BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://cjns.gums.ac.ir/article-1-325-en.html
2- Department of Psychology, Islamic Azad University, Tonekabon Branch, Tonekabon, Iran
3- Department of Psychology, Karaj Branch, Islamic Azad University, Karaj, Iran
Introduction
Obsessive-Compulsive Disorder (OCD) is a disabling neuropsychiatric condition with very serious impairments and prevalence of 1%-3% [1, 2]. Studies indicated an association between OCD and various functional impairments and decreased quality of life [3]. OCD could lead to functional impairments in multiple ways [4]; it has adverse consequences, such as personal, interpersonal, and quality of life problems for patients [5, 6].
Numerous patients with OCD demonstrate major deficiencies in the ability to truly assess the risk probability; they are suspicious of everything and experience morbid obsessions. Therefore, they engage in mental ruminations and rituals to reduce their worries. These conducts could lead to the generation of the negative symptoms of obsession, namely depression, emotional coldness, and reduced communication levels with others in the long term. The positive symptoms of obsession or ineffective symptoms, i.e. very time-consuming and prevent individuals from accomplishing their goals and values of life. These characteristics include repetitive behaviors, such as washing, checking, and counting, and could endanger the individual performance in personal, professional, and social aspects [2, 7, 8].
Considering the dramatic effect of OCD on patients’ quality of life [5], its relatively high global prevalence [9], the increased frequency of its diagnosis [10], and the existence of therapeutic challenges, including the recurrence of this disorder after treatment [11], it is always necessary to examine the OCD and its related dimensions.
An important issue about OCD is the common types of obsessions and compulsions. In this regard, Goodman et al. prepared a checklist of Yale-Brown Obsessive Compulsive Scale (Y-BOCS), consisting of >50 cases of obsessions and compulsions [12, 13]. Baer considered three factors, namely symmetry/hoarding, contamination/checking, and pure obsessions [14]. In another study, Leckman discussed 4 factors of checking symmetry, cleanliness, and hoarding [15]. Abramowitz et al. also suggested 5 clusters, including harming, contamination, hoarding, unacceptable thoughts, and symmetry [16]. In addition, Williams et al. expressed 4 symptom dimensions, consisting of contamination/cleaning, doubt about the damage/checking, unacceptable thoughts/the coercion of thoughts, and symmetry/order for OCD [17].
Furthermore, Heyman, Mataix-Cols, and Fineberg expressed cases, including the fear of causing harm to someone, the fear of self-harm, the fear of contamination, the need for symmetry or exactness, sexual and religious obsessions, the fear of behaving unacceptably, and the fear of making a mistake, as the most common obsessions [18]. They also cited behaviors, such as cleaning, hand washing, checking, ordering and arranging, hoarding, asking for reassurance, and mental acts, including counting, repeating words silently, ruminations, and neutralizing thoughts, as the most frequent compulsions. Given some differences in the content of OCD symptoms in various cultures [19, 20], differences in the consequences of treatment, and various symptom dimensions of OCD, as well as the clinical importance of awareness of OCD symptoms [17], it is always necessary to investigate the prevalence of different types of OCD symptoms among multiple groups and at different times.
Considering the limited studies on the prevalence of OCD dimensions, one purpose of the present study was exploring the prevalence of different symptoms of OCD. Notably, genetic factors are among the most essential dimensions of OCD [21]. Studies on twins and family reviews indicated the significance of inheritance and the crucial roles of genes in causing this disorder. The risk of OCD in first-degree relatives of the affected patients is 4 times higher than the general population; its inheritance rate is up to 65% [22-24]. Heredity is a very prominent subject in OCD that requires attention [25].
In this regard, several genes, such as glutamate [26], serotonin [27-29], Catechol-O-Methyl Transferase (COMT), and Monoamine Oxidase A (MAO-A [30, 31] are candidates in OCD. Furthermore, the Brain-Derived Neurotrophic Factor (BDNF) is a critical gene in OCD [32, 33], i.e. expressed in the brain tissue. This gene is on the short arm of chromosome 11 (11p14.1) and a Single Nucleotide Polymorphism (SNP) of BDNF gene (SNP, rs6265), i.e. the outcome of the substitution of Valine (Val) amino acid with Methionine (Met) in codon 66 (Val66Met) [34, 35].
Studies reported that the BDNF level is lower in patients with OCD [36]. Wang et al. [32] in a Chinese Han population sample indicated that BDNF plasma/serum levels were lower in a group of patients with OCD, compared to the healthy samples. Wang et al. [37] stated that the BDNF significantly impacted the physiological damage of OCD. Taj et al. [38] also argued that the BDNF was associated with OCD and played a protective role in this regard.
Marquez et al. [39] reported that the BDNF gene could be influential in causing OCD. Hemmings et al. [40] also considered a dysfunctional role of BDNF in this disorder. Moreover, Val66Met is a BDNF gene polymorphism that could significantly affect OCD [41]. Marquez et al. [39] mentioned the difference between Val66Met polymorphisms in individuals with ODC. Studies by Wang et al. [32], Katerberg et al. [42], and Tukel et al. [43] also supported the importance of Val66Met polymorphism in OCD. In line with few studies in Iran, e.g. a study by Abdol-Hosseinzadeh et al. [44], the present study aimed to investigate the role of Val66Met polymorphism in BDNF gene in OCD among the Iranian population.
In summary, the present study aimed to explore the prevalence rate of various symptoms, the severity of OCD, as well as the BDNF gene polymorphisms in the gene position (rs6265) in individuals with OCD in Iran.
