Fri, Apr 26, 2024
Volume 7, Issue 4 (Autumn 2021)
Caspian J Neurol Sci 2021, 7(4): 193-201 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hajizadeh N, Farjah G H, Karimipour M, Pourheidar B. Protective Effect of Catechin Hydrate on Spinal Cord Ischemia-reperfusion Injury in Rats. Caspian J Neurol Sci 2021; 7 (4) :193-201
URL: http://cjns.gums.ac.ir/article-1-465-en.html
URL: http://cjns.gums.ac.ir/article-1-465-en.html
1- Department of Anatomy, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran.
2- Department of Anatomy, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran. , farjah.gh@umsu.ac.ir; hfarjah@hotmail.com
2- Department of Anatomy, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran. , farjah.gh@umsu.ac.ir; hfarjah@hotmail.com
Full-Text [PDF 1758 kb]
(475 Downloads)
| Abstract (HTML) (1295 Views)
Full-Text: (474 Views)
Introduction
The aortic artery must be clamped during some surgical procedures for reasons, such as an aneurysm and abdominal injuries [1]. The clamp of the abdominal aortic artery causes many undesirable pathologic changes in different parts of the body [1]. Two physiological problems of Ischemia-Reperfusion (I-R) will occur during clamping (Ischemia) and after the resumption of blood circulation (reperfusion) [2]. I-R injury can lead to certain clinical expressions, such as brain and digestive system disorders, infection, heart problems, and dysfunction of various organs of the body [3]. In neural cells, inflammation, oxidative stress, and cell apoptosis are the common outcomes of the I-R injury [4]. To minimize neurological complications (paraparesis or paraplegia), numerous efforts have been made. In this context, the use of materials with antioxidant and anti-inflammatory properties, such as saffron [5], and spinach [6] has been considered.
Tea is one of the most popular drinks in the world. It is rich in an important polyphenol called Catechin, which represents nearly 30%-42% of the weight dry of green tea [7]. This substance, also found in many other foods, has antioxidant and anti-inflammatory properties to treat numerous diseases [8]. The protective effect of catechin against I-R injuries has been studied in various organs, such as the kidneys [9] and the heart [10]. Moreover, catechin inhibits the activation of microglia in neurological disorders [11].
The Catechin Hydrate (CH) is a major type of catechin [12], with strong antioxidant and anti-inflammatory properties that scavenges free radicals [13]. It is the major phenolic compound of green tea (65% of dry weight) [8].
There is no investigation on the neuroprotective action of CH on I-R spinal cord damage. Therefore, we decided to study the effect of catechin on spinal cord I-R injury in male rats.
Materials and Methods
Experimental designs
Thirty-five male albino Sprague-Dawley rats (250 - 300 g) were chosen for this study. The animals were housed in a quiet room under a 12-h light/dark cycle and at room temperature of 21-23ºC. They had free access to standard laboratory food and tap water. The rats were randomized into 5 groups: intact (no injections or surgery), sham (the abdominal aorta was exposed), I-R (following a daily gavage of 1 mg/kg of 0.5% dimethyl sulfoxide (DMSO) for 21 days, the abdominal aorta was subjected to I-R), low-dose CH (following a daily gavage of 10 mg/kg of CH for 21 days, the abdominal aorta was subjected to I-R), high-dose CH (following a daily gavage of 20 mg/kg of CH for 21 days, the abdominal aorta was subjected to I-R) [14]. CH was dissolved in 0.5% DMSO to be administrated through gavage. Before the surgery, all animals were evaluated for neurological function. CH powder (CAS Number: 225937-10-0) was purchased from Sigma Aldrich.
Surgical procedure
Ketamine and xylazine (at the doses of 90 and 10 mg/kg, respectively; IP) were injected for pre-operation anesthesia, followed by heparin (400 IU/kg). In sterile conditions, the skin and abdomen wall muscles were opened from the median line. For the sham surgery group, the surgery was completed after observing the abdominal aorta. For other surgical groups, the abdominal aorta was clamped in two zones with the aid of arterial micro-clips (ischemia for 60 min): under the left renal artery and above of the abdominal aortic bifurcation. Meanwhile, the pulse of the femoral artery could not be sensed through repeated touch, which signified that blood flow stopped at the lower extremities. During surgery, the animal’s body heat was remained constant within a normal range (37.5°C±0.5°C) with a lamp overhead. After an ischemic period, perfusion was started by removing the micro-clips [15]. Finally, the abdominal muscles and skin were sutured. In the gavage groups, catechin (10 and 20 mg/kg) and 0.5% DMSO (1 mg/kg) were administered orally for 21 days prior to the surgery. During the study, the animals were kept under standard conditions (12/12 h light /dark cycle and easy access to water and food).
Neurologic evaluation
For the present study, the Motor Deficit Index (MDI) was used. This index is calculated by the sum of scores of ambulation and placing/stepping. The number 0 was attributed to completely normal rats and the highest score was 6, which belonged to the rats with the most serious neurological defects. The rats with a score less than 3 were considered non-paraplegic and those with a score 3 or greater were known as paraplegia [16]. Rats were assessed in two stages: before the surgery and 72 h after I-R spinal cord.
Biochemical measurements
After determining MDI, the rats were anesthetized with a dose of 100 mg/kg of ketamine, and blood samples were taken directly through the left ventricle. Samples were immediately placed on ice and centrifuged (1500 g; 4°C; 20 min) to extract the plasma. Then, the samples were moved to the refrigerator (-80°C). Biochemical measurements were performed to assess the level of Malondialdehyde (MDA), catalase, Total Antioxidant Capacity (TAC), and Superoxide Dismutase (SOD) in plasma using colorimetric assay kits (Elabscience, Wuhan, China). The hydroxylamine method was used for the calculation of the SOD levels. Hydroxylamine was oxidized by the superoxide anion production, changed to nitrite and purplish red, and measured at a wavelength of 550 nm. Fluorescence recovery after photobleaching (FRAP method) was used to calculate the TAC and measured at a wavelength of 520 nm. The plasma level of CAT was measured at a wavelength of 405 nm after the generation of a yellowish complex. The MDA and thiobarbituric acid reagent (TBA) yielded the pink plasma and were measured at a wavelength of 532 nm [17].
Histological study
Initially, the rats were perfused via the heart (10% formalin), and then the fourth lumbar segment was removed from the spinal cord, and placed in fixation solution for 2 days. From tissue samples, cross-sections were prepared with a thickness of 5 µm and stained with Hematoxylin and Eosin (H-E). Normal motor neurons (prominent nucleoli, loose chromatin, and Nissl substance) were counted in the anterior horns of the spinal cord. Microvacuolation expansion in the anterior and lateral funiculi was considered a white matter injury. The scoring based on the microvacuolation formation was as follows: free (score 0), 1%-10% (score 1), 10%-50% (score 2), and more than about 50 % (score 3) [2].
Statistical analysis
The data collected were presented as Mean±SEM. Statistical analysis was performed by SPSS v. 16 software. Tukey’s post hoc test and ANOVA were used for comparisons. Kruskal-Wallis test was used to analyze MDI and white matter damage among the experimental groups and the Mann-Whitney U test was used to determine significant differences between the two groups. In comparisons, where a P-value was smaller than 0.05, a significant difference was considered.
Results
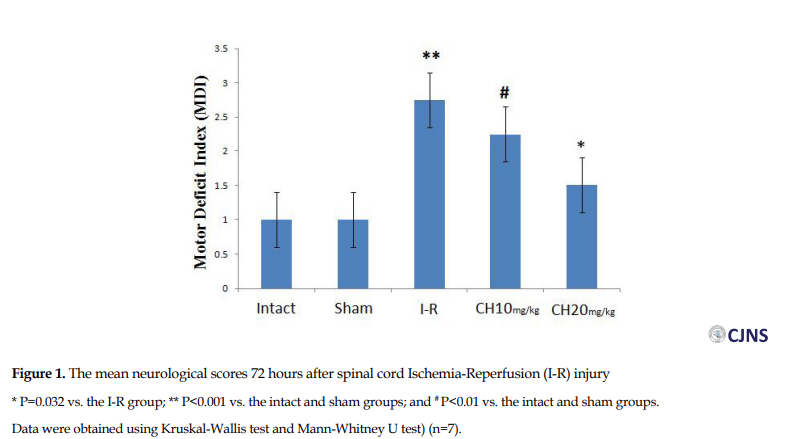
Seventy-two hours following spinal cord I-R, the average MDI score was significantly lower in the CH (20 mg/kg) group (1.5±0.5) compared to the I-R group (2.75±0.4) (P=0.032). The MDI scores in the intact and sham surgery groups were significantly lower than in the I-R group (P<0.001). In spite of the decrease in MDI score in the CH (10 mg/kg) group compared to the I-R group, the difference was not significant (Figure 1).
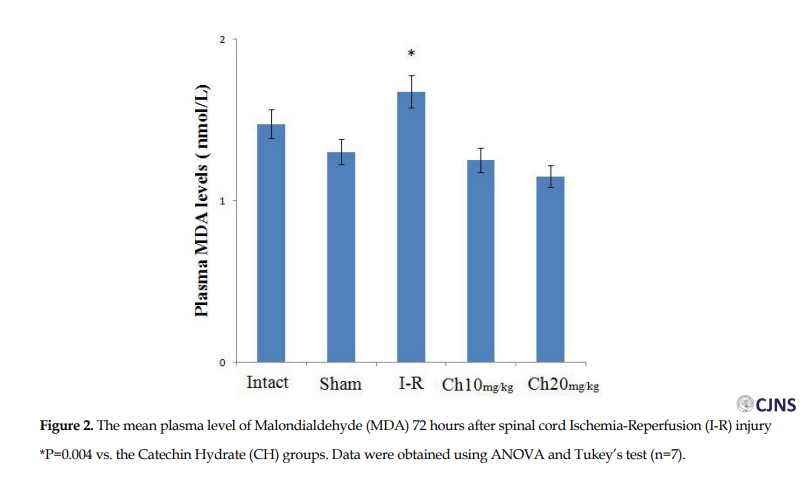
The plasma levels of MDA in the CH (20 mg/kg) (1.15±0.025) and CH (10 mg/kg) (1.25±0.064 nmol/L) groups were significantly less than the I-R (1.675±0.05 nmol/L) group (P=0.004). Additionally, there was a significant difference between the CH groups (Figure 2).
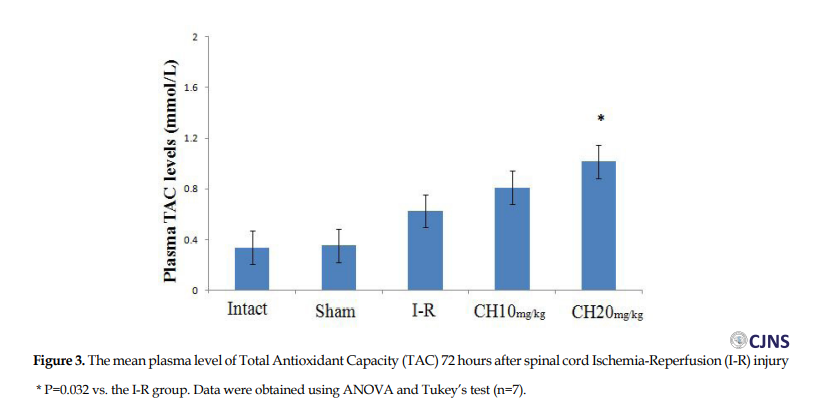
Plasma level of TAC in the CH (20 mg/kg) group (1.017±0.08 mmol/L) was significantly greater than the I-R (0.627±0.12 mmol/L) group (P=0.032). However, there was no significant difference between the CH (10 mg/kg) and the I-R groups (Figure 3).
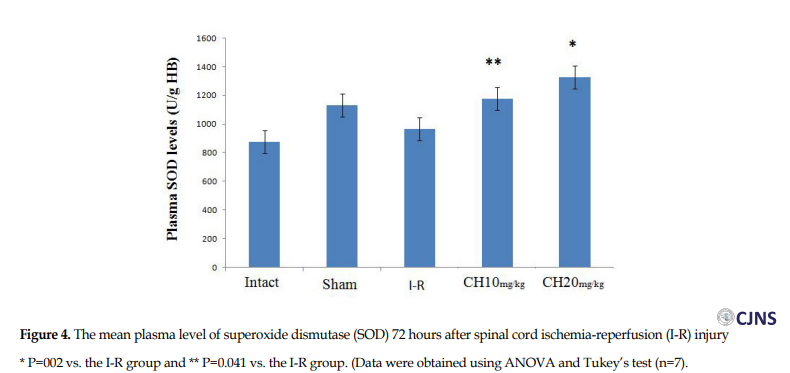
The plasma level of SOD in the CH (20 mg/kg) (1327±83 U/g HB) group was significantly greater than in the I-R group (965±3 U/g HB) (P=0.002). Also, there was a significant difference between the CH (10 mg/kg) group (1175±52 U/ mg HB) and the I-R group (P=0.041) (Figure 4).
.PNG)
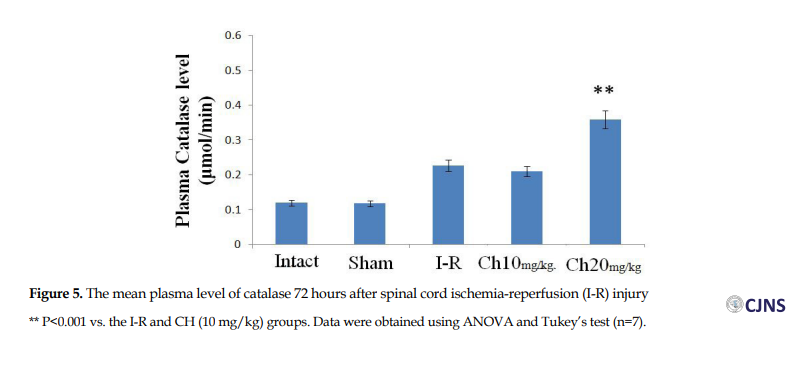
The plasma level of CAT was significantly greater in the CH (20 mg/kg) group (0.359±0.005 µmol/min) than in the I-R group (0.227±0.019 µmol/min) (P<0.001). There was also a significant difference between the CH (20 mg/kg) and CH (10 mg/kg) (0.21±0.033 µmol/min) groups (P<0.001) (Figure 5).
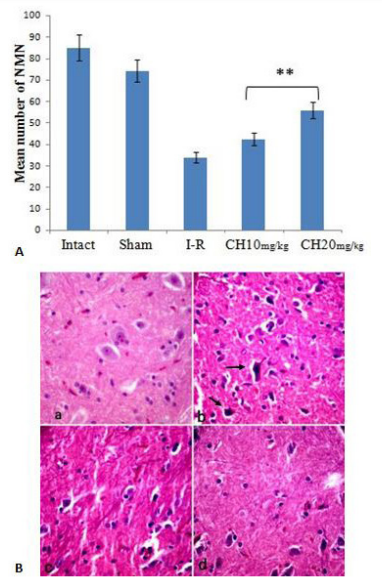
The mean number of normal motor neurons was significantly different between the CH (20 mg/kg) (56±0.7) group than the CH (10 mg/kg) (42.5±1.25) and I-R (34±1.08) groups (P<0.001). This means the loss of 7%, 29%, and 44% in the number of normal motor neurons in these groups, respectively (Figure 6A, B).
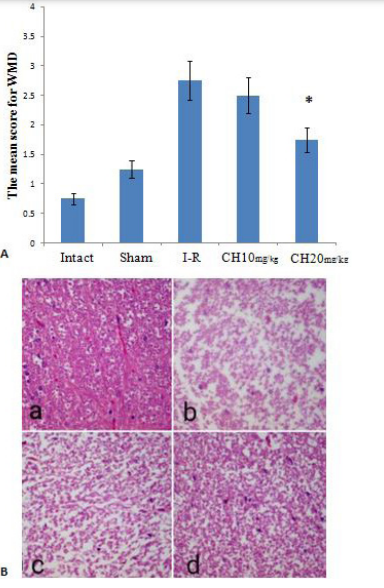
The average white matter damage scores in the CH (20 mg/kg) group (1.75±0.5) were significantly lower than in the I-R group (2.75±0.5) (P=0.015). However, there was no significant difference between the I-R and the CH (10 mg/kg) (2.5±0.57) groups (P>0.05) (Figure 7A and B).
Discussion
The finding of this study indicated that in the CH groups hind limb function improved 72h after the I-R spinal cord injury so that the number of damaged neurons and the amount of white matter damage were smaller. This is the first study to examine the protective effects of CH on I-R spinal cord injury.
In this study, the positive effects of CH against I-R damage to the rat’s spinal cord were confirmed by biochemical, histological, and neurological examinations. Following an I-R spinal cord injury, oxidative agents increase the permeability of the blood-brain barrier (BBB) [18], leading to an increase in the permeability of catechins [19]. One of the benefits of using CH is its antioxidant properties, which can be explained by the following mechanism: because the catechins are known as the sweepers of the Reactive Oxygen Species (ROS), they play an important role in the prevention of cellular oxidative stress. ROS has a negative effect on neuronal function by inducing oxidative stress [20]. In this article, the improvement of histological conditions of cells in the CH groups (especially in the CH (20 mg/kg) group) was observed by increasing the levels of SOD, TAC, and CAT. SOD and CAT destroy superoxide radicals, break down the chain of oxidizing agents, and transform them into harmless molecules [21]. The present study showed that the mean number of normal motor neurons in the CH 20 (mg/kg) group was higher than in the I-R group, and this may be due to the antioxidant properties of the CH (especially, SOD and CAT). A previous study found that increasing the amount of SOD and CAT increased the resistance of astrocytes to I-R damage and as a result, damage to the white matter and of vacuole formation reduces [22]. In addition, increasing the TAC levels emphasize an enhancement in cellular health and a reduction in free radical production [23].
Besides their antioxidant properties, catechins have attracted our attention due to their anti-inflammatory properties [24]. Fan et al. (2017) reported that catechins help to improve inflammatory bowel disease, by increasing the production of factors affecting the immune system (such as macrophages, T lymphocytes, and neutrophils) [8].
In our study, a reduction in MDA levels in the CH groups was effective in reducing I-R spinal cord injury. MDA is the final result of lipid peroxidation caused by oxidative stress in the cell. It plays a major role in cell infection and causes a number of clinical abnormalities in the body [25]. One of the interesting results of the present study was a decrease in the MDA levels in the CH (20 mg/kg) group compared to the intact group. Catechin appears to effectively reduce the plasma level of MDA. Another finding of this study was the dose-dependent effect of CH. In a similar study, the effect of CH on traumatic brain injury at a daily dose of 20 mg/kg was greater than other doses [14].
In this study, there may be challenges to the effectiveness of CH in reducing I-R spinal cord injury. First, it is possible to make numerous changes to the given dose. For example, different doses may be prescribed in different periods. Secondly, the rats lived only three days after ischemia. Therefore, it is not known if the results will be acceptable for a longer period. Thirdly, the duration of ischemia was 60 minutes and there is no information on the effects of CH by increasing the ischemia time.
In a similar study, CH was administrated to rats for 21 days, and then, the middle cerebral artery was blocked for 2 hours. They concluded that CH increased the activity of antioxidant enzymes and decreased cytokines levels, which in turn has a positive effect on nerve function by reducing the volume of the damaged area of the brain. These effects were significant compared to the group that did not receive CH [26].
In this study, plasma levels of SOD, TAC, and CAT increased, especially in the high-dose catechin group. Previous studies have emphasized the antioxidant and anti-inflammatory properties of catechins in reducing I-R side effects on the liver [27], brain [28], kidney [15], and stomach [29]. In addition, catechins have neuroprotective effects in different models of neural injury [30]. Therefore, the death of nerve cells in the catechin group was delayed due to its antioxidant properties [20].
Conclusion
Our research indicated that CH can protect neurons from I-R injury. However, the molecular mechanisms of the neuroprotective effect of CH are still unclear and further studies are needed.
Ethical Considerations
Compliance with ethical guidelines
This research was approved by the Ethical Board of Urmia University of Medical Sciences (Approval No.: IR. UMSU. REC. 1398.211). All study procedures were done in compliance with the ethical guidelines of the Declaration of Helsinki, 2013.
Funding
This study was a part of the MSc. thesis of first author at the Department of Anatomy, School of Medicine, Urmia University of Medical Sciences, Urmia (Registered thesis number: 1398.212). It was supported by the Urmia University of Medical Sciences, Urmia, Iran.
Authors' contributions
Drafting: Gholam Hossein Farjah, Naser Hajizadeh; Methodology: Gholam Hossein Farjah, Mojtaba Karimioiur; Writing and editing: Naser Hajizadeh, Gholam Hossein Farjah, Bagher Pourheidar; Data collection: Naser Hajizadeh; Data Analysis: All authors.
Conflict of interest
The authors declare no conflicts of interests.
Refrences:
The aortic artery must be clamped during some surgical procedures for reasons, such as an aneurysm and abdominal injuries [1]. The clamp of the abdominal aortic artery causes many undesirable pathologic changes in different parts of the body [1]. Two physiological problems of Ischemia-Reperfusion (I-R) will occur during clamping (Ischemia) and after the resumption of blood circulation (reperfusion) [2]. I-R injury can lead to certain clinical expressions, such as brain and digestive system disorders, infection, heart problems, and dysfunction of various organs of the body [3]. In neural cells, inflammation, oxidative stress, and cell apoptosis are the common outcomes of the I-R injury [4]. To minimize neurological complications (paraparesis or paraplegia), numerous efforts have been made. In this context, the use of materials with antioxidant and anti-inflammatory properties, such as saffron [5], and spinach [6] has been considered.
Tea is one of the most popular drinks in the world. It is rich in an important polyphenol called Catechin, which represents nearly 30%-42% of the weight dry of green tea [7]. This substance, also found in many other foods, has antioxidant and anti-inflammatory properties to treat numerous diseases [8]. The protective effect of catechin against I-R injuries has been studied in various organs, such as the kidneys [9] and the heart [10]. Moreover, catechin inhibits the activation of microglia in neurological disorders [11].
The Catechin Hydrate (CH) is a major type of catechin [12], with strong antioxidant and anti-inflammatory properties that scavenges free radicals [13]. It is the major phenolic compound of green tea (65% of dry weight) [8].
There is no investigation on the neuroprotective action of CH on I-R spinal cord damage. Therefore, we decided to study the effect of catechin on spinal cord I-R injury in male rats.
Materials and Methods
Experimental designs
Thirty-five male albino Sprague-Dawley rats (250 - 300 g) were chosen for this study. The animals were housed in a quiet room under a 12-h light/dark cycle and at room temperature of 21-23ºC. They had free access to standard laboratory food and tap water. The rats were randomized into 5 groups: intact (no injections or surgery), sham (the abdominal aorta was exposed), I-R (following a daily gavage of 1 mg/kg of 0.5% dimethyl sulfoxide (DMSO) for 21 days, the abdominal aorta was subjected to I-R), low-dose CH (following a daily gavage of 10 mg/kg of CH for 21 days, the abdominal aorta was subjected to I-R), high-dose CH (following a daily gavage of 20 mg/kg of CH for 21 days, the abdominal aorta was subjected to I-R) [14]. CH was dissolved in 0.5% DMSO to be administrated through gavage. Before the surgery, all animals were evaluated for neurological function. CH powder (CAS Number: 225937-10-0) was purchased from Sigma Aldrich.
Surgical procedure
Ketamine and xylazine (at the doses of 90 and 10 mg/kg, respectively; IP) were injected for pre-operation anesthesia, followed by heparin (400 IU/kg). In sterile conditions, the skin and abdomen wall muscles were opened from the median line. For the sham surgery group, the surgery was completed after observing the abdominal aorta. For other surgical groups, the abdominal aorta was clamped in two zones with the aid of arterial micro-clips (ischemia for 60 min): under the left renal artery and above of the abdominal aortic bifurcation. Meanwhile, the pulse of the femoral artery could not be sensed through repeated touch, which signified that blood flow stopped at the lower extremities. During surgery, the animal’s body heat was remained constant within a normal range (37.5°C±0.5°C) with a lamp overhead. After an ischemic period, perfusion was started by removing the micro-clips [15]. Finally, the abdominal muscles and skin were sutured. In the gavage groups, catechin (10 and 20 mg/kg) and 0.5% DMSO (1 mg/kg) were administered orally for 21 days prior to the surgery. During the study, the animals were kept under standard conditions (12/12 h light /dark cycle and easy access to water and food).
Neurologic evaluation
For the present study, the Motor Deficit Index (MDI) was used. This index is calculated by the sum of scores of ambulation and placing/stepping. The number 0 was attributed to completely normal rats and the highest score was 6, which belonged to the rats with the most serious neurological defects. The rats with a score less than 3 were considered non-paraplegic and those with a score 3 or greater were known as paraplegia [16]. Rats were assessed in two stages: before the surgery and 72 h after I-R spinal cord.
Biochemical measurements
After determining MDI, the rats were anesthetized with a dose of 100 mg/kg of ketamine, and blood samples were taken directly through the left ventricle. Samples were immediately placed on ice and centrifuged (1500 g; 4°C; 20 min) to extract the plasma. Then, the samples were moved to the refrigerator (-80°C). Biochemical measurements were performed to assess the level of Malondialdehyde (MDA), catalase, Total Antioxidant Capacity (TAC), and Superoxide Dismutase (SOD) in plasma using colorimetric assay kits (Elabscience, Wuhan, China). The hydroxylamine method was used for the calculation of the SOD levels. Hydroxylamine was oxidized by the superoxide anion production, changed to nitrite and purplish red, and measured at a wavelength of 550 nm. Fluorescence recovery after photobleaching (FRAP method) was used to calculate the TAC and measured at a wavelength of 520 nm. The plasma level of CAT was measured at a wavelength of 405 nm after the generation of a yellowish complex. The MDA and thiobarbituric acid reagent (TBA) yielded the pink plasma and were measured at a wavelength of 532 nm [17].
Histological study
Initially, the rats were perfused via the heart (10% formalin), and then the fourth lumbar segment was removed from the spinal cord, and placed in fixation solution for 2 days. From tissue samples, cross-sections were prepared with a thickness of 5 µm and stained with Hematoxylin and Eosin (H-E). Normal motor neurons (prominent nucleoli, loose chromatin, and Nissl substance) were counted in the anterior horns of the spinal cord. Microvacuolation expansion in the anterior and lateral funiculi was considered a white matter injury. The scoring based on the microvacuolation formation was as follows: free (score 0), 1%-10% (score 1), 10%-50% (score 2), and more than about 50 % (score 3) [2].
Statistical analysis
The data collected were presented as Mean±SEM. Statistical analysis was performed by SPSS v. 16 software. Tukey’s post hoc test and ANOVA were used for comparisons. Kruskal-Wallis test was used to analyze MDI and white matter damage among the experimental groups and the Mann-Whitney U test was used to determine significant differences between the two groups. In comparisons, where a P-value was smaller than 0.05, a significant difference was considered.
