Fri, Apr 26, 2024
Volume 7, Issue 4 (Autumn 2021)
Caspian J Neurol Sci 2021, 7(4): 202-208 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mohammadzadeh Jouryabi A, Imantalab V, Sedighinejad A, Emir Alavi C, Biazar G, Mansour Ghanaie M, et al . Post-dural Puncture Headache in Cesarean Section; Frequency and Risk Factors: A Report From the North of Iran. Caspian J Neurol Sci 2021; 7 (4) :202-208
URL: http://cjns.gums.ac.ir/article-1-466-en.html
URL: http://cjns.gums.ac.ir/article-1-466-en.html
Ali Mohammadzadeh Jouryabi1 
 , Vali Imantalab1
, Vali Imantalab1 

 , Abbas Sedighinejad1
, Abbas Sedighinejad1 

 , Cyrus Emir Alavi1
, Cyrus Emir Alavi1 

 , Gelareh Biazar *
, Gelareh Biazar * 

 2, Mandana Mansour Ghanaie3
2, Mandana Mansour Ghanaie3 

 , Mahin Tayefeh Ashrafiyeh1
, Mahin Tayefeh Ashrafiyeh1 

 , Faezeh Emami1
, Faezeh Emami1 


 , Vali Imantalab1
, Vali Imantalab1 

 , Abbas Sedighinejad1
, Abbas Sedighinejad1 

 , Cyrus Emir Alavi1
, Cyrus Emir Alavi1 

 , Gelareh Biazar *
, Gelareh Biazar * 

 2, Mandana Mansour Ghanaie3
2, Mandana Mansour Ghanaie3 

 , Mahin Tayefeh Ashrafiyeh1
, Mahin Tayefeh Ashrafiyeh1 

 , Faezeh Emami1
, Faezeh Emami1 

1- Department of Anesthesiology, Anesthesiology Research Center, Al-Zahra Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Anesthesiology, Anesthesiology Research Center, Al-Zahra Hospital, Guilan University of Medical Sciences, Rasht, Iran. , gelarehbiazar1386@gmail.com
3- Department of Obstetrics and Gynecology, Reproductive Health Research Center, Al-Zahra Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Anesthesiology, Anesthesiology Research Center, Al-Zahra Hospital, Guilan University of Medical Sciences, Rasht, Iran. , gelarehbiazar1386@gmail.com
3- Department of Obstetrics and Gynecology, Reproductive Health Research Center, Al-Zahra Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
Full-Text [PDF 1042 kb]
(584 Downloads)
| Abstract (HTML) (1293 Views)
Full-Text: (589 Views)
Introduction
Cesarean Section (CS) is considered when the mother or baby or both are at risk and there are no acceptable conditions for normal vaginal delivery. Spinal Anesthesia (SA) has been used widely in CS [1, 2]. Because of several advantages, such as preventing general anesthesia-related neurotoxicity, which has strongly been recently focused on, cost-effectiveness, ease to perform, early-onset, lower risk of aspiration, and avoidance of airway manipulation, this method is preferred for non-complicated elective cesarean delivery [3-5]. However, despite several advantages of SA, it is sometimes complicated by Post-Dural Puncture Headache (PDPH) [6]. Headaches can be more complicated than most people realize. Different kinds of headaches can have their set of symptoms, happening for unique reasons, and need different treatments [7, 8]. Performing SA, following a dural puncture, a marked Cerebrospinal Fluid (CSF) leakage may occur, resulting in a significant intracranial pressure drop [9]. As CSF production is slower than the loss, it could not immediately be replaced, resulting in a downward stretching of the intracranial pain-sensitive structures that causes headache and pain in the neck and shoulders. The other postulated mechanism is the vasodilation of meningeal vessels through adenosine release [10]. So far, no standard preventive guideline has been recommended. However, it is expected that this complication responds well to rest, hydration, caffeine, and simple analgesics, such as paracetamol [11, 12]
According to the International Classification of Headache Disorders, the diagnostic criteria for PDPH, are the dull throbbing pain with a frontal-occipital distribution, get worsening when sitting or standing, and relieving when lying down. PDPH develops within 5 days after dural puncture and disappears spontaneously within one week or up to 48 h after an epidural blood patch. Rarely, it may last for months or even years. The headache may be accompanied by neck stiffness, tinnitus, hyperacusis, photophobia, and nausea [13]. The incidence of PDPH is reported from 0.4%-25% [14]. This complication may affect the patient’s acceptance of the method and causes an unpleasant anesthesia experience, increased risk of chronic headache, decreased mother ability to take care of the baby, prevention of early ambulation, and consequently, the risk of pulmonary emboli and deep vein thrombosis [15, 16].
Female gender, pregnancy, needle size, needle tip shape, type of needle tip, younger age, multiple punctures, and needle bevel direction are well-described risk factors for this complication [9, 17, 18]. The issue as a common and annoying problem has been investigated in some centers; however, limited data are available on some other influencing factors, such as the history of headaches and individuals habits [1, 19-21]. Moreover, because of the differences among studies regarding their studied samples, characteristics of the treatment center, for example, private centers compared to educational hospitals, and the medical team experience, their results could not be generalized to the other centers. Therefore, due to the importance of the issue, each hospital should be aware of its conditions and perform interventions regarding some influencing factors if required and possible. In this research, we prospectively investigated the frequency of PDPH and some influencing factors in parturients undergoing CS under SA.
Materials and Methods
After the permission of the Ethics Committee of Guilan University of Medical Sciences (GUMS), this descriptive cross-sectional prospective study was conducted at Alzahra hospital, an academic referral center affiliated with the GUMS. During the study period (six months) from May 2019 to October 2019, 147 eligible cases were enrolled. Then, questionnaires containing questions about patients’ demographic data and some considered PDPH-related factors were filled out via face-to-face interviews.
Inclusion criteria: Women aged 18-45 years with stable hemodynamic status undergoing CS under SA were enrolled in the survey. Exclusion criteria: Patients suffering from chronic pain (suffering from persistent pain for more than 12 weeks and more than 15 days in a month despite medication or treatment), those with psychiatric diseases (suffering from a mental illness diagnosed by a psychiatrist that greatly disturbs his/her thinking, moods, and/or behavior), cases treated with analgesic drugs before SA (regular consumption), changing anesthesia plane to general anesthesia, and any contraindication for SA were considered as exclusion criteria.
Sample size
According to Nazemi et al. who reported the rate of 6% for PDPH and considering the significance level of 0.05 and the accuracy of 0.05, a sample size of 147 subjects was considered in this study [22].
During the study, 677 pregnant women were interviewed, of whom 124 cases refused to participate due to personal reasons, 21 cases were excluded due to the need for GA, and 167 cases did not meet the mentioned inclusion criteria as most of them were not in a stable status and not cooperative. A large number of patients were not in stable conditions and not communicative and 218 cases refused to answer our phone calls during the follow-ups after discharge from the hospital. Finally, 147 eligible cases were studied.
After obtaining informed consent, eligible cases were interviewed and demographic data, maternal medical history, and physical examination were documented by senior anesthesia residents under supervision. In the operating room, the standard monitoring was performed and preloading with 500 mL of crystalloids was started. In stable hemodynamic status, the spinal block was performed with a 25-gauge Quincke needle in a sitting position. To perform a subarachnoid block in CS, both sitting and lateral positions are commonly used. However, in a sitting position, the landmarks are easily identified; thus, it is technically safer and preferred in terms of the need for fewer attempts and more maternal satisfaction [23, 24].
After confirmation of the dural puncture with clear CSF flow, 2.5 ml of bupivacaine 0.5% was injected and the subject laid flat quickly. Any adverse effects, such as hypotension, nausea and vomiting, and shivering were documented, as well. The same protocol was considered for all women. The procedure was performed by the on-duty anesthesia resident regardless of his level. Another resident not present on that day followed up the patients for up to 7 days. In the hospital, by face-to-face visits and after discharge from the hospital by making phone calls, the cases were followed up on days one, three, and seven. They were also recommended to return to the hospital or call in any complicated state not restricted to the mentioned days. They were questioned about any type of headache experience and its characteristics.
According to the PDPH definition, cases with frontal or occipital HA, worsening while standing, and alleviated by lying flat were diagnosed as PDPH. The patients were advised to drink liquids and rest in the supine position and take two Novafen capsules containing acetaminophen 325 mg plus caffeine 40 mg plus ibuprofen 200 mg, manufactured by Alhavi Pharmaceutical Company, every 8 hours if needed. The patients could score their pain severity from 0 to 10; 0 represented “no pain”, while 10 represented the highest degree of pain. The scores 8-10 were considered as severe pain. Moreover, if patients’ symptoms were resistant to the conventional pharmacological and non-pharmacological treatments it was marked as persistent HA and the patient was recommended to refer to the hospital for more evaluation. In conditions with no improvement, the epidural patch was considered.
Statistical analysis
The collected data were analyzed by SPSS v. 21 using Chi-squared and Fisher’s exact tests. The data were presented as Mean±SD. Statistically, a P-value of less than 0.05 was considered significant.
Results
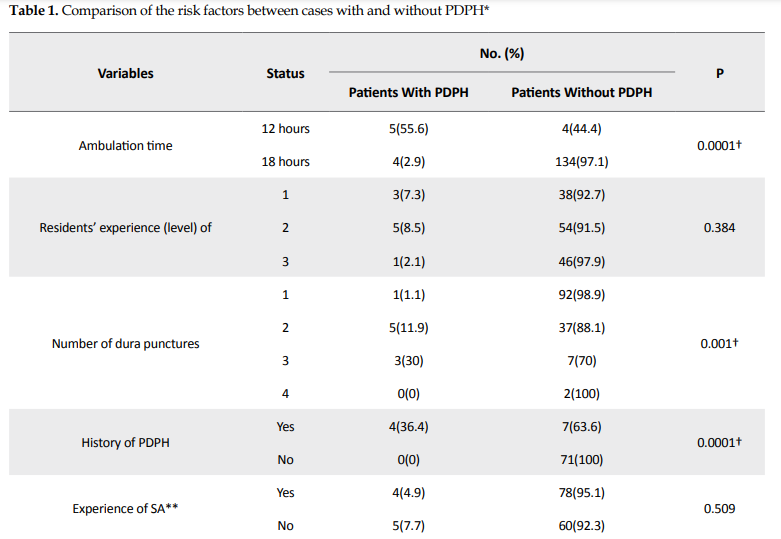
The data of 147 eligible cases were analyzed. The Mean±SD age of our cases was 30.68±5.95 years (range: 17-46 years), their mean body mass index (BMI) was 33.33±6.2 kg/m² (range: 22.07-48.54 kg/m²), 12 cases (8.16%) developed HA, and 9 cases (6.1%) were diagnosed with PDPH. None of them experienced a severe or persistent HA and recovered within 4 to 5 days. Also, 27.9 % of SA were done by junior residents and 72.1% by senior residents. HA mostly was resolved by rest and hydration and only 3 patients required medications. No significant relationship was found between PDPH development and the level of residents (P=0.384), BMI (P=0.106), age (P=0.093), and the experience of SA (P=0.509). However, the occurrence of PDPH was statistically related to early ambulation (P=0.0001), the number of attempts (P=0.001), and the history of HA (P=0.0001) (Table 1).
_cesearian.PNG)
Discussion
In this study, the frequency of PDPH was 6.1%, which is almost close to the minimum border of the reported range (0.4- 25%) [25]. It was the first research focusing on this issue in a referral academic center in the North of Iran, with an annual admission rate of more than 4000 CS cases. We found that the history of previous PDPH, early ambulation, and the number of attempts could significantly affect the PDPH occurrence. However, the resident’s experience, having a history of SA, and BMI were not found as predisposing factors.
It was expected that the number of attempts could be a predictor for PDPH due to multiple punctures and more CSF leakage. However, considering that the resident’s experience was not an affecting factor, it seems that multi-puncture was not the result of inexperience. Etezadi et al. in a similar study reported the incidence of 10.8% for PDPH, which was not significantly affected by BMI, age, history of HA, and coffee or tea consumption. They declared that this adverse event might be partly independent and unpredictable [1]. Davoudi et al. reported that an overall incidence of 12.7% for PDPH and also found that the frequency of PDPH in CS was significantly correlated with sitting position compared to lateral decubitus [26]. Gisore et al. from Kenya reported that the overall incidence of 20.35% for PDPH in their academic hospitals. They concluded that the use of Quinke needles was the main reason for the high incidence rate of PDPH [6]. Mohammad et al. reported the incidence of the rate of 32.58% for PDPH in CS under SA from an academic center. They recommended the use of pencil-point needles or smaller sizes to achieve better outcomes [27]. Khraise et al. reported that repeated punctures and the history of tension headache were the main risk factors for PDPH in the woman who underwent CS under SA [9]. Vallejo et al. found that the type and size of the needle were important influencing factors in PDPH, while Khraise et al. did not find any direct effect [9]. A significant correlation between BMI and PDPH development was reported by Faure et al. [10]. Khraise et al. in line with our study did not support this observation [9]. Seeberger et al. demonstrated an inverse correlation between age and the incidence of PDPH [28]. In line with our findings, Rasooli et al. [10] in a teaching hospital reported that early ambulation led to a higher incidence of PDPH. The similarity between the methods of the two studies should be noted; both were performed in academic hospitals and the residents’ experience, the drugs, and ASA class were the same. However, the frequency of PDPH was 1.27% in their study, markedly lower compared to ours. This superiority might be due to the differences between methods. In their study, the procedures were performed by attending and residents compared to our research, in which only residents were involved under the supervision of specialists. Additionally, the assessment accuracy might be different [10].
Tarekegn et al. from Ethiopia reported the prevalence of 42.6% for PDPH in cases undergoing CS under SA, which is almost seven times higher than our study. Larger used needles and repeated punctures were the reasons for this higher rate of incidence [29]. They also did not come across the previously confirmed risk factors for PDPH development, such as lower BMI, history of PDPH, and younger age. Siddiqui et al. from Pakistan reported an incidence of 8.7% for this complication in an academic center. It should be noted that emergency cases in hypovolemic and dehydrated status were not excluded and underwent SA by inexperienced residents during odd hours [30]. Firdous et al. found that the paramedian approach had an inverse association with PDPH occurrence [31]. In contrast, Uluer et al. reported no significant relationship between this variable and PDPH [14]. It should be noted that the inclusion criteria were different between the two studies. In contrast to Firdous et al., Uluer et al. only enrolled those with the ASA class II, and BMI >35 kg/m2, and cases with multiple gestations and any abdominal mass or ascites were excluded. Checkol et al. in a meta-analysis reported that the prevalence of PDPH following SA in CS was 23.47% and reported a relationship between normal BMI, multiple attempts, and needle size less than or equal to 22 gauge and the occurrence of PDPH [32].
In the literature, contrary results have been reported regarding predisposing factors for PDPH. This discrepancy emphasizes the fact that the real pathophysiology of PDPH has not been completely understood yet and also the necessity of performing this study in different areas. Moreover, it should be noted that the individuals’ definition and perception of pain are different and could be influencing factors. Also, people have different pain thresholds and sensitivity and willingness to report pain, according to gender, age, and race, which are different in studies [33, 34]. This study was limited to a single center with a small sample size. Additionally, the significant number of drops could be considered a major limitation.
Conclusion
Based on our findings, the frequency of PDPH was found to be 6.1%, which could be an acceptable result as it is in the ranged 0.4%-25%. We found that the occurrence of PDPH in a parturient candidate for CS under SA was associated with the history of HA, early ambulation, and the number of dura punctures. Women at risk for PDPH are suggested to be objectively screened before CS under SA.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethical Institutional Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1398.398).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conception and design: Gelareh Biazar and Vali Imantalab; Analysis and interpretation: Cyrus Emir Alavi; Drafting of the article: Mahin Tayefeh Ashrafiyeh; Critical revision of the article for important intellectual content: Ali Mohammadzadeh Jouryabi; Final approval: Abbas Sedighinejad; Collection and assembly of data: Faezeh Emami; Supervision: Mandana Mansour Ghanaie.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Anesthesiology Research Center members Mohadese Ahmadi and Mahin Tayefeh Ashrafie for their collaboration in this study.
Refrences:
Cesarean Section (CS) is considered when the mother or baby or both are at risk and there are no acceptable conditions for normal vaginal delivery. Spinal Anesthesia (SA) has been used widely in CS [1, 2]. Because of several advantages, such as preventing general anesthesia-related neurotoxicity, which has strongly been recently focused on, cost-effectiveness, ease to perform, early-onset, lower risk of aspiration, and avoidance of airway manipulation, this method is preferred for non-complicated elective cesarean delivery [3-5]. However, despite several advantages of SA, it is sometimes complicated by Post-Dural Puncture Headache (PDPH) [6]. Headaches can be more complicated than most people realize. Different kinds of headaches can have their set of symptoms, happening for unique reasons, and need different treatments [7, 8]. Performing SA, following a dural puncture, a marked Cerebrospinal Fluid (CSF) leakage may occur, resulting in a significant intracranial pressure drop [9]. As CSF production is slower than the loss, it could not immediately be replaced, resulting in a downward stretching of the intracranial pain-sensitive structures that causes headache and pain in the neck and shoulders. The other postulated mechanism is the vasodilation of meningeal vessels through adenosine release [10]. So far, no standard preventive guideline has been recommended. However, it is expected that this complication responds well to rest, hydration, caffeine, and simple analgesics, such as paracetamol [11, 12]
According to the International Classification of Headache Disorders, the diagnostic criteria for PDPH, are the dull throbbing pain with a frontal-occipital distribution, get worsening when sitting or standing, and relieving when lying down. PDPH develops within 5 days after dural puncture and disappears spontaneously within one week or up to 48 h after an epidural blood patch. Rarely, it may last for months or even years. The headache may be accompanied by neck stiffness, tinnitus, hyperacusis, photophobia, and nausea [13]. The incidence of PDPH is reported from 0.4%-25% [14]. This complication may affect the patient’s acceptance of the method and causes an unpleasant anesthesia experience, increased risk of chronic headache, decreased mother ability to take care of the baby, prevention of early ambulation, and consequently, the risk of pulmonary emboli and deep vein thrombosis [15, 16].
Female gender, pregnancy, needle size, needle tip shape, type of needle tip, younger age, multiple punctures, and needle bevel direction are well-described risk factors for this complication [9, 17, 18]. The issue as a common and annoying problem has been investigated in some centers; however, limited data are available on some other influencing factors, such as the history of headaches and individuals habits [1, 19-21]. Moreover, because of the differences among studies regarding their studied samples, characteristics of the treatment center, for example, private centers compared to educational hospitals, and the medical team experience, their results could not be generalized to the other centers. Therefore, due to the importance of the issue, each hospital should be aware of its conditions and perform interventions regarding some influencing factors if required and possible. In this research, we prospectively investigated the frequency of PDPH and some influencing factors in parturients undergoing CS under SA.
Materials and Methods
After the permission of the Ethics Committee of Guilan University of Medical Sciences (GUMS), this descriptive cross-sectional prospective study was conducted at Alzahra hospital, an academic referral center affiliated with the GUMS. During the study period (six months) from May 2019 to October 2019, 147 eligible cases were enrolled. Then, questionnaires containing questions about patients’ demographic data and some considered PDPH-related factors were filled out via face-to-face interviews.
Inclusion criteria: Women aged 18-45 years with stable hemodynamic status undergoing CS under SA were enrolled in the survey. Exclusion criteria: Patients suffering from chronic pain (suffering from persistent pain for more than 12 weeks and more than 15 days in a month despite medication or treatment), those with psychiatric diseases (suffering from a mental illness diagnosed by a psychiatrist that greatly disturbs his/her thinking, moods, and/or behavior), cases treated with analgesic drugs before SA (regular consumption), changing anesthesia plane to general anesthesia, and any contraindication for SA were considered as exclusion criteria.
Sample size
According to Nazemi et al. who reported the rate of 6% for PDPH and considering the significance level of 0.05 and the accuracy of 0.05, a sample size of 147 subjects was considered in this study [22].
During the study, 677 pregnant women were interviewed, of whom 124 cases refused to participate due to personal reasons, 21 cases were excluded due to the need for GA, and 167 cases did not meet the mentioned inclusion criteria as most of them were not in a stable status and not cooperative. A large number of patients were not in stable conditions and not communicative and 218 cases refused to answer our phone calls during the follow-ups after discharge from the hospital. Finally, 147 eligible cases were studied.
After obtaining informed consent, eligible cases were interviewed and demographic data, maternal medical history, and physical examination were documented by senior anesthesia residents under supervision. In the operating room, the standard monitoring was performed and preloading with 500 mL of crystalloids was started. In stable hemodynamic status, the spinal block was performed with a 25-gauge Quincke needle in a sitting position. To perform a subarachnoid block in CS, both sitting and lateral positions are commonly used. However, in a sitting position, the landmarks are easily identified; thus, it is technically safer and preferred in terms of the need for fewer attempts and more maternal satisfaction [23, 24].
After confirmation of the dural puncture with clear CSF flow, 2.5 ml of bupivacaine 0.5% was injected and the subject laid flat quickly. Any adverse effects, such as hypotension, nausea and vomiting, and shivering were documented, as well. The same protocol was considered for all women. The procedure was performed by the on-duty anesthesia resident regardless of his level. Another resident not present on that day followed up the patients for up to 7 days. In the hospital, by face-to-face visits and after discharge from the hospital by making phone calls, the cases were followed up on days one, three, and seven. They were also recommended to return to the hospital or call in any complicated state not restricted to the mentioned days. They were questioned about any type of headache experience and its characteristics.
According to the PDPH definition, cases with frontal or occipital HA, worsening while standing, and alleviated by lying flat were diagnosed as PDPH. The patients were advised to drink liquids and rest in the supine position and take two Novafen capsules containing acetaminophen 325 mg plus caffeine 40 mg plus ibuprofen 200 mg, manufactured by Alhavi Pharmaceutical Company, every 8 hours if needed. The patients could score their pain severity from 0 to 10; 0 represented “no pain”, while 10 represented the highest degree of pain. The scores 8-10 were considered as severe pain. Moreover, if patients’ symptoms were resistant to the conventional pharmacological and non-pharmacological treatments it was marked as persistent HA and the patient was recommended to refer to the hospital for more evaluation. In conditions with no improvement, the epidural patch was considered.
Statistical analysis
The collected data were analyzed by SPSS v. 21 using Chi-squared and Fisher’s exact tests. The data were presented as Mean±SD. Statistically, a P-value of less than 0.05 was considered significant.
Results
The data of 147 eligible cases were analyzed. The Mean±SD age of our cases was 30.68±5.95 years (range: 17-46 years), their mean body mass index (BMI) was 33.33±6.2 kg/m² (range: 22.07-48.54 kg/m²), 12 cases (8.16%) developed HA, and 9 cases (6.1%) were diagnosed with PDPH. None of them experienced a severe or persistent HA and recovered within 4 to 5 days. Also, 27.9 % of SA were done by junior residents and 72.1% by senior residents. HA mostly was resolved by rest and hydration and only 3 patients required medications. No significant relationship was found between PDPH development and the level of residents (P=0.384), BMI (P=0.106), age (P=0.093), and the experience of SA (P=0.509). However, the occurrence of PDPH was statistically related to early ambulation (P=0.0001), the number of attempts (P=0.001), and the history of HA (P=0.0001) (Table 1).

_cesearian.PNG)
Discussion
In this study, the frequency of PDPH was 6.1%, which is almost close to the minimum border of the reported range (0.4- 25%) [25]. It was the first research focusing on this issue in a referral academic center in the North of Iran, with an annual admission rate of more than 4000 CS cases. We found that the history of previous PDPH, early ambulation, and the number of attempts could significantly affect the PDPH occurrence. However, the resident’s experience, having a history of SA, and BMI were not found as predisposing factors.
It was expected that the number of attempts could be a predictor for PDPH due to multiple punctures and more CSF leakage. However, considering that the resident’s experience was not an affecting factor, it seems that multi-puncture was not the result of inexperience. Etezadi et al. in a similar study reported the incidence of 10.8% for PDPH, which was not significantly affected by BMI, age, history of HA, and coffee or tea consumption. They declared that this adverse event might be partly independent and unpredictable [1]. Davoudi et al. reported that an overall incidence of 12.7% for PDPH and also found that the frequency of PDPH in CS was significantly correlated with sitting position compared to lateral decubitus [26]. Gisore et al. from Kenya reported that the overall incidence of 20.35% for PDPH in their academic hospitals. They concluded that the use of Quinke needles was the main reason for the high incidence rate of PDPH [6]. Mohammad et al. reported the incidence of the rate of 32.58% for PDPH in CS under SA from an academic center. They recommended the use of pencil-point needles or smaller sizes to achieve better outcomes [27]. Khraise et al. reported that repeated punctures and the history of tension headache were the main risk factors for PDPH in the woman who underwent CS under SA [9]. Vallejo et al. found that the type and size of the needle were important influencing factors in PDPH, while Khraise et al. did not find any direct effect [9]. A significant correlation between BMI and PDPH development was reported by Faure et al. [10]. Khraise et al. in line with our study did not support this observation [9]. Seeberger et al. demonstrated an inverse correlation between age and the incidence of PDPH [28]. In line with our findings, Rasooli et al. [10] in a teaching hospital reported that early ambulation led to a higher incidence of PDPH. The similarity between the methods of the two studies should be noted; both were performed in academic hospitals and the residents’ experience, the drugs, and ASA class were the same. However, the frequency of PDPH was 1.27% in their study, markedly lower compared to ours. This superiority might be due to the differences between methods. In their study, the procedures were performed by attending and residents compared to our research, in which only residents were involved under the supervision of specialists. Additionally, the assessment accuracy might be different [10].
Tarekegn et al. from Ethiopia reported the prevalence of 42.6% for PDPH in cases undergoing CS under SA, which is almost seven times higher than our study. Larger used needles and repeated punctures were the reasons for this higher rate of incidence [29]. They also did not come across the previously confirmed risk factors for PDPH development, such as lower BMI, history of PDPH, and younger age. Siddiqui et al. from Pakistan reported an incidence of 8.7% for this complication in an academic center. It should be noted that emergency cases in hypovolemic and dehydrated status were not excluded and underwent SA by inexperienced residents during odd hours [30]. Firdous et al. found that the paramedian approach had an inverse association with PDPH occurrence [31]. In contrast, Uluer et al. reported no significant relationship between this variable and PDPH [14]. It should be noted that the inclusion criteria were different between the two studies. In contrast to Firdous et al., Uluer et al. only enrolled those with the ASA class II, and BMI >35 kg/m2, and cases with multiple gestations and any abdominal mass or ascites were excluded. Checkol et al. in a meta-analysis reported that the prevalence of PDPH following SA in CS was 23.47% and reported a relationship between normal BMI, multiple attempts, and needle size less than or equal to 22 gauge and the occurrence of PDPH [32].
In the literature, contrary results have been reported regarding predisposing factors for PDPH. This discrepancy emphasizes the fact that the real pathophysiology of PDPH has not been completely understood yet and also the necessity of performing this study in different areas. Moreover, it should be noted that the individuals’ definition and perception of pain are different and could be influencing factors. Also, people have different pain thresholds and sensitivity and willingness to report pain, according to gender, age, and race, which are different in studies [33, 34]. This study was limited to a single center with a small sample size. Additionally, the significant number of drops could be considered a major limitation.