Materials and Methods
This was case-control research. The sample size of the study was measured using the literature review and based on a study by Hemmings (n=40/group). In the study, 83 outpatients (34 men & 49 women) with OCD who referred to psychology and counseling centers in Tehran City, Iran (from summer 2018 to summer 2019) were selected by the convenience sampling method. The study participants were selected after the confirmation of the clinical diagnosis by a psychiatrist and based on the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM5) criteria. The study inclusion criteria were as follows: receiving OCD diagnosis based on the DSM-5 [45]; the required score obtained on the Y-BOCS; the age range of 18-60 years, and providing personal consent to participate in the research.
The study exclusion criteria were the lack of physical health; a history of major psychiatric disorders; and substance dependence. Measurement tools included definitive psychiatric diagnosis, unorganized interview [46], and Y-BOCS. The checklist’s questions included the DSM-5 diagnostic criteria, i.e. probed by a clinical psychologist. In addition, the control group consisted of 83 individuals (34 men & 49 women) with no history of psychiatric disorders. They were selected by the convenience sampling method and using a diagnostic interview by a clinical psychologist and clinical examinations by a physician. Besides, they matched the OCD group in terms of demographic characteristics, including age, gender, educational level, and marital status. The study inclusion criteria for the control group include the age range of 18-60 years; adequate physical health to complete the research, and personal consent for study participation. Furthermore, the study exclusion criteria were the same as the OCD group.
The demographic data questionnaire was used to collect information about gender, age, educational level, marital status, and the number of children. The Y-BOCS was employed to rate the obsessions and compulsions [12, 13]. This questionnaire is a semi-structured interview to assess the severity of obsessions and compulsions, regardless of the number and content of current obsessions and compulsions. Unlike other questionnaires in this field, Y-BOCS has a high sensitivity to therapeutic changes and is widely used for evaluating the effectiveness of OCD treatment; it is known as the “golden standard” to assess the severity of OCD symptoms at the end of treatment [47]. Y-BOCS consists of two parts; the Symptom Checklist (SC) and Severity Scale (SS). The SC items are answered by the self-report manner on a five-point Likert-type scale. In the SS, each obsession and compulsion is estimated in 5 dimensions, namely time/frequency, interference, distress, resistance, and the extent of control. The Y-BOCS dedicates three scores for the severity of obsessions, the severity of compulsions, and a total score, including all items. The correlation coefficient of this tool was obtained as 0.80 to 0.99, i.e. equal to 0.81 to 0.99 for a two-week interval. The Cronbach’s alpha coefficient of it was reported to be 0.69 for its internal consistency [22, 23]. Rajezi Esfahani et al. also reported that the Persian version of the questionnaire has the necessary validity and reliability in the Iranian population [48]. According to his report, the internal consistency of the two parts of the SC and SS lists was 0.97 and 0.95, respectively; their split-half validity was equal to 0.93 and 0.89, respectively; and the test-retest validity of this scale was 0.99.
Initially, the necessary measures were taken to receive the code of ethics from the Ethics Committee of Islamic Azad University of Karaj (Code: IR.IAU.K.REC.1396.88). The convenience sampling method was performed to select the study participants and collect the required data after coordination with psychological clinics and pre-determined counseling in Tehran City, Iran. A non-organized clinical interview was first conducted by a clinical psychologist with patients; during which, some information was recorded by the researcher to select the study samples according to the diagnosis in the patients’ records. During the interviews, the diagnostic checklists were also completed by the investigated individuals. If the diagnosis from the checklist was consistent with the psychiatrist’s opinion or the diagnosis in the patient’s record, they were selected as the study participants. Accordingly, the Y-BOCS and genetic assessment were individually performed in them. SPSS and genetic data SNPAlyze were used to analyze the achieved data. Hardy-Weinberg equilibrium was used for genotypic distribution using the χ2 test (P=0.3), indicating the equilibrium in the study population.
The sampling (peripheral blood) was conducted in a test tube, containing 6M EDTA anticoagulant. After the psychiatrist’s confirmation of the clinical diagnosis in this group, 5-10 mL of venous blood was obtained from the study patients and poured into tubes, containing EDTA anticoagulant (EDTA 6 molar).
DNA extraction was performed from peripheral blood by a standard method. Accordingly, DNA extraction from the peripheral blood was measured by the salting-out method. The cellular DNA decomposition and separation from other cellular components is the first step in DNA purification. The High Pure Polymerase Chain Reaction (PCR) Template Preparation Kit (made in Roche, Swiss) was used to extract DNA. The proliferation of DNA sequences was performed by the PCR, i.e. a sensitive method for multiplying the sequences of DNA in the laboratory. Furthermore, a single nucleotide polymorphism; rs6265, was selected from the BDNF gene. The single-nucleotide polymorphism was selected based on a series of previous studies and according to databases, dbSNP (http://www.ncbi.nlm.nih.gov/SNP/), and SNPper (http://snpper.chip.org/). Rs6265 in the BDNF gene was proliferated by PCR. The primer design was performed using primer 3. The Sanger sequencing, by the electrophoresis, was used at the end of the DNA synthesis stage; the electrophoresis method on agarose gel was used for the separation of nucleic acid parts, resulting from enzymatic digestion in the RFLP method.
Results
Table 1 reports the demographic information of the study participants with OCD and healthy controls.
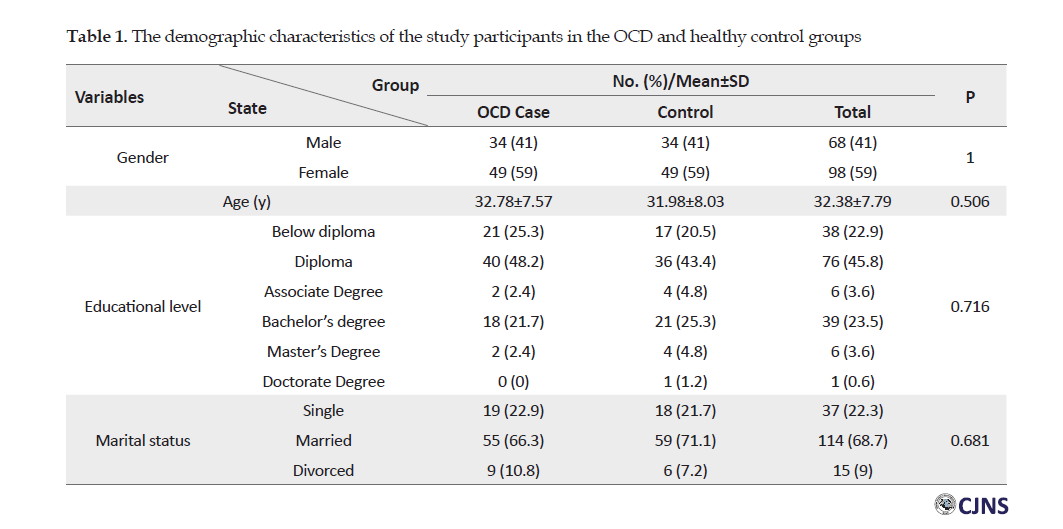
The Chi-squared test data indicated no significant relationship in gender, educational level, and marital status between the two groups of individuals with and without OCD (P>0.05). The t-test data indicated no significant difference in the mean values of the age of the individuals with and without OCD (P=0.716). Figure 1 shows symptom dimensions in the OCD group.
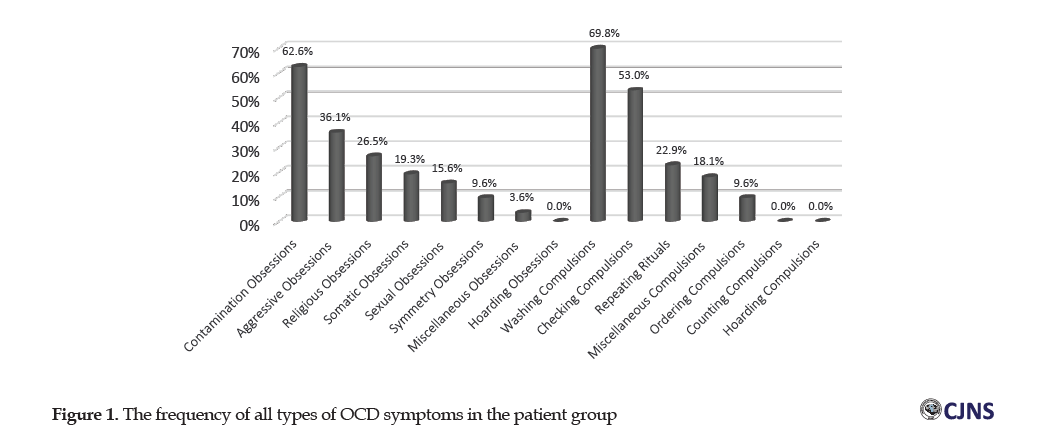
According to Figure 1, the contamination obsession with 62.6% of cases, constituted the highest frequency of obsession in 83 patients with OCD, followed by an aggressive obsession with 36.1% and religious obsession with 26.5%. The collected results also indicated that the highest proportion of compulsion belonged to the washing group with 69.8%, then control compulsion with 53%, and repetition compulsion with 22.9%.
Table 2 presents the types of genotypic and allelic frequencies in the BDNF gene polymorphisms in the two groups of patients with OCD and healthy controls
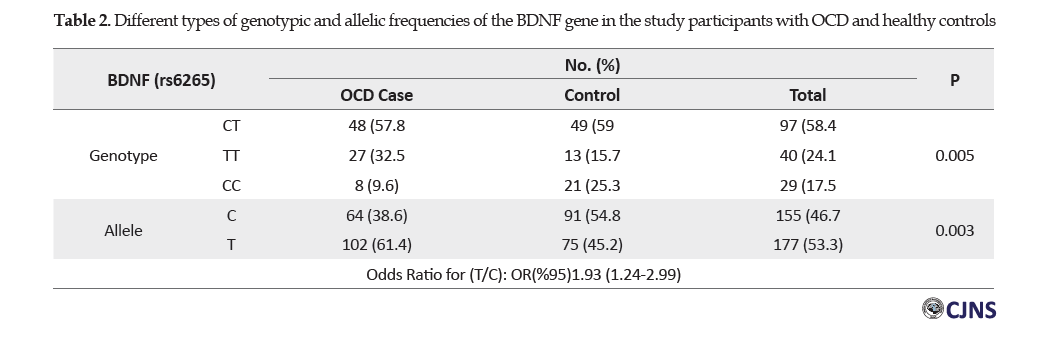
The Chi-squared test results indicated a significant relationship in the genotypic frequencies of various polymorphisms of the BDNF gene between the study groups (P=0.005). In this regard, the genotypic frequency of TT was more common in individuals with OCD, compared to the other genotypes. There was also a significant relationship in different alleles of the BDNF gene between the study groups (P=0.003). In this regard, allele T was more frequent in the research participants with OCD, compared to those with allele C. The Odds Ratio (OR) was equal to 1.93 for allele T, compared to allele C at a confidence interval of 95%. Therefore, allele T increased the risk of obsession, compared to allele C. Besides, the frequency of allele T was greater in the OCD group, compared to the healthy controls, indicating that allele T increased the obsession frequency.
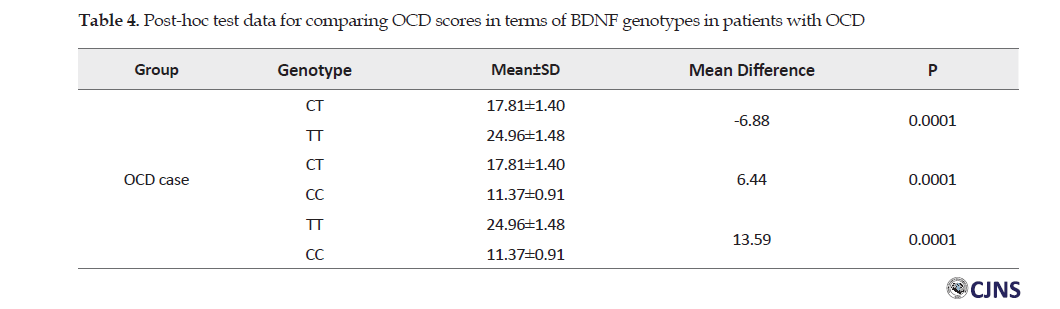
The one-way Analysis of Variance (ANOVA) indicated a significant difference between the values of the severity of OCD in terms of different types of BDNF genotype in patients with OCD (P=0.0001). In this regard, the TT genotype provided the highest statistical mean severity of OCD (Table 3).
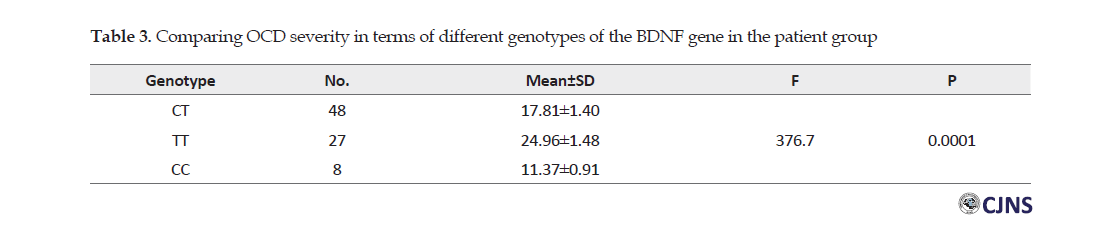
Scheffe Test indicated findings highlighted a significant difference between the scores of the severity of OCD in different genotypes in the patient group (P>0.0001). The mean difference between TT and CC genotypes was much greater.
Discussion
The present study data suggested that the highest obsessive mentality belonged to contamination, aggression, religion, physical, sexual, and symmetry aspects. The obtained results also indicated that washing, control, and repetition were the most frequent compulsions among the investigated patients with OCD. More specifically, the research findings indicated a higher prevalence of contamination obsession and washing compulsion in the stud participants. In this regard, Zambaldi et al. [49] considered the contamination obsession as a common obsession and the contamination compulsion as a common compulsion.
In Iran, Ghasemzadeh et al. [50] stated that doubt was the most common obsession; washing compulsion was addressed as the most frequent compulsion in the research sample. Considering the greater number of female participants, gender should be considered in interpreting and comparing the present study findings. In conclusion, the achieved results indicated that the greatest obsession was contamination (62.2%) and the highest practical compulsion was washing (69.8%) in the explored patients with OCD.
Furthermore, we studied the association of BDNF Val66Met polymorphism and the occurrence and severity of OCD symptoms. The neurotrophic factor derived from the brain is the most abundant neurotrophin in the mammalian brain and plays a role in the growth and survival of neurons [51]. The lower level of BDNF in the blood serum of patients with OCD supports that dysfunction in the expression of neurotrophin is a pathogenetic mechanism for this disorder [52].
The collected data indicated that allele T in this polymorphism was associated with an increased risk of OCD development in the research sample (χ2=4.7, P=0.003; OR (95%) 1.93 [1.24-2.99]). In humans, the methionine allele (T) of the BDNF gene is the characteristic of a subset of individuals at risk for OCD [53] (Table 4).
Emotional trauma during childhood in methionine allele carriers significantly increases the risk of obsession (P=0.024) [54]. The presence of a methionine allele weakens the BDNF gene activity and may cause poorer functions of the brain in patients with OCD [45]. The presence of a methionine allele makes a significant difference in the brain volume, i.e. the smaller gray matter volume [53], smaller hippocampal volume [55], the smaller anterior prefrontal cortex in women [56], and smaller amygdala volume in the aging population [57]. In terms of pathophysiology, for the brain, the basal ganglia (putamen & acumens), thalamus, and amygdala are associated with OCD [58]. Structural changes in the brain, due to the presence of various polymorphisms of this gene, could be considered as a cause of this disorder. Some scholars indicated that the gene was associated with the severity of positive symptoms in obsessions (P=0.02); however, methionine allele in Val66Met locus provides a protective effect in individuals with OCD [38].
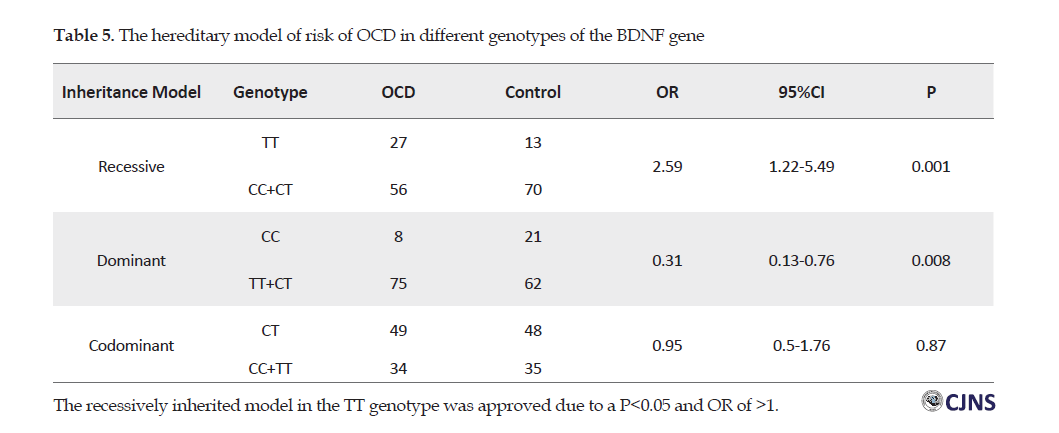
Based on the present study findings, the genotypic frequency of Val66Met of the BDNF gene differed between individuals with OCD and healthy participants (Table 5).
In other words, the genotypic frequencies of CC and CT were higher in the healthy controls, compared to patients with OCD. Furthermore, the greatest difference between healthy participants and patients with OCD belonged to the CC levels; indicating the greater importance of homozygote CC, compared to the other cases. Studies on the association of BDNF gene and the susceptibility to OCD signified that the polymorphisms of the gene affected the sequence of BDNF protein amino acid [59] and played a pathogenetic role in various conditions, such as Huntington’s disease, Alzheimer’s disease, OCD, and depression, by creating a defect in the BDNF signaling [60]. The lower serum level of BDNF reflects a decrease in the cerebral expression and could be used as a neurobiological indicator in these pathological processes [61]. According to the results of the present study and similar investigations, Val66Met polymorphisms, as a coding factor of BDNF could act as a mediator to create the subgroups of OCD and these confounding factors.
The results also indicated the importance of single-nucleotide polymorphisms (rs6265) of the BDNF gene in the emergence of OCD symptoms and its severity. In addition, homozygous carriers (CC) indicated the minimum severity of OCD (11.37±0.91). Moreover, homozygous carriers (TT) indicated the maximum severity of the OCD (24.96±1.48) (P=0.0001) that could indicate the protective role of allele C in the severity of OCD symptoms.
Conclusion
The symptoms dimensions of OCD in the explored Iranian sample were similar to other populations; the contamination (62.6%) and the cleanliness (69.8%) were the most prevalent obsessions and compulsions, respectively. Moreover, the present research findings suggested an association between BDNF val66Met polymorphism genotype and OCD in the Iranian sample. There was a significant relationship between the TT genotype of BDNF val66Met polymorphism and OCD. The inheritance hypothesis for the TT genotype was the recessive model.
Finally, the present study had some limitations. For instance, we could address the sample size and the lack of controlling or studying confounding factors, like the study participants’ socioeconomic status that could affect the OCD.
Ethical Considerations
Compliance with ethical guidelines
The study procedures complied with the ethical guidelines of the Declaration of Helsinki 2013. Moreover, this study was approved by the Ethics Committee of Karaj Branch, Islamic Azad University (Code: IR.IAU.K.REC.1396.88). All study participants were ensured that their data would be confidentially managed and solely used for the present study.
Funding
This article was extracted from the PhD. thesis of Shahrzad Hoveyda in the Department of Health Psychology, Karaj Branch, Islamic Azad University, Karaj.
Authors contributions
Draft, writing review and editing: Shahrzad Hoveyda, Javad Khalatbari, Javid Peymani, Hasan Ahadi; Supervision: Javad Khalatbari, Javid Peymani, Hasan Ahadi; Data collecting: Shahrzad Hoveyda.
Conflict of interest
The author declared no conflicts of interest.
Acknowledgements
The authors are grateful to the Vice Chancellorship of Research and Technology of Karaj Branch, Islamic Azad University, Karaj, Iran.
References
1.Wang Sh, Xu X, Yan P, Song M, Li J, Wang Sh. Is Brain-Derived Neurotrophic Factor (BDNF) Val66met polymorphism associated with obsessive-compulsive disorder? A meta-analysis. Psychiatr Danub. 2019; 31(2):141-7. [DOI:10.24869/psyd.2019.141] [PMID]
2.Hirschtritt ME, Bloch MH, Mathews CA. Obsessive-compulsive disorder: Advances in diagnosis and treatment. JAMA. 2017; 317(13):1358-67. [DOI:10.1001/jama.2017.2200] [PMID]
3.Sahoo P, Sethy RR, Ram D. Functional impairment and quality of life in patients with obsessive compulsive disorder. Indian J Psychol Med. 2017; 39(6):760-5. [DOI:10.4103/IJPSYM.IJPSYM_53_17] [PMID] [PMCID]
4.Markarian Y, Larson MJ, Aldea MA, Baldwin SA, Good D, Berkeljon A, et al. Multiple pathways to functional impairment in obsessive-compulsive disorder. Clin Psychol Rev. 2010; 30(1):78-88. [DOI:10.1016/j.cpr.2009.09.005] [PMID]
5.Eisen JL, Mancebo MA, Pinto A, Coles ME, Pagano ME, Stout R, et al. Impact of obsessive-compulsive disorder on quality of life. Compr Psychiatry. 2006; 47(4):270-5. [DOI:10.1016/j.comppsych.2005.11.006] [PMID] [PMCID]
6.Grabe HJ, Meyer Ch, Hapke U, Rumpf HJ, Freyberger HJ, Dilling H, et al. Prevalence, quality of life and psychosocial function in obsessive-compulsive disorder and subclinical obsessive-compulsive disorder in northern Germany. Eur Arch Psychiatry Clin Neurosci. 2000; 250(5):262-8. [DOI:10.1007/s004060070017] [PMID]
7.Greisberg S, McKay D. Neuropsychology of obsessive-compulsive disorder: A review and treatment implications. Clin Psychol Rev. 2003; 23(1):95-117. [DOI:10.1016/s0272-7358(02)00232-5]
8.Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: A critical review. Biol Psychol. 2004; 65(3):185-236. [DOI:10.1016/j.biopsycho.2003.07.007] [PMID]
9.Sasson Y, Zohar J, Chopra M, Lustig M, Iancu I, Hendler T. Epidemiology of obsessive-compulsive disorder: A world view. J Clin Psychiatry. 1997; 58 Suppl 12:7-10. [PMID]
10.Stoll AL, Tohen M, Baldessarini RJ. Increasing frequency of the diagnosis of obsessive-compulsive disorder. Am J Psychiatry. 1992; 149(5):638-40. [DOI:10.1176/ajp.149.5.638] [PMID]
11.Pittenger Ch, Kelmendi B, Bloch M, Krystal JH, Coric V. Clinical treatment of obsessive compulsive disorder. Psychiatry (Edgmont). 2005; 2(11):34-43. [PMID] [PMCID]
12.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive - Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989; 46(11):1006-11. [DOI:10.1001/archpsyc.1989.01810110048007] [PMID]
13.Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown Obsessive-Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989; 46(11):1012-6. [DOI:10.1001/archpsyc.1989.01810110054008] [PMID]
14.Baer L. Factor analysis of symptom subtypes of obsessive compulsive disorder and their relation to personality and tic disorders. J Clin Psychiatry. 1994; 55 Suppl:18-23. [PMID]
15.Leckman JF, Grice DE, Boardman J, Zhang H, Vitale A, Bondi C, et al. Symptoms of obsessive-compulsive disorder. Am J of Psychiatry. 1997; 154(7):911-7. [DOI:10.1176/ajp.154.7.911] [PMID]
16.Abramowitz JS, Franklin ME, Schwartz SA, Furr JM. Symptom presentation and outcome of cognitive-behavioral therapy for obsessive-compulsive disorder. J Consult Clin Psychol. 2003; 71(6):1049-57. [DOI:10.1037/0022-006X.71.6.1049] [PMID]
17.Williams MT, Mugno B, Franklin M, Faber S. Symptom dimensions in obsessive-compulsive disorder: Phenomenology and treatment outcomes with exposure and ritual prevention. Psychopathology. 2013; 46(6):365-76. [DOI:10.1159/000348582] [PMID] [PMCID]
18.Heyman I, Mataix-Cols D, Fineberg NA. Obsessive-compulsive disorder. BMJ. 2006; 333(7565):424-9. [DOI:10.1136/bmj.333.7565.424] [PMID] [PMCID]
19.Eǧrilmez A, Gülseren L, Gülseren Ş, Kültür S. Phenomenology of obsessions in a Turkish series of OCD patients. Psychopathology. 1997; 30(2):106-10. [DOI:10.1159/000285037] [PMID]
20.Shooka A, Al-Haddad MK, Raees A. OCD in Bahrain: A phenomenological profile. Int J Soc Psychiatry. 1998; 44(2):147-54. [DOI:10.1177/002076409804400207] [PMID]
21.Pury A, Nestadt G, Samuels JF, Viswanath B. Genetics of obsessive-compulsive disorder. Indian J Psychiatry. 2019; 61(7):37-42. [DOI:10.4103/psychiatry.IndianJPsychiatry_518_18] [PMID] [PMCID]
22.van Grootheest DS, Cath DC, Beekman AT, Boomsma DI. Twin studies on obsessive-compulsive disorder: A review. Twin Res Hum Genet. 2005; 8(5):450-8. [DOI:10.1375/twin.8.5.450] [PMID]
23.Costas J, Carrera N, Alonso P, Gurriarán X, Segalàs C, Real E, et al. Exon-focused genome-wide association study of obsessive-compulsive disorder and shared polygenic risk with schizophrenia. Transl Psychiatry. 2016; 6(3):e768. [DOI:10.1038/tp.2016.34] [PMID] [PMCID]
24.Zilhão NR, Smit DJA, den Braber A, Dolan CV, Willemsen G, Boomsma DI, et al. Genetic and environmental contributions to stability in adult obsessive compulsive behavior. Twin Res Hum Genet. 2015; 18(1):52-60. [DOI:10.1017/thg.2014.77] [PMID]
25.Nestadt G, Grados M, Samuels JF. Genetics of obsessive-compulsive disorder. Psychiatr Clin North Am. 2010; 33(1):141-58. [DOI:10.1016/j.psc.2009.11.001] [PMID] [PMCID]
26.Rajendram R, Kronenberg S, Burton CL, Arnold PD. Glutamate genetics in obsessive-compulsive disorder: A review. J Can Acad Child Adolesc Psychiatry. 2017; 26(3):205-13. [PMID] [PMCID]
27.Nicolini H. Serotonin transporter gene polymorphisms & obsessive-compulsive disorder. Indian J Med Res. 2010; 132(6):663-6. [PMID] [PMCID]
28.Walitza S, Marinova Z, Grünblatt E, Lazic SE, Remschmidt H, Vloet TD, et al. Trio study and meta-analysis support the association of genetic variation at the serotonin transporter with early-onset obsessive-compulsive disorder. Neurosci Lett. 2014; 580:100-3. [DOI:10.1016/j.neulet.2014.07.038] [PMID] [PMCID]
29.Mak L, Streiner DL, Steiner M. Is serotonin transporter polymorphism (5-HTTLPR) allele status a predictor for obsessive-compulsive disorder? A meta-analysis. Arch Womens Ment Health. 2015; 18(3):435-45. [DOI:10.1007/s00737-015-0526-z] [PMID]
30.Pooley EC, Fineberg N, Harrison PJ. The met158 allele of catechol-O-methyltransferase (COMT) is associated with obsessive-compulsive disorder in men: Case-control study and meta-analysis. Mol Psychiatry. 2007; 12(6):556-61. [DOI:10.1038/sj.mp.4001951] [PMID]
31.Sampaio AS, Hounie AG, Petribú K, Cappi C, Morais I, Vallada H, et al. COMT and MAO-A polymorphisms and obsessive-compulsive disorder: A family-based association study. PLoS One. 2015; 10(3):e0119592. [DOI:10.1371/journal.pone.0119592] [PMID] [PMCID]
32.Wang Y, Zhang H, Li Y, Wang Z, Fan Q, Yu Sh, et al. BDNF Val66Met polymorphism and plasma levels in Chinese Han population with obsessive-compulsive disorder and generalized anxiety disorder. J Affect Disord. 2015; 186:7-12. [DOI:10.1016/j.jad.2015.07.023] [PMID]
33.Zai G, Zai C, Arnold PD, Freeman N, Burroughs E, Kennedy JL, et al. Meta-analysis and association of Brain-Derived Neurotrophic Factor (BDNF) gene with obsessive-compulsive disorder. Psychiatr Genet. 2015; 25(2):95-6. [DOI:10.1097/YPG.0000000000000077] [PMID]
34.González-Castro TB, Pool-García Sh, Tovilla-Zárate CA, Juárez-Rojop IE, Isela E, López-Narváez ML, et al. Association between BDNF Val66Met polymorphism and generalized anxiety disorder and clinical characteristics in a Mexican population, a case-control study. Medicine. 2019; 98(11):e14838. [DOI:10.1097/MD.0000000000014838] [PMID] [PMCID]
35.Borodinova AA, Salozhin SV. [Diversity of proBDNF and mBDNF functions in the central nervous system (Russian)]. Zh Vyssh Nerv Deiat Im I P Pavlova. 2016; 66(1):3-23. [PMID]
36.Suliman Sh, Hemmings SMJ, Seedat S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: Systematic review and meta-regression analysis. Front Integr Neurosci. 2013; 7:55. [DOI:10.3389/fnint.2013.00055] [PMID] [PMCID]
37.Wang Y, Mathews CA, Li Y, Lin Z, Xiao Z. Brain-Derived Neurotrophic Factor (BDNF) plasma levels in drug-naïve OCD patients are lower than those in healthy people, but are not lower than those in drug-treated OCD patients. J Affect Disord. 2011; 133(1-2):305-10. [DOI:10.1016/j.jad.2011.04.002] [PMID]
38.Taj M J RJ, Ganesh S, Shukla T, Deolankar S, Nadella RK, Sen S, et al. BDNF gene and obsessive compulsive disorder risk, symptom dimensions and treatment response. Asian J Psychiatr. 2018; 38:65-9. [DOI:10.1016/j.ajp.2017.10.014] [PMID]
39.Márquez L, Camarena B, Hernández S, Lóyzaga C, Vargas L, Nicolini H. Association study between BDNF gene variants and Mexican patients with obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2013; 23(11):1600-5. [DOI:10.1016/j.euroneuro.2013.08.001] [PMID]
40.Hemmings SMJ, Kinnear CJ, Van der Merwe L, Lochner Ch, Corfield VA, Moolman-Smook JC, et al. Investigating the role of the Brain-Derived Neurotrophic Factor (BDNF) val66met variant in Obsessive-Compulsive Disorder (OCD). World J Biol Psychiatry. 2008; 9(2):126-34. [DOI:10.1080/15622970701245003] [PMID]
41.Tsai SJ. Critical issues in BDNF Val66Met genetic studies of neuropsychiatric disorders. Front Mol Neurosci. 2018; 11:156. [DOI:10.3389/fnmol.2018.00156] [PMID] [PMCID]
42.Katerberg H, Lochner Ch, Cath DC, de Jonge P, Bochdanovits Z, Moolman‐Smook JC, et al. The role of the Brain‐Derived Neurotrophic Factor (BDNF) val66met variant in the phenotypic expression of Obsessive‐Compulsive Disorder (OCD). Am J Med Genet B Neuropsychiatr Genet. 2009; 150B(8):1050-62. [DOI:10.1002/ajmg.b.30930] [PMID]
43.Tükel R, Gürvit H, Özata B, Öztürk N, Ertekin BA, Ertekin E, et al. Brain‐derived neurotrophic factor gene Val66Met polymorphism and cognitive function in obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2012; 159B(7):850-8. [DOI:10.1002/ajmg.b.32092] [PMID]
44.Abdolhosseinzadeh S, Alizadeh N, Shams J, Asadi S, Ahmadiani A. BDNF association study with obsessive-compulsive disorder, its clinical characteristics, and response to fluvoxamine-treatment in Iranian patients. Exp Clin Psychopharmacol. 2020; 28(2):216-24. [DOI:10.1037/pha0000297] [PMID]
45.First MB, Williams JBW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5 Disorders, Clinical Trials Version (SCID-5-CT). 1st edition. Washington: American Psychiatric Association; 2015.
46.Helzer JE, Robins LN. The diagnostic interview schedule: Its development, evolution, and use. Soc Psychiatry Psychiatr Epidemiol. 1988; 23(1):6-16. [DOI:10.1007/BF01788437] [PMID]
47.Steketee G, Frost RO. Measurement of risk-taking in obsessive-compulsive disorder. Behav Cogn Psychother. 1994; 22(4):287-98. [DOI:10.1017/S1352465800013175]
48.Rajezi Esfahani S, Motaghipour Y, Kamkari K, Zahiredin AR, Janbozorgi M. [Reliability and validity of the Persian version of the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (Persian)]. Iran J Psychiatry Clin Psychol. 2012; 17(4):297-303. http://ijpcp.iums.ac.ir/article-1-1453-en.html
49.Zambaldi CF, Cantilino A, Montenegro AC, Paes JA, de Albuquerque TLC, Sougey EB. Postpartum obsessive-compulsive disorder: Prevalence and clinical characteristics. Compr Psychiatry. 2009; 50(6):503-9. [DOI:10.1016/j.comppsych.2008.11.014] [PMID]
50.Ghassemzadeh H, Mojtabai R, Khamseh A, Ebrahimkhani N, Issazadegan AA, Saif-Nobakht Z. Symptoms of obsessive-compulsive disorder in a sample of Iranian patients. Int J Soc Psychiatry. 2002; 48(1):20-8. [DOI:10.1177/002076402128783055] [PMID]
51.Dotta-Panichi RM, Bins HD, Tramontina JF, Ceresér KM, de Aguiar BW, Paz AC, et al. Serum concentrations of brain-derived neurotrophic factor and mental disorders in imprisoned women. Braz J Psychiatry. 2015; 37(2):113-20. [DOI:10.1590/1516-4446-2014-1421] [PMID]
52.Maina G, Rosso G, Zanardini R, Bogetto F, Gennarelli M, Bocchio-Chiavetto L. Serum levels of brain-derived neurotrophic factor in drug-naïve obsessive-compulsive patients: A case-control study. J Affect Disord. 2010; 122(1-2):174-8. [DOI:10.1016/j.jad.2009.07.009] [PMID]
53.Mueller SC, Aouidad A, Gorodetsky E, Goldman D, Pine DS, Ernst M. Gray matter volume in adolescent anxiety: An impact of the brain-derived neurotrophic factor Val66Met polymorphism? J Am Acad Child Adolesc Psychiatry. 2013; 52(2):184-95. [DOI:10.1016/j.jaac.2012.11.016] [PMID] [PMCID]
54.Hemmings SMJ, Lochner Ch, van der Merwe L, Cath DC, Seedat S, Stein DJ. BDNF Val66Met modifies the risk of childhood trauma on obsessive-compulsive disorder. J Psychiatr Res. 2013; 47(12):1857-63. [DOI:10.1016/j.jpsychires.2013.08.012] [PMID]
55.Carballedo A, Morris D, Zill P, Fahey C, Reinhold E, Meisenzahl E, et al. Brain-derived neurotrophic factor Val66Met polymorphism and early life adversity affect hippocampal volume. Am J Med Genet B Neuropsychiatr Genet. 2013; 162(2):183-90. [DOI:10.1002/ajmg.b.32130] [PMID]
56.Nemoto K, Ohnishi T, Mori T, Moriguchi Y, Hashimoto R, Asada T, et al. The Val66Met polymorphism of the brain derived neurotrophic factor gene affects age-related brain morphology. Neurosci Lett. 2006; 397(1-2):25-9. [DOI:10.1016/j.neulet.2005.11.067] [PMID]
57.Sublette ME, Baca-Garcia E, Parsey RV, Oquendo MA, Rodrigues SM, Galfalvy H, et al. Effect of BDNF val66met polymorphism on age-related amygdala volume changes in healthy subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2008; 32(7):1652-5. [DOI:10.1016/j.pnpbp.2008.06.009] [PMID] [PMCID]
58.Hibar DP, Cheung JW, Medland SE, Mufford MS, Jahanshad N, Dalvie Sh, et al. Significant concordance if genetic variation that increase both the risk for obsessive-compulsive disorder and the volumes of the nucleus accumbens and putamen. Br J Psychiatry. 2018; 213(1):430-6. [DOI:10.1192/bjp.2018.62] [PMID] [PMCID]
59.Hall D, Dhilla A, Charalambous A, Gogos JA, Karayiorgou M. Sequence variants of the Brain-Derived Neurotrophic Factor (BDNF) gene are strongly associated with obsessive-compulsive disorder. Am J Hum Genet. 2003; 73(2):370-6. [DOI:10.1086/377003] [PMID] [PMCID]
60.Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. In: Lewin G, Carter B, editors. Neurotrophic Factors, Handbook of Experimental Pharmacology. Vol. 220. Berlin/Heidelberg: Springer; 2014. pp. 223-50. [DOI:10.1007/978-3-642-45106-5_9] [PMID]
61.Levada OA, Cherednichenko NV. [Brain-Derived Neurotrophic Factor (BDNF): Neurobiology and marker value in neuropsychiatry (Russian)]. Lik Sprava. 2015; (3-4):15-25. [PMID]
Received: 2020/08/25 | Accepted: 2020/06/12 | Published: 2020/06/12
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