Results
Seventy-two hours following spinal cord I-R, the average MDI score was significantly lower in the CH (20 mg/kg) group (1.5±0.5) compared to the I-R group (2.75±0.4) (P=0.032). The MDI scores in the intact and sham surgery groups were significantly lower than in the I-R group (P<0.001). In spite of the decrease in MDI score in the CH (10 mg/kg) group compared to the I-R group, the difference was not significant (Figure 1).

The plasma levels of MDA in the CH (20 mg/kg) (1.15±0.025) and CH (10 mg/kg) (1.25±0.064 nmol/L) groups were significantly less than the I-R (1.675±0.05 nmol/L) group (P=0.004). Additionally, there was a significant difference between the CH groups (Figure 2).

Plasma level of TAC in the CH (20 mg/kg) group (1.017±0.08 mmol/L) was significantly greater than the I-R (0.627±0.12 mmol/L) group (P=0.032). However, there was no significant difference between the CH (10 mg/kg) and the I-R groups (Figure 3).

The plasma level of SOD in the CH (20 mg/kg) (1327±83 U/g HB) group was significantly greater than in the I-R group (965±3 U/g HB) (P=0.002). Also, there was a significant difference between the CH (10 mg/kg) group (1175±52 U/ mg HB) and the I-R group (P=0.041) (Figure 4).



.PNG)


The plasma level of CAT was significantly greater in the CH (20 mg/kg) group (0.359±0.005 µmol/min) than in the I-R group (0.227±0.019 µmol/min) (P<0.001). There was also a significant difference between the CH (20 mg/kg) and CH (10 mg/kg) (0.21±0.033 µmol/min) groups (P<0.001) (Figure 5).
The mean number of normal motor neurons was significantly different between the CH (20 mg/kg) (56±0.7) group than the CH (10 mg/kg) (42.5±1.25) and I-R (34±1.08) groups (P<0.001). This means the loss of 7%, 29%, and 44% in the number of normal motor neurons in these groups, respectively (Figure 6A, B).
The average white matter damage scores in the CH (20 mg/kg) group (1.75±0.5) were significantly lower than in the I-R group (2.75±0.5) (P=0.015). However, there was no significant difference between the I-R and the CH (10 mg/kg) (2.5±0.57) groups (P>0.05) (Figure 7A and B).
Discussion
The finding of this study indicated that in the CH groups hind limb function improved 72h after the I-R spinal cord injury so that the number of damaged neurons and the amount of white matter damage were smaller. This is the first study to examine the protective effects of CH on I-R spinal cord injury.
In this study, the positive effects of CH against I-R damage to the rat’s spinal cord were confirmed by biochemical, histological, and neurological examinations. Following an I-R spinal cord injury, oxidative agents increase the permeability of the blood-brain barrier (BBB) [18], leading to an increase in the permeability of catechins [19]. One of the benefits of using CH is its antioxidant properties, which can be explained by the following mechanism: because the catechins are known as the sweepers of the Reactive Oxygen Species (ROS), they play an important role in the prevention of cellular oxidative stress. ROS has a negative effect on neuronal function by inducing oxidative stress [20]. In this article, the improvement of histological conditions of cells in the CH groups (especially in the CH (20 mg/kg) group) was observed by increasing the levels of SOD, TAC, and CAT. SOD and CAT destroy superoxide radicals, break down the chain of oxidizing agents, and transform them into harmless molecules [21]. The present study showed that the mean number of normal motor neurons in the CH 20 (mg/kg) group was higher than in the I-R group, and this may be due to the antioxidant properties of the CH (especially, SOD and CAT). A previous study found that increasing the amount of SOD and CAT increased the resistance of astrocytes to I-R damage and as a result, damage to the white matter and of vacuole formation reduces [22]. In addition, increasing the TAC levels emphasize an enhancement in cellular health and a reduction in free radical production [23].
Besides their antioxidant properties, catechins have attracted our attention due to their anti-inflammatory properties [24]. Fan et al. (2017) reported that catechins help to improve inflammatory bowel disease, by increasing the production of factors affecting the immune system (such as macrophages, T lymphocytes, and neutrophils) [8].
In our study, a reduction in MDA levels in the CH groups was effective in reducing I-R spinal cord injury. MDA is the final result of lipid peroxidation caused by oxidative stress in the cell. It plays a major role in cell infection and causes a number of clinical abnormalities in the body [25]. One of the interesting results of the present study was a decrease in the MDA levels in the CH (20 mg/kg) group compared to the intact group. Catechin appears to effectively reduce the plasma level of MDA. Another finding of this study was the dose-dependent effect of CH. In a similar study, the effect of CH on traumatic brain injury at a daily dose of 20 mg/kg was greater than other doses [14].
In this study, there may be challenges to the effectiveness of CH in reducing I-R spinal cord injury. First, it is possible to make numerous changes to the given dose. For example, different doses may be prescribed in different periods. Secondly, the rats lived only three days after ischemia. Therefore, it is not known if the results will be acceptable for a longer period. Thirdly, the duration of ischemia was 60 minutes and there is no information on the effects of CH by increasing the ischemia time.
In a similar study, CH was administrated to rats for 21 days, and then, the middle cerebral artery was blocked for 2 hours. They concluded that CH increased the activity of antioxidant enzymes and decreased cytokines levels, which in turn has a positive effect on nerve function by reducing the volume of the damaged area of the brain. These effects were significant compared to the group that did not receive CH [26].
In this study, plasma levels of SOD, TAC, and CAT increased, especially in the high-dose catechin group. Previous studies have emphasized the antioxidant and anti-inflammatory properties of catechins in reducing I-R side effects on the liver [27], brain [28], kidney [15], and stomach [29]. In addition, catechins have neuroprotective effects in different models of neural injury [30]. Therefore, the death of nerve cells in the catechin group was delayed due to its antioxidant properties [20].
Conclusion
Our research indicated that CH can protect neurons from I-R injury. However, the molecular mechanisms of the neuroprotective effect of CH are still unclear and further studies are needed.
Ethical Considerations
Compliance with ethical guidelines
This research was approved by the Ethical Board of Urmia University of Medical Sciences (Approval No.: IR. UMSU. REC. 1398.211). All study procedures were done in compliance with the ethical guidelines of the Declaration of Helsinki, 2013.
Funding
This study was a part of the MSc. thesis of first author at the Department of Anatomy, School of Medicine, Urmia University of Medical Sciences, Urmia (Registered thesis number: 1398.212). It was supported by the Urmia University of Medical Sciences, Urmia, Iran.
Authors' contributions
Drafting: Gholam Hossein Farjah, Naser Hajizadeh; Methodology: Gholam Hossein Farjah, Mojtaba Karimioiur; Writing and editing: Naser Hajizadeh, Gholam Hossein Farjah, Bagher Pourheidar; Data collection: Naser Hajizadeh; Data Analysis: All authors.
Conflict of interest
The authors declare no conflicts of interests.
Refrences:
- Zammert M, Gelman S. The pathophysiology of aortic cross-clamping. Best Pract Res Clin Anaesthesiol. 2016; 30(3):257-69. [DOI:10.1016/j.bpa.2016.07.006] [PMID]
- Kanellopoulos GK, XU XM, Hsu CY, Lu X, Sundt TM, Kouchoukos NT. White matter injury in spinal cord ischemia: Protection by AMPA/Kainate glutamate receptor antagonism. Stroke. 2000; 31(8):1945-52. [DOI:10.1161/01.STR.31.8.1945] [PMID]
- Wu MY, Yiang GT, Liao WT, Tsai APY, Cheng YL, Cheng PW, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem. 2018; 46(4):1650-1667. [DOI:10.1159/000489241] [PMID]
- Gökce EC, Kahveci R, Gökce A, Cemil B, Aksoy N, Sargon MF, et al. Neuroprotective effects of thymoquinone against spinal cord ischemia-reperfusion injury by attenuation of inflammation, oxidative stress, and apoptosis. J Neurosurg Spine. 2016; 24(6):949-59. [DOI:10.3171/2015.10.SPINE15612] [PMID]
- Farjah GH, Salehi S, Ansari MH, Pourheidar B. Protective effect of Crocus Sativus L. (Saffron) extract on spinal cord ischemia reperfusion injury in rats. Iran J Basic Med Sci. 2017; 20(3):334-7. [DOI:10.22038/IJBMS.2017.8364] [PMID] [PMCID]
- Farjah GH, Mohammad Pour M, Khadem-Ansari MH, Karimpour M, Pourheidar B. Protective effect of aqueous spinach (Spinacia oleracea L.) extract on spinal cord ischemia-reperfusion injury in rats. Vet Res Forum. 2018; 9(2):187-91. [DOI:10.30466/VRF.2018.30825] [PMID] [PMCID]
- Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007; 81(7):519-33. [DOI:10.1016/j.lfs.2007.06.011] [PMID] [PMCID]
- Fan FY, Sang LX, Jiang M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules. 2017; 22(3):484. [DOI:10.3390/molecules22030484] [PMID] [PMCID]
- Ohkita M, Hayashi H, Ito K, Shigematsu N, Tanaka R, Tsutsui H, et al. Preventive effects of grape extract on ischemia/reperfusion-induced acute kidney injury in mice. Biol Pharm Bull. 2019; 42(11):1883-90. [DOI:10.1248/bpb.b19-00462] [PMID]
- Tu S, Xiao F, Min X, Chen H, Fan X, Cao K. Catechin attenuates coronary heart disease in a rat model by inhibiting inflammation. Cardiovasc Toxicol. 2018; 18(5):393-9. [DOI:10.1007/s12012-018-9449-z] [PMID]
- Farkhondeh T, Pourbagher-Shahri AM, Ashrafizadeh M, Folgado SL, Rajabpour-Sanati A, Khazdair MR, et al. Green tea catechins inhibit microglial activation which prevents the development of neurological disorders. Neural Regen Res. 2020; 15(10):1792-8. [DOI:10.4103/1673-5374.280300] [PMID] [PMCID]
- Umar KM, Abdulkarim S, Radu S, Abdul Hamid A, Saari N. Engineering the production of major catechins by Escherichia coli carrying metabolite genes of Camellia sinensis. Sci World J. 2012; 2012:529031. [DOI:10.1100/2012/529031] [PMID] [PMCID]
- Shahid A, Ali R, Ali N, Hasan SK, Bernwal P, Afzal SM, et al. Modulatory effects of catechin hydrate against genotoxicity, oxidative stress, inflammation and apoptosis induced by benzo (a) pyrene in mice. Food Chem Toxical. 2016; 92:64-74. [DOI:10.1016/j.fct.2016.03.021] [PMID]
- Jiang Z, Zhang J, Cai Y, Huang J, You L. Catechin attenuates traumatic brain injury‐induced blood-brain barrier damage and improves longer-term neurological outcomes in rats. Exp Physiol. 2017; 102(10):1269-77. [DOI:10.1113/EP086520] [PMID]
- Mohammadpour M, Farjah G, Karimipour M, Pourheidar B, Khadem Ansari M. Protective effect of lutein on spinal cord ischemia-reperfusion injury in rats. Iran J Basic Med Sci. 2019; 22(4):412-7. [DOI:10.22038/ijbms.2018.30039.7239] [PMID] [PMCID]
- Taira Y, Marsala M. Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke. 1996; 27(10):1850-8. [DOI:10.1161/01.STR.27.10.1850] [PMID]
- Karimipour M, Farjah GH, Molazadeh F, Ansari M, Pourheidar B. Protective effect of contralateral, ipsilateral, and bilateral remote ischemic preconditioning on spinal cord ischemia reperfusion injury in rats. Turk Neurosurg. 2019; 29(6):933-9. [DOI:10.5137/1019-5149.JTN.26237-19.3] [PMID]
- Zhai L, Liu M, Wang T, Zhang H, Li S, Guo Y. Picroside ll protects the blood-brain barrier by inhibiting the oxidative signaling pathway in cerebral ischemia-reperfusion injury. PloS One. 2017; 12(4):e0174414. [DOI:10.1371/journal.pone.0174414] [PMID] [PMCID]
- Unno K, Pervin M, Nakagawa A, Iguchi K, Hara A, Takagaki A, et al. Blood brain barrier permeability of green tea catechins metabolites and their neuritogenic activity in human neuroblastoma SH-SY5Y cells. Mol Nutr Food Res. 2017; 61(12):28891114. [DOI:10.1002/mnfr.201700294] [PMID]
- Bernatoniene J, Kopustinskiene DM. The role of catechins in cellular responses to oxidative stress. Molecules. 2018; 23(4):965. [DOI:10.3390/molecules23040965] [PMID] [PMCID]
- Sanchez-Carbente MR, Castro-Obregon S, Covarrubis L, Narvaez V. Motoneuronal death during spinal cord development is mediated by oxidative stress. Cell Death Differ. 2005; 12(3):279-91 [DOI:10.1038/sj.cdd.4401560] [PMID]
- Dankova M, Domarakova I, Fagova Z, Stebnicky M, Kunova A, Mechirova E. Bradykinin and noradrenaline preconditioning influences level of antioxidant enzymes SOD, CuZn-SOD, Mn-SOD and catalase in the white matter of spinal cord in rabbits after ischemia/reperfusion. Eur J Histochem. 2019; 63(4):3045. [DOI:10.4081/ejh.2019.3045] [PMID] [PMCID]
- Zińczuk J, Maciejczyk M, Zaręba K, Romaniuk W, Markowski A, Kędra B, et al. antioxidant barrier, redox status, and oxidative damage to biomolecules in patients with colorectal cancer. Can malondialdehyde and catalase be markers of colorectal cancer advancement? Biomolecules.2019; 9(10):637. [DOI:10.3390/biom9100637] [PMID] [PMCID]
- Nakano E, Kamei D, Murase R, Taki I, Karasawa K, Fukuhara K, et al. Anti-inflammatory effects of new catechin derivatives in a hapten-induced mouse contact dermatitis model. Eur J Pharmacol. 2019; 845:40-47. [DOI:10.1016/j.ejphar.2018.12.036] [PMID]
- Busch CJ, Binder CJ. Malondialdehyde epitopes as mediatores of sterile inflammation. Biochim Biophys Acta Mol Cell Biol Lipids. 2017; 1862(4):398-406. [DOI:10.1016/j.bbalip.2016.06.016] [PMID]
- Ashafaq M, Reza SS, Khan MM, Ahmad A, Javad H, Ahmad ME, et al. Catehin hydrate ameliorates redox imbalance and limits inflammatory response in focal cerebral ischemia. Neurochem Res. 2012; 37(8):1747-60. [DOI:10.1007/s11064-012-0786-1] [PMID]
- Tak E, Park G-C, Kim S-H, Jun DY, Lee J, Hwang S, et al. Epigallocatechin-3-gallate protects against hepatic ischaemia-reperfusion injury by reducing oxidative stress and apoptotic cell death. J Int Med Res. 2016; 44(6):1248-62. [DOI:10.1177/0300060516662735] [PMID] [PMCID]
- Lv J, Feng M, Zhang L, Wan X, Zeng YC, Liang PF, et al. Protective effect of epigallocatechin gallate, a major constituent of green tea, against renal ischemia-reperfusion injury in rats. Int Urol Nephrol. 2015; 47(8):1429-35. [DOI:10.1007/s11255-015-1030-0] [PMID] [PMCID]
- Rao CV, Vijayakumar M. Protective effect of (+) - catechin against gastric mucosal injury induced by ischaemia-eperfusion in rats. J Pharm Pharmacol. 2007; 59(8):1103-7. [DOI:10.1211/jpp.59.8.0007] [PMID]
- Khalatbary AR, Khademi E. The green tea polyphenolic catechin epigallocatechin gallate and neuroprotection. Nutr Neurosci. 2020; 23(4):281-94. [DOI:10.1080/1028415X.2018.1500124] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2021/06/11 | Accepted: 2021/08/29 | Published: 2021/10/1
Received: 2021/06/11 | Accepted: 2021/08/29 | Published: 2021/10/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