Conclusion
Based on our findings, the frequency of PDPH was found to be 6.1%, which could be an acceptable result as it is in the ranged 0.4%-25%. We found that the occurrence of PDPH in a parturient candidate for CS under SA was associated with the history of HA, early ambulation, and the number of dura punctures. Women at risk for PDPH are suggested to be objectively screened before CS under SA.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethical Institutional Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1398.398).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conception and design: Gelareh Biazar and Vali Imantalab; Analysis and interpretation: Cyrus Emir Alavi; Drafting of the article: Mahin Tayefeh Ashrafiyeh; Critical revision of the article for important intellectual content: Ali Mohammadzadeh Jouryabi; Final approval: Abbas Sedighinejad; Collection and assembly of data: Faezeh Emami; Supervision: Mandana Mansour Ghanaie.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Anesthesiology Research Center members Mohadese Ahmadi and Mahin Tayefeh Ashrafie for their collaboration in this study.
Refrences:
- Etezadi F, Yousefshahi F, Khajavi M, Tanha F, Dahmarde A, Najafi A. Post dural puncture headache after cesarean section, a teaching hospital experience. J Family Reprod Health. 2012; 6(1):17-21. https://jfrh.tums.ac.ir/index.php/jfrh/article/view/137
- Zangouei A, Zahraei SAH, Sabertanha A, Nademi A, Golafshan Z, Zangoue M. Effect of low-dose intravenous ketamine on prevention of headache after spinal anesthesia in patients undergoing elective cesarean section: A double-blind clinical trial study. Anesth Pain Med. 2019; 9(6):e97249. [DOI:10.5812/aapm.97249] [PMID] [PMCID]
- Sedighinejad A, Soltanipour S, Rimaz S, Biazar G, Chaibakhsh Y, Kouhi MB. General anesthesia-related neurotoxicity in the developing brain and current knowledge and practice of physicians at Guilan academic hospitals. Anesth Pain Med. 2019; 9(4):e92366. [DOI:10.5812/aapm.92366] [PMID] [PMCID]
- Biazar G, Farzi F, Naderi Nabi B, Atrkarroushan Z, Chaibakhsh Y, Rostami Lima S. General anesthesia-related neurotoxicity: Status of pediatric surgeries at an academic hospital in the north of Iran. J Compr Pediatr. 2019; 10(4):e92316. [DOI:10.5812/compreped.92316]
- Iddrisu M, Khan ZH. Anesthesia for cesarean delivery: General or regional anesthesia-a systematic review. Ain-Shams J Anesthesiol. 2021; 13:1. [DOI:10.1186/s42077-020-00121-7]
- Gisore E, Mung’ayi V, Sharif T. Incidence of post dural puncture headache following caesarean section under spinal anaesthesia at the Aga Khan University Hospital, Nairobi. East Afr Med J. 2010; 87(6):227-30. [DOI:10.4314/eamj.v87i6.63078] [PMID]
- Saberi A, Nemati S, Shakib RJ, Kazemnejad E, Maleki M. Association between allergic rhinitis and migraine. J Res Med Sci. 2012; 17(6):508 -12. [PMCID]
- Naderinabi B, Saberi A, Hashemi M, Haghighi M, Biazar G, Gharehdaghi FA, et al. Acupuncture and botulinum toxin A injection in the treatment of chronic migraine: A randomized controlled study. Caspian J Intern Med. 2017; 8(3):196-204. [DOI:10.22088/cjim.8.3.196] [PMID] [PMCID]
- Khraise WN, Allouh MZ, El-Radaideh KM, Said RS, Al-Rusan AM. Assessment of risk factors for postdural puncture headache in women undergoing cesarean delivery in Jordan: A retrospective analytical study. Local Reg Anesth. 2017; 10:9-13. [DOI:10.2147/LRA.S129811] [PMID] [PMCID]
- Rasooli S, Moslemi F, Baybordi A. Post-dural puncture headache in the obstetric patient: Needle size, number of dural puncture and timing of ambulation. Int J Women’s Health Reprod Sci. 2015; 3(3):163-7. [DOI:10.15296/ijwhr.2015.34]
- Gaiser R. Postdural puncture headache. Curr Opin Anaesthesiol. 2006; 19(3):249-53. [DOI:10.1097/01.aco.0000192809.71408.ba] [PMID]
- Mashak B, Hashemnejad M, Kabir K, Refaei M, Saeieh S, Torkashvand S, et al. The effect of ginger on preventing post-spinal puncture headache in patients undergoing cesarean section. Int J Women’s Health Reprod. 2019; 7(2):204-10. [DOI:10.15296/ijwhr.2019.34]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013; 33(9):629-808. [DOI:10.1177/0333102413485658] [PMID]
- Uluer M, Sargin M, Akin F, Uluer E, Sahin O. A randomized study to evaluate post-dural puncture headache after cesarean section: Comparison with median and paramedian approaches. Niger J Clin Pract. 2019; 22(11):1564-9. [DOI:10.4103/njcp.njcp_100_19] [PMID]
- Perez ER, Sanchez MG, Rocuts A, Jimenez E. Use of a triple prophylactic strategy to prevent post-dural puncture headache: An observational study. Cureus. 2020; 12(2):e7052. [DOI:10.7759/cureus.7052]
- Mondal P, Chakraborty I, Acharjee A. Incidence of post dural puncture headache in caesarean section: A comparison of 25 G quincke and 25 G whitacre needle. Paripex Indian J. 2020; 8(12):73-6. https://www.worldwidejournals.com/paripex/recent_issues_pdf
- Hashemi M, Akhlagh SH, Shadegan SH, Taheri M, Farbood A, Dadkhah P, et al. The impact of increased body mass index on the incidence and severity of post-spinal headache after cesarian section. J Res Med Dent Sci. 2019; 7(2):1-5. https://www.jrmds.in/articles/the-impact-of-increased-body-mass-index-on-the-incidence-and-severity-of-postspinal-headache-after-cesarean-section-18173.html
- Mosaffa F, Karimi K, Madadi F, Khoshnevis SH, Besheli LD, Eajazi A. Post-dural puncture headache: A comparison between median and paramedian approaches in orthopedic patients. Anesth Pain Med. 2011; 1(2):66-9. [DOI:10.5812/kowsar.22287523.2159] [PMID] [PMCID]
- Babaei K, Khaleghipoor M, Saadati SM, Ghodsi A, Sadeghi N, Nikoo N. The effect of fluid therapy before spinal anesthesia on prevention of headache after cesarean section: A clinical trial. Cureus. 2020; 12(11):e11772. [DOI:10.7759/cureus.11772] [PMID] [PMCID]
- Fenta E, Kibret S, Hunie M, Teshome D. Dexamethasone and post-dural puncture headache in women who underwent cesarean delivery under spinal anesthesia: A systemic review and meta-analysis of randomized controlled trials. Ann Med Surg (Lond). 2021; 62:104-13. [DOI:10.1016/j.amsu.2021.01.024] [PMID] [PMCID]
- Dehghanpisheh L, Bayani S, Azemati S, Rakhshan M. The effect of intravenous administration of ondansetron compared to aminophylline on incidence and severity of Post-Dural Puncture Headache (PDPH) in cesarean section surgeries. Biomed Res. 2019; 30(6):1-6. https://www.biomedres.info/biomedical-research-.pdf
- Nazemi S, Hamzei A, Pasban NS, Moslem A, Ghafarzadeh NB. [Incidence of headache after spinal anesthesia in cesarean and its related factors (Persian)]. Ofogh-E-Danesh. 2013; 19(2):112-5. https://www.sid.ir/en/journal/ViewPaper.aspx?id=336796
- Achary AR, Puthenveettil N, Rajan S, Kumar L. A comparison of time to achieve T5 blockade in lateral versus sitting position during elective cesarean section under spinal anesthesia: A randomized control trial. J Obstet Anaesth Crit Care. 2020; 10(1):21-5. [DOI:10.4103/joacc.JOACC_47_19]
- Tan ED, Günaydın B. Comparison of maternal and neonatal effects of combined spinal epidural anaesthesia in either the sitting or lateral position during elective cesarean section. Turk J Anaesthesiol Reanim. 2014; 42(1):23-32. [DOI:10.5152/TJAR.2013.55] [PMID] [PMCID]
- Masoudifar M, Aghadavoudi O, Adib S. Effect of venous dexamethasone, oral caffeine and acetaminophen on relative frequency and intensity of postdural puncture headache after spinal anesthesia. Adv Biomed Res. 2016; 5:66. [DOI:10.4103/2277-9175.180635] [PMID] [PMCID]
- Davoudi M, Tarbiat M, Ebadian MR, Hajian P. Effect of position during spinal anesthesia on postdural puncture headache after cesarean section: A prospective, single-blind randomized clinical trial. Anesth Pain Med. 2016; 6(4):e35486. [DOI:10.5812/aapm.35486] [PMID] [PMCID]
- Ali HM, Mohamed MY, Ahmed YM. Postdural puncture headache after spinal anesthesia in cesarean section: Experience in six months in 2736 patients in Kasr El aini teaching hospital-Cairo University. Egypt J Anaesth. 2014; 30(4):383-6. [DOI:10.1016/j.egja.2014.06.001]
- Seeberger MD, Kaufmann M, Staender S, Schneider M, Scheidegger D. Repeated dural punctures increase the incidence of postdural puncture headache. Anesth Analg. 1996; 82(2):302-5. [DOI:10.1097/00000539-199602000-00015] [PMID]
- Tarekegn F, Eshetie S, Aregawi A, Moges K. Assessment of the prevalence and associated risk factors of post dural puncture headache PDPH after cesarean section delivery under spinal anesthesia. J Anesth Crit Care Open Access. 2017; 8(6):00330. [DOI:10.15406/jaccoa.2017.08.00330]
- Siddiqui AS, Salim B, Hashemy N, Siddiqui SZ. Post-dural puncture headache after spinal anaesthesia for caesarean section. J Surg Pak. 2015; 20(1):10-4. http://old.jsp.org.pk/Issues/JSP%2020%20(1)%20Jan-%20Mar%202015%20PDF/Ali%20Sarfaraz%20Siddiqui%20OA.pdf
- Firdous T, Siddiqui MA, Siddiqui SM. Frequency of post dural puncture headache in patients undergoing elective cesarean section under spinal anesthesia with median versus paramedian approach. Anaesth Pain Intensive Care. 2019; 20(2):165-70. https://mail.apicareonline.com/index.php/APIC/article/view/253
- Chekol B, Yetneberk T, Teshome D. Prevalence and associated factors of post dural puncture headache among parturients who underwent cesarean section with spinal anesthesia: A systemic review and meta-analysis, 2021. Ann Med Surg (Lond). 2021; 66:102456. [DOI:10.1016/j.amsu.2021.102456] [PMID] [PMCID]
- Wandner LD, Scipio CD, Hirsh AT, Torres CA, Robinson ME. The perception of pain in others: How gender, race, and age influence pain expectations. J Pain. 2012; 13(3):220-7. [DOI:10.1016/j.jpain.2011.10.014] [PMID] [PMCID]
- Zhou Y, Han S. Neural dynamics of pain expression processing: Alpha-band synchronization to same-race pain but desynchronization to other-race pain. Neuroimage. 2021; 224:117400. [DOI:10.1016/j.neuroimage.2020.117400] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2021/06/10 | Accepted: 2021/08/13 | Published: 2021/10/1
Received: 2021/06/10 | Accepted: 2021/08/13 | Published: 2021/10/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



