BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://cjns.gums.ac.ir/article-1-34-en.html
2- Neurology Department, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran ; milad.javadpoor@yahoo.com
3- Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran
4- Biostatistics Department, Faculty of Nursery, Guilan University of Medical Sciences, Rasht, Iran
ABSTRACCT
Background: The role of estrogen and progesterone in stroke is suggested in previous studies, but yet is controversial.
Objectives: Comparing the plasma levels of estrogen and progesterone and their ratio in ischemic stroke patients and healthy population.
Materials and Methods: This cross-sectional comparative study was conducted from March to September of 2013. Sixty-six female patients older than 60 years with ischemic stroke who referred within the first 12 hour of their neurologic symptoms were included. A total of 48 women who were in the same age range comprised the control group. The serum levels of progesterone and 17-β-estradiol concentrations were measured. The data were analysed by Chi2 test, univariate and logistic regression models in SPSS software 19.
Results: There was no significant difference between case and control groups in serum levels of estrogen (28.66 ± 13.60 vs. 35.72 ± 28.36) and progesterone (1.07 ± 0.83 vs. 1.40 ± 1.87) and their ratio (p > 0.05). But in multivariate analysis, estrogen (p = 0.033), progesterone (p = 0.02) and the interaction between them (p = 0.013) remained as associated factors.
In addition, the higher ratio of estrogen to progesterone indicates the less ability of patients, which one unit increase in this ratio results in 0.072 reduction in NIHSS (National Institutes of Health Stroke Scale) score.
Conclusions: The level of estrogen and progesterone and interaction between them are associated with ischemic stroke. The ratio of estrogen to progesterone has reverse association with score of the ability of patient according to NIHSS.
Keywords: Estrogens; Progesterone; Stroke
Introduction
Worldwide, cerebral stroke is considered the most common neurologic disorder and cause of disability, and is the third common cause of death (1, 2). Almost 20 million individuals annually are reported to be involved by stroke, with a survival rate of nearly 75% (3). Nearly 30% of survived patients, need assistance in daily routine activities, 20% need assistance with mobility, and 16% need care in a facility (4).
Of all strokes, ischemic type of stroke is estimated to be 78% of all and leads to death within 30 days after occurrence in 8–12% of individuals, especially those between 45 and 65 years of age (4, 5).
In Iran, stroke epidemiologic data, its patterns and risk factors are brief and dispersed (6). The first occurrence of ischemic stroke was recounted in a survey in the north-eastern area of Iran to be 43 in 100,000 people in a year and after adjusting for age in another study in this area, it was proposed that stroke has a higher incidence rate than Europe (7). In addition, according to this report stroke occurs one decade sooner rather than there, was as nearly seventh decade of life (6, 8).
Notwithstanding stroke is more prevalent among men in the age range of 15–45 years, but in the total age spectrum, is more common among women (31–53%) (9). Menopausal women are proposed to have a higher incidence of ischemic stroke with worse prognosis rather than men (10, 11). There are some theories indicate that the difference in incidence and prognosis of stroke between men and women is due to hormonal diversity, especially the impact of estrogen. Although the protective role of estrogen against ischemic injury has been determined, endogen level of estradiol is shown to be a risk factor for stroke in menopausal women, and this relationship is stronger among dyslipidemic and insulin resistant patients (2, 12-17). The serum progesterone level and its proportion to estrogen level are reported to be considerably lower among ischemic stroke patients than controls (18).
In addition, the effect of exogenous estrogen and progesterone was assessed in individuals who use contraceptives and hormone replacement therapy (HRT). Oral contraceptives with high doses of estrogen were proved to be a risk factor of stroke, and its recent combinations with ethinyl estradiol in a dose of less than 50 μg, carry lower risk of stroke, particularly among non-smokers without arterial hypertension (4, 19). The conclusions from surveys concerning the effect of HRT on the occurrence of stroke are somewhat contradictory (20-23).
Still now there are debates regarding the impact of endogenous estrogen and progesterone or their interaction, on the pathogenesis of ischemic stroke as well as serum level differences among stroke patients and non-stroke subjects. This subject was investigated throughout present study.
Materials and Methods
This comparative cross-sectional study, which was approved by the ethics committee of vice-chancellorship of Guilan University of Medical Sciences, was conducted through six months of 2013 in an educational hospital in Iran.
Patients:
During this ascertained research period, all female patients over the age of 60 years, who were reffered within the first 12 hours, and diagnosed by a neurologist as having had an acute ischemic stroke participated in this study. Either the patients or their legal responsibles signed the informed consent. Patients who reported primary or traumatic intracranial haemorrhage, previous stroke or other neurologic disorders were excluded.
The same number of females without stroke were adjusted for age and stroke risk factors then included in the control group. The exclusion criteria for this group were defined as present or previous neurologic disorders and hepatic or renal dysfunction.
Data gathering:
All participants were examined and the stroke severity of patients was determined according to NIHSS. Brain CT scan and/or MRI routinely performed for all patients in order to confirm the diagnosis. All data including demographics, past medical history concerning diabetes mellitus, arterial hypertension, hyperlipidemia, and Body Mass Index (BMI) as well as the reports of biochemistry, neuroimaging and NIHSS were recorded in a previously designed checklist. Blood samples of 5cc were taken from controls and all identified patients acquiring medical care within the first 12 hours of attack. Serum levels of progesterone and 17β-estradiol (estrogen) were measured by comparative quantitative immunoassay analysis.
Data analysis:
The data were analysed by Chi2 test, univariate and multivariate logistic regression models in SPSS software version 19.
Considering the large effort, the perfect unifying and matching of groups wasn’t possible, so they were adjusted through analysis.
Results
There were a total of sixty- six female patients with ischemic stroke and 59 female controls who participated in the present study. Because of the large age dispersion among the controls compared with that of the patients and lack of similarity in stroke risk factors as well as having no desire to participate in related tests, 48 individuals remained in the control group. The mean age of stroke group patients was 73.65 ± 7.01 years and of control subjects as 72.00 ± 6.18 years with no significant statistical difference (p = 0.195).
Comparing the risk factors of stroke, the admitting systolic and diastolic blood pressure among patients were significantly higher than controls (p = 0.0001), but the serum levels of total cholesterol (194.45 ± 58.49 vs.184.70 ± 48.46 mg/dl p = 0.348) and triglyceride (140.54 ± 60.10 vs. 133.70 ± 52.15 mg/dl p = 0.621) were the same in both participant groups. The statistical differences in terms of the prevalence of diabetes mellitus (30.3% vs. 12.5%, p = 0.025), arterial hypertension (75.8% vs. 50%, p = 0.004) as well as BMI (p = 0.009), were determined. There were no statistical differences with hyperlipidemia (30.3% vs. 20.8%, p = 0.257).
The main subjects of research, serum levels of endogenous estrogen, progesterone and their proportions were not different statistically (p > 0.05) (table 1).
|
Table 1: Comparison the serum levels of estrogen, progesterone and their ratio between studied groups |
|||||
|
Studied Group |
N |
Mean |
SD |
p |
|
|
Estrogen Serum Level (mg/dl) |
Ischemic stroke |
66 |
28.66 |
13.60 |
.115 |
|
Control |
48 |
35.72 |
28.36 |
||
|
Progesterone Serum Level (mg/dl) |
Ischemic stroke |
66 |
1.07 |
.836 |
.700 |
|
Control |
48 |
1.40 |
1.87 |
||
|
Progesterone to Estrogen Ratio |
Ischemic stroke |
66 |
.051 |
.056 |
.206 |
|
Control |
48 |
.041 |
.036 |
||
|
Estrogen to Progesterone Ratio |
Ischemic stroke |
66 |
39.73 |
29.18 |
.535 |
|
Control |
48 |
42.85 |
28.31 |
||
The backward stepwise logistic regression model and multivariate analysis were used in order to control the confounding effect of other major parameters associated with stroke. In this model that was made with enteral p-value less than 0.1 and removal p-value more than 0.05, the serum level of estrogen (p= 0.033), progesterone (p= 0.02) and interaction between them (p= 0.013) remained in the final model as related factors to stroke (table 2).
|
Table 2: Multivariate analysis of association between main parameters of study and stroke |
|||||||
|
B |
S.E. |
Sig. |
Exp(B) |
95% C.I. for EXP(B) |
|||
|
Lower |
Upper |
||||||
|
Final Model |
Estrogen |
.049 |
.023 |
.033 |
1.050 |
1.004 |
1.098 |
|
Progesterone |
2.034 |
.874 |
.020 |
7.648 |
1.380 |
42.402 |
|
|
Estrogen by Progesterone |
-.041 |
.016 |
.013 |
.960 |
.930 |
.991 |
|
|
Constant |
-26.414 |
6.802 |
.000 |
.000 |
|||
A significant positive correlation was found to exist between the patient ability based on NIHSS and serum level of progesterone (p = 0.022, r = 0.282) and the proportion of progesterone to estrogen (p = 0.001, r = 0.395) (Diagram 1, 2). There was also a reciprocal correlation but not statistically significant between the patient’s NIHSS and serum level of estrogen (p = 0.899, r = 0.016).
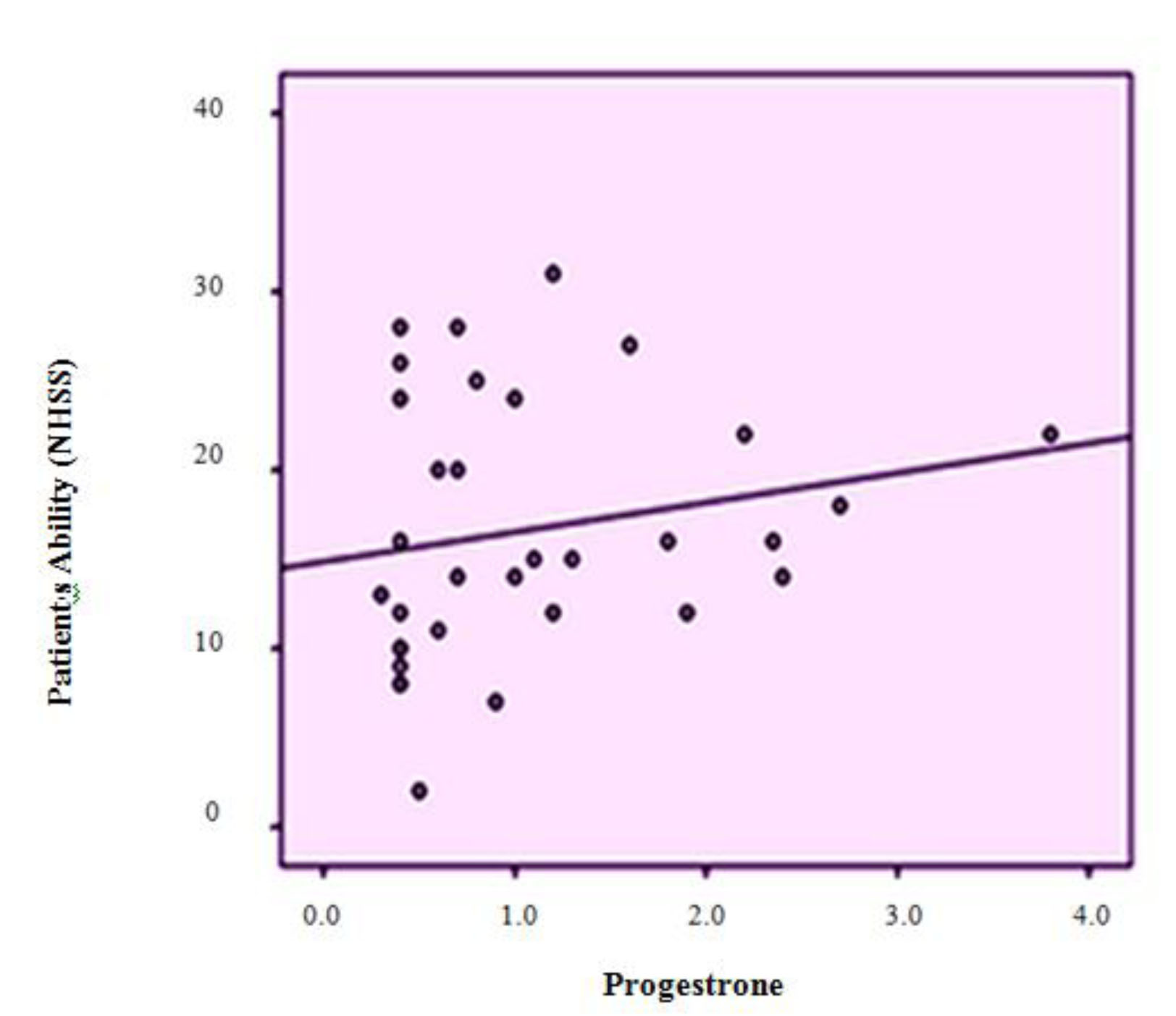
Diagram 1: Dispersion diagram for correlation between patients’ ability (NIHSS) and the serum level of progesterone
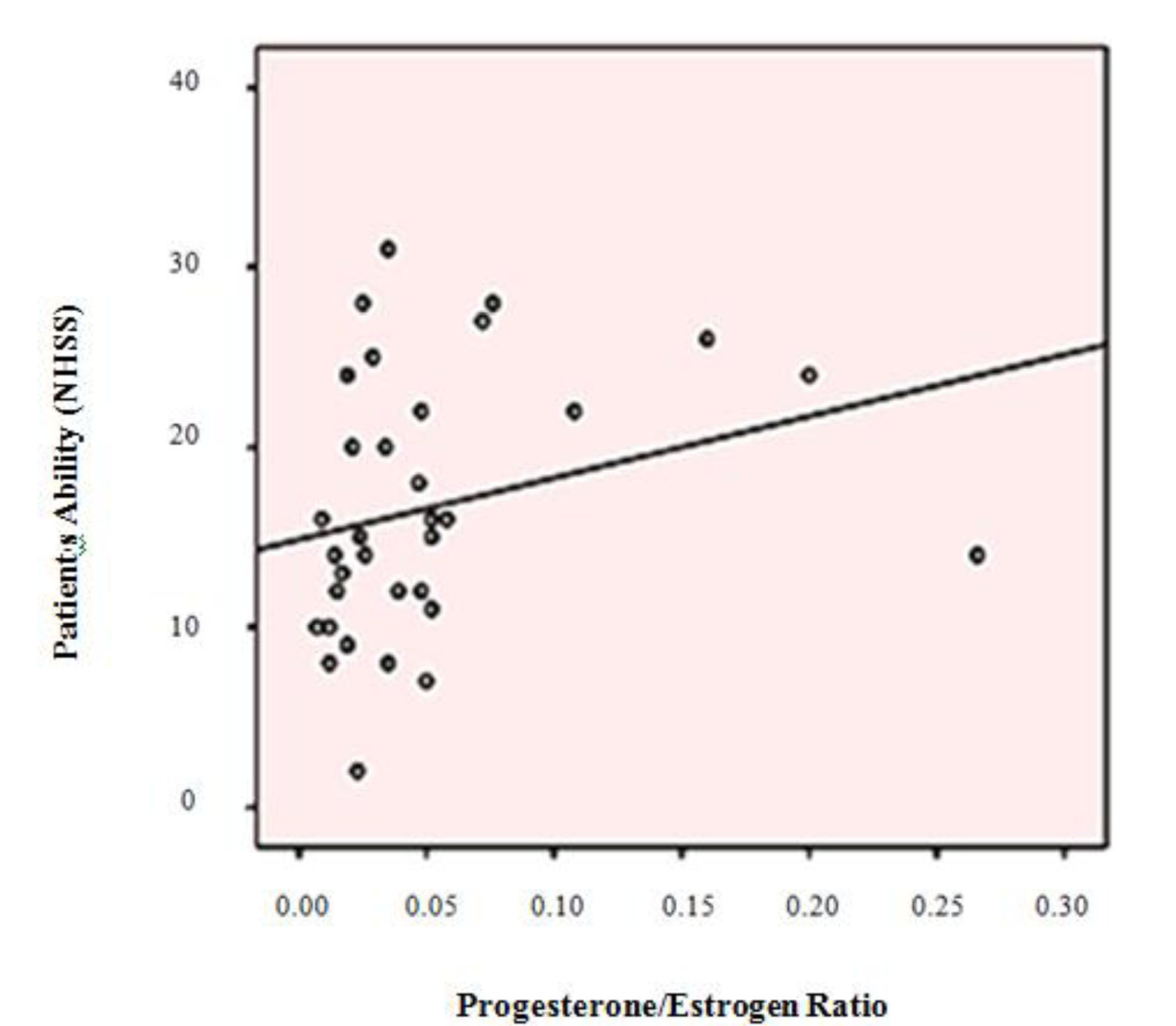
Diagram 2: Dispersion diagram for correlation between patients’ ability (NIHSS) and the ratio of progesterone to estrogen
This relationship was also analysed by a stepwise multivariate regression linear model and conclusively, the proportion of estrogen to progesterone was determined to be the only associated factor with the stroke patient’s ability (p = 0.016), as by its increasing as one unit, NIHSS decreases as 0.072 unit (95% CI: 0.014-0.131) (SE: 0.029).
Discussion
The present study concludes there was no obvious difference in terms of serum level of estrogen, progesterone and their ratio between studied groups, so by controlling the effect of confounding parameters in the multivariate analysis, both estrogen and progesterone and their interaction were
introduced as stroke-associated factors. With increasing the serum level of estrogen as much as 1 mg/dl, the chance of stroke increases as 1.05 folds, also by increasing progesterone serum level as much as 1 mg/dl the risk increases to 7.6 folds. It is fascinating that their contemporaneous increment lessens the odds of stroke.
A significant positive correlation was also concluded between admission NIHSS, and the level of progesterone and its ratio to estrogen. Nonetheless this correlation may disappear for instance after three months by improvement of patient’s ability throughout time which wasn’t assessed in survey.
Although NIHSS was found to have a reciprocal association with estrogen, it wasn’t statistically significant. The only stroke associated factor remaining in the final model of logistic regression multivariate analysis was the proportion of estrogen to progesterone level. So that 1 unit increasing of it resulted in 0.072 unit decrement of NIHSS.
Past studies executed in this manner investigated the effect of hormone replacement therapy (HRT) during the menopausal period which declared increasing, decreasing or no effect on the chance of stroke (20-23, 26), or introduced contraceptives as a strong risk factor for stroke (4, 19).
Only a few studies assessed the effect of endogenous hormones (18). So we aimed to assess the pure effect of their endogenous source on stroke occurrence.
In the way of the present study, the control group was selected from the similar age and genus of non-stroke patients in the same period of time. During the primary analysis, most of atherosclerosis risk factors including systolic and diastolic blood pressure, and the prevalence of arterial hypertension, diabetes mellitus and BMI in patient’s group were significantly higher than the controls. By eliminating their confounding effect using linear logistic regression model the serum level of estrogen and progesterone and their interaction were suggested as the associated factors to stroke.
Lee et al. proved that the chance of stroke among post-menopausal women having higher levels of free estradiol is two to three times higher than low estradiol subjects. Although throughout the present study, an association was provided between serum estrogen level (with no separation between its total and free form) and stroke, no considerable effect was determined. With the
increase of estradiol level 1 mg/dl; the odds of stroke will slightly change as much as 1.05 folds. An explanation about the Lee et al survey is essential, as it used more expanded sample volume in a prospective study and the serum level of free steroidal was measured in a female population who were followed for eight years (7). It seems the long term effect of endogenous estrogen was assessed in progress of aforementioned study. The same results might be found by long term following of the patients. Of course, as well as estrogen, the serum level of progesterone and their proportion and interaction were analysed in present study. In conclusion the latter parameters showed significant effect.
Sheikh et al. concluded other results in an Iranian survey dated 2009, in which the serum level of progesterone and its proportion to estrogen were lower among stroke patients compared with non-stroke patients. This investigation was carried out by the small sample volume and both males and females were included (18). In this study the impact from these confounding factors was eliminated, by conducting it only among the female population over 60 years of age, yielding a higher accuracy.
Viscoli et al. conducted a study in 2001, on 664 post-menopausal women (mean age of 71 years) who recently experienced an ischemic stroke or transient ischemic attack. They received estrogen as 1 mg/day. During a 2.8 year follow up, the risk of the fatal or disabling stroke was increased. This supports the results of us and Lee et al. (22). But Fakleborn and Finucane in 1993 confirmed the protective effect of postmenopausal estrogen therapy (20, 24). Some researches assessed the potential role of free estrogen in induction of cardiovascular disease and in conclusion no impact was found out (25-27). Therefore the
other mechanisms for estrogen effect on stroke have to be provided.
Dyslipidemia, diabetes mellitus, insulin resistance and biologic mediators of inflammation are proposed to be the factors associated with stroke and endogenous estradiol or other hormones. Lee et al. considered the strongest association of stroke with free estradiol index rather than total estradiol level. Fatty tissue is a potential resource of producing endogenous estrogen for post-menopausal women. Association between stroke and estradiol is even stronger among those with central obesity. Central obesity increases the production of endogenous estradiol and its circulatory level. The waist size was introduced as an effective modifier for abovementioned association (17). Although the present survey didn’t assess the modifying effect of waist size on stroke, the BMI data as a confounding factor was entered into the analysis and the sheer effect of these hormones was extracted.
In addition to protection against stroke, eliminating use of these hormones would be a way of improving the patient’s health. The association between capability score (NIHSS) and endogenous hormone level were determined. The Sheikh et al. study measured the admission severity of stroke by Canadian Neurologist Score (CNS), but no final analysis was done in order to establish its association with the serum level of the studied hormones and only a comparison was made between males and females in term of stroke severity (18). We found an obvious correlation between the proportion of estrogen to progesterone and NIHSS and so patient’s ability with downward slopping, i.e. by increasing the estrogen to progesterone ratio, their scores lessen and so the patient’s capabilities increases, which is in coherency with other documents indicating the
neuro-protective effect of estrogen. It was said that estrogen, prevent neuro-apoptosis by decreasing enzyme activity and DNA destruction, and also impress on tissue viability, cerebral blood flow, immune response to stroke (12, 2).
Alkayed et al. survey on the rats engrossingly demonstrated the lower size of infarction and the further blood flow among females rather than males. This difference was eliminated by ovarectomy which indicates the protection effect of estrogen (28). Also Krause survey showed estrogen can protect cerebral endothelial cell, decreases the production of free radicals and stimulate of angiogenesis (29). Whereas some investigations insist on neuro-protective effect of progesterone by protection of blood-brain-barrier, decreasing cytokines and limitation of cellular necrosis and apoptosis. Meanwhile some of them prescribed progesterone in order to improve and obtain acceptable results i.e. smaller size and better prognosis of stroke (30, 32, 33). Singh and Alkeyed et al. also supported the improving and protective role of progesterone (31, 33).
It seems that estrogen studies are coherent with our study, but progesterone studies aren’t, and it may be due to either interaction between these hormones - not only their pure effect - or the stronger effect of estrogen.
Conclusion
High levels of estrogen or progesterone alone increase the risk of stroke, but their interaction decreases its risk. Also the patients’ ability according to NIHSS is coherent with the estrogen to progesterone ratio; by considering the neuro-protection effects of both, it defines the stronger effect of estrogen in this way.
Acknowledgement
This investigation was based on a thesis submitted by Milad Javadpoor to the faculty of medicine of Guilan University of Medical Sciences (GUMS) in Rasht, Iran.
Conflict of Interest
No conflict of interest.
References
- Eelco EMW, Jennifer EF. Anoxic-Ischemic Encephalopathy in: Bradley W G, Daroff RB, et al. Neurology in Clinical Practice. 6th ed. ELSEVIR; 1314-20.
- Bushnell CD, Hurn P, Colton C, Miller VM, del Zoppo G, Elkind MS, et al. Advancing the Study of Stroke in Women: Summary and Recommendations for Future Research from an NINDS-sponsored Multidisciplinary Working Group. Stroke 2006; 37(9): 2387– 99.
- Dalal P, Bhattacharjee M, Vairale J, Bhat P. UN Millennium Development Goals: Can We Halt the Stroke Epidemic in India? Ann Indian Acad Neurol 2007; 10:130-6.
- Tran J, Mirzaei M, Anderson L, Leeder SR. The Epidemiology of Stroke in the Middle East and North Africa. J Neurol Sci 2010; 295(1-2):38- 40.
- Hosseini A, Sobhani-rad D, Ghandehari K, Benamer HT. Frequency and Clinical Patterns of Stroke in Iran-Systematic and Critical Review. BMC Neurol 2010; 10:72.
- Fang CW, Ma MC, Lin HJ, Chen CH. Ambient Temperature and Spontaneous Intracerebral Haemorrhage: A Cross-Sectional Analysis in Tainan, Taiwan. BMJ Open 2012; 2(3): e000842.
- Farajzadeh M, Darand M. Analizing the Influence of Air Temperature on the Cardiovascular, Respiratory and Stroke Mortality in Tehran: Iran. J Environ Health Sci Eng 2009; 6(4):261-70.
- Di Carlo A. Human and Economic Burden of Stroke. Age and Ageing 2009; 38(1):4-5.
- Ohshige K, Hori Y, Tochikubo O, Sugiyama M. Influence of Weather on Emergency Transport Events Coded as Stroke: Population-based Study in Japan. Int J Biometeorol 2006; 50(5):305-11.
- Harrod CG, Batjer HH, Bendok BR. Deficiencies in Estrogen-Mediated Regulation of Cerebrovascular Homeostasis May Contribute to an Increased Risk of Cerebral Aneurysm Pathogenesis and Rupture in Menopausal and Postmenopausal Women. Med Hypotheses 2006; 66(4):736– 56.
- Cue L, Diaz F, Briegel K, Patel HH, Raval AP. Periodic Estrogen Receptor-Beta Activation: A Novel Approach to Prevent Ischemic Brain Damage. Neurochem Res 2014; Jun 7.
- Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol Attenuates Programmed Cell Death after Stroke-Like Injury. J Neurosci 2003;23(36):11420 – 6.
- Croft P, Hannaford P. Risk Factors for Acute Myocardial Infarction in Women: Evidence from the Royal College of General Practitioners' Oral Contraception Study. BMJ 1989; 298:165.
- Rosenberg L, Palmer JR, Rao RS, Shapiro S. Low-Dose Oral Contraceptive Use and the Risk of Myocardial Infarction. Arch Intern Med 2001; 161(8):1065-70.
- Acute Myocardial Infarction and Combined Oral Contraceptives: Results of an International Multicentre Case-Control Study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 1997; 349(9060):1202-9.
- Sidney S, Siscovick DS, Petitti DB, Schwartz SM, Quesenberry CP, Psaty BM, et al. Myocardial Infarction and Use of Low-Dose Oral Contraceptives: A Pooled Analysis of 2 US Studies. Circulation 1998; 98(11):1058-63.
- Lee J, Yaffe K, Lui L, Cauley J, Taylor B, Browner W, et al. Prospective Study of Endogenous Circulating Estradiol and Risk of Stroke in Older Women. Arch Neurol 2010; 67(2):195-201.
- Sheikh N, Tavilani H, Rezaie AL. Decreased Endogenous Progesterone and Ratio of Progesterone to Estrogen in Stroke Ischemia. Afr J Biotechnol 2010; 9(5): 732-4.
- Gillum LA, Mamidipudi SK, Johnston SC. Ischemic Stroke Risk with Oral Contraceptives: A Meta-Analysis. JAMA 2000; 284(1):72-8
- Falkeborn M, Persson I, Terent A, Adami HO, Lithell H, Bergström R. Hormone Replacement Therapy and the Risk of Stroke. Follow-up of a Population-Based Cohort in Sweden. Arch Intern Med 1993; 153(10):1201 –9.
- Petitti DB, Sidney S, Quesenberry CP Jr, Bernstein A. Ischemic Stroke and Use of Estrogen and Estrogen/Progestogen as Hormone Replacement Therapy. Stroke 1998; 29(1):23-8.
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A Clinical Trial of Estrogen-Replacement Therapy after Ischemic Stroke. N Engl J Med 2001; 345(17) 25: 1243-49.
- Simon JA, Hsia J, Cauley JA, Richards C, Harris F, Fong J, et al. Postmenopausal Hormone Therapy and Risk of Stroke: The Heart and Estrogen-progestin Replacement Study (HERS). Circulation 2001; 103(5):638-42.
- Finucane FF, Madans JH, Bush TL, Wolf PH, Kleinman JC. Decreased Risk of Stroke Among Postmenopausal Hormone Users. Results from a National Cohort. Arch Intern Med 1993; 153(1):73-9.
- Barrett-Connor E, Goodman-Gruen D. Prospective Study of Endogenous Sex Hormones and Fatal Cardiovascular Disease in Postmenopausal Women. BMJ 1995; 311(7014):1193-6.
- Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, Cook NR, et al. Sex Hormone Levels and Risk of Cardiovascular Events in Postmenopausal Women. Circulation 2003; 108(14):1688-93.
- Goodman-Gruen D, Barrett-Connor E. A Prospective Study of Sex Hormone binding Globulin and Fatal Cardiovascular Disease in Rancho Bernardo Men and Women. J Clin Endocrinol Metab 1996; 81(8):2999-3003.
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-Linked Brain Injury in Experimental Stroke. Stroke 1998; 29(1):159-66.
- Krause DN, Duckles SP, Pelligrino DA. Influence of Sex Steroid Hormones on Cerebrovascular Function. J Appl Physiol (1985). 2006; 101(4):1252-61.
- Simpkins J, Yang S, Wen Y, Singh M. Estrogens, Progestins, Menopause and Neurodegeneration: Basic and Clinical Studies. Cell Mol Life Sci 2005; 62(3):271-80.
- Singh M. Progesterone-Induced Neuroprotection. Endocrine 2006; 29(2):271-4.
- Stein DG. Progesterone Exerts Neuroprotective Effects after Brain Injury. Brain Res Rev 2008; 57(2):386-97.
- Alkeyed NJ, Murphy SJ, Traystman R, Hurn PD, Miller VM. Neuroprotective Effects of Female Gonadal Steroids in Reproductively Senescent Female Rats. Stroke 2000; 31(1):161-8.
Received: 2015/03/21 | Accepted: 2015/03/21 | Published: 2015/03/21
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




