Sat, Apr 27, 2024
Volume 6, Issue 1 (Winter 2020)
Caspian J Neurol Sci 2020, 6(1): 9-15 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Izadi S, Ahmadi M, Poursadeghfard M. Predictors and Conversion Rate of Clinically Isolated Syndrome to Clinically Definite Multiple Sclerosis: A Follow-up Study in Patients Living in the Southern Part of Iran. Caspian J Neurol Sci 2020; 6 (1) :9-15
URL: http://cjns.gums.ac.ir/article-1-302-en.html
URL: http://cjns.gums.ac.ir/article-1-302-en.html
1- Clinical Neurology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
2- Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran
3- Clinical Neurology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran , poursadegh@sums.ac.ir
2- Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran
3- Clinical Neurology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran , poursadegh@sums.ac.ir
Full-Text [PDF 1120 kb]
(826 Downloads)
| Abstract (HTML) (2184 Views)
Full-Text: (736 Views)
Introduction
The Clinically Isolated Syndrome (CIS) is a term to describe the first neurologic episode suggestive of a demyelinating inflammatory disorder of the Central Nervous System (CNS) [1]. Approximately 90% of patients with Multiple Sclerosis (MS) initially present with CIS [2]. The majority of CIS patients develop a monofocal presentation with symptoms and signs, indicating a lesion in the optic pathway, spinal cord, brainstem, cerebellum, or less commonly, the cerebral hemisphere. However, some patients with CIS have clinical evidence of two or more symptoms or signs, affecting two or more locations of CNS (multifocal presentation) [1, 2].
Even though some CIS patients may not develop new symptoms consistent with MS, eventually, a considerable number of CIS patients experience the second clinical attack [3]. In other words, in the long term, a CIS patient can have either no further relapse or convert to Clinically Definite MS (CDMS). Predicting CIS patients who will convert to CDMS or those who will have a monophasic course is an important issue. Studies have shown that gender, ethnicity, the age of the first attack, season, clinical manifestations, and other factors can affect the prognosis of CIS [4, 5]. It has been shown that baseline MRI findings and the age of the onset have the most predictive value in evaluating the risk of CIS conversion to CDMS [6].
The current treatment approach to MS is the initiation of Disease-Modifying Drugs (DMD), including beta-interferon therapy in the early phase of the disease, i.e., when a patient presents with CIS [7]. Many studies have suggested that starting a DMD at the time of a CIS can be beneficial to reduce the rate of development of MS [7, 8]. Information obtained from long-term clinical follow up can be helpful when considering prophylactic treatment at the time of a first clinical presentation.
The clinical course of CIS is variable in each patient, and identifying who will remain as CIS and who will develop MS over the mid- to long-term period is very important. Because ethnic and environmental factors can influence the conversion of CIS to CDMS, regional studies are mandatory.
Many longitudinal studies have been conducted on the clinical course of CIS in western countries, but few studies in Asian countries. Epidemiological and clinical characteristics of MS in Iran, as a vast Asian country, are relatively well recognized. Clinical characteristics are generally similar to western countries. But its prevalence and incidence are heterogeneous in different provinces of Iran [9-11]. Since there was no regional study to assess the CIS patients in the southern part of Iran, we decided to perform this study in Fars Province, in south Iran, one of the largest provinces of Iran with more than 4651718 people and more than 3354 MS patients [11].
The aims of this study were:1) to determine the demographic, clinical and radiologic characteristics of Iranian patients with CIS; 2) to estimate the rate of conversion from CIS to CDMS, and; 3) to investigate variables predicting the conversion of CIS to CDMS in a cohort of Iranian patients.
Materials and Methods
All patients with a diagnosis of the demyelinating disorder in Fars Province were registered by Fars Multiple Sclerosis Society (FMSS) of Shiraz University of Medical Sciences. A total of 143 CIS patients registered to FMSS were enrolled for this study from 2006 until 2012, and all patients were followed for 5 years in a retrospective follow-up study. Conversion from CIS to CDMS was recorded, according to McDonald 2010 criteria [12]. The inclusion criteria were as follows: 1. first episode of neurologic symptom suggestive of inflammatory demyelinating disease without previous history of possible demyelinating events; 2. a clinical syndrome lasting for more than 24 h. Patients with progressive symptoms at the onset, those who did not satisfy the CIS definition, those with incomplete baseline demographic, clinical, or MRI data, or those who had symptoms or signs suggestive of other inflammatory disorders (e.g., Acute Disseminated Encephalomyelitis [ADEM], Neuromyelitis Optica [NMO], or vasculitis) were excluded.
Demographic and clinical data, including gender, age at onset, smoking, types of clinical presentation, blood group, and radiological data (brain and spinal cord lesions) were collected from this database. Based on the age of onset, the patients were divided into two groups: patients aged <30 years and patients aged ≥30 years. The initial clinical presentation was classified into four main categories: optic neuritis, brainstem-cerebellar, spinal cord, and cerebral syndrome.
Brain and spinal cord MRI were performed in all patients using a 1.5-T MR imaging unit (Siemens, Germany). We utilized T2/FLAIR-weighted scans to determine the presence of brain and spinal cord lesions. Two different categories for the number of lesions were considered: fewer than 3 lesions and 3 or more lesions.
The diagnosis of CDMS was established whenever the patient experienced a second clinical neurological event lasting for at least 24 h. The establishment of the second clinical attack was done by a review of the patients’ documents and hospital records of the patients. Additionally, the telephone call interview was done with all patients, and the history of the second clinical attack that lasted more than 24 h was recorded.
At the end of the study, the patients were categorized into two possible outcomes: 1. converted to CDMS, diagnosed according to the second clinical attack not attributable to other diseases; 2. remained as CIS (not converted to CDMS).
Statistical analysis
Statistical analysis was performed in SPSS V. 17 software. The numerical values of the sample are presented by the mean and standard deviation. The significance of the differences between the converter group and the non-converter group was determined using the Chi-squared test. The patient’s age, age at CIS onset, gender, blood group, clinical presentation, and the number of MRI lesions at the baseline were the variables used for the univariate and multivariate analyses to predict CIS conversion to CDMS. P<0.05 was considered statistically significant.
Results
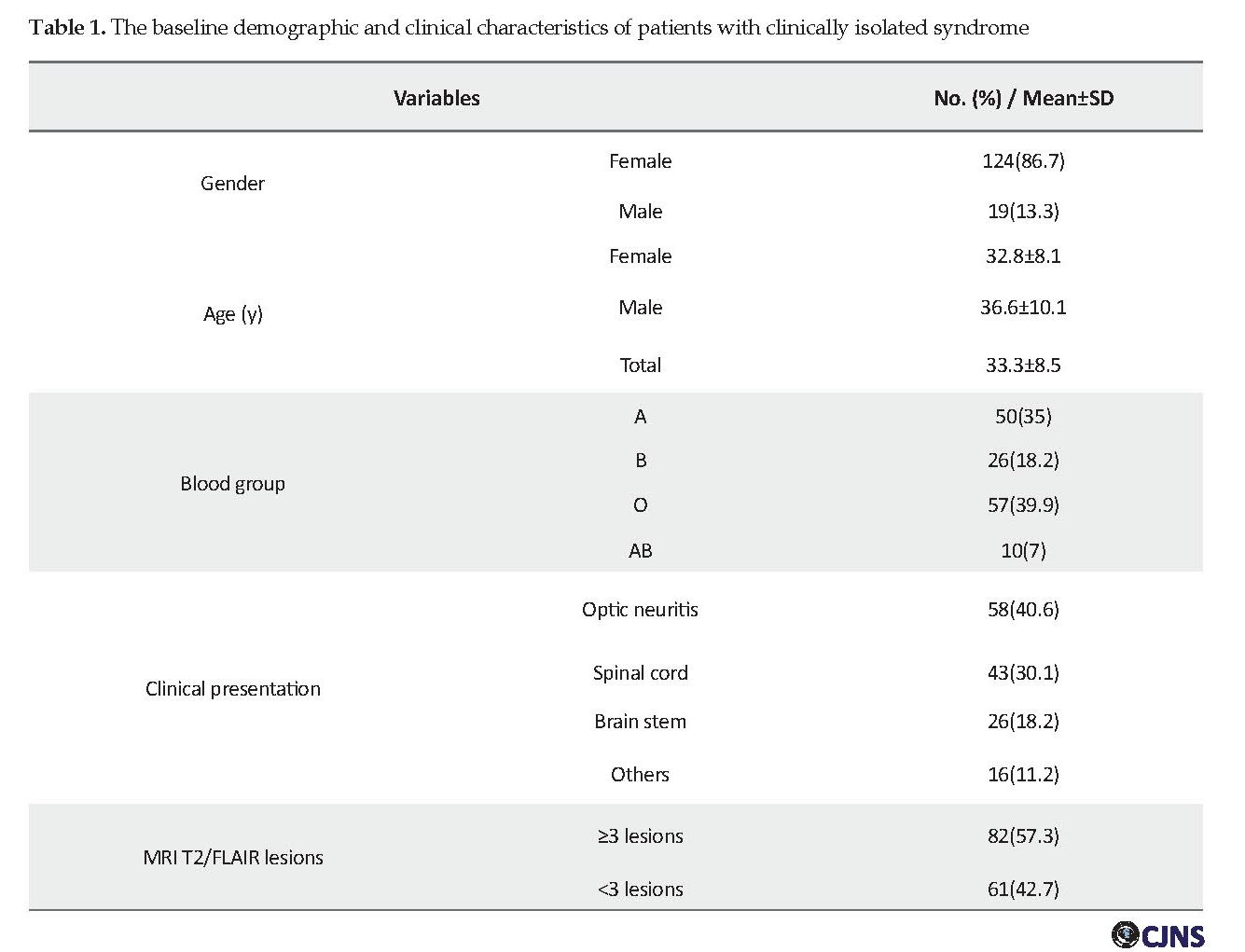
A total of 143 CIS patients were included in the study. The mean time of conversion was 3.4±1.1 years. About 86.7% (124) of patients were female, and 19 (13.3%) were male, and female to male sex ratio was 6.52:1. The Mean±SD age of the patients and the mean age at the onset was 33.3±8.5 years and 28.7±6.2 years, respectively.
The Clinically Isolated Syndrome (CIS) is a term to describe the first neurologic episode suggestive of a demyelinating inflammatory disorder of the Central Nervous System (CNS) [1]. Approximately 90% of patients with Multiple Sclerosis (MS) initially present with CIS [2]. The majority of CIS patients develop a monofocal presentation with symptoms and signs, indicating a lesion in the optic pathway, spinal cord, brainstem, cerebellum, or less commonly, the cerebral hemisphere. However, some patients with CIS have clinical evidence of two or more symptoms or signs, affecting two or more locations of CNS (multifocal presentation) [1, 2].
Even though some CIS patients may not develop new symptoms consistent with MS, eventually, a considerable number of CIS patients experience the second clinical attack [3]. In other words, in the long term, a CIS patient can have either no further relapse or convert to Clinically Definite MS (CDMS). Predicting CIS patients who will convert to CDMS or those who will have a monophasic course is an important issue. Studies have shown that gender, ethnicity, the age of the first attack, season, clinical manifestations, and other factors can affect the prognosis of CIS [4, 5]. It has been shown that baseline MRI findings and the age of the onset have the most predictive value in evaluating the risk of CIS conversion to CDMS [6].
The current treatment approach to MS is the initiation of Disease-Modifying Drugs (DMD), including beta-interferon therapy in the early phase of the disease, i.e., when a patient presents with CIS [7]. Many studies have suggested that starting a DMD at the time of a CIS can be beneficial to reduce the rate of development of MS [7, 8]. Information obtained from long-term clinical follow up can be helpful when considering prophylactic treatment at the time of a first clinical presentation.
The clinical course of CIS is variable in each patient, and identifying who will remain as CIS and who will develop MS over the mid- to long-term period is very important. Because ethnic and environmental factors can influence the conversion of CIS to CDMS, regional studies are mandatory.
Many longitudinal studies have been conducted on the clinical course of CIS in western countries, but few studies in Asian countries. Epidemiological and clinical characteristics of MS in Iran, as a vast Asian country, are relatively well recognized. Clinical characteristics are generally similar to western countries. But its prevalence and incidence are heterogeneous in different provinces of Iran [9-11]. Since there was no regional study to assess the CIS patients in the southern part of Iran, we decided to perform this study in Fars Province, in south Iran, one of the largest provinces of Iran with more than 4651718 people and more than 3354 MS patients [11].
The aims of this study were:1) to determine the demographic, clinical and radiologic characteristics of Iranian patients with CIS; 2) to estimate the rate of conversion from CIS to CDMS, and; 3) to investigate variables predicting the conversion of CIS to CDMS in a cohort of Iranian patients.
Materials and Methods
All patients with a diagnosis of the demyelinating disorder in Fars Province were registered by Fars Multiple Sclerosis Society (FMSS) of Shiraz University of Medical Sciences. A total of 143 CIS patients registered to FMSS were enrolled for this study from 2006 until 2012, and all patients were followed for 5 years in a retrospective follow-up study. Conversion from CIS to CDMS was recorded, according to McDonald 2010 criteria [12]. The inclusion criteria were as follows: 1. first episode of neurologic symptom suggestive of inflammatory demyelinating disease without previous history of possible demyelinating events; 2. a clinical syndrome lasting for more than 24 h. Patients with progressive symptoms at the onset, those who did not satisfy the CIS definition, those with incomplete baseline demographic, clinical, or MRI data, or those who had symptoms or signs suggestive of other inflammatory disorders (e.g., Acute Disseminated Encephalomyelitis [ADEM], Neuromyelitis Optica [NMO], or vasculitis) were excluded.
Demographic and clinical data, including gender, age at onset, smoking, types of clinical presentation, blood group, and radiological data (brain and spinal cord lesions) were collected from this database. Based on the age of onset, the patients were divided into two groups: patients aged <30 years and patients aged ≥30 years. The initial clinical presentation was classified into four main categories: optic neuritis, brainstem-cerebellar, spinal cord, and cerebral syndrome.
Brain and spinal cord MRI were performed in all patients using a 1.5-T MR imaging unit (Siemens, Germany). We utilized T2/FLAIR-weighted scans to determine the presence of brain and spinal cord lesions. Two different categories for the number of lesions were considered: fewer than 3 lesions and 3 or more lesions.
The diagnosis of CDMS was established whenever the patient experienced a second clinical neurological event lasting for at least 24 h. The establishment of the second clinical attack was done by a review of the patients’ documents and hospital records of the patients. Additionally, the telephone call interview was done with all patients, and the history of the second clinical attack that lasted more than 24 h was recorded.
At the end of the study, the patients were categorized into two possible outcomes: 1. converted to CDMS, diagnosed according to the second clinical attack not attributable to other diseases; 2. remained as CIS (not converted to CDMS).
Statistical analysis
Statistical analysis was performed in SPSS V. 17 software. The numerical values of the sample are presented by the mean and standard deviation. The significance of the differences between the converter group and the non-converter group was determined using the Chi-squared test. The patient’s age, age at CIS onset, gender, blood group, clinical presentation, and the number of MRI lesions at the baseline were the variables used for the univariate and multivariate analyses to predict CIS conversion to CDMS. P<0.05 was considered statistically significant.
Results
A total of 143 CIS patients were included in the study. The mean time of conversion was 3.4±1.1 years. About 86.7% (124) of patients were female, and 19 (13.3%) were male, and female to male sex ratio was 6.52:1. The Mean±SD age of the patients and the mean age at the onset was 33.3±8.5 years and 28.7±6.2 years, respectively.
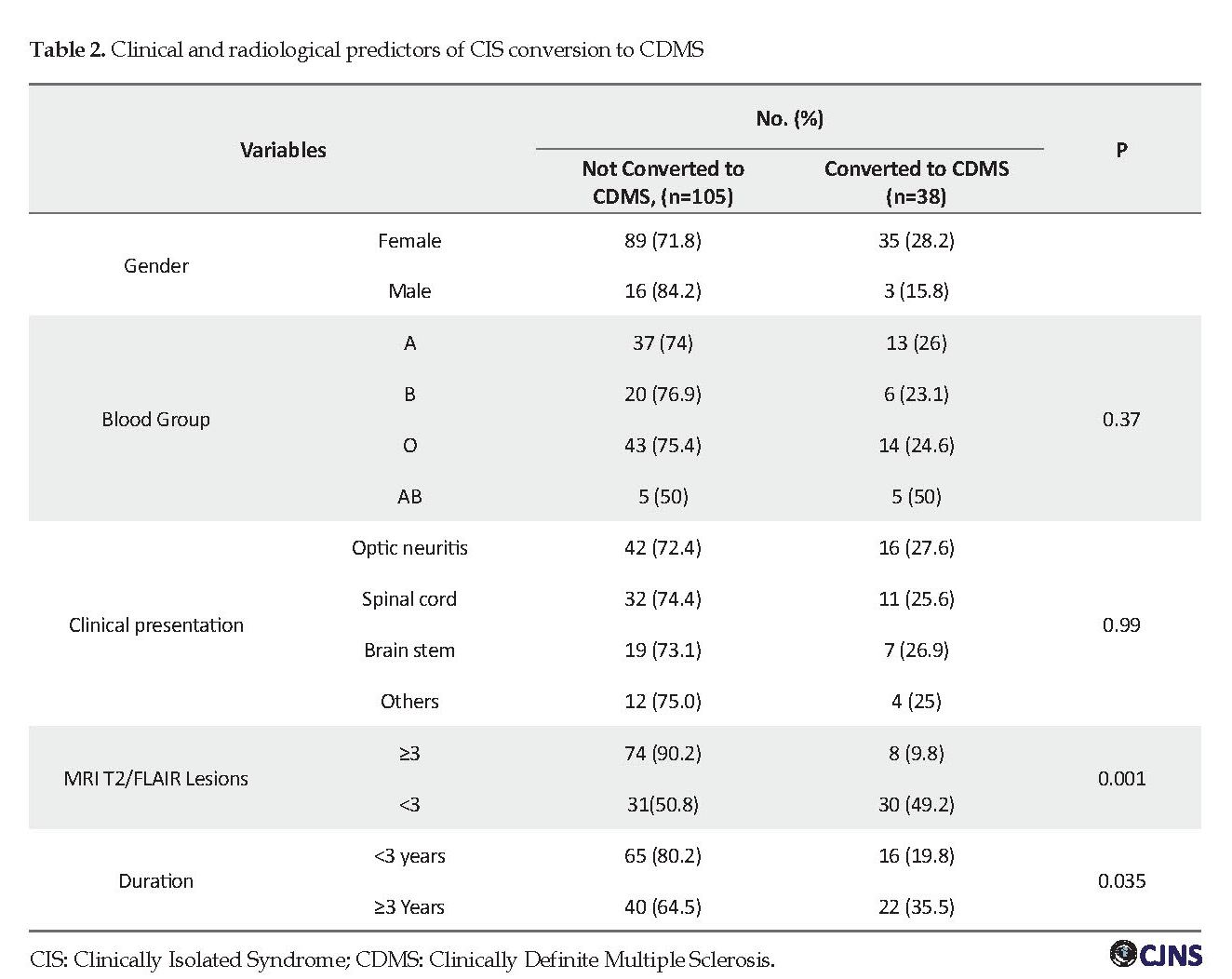
The initial clinical presentation of CIS was optic neuritis (ON) in 58 patients (40.6%), spinal cord syndrome in 43 (30.1%), and a cerebellar or brainstem syndrome in 26 (18.2%). Also, 16 patients (11.2%) presented with other clinical manifestations, including cerebral and multifocal onset symptoms. The demographic and clinical features of CIS patients are presented in Table 1. Thirty-eight patients (26.6%) developed the second clinical attack and converted to CDMS, and the remaining 105 patients had no further attacks until the end of the study. Using a univariate and multivariable Cox regression model, each variable was tested as a predictor of conversion from CIS to CDMS (Table 2).
With respect to baseline T2/FLAIR MRI lesions, 57.3% of patients had fewer than 3 lesions, while 42.7% of patients had 3 or more lesions (Table 1). The conversion rate to CDMS was 49.2% (30/61) for patients who had ≥3 lesions at the onset of CIS, but it was only 9.8% (8/72) in patients who had 3< lesions (Table 2). The risk of conversion was significantly higher in patients with ≥3 T2 MRI lesions (OR=8.95, 95% CI=3.69-21.7, P<0.001).
Female patients had higher conversion rate than males, but it was not statistically significant (OR=2.09, 95% CI=0.57-7.64, P=0.26).
Individuals with blood group O were at higher risk, but it was not statistically significant (0R=3.07, 95% CI=0.77-12.1, P=0.26). It should be mentioned that only a small number of patients had this blood group. No association was seen between the risk of conversion and other blood groups.
Interestingly, only 4% of our patients were smoker; therefore, it was not possible to assess the relationship between smoking and conversion to CDMS.
The conversion rate was 27.6% (16/58) in CIS patients with ON, and 25.6% (11/43) in patients with spinal cord presentation. The rate of conversion of CIS to CDMS was not significant when the initial presentations were assessed (Table 2). The mean age at the onset was lower in CIS patients who converted to CDMS (27.6 vs. 29.4 years), but it was not statistically significant (P=0.23).
Discussion
CIS characteristics
The most frequent initial clinical presentation of our patients was ON (40.6%) followed by spinal cord and brain stem presentation (30.1% and 18.2%, respectively). The type of clinical presentation of CIS is not similar in different geographical areas. In western studies, spinal cord syndromes account for 20%-30% of CIS presentations. For example, in the CHAMPS Study Group, 22% of patients presented with spinal cord CIS [13]. In a study in China, this figure reached 57% [14]. Wallace and his colleagues in a review article reported that partial spinal cord syndrome as the most common CIS syndrome, and ON is the first presentation of CIS in about 20% of patients, and brainstem syndromes account for about 25% of cases of CIS [15].
The gender ratio in our cohort (F: M ratio 6.52:1) is significantly higher than worldwide figures [16]. In the previous studies from Iran, the reported F:M ratio of MS patients was also significantly higher than worldwide figures [9, 10]. Etemadifar et al. have suggested that low sun exposure and its associated vitamin D deficiency, as a contributing factor in the prevalence of MS and CIS, is significantly higher among Iranian females than males [17].
Conversion rate
In our study, 26.6% of our CIS patients developed a definite second clinical attack and converted to CDMS within the study period. Even in a long-term follow-up, a considerable proportion of patients will not convert to CDMS. The reported rate of conversion to CDMS varies in different studies in western and eastern countries. Depending on the clinical and radiological baseline characteristics and also, more importantly, the duration of follow up, the risk of developing CDMS is estimated to be 20%-85% [4, 15]. The Mean±SD time of conversion in our study was 3.4±1.1 years, with 5 years of supervision for each patient. In one study in China, the conversion rate was 24% in a 3-year follow up [14]. In another study, the reported 2-year conversion rate was 28% [17]. Kuhle et al. in a large multicenter study in 17 western countries, reported that at a median follow-up of 4.31 years, 59.5% CIS of cases converted to CDMS [5]. These differences in conversion rates might be explained to some extent by the duration of follow up and type and method of study. Also, geographical and racial variations may be the contributing factors.
Predictors of conversion
One of the essential questions in the evaluation of patients with CIS is to determine the predictors of further clinical attack and conversion to CDMS. Several predictors of conversion of CIS to CDMS were identified in observational studies and also in clinical trial studies.
It is said that initial clinical findings could be used in MS patients to predict the prognosis, and also it may be a predictive factor for the conversion of CIS to CDMS. In patients with spinal cord CIS and brainstem syndromes, conversion rate to CDMS has been reported to vary between 41%-61% and 53%-60%, respectively [18, 19]. In our study, the conversion rate was 25.6% for spinal CIS and 26.9% for CIS patients with brainstem syndrome.
In the study of Miller and his colleagues, ON was associated with a lower risk of conversion to CDMS than other types of CIS [2]. In the optic neuritis treatment trial, the conversion rate to CDMS was 30% following the first episode of ON in five years [20]. But, our study and some previous studies did not find a significant relationship between initial clinical presentation and conversion to CDMS [17, 21-23].
The effect of gender as a predictor of conversion is conflicting and unclear. In a meta-analysis study, the risk of conversion of CIS to CDMS was higher in women, but it was not statistically significant. In this study, the relative risk of females developing CDMS following CIS was 1.2 [21]. In our study, 28.5% of the females and 15.8% of the males converted to CDMS, but it was not statistically significant (P=0.25).
In the Iranian population, due to cultural and religious factors, the number of female smokers is very low (less than 5%). Therefore, due to the small number of this subgroup, we cannot study the effect of smoking on the conversion rate of CIS. Di Pauli et al. reported that the risk of CDMS is higher among patients with CIS who smoke compared with non-smokers [24].
Even though our results revealed that the younger age of onset (<30 years) is associated with a higher risk of developing a second attack, it was not statistically significant. But, in another study, the age of the onset of CIS was a significant and independent predictor of conversion of CIS to CDMS [17].
It was mentioned that the number of MRI lesions at baseline was a strong predictor of conversion of CIS to CDMS. Our result revealed that conversion rate in patients with 3 or more baseline T2/FLAIR lesions was 49.2% versus only 9.8% in patients with less than 3 lesions (OR=8.95, 95% CI=3.69–21.7, P<0.001). This finding is inconsistent with those of other studies [4, 5]. As seen in our research, some other studies reported that up to half of CIS patients with an abnormal baseline MRI would not have developed further clinical attacks for several years [4, 6].
The distribution of the ABO blood groups was similar in our CIS patients with previous studies, which reported the frequency of ABO blood group in MS population (O: 45.9% and A: 37.7%) [25]. In our study, there was no significant difference between blood groups in CIS patients who converted and those who did not convert to CDMS. We could not find any study about blood group and risk of conversion to MS in CIS patients.
Conclusion
In our patients with CIS, 26.6% converted to CDMS after 5 years follow-up and a mean time conversion of 3.4 years. The conversion rate in this Iranian population was similar to that of western countries. The most important risk factors for conversion to CDMS were the number of baseline MRI lesions and disease duration.
Ethical Considerations
Compliance with ethical guidelines
All the study procedures were done in compliance with the ethical guidelines of the Declaration of Helsinki 2013.
Funding
The present article was extracted from the thesis written by Mysam Ahmadi and was financially supported by Shiraz University of Medical Sciences (Grants No:10759).
Authors contributions
Conceptualization: Sadegh Izadi, Maryam Poursadeghfard; Methodology, investigation and writing – original draft: Meysam Ahmadi; Funding acquisition: Sadegh Izadi; Writing – review & editing, resources and supervision: Maryam Poursadeghfard
Conflict of interest
The authors declared no conflict of interest.
Acknowlegements
The authors would like to thank Shiraz University of Medical Sciences, Shiraz, Iran, and also Center for Development of Clinical Research of Nemazee Hospital and Dr. Nasrin Shokrpour for editorial assistance.
References
Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol. 2012; 11:157-69. [DOI:10.1016/S1474-4422(11)70274-5]
Miller D, Barkhof F, Montalban X, Thompson A, Filippi M. Clinically isolated syndromes suggestive of multiple sclerosis, part I: Natural history, pathogenesis, diagnosis and prognosis. Lancet Neurol. 2005; 4(5):281-8. [DOI:10.1016/S1474-4422(05)70071-5]
Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis. Mult Scler. 2003; 9(3):260-74. [DOI:10.1191/1352458503ms914oa] [PMID]
Arrambide G, Sastre-Garriga J. Predictive markers of disease evolution after a CIS in every day practice. J Neurol Sci. 2014; 343(1-2):8-14. [DOI:10.1016/j.jns.2014.05.023] [PMID]
Kuhle J, Disanto G, Dobson R, Adiutori R, Bianchi L, Topping J, et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult Scler J. 2015; 21(8):1013-24. [DOI:10.1177/1352458514568827] [PMID]
Tintoré M, Rovira A, Río J, Nos C, Grivé E, Téllez N, et al. Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurol. 2006; 67(6):968-72. [DOI:10.1212/01.wnl.0000237354.10144.ec] [PMID]
O’Connor P, Kinkel RP, Kremenchutzky M. Efficacy of intramuscular interferon beta-1a in patients with clinically isolated syndrome: Analysis of subgroups based on new risk criteria. Mult Scler J. 2009; 15(6):728-34. [DOI:10.1177/1352458509103173] [PMID]
Comi G, Filippi M, Barkhof F, Durelli L, Edan G, Fernández O, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis. Lancet. 2001; 357(9268):1576-82. [DOI:10.1016/S0140-6736(00)04725-5]
Etemadifar M, Izadi S, Nikseresht A, Sharifian M, Sahraian MA, Nasr Z. Estimated prevalence and incidence of multiple sclerosis in Iran. Eur Neurol. 2015; 72(5-6):370-4. [DOI:10.1159/000365846] [PMID]
Izadi S, Nikseresht A, Sharifian M, Saharian MA, Hamidian-Jahromi A, Aghighi M, et al. [Significant increase in the prevalence of multiple sclerosis in Iran in 2011 (Persian)]. Iran J Med Sci. 2014; 39(2):152-3.
Izadi S, Nikseresht AR, Poursadeghfard M, Borhanihaghighi, A, Heydari, T. [Prevalence and incidence of multiple sclerosis in Fars Province, Southern Iran (Persian)]. Iran J Med Sci. 2015; 40(5):390-5.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann of Neurol. 2011; 69(2):292-302. [DOI:10.1002/ana.22366] [PMID] [PMCID]
Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. Eng J Med. 2000; 343(13):898-904. [DOI:10.1056/NEJM200009283431301] [PMID]
Liu Y, Duan Y, Yu C, Qin W, Chen H, Dong H, et al. Clinical isolated syndrome: A 3-year follow-up study in China. Clin Neurol Neurosurg. 2011; 113(8):658-60. [DOI:10.1016/j.clineuro.2011.05.013] [PMID]
Brownlee WJ, Miller DH. Clinically isolated syndromes and the relationship to multiple sclerosis. J Clin Neurosci. 2014; 21(12):2065-71 [DOI:10.1016/j.jocn.2014.02.026] [PMID]
Etemadifar M, Janghorbani M, Shaygannejad V, Ashtari F. Prevalence of multiple sclerosis in Isfahan, Iran. Neuroepidemiology. 2006; 27(1):39-44. [DOI:10.1159/000094235] [PMID]
Mowry EM, Pesic M, Grimes B, Deen SR, Bacchetti P, Waubant E. Clinical predictors of early second event in patients with clinically isolated syndrome. J Neurol. 2009; 256(7):1061-6. [DOI:10.1007/s00415-009-5063-0] [PMID] [PMCID]
Poser S, Bauer HJ, Poser W. Prognosis of multiple sclerosis. Results from an epidemiological area in Germany. Acta Neurol Scand. 1982; 65(4): 347-54. [DOI:10.1111/j.1600-0404.1982.tb03091.x] [PMID]
Young J, Quinn S, Hurrell M, Taylor B. Clinically isolated acute transverse myelitis: Prognostic features and incidence. Mult Scler J. 2009; 15(11):1295-302. [DOI:10.1177/1352458509345906] [PMID]
The optic neuritis study group. Multiple sclerosis risk after optic neuritis: Final optic neuritis treatment trial follow-up. Arch Neurol. 2008; 65(6): 727-32. [DOI:10.1001/archneur.65.6.727]
Dobson R, Ramagopalan S, Giovannoni G. The effect of gender in Clinically Isolated Syndrome (CIS): A meta-analysis. Mult Scler J. 2012; 18(5):600-4. [DOI:10.1177/1352458511426740] [PMID]
Tintoré M, Rovira A, Rio J, Nos C, Grivé E, Téllez N, et al. Is optic neuritis more benign than other first attacks in multiple sclerosis? Ann Neurol. 2005; 57(2):210-5. [DOI:10.1002/ana.20363] [PMID]
Polman C, Kappos L, Freedman MS, Edan G, Hartung HP, Miller DH, et al. Subgroups of the BENEFIT study: Risk of developing MS and treatment effect of interferon beta-1b. J Neurol. 2008; 255(4):480-7. [DOI:10.1007/s00415-007-0733-2] [PMID]
Di Pauli F, Reind M, Ehling R, Schautzer F, Gneiss C, Lutterotti A, et al. Smoking is a risk factor for early conversion to clinically definite multiple sclerosis. Mult Scler J. 2008; 14(8):1026-30. [DOI:10.1177/1352458508093679] [PMID]
Warner HB, Merz GS, Carp RI. Blood group frequencies in multiple sclerosis populations in the United States. Neurol. 1980; 30(6):671-3. [DOI:10.1212/WNL.30.6.671] [PMID]
Female patients had higher conversion rate than males, but it was not statistically significant (OR=2.09, 95% CI=0.57-7.64, P=0.26).
Individuals with blood group O were at higher risk, but it was not statistically significant (0R=3.07, 95% CI=0.77-12.1, P=0.26). It should be mentioned that only a small number of patients had this blood group. No association was seen between the risk of conversion and other blood groups.
Interestingly, only 4% of our patients were smoker; therefore, it was not possible to assess the relationship between smoking and conversion to CDMS.
The conversion rate was 27.6% (16/58) in CIS patients with ON, and 25.6% (11/43) in patients with spinal cord presentation. The rate of conversion of CIS to CDMS was not significant when the initial presentations were assessed (Table 2). The mean age at the onset was lower in CIS patients who converted to CDMS (27.6 vs. 29.4 years), but it was not statistically significant (P=0.23).
Discussion
CIS characteristics
The most frequent initial clinical presentation of our patients was ON (40.6%) followed by spinal cord and brain stem presentation (30.1% and 18.2%, respectively). The type of clinical presentation of CIS is not similar in different geographical areas. In western studies, spinal cord syndromes account for 20%-30% of CIS presentations. For example, in the CHAMPS Study Group, 22% of patients presented with spinal cord CIS [13]. In a study in China, this figure reached 57% [14]. Wallace and his colleagues in a review article reported that partial spinal cord syndrome as the most common CIS syndrome, and ON is the first presentation of CIS in about 20% of patients, and brainstem syndromes account for about 25% of cases of CIS [15].
The gender ratio in our cohort (F: M ratio 6.52:1) is significantly higher than worldwide figures [16]. In the previous studies from Iran, the reported F:M ratio of MS patients was also significantly higher than worldwide figures [9, 10]. Etemadifar et al. have suggested that low sun exposure and its associated vitamin D deficiency, as a contributing factor in the prevalence of MS and CIS, is significantly higher among Iranian females than males [17].
Conversion rate
In our study, 26.6% of our CIS patients developed a definite second clinical attack and converted to CDMS within the study period. Even in a long-term follow-up, a considerable proportion of patients will not convert to CDMS. The reported rate of conversion to CDMS varies in different studies in western and eastern countries. Depending on the clinical and radiological baseline characteristics and also, more importantly, the duration of follow up, the risk of developing CDMS is estimated to be 20%-85% [4, 15]. The Mean±SD time of conversion in our study was 3.4±1.1 years, with 5 years of supervision for each patient. In one study in China, the conversion rate was 24% in a 3-year follow up [14]. In another study, the reported 2-year conversion rate was 28% [17]. Kuhle et al. in a large multicenter study in 17 western countries, reported that at a median follow-up of 4.31 years, 59.5% CIS of cases converted to CDMS [5]. These differences in conversion rates might be explained to some extent by the duration of follow up and type and method of study. Also, geographical and racial variations may be the contributing factors.
Predictors of conversion
One of the essential questions in the evaluation of patients with CIS is to determine the predictors of further clinical attack and conversion to CDMS. Several predictors of conversion of CIS to CDMS were identified in observational studies and also in clinical trial studies.
It is said that initial clinical findings could be used in MS patients to predict the prognosis, and also it may be a predictive factor for the conversion of CIS to CDMS. In patients with spinal cord CIS and brainstem syndromes, conversion rate to CDMS has been reported to vary between 41%-61% and 53%-60%, respectively [18, 19]. In our study, the conversion rate was 25.6% for spinal CIS and 26.9% for CIS patients with brainstem syndrome.
In the study of Miller and his colleagues, ON was associated with a lower risk of conversion to CDMS than other types of CIS [2]. In the optic neuritis treatment trial, the conversion rate to CDMS was 30% following the first episode of ON in five years [20]. But, our study and some previous studies did not find a significant relationship between initial clinical presentation and conversion to CDMS [17, 21-23].
The effect of gender as a predictor of conversion is conflicting and unclear. In a meta-analysis study, the risk of conversion of CIS to CDMS was higher in women, but it was not statistically significant. In this study, the relative risk of females developing CDMS following CIS was 1.2 [21]. In our study, 28.5% of the females and 15.8% of the males converted to CDMS, but it was not statistically significant (P=0.25).
In the Iranian population, due to cultural and religious factors, the number of female smokers is very low (less than 5%). Therefore, due to the small number of this subgroup, we cannot study the effect of smoking on the conversion rate of CIS. Di Pauli et al. reported that the risk of CDMS is higher among patients with CIS who smoke compared with non-smokers [24].
Even though our results revealed that the younger age of onset (<30 years) is associated with a higher risk of developing a second attack, it was not statistically significant. But, in another study, the age of the onset of CIS was a significant and independent predictor of conversion of CIS to CDMS [17].
It was mentioned that the number of MRI lesions at baseline was a strong predictor of conversion of CIS to CDMS. Our result revealed that conversion rate in patients with 3 or more baseline T2/FLAIR lesions was 49.2% versus only 9.8% in patients with less than 3 lesions (OR=8.95, 95% CI=3.69–21.7, P<0.001). This finding is inconsistent with those of other studies [4, 5]. As seen in our research, some other studies reported that up to half of CIS patients with an abnormal baseline MRI would not have developed further clinical attacks for several years [4, 6].
The distribution of the ABO blood groups was similar in our CIS patients with previous studies, which reported the frequency of ABO blood group in MS population (O: 45.9% and A: 37.7%) [25]. In our study, there was no significant difference between blood groups in CIS patients who converted and those who did not convert to CDMS. We could not find any study about blood group and risk of conversion to MS in CIS patients.
Conclusion
In our patients with CIS, 26.6% converted to CDMS after 5 years follow-up and a mean time conversion of 3.4 years. The conversion rate in this Iranian population was similar to that of western countries. The most important risk factors for conversion to CDMS were the number of baseline MRI lesions and disease duration.
Ethical Considerations
Compliance with ethical guidelines
All the study procedures were done in compliance with the ethical guidelines of the Declaration of Helsinki 2013.
Funding
The present article was extracted from the thesis written by Mysam Ahmadi and was financially supported by Shiraz University of Medical Sciences (Grants No:10759).
Authors contributions
Conceptualization: Sadegh Izadi, Maryam Poursadeghfard; Methodology, investigation and writing – original draft: Meysam Ahmadi; Funding acquisition: Sadegh Izadi; Writing – review & editing, resources and supervision: Maryam Poursadeghfard
Conflict of interest
The authors declared no conflict of interest.
Acknowlegements
The authors would like to thank Shiraz University of Medical Sciences, Shiraz, Iran, and also Center for Development of Clinical Research of Nemazee Hospital and Dr. Nasrin Shokrpour for editorial assistance.
References
Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol. 2012; 11:157-69. [DOI:10.1016/S1474-4422(11)70274-5]
Miller D, Barkhof F, Montalban X, Thompson A, Filippi M. Clinically isolated syndromes suggestive of multiple sclerosis, part I: Natural history, pathogenesis, diagnosis and prognosis. Lancet Neurol. 2005; 4(5):281-8. [DOI:10.1016/S1474-4422(05)70071-5]
Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis. Mult Scler. 2003; 9(3):260-74. [DOI:10.1191/1352458503ms914oa] [PMID]
Arrambide G, Sastre-Garriga J. Predictive markers of disease evolution after a CIS in every day practice. J Neurol Sci. 2014; 343(1-2):8-14. [DOI:10.1016/j.jns.2014.05.023] [PMID]
Kuhle J, Disanto G, Dobson R, Adiutori R, Bianchi L, Topping J, et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult Scler J. 2015; 21(8):1013-24. [DOI:10.1177/1352458514568827] [PMID]
Tintoré M, Rovira A, Río J, Nos C, Grivé E, Téllez N, et al. Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurol. 2006; 67(6):968-72. [DOI:10.1212/01.wnl.0000237354.10144.ec] [PMID]
O’Connor P, Kinkel RP, Kremenchutzky M. Efficacy of intramuscular interferon beta-1a in patients with clinically isolated syndrome: Analysis of subgroups based on new risk criteria. Mult Scler J. 2009; 15(6):728-34. [DOI:10.1177/1352458509103173] [PMID]
Comi G, Filippi M, Barkhof F, Durelli L, Edan G, Fernández O, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis. Lancet. 2001; 357(9268):1576-82. [DOI:10.1016/S0140-6736(00)04725-5]
Etemadifar M, Izadi S, Nikseresht A, Sharifian M, Sahraian MA, Nasr Z. Estimated prevalence and incidence of multiple sclerosis in Iran. Eur Neurol. 2015; 72(5-6):370-4. [DOI:10.1159/000365846] [PMID]
Izadi S, Nikseresht A, Sharifian M, Saharian MA, Hamidian-Jahromi A, Aghighi M, et al. [Significant increase in the prevalence of multiple sclerosis in Iran in 2011 (Persian)]. Iran J Med Sci. 2014; 39(2):152-3.
Izadi S, Nikseresht AR, Poursadeghfard M, Borhanihaghighi, A, Heydari, T. [Prevalence and incidence of multiple sclerosis in Fars Province, Southern Iran (Persian)]. Iran J Med Sci. 2015; 40(5):390-5.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann of Neurol. 2011; 69(2):292-302. [DOI:10.1002/ana.22366] [PMID] [PMCID]
Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. Eng J Med. 2000; 343(13):898-904. [DOI:10.1056/NEJM200009283431301] [PMID]
Liu Y, Duan Y, Yu C, Qin W, Chen H, Dong H, et al. Clinical isolated syndrome: A 3-year follow-up study in China. Clin Neurol Neurosurg. 2011; 113(8):658-60. [DOI:10.1016/j.clineuro.2011.05.013] [PMID]
Brownlee WJ, Miller DH. Clinically isolated syndromes and the relationship to multiple sclerosis. J Clin Neurosci. 2014; 21(12):2065-71 [DOI:10.1016/j.jocn.2014.02.026] [PMID]
Etemadifar M, Janghorbani M, Shaygannejad V, Ashtari F. Prevalence of multiple sclerosis in Isfahan, Iran. Neuroepidemiology. 2006; 27(1):39-44. [DOI:10.1159/000094235] [PMID]
Mowry EM, Pesic M, Grimes B, Deen SR, Bacchetti P, Waubant E. Clinical predictors of early second event in patients with clinically isolated syndrome. J Neurol. 2009; 256(7):1061-6. [DOI:10.1007/s00415-009-5063-0] [PMID] [PMCID]
Poser S, Bauer HJ, Poser W. Prognosis of multiple sclerosis. Results from an epidemiological area in Germany. Acta Neurol Scand. 1982; 65(4): 347-54. [DOI:10.1111/j.1600-0404.1982.tb03091.x] [PMID]
Young J, Quinn S, Hurrell M, Taylor B. Clinically isolated acute transverse myelitis: Prognostic features and incidence. Mult Scler J. 2009; 15(11):1295-302. [DOI:10.1177/1352458509345906] [PMID]
The optic neuritis study group. Multiple sclerosis risk after optic neuritis: Final optic neuritis treatment trial follow-up. Arch Neurol. 2008; 65(6): 727-32. [DOI:10.1001/archneur.65.6.727]
Dobson R, Ramagopalan S, Giovannoni G. The effect of gender in Clinically Isolated Syndrome (CIS): A meta-analysis. Mult Scler J. 2012; 18(5):600-4. [DOI:10.1177/1352458511426740] [PMID]
Tintoré M, Rovira A, Rio J, Nos C, Grivé E, Téllez N, et al. Is optic neuritis more benign than other first attacks in multiple sclerosis? Ann Neurol. 2005; 57(2):210-5. [DOI:10.1002/ana.20363] [PMID]
Polman C, Kappos L, Freedman MS, Edan G, Hartung HP, Miller DH, et al. Subgroups of the BENEFIT study: Risk of developing MS and treatment effect of interferon beta-1b. J Neurol. 2008; 255(4):480-7. [DOI:10.1007/s00415-007-0733-2] [PMID]
Di Pauli F, Reind M, Ehling R, Schautzer F, Gneiss C, Lutterotti A, et al. Smoking is a risk factor for early conversion to clinically definite multiple sclerosis. Mult Scler J. 2008; 14(8):1026-30. [DOI:10.1177/1352458508093679] [PMID]
Warner HB, Merz GS, Carp RI. Blood group frequencies in multiple sclerosis populations in the United States. Neurol. 1980; 30(6):671-3. [DOI:10.1212/WNL.30.6.671] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2020/05/23 | Accepted: 2020/05/23 | Published: 2020/05/23
Received: 2020/05/23 | Accepted: 2020/05/23 | Published: 2020/05/23
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |