Thu, Dec 11, 2025
Volume 8, Issue 2 (Spring 2022)
Caspian J Neurol Sci 2022, 8(2): 124-132 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Lotfinesab S, Doulah A, Rafieirad M. The Combined Effect of Aerobic Exercise and α-Pinene on Pentylenetetrazole-Induced Seizure in Male Rats. Caspian J Neurol Sci 2022; 8 (2) :124-132
URL: http://cjns.gums.ac.ir/article-1-523-en.html
URL: http://cjns.gums.ac.ir/article-1-523-en.html
1- Department of Physical Education and Sport Sciences, Faculty of Humanities, Ahvaz Branch, Islamic Azad University, Ahvaz, Iran.
2- Department of Biology, Faculty of Basic Sciences, Ahvaz Branch, Islamic Azad University, Ahvaz, Iran.
3- Department of Biology, Faculty of Basic Sciences, Izeh Branch, Islamic Azad University, Izeh, Iran.
2- Department of Biology, Faculty of Basic Sciences, Ahvaz Branch, Islamic Azad University, Ahvaz, Iran.
3- Department of Biology, Faculty of Basic Sciences, Izeh Branch, Islamic Azad University, Izeh, Iran.
Full-Text [PDF 1507 kb]
(830 Downloads)
| Abstract (HTML) (1896 Views)
Full-Text: (841 Views)
Introduction
Long-term and continuous antiepileptic drugs may result in numerous side effects. Hence, it is essential to search for traditional and plant medicines with minimum side effects [1]. The incidence of epilepsy is 7-9 per 1000 population. It occurs in all ages, races, and genders. Epileptic seizures occur due to an imbalance in the inhibition and stimulation of neuronal connections. This imbalance is caused by the sudden and uncontrolled discharge of neurons in the central nervous system [2].
Epilepsy is a set of central nervous system disorders that manifest as abrupt, transient, repetitive, and unpredictable seizures with sensory, motor, and autonomous origin [3]. Seizure is caused by factors such as infection, ischemia, and brain stroke [4], creating inflammation in the central nervous system. This inflammation can be followed by some neurological disorders. [5].
In addition to causing many limitations in daily activities, uncontrolled epilepsy can induce irreversible impairments in the brain cells [6]. Nowadays, techniques with three mechanisms of effect, including enhancement of GABAergic activity, reduction of glutamate stimulatory flow, and modulation of ionic currents, especially sodium, calcium, and chloride ions, are used for treating epilepsy [7].
A common cause of epilepsy and seizure in humans and animals is the weakened GABAergic system [8]. Pentylenetetrazol (PTZ) induces seizure by inhibiting the chloride ion flow mediated by γ-Aminobutyric Acid (GABA) on the GABAA receptor and consequently reducing the chloride ion inflow. GABAA is an inhibitory neurotransmitter receptor in the central nervous system of vertebrates. When it is active, the receptor chloride ion channel is opened, allowing chloride ion flow and neuronal hyperpolarization. This receptor has several allosteric binding sites through which various drugs can act and regulate the GABA-induced chloride ion flow [9]. Therefore, drugs that reinforce the GABAergic system activity through GABAA receptors can effectively prevent PTZ-induced epilepsy [3].
Benzodiazepines, including diazepam, operate via such a mechanism, too [8]. Plant medicines contain numerous active compounds [3]. According to research, it is logical to conduct studies on plants that have been claimed to exert beneficial effects on the nervous system or to have antiepileptic effects, as mentioned in some studies [10]. α-Pinene is a member of bicyclic monoterpenes that is highly important commercially. As a major active compound in many plants, α-pinene also inhibits acetylcholinesterase enzyme activity [11]. Pharmacologic studies have shown that the extracts of these plants have a wide range of activities such as anti-depression [12], antiepileptic [13], and antioxidant activities [14].
Regular physical activity, which can affect all body organs and systems, is considered a necessity for a healthy lifestyle. Some studies have indicated that exercise plays a crucial role in the central nervous system functioning [15]. Other reports have shown that exercise can significantly reduce seizure frequency [16]. Studies have also reported that the prevalence of seizures is reduced during and after exercise in epileptic patients. Some believe that deep breathing during exercise decreases the incidence of epileptic seizures by reducing CO2 [17]. Exercise reduces stress and increases freshness, thereby reducing epileptic seizures. Enhanced concentration during exercise is also an important reason for reducing epileptic seizures [17]. Hence, the present research aimed to explore the pretreatment effect of aerobic exercise and α-pinene on the PTZ-induced seizure in mature male rats.
Materials and Methods
A total of 40 male Wistar rats (weight: 200-250 g) were prepared from Ahvaz Jundishapur University of Medical Sciences and used in this study. The rats were kept in individual cages under standard 12/12 dark/light conditions at 21±2°C, with free access to water and food. They were randomly divided into five groups (n=8) as follows: 1) control group (received normal saline), 2) positive control group (DZP group: received 1 mg/kg diazepam as an antiepileptic drug), 3) α-pinene (Sigma Company, American( group (received 200 mg/kg α-pinene), 4) swimming exercise group (EXE group: performed 30 min before epilepsy induction), and 5) α-pinene plus swimming exercise (α-pinene + EXE: performed 30 min before epilepsy induction and received 200 mg/kg α-pinene) (Figure 1).

Thirty minutes after administering these materials and or exercise, 85 mg/kg PTZ (Sigma Co.) was administered intraperitoneally to each rat [18]. The epileptic behavior of animals, including delay of onset of seizure, clonic seizure duration, tonic seizure duration, tonic-clonic seizure duration, and the total time of seizure immediately and half an hour later, was recorded [18]. The swimming exercise protocol included four weeks of swimming exercise in 25-30°C water, five sessions per week, each session for 30 min [19] (Figure 1).
Data were presented as mean±SEM and analyzed using the 1-way ANOVA and post hoc Tukey test in Excel and SPSS software. P<0.05 was considered statistically significant.
Results
The results of the ANOVA test showed that diazepam (positive control group) increased the delay of onset of seizure (P<0.001) (Figure 2) and decreased clonic seizure duration (P<0.05), tonic-clonic seizure duration (P<0.001) (Figure 3), and the total time of seizure (P<0.001) (Figure 4) significantly compared to the negative control group (saline administration).
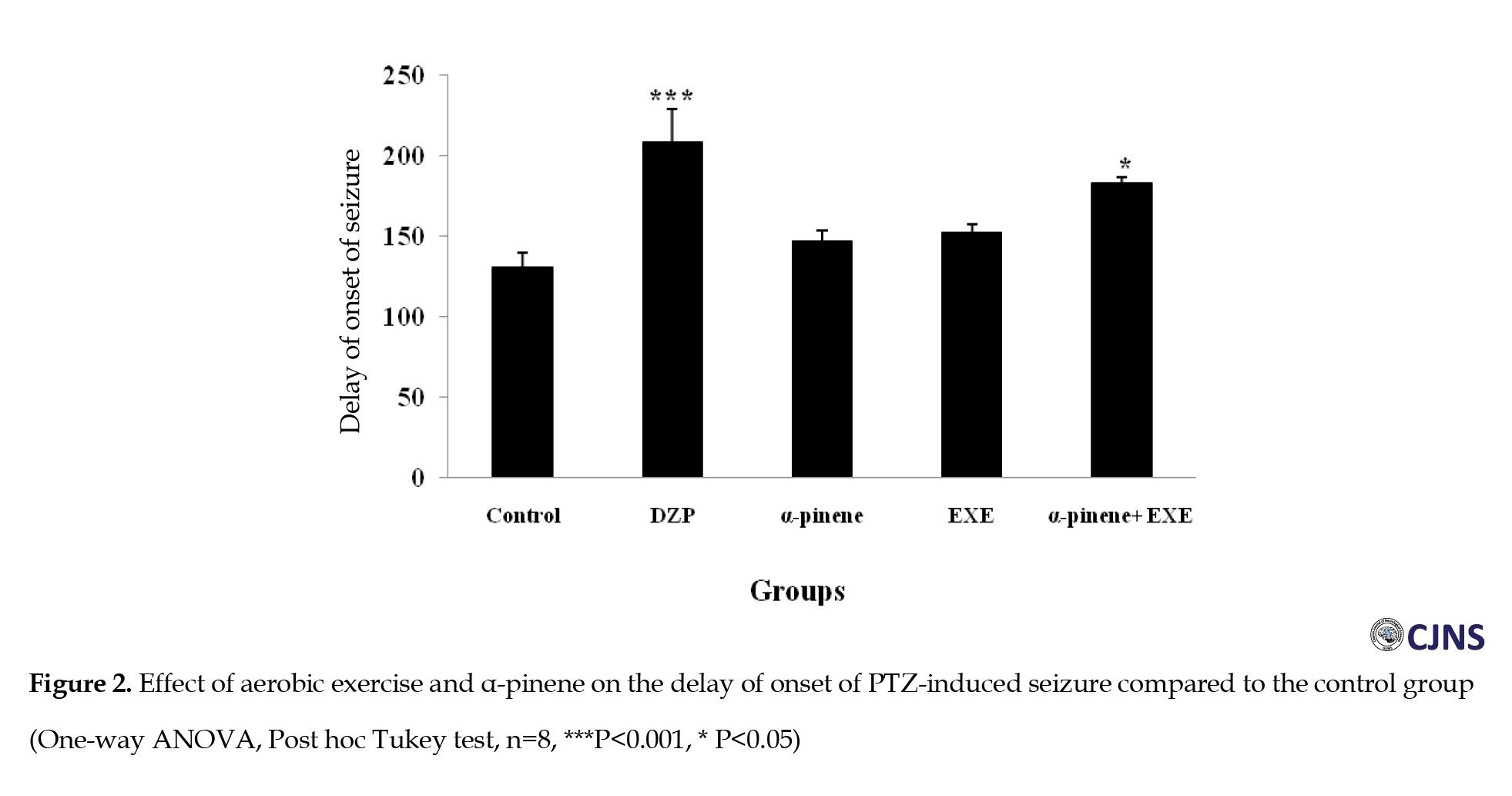
Moreover, diazepam increased the tonic seizure duration compared to the control group, but the difference was significant statistically (Figure 5). Intraperitoneal administration of α-pinene (100 and 200 mg/kg) significantly reduced the tonic and clonic-tonic seizure duration (P<0.001) and total seizure duration (P<0.05) compared with the negative control group (Figures 4, 5 and 6). Furthermore, administration of 200 mg/kg α-pinene had no significant effect on the delay of onset of seizure and clonic seizure duration compared to the negative control group (Figures 2 and 3).
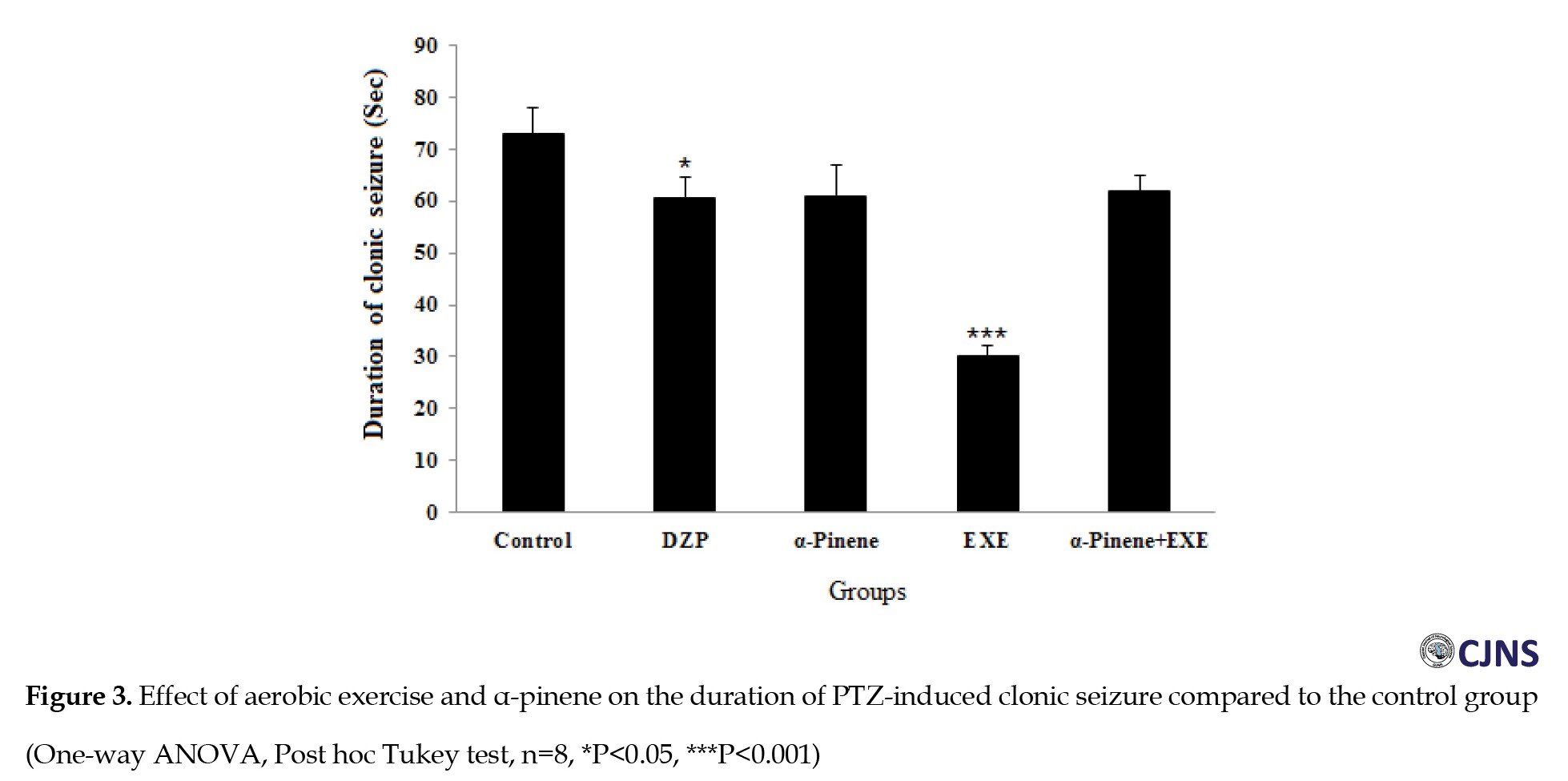
As indicated, four weeks of swimming exercise alone significantly decreased tonic seizure, clonic seizure, clonic-tonic seizure, and the total seizure duration (P<0.001) compared to the control group (Figures 3, 4, 5 and 6).
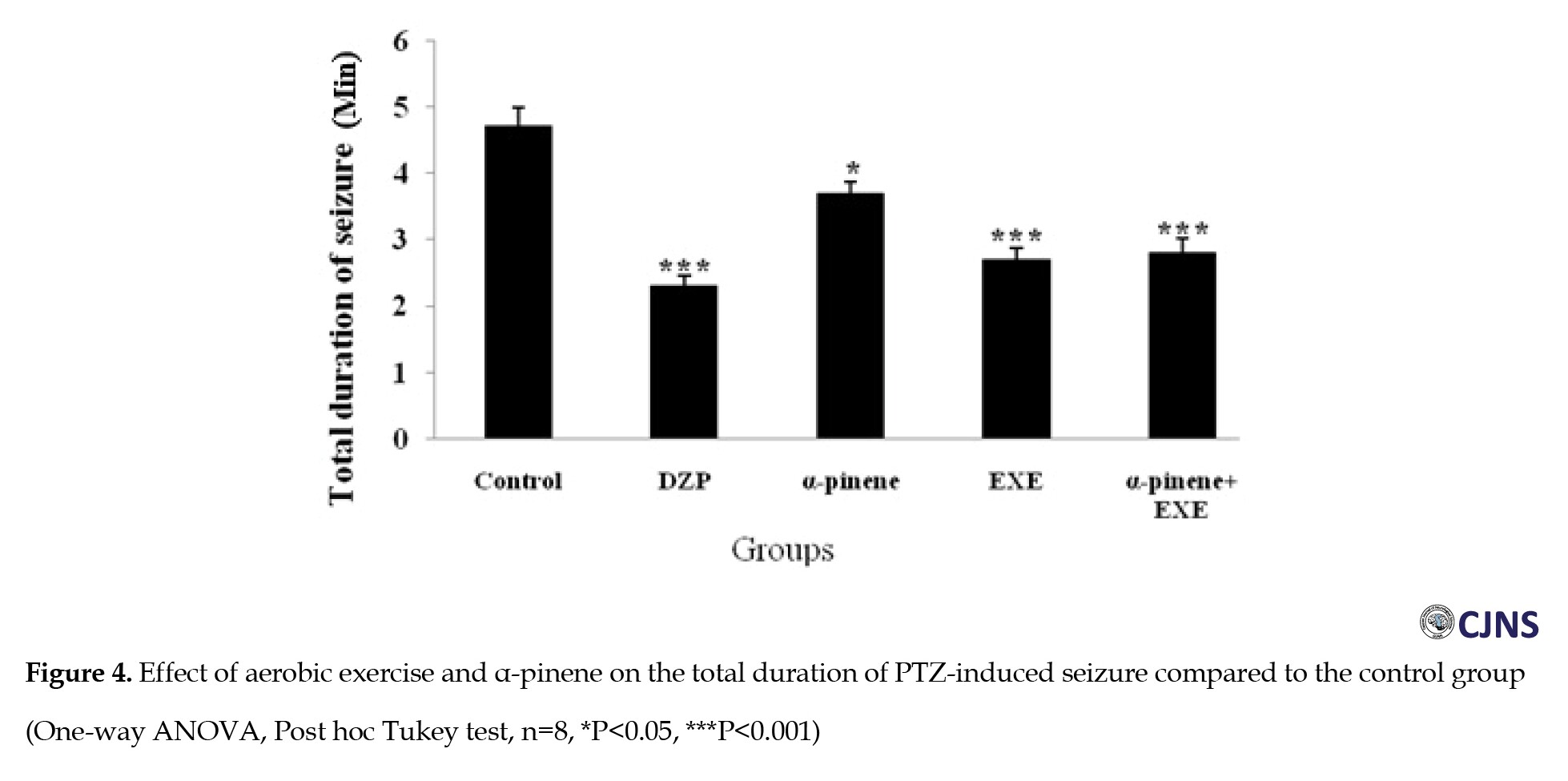
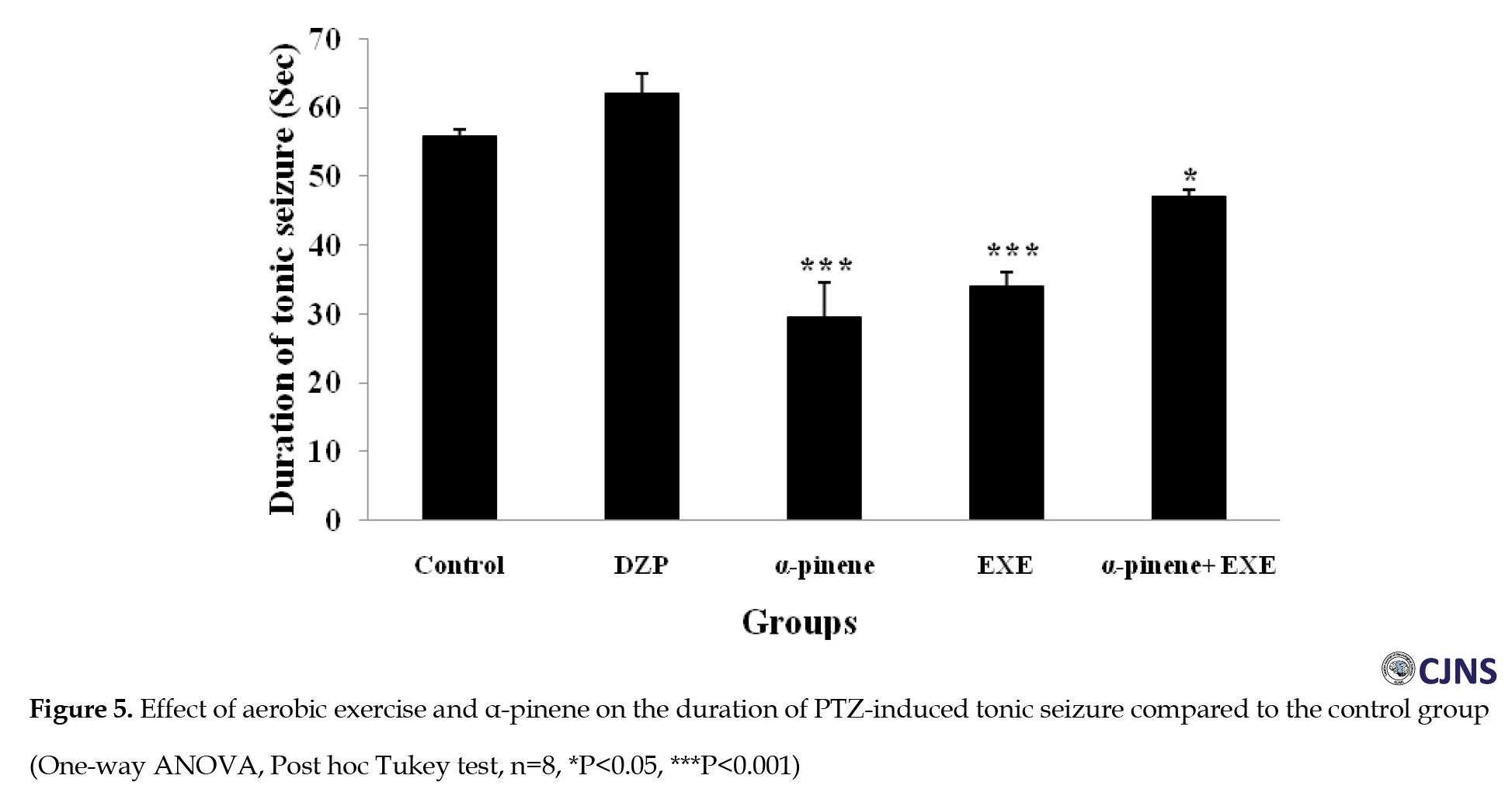
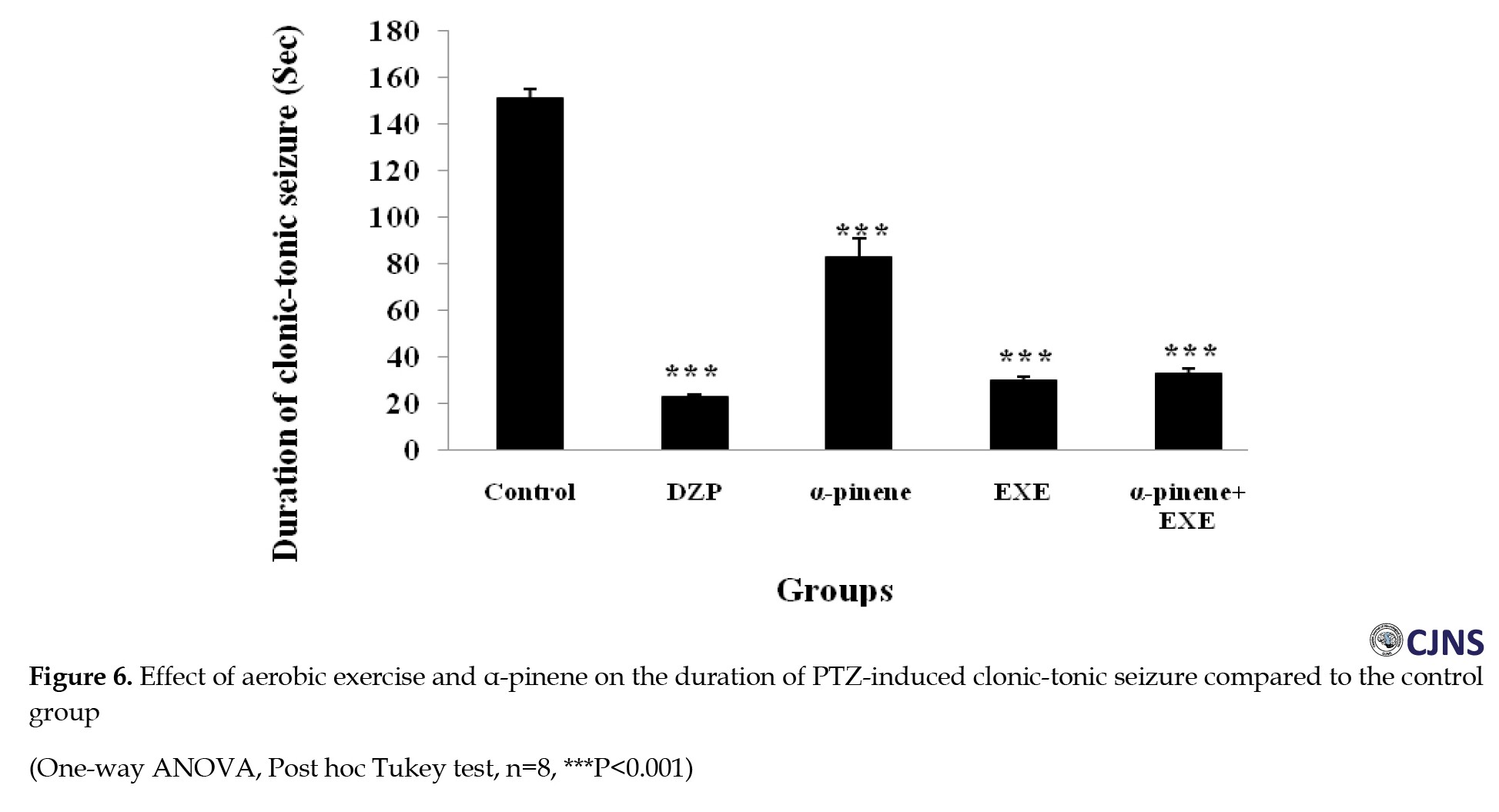
In addition, four weeks of swimming exercise alone increased the delay of onset of seizures compared to the control group, but the difference was not statistically significant (Figure 2). Furthermore, combined α-pinene use and swimming exercise significantly increased the delay of onset of seizure and decreased duration of the tonic seizure (P<0.05), and clonic-tonic seizure (P<0.001), and the total duration of the seizure (P<0.001) compared to the control group (Figures 2, 4, 5 and 6). Moreover, swimming exercises together with α-pinene reduced clonic seizures compared with the control group, but the difference was not statistically significant (Figure 3).
Discussion
Studies have shown that exercise decreases seizure frequency and neuronal activity. This effect is associated with neurotransmitters, whose release during an exercise activity is increased. Many studies have shown that the level of catecholamines increases after acute exercise activity [6]. Regular exercise activities enhance the norepinephrine level and metabolites in different brain areas [20]. It has been reported that norepinephrine has an inhibitory effect on the development of seizures, and its reduction facilitates epileptic activities [21].
Furthermore, dopamine, one of the most abundant catecholamines, has been recently found to contribute to the pathophysiology of epilepsy and modulation of epileptic attacks [22]. Exercise alters the dopamine level in the brain of animals [23]. In this regard, the connection point of D1 and D2 receptors of dopamine has been found to reduce in the temporal lobe of epileptic patients [24]. Another neurotransmitter involved in epilepsy is GABA, the most important inhibitory neurotransmitter in the central nervous system. The activity of the GABA system is diminished in epileptic patients [25].
The present study investigated the pretreatment effect of aerobic exercise and α-pinene on PTZ-induced epilepsy in male rats. The exercise plus α-pinene use significantly decreased delay of onset of seizures, tonic seizures, clonic seizures, clonic-tonic seizures, and the total duration of the seizure. The results of this study are in line with those showing the effect of aerobic exercise on decreasing the seizure rate. Arida et al. [26] reported that physical activity reduced epileptic seizures in epileptic rats. These researchers also studied the effect of aquatic exercise on epileptic seizures and found that aquatic physical activity reduced epileptic disorders. Training can stimulate GABA neuropeptide, which suppresses hippocampal excitability [27].
Evidence also shows that cerebral neurotransmitters are influenced by exercise. The inhibitory effect of noradrenaline on the kindling also suggests that altered neurotransmitter systems due to physical activity can modulate inhibition/excitation balance, which reduces the development and frequency of seizures [28]. For instance, noradrenaline is increased in the rats with physical activity and has inhibitory effects on kindling. Its elimination has also been found to facilitate the development of seizures in the hippocampus of kindled rats [29].
A study on epileptic rats indicated that aerobic exercises have no impact on reducing epileptic disorders, which is not in agreement with the results of the present study. Based on the discussion mentioned above, it seems that the discrepancy between the results of the present study and those of Vannucci Campus et al. [30] regarding the effect of aerobic exercise on decreasing seizures in epileptic female rats is due to the intensity of exercises, especially in the final weeks of treatment, because these exercises have been classified as high-intensity exercises.
Studies have shown that PTZ-kindling experimental epilepsy reduces healthy neurotransmitters in the hippocampal dentate gyrus, while exercise postpones the morphological changes of healthy neurons in the hippocampal dentate gyrus [31]. A study showed that aerobic exercise decreases seizure intensity in male rats, with no difference between the intensities of seizures in the pregnant and non-pregnant rats [32]. Another study in Nigeria indicated that physiotherapists play a pivotal role in rehabilitating epileptic children. Regular physical exercise has psychological and physiological advantages for epileptic children [33].
Studies have reported that Yoga affects electroencephalogram stability and the autonomic nervous system; hence, it can be used as a non-pharmaceutical intervention to improve health and reduce the adverse effects of epilepsy [34].
In one study, the researchers examined the relationship between exercise and seizure frequency. In Scandinavia, while 10% of the population with epilepsy experienced more seizures due to strenuous physical activity, the other 30%-40% with epilepsy experienced a moderate reduction in seizures following regular physical activity. Subsequent studies have shown the possible role of flavonoids on the central nervous system [35].
According to studies, α-pinene is one of the main constituents of Artemisia annua, which shows anticonvulsant effects through the binding activity of benzodiazepine receptors and modulation of oxidative stress and inflammatory process [36].
A current study by Etemadi Kermani et al. has shown that choyl extract can affect the seizure process caused by pentylenetetrazole and reduce the seizure time [37].
Also, according to Behzad Nia et al., the hydroalcoholic extract of mountain tea has anticonvulsant properties. This effect may be due to the presence of the operating system of this extract on the central benzodiazepine [38].
Pereira et al. [39] reported that acute administration of coumarins increases the release of prefrontal cortical GABA, probably by affecting the GABAA receptor. Since aminobutyric plays a key role in inhibiting neurons, coumarin can improve epileptic seizures. Keisalari et al. [40] evaluated the effect of Ferula assa-foetida hydroalcoholic extract on PTZ-induced antiepileptic and antioxidant activities in male mice. They reported the presence of monoterpenes such as α-pinene and β-pinene in Ferula assa-foetida, which have suppressive effects on PTZ-induced seizures.
GABA receptor analogs such as α-pinene and verbenone have been reported in many plant compounds, including choy [14]. Alphapenic has a kinetic effect on gabadard receptors and increases postsynaptic chloride ion current [41]. Also, some penin analogs can cause idiopathic epilepsy in power mice and are known to amplify the chloride current induced by GABAA receptors. Linalool is a monoterpene present as the main compound in several aromatic plant species, including choy [42], and exerts its anticonvulsant effect through the glutamatergic system [9]. In general, according to the results of the present study and other studies, exercise reduces stress and increases freshness, which in turn reduces the epileptic seizures [43]. A wide range of evidence shows that abnormalities of the neurotransmitter systems such as serotonin, noradrenaline, dopamine, glutamate, and GABA are observed in mood disorders and epilepsy. Physical activity can modulate several neurotransmitter systems [44].
According to the present study and other studies, exercise reduces stress, increases freshness, and decreases epileptic seizures [43]. A wealth of evidence shows that abnormalities of the neurotransmitter systems such as serotonin, noradrenaline, dopamine, glutamate, and GABA are observed in mood disorders and epilepsy. Physical activity can modulate several neurotransmitter systems [45].
Conclusion
Since α-pinene is the main compound of plant extracts, it can be used in preventive activities and exercise against neurodegenerative diseases.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were performed in compliance with the ethical guidelines of the Declaration of Helsinki 2013. During the study, keeping and conducting experiments and killing animals followed the standard work methods and principles of ethics with animals, approved by the Research Department of the Ahvaz branch of Islamic Azad University on September 14, 2018 (No. 10621423962038).
Funding
This article was extracted from MSc thesis of the first author, Department of Physical Education and Sport Sciences, Faculty of Humanities, Ahvaz Branch, Islamic Azad University, Ahvaz, Iran.
Authors contributions
Conceptualization, methodology, investigation, and funding acquisition: Abdolhassan Doulah, Maryam Rafieirad, and Sadegh Lotfinesab; Writing the original draft, Supervision, writing, review, and editing: Abdolhassan Doulah and Maryam Rafieirad; Resources: Abdolhassan Doulah and Sadegh Lotfinesab.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Research Deputy of Ahvaz Branch, Islamic Azad University, for its support in carrying out this study.
References
Long-term and continuous antiepileptic drugs may result in numerous side effects. Hence, it is essential to search for traditional and plant medicines with minimum side effects [1]. The incidence of epilepsy is 7-9 per 1000 population. It occurs in all ages, races, and genders. Epileptic seizures occur due to an imbalance in the inhibition and stimulation of neuronal connections. This imbalance is caused by the sudden and uncontrolled discharge of neurons in the central nervous system [2].
Epilepsy is a set of central nervous system disorders that manifest as abrupt, transient, repetitive, and unpredictable seizures with sensory, motor, and autonomous origin [3]. Seizure is caused by factors such as infection, ischemia, and brain stroke [4], creating inflammation in the central nervous system. This inflammation can be followed by some neurological disorders. [5].
In addition to causing many limitations in daily activities, uncontrolled epilepsy can induce irreversible impairments in the brain cells [6]. Nowadays, techniques with three mechanisms of effect, including enhancement of GABAergic activity, reduction of glutamate stimulatory flow, and modulation of ionic currents, especially sodium, calcium, and chloride ions, are used for treating epilepsy [7].
A common cause of epilepsy and seizure in humans and animals is the weakened GABAergic system [8]. Pentylenetetrazol (PTZ) induces seizure by inhibiting the chloride ion flow mediated by γ-Aminobutyric Acid (GABA) on the GABAA receptor and consequently reducing the chloride ion inflow. GABAA is an inhibitory neurotransmitter receptor in the central nervous system of vertebrates. When it is active, the receptor chloride ion channel is opened, allowing chloride ion flow and neuronal hyperpolarization. This receptor has several allosteric binding sites through which various drugs can act and regulate the GABA-induced chloride ion flow [9]. Therefore, drugs that reinforce the GABAergic system activity through GABAA receptors can effectively prevent PTZ-induced epilepsy [3].
Benzodiazepines, including diazepam, operate via such a mechanism, too [8]. Plant medicines contain numerous active compounds [3]. According to research, it is logical to conduct studies on plants that have been claimed to exert beneficial effects on the nervous system or to have antiepileptic effects, as mentioned in some studies [10]. α-Pinene is a member of bicyclic monoterpenes that is highly important commercially. As a major active compound in many plants, α-pinene also inhibits acetylcholinesterase enzyme activity [11]. Pharmacologic studies have shown that the extracts of these plants have a wide range of activities such as anti-depression [12], antiepileptic [13], and antioxidant activities [14].
Regular physical activity, which can affect all body organs and systems, is considered a necessity for a healthy lifestyle. Some studies have indicated that exercise plays a crucial role in the central nervous system functioning [15]. Other reports have shown that exercise can significantly reduce seizure frequency [16]. Studies have also reported that the prevalence of seizures is reduced during and after exercise in epileptic patients. Some believe that deep breathing during exercise decreases the incidence of epileptic seizures by reducing CO2 [17]. Exercise reduces stress and increases freshness, thereby reducing epileptic seizures. Enhanced concentration during exercise is also an important reason for reducing epileptic seizures [17]. Hence, the present research aimed to explore the pretreatment effect of aerobic exercise and α-pinene on the PTZ-induced seizure in mature male rats.
Materials and Methods
A total of 40 male Wistar rats (weight: 200-250 g) were prepared from Ahvaz Jundishapur University of Medical Sciences and used in this study. The rats were kept in individual cages under standard 12/12 dark/light conditions at 21±2°C, with free access to water and food. They were randomly divided into five groups (n=8) as follows: 1) control group (received normal saline), 2) positive control group (DZP group: received 1 mg/kg diazepam as an antiepileptic drug), 3) α-pinene (Sigma Company, American( group (received 200 mg/kg α-pinene), 4) swimming exercise group (EXE group: performed 30 min before epilepsy induction), and 5) α-pinene plus swimming exercise (α-pinene + EXE: performed 30 min before epilepsy induction and received 200 mg/kg α-pinene) (Figure 1).

Thirty minutes after administering these materials and or exercise, 85 mg/kg PTZ (Sigma Co.) was administered intraperitoneally to each rat [18]. The epileptic behavior of animals, including delay of onset of seizure, clonic seizure duration, tonic seizure duration, tonic-clonic seizure duration, and the total time of seizure immediately and half an hour later, was recorded [18]. The swimming exercise protocol included four weeks of swimming exercise in 25-30°C water, five sessions per week, each session for 30 min [19] (Figure 1).
Data were presented as mean±SEM and analyzed using the 1-way ANOVA and post hoc Tukey test in Excel and SPSS software. P<0.05 was considered statistically significant.
Results
The results of the ANOVA test showed that diazepam (positive control group) increased the delay of onset of seizure (P<0.001) (Figure 2) and decreased clonic seizure duration (P<0.05), tonic-clonic seizure duration (P<0.001) (Figure 3), and the total time of seizure (P<0.001) (Figure 4) significantly compared to the negative control group (saline administration).

Moreover, diazepam increased the tonic seizure duration compared to the control group, but the difference was significant statistically (Figure 5). Intraperitoneal administration of α-pinene (100 and 200 mg/kg) significantly reduced the tonic and clonic-tonic seizure duration (P<0.001) and total seizure duration (P<0.05) compared with the negative control group (Figures 4, 5 and 6). Furthermore, administration of 200 mg/kg α-pinene had no significant effect on the delay of onset of seizure and clonic seizure duration compared to the negative control group (Figures 2 and 3).

As indicated, four weeks of swimming exercise alone significantly decreased tonic seizure, clonic seizure, clonic-tonic seizure, and the total seizure duration (P<0.001) compared to the control group (Figures 3, 4, 5 and 6).



In addition, four weeks of swimming exercise alone increased the delay of onset of seizures compared to the control group, but the difference was not statistically significant (Figure 2). Furthermore, combined α-pinene use and swimming exercise significantly increased the delay of onset of seizure and decreased duration of the tonic seizure (P<0.05), and clonic-tonic seizure (P<0.001), and the total duration of the seizure (P<0.001) compared to the control group (Figures 2, 4, 5 and 6). Moreover, swimming exercises together with α-pinene reduced clonic seizures compared with the control group, but the difference was not statistically significant (Figure 3).
Discussion
Studies have shown that exercise decreases seizure frequency and neuronal activity. This effect is associated with neurotransmitters, whose release during an exercise activity is increased. Many studies have shown that the level of catecholamines increases after acute exercise activity [6]. Regular exercise activities enhance the norepinephrine level and metabolites in different brain areas [20]. It has been reported that norepinephrine has an inhibitory effect on the development of seizures, and its reduction facilitates epileptic activities [21].
Furthermore, dopamine, one of the most abundant catecholamines, has been recently found to contribute to the pathophysiology of epilepsy and modulation of epileptic attacks [22]. Exercise alters the dopamine level in the brain of animals [23]. In this regard, the connection point of D1 and D2 receptors of dopamine has been found to reduce in the temporal lobe of epileptic patients [24]. Another neurotransmitter involved in epilepsy is GABA, the most important inhibitory neurotransmitter in the central nervous system. The activity of the GABA system is diminished in epileptic patients [25].
The present study investigated the pretreatment effect of aerobic exercise and α-pinene on PTZ-induced epilepsy in male rats. The exercise plus α-pinene use significantly decreased delay of onset of seizures, tonic seizures, clonic seizures, clonic-tonic seizures, and the total duration of the seizure. The results of this study are in line with those showing the effect of aerobic exercise on decreasing the seizure rate. Arida et al. [26] reported that physical activity reduced epileptic seizures in epileptic rats. These researchers also studied the effect of aquatic exercise on epileptic seizures and found that aquatic physical activity reduced epileptic disorders. Training can stimulate GABA neuropeptide, which suppresses hippocampal excitability [27].
Evidence also shows that cerebral neurotransmitters are influenced by exercise. The inhibitory effect of noradrenaline on the kindling also suggests that altered neurotransmitter systems due to physical activity can modulate inhibition/excitation balance, which reduces the development and frequency of seizures [28]. For instance, noradrenaline is increased in the rats with physical activity and has inhibitory effects on kindling. Its elimination has also been found to facilitate the development of seizures in the hippocampus of kindled rats [29].
A study on epileptic rats indicated that aerobic exercises have no impact on reducing epileptic disorders, which is not in agreement with the results of the present study. Based on the discussion mentioned above, it seems that the discrepancy between the results of the present study and those of Vannucci Campus et al. [30] regarding the effect of aerobic exercise on decreasing seizures in epileptic female rats is due to the intensity of exercises, especially in the final weeks of treatment, because these exercises have been classified as high-intensity exercises.
Studies have shown that PTZ-kindling experimental epilepsy reduces healthy neurotransmitters in the hippocampal dentate gyrus, while exercise postpones the morphological changes of healthy neurons in the hippocampal dentate gyrus [31]. A study showed that aerobic exercise decreases seizure intensity in male rats, with no difference between the intensities of seizures in the pregnant and non-pregnant rats [32]. Another study in Nigeria indicated that physiotherapists play a pivotal role in rehabilitating epileptic children. Regular physical exercise has psychological and physiological advantages for epileptic children [33].
Studies have reported that Yoga affects electroencephalogram stability and the autonomic nervous system; hence, it can be used as a non-pharmaceutical intervention to improve health and reduce the adverse effects of epilepsy [34].
In one study, the researchers examined the relationship between exercise and seizure frequency. In Scandinavia, while 10% of the population with epilepsy experienced more seizures due to strenuous physical activity, the other 30%-40% with epilepsy experienced a moderate reduction in seizures following regular physical activity. Subsequent studies have shown the possible role of flavonoids on the central nervous system [35].
According to studies, α-pinene is one of the main constituents of Artemisia annua, which shows anticonvulsant effects through the binding activity of benzodiazepine receptors and modulation of oxidative stress and inflammatory process [36].
A current study by Etemadi Kermani et al. has shown that choyl extract can affect the seizure process caused by pentylenetetrazole and reduce the seizure time [37].
Also, according to Behzad Nia et al., the hydroalcoholic extract of mountain tea has anticonvulsant properties. This effect may be due to the presence of the operating system of this extract on the central benzodiazepine [38].
Pereira et al. [39] reported that acute administration of coumarins increases the release of prefrontal cortical GABA, probably by affecting the GABAA receptor. Since aminobutyric plays a key role in inhibiting neurons, coumarin can improve epileptic seizures. Keisalari et al. [40] evaluated the effect of Ferula assa-foetida hydroalcoholic extract on PTZ-induced antiepileptic and antioxidant activities in male mice. They reported the presence of monoterpenes such as α-pinene and β-pinene in Ferula assa-foetida, which have suppressive effects on PTZ-induced seizures.
GABA receptor analogs such as α-pinene and verbenone have been reported in many plant compounds, including choy [14]. Alphapenic has a kinetic effect on gabadard receptors and increases postsynaptic chloride ion current [41]. Also, some penin analogs can cause idiopathic epilepsy in power mice and are known to amplify the chloride current induced by GABAA receptors. Linalool is a monoterpene present as the main compound in several aromatic plant species, including choy [42], and exerts its anticonvulsant effect through the glutamatergic system [9]. In general, according to the results of the present study and other studies, exercise reduces stress and increases freshness, which in turn reduces the epileptic seizures [43]. A wide range of evidence shows that abnormalities of the neurotransmitter systems such as serotonin, noradrenaline, dopamine, glutamate, and GABA are observed in mood disorders and epilepsy. Physical activity can modulate several neurotransmitter systems [44].
According to the present study and other studies, exercise reduces stress, increases freshness, and decreases epileptic seizures [43]. A wealth of evidence shows that abnormalities of the neurotransmitter systems such as serotonin, noradrenaline, dopamine, glutamate, and GABA are observed in mood disorders and epilepsy. Physical activity can modulate several neurotransmitter systems [45].
Conclusion
Since α-pinene is the main compound of plant extracts, it can be used in preventive activities and exercise against neurodegenerative diseases.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were performed in compliance with the ethical guidelines of the Declaration of Helsinki 2013. During the study, keeping and conducting experiments and killing animals followed the standard work methods and principles of ethics with animals, approved by the Research Department of the Ahvaz branch of Islamic Azad University on September 14, 2018 (No. 10621423962038).
Funding
This article was extracted from MSc thesis of the first author, Department of Physical Education and Sport Sciences, Faculty of Humanities, Ahvaz Branch, Islamic Azad University, Ahvaz, Iran.
Authors contributions
Conceptualization, methodology, investigation, and funding acquisition: Abdolhassan Doulah, Maryam Rafieirad, and Sadegh Lotfinesab; Writing the original draft, Supervision, writing, review, and editing: Abdolhassan Doulah and Maryam Rafieirad; Resources: Abdolhassan Doulah and Sadegh Lotfinesab.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Research Deputy of Ahvaz Branch, Islamic Azad University, for its support in carrying out this study.
References
- Guerreiro CA. Guidelines for drug treatment of epilepsy: A critical review. Arq Neuropsiquiatr. 2008; 66(3A):5919. [PMID]
- Mongabadi S, Firoozabadi SM, Javan M, Shojaei A, Mirnajafi-Zadeh J. Effect of different frequencies of repetitive transcranial magnetic stimulation on acquisition of chemical kindled seizures in rats. Neurological Sciences. 2013; 34:1897–1903 [DOI:10.1007/s10072-013-1401-1]
- Porter RJ, Meldrum BS. Antiseizure drugs. In: Katzung BG, editor. Basic and clinical pharmacology. New York: McGraw Hill; 2018. https://www.google.com/books/edition/Basic_Clinical_Pharmacology/7nCutQEACAAJ?hl=en
- McNamara JO. Cellular and molecular basis of epilepsy. J Neurosci. 1994; 14(6):3413-25. [DOI:10.1523/JNEUROSCI.14-06-03413.1994] [PMID] [PMCID]
- Matyszak MK. Inflammation in the CNS: Balance between immunological privilege and immune responses. Prog Neurobiol 1998; 56(1):19-35. [DOI:10.1016/S0301-0082(98)00014-8]
- Carvey PM. Drug action in the central nervous system. Oxford: Oxford University Press; 1998. https://www.google.com/books/edition/Drug_Action_in_the_Central_Nervous_Syste/a_psAAAAMAAJ?hl=en
- Sierra-Paredes G, Sierra-Marcuno G. Extrasynaptic GABA and glutamate receptors in epilepsy. CNS Neurol Disord Drug Targets. 2007; 6(4):288-300. [DOI:10.2174/187152707781387251] [PMID]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986; 24(3):525-9. [DOI:10.1016/0091-3057(86)90552-6]
- Elisabetsky E, Silva Brum LF, Souza DO. Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine. 1999; 6(2):107-13. [DOI:10.1016/S0944-7113(99)80044-0]
- Bienvenu E, Amabeoku GJ, Eagles PK, Scott G, Springfield EP. Anticonvulsant activity of aqueous extract of Leonotis Leonurus. Phytomedicine. 2002; 9(3):217-23. [DOI:10.1078/0944-7113-00103] [PMID]
- McPartland JM, L Pruitt PL. Side effects of pharmaceuticals not elicited by comparable herbal medicines: The case of tetrahydrocannabinol and marijuana. Altern Ther Health Med. 1999; 5(4):57-62. [PMID]
- Evans WC. Trease and Evans' Pharmacognosy (Evans, Trease and Evans Pharmacognosy). Philadelphia: Saunders Company; 1996. https://www.abebooks.com/9780702029332/Trease-Evans-Pharmacognosy-William-Charles-0702029335/plp
- Limberger RP, Aleixo AM, Fett-Neto AG, Henriques AT. Bioconversion of (+)- and (-)-α-pinene to (+)- and (-)-verbenone by plant cell cultures of Psychotria brachyceras and Rauvolfia sellowii. Electron J Biotechnol. 2007; 10(4):500-7. [DOI:10.2225/vol10-issue4-fulltext-8]
- Basile A, Sorbo S, Spadaro V, Bruno M, Maggio A, Faraone N, et al. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae). Molecules. 2009; 14(3):939-52. [DOI:10.3390/molecules14030939] [PMID] [PMCID]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002; 25(6):295-301. [DOI:10.1016/S0166-2236(02)02143-4]
- Peixinho-Pena LF, Fernandes J, de Almeida AA, Novaes Gomes FG, Cassilhas R, Venancio DP, et al. A strength exercise program in rats with epilepsy is protective against seizures. Epilepsy Behav. 2012; 25(3):323-8. [DOI:10.1016/j.yebeh.2012.08.011] [PMID]
- Tchekalarova J, Shishmanova M, Atanasova D, Stefanova M, Alova L, Lazarov N, et al. Effect of endurance training on seizure susceptibility, behavioral changes and neuronal damage after ainite-induced status epilepticus in spontaneously hypertensive rats. Brain Res. 2015; 1625:39-53. [DOI:10.1016/j.brainres.2015.08.022] [PMID]
- Sadate Hosseini P, Rafieirad M, Esmaeili S. The effect of oleuropein on working and passive avoidance memory in the pentylenetetrazole-induced seizure animal model. J Bas Res Med Sci. 2019; 6(1):41-8. https://jbrms.medilam.ac.ir/browse.php?sid=1&a_id=414&slc_lang=&ftxt=1
- Zar A, Hoseini A, Ahmadi F, Rezaei M. [Effects of ginger together with swimming training on blood fat profiles in adult diabetic rats with streptozotocin (Persian)]. Iran J Nutr Sci Food Technol. 2016; 11(2):65-74. http://nsft.sbmu.ac.ir/article-1-2181-en.html
- Dunn AL, Reigle TG, Youngstedt SD, Armstrong RB, Dishman RK. Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Med Sci Sports Exerc.1996; 28(2):204-9. [PMID]
- Bortolotto ZA, Cavalheiro EA. Effect of DSP4 on hippocampal kindling in rats. Pharmacol Biochem Behav. 1986; 24(3):777-9. [PMID]
- Haut SR, Albin RL. Dopamine and epilepsy: Hints of complex subcortical roles. Neurology. 2008; 71(11):784-5.[DOI:10.1212/01.wnl.0000325637.38931.27] [PMID]
- Meeusen R, Smolders I, Sarre S, de Meirleir K, Keizer H, Serneels M, et al. Endurance training effects on neurotransmittcr release in rat striatum: An in vivo microdialysis study. Acta Physiol Scand. 1997; 159(4):335-41.[DOI:10.1046/j.1365-201X.1997.00118.x] [PMID]
- Yakushev IY, Dupont E, Buchholz HG, Tillmanns J, Debus F, Cumming P, et al. In vivo imaging of dopamine receptors in a model of temporal lobe epilepsy. Epilepsia. 2010; 51(3):415-22. [DOI:10.1111/j.1528-1167.2009.02272.x] [PMID]
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001; 42(Suppl 3):8-12. [DOI:10.1046/j.1528-1157.2001.042suppl.3008.x] [PMID]
- Arida RM, de Jesus Vieira A, Cavalheiro EA. Effect ofphysical exercise on kindling development. Epilepsy Res. 1998; 30(2):127 32. [DOI:10.1016/S0920-1211(97)00102-2]
- Arida RM, Sanabria ER, da Silva AC, Faria LC, Scorza FA, Cavalheiro EA. Physical training reverts hippocampal electrophysiological changes in ratssubmitted to the pilocarpine model of epilepsy. Physiol Behav. 2004; 83(1):165-71.[DOI:10.1016/j.physbeh.2004.08.008] [PMID]
- Arida RM, de Almeida AC, Cavalheiro EA, Scorza FA. Experimental and clinical findings from physical exercise as complementary therapy for epilepsy. Epilepsy Behav. 2013; 26(3):273-8. [DOI:10.1016/j.yebeh.2012.07.025] [PMID]
- Arida RM, Cavalheiro EA, da Silva AC, Scorza FA. Physical activity and epilepsy: Proven and predicted benefits. Sports Med. 2008; 38(7):607-15. [DOI:10.2165/00007256-200838070-00006] [PMID]
- Vannucci Campos D, Lopim GM, da Silva DA, de Almeida AA, Amado D, Arida RM. Epilepsy and exercise: An experimental study in female rats. Physiol Behav. 2017; 171:120-6. [DOI:10.1016/j.physbeh.2016.12.040] [PMID]
- Overall RW, Walker TL, Leiter O, Lenke S, Ruhwald S, Kempermann G. Delayed and transient increase of adult hippocampal neurogenesis by physical exercise in DBA/2 mice. PLoS One. 2013; 8(12):e83797. [DOI:10.1371/journal.pone.0083797] [PMID] [PMCID]
- Kim HJ, Kim IK, Song W, Lee J, Park S. The synergic effect of regular exercise and resveratrol on kainate-induced oxidative stress and seizure activity in mice. Neurochem Res. 2013; 38(1):117-22. [DOI:10.1007/s11064-012-0897-8] [PMID]
- Yakasai AM, Danazumi MS, Zakari UU, Usman IL, Abdullahi A, Shehu UT. Knowledge and current practices of physiotherapists on the physical activity and exercise in the rehabilitation of children with epileptic seizures. Epilepsy Behav. 2020; 104(Pt A):106891. [DOI:10.1016/j.yebeh.2019.106891] [PMID]
- Shawahna R, Abdelhaq I. Exploring perceived benefits, motives, barriers, and recommendations for prescribing yoga exercises as a nonpharmacological intervention for patients with epilepsy: A qualitative study from Palestine.Epilepsy Behav. 2020; 106:107041. [DOI:10.1016/j.yebeh.2020.107041] [PMID]
- Zarei M, Golmohammadi R, Beheshti-Nasr SM. [The effect of aerobic exercise training on epileptic behaviors in the kindled and non-kindled rats with pentylenetetrazole (PTZ) (Persian)]. J Isfahan Med Sch. 2020; 38(573):278-85. http://jims.mui.ac.ir/index.php/jims/article/view/13065
- Daneshkhah M, Setorki M. Protective effects of artemisia persica essential oil against pentylenetetrazol-induced seizure in male mice with emphasizing its mechanism of action. Iran Red Crescent Med J. 2019; 21(2):e85021. https://www.sid.ir/en/Journal/ViewPaper.aspx?ID=683958
- EtemadiKermani Sh, Rafieerad M.[The effect of intraperitoneal injection of ferulae angulatahydroalcoholic extract on pentylenetetrazole- induced epilepsy in male mice (Persian)].Jundishapur Sci Med J. 2018; 17(1):107-14. [DOI:10.22118/JSMJ.2018.59644]
- Behzad Nia H, Naseri A, Emamhadi, M, Ghadarjani S, Aghaei I, Dehpour AR. Effect of hydroalcoholic extract of satchys lavandulifolia on pentylenetetrazole-induced seizures in male mice: The role of gabaergic and opioidergic systems. Basic Clin Neurosci. 2022; 13(1):1-18. [DOI:10.32598/bcn.2021.2299.1]
- Pereira EC, Lucetti DL, Barbosa-Filho JM, de Brito EM, Monteiro VS, Patrocínio MC, et al. Coumarin effects on amino acid levels in mice prefrontal cortex and hippocampus.Neurosci Lett. 2009; 454(2):139-42. [DOI:10.1016/j.neulet.2009.03.009]
- Achliya GS, Wadodkar SG, Dorle AK. Evaluation of sedative and anticonvulsant activities of Unmadnashak Ghrita. J Ethnopharmacol. 2004; 94(1):77-83. [DOI:10.1016/j.jep.2004.04.020] [PMID]
- Kiasalari Z, Khalili M, Roghani M, Heidari H, Azizi Y. Antiepileptic and antioxidant effect of hydroalcoholic extract of Ferula assa foetida gum on pentylentetrazole-induced kindling in male mice. Basic Clin. Neurosci. 2013; 4(4):299–306. [PMID]
- Aoshima H, Hamamoto K. Potentiation of GABAA receptors expressed in Xenopus oocytes by perfume and phytoncid. Biosci Biotechnol Biochem. 1999; 63(4):743-8. [PMID]
- Shahbazi Y, Shavisi N, Karami N, Kakaei S. Chemical composition and invitro antibacterial activity of ferulago angulata (Schlecht.) boiss essential oil. Pharma Sci. 2015; 21(1):6-11. [DOI:10.15171/PS.2015.10]
- Tavakoli M, Neshat Doost HT, Molavi H, Barekatain M, Karmi Nouri R, Mehvari J. [Evaluation of memory in refractory temporal lobe epilepsy (Persian)]. J Res Behav Sci. 2011; 9(1):63-9. http://rbs.mui.ac.ir/article-1-186-en.html
- Zhang B, Zhang JW, Wang WP, Dong RF, Tian S, Zhang C. Effect of lamotrigine on epilepsyinduced cognitive impairment and hippocampal neuronal apoptosis in pentylenetetrazole-kindled animal model. Synapse. 2017; 71(2). [PMID]
Type of Study: Research |
Subject:
Special
Received: 2022/04/17 | Accepted: 2022/04/21 | Published: 2022/04/21
Received: 2022/04/17 | Accepted: 2022/04/21 | Published: 2022/04/21
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






