with stroke during COVID-19 (66.2%) pandemic versus pre-pandemic (33.8%). Those who experienced seizures due to brain infection had a higher percentage during COVID-19 (69%) than during the COVID-19 era (31%). Additionally, the frequency of some seizure etiologies, such as unknown (40.7%) and genetic (42%), decreased during COVID-19 compared to the same period the year before. More details are indicated in Table 1.
Interestingly, out of 149 seizure patients during COVID-19, 64(43.0%) demonstrated positive results in COVID-19 tests. Unexpectedly, there was no significant relationship between the presence of coronavirus infection and seizure characteristics in hospitalized patients having seizures. Coronavirus infection was not also related to gender in the patients with seizures hospitalized in the NU during the initial peaks of the COVID-19 era. Table 2 depicts more information.

Discussion
In this project, we witnessed a drastic reduction in total admission in the NU of Poursina Hospital during the COVID-19 pandemic. However, a notable increase in seizure frequency was reported during the COVID-19 pandemic period as compared with the same period last year. The hospital burden of seizure increases despite the decrease in the absolute total number of seizures during the COVID-19 pandemic might be due to more severe cases during this period.
But in a retrospective cohort study in the UK, this increase is not related to the hospitalization changes and the incidence of new seizures or epilepsy diagnoses in the 6 months after COVID-19 was higher than in matched patients with influenza. This difference was more marked in people not hospitalized, highlighting the risk of epilepsy and seizures in COVID-19 regardless of severity [17].
Furthermore, the frequency of type, etiology, history of seizure, and gender have changed during the COVID-19 pandemic. Particularly, stroke patients who manifested seizures doubled during the COVID-19 period versus before it. It is plausible that those with a good prognosis in an emergency were discharged. Thus, those with poorer outcomes and more susceptibility to seizure development were hospitalized during COVID-19, which increased the seizure burden. COVID-19 also has been suggested as a stroke risk factor [6, 7, 9]. There is a complex interplay between seizure, stroke, and COVID-19. Another possibility is that stressful circumstances induced by the COVID-19 pandemic might lessen medical examination rates and slow nursing care and proper medical management of hospitalized stroke patients in NU, leading to more complications simultaneously. On the other hand, it may lead to overlook some seizures.
A previous study postulated that COVID-19 could be directly associated with seizures in patients with neurological manifestations [20]. The results of our study also displayed that nearly half of the seizure patients during the COVID-19 era were concurrently affected by COVID-19-induced infection. Additionally, the same study indicated that age was an influential factor in the development of seizures in patients with severe acute respiratory syndrome COVID-19 infection. Older people were more likely to have seizures. But in our study, age did not affect seizure prevalence. Likewise, female patients with coronavirus infection seem more susceptible than male ones to seizure development [20]. Although we did not investigate the risk of seizure occurrence between the patients with and without coronavirus infection, our analysis of the seizure patients demonstrated a substantial association of gender but not age with the COVID-19 period.
Based on the literature, the relationship between coronavirus infection and seizure has been very controversial. Sometimes it has been claimed that seizures were unrelated to the coronavirus [21]. However, some evidence has suggested that coronavirus infection might be associated with some neurological manifestations, such as seizures, impaired level of consciousness, and encephalitis. Indeed, the coronavirus cannot cause or provoke seizures in susceptible individuals, such as patients with epilepsy. However, fever is a known precipitating factor in some patients with epilepsy [22]. The destructive effects of COVID-19 on the central nervous system are mainly caused by a cytokine storm produced by either the entry of pro-inflammatory cytokines from the periphery into the CNS or the production of these cytokines by activated microglia. It can result in neurotransmitter modulation and ion channel dysfunction, leading to neuronal hyperexcitability, presenting as seizures. Pro-inflammatory cytokines, such as tumor necrosis factor-alpha, interleukin 1-beta (IL-1B), interleukin 6 (IL-6), free radicals, nitric oxide, and others are released, leading to acute or chronic inflammation, causing neuronal hyperexcitability and potentially resulting in seizures. These cytokines aggravate apoptosis and neuronal necrosis in the CNS, and they also stimulate glutamate release while inhibiting GABA release in the hippocampus and cerebral cortex (IL-1B, TNF-α). Additionally, using α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) and N-methyl-D-aspartate (NMDA) receptors, these cytokines increase calcium entry into the neurons, leading to neuronal hyper-excitability (TNF-α). Lastly, they release neurotoxic compounds through different autocrine/paracrine mechanisms (IL-6). Secondary seizures may be initiated after strokes, elevated levels of IL-6, electrolyte imbalance, increased oxidative stress, hypoxia, and mitochondrial dysfunction in COVID-19 patients [16, 23].
In our study, the other results were that the frequency of patients with seizure history markedly attenuated during the COVID-19 pandemic compared to pre-pandemic. This result might be due to less willingness of patients with a seizure history for hospitalization during the COVID-19 lockdown versus before the pandemic for the psycho-social effects of the COVID-19 pandemic. Nevertheless, a dramatic enhancement of hospitalization was discovered in patients without a seizure history. An explanation for this result might be due to a referral bias and the higher degree of anxiety related to the first seizure. A higher percentage of malignant patients with more critical underlying etiology were referred to the hospital and transferred to NU. Subsequently, the probability of new-onset seizures induced by the above disorders was increased during COVID-19.
In particular, a remarkable elevation of convulsive status epilepticus during COVID-19 compared to the pre-pandemic. Also, the COVID-19 era was associated with more seizure cases. These findings confirm the previous theory that patients with malignant seizures were probably prevalently hospitalized during the pandemic versus the pre-pandemic period. However, generalized tonic-colonic seizure similarly was the most prevalent type of seizure during the two time periods of this study.
Interestingly, we found no profound association of coronavirus infection with seizure types, etiology, and history. It seems that seizure characteristics were not related to coronavirus infection.
It is suggested that a prospective study with large sample size is designed to follow the seizure development in patients with COVID-19. A similar multicenter investigation is also recommended. Finally, it is seriously suggested to investigate the medications and treatment processes during the COVID-19 pandemic in hospitalized seizure patients in future studies.
Conclusions
We found that the frequency of seizures in hospitalized patients during COVID-19 was substantially higher than before the COVID-19 pandemic. Less than half of seizure patients hospitalized during the COVID-19 pandemic had a positive COVID-19. Gender but not age as a demographic parameter in seizure patients differed between the two time periods of study. The most prevalent causes of seizures and stroke before and during the COVID-19 pandemic are unknown. The more common type of seizure during the two periods of the study was generalized tonic-clonic seizure. COVID-19 era may increase the appearance of malignant seizures such as convulsive status epilepticus by 4-fold. It seems that lockdown due to the COVID-19 pandemic might intensify the hospital burden of seizure but not its incidence.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki (2013).The study was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1399.559).
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
Conceptualization: Babak Bakhshayesh Eghbali, Alia Saberi, Seyede Zahra Jalali Emam, Sima Fallah Arzpayma, and Roghaye Zare; Methodology and data analysis: Roghaye Zare and Mohammad Ali Yazdanipour; Data collection: Alia Saberi, Babak Bakhshayesh Eghbali, Seyede Zahra Jalali Emam, and Sima Fallah Arzpayma; Writing the original draft, editing, and review: Alia Saberi, Babak Bakhshayesh Eghbali, Mohammad Ali Yazdanipour, and Sara Ramezani; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We acknowledge the assistance of Neuroscience Research Center and Clinical Research Development Unit of Poursina Hospital.
References
The novel coronavirus disease 2019 (COVID-19) pandemic has been since December 2019 [1]. Based on May 18, 2022, World Health Organization (WHO) situation report, there have been 520372000 confirmed cases of COVID-19, including 6270000 deaths worldwide [2]. Given the COVID-19 pandemic continues, a better understanding of the neurological manifestations and accompanying symptoms of COVID-19 is a serious priority [3]. Different neurological manifestations have been reported that are caused or provoked by COVID-19, including stroke, cerebral vein thrombosis, myelitis, and peripheral neuropathy [4, 5, 6, 7, 8, 9, 10, 11].
Evidence has signified the incidence of acute seizure as one of the well-known complications in critically-ill patients with sepsis, presumably affected by COVID-19 [12]. Likewise, some consequences of COVID-19, such as multi-organ failure and severe metabolic and electrolyte disturbance, may induce a subclinical and clinical acute symptomatic seizure in some patients [13, 14]. It has been reported that the first wave of the COVID-19 pandemic led to worsening seizure control in patients with epilepsy [15]. Also, there are some reports of COVID-19 presenting with a seizure [16].
In assessing 2 cohort studies in the UK, the incidence of new seizures or epilepsy diagnoses in the 6 months after COVID-19 was low but higher than in matched patients with influenza. This difference was more obvious among patients not hospitalized, indicating the risk of epilepsy and seizures in COVID-19 with different severity [17].
On the other hand, based on the evidence, epilepsy may bring about high hospital burdens such as increased costs and length of hospitalization [18].
However, the impact of the COVID-19 period on seizure burden changes in hospitalized patients is still unclear. Hence, this study aimed to investigate the burden and characteristics of seizures in hospitalized patients during the COVID-19 pandemic compared to last year.
Materials and Methods
In this observational cross-sectional comparative study, patients hospitalized in the neurology unit (NU) of an academic hospital in the north of Iran from February to August 2019 and 2020 were included in the emergency unit (EU). The emergency physician admitted those with recurrent or prolonged convulsive seizures, abnormal neurologic exam, ongoing seizure, or within <90 minutes of seizure termination. Otherwise, patients are usually discharged to the rapid-access outpatient neurology clinic. The admitted patients in the EU were then stabilized, treated as necessary, and then visited by the neurology consultant to decide early discharge versus admission to NU. We also included patients with seizures during hospitalization due to other neurologic conditions.
Probable coronavirus infection was defined when the clinical symptoms were accompanied by either a positive result of SARS-CoV-2 RT-PCR (reverse transcription-polymerase chain reaction) assay [19] or COVID-19 manifestations on chest computerized tomography (CT) findings.
Data including gender, age, seizure history, type, and etiology during hospitalization were recorded. A neurological examination was done on each patient to diagnose the seizure. In addition, routine neuro-radiological techniques consisting of CT scan or structural magnetic resonance imaging (MRI) were carried out to determine structural abnormality of the brain. Furthermore, conventional EEG was accomplished for all patients according to the international 10-20 system. The patients suspected of having neuro infection and subarachnoid hemorrhage were tested using a lumbar puncture. Routine laboratory experiments such as blood gases and cells, electrolytes, liver and renal function, C-reactive protein, and coagulant profile were performed for all patients. Demographic, clinical, and paraclinical data were collected and entered into the statistical software.
Statistical analysis
All analyses and calculations were performed in SPSS software, version 16 (SPSS Inc., Chicago, IL). Continuous variables are presented as Mean±SD, whereas categorical variables are expressed as numbers and percentages. The independent sample t-test, chi-square test, and Fisher exact test were applied to analyze the quantitative and categorical data differences between variables. The significant level was considered at P<0.05.
Results
A total of 1525 patients were hospitalized in the NU of Poursina Hospital in Rasht City, Iran, before COVID-19 and 985 patients during COVID-19. In particular, 149 patients with seizures (15.1%) were admitted between February and August 2020 during COVID-19, and 179 patients with seizures (11.7%) were admitted in the same period the year before (Figure 1). This increase in seizure frequency during COVID-19 versus pre-pandemic was statistically significant (P=0.014).
According to the findings in Table 1, there was no significant difference in the mean age between seizure patients hospitalized in NU during COVID-19 (51.3±18.9 years) and the same period last year (52.3±19.3 years).

However, a considerable difference was observed between the two study periods regarding gender (P=0.012). Notably, a drastic decrease in the frequency of female patients with seizures was reported during the COVID-19 pandemic (36.9%) versus the pre-pandemic period (63.1%). Also, the previous history of seizure was significantly related to the study period, and it was more frequent before the COVID-19 pandemic (68.8% vs 31.3%, P<0.001). The results showed that the distribution of seizure types was significantly different between the COVID-19 pandemic and the same period the year before (P=0.005). The percentage of convulsive status epilepticus (CSE) seizures during the COVID-19 pandemic (70%) was substantially elevated relative to pre-pandemic (40%). Furthermore, the frequency of some types of seizure, such as focal impaired awareness motor (FIAM), focal aware motor (FAM), and generalized tonic-clonic (GTC) before the COVID-19 pandemic were 60%, 75%, and 56.8%, respectively that hugely declined during the COVID-19 period. Based on the results of this study, a significant difference was disclosed between the two periods of the study in terms of seizure etiology (P=0.032). A remarkable increase was seen in the frequency of seizure patients with stroke during COVID-19 (66.2%) pandemic versus pre-pandemic (33.8%). Those who experienced seizures due to brain infection had a higher percentage during COVID-19 (69%) than during the COVID-19 era (31%). Additionally, the frequency of some seizure etiologies, such as unknown (40.7%) and genetic (42%), decreased during COVID-19 compared to the same period the year before. More details are indicated in Table 1.
Interestingly, out of 149 seizure patients during COVID-19, 64(43.0%) demonstrated positive results in COVID-19 tests. Unexpectedly, there was no significant relationship between the presence of coronavirus infection and seizure characteristics in hospitalized patients having seizures. Coronavirus infection was not also related to gender in the patients with seizures hospitalized in the NU during the initial peaks of the COVID-19 era. Table 2 depicts more information.

Discussion
In this project, we witnessed a drastic reduction in total admission in the NU of Poursina Hospital during the COVID-19 pandemic. However, a notable increase in seizure frequency was reported during the COVID-19 pandemic period as compared with the same period last year. The hospital burden of seizure increases despite the decrease in the absolute total number of seizures during the COVID-19 pandemic might be due to more severe cases during this period.
But in a retrospective cohort study in the UK, this increase is not related to the hospitalization changes and the incidence of new seizures or epilepsy diagnoses in the 6 months after COVID-19 was higher than in matched patients with influenza. This difference was more marked in people not hospitalized, highlighting the risk of epilepsy and seizures in COVID-19 regardless of severity [17].
Furthermore, the frequency of type, etiology, history of seizure, and gender have changed during the COVID-19 pandemic. Particularly, stroke patients who manifested seizures doubled during the COVID-19 period versus before it. It is plausible that those with a good prognosis in an emergency were discharged. Thus, those with poorer outcomes and more susceptibility to seizure development were hospitalized during COVID-19, which increased the seizure burden. COVID-19 also has been suggested as a stroke risk factor [6, 7, 9]. There is a complex interplay between seizure, stroke, and COVID-19. Another possibility is that stressful circumstances induced by the COVID-19 pandemic might lessen medical examination rates and slow nursing care and proper medical management of hospitalized stroke patients in NU, leading to more complications simultaneously. On the other hand, it may lead to overlook some seizures.
A previous study postulated that COVID-19 could be directly associated with seizures in patients with neurological manifestations [20]. The results of our study also displayed that nearly half of the seizure patients during the COVID-19 era were concurrently affected by COVID-19-induced infection. Additionally, the same study indicated that age was an influential factor in the development of seizures in patients with severe acute respiratory syndrome COVID-19 infection. Older people were more likely to have seizures. But in our study, age did not affect seizure prevalence. Likewise, female patients with coronavirus infection seem more susceptible than male ones to seizure development [20]. Although we did not investigate the risk of seizure occurrence between the patients with and without coronavirus infection, our analysis of the seizure patients demonstrated a substantial association of gender but not age with the COVID-19 period.
Based on the literature, the relationship between coronavirus infection and seizure has been very controversial. Sometimes it has been claimed that seizures were unrelated to the coronavirus [21]. However, some evidence has suggested that coronavirus infection might be associated with some neurological manifestations, such as seizures, impaired level of consciousness, and encephalitis. Indeed, the coronavirus cannot cause or provoke seizures in susceptible individuals, such as patients with epilepsy. However, fever is a known precipitating factor in some patients with epilepsy [22]. The destructive effects of COVID-19 on the central nervous system are mainly caused by a cytokine storm produced by either the entry of pro-inflammatory cytokines from the periphery into the CNS or the production of these cytokines by activated microglia. It can result in neurotransmitter modulation and ion channel dysfunction, leading to neuronal hyperexcitability, presenting as seizures. Pro-inflammatory cytokines, such as tumor necrosis factor-alpha, interleukin 1-beta (IL-1B), interleukin 6 (IL-6), free radicals, nitric oxide, and others are released, leading to acute or chronic inflammation, causing neuronal hyperexcitability and potentially resulting in seizures. These cytokines aggravate apoptosis and neuronal necrosis in the CNS, and they also stimulate glutamate release while inhibiting GABA release in the hippocampus and cerebral cortex (IL-1B, TNF-α). Additionally, using α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) and N-methyl-D-aspartate (NMDA) receptors, these cytokines increase calcium entry into the neurons, leading to neuronal hyper-excitability (TNF-α). Lastly, they release neurotoxic compounds through different autocrine/paracrine mechanisms (IL-6). Secondary seizures may be initiated after strokes, elevated levels of IL-6, electrolyte imbalance, increased oxidative stress, hypoxia, and mitochondrial dysfunction in COVID-19 patients [16, 23].
In our study, the other results were that the frequency of patients with seizure history markedly attenuated during the COVID-19 pandemic compared to pre-pandemic. This result might be due to less willingness of patients with a seizure history for hospitalization during the COVID-19 lockdown versus before the pandemic for the psycho-social effects of the COVID-19 pandemic. Nevertheless, a dramatic enhancement of hospitalization was discovered in patients without a seizure history. An explanation for this result might be due to a referral bias and the higher degree of anxiety related to the first seizure. A higher percentage of malignant patients with more critical underlying etiology were referred to the hospital and transferred to NU. Subsequently, the probability of new-onset seizures induced by the above disorders was increased during COVID-19.
In particular, a remarkable elevation of convulsive status epilepticus during COVID-19 compared to the pre-pandemic. Also, the COVID-19 era was associated with more seizure cases. These findings confirm the previous theory that patients with malignant seizures were probably prevalently hospitalized during the pandemic versus the pre-pandemic period. However, generalized tonic-colonic seizure similarly was the most prevalent type of seizure during the two time periods of this study.
Interestingly, we found no profound association of coronavirus infection with seizure types, etiology, and history. It seems that seizure characteristics were not related to coronavirus infection.
It is suggested that a prospective study with large sample size is designed to follow the seizure development in patients with COVID-19. A similar multicenter investigation is also recommended. Finally, it is seriously suggested to investigate the medications and treatment processes during the COVID-19 pandemic in hospitalized seizure patients in future studies.
Conclusions
We found that the frequency of seizures in hospitalized patients during COVID-19 was substantially higher than before the COVID-19 pandemic. Less than half of seizure patients hospitalized during the COVID-19 pandemic had a positive COVID-19. Gender but not age as a demographic parameter in seizure patients differed between the two time periods of study. The most prevalent causes of seizures and stroke before and during the COVID-19 pandemic are unknown. The more common type of seizure during the two periods of the study was generalized tonic-clonic seizure. COVID-19 era may increase the appearance of malignant seizures such as convulsive status epilepticus by 4-fold. It seems that lockdown due to the COVID-19 pandemic might intensify the hospital burden of seizure but not its incidence.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki (2013).The study was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1399.559).
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
Conceptualization: Babak Bakhshayesh Eghbali, Alia Saberi, Seyede Zahra Jalali Emam, Sima Fallah Arzpayma, and Roghaye Zare; Methodology and data analysis: Roghaye Zare and Mohammad Ali Yazdanipour; Data collection: Alia Saberi, Babak Bakhshayesh Eghbali, Seyede Zahra Jalali Emam, and Sima Fallah Arzpayma; Writing the original draft, editing, and review: Alia Saberi, Babak Bakhshayesh Eghbali, Mohammad Ali Yazdanipour, and Sara Ramezani; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We acknowledge the assistance of Neuroscience Research Center and Clinical Research Development Unit of Poursina Hospital.
References
- Palladino F, Merolla E, Solimeno M, de Leva MF, Lenta S, Di Mita O, et al. Is Covid-19 lockdown related to an increase of accesses for seizures in the emergency department? An observational analysis of a paediatric cohort in the Southern Italy. Neurol Sci. 2020; 41(12):3475-83. [DOI:10.1007/s10072-020-04824-5] [PMID] [PMCID]
- World Health Organization (WHO). WHO Coronavirus (COVID-19) dashboard. 2022 [2023 June 8]. Available from: [Link]
- Yu H, Sun T, Feng J. Complications and pathophysiology of COVID-19 in the nervous system. Front Neurol. 2020; 11:573421. [DOI:10.3389/fneur.2020.573421] [PMID] [PMCID]
- Shahjouei S, Naderi S, Li J, Khan A, Chaudhary D, Farahmand G, et al. Risk of stroke in hospitalized SARS-CoV-2 infected patients: A multinational study. EBioMedicine. 2020; 59:102939. [DOI:10.1016/j.ebiom.2020.102939] [PMID] [PMCID]
- Saberi A, Ghayeghran A, Hatamian H, Hosseini-Nejad M, Bakhshayesh Eghbali B. COVID-19-associated myelitis, para/post infectious or infectious myelitis: A Case report from the north of Iran. Caspian J Neurol Sci. 2020; 6(2):132-8 [DOI:10.32598/cjns.6.21.1]
- Shahjouei S, Tsivgoulis G, Farahmand G, Koza E, Mowla A, Sadr AV, et al. SARS-CoV-2 and stroke characteristics: A report from the multinational COVID-19 stroke study Group. Stroke. 2021; 52(5):e117-30 [DOI:10.1161/STROKEAHA.120.032927]
- Shahjouei S, Anyaehie M, Koza E, Tsivgoulis G, Naderi S, Mowla A, et al. SARS-CoV-2 is a culprit for some, but not all acute ischemic strokes: A report from the multinational COVID-19 stroke study group. J Clin Med. 2021; 10(5):931. [DOI:10.3390/jcm10050931] [PMID] [PMCID]
- Alijani B, Saberi A, Niyasti P, Dogahe MH. Transverse myelitis following covid-19 infection. What is the mechanism? A case report and literature review. Rom J Neurol. 2021; 20(2):255-63. [DOI:10.37897/RJN.2021.2.22]
- Kazemi S, Pourgholaminejad A, Saberi A. Stroke associated with sars-cov-2 infection and its pathogenesis: A systematic review. Basic Clin Neurosci. 2021; 12(5):569-86. [DOI:10.32598/bcn.2021.3277.1] [PMID] [PMCID]
- Besharati A, Saberi A, Ghorbani Shirkouhi S, Ashraf A, Hatamian H, Eslami Kenarsari H, et al. Guillain-barré syndrome during the COVID-19 pandemic and pre-pandemic periods. Caspian J Neurol Sci. 2022; 8(1):33-8 [DOI:10.32598/cjns.8.28.213.2]
- Pourgholaminejad A, Kazemi S, Saberi A. Virology, pathophysiology and neuroinvasion mechanisms of SARS-CoV-2: A mini literature review. Rom J Neurol. 2021; 20(3):288-91.[DOI:10.37897/RJN.2021.3.3]
- Wang ST, Ballout AA, Mirza U, Sonti AN, Husain A, Kirsch C, et al. Acute seizures occurring in association with SARS-CoV-2. Front Neurol. 2020; 11:576329. [DOI:10.3389/fneur.2020.576329] [PMID] [PMCID]
- Emami A, Fadakar N, Akbari A, Lotfi M, Farazdaghi M, Javanmardi F, et al. Seizure in patients with COVID-19. Neurol Sci. 2020; 41(11):3057-61. [DOI:10.1007%2Fs10072-020-04731-9] [PMID] [PMCID]
- Nalleballe K, Reddy Onteddu S, Sharma R, Dandu V, Brown A, Jasti M, et al. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav Immun. 2020; 88:71-4. [DOI:10.1016/j.bbi.2020.06.020] [PMID] [PMCID]
- Andraus M, Thorpe J, Tai XY, Ashby S, Hallab A, Ding D, et al. Impact of the COVID-19 pandemic on people with epilepsy: Findings from the Brazilian arm of the COV-E study. Epilepsy Behav. 2021 ;123:108261. [DOI:10.1016/j.yebeh.2021.108261] [PMID] [PMCID]
- Raza SM, Ebrahim F, Ekea H, Ali SK. COVID-19 presenting as a seizure: A kenyan case report. Cureus. 2022; 14(4):e24431. [DOI:10.7759/cureus.24431] [PMID] [PMCID]
- Taquet M, Devinsky O, Cross JH, Harrison PJ, Sen A. Incidence of epilepsy and seizures over the first 6 months after a COVID-19 diagnosis. A Retrospective Cohort Study. 2023;100(8):e790-e9 [DOI:10.1212/wnl.0000000000201595] [PMID] [PMCID]
- Yu N, Lin XJ, Zhang SG, Di Q. Analysis of the reasons and costs of hospitalization for epilepsy patients in East China. Seizure. 2019; 72:40-45. [DOI:10.1016/j.seizure.2019.09.013] [PMID]
- Lu RJ, Zhao L, Huang BY, Ye F, Wang WL, Tan WJ. Real-time reverse transcription-polymerase chain reaction assay panel for the detection of severe acute respiratory syndrome coronavirus 2 and its variants. Chin Med J. 2021; 134(17):2048-53. [DOI:10.1097/cm9.0000000000001687] [PMID] [PMCID]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020; 77(6):683-90. [DOI:10.1001/jamaneurol.2020.1127] [PMID] [PMCID]
- Lu L, Xiong W, Liu D, Liu J, Yang D, Li N, et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study. Epilepsia. 2020; 61(6):e49-53. [DOI:10.1111/epi.16524]
- Sohal S, Mansur M. COVID-19 presenting with seizures. IDCases. 2020; 20:e00782. [DOI:10.1016/j.idcr.2020.e00782] [PMID] [PMCID]
- Nikbakht F, Mohammadkhanizadeh A, Mohammadi E. How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms. Mult Scler Relat Disord. 2020; 46:102535. [DOI:10.1016/j.msard.2020.102535] [PMID] [PMCID]
Type of Study: Research |
Subject:
Special
Received: 2023/07/5 | Accepted: 2023/07/28 | Published: 2023/07/28
Received: 2023/07/5 | Accepted: 2023/07/28 | Published: 2023/07/28
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




