Fri, Apr 26, 2024
Volume 5, Issue 1 (Winter 2019)
Caspian J Neurol Sci 2019, 5(1): 34-40 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jaeri S, Machin A. Clinical Improvement with Non-Surgical Management of Tuberculous Spondylitis. Caspian J Neurol Sci 2019; 5 (1) :34-40
URL: http://cjns.gums.ac.ir/article-1-243-en.html
URL: http://cjns.gums.ac.ir/article-1-243-en.html
1- Department of Neurology, Faculty of Medicine, Airlangga University, Surabaya, Indonesia , jw.santoso@gmail.com
2- Department of Neurology, Faculty of Medicine, Airlangga University, Surabaya, Indonesia
2- Department of Neurology, Faculty of Medicine, Airlangga University, Surabaya, Indonesia
Full-Text [PDF 1363 kb]
(1073 Downloads)
| Abstract (HTML) (2980 Views)
Full-Text: (807 Views)
Introduction
Tuberculosis (TB) is the second most common fatal infectious disease in the world after AIDS (acquired immunodeficiency syndrome) [1]. In 2012, 8.6 million cases of TB have been identified worldwide, which have resulted in more than 2.9 million deaths [2, 3]. Extrapulmonary TB accounts for 15% to 20% of all cases and spinal TB accounts for 50% of all skeletal tuberculosis cases and 1% of all tuberculosis because of the rich vascular supply of the vertebra [2, 4-6]. Tuberculous Spondylitis (TS), commonly known as Pott’s disease, usually arises secondary to direct inoculation of bacteria following a traumatic injury or during surgical procedures; spreading hematogenously via venous spread, Batson’s paravertebral venous plexus; or by lymphatic drainage to the paraaortic lymph nodes, immediately or later from the primary infection site [4, 6]. The preferred sites of infection are the thoracic segments, followed by the lumbar area [4, 7, 8].
The general symptoms of TS are fever, loss of appetite, weight loss, and night sweats which are seen in only 20% to 30% of cases [9]. These signs and symptoms are similar to other infectious or neoplastic processes in the same location [10]. Occasionally, TS patients may cry at nights because muscle spasms relax and allow the movement of the inflamed surfaces. A small gibbus may be detected upon palpation. Later on, neurological deficits may appear such as local and radicular pain as well as motor, sensory, and sphincter disturbances. According to one review study on TS, the incidence of neurological deficits in TS varies from 23% to 76% and paraplegia occurs in 4% to 38% of cases [9, 10].
The TS patients typically complain of persistent severe backache and tenderness in the region of the infected vertebrae, which is usually resistant to analgesics[2, 10]. The average duration of symptoms prior to diagnosis is one year, but it may range from weeks to years [10]. The neurological deficits increase sequentially as cord compression increases [11]. Cord compression is due to an abscess and granulation tissue, sequestrums, and the posterior bony edge of the vertebral body at the kyphosis level, and bony canal stenosis of the deformed spine above that level [11].
The treatment goals of TS are first to eradicate the infection and improve the general wellbeing of the patient. Second, the affected spine should be stabilized, and the spinal deformities be corrected, and finally prevent or treat paralysis. The management of TS consists of supportive care, including nutritional therapy, chemotherapy using antituberculous agents, and operation [2]. In developing countries, a large mismatch is seen between the disease burden and the available surgical facilities. There is little information in the literature about systemic non-surgical treatment in the condition of cord compression in TS. With the literature supporting good neurological outcomes following chemotherapy in patients of TS, some of these patients can be considered for non-surgical treatment, especially the ones who do well with supervised prescription of antituberculous agents [12].
An ideal classification system should assess the functional status of the tetra/paraplegic patient and reflect the severity of cord compression. Unfortunately, there are no universally accepted staging systems in TS, though several systems have been reported. Frankel suggested the classification from grade A to F with the A refers to the worst case. ASIA scale classification reflects the TS neurological deficits severity by scores depending upon the level of involvement and degree of cord compression at the involved level [11].
Likewise, Oguz et al. proposed their therapeutic classification of the TS based on the clinical stage of the disease processes [13]. Classification suggested by Tuli and modified by Jain seems the most rational one which includes all cases of paraplegia and reflects the severity of the cord compression as a score reflecting the sensory and motor deficits [11]. However, these classifications cannot be used as a guide for the indication of surgery. This article reports two cases of TS who were treated non-surgically with clinical and radiological improvement, to propose that clinico-radiological signs of spinal cord compression are not an emergency indication for surgery.
Case Presentation
Case 1
A 34-year-old housewife woman presented with the complaint of weakness on both legs, worsen gradually for a month duration. Simultaneously she had descending paresthesia from her chest to both feet since 2 months ago, followed by disturbance of sweating from chest to both feet. There were no difficulties with urinary and defecation. On neurological examination, her higher mental functions and cranial nerves were normal. Her power of upper limb was 5 out of 5 on both sides with a normal tone and deep tendon reflexes. The power of the lower limbs was 0 with normal tone, exaggerated reflexes, and the presence of pathological reflexes on both sides. She had hypesthesia below thoracic myelum 7-8 (T7-8) corresponding to thoracic vertebral level 5-6 (T5-6).
Tuberculosis (TB) is the second most common fatal infectious disease in the world after AIDS (acquired immunodeficiency syndrome) [1]. In 2012, 8.6 million cases of TB have been identified worldwide, which have resulted in more than 2.9 million deaths [2, 3]. Extrapulmonary TB accounts for 15% to 20% of all cases and spinal TB accounts for 50% of all skeletal tuberculosis cases and 1% of all tuberculosis because of the rich vascular supply of the vertebra [2, 4-6]. Tuberculous Spondylitis (TS), commonly known as Pott’s disease, usually arises secondary to direct inoculation of bacteria following a traumatic injury or during surgical procedures; spreading hematogenously via venous spread, Batson’s paravertebral venous plexus; or by lymphatic drainage to the paraaortic lymph nodes, immediately or later from the primary infection site [4, 6]. The preferred sites of infection are the thoracic segments, followed by the lumbar area [4, 7, 8].
The general symptoms of TS are fever, loss of appetite, weight loss, and night sweats which are seen in only 20% to 30% of cases [9]. These signs and symptoms are similar to other infectious or neoplastic processes in the same location [10]. Occasionally, TS patients may cry at nights because muscle spasms relax and allow the movement of the inflamed surfaces. A small gibbus may be detected upon palpation. Later on, neurological deficits may appear such as local and radicular pain as well as motor, sensory, and sphincter disturbances. According to one review study on TS, the incidence of neurological deficits in TS varies from 23% to 76% and paraplegia occurs in 4% to 38% of cases [9, 10].
The TS patients typically complain of persistent severe backache and tenderness in the region of the infected vertebrae, which is usually resistant to analgesics[2, 10]. The average duration of symptoms prior to diagnosis is one year, but it may range from weeks to years [10]. The neurological deficits increase sequentially as cord compression increases [11]. Cord compression is due to an abscess and granulation tissue, sequestrums, and the posterior bony edge of the vertebral body at the kyphosis level, and bony canal stenosis of the deformed spine above that level [11].
The treatment goals of TS are first to eradicate the infection and improve the general wellbeing of the patient. Second, the affected spine should be stabilized, and the spinal deformities be corrected, and finally prevent or treat paralysis. The management of TS consists of supportive care, including nutritional therapy, chemotherapy using antituberculous agents, and operation [2]. In developing countries, a large mismatch is seen between the disease burden and the available surgical facilities. There is little information in the literature about systemic non-surgical treatment in the condition of cord compression in TS. With the literature supporting good neurological outcomes following chemotherapy in patients of TS, some of these patients can be considered for non-surgical treatment, especially the ones who do well with supervised prescription of antituberculous agents [12].
An ideal classification system should assess the functional status of the tetra/paraplegic patient and reflect the severity of cord compression. Unfortunately, there are no universally accepted staging systems in TS, though several systems have been reported. Frankel suggested the classification from grade A to F with the A refers to the worst case. ASIA scale classification reflects the TS neurological deficits severity by scores depending upon the level of involvement and degree of cord compression at the involved level [11].
Likewise, Oguz et al. proposed their therapeutic classification of the TS based on the clinical stage of the disease processes [13]. Classification suggested by Tuli and modified by Jain seems the most rational one which includes all cases of paraplegia and reflects the severity of the cord compression as a score reflecting the sensory and motor deficits [11]. However, these classifications cannot be used as a guide for the indication of surgery. This article reports two cases of TS who were treated non-surgically with clinical and radiological improvement, to propose that clinico-radiological signs of spinal cord compression are not an emergency indication for surgery.
Case Presentation
Case 1
A 34-year-old housewife woman presented with the complaint of weakness on both legs, worsen gradually for a month duration. Simultaneously she had descending paresthesia from her chest to both feet since 2 months ago, followed by disturbance of sweating from chest to both feet. There were no difficulties with urinary and defecation. On neurological examination, her higher mental functions and cranial nerves were normal. Her power of upper limb was 5 out of 5 on both sides with a normal tone and deep tendon reflexes. The power of the lower limbs was 0 with normal tone, exaggerated reflexes, and the presence of pathological reflexes on both sides. She had hypesthesia below thoracic myelum 7-8 (T7-8) corresponding to thoracic vertebral level 5-6 (T5-6).
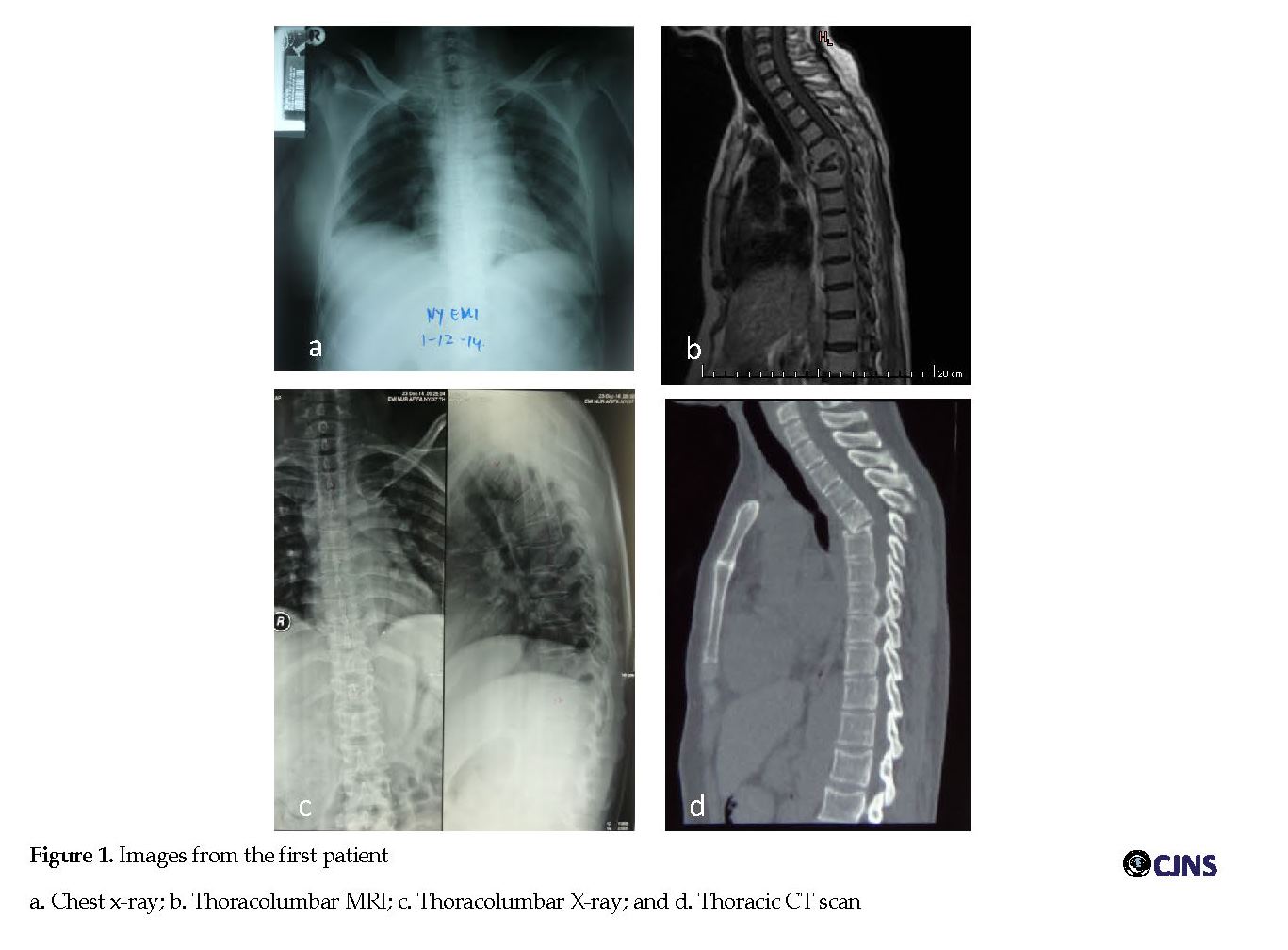
Her general physical examination and other system examinations were found normal. A provisional diagnosis of thoracic myelopathy was made and the patient was investigated. Her complete blood count, renal profile, liver function tests, HIV and hepatitis B surface antigen were not indicative of any disease. Her chest X-ray was normal (Figure 1 a). The Erythrocyte Sedimentation Rate (ESR) was moderately high. Magnetic Resonance Imaging (MRI) of the thoracolumbar area revealed a collapse of vertebral body level T5 anteriorly formed gibbus and compression of spinal cord at level T4-5 with cord edema. Another finding was intraosseous abscess at T4 and paravertebral and epidural abscesses at T4-5 (Figure 1 b). Thoracolumbar X-ray showed a collapse on the vertebral body at level T5 with kyphotic angle of 33o and irregularity on both superior and inferior endplate (Figure 1 c).
The diagnosis was TS. The patient then was given antituberculosis drugs, started with Isoniazid (INH) 300 mg/d, Rifampicin (RF) 450 mg/d, Pyrazinamide (PRZ) 1500 mg/d and streptomycin intramuscularly 1 g/d for 2 months, followed by INH, RF, and PRZ for another 10 months. After 8 months follow up, the patient’s neurological examination gradually improved, she could stand from sitting position and walk without support. In the long-term follow up, she developed a 36o kyphotic deformity as depicted on the thoracic CT scan (taken after 9 months follow up) (Figure 1 d).
Case 2
A 26-year-old woman, a shoe factory worker, was hospitalized because of the weakness on both legs, worsen gradually since 5 days ago. She also had numbness from the abdomen to both feet since 2 weeks before admission. She had no difficulties in urination or defecation but she had dry skin because of sweating disturbance. The patient also had back pain for 10 months since ten months ago. On the neurological examination, her higher mental functions and cranial nerves were normal. Her upper limbs power was 5 out of 5 on both sides with a normal tone and deep tendon reflexes.
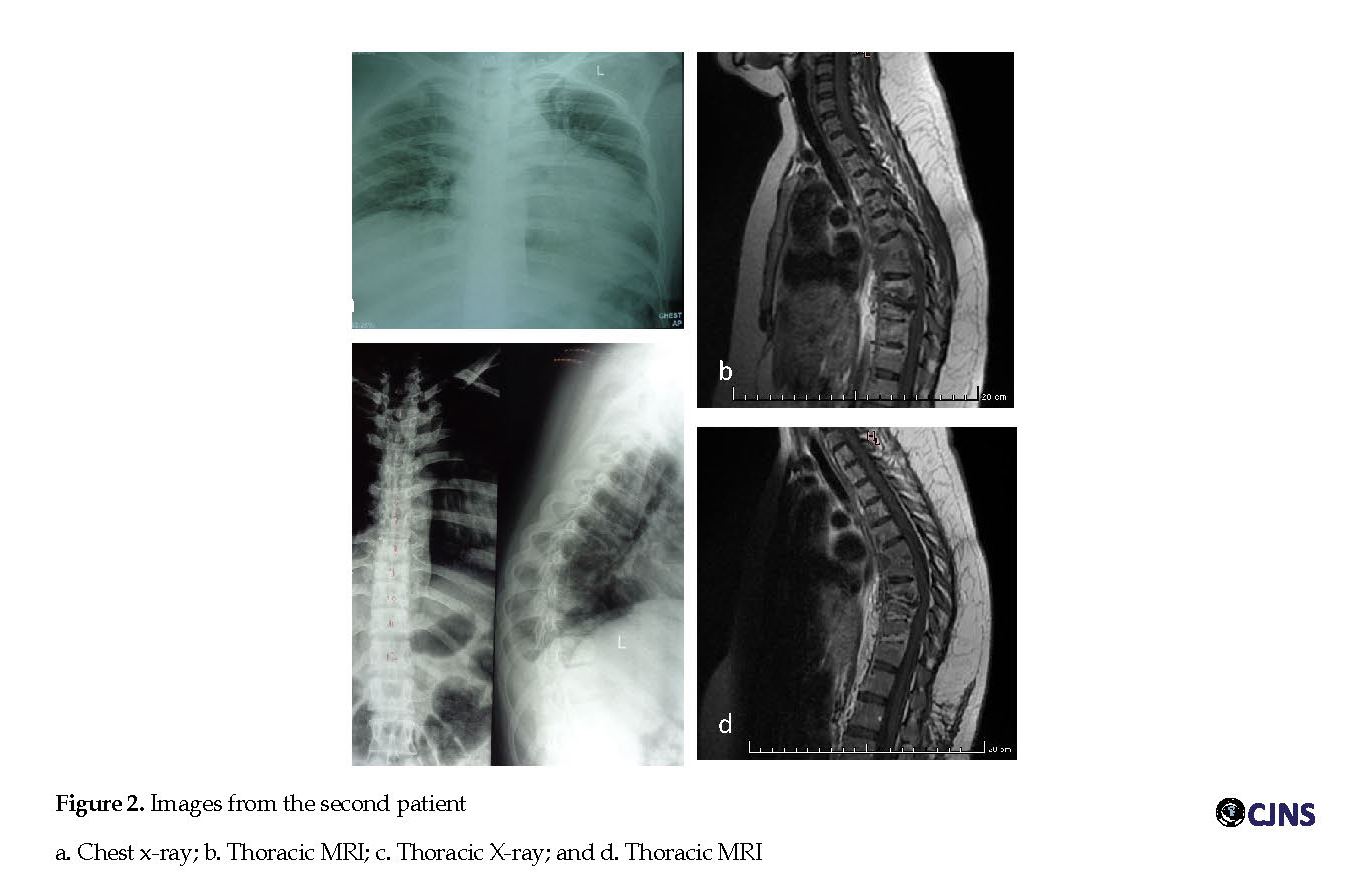
Her lower limbs power was low with score 3 on the right side and 2 on the left side with normal tone, increased reflexes, the presence of pathological reflexes, and ill-sustained clonus on both sides. She had numbness below myelum segment T12 corresponding to vertebral level T10, and knock pain on vertebra at level T7-8. Her general physical examination and other system examinations were normal. Her complete blood count, renal profile, liver function tests, HIV and hepatitis B surface antigen results were not indicative of any disease. Her chest X-ray was normal (Figure 2 a.) but her ESR was high. Also, MRI of cervicothoracic showed destruction of vertebral body level T5 to T10, with intraosseous lesion and irregular endplates at those levels, paravertebral soft tissue mass at level T6-9 on the right side, which showed rim contrast enhancement.
Case 2
A 26-year-old woman, a shoe factory worker, was hospitalized because of the weakness on both legs, worsen gradually since 5 days ago. She also had numbness from the abdomen to both feet since 2 weeks before admission. She had no difficulties in urination or defecation but she had dry skin because of sweating disturbance. The patient also had back pain for 10 months since ten months ago. On the neurological examination, her higher mental functions and cranial nerves were normal. Her upper limbs power was 5 out of 5 on both sides with a normal tone and deep tendon reflexes.
Her lower limbs power was low with score 3 on the right side and 2 on the left side with normal tone, increased reflexes, the presence of pathological reflexes, and ill-sustained clonus on both sides. She had numbness below myelum segment T12 corresponding to vertebral level T10, and knock pain on vertebra at level T7-8. Her general physical examination and other system examinations were normal. Her complete blood count, renal profile, liver function tests, HIV and hepatitis B surface antigen results were not indicative of any disease. Her chest X-ray was normal (Figure 2 a.) but her ESR was high. Also, MRI of cervicothoracic showed destruction of vertebral body level T5 to T10, with intraosseous lesion and irregular endplates at those levels, paravertebral soft tissue mass at level T6-9 on the right side, which showed rim contrast enhancement.
This lesion caused the narrowing intervertebral disk at those levels (Figure 2 b). The diagnosis was TS. Thoracolumbar X-ray showed a compression fracture of vertebral body at level T7, 8, 9 with normal curve and irregularity on inferior endplate level T8 and superior endplate at level T9 (Figure 2 c). Nerve conduction velocity and somatosensory evoked potential was impressed in normal response. The patient was treated with antituberculosis medication, INH 300 mg/d; RF 600 mg/d; PRZ 1500 mg/d and streptomycin intramuscularly 1 g/d for 2 months, followed by INH, RF, and PRZ for 10 months. There was an improvement on the power of lower limb from 3 on the right and 2 on the left side to 4 on both sides. She could stand from sitting position without support over a period of 1 month after admission, and walk with assistance after 4 months follow up. The improvement was not only clinical but based on MRI images which demonstrated reduction of intraosseous and paravertebral abscesses compared to the previous images (Figure 2 d).
Discussion
Regarding the treatment goals, antituberculous treatment in patients with TS should be started as early as possible. Even though the World Health Organization (WHO) recommends a category-based treatment for TB [9], there is no standard anti-tuberculosis regimen because of some limitations such as lack of identification of MTB (Mycobacterium Tuberculosis) strains [14]. To effectively eradicate this disease, a medication regimen must consist of highly active agents such as Rifampin (RF), Isoniazid (INH), Pyrazinamide (PRZ), Ethambutol (E) and streptomycin that are capable of reaching the organisms within the various regions and tissues. Rifampin is bactericidal against all three strains of TB, isoniazid is bactericidal against extracellular and intracellular organisms, pyrazinamide is bactericidal against intracellular organisms and works well in an acidic environment [14].
Our patients received the antituberculosis agent: INH, RF, PRZ, and streptomycin intramuscularly for 2 months, followed by INH, RF, and PRZ administration for another 10 months. Both also planned to do surgery for debridement and stabilization of the lesions. These plans were performed for the second patient despite her improvement but canceled for the first patient because of improvement of her neurological deficits despite her kyphosis which was still present. These two cases demonstrate that administrating antituberculous agent therapy alone could result in good response to neurological deficits and the choice of surgery could be waiting after taking the results of medication.
Various studies have shown that the majority (82-95%) of the patients with TS respond very well to chemotherapy treatments [9]. Almost all anti-tuberculosis drugs penetrate well into the target lesion [9]. Most patients respond well to the antituberculous agents; however, paradoxical response happens in 6% to 30% of cases from 2 weeks to a few months after starting the medication, which is detected by clinical or radiological worsening of preexisting TB lesions or the development of the new lesions [8]. The pathophysiology of this response in HIV-positive patients is the phenomenon of “immune restitution” and antituberculous agent-induced disinhibition of the cell-mediated immunity that normally accompanies the TB infections [15].
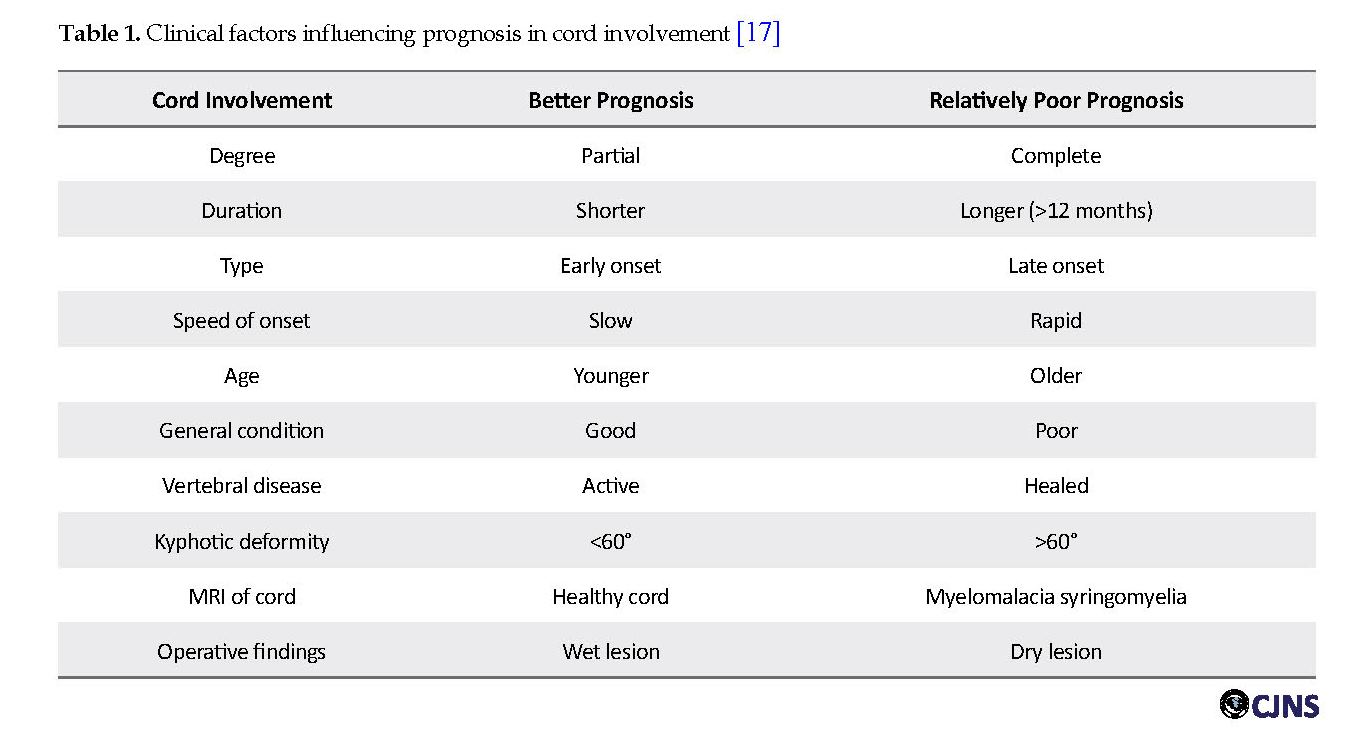
The prognosis for neurologic recovery is good for 75% to 95% of appropriately treated patients with TS. However, many factors affect recovery from paraplegia resulted from TS [10]. These include the patient’s general physical condition, including immunological status, age, spinal cord status, level and number of vertebrae involved, degree of the spinal deformity (almost no recovery even after radical decompression operation in patients with kyphosis of over 60o), duration and degree of the paraplegia, time to onset of treatment, kind of treatment and drug sensitivity [2, 16, 17]. Table 1 presents the clinical factors influencing the prognosis of TS [17].
The success of chemotherapy on TS depends on many factors. The effective institution of chemotherapy and good general supportive care are the key points to early eradication and minimal sequelae. Various studies have shown that 82% to 95% of patients of TS respond very well to chemotherapy treatment, but sometimes a paradoxical response could occur. In addition, the patient’s education and cooperation are vital and should receive proper attention in implementing non-operative treatment, especially in the case of spinal cord compression [12].
TS therapy can be supervised by clinical evaluation, as well as imaging and blood investigation. From a clinical perspective, improvement is documented when significant changes in signs and symptoms, including neurological deficits, are seen. In addition, radiological improvement happens when significant regression is observed in the epidural abscess/granulation tissue images in the immediate follow-up period. Later on, marrow reconversion and fatty reconstitution of the diseased bone should be seen at the final follow-up images [12].
Conclusion
Radiological signs of cord compression and neurological problems should not be taken as an emergency surgical indication in the management of TS. Chemotherapy can alone yield excellent results. However, the patient’s education and cooperation is vital and should receive proper attention in implementing non-operative treatment, especially in the case of spinal cord compression.
Ethical Considerations
Compliance with ethical guidelines
Informed consent was taken from both patients before enrollment in the study.
Funding
This case report was funded by the Government with National Health Insurance.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We acknowledge the department. of Radiology, Dr. Soetomo Hospital Surabaya, Indonesia for the radiographic examination and for the expertise.
References
McLaughlin A. Extrapulmonary tuberculosis Three Cases in The Spine. Aust Fam Physician. 2013; 42(4):204-6.
Moon MS. Tuberculosis of spine: Current views in diagnosis and management. Asian Spine J. 2014; 8(1):97-111. [DOI:10.4184/asj.2014.8.1.97] [PMID] [PMCID]
Dinihari TN, Siagian V. Pedoman nasional pengobatan tuberculosis. Jakarta: Kementerian Kesehatan Republik Indonesia; 2014.
Pigrau-Serrallach C, Rodriguez-Pardo D. Bone and joint tuberculosis. Eur Spine J. 2013; 22(Suppl 4):S556-S66. [DOI:10.1007/s00586-012-2331-y] [PMID] [PMCID]
Muhammad T, Baloch NA, Khan A. Management of spinal tuberculosis-a metropolitan city based survey among orthopaedic and neurosurgeons. J Pak Med Assoc. 2015; 65(12):1256-60. [PMID]
Lee KY. Comparison of pyogenic spondylitis and tuberculous spondylitis. Asian Spine J. 2014; 8(2):216-23. [DOI:10.4184/asj.2014.8.2.216] [PMID] [PMCID]
Rivas-Garcia A, Sarria-Estrada S, Torrents-Odin C, Casas-Gomila L, Franquet E. Imaging finding of Pott’s disease. Eur Spine J. 2013; 22(Suppl 4):S567-S78. [DOI:10.1007/s00586-012-2333-9] [PMID] [PMCID]
Cheung WY, Luk KDK. Clinical and Radiological Outcomes After Conservative Treatment of TB Spondylitis: Is The 15 Years’ Follow up in The MRC Study Long Enough? Eur Spine J. 2013; 22(Suppl 4):S594-S602. [DOI:10.1007/s00586-012-2332-x] [PMID] [PMCID]
Garg RK, Somvanshi DS. Spinal tuberculosis: A review. J Spinal Cord Med. 2011; 34(5):440-54. [DOI:10.1179/2045772311Y.0000000023] [PMID] [PMCID]
Anonym. Infectious and noninfectious inflammatory diseases affecting the spine. In: Byrne TN, Benzel EC, Waxman SG, editors. Diseases of the Spine and Spinal Cord. London: Oxford University Press; 2000.
Jain AK, Kumar J. Tuberculosis of Spine. Eur Spine J. 2013; 22(Suppl 4):S624-S33. [DOI:10.1007/s00586-012-2335-7] [PMID] [PMCID]
Patil SS, Mohite S, Varma R, Bhojraj SY, Nene AM. Non-surgical management of cord compression in tuberculosis: A series of surprises. Asian Spine J. 2014; 8(3):315-21. [DOI:10.4184/asj.2014.8.3.315] [PMID] [PMCID]
Oguz E, Sehirlioglu A, Altinmakas M, Ozturk C, Komurcu M, Solakoglu C, et al. A new classification and guide for surgical treatment of spinal tuberculosis. Int Orthop. 2008; 32(1):127-33. [DOI:10.1007/s00264-006-0278-5] [PMID] [PMCID]
Dobbs TE, Webb RM. Chamotherapy of tuberculosis. In: Schlossberg D, editor. Tuberculosis and Nontuberculous Mycobacterial Infections. Washington DC: ASM Press; 2011.
Velivela K, Rajesh A. Paradoxical response in spinal tuberculosis: Lesson learnt. J Neurosci Rural Pract. 2016; 7(2):206-9. [DOI:10.4103/0976-3147.178659] [PMID] [PMCID]
Sharma A, Chabra H, Chabra T, Mahajan R, batra S, Sangondimath G. Demographics of tuberculosis of spine and factors affecting neurological improvement in patients suffering from tuberculosis of spine: A retro-spective analysis of 312 cases. Spinal Cord. 2017; 55(1):59-63. [DOI:10.1038/sc.2016.85] [PMID]
Tuli SM. Historical Aspects of Pott’s Disease (Spinal Tuberculosis). Eur Spine J. 2013; 22(Suppl 4):S529-S38. [DOI:10.1007/s00586-012-2388-7] [PMID] [PMCID]
Discussion
Regarding the treatment goals, antituberculous treatment in patients with TS should be started as early as possible. Even though the World Health Organization (WHO) recommends a category-based treatment for TB [9], there is no standard anti-tuberculosis regimen because of some limitations such as lack of identification of MTB (Mycobacterium Tuberculosis) strains [14]. To effectively eradicate this disease, a medication regimen must consist of highly active agents such as Rifampin (RF), Isoniazid (INH), Pyrazinamide (PRZ), Ethambutol (E) and streptomycin that are capable of reaching the organisms within the various regions and tissues. Rifampin is bactericidal against all three strains of TB, isoniazid is bactericidal against extracellular and intracellular organisms, pyrazinamide is bactericidal against intracellular organisms and works well in an acidic environment [14].
Our patients received the antituberculosis agent: INH, RF, PRZ, and streptomycin intramuscularly for 2 months, followed by INH, RF, and PRZ administration for another 10 months. Both also planned to do surgery for debridement and stabilization of the lesions. These plans were performed for the second patient despite her improvement but canceled for the first patient because of improvement of her neurological deficits despite her kyphosis which was still present. These two cases demonstrate that administrating antituberculous agent therapy alone could result in good response to neurological deficits and the choice of surgery could be waiting after taking the results of medication.
Various studies have shown that the majority (82-95%) of the patients with TS respond very well to chemotherapy treatments [9]. Almost all anti-tuberculosis drugs penetrate well into the target lesion [9]. Most patients respond well to the antituberculous agents; however, paradoxical response happens in 6% to 30% of cases from 2 weeks to a few months after starting the medication, which is detected by clinical or radiological worsening of preexisting TB lesions or the development of the new lesions [8]. The pathophysiology of this response in HIV-positive patients is the phenomenon of “immune restitution” and antituberculous agent-induced disinhibition of the cell-mediated immunity that normally accompanies the TB infections [15].
The prognosis for neurologic recovery is good for 75% to 95% of appropriately treated patients with TS. However, many factors affect recovery from paraplegia resulted from TS [10]. These include the patient’s general physical condition, including immunological status, age, spinal cord status, level and number of vertebrae involved, degree of the spinal deformity (almost no recovery even after radical decompression operation in patients with kyphosis of over 60o), duration and degree of the paraplegia, time to onset of treatment, kind of treatment and drug sensitivity [2, 16, 17]. Table 1 presents the clinical factors influencing the prognosis of TS [17].
The success of chemotherapy on TS depends on many factors. The effective institution of chemotherapy and good general supportive care are the key points to early eradication and minimal sequelae. Various studies have shown that 82% to 95% of patients of TS respond very well to chemotherapy treatment, but sometimes a paradoxical response could occur. In addition, the patient’s education and cooperation are vital and should receive proper attention in implementing non-operative treatment, especially in the case of spinal cord compression [12].
TS therapy can be supervised by clinical evaluation, as well as imaging and blood investigation. From a clinical perspective, improvement is documented when significant changes in signs and symptoms, including neurological deficits, are seen. In addition, radiological improvement happens when significant regression is observed in the epidural abscess/granulation tissue images in the immediate follow-up period. Later on, marrow reconversion and fatty reconstitution of the diseased bone should be seen at the final follow-up images [12].
Conclusion
Radiological signs of cord compression and neurological problems should not be taken as an emergency surgical indication in the management of TS. Chemotherapy can alone yield excellent results. However, the patient’s education and cooperation is vital and should receive proper attention in implementing non-operative treatment, especially in the case of spinal cord compression.
Ethical Considerations
Compliance with ethical guidelines
Informed consent was taken from both patients before enrollment in the study.
Funding
This case report was funded by the Government with National Health Insurance.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We acknowledge the department. of Radiology, Dr. Soetomo Hospital Surabaya, Indonesia for the radiographic examination and for the expertise.
References
McLaughlin A. Extrapulmonary tuberculosis Three Cases in The Spine. Aust Fam Physician. 2013; 42(4):204-6.
Moon MS. Tuberculosis of spine: Current views in diagnosis and management. Asian Spine J. 2014; 8(1):97-111. [DOI:10.4184/asj.2014.8.1.97] [PMID] [PMCID]
Dinihari TN, Siagian V. Pedoman nasional pengobatan tuberculosis. Jakarta: Kementerian Kesehatan Republik Indonesia; 2014.
Pigrau-Serrallach C, Rodriguez-Pardo D. Bone and joint tuberculosis. Eur Spine J. 2013; 22(Suppl 4):S556-S66. [DOI:10.1007/s00586-012-2331-y] [PMID] [PMCID]
Muhammad T, Baloch NA, Khan A. Management of spinal tuberculosis-a metropolitan city based survey among orthopaedic and neurosurgeons. J Pak Med Assoc. 2015; 65(12):1256-60. [PMID]
Lee KY. Comparison of pyogenic spondylitis and tuberculous spondylitis. Asian Spine J. 2014; 8(2):216-23. [DOI:10.4184/asj.2014.8.2.216] [PMID] [PMCID]
Rivas-Garcia A, Sarria-Estrada S, Torrents-Odin C, Casas-Gomila L, Franquet E. Imaging finding of Pott’s disease. Eur Spine J. 2013; 22(Suppl 4):S567-S78. [DOI:10.1007/s00586-012-2333-9] [PMID] [PMCID]
Cheung WY, Luk KDK. Clinical and Radiological Outcomes After Conservative Treatment of TB Spondylitis: Is The 15 Years’ Follow up in The MRC Study Long Enough? Eur Spine J. 2013; 22(Suppl 4):S594-S602. [DOI:10.1007/s00586-012-2332-x] [PMID] [PMCID]
Garg RK, Somvanshi DS. Spinal tuberculosis: A review. J Spinal Cord Med. 2011; 34(5):440-54. [DOI:10.1179/2045772311Y.0000000023] [PMID] [PMCID]
Anonym. Infectious and noninfectious inflammatory diseases affecting the spine. In: Byrne TN, Benzel EC, Waxman SG, editors. Diseases of the Spine and Spinal Cord. London: Oxford University Press; 2000.
Jain AK, Kumar J. Tuberculosis of Spine. Eur Spine J. 2013; 22(Suppl 4):S624-S33. [DOI:10.1007/s00586-012-2335-7] [PMID] [PMCID]
Patil SS, Mohite S, Varma R, Bhojraj SY, Nene AM. Non-surgical management of cord compression in tuberculosis: A series of surprises. Asian Spine J. 2014; 8(3):315-21. [DOI:10.4184/asj.2014.8.3.315] [PMID] [PMCID]
Oguz E, Sehirlioglu A, Altinmakas M, Ozturk C, Komurcu M, Solakoglu C, et al. A new classification and guide for surgical treatment of spinal tuberculosis. Int Orthop. 2008; 32(1):127-33. [DOI:10.1007/s00264-006-0278-5] [PMID] [PMCID]
Dobbs TE, Webb RM. Chamotherapy of tuberculosis. In: Schlossberg D, editor. Tuberculosis and Nontuberculous Mycobacterial Infections. Washington DC: ASM Press; 2011.
Velivela K, Rajesh A. Paradoxical response in spinal tuberculosis: Lesson learnt. J Neurosci Rural Pract. 2016; 7(2):206-9. [DOI:10.4103/0976-3147.178659] [PMID] [PMCID]
Sharma A, Chabra H, Chabra T, Mahajan R, batra S, Sangondimath G. Demographics of tuberculosis of spine and factors affecting neurological improvement in patients suffering from tuberculosis of spine: A retro-spective analysis of 312 cases. Spinal Cord. 2017; 55(1):59-63. [DOI:10.1038/sc.2016.85] [PMID]
Tuli SM. Historical Aspects of Pott’s Disease (Spinal Tuberculosis). Eur Spine J. 2013; 22(Suppl 4):S529-S38. [DOI:10.1007/s00586-012-2388-7] [PMID] [PMCID]
Type of Study: case report |
Subject:
General
Received: 2018/06/19 | Accepted: 2018/11/1 | Published: 2019/01/1
Received: 2018/06/19 | Accepted: 2018/11/1 | Published: 2019/01/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |