Sat, Apr 27, 2024
Volume 5, Issue 1 (Winter 2019)
Caspian J Neurol Sci 2019, 5(1): 1-6 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jazini M, Roghanian R, Mirmosayyeb O, Shaygannejad V, Zarkesh Esfahani S H. Assessment the Possible Association Between Neuromyelitis Optica and Cytomegalovirus as a Provocative Factor. Caspian J Neurol Sci 2019; 5 (1) :1-6
URL: http://cjns.gums.ac.ir/article-1-249-en.html
URL: http://cjns.gums.ac.ir/article-1-249-en.html
Mahboubeh Jazini1 

 , Rasoul Roghanian *
, Rasoul Roghanian * 

 2, Omid Mirmosayyeb3
2, Omid Mirmosayyeb3 

 , Vahid Shaygannejad3
, Vahid Shaygannejad3 

 , Sayyed Hamid Zarkesh Esfahani1
, Sayyed Hamid Zarkesh Esfahani1 




 , Rasoul Roghanian *
, Rasoul Roghanian * 

 2, Omid Mirmosayyeb3
2, Omid Mirmosayyeb3 

 , Vahid Shaygannejad3
, Vahid Shaygannejad3 

 , Sayyed Hamid Zarkesh Esfahani1
, Sayyed Hamid Zarkesh Esfahani1 


1- Department of Biology, Faculty of Sciences, University of Isfahan, Isfahan, Iran
2- Department of Biology, Faculty of Sciences, University of Isfahan, Isfahan, Iran , r.roghanian@sci.ui.ac.ir
3- Isfahan Neuroscience Research Center, Al-Zahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran
2- Department of Biology, Faculty of Sciences, University of Isfahan, Isfahan, Iran , r.roghanian@sci.ui.ac.ir
3- Isfahan Neuroscience Research Center, Al-Zahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran
Full-Text [PDF 1251 kb]
(735 Downloads)
| Abstract (HTML) (2426 Views)
Discussion
Since infectious agents, such as viruses, can contribute to the development of neurological diseases and the relatively high prevalence of autoimmune diseases, a proper understanding of the relationship between viral infection and NMO disease is important in its treatment and prevention. The purpose of this study was to investigate the possible relationship between CMV viral infection and the NMO disease. For the first time in Iran, this study examined the relationship between CMV and NMO disease by molecular methods and through real-time PCR technique.
Full-Text: (569 Views)
Introduction
Neuromyelitis Optica (NMO), a combination of myelitis and optic neuritis, is a demyelinating autoimmune disease [1-3]. The disease was first introduced by Eugene Devic in 1894. NMO is similar to Multiple Sclerosis (MS) with respect to some clinical symptoms. That is why physicians first classified NMO as a type of MS. However in 2004, specific antibodies against aquaporin-4 were discovered in the serum of NMO patients, which could distinguish between the two diseases. This antibody, known as IgG antibody against Aquaporin-4 (AQP4-IgG), plays a key role in the pathogenesis of this disease and is capable of detecting the extracellular epithelium of the water channel of astrocyte cells called aquaporin-4 which attack the epithelium and leading to its destruction [4, 5, 6].
In fact, the main cause of anti-aquaporin antibodies damage is the inflammatory changes of the cerebrospinal fluid barrier. Therefore, viral and bacterial infections can be considered as environmental factors in the development of inflammation in the white and gray matter of the brain. After the inflammation, anti-aquaporin-4 antibodies from the cerebrospinal barrier damage the tissues rich in aquaporin-4 and increases the presentation of autoantigen and excessive activation of T and B cells [7, 8].
According to Koga et al. study on the cause of the disease, out of 24 infectious agents studied, mumps and herpes viruses were more associated with this disease. In 2007, a case report was presented by Tran et al. in which a man was involved by Neuromyelitis Optica following Cytomegalovirus (CMV) primo-infection [9, 10].
This study aimed to investigate the relationship between CMV infection and NMO disease by the molecular method and real-time PCR testing. Considering the sophisticated etiology of the NMO, the role of infectious agents in its development, and the increasing incidence of such disease in Isfahan, Iran, it was necessary to investigate the possible association between some infectious agents and NMO. So far no studies have been done in Iran to investigate the relationship between CMV and NMO disease by molecular methods and real-time PCR.
Materials and Methods
The study samples
This descriptive cross-sectional study was conducted between June and September of 2016. The study samples were the patients with NMO and MS referring to an academic MS clinic in Isfahan, Iran as the experimental group and healthy individuals as the control group. The study was approved by the Ethics Committee of Isfahan University of Medical Sciences (approval code: 931811394001). Informed consent was obtained from all participants. In this study, 25 patients with NMO, 30 patients with MS, and 30 healthy individuals were recruited. The blood samples of healthy people were taken from a blood bank and their demographic data were collected. Diagnosis of NMO and MS diseases was done by a neurologist according to Wingerchuk NMO Criteria and McDonald’s Criteria, respectively [11].
The study procedure
First, the DNA was extracted from the serum samples of NMO and MS subjects as well as the healthy individuals group by the RIBO-prep nucleic acid extraction kit (Russia). The extraction steps were carried out according to the instructions provided. The serum sample (100 μL) and lubricant buffer (300 μL) were centrifuged for 5 s at 5000 rpm and placed at 65°C for 5 min. After completion, 400 μL of the precipitator buffer was added and centrifuged at 13000 rpm. The supernatant was transferred to the pellet and the remaining sediment was centrifuged for 1.5 min at a speed of 13000 rpm with 400 μL of washing solution 3.
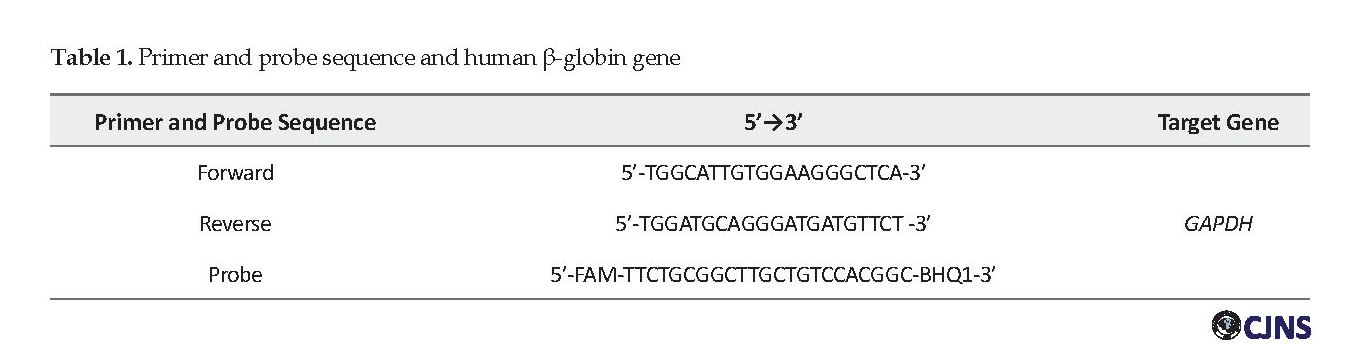
The supernatant was carefully removed using an aspirator. About 200 μL of the washing solution 4 was added to the remaining precipitate and centrifuged for 1.5 min at 13000 rpm and incubated at 65°C for 5 min. After 5 minutes, 50 μL of RNA buffer was added and verified and incubated at 65°C for 5 min. After incubation of the vortex, it was centrifuged at 15000 rpm for 1.5 min. In this case, the supernatant contains extracted DNA and stored at -20°C until real-time PCR is performed. The real-time PCR method was used to search the DNA of the cytomegalovirus virus in study samples. In this regard, human GAPDH gene was used as an extraction control. The sequence and characteristics of the primers used to control extraction with the human β-globin gene designed by Beacon Designer 7 are presented in Table 1.
Real-time PCR reaction was performed on human β-globin genes in a total volume of 25 μL for each reaction. The reaction mixture consisted of 5 μL buffer X 10, 5 μL MgCl2 2.5, 1 μL dNTP, 0.1 μL primer and probe, 0.2 μL Taq polymerase enzyme, 10.8 μL of water without nucleases, followed by 5 μL DNA added to each tube. For real-time PCR, the reaction mixture was placed at 95°C for 3 min, then 45 cycles were performed with the following characteristics.
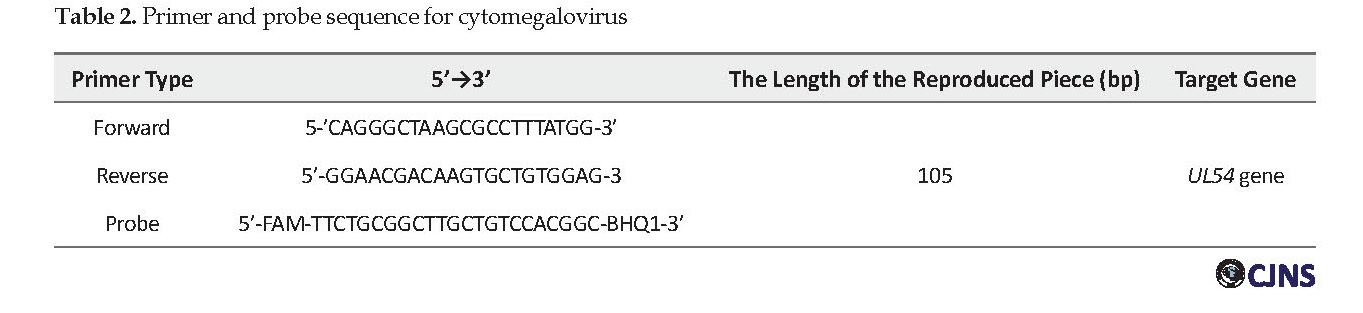
The initial phase was 15 s at 95°C and annealing-extension for 60 s at 58°C. The number of cycles used was 45. UL54 gene amplification was used to detect cytomegalovirus virus, the sequence and characteristics of the primers used to replicate the virus are shown in Table 2. The primer and probe sequence was designed using the Beacon Designer 7 software. The real-time PCR reaction of the UL54 gene was used to detect cytomegalovirus virus in the final volume of 25 μL for each reaction. The reaction mixture consisted of 5 μL buffer 10X, 5 μL MgCl2 2.5, 1 μL dNTP, 5 μL primer and probe, 2 μL of Taq polymerase enzyme, 10.8 μL of water without nuclease, followed by 5 μL DNA added to each tube. To conduct real-time PCR, the reaction mixture was placed at 95°C for 3 min, then 45 cycles were performed with the following specifications: the initial phase was 15 s at 95°C and annealing-extension for 60 s at 58°C. The number of cycles was 45.
Neuromyelitis Optica (NMO), a combination of myelitis and optic neuritis, is a demyelinating autoimmune disease [1-3]. The disease was first introduced by Eugene Devic in 1894. NMO is similar to Multiple Sclerosis (MS) with respect to some clinical symptoms. That is why physicians first classified NMO as a type of MS. However in 2004, specific antibodies against aquaporin-4 were discovered in the serum of NMO patients, which could distinguish between the two diseases. This antibody, known as IgG antibody against Aquaporin-4 (AQP4-IgG), plays a key role in the pathogenesis of this disease and is capable of detecting the extracellular epithelium of the water channel of astrocyte cells called aquaporin-4 which attack the epithelium and leading to its destruction [4, 5, 6].
In fact, the main cause of anti-aquaporin antibodies damage is the inflammatory changes of the cerebrospinal fluid barrier. Therefore, viral and bacterial infections can be considered as environmental factors in the development of inflammation in the white and gray matter of the brain. After the inflammation, anti-aquaporin-4 antibodies from the cerebrospinal barrier damage the tissues rich in aquaporin-4 and increases the presentation of autoantigen and excessive activation of T and B cells [7, 8].
According to Koga et al. study on the cause of the disease, out of 24 infectious agents studied, mumps and herpes viruses were more associated with this disease. In 2007, a case report was presented by Tran et al. in which a man was involved by Neuromyelitis Optica following Cytomegalovirus (CMV) primo-infection [9, 10].
This study aimed to investigate the relationship between CMV infection and NMO disease by the molecular method and real-time PCR testing. Considering the sophisticated etiology of the NMO, the role of infectious agents in its development, and the increasing incidence of such disease in Isfahan, Iran, it was necessary to investigate the possible association between some infectious agents and NMO. So far no studies have been done in Iran to investigate the relationship between CMV and NMO disease by molecular methods and real-time PCR.
Materials and Methods
The study samples
This descriptive cross-sectional study was conducted between June and September of 2016. The study samples were the patients with NMO and MS referring to an academic MS clinic in Isfahan, Iran as the experimental group and healthy individuals as the control group. The study was approved by the Ethics Committee of Isfahan University of Medical Sciences (approval code: 931811394001). Informed consent was obtained from all participants. In this study, 25 patients with NMO, 30 patients with MS, and 30 healthy individuals were recruited. The blood samples of healthy people were taken from a blood bank and their demographic data were collected. Diagnosis of NMO and MS diseases was done by a neurologist according to Wingerchuk NMO Criteria and McDonald’s Criteria, respectively [11].
The study procedure
First, the DNA was extracted from the serum samples of NMO and MS subjects as well as the healthy individuals group by the RIBO-prep nucleic acid extraction kit (Russia). The extraction steps were carried out according to the instructions provided. The serum sample (100 μL) and lubricant buffer (300 μL) were centrifuged for 5 s at 5000 rpm and placed at 65°C for 5 min. After completion, 400 μL of the precipitator buffer was added and centrifuged at 13000 rpm. The supernatant was transferred to the pellet and the remaining sediment was centrifuged for 1.5 min at a speed of 13000 rpm with 400 μL of washing solution 3.
The supernatant was carefully removed using an aspirator. About 200 μL of the washing solution 4 was added to the remaining precipitate and centrifuged for 1.5 min at 13000 rpm and incubated at 65°C for 5 min. After 5 minutes, 50 μL of RNA buffer was added and verified and incubated at 65°C for 5 min. After incubation of the vortex, it was centrifuged at 15000 rpm for 1.5 min. In this case, the supernatant contains extracted DNA and stored at -20°C until real-time PCR is performed. The real-time PCR method was used to search the DNA of the cytomegalovirus virus in study samples. In this regard, human GAPDH gene was used as an extraction control. The sequence and characteristics of the primers used to control extraction with the human β-globin gene designed by Beacon Designer 7 are presented in Table 1.
Real-time PCR reaction was performed on human β-globin genes in a total volume of 25 μL for each reaction. The reaction mixture consisted of 5 μL buffer X 10, 5 μL MgCl2 2.5, 1 μL dNTP, 0.1 μL primer and probe, 0.2 μL Taq polymerase enzyme, 10.8 μL of water without nucleases, followed by 5 μL DNA added to each tube. For real-time PCR, the reaction mixture was placed at 95°C for 3 min, then 45 cycles were performed with the following characteristics.
The initial phase was 15 s at 95°C and annealing-extension for 60 s at 58°C. The number of cycles used was 45. UL54 gene amplification was used to detect cytomegalovirus virus, the sequence and characteristics of the primers used to replicate the virus are shown in Table 2. The primer and probe sequence was designed using the Beacon Designer 7 software. The real-time PCR reaction of the UL54 gene was used to detect cytomegalovirus virus in the final volume of 25 μL for each reaction. The reaction mixture consisted of 5 μL buffer 10X, 5 μL MgCl2 2.5, 1 μL dNTP, 5 μL primer and probe, 2 μL of Taq polymerase enzyme, 10.8 μL of water without nuclease, followed by 5 μL DNA added to each tube. To conduct real-time PCR, the reaction mixture was placed at 95°C for 3 min, then 45 cycles were performed with the following specifications: the initial phase was 15 s at 95°C and annealing-extension for 60 s at 58°C. The number of cycles was 45.
In this study, the accuracy of the positive and negative control cases was evaluated using the QIAGEN kit. After completing real-time PCR, the obtained data were shown on the timeline as a graph. Demographic and other study data were analyzed by the Chi-square test in SPSS version 16. P values less than 0.05 were considered significant.
Results
A total of 85 subjects in three groups of NMO group with 25 samples, MS group with 30 samples, and healthy individuals with 30 samples were enrolled in this study. They were matched with regard to their age. The Mean±SD age of the NMO group was 32±8.75 years. The Mean±SD age of MS group was 34.10±10.5 years and the Mean±SD age of healthy individuals was 30.14±11.51 years with no significant difference between groups (P=0.33). There was no significant difference between the study groups in term of gender, too (P=0.599).
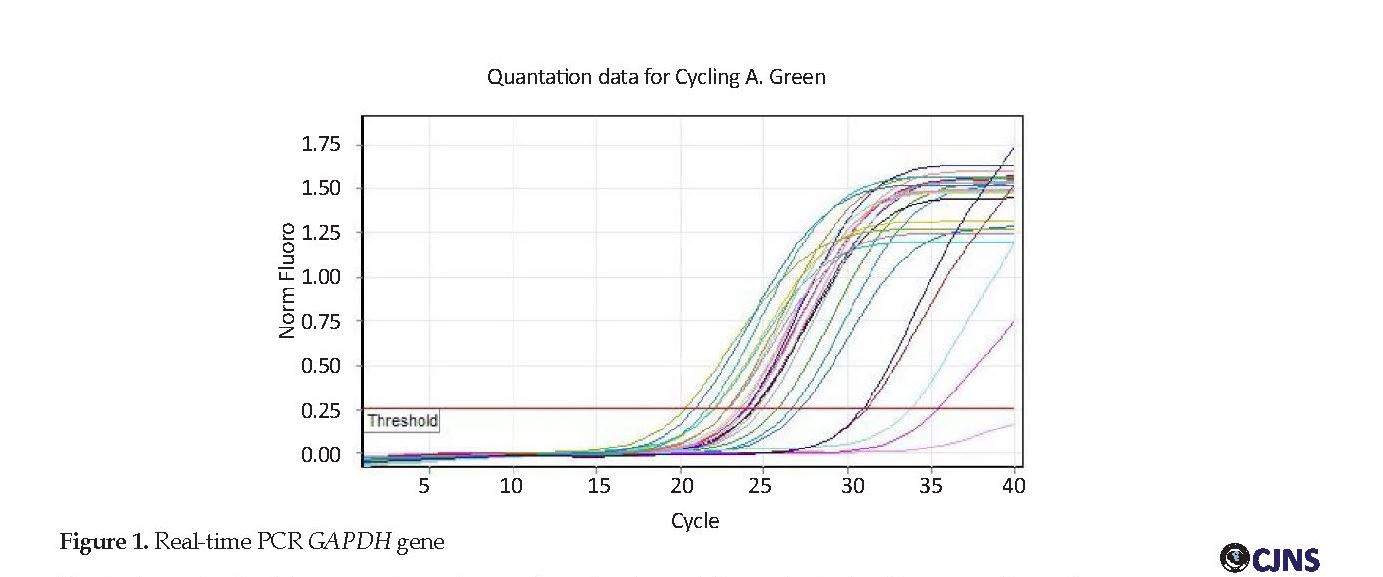
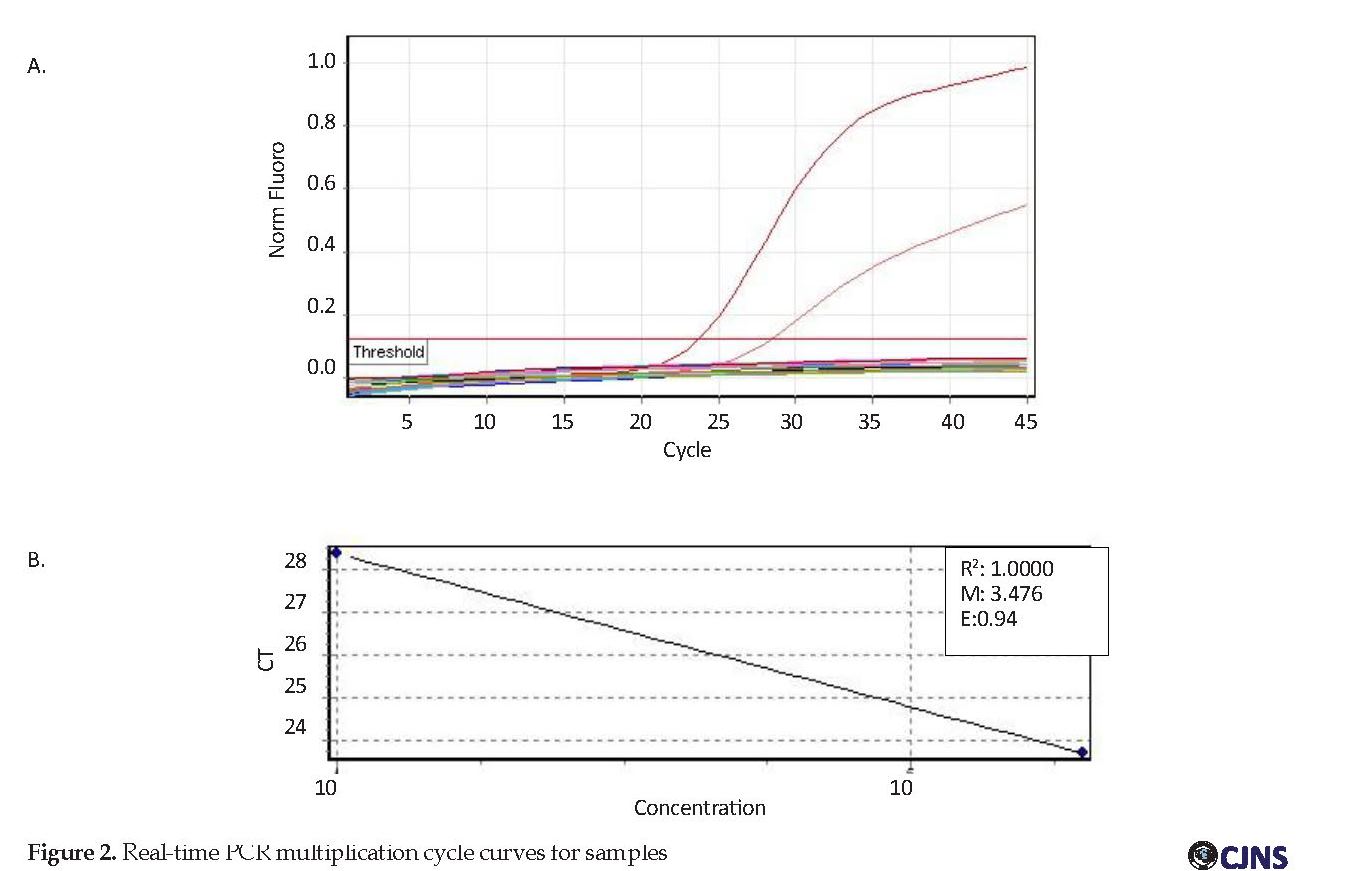
DNA samples were duplicated by human β-globin gene primers to be ensured of the DNA and its structure. Human primers GAPDH PCR were performed; the results of which are shown in Figure 1. The results of serum samples of the patients with NMO, MS as well as healthy individuals. All of the samples were DNA negative. According to Figure 2, the standard samples had a higher threshold line indicating their positive value while the study samples were below the threshold line, which indicates their negative result. Because of 94% efficiency of the test, indicating the high efficiency of the real-time PCR technique, none of the samples was positive despite the accuracy of this technique and the use of internal control to verify the DNA extraction.
Results
A total of 85 subjects in three groups of NMO group with 25 samples, MS group with 30 samples, and healthy individuals with 30 samples were enrolled in this study. They were matched with regard to their age. The Mean±SD age of the NMO group was 32±8.75 years. The Mean±SD age of MS group was 34.10±10.5 years and the Mean±SD age of healthy individuals was 30.14±11.51 years with no significant difference between groups (P=0.33). There was no significant difference between the study groups in term of gender, too (P=0.599).
DNA samples were duplicated by human β-globin gene primers to be ensured of the DNA and its structure. Human primers GAPDH PCR were performed; the results of which are shown in Figure 1. The results of serum samples of the patients with NMO, MS as well as healthy individuals. All of the samples were DNA negative. According to Figure 2, the standard samples had a higher threshold line indicating their positive value while the study samples were below the threshold line, which indicates their negative result. Because of 94% efficiency of the test, indicating the high efficiency of the real-time PCR technique, none of the samples was positive despite the accuracy of this technique and the use of internal control to verify the DNA extraction.
Discussion
Since infectious agents, such as viruses, can contribute to the development of neurological diseases and the relatively high prevalence of autoimmune diseases, a proper understanding of the relationship between viral infection and NMO disease is important in its treatment and prevention. The purpose of this study was to investigate the possible relationship between CMV viral infection and the NMO disease. For the first time in Iran, this study examined the relationship between CMV and NMO disease by molecular methods and through real-time PCR technique.
The result of the real-time PCR was negative for all specimens considering its accuracy and sensitivity and in particular the use of higher specificity primers and probes for detecting this virus. In 2007, Tran et al. introduced a 34-year-old male with Neuromyelitis Optica and rhabdomyolysis comorbid with CMV infection. They reported that CMV infection stimulated rhabdomyolysis, followed by myelitis and optic neurons. In this patient, a specific IgM titer was detected against the CMV virus, but PCR was performed to detect the DNA of the CMV virus, which was consistent with the study conducted by Tran et al. [10].
Conclusion
No significant association was found between NMO disease and CMV infection, but the importance of viruses as an etiology in autoimmune diseases is a challenging topic and requires further research. A similar project in a longer duration with more serum samples and other infectious disease-related factors are suggested for drawing better conclusions about the relationship between infectious agents and NMO disease.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Isfahan University of Medical Sciences (approval code: 931811394001). Informed consent was obtained from all participants.
Funding
This paper was funded by Graduate Studies at the University of Isfahan in the research laboratories of the Department of Biology (No. 931811394001).
Authors contributions
Conceptualization: Rasoul Roghanian, Sayyed Hamid Zarkesh Esfahani, Vahid Shaygannejad; Methodology: Rasoul Roghanian, Sayyed Hamid Zarkesh Esfahani, Omid Mirmosayyeb; Investigation: Mahboubeh Jazini; Writing– original draft preparation: Mahboubeh Jazini; Writing–review & editing: Rasoul Roghanian, Omid Mirmosayyeb; Funding acquisition: Graduate Studies at the University of Isfahan; Resources: ahid Shaygannejad, Omid Mirmosayyeb; and Supervision: Rasoul Roghanian, Sayyed Hamid Zarkesh Esfahani, Vahid Shaygannejad.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
Hereby, we appreciate laboratory of Kashani hospital for preparing samples.
References
Mitsdoerffer M, Kuchroo V, Korn T. Immunology of Neuromyelitis Optica: A T cell–B cell collaboration. Ann N Y Acad Sci. 2013; 1283(1):57-66. [DOI:10.1111/nyas.12118] [PMID] [PMCID]
AnChiu C, Xian W, Moss. Flying in silence: Echolocating bats cease vocalizing to avoid sonar jamming. Proc Natl Acad Sci USA. 2008; 105(35):13116-21. [DOI:10.1073/pnas.0804408105] [PMID] [PMCID]
Melamed E, Levy M, Waters PJ, Sato DK, Bennett JL, Jhon GR, et al. Update on biomarkers in Neuromyelitis Optica. Neurol Neuroimmunol Neuroinflamm. 2015; 2(4):e134.
Weinshenker BG, Wingerchuk DM, Pittok SJ, Lucchinetti CF, Lennon VA. NMO-IgG: A specific biomarker for Neuromyelitis Optica. Dis Markers. 2006; 22(4):197-206. [DOI:10.1155/2006/586306] [PMID] [PMCID]
Jarius S, Wildemann B. The history of Neuromyelitis Optica. J Neuroinflammation. 2013; 10:797. [DOI:10.1186/1742-2094-10-8] [PMID] [PMCID]
Sofroniew MV, Vinters HV. Astrocytes: Biology and pathology. Acta Neuropathol. 2010; 119(1):7-35. [DOI:10.1007/s00401-009-0619-8] [PMID] [PMCID]
Kalluri SR, Illes Z, Srivastava R, Cree B, Menge T, Bennett JL, et al. Quantification and functional characterization of antibodies to native aquaporin 4 in Neuromyelitis Optica. Arch Neurol. 2010; 67(10):1201-8. [DOI:10.1001/archneurol.2010.269] [PMID]
Moore F, Wolfson C. Human herpes virus 6 and Multiple Sclerosis. Acta Neurol Scand. 2002. 106(2):63-83. [DOI:10.1034/j.1600-0404.2002.01251.x] [PMID]
Koga M, Takahashi T, Kawai M, Fujihara K, Kanda T. A serological analysis of viral and bacterial infections associated with Neuromyelitis Optica. J Neurol Sci. 2011; 300(1):19-22. [DOI:10.1016/j.jns.2010.10.013] [PMID]
Tran C, Du Pasquier R, Cavassini M, Guex‐Crosier Y, Meuli R, Ciuffreda D, et al. Neuromyelitis Optica following CMV primo‐infection. J Intern Med. 2007; 261(5):500-3. [DOI:10.1111/j.1365-2796.2007.01794.x] [PMID]
Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm. 2015; 2(1):1-7. [DOI:10.1212/NXI.0000000000000062] [PMID] [PMCID]
No significant association was found between NMO disease and CMV infection, but the importance of viruses as an etiology in autoimmune diseases is a challenging topic and requires further research. A similar project in a longer duration with more serum samples and other infectious disease-related factors are suggested for drawing better conclusions about the relationship between infectious agents and NMO disease.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Isfahan University of Medical Sciences (approval code: 931811394001). Informed consent was obtained from all participants.
Funding
This paper was funded by Graduate Studies at the University of Isfahan in the research laboratories of the Department of Biology (No. 931811394001).
Authors contributions
Conceptualization: Rasoul Roghanian, Sayyed Hamid Zarkesh Esfahani, Vahid Shaygannejad; Methodology: Rasoul Roghanian, Sayyed Hamid Zarkesh Esfahani, Omid Mirmosayyeb; Investigation: Mahboubeh Jazini; Writing– original draft preparation: Mahboubeh Jazini; Writing–review & editing: Rasoul Roghanian, Omid Mirmosayyeb; Funding acquisition: Graduate Studies at the University of Isfahan; Resources: ahid Shaygannejad, Omid Mirmosayyeb; and Supervision: Rasoul Roghanian, Sayyed Hamid Zarkesh Esfahani, Vahid Shaygannejad.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
Hereby, we appreciate laboratory of Kashani hospital for preparing samples.
References
Mitsdoerffer M, Kuchroo V, Korn T. Immunology of Neuromyelitis Optica: A T cell–B cell collaboration. Ann N Y Acad Sci. 2013; 1283(1):57-66. [DOI:10.1111/nyas.12118] [PMID] [PMCID]
AnChiu C, Xian W, Moss. Flying in silence: Echolocating bats cease vocalizing to avoid sonar jamming. Proc Natl Acad Sci USA. 2008; 105(35):13116-21. [DOI:10.1073/pnas.0804408105] [PMID] [PMCID]
Melamed E, Levy M, Waters PJ, Sato DK, Bennett JL, Jhon GR, et al. Update on biomarkers in Neuromyelitis Optica. Neurol Neuroimmunol Neuroinflamm. 2015; 2(4):e134.
Weinshenker BG, Wingerchuk DM, Pittok SJ, Lucchinetti CF, Lennon VA. NMO-IgG: A specific biomarker for Neuromyelitis Optica. Dis Markers. 2006; 22(4):197-206. [DOI:10.1155/2006/586306] [PMID] [PMCID]
Jarius S, Wildemann B. The history of Neuromyelitis Optica. J Neuroinflammation. 2013; 10:797. [DOI:10.1186/1742-2094-10-8] [PMID] [PMCID]
Sofroniew MV, Vinters HV. Astrocytes: Biology and pathology. Acta Neuropathol. 2010; 119(1):7-35. [DOI:10.1007/s00401-009-0619-8] [PMID] [PMCID]
Kalluri SR, Illes Z, Srivastava R, Cree B, Menge T, Bennett JL, et al. Quantification and functional characterization of antibodies to native aquaporin 4 in Neuromyelitis Optica. Arch Neurol. 2010; 67(10):1201-8. [DOI:10.1001/archneurol.2010.269] [PMID]
Moore F, Wolfson C. Human herpes virus 6 and Multiple Sclerosis. Acta Neurol Scand. 2002. 106(2):63-83. [DOI:10.1034/j.1600-0404.2002.01251.x] [PMID]
Koga M, Takahashi T, Kawai M, Fujihara K, Kanda T. A serological analysis of viral and bacterial infections associated with Neuromyelitis Optica. J Neurol Sci. 2011; 300(1):19-22. [DOI:10.1016/j.jns.2010.10.013] [PMID]
Tran C, Du Pasquier R, Cavassini M, Guex‐Crosier Y, Meuli R, Ciuffreda D, et al. Neuromyelitis Optica following CMV primo‐infection. J Intern Med. 2007; 261(5):500-3. [DOI:10.1111/j.1365-2796.2007.01794.x] [PMID]
Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm. 2015; 2(1):1-7. [DOI:10.1212/NXI.0000000000000062] [PMID] [PMCID]
Type of Study: Research |
Subject:
Special
Received: 2018/06/10 | Accepted: 2018/11/23 | Published: 2019/01/1
Received: 2018/06/10 | Accepted: 2018/11/23 | Published: 2019/01/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |