Wed, May 8, 2024
Volume 5, Issue 1 (Winter 2019)
Caspian J Neurol Sci 2019, 5(1): 23-27 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghorbani S, Hatamian H, Mahmoudzadeh A, Raeisi S, Eslami M, Etemadifar M et al . The Association Between Salt and Potassium Intake with Multiple Sclerosis. Caspian J Neurol Sci 2019; 5 (1) :23-27
URL: http://cjns.gums.ac.ir/article-1-222-en.html
URL: http://cjns.gums.ac.ir/article-1-222-en.html
Sarah Ghorbani1 

 , Hamidreza Hatamian2
, Hamidreza Hatamian2 
 , Amirhossain Mahmoudzadeh1
, Amirhossain Mahmoudzadeh1 
 , Sina Raeisi1
, Sina Raeisi1 
 , Mohammadjavad Eslami1
, Mohammadjavad Eslami1 
 , Masoud Etemadifar *
, Masoud Etemadifar * 

 3, Fatemeh Shafaei4
3, Fatemeh Shafaei4 



 , Hamidreza Hatamian2
, Hamidreza Hatamian2 
 , Amirhossain Mahmoudzadeh1
, Amirhossain Mahmoudzadeh1 
 , Sina Raeisi1
, Sina Raeisi1 
 , Mohammadjavad Eslami1
, Mohammadjavad Eslami1 
 , Masoud Etemadifar *
, Masoud Etemadifar * 

 3, Fatemeh Shafaei4
3, Fatemeh Shafaei4 

1- Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
2- Department of Neurology, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran
3- Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran , etemadifar@med.mui.ac.ir
4- Neuroscience Research Center, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran
2- Department of Neurology, School of Medicine, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran
3- Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran , etemadifar@med.mui.ac.ir
4- Neuroscience Research Center, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran
Full-Text [PDF 857 kb]
(3038 Downloads)
| Abstract (HTML) (3295 Views)
Full-Text: (841 Views)
Introduction
Multiple Sclerosis (MS) is a chronic inflammatory disorder of the central nervous system leading to both axonal damage and demyelination. This disease is more common in young adults [1, 2]. Common presentations of MS include sensory disturbance, visual loss, diplopia, limb weakness, and vertigo [3]. The number of patients with MS is increasing in developing countries such as Iran [4-6]. It seems that MS usually develops in genetically susceptible people as a result of environmental exposure. Several environmental factors, such as viral infection, vitamin D deficiency, smoking, obesity, sunlight exposure, and diet have been postulated as potential risk factors for the increasing incidence of autoimmune diseases such as MS [7-9].
The mean sodium intake in most Asian countries is over 100 mmol/d, while the recommended salt intake value by WHO is 80-90 mmol/d [10] and in many societies the mean potassium consumption is below 70-80 mmol/d, which is the value recommended by the WHO (1988, 2003). Previous research studies have shown that high salt intake is associated with the development of autoimmune diseases and aggravations of MS symptoms in patients, while potassium supplementation has a diverse effect [11, 12].
T helper 17 (Th 17) cells produce Interleukin (IL)-17, which is a pro-inflammatory cytokine. It plays a critical role in the pathogenesis of autoimmune diseases. High physiological salt concentrations have a direct effect on the induction of Th 17 cells and IL-17, which initiate pro-inflammatory signatures. However, potassium supplements block IL-17 production in T lymphocytes [11-14]. In this research, we performed a case-control study to investigate whether NaCl uptake in MS patients is one of the underlying factors of the disease.
Materials and Methods
A total of 23 patients with Relapsing-Remitting Multiple Sclerosis (RRMS) were randomly selected from Alzahra Clinic of MS, Isfahan, Iran. Then, 23 healthy controls matched with the cases based on gender and age were selected, too. The participants were recruited between October and June 2016, and all of them signed informed consent forms. Furthermore, the Ethics Committee of Isfahan Research Committee of Multiple Sclerosis approved this project.
Study samples
The MS patients, aged 15-46 years, would be included in this study if they were clinically diagnosed with MS with a relapsing-remitting course for more than 1 year. Exclusion criteria were the presence of renal disease, diabetes insipidus, and adrenal disorders. In addition, the patients using diuretics were excluded from this study.
Sodium chloride (NaCl) and Potassium measurements
The levels of sodium chloride (NaCl) and potassium were measured in 24 hours of urine. To confirm that the high level of sodium in urine is not due to the elevated level of sodium in the blood, we also measured the level of blood sodium. In addition, the blood creatinine level and Glomerular Filtration Rate (GFR) were also measured in all cases and controls to rule out kidney diseases. All the measurements were performed in one laboratory and by the same technicians.
Statistical analysis
All analyses were performed in SPSS (version 23.0). We used the Chi-square test to compare NaCl and potassium level in 24 hours of urine between cases and controls. Conditional logistic regression was performed to determine the effect of daily sodium and potassium intake on the development of MS. The level of significance was set at P<0.050 in all analysis.
Results
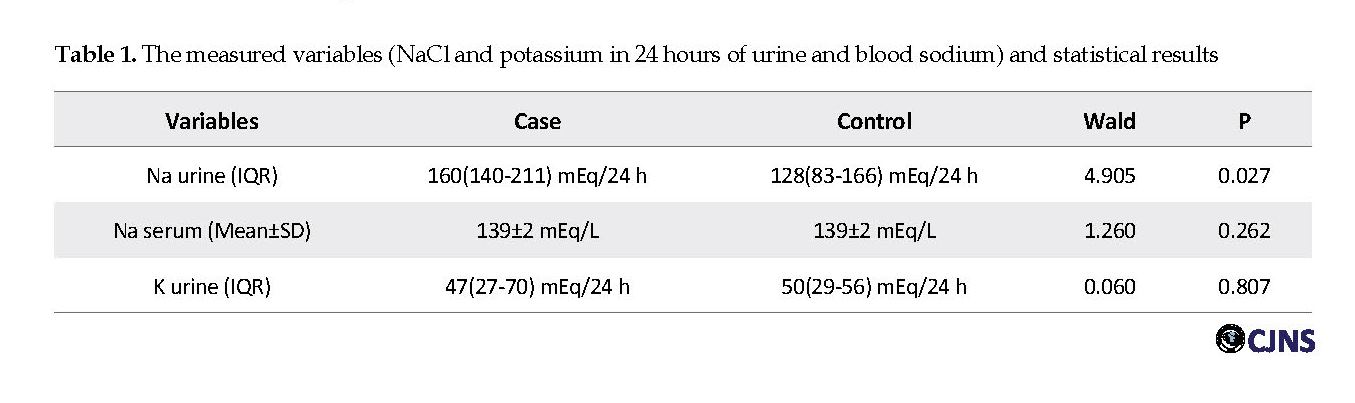
In this study, a total of 46 subjects (23 cases and 23 matched controls for sex and age) were included. The Mean±SD age of cases and controls was 27±7 years. In each group, 5 (22%) males and 18 (78%) females were studied. Serum sodium concentrations were in the normal range, and none of the cases or controls had hyper- or hypo-natremia (Mean±SD: 139±2 mEq/L vs. 139±2 mEq/L, respectively) (P=0.262). Urine sodium level was relatively higher in cases compared to controls (Interquartile Range [IQR]; 160 [140-211] mEq/24 h vs. 128 [83-166] mEq/24 h), which is probably due to higher sodium intake in the patients. The difference in the level of urine sodium between the two groups of cases and controls was significant (P=0.027). Urine potassium concentration was lower in cases compared to controls (IQR; 47[27-70] mEq/24 h vs. 50[29-56] mEq/24 h]. However, this difference was not significant (P=0.807). Table 1 presents the level of the measured variables and the significant differences between the two groups.
Discussion
There are several reasons to postulate that sodium and potassium intake is involved in MS. Recent studies have shown the complex effects of sodium on different cellular components which are involved in central nervous system autoimmunity [15].
First of all, the high salt condition can activate the MAPK (p38/Mitogen-Activated Protein Kinase) pathway leading to upregulation of the transcriptional factor NFAT5 (Nuclear Factor of Activated T-cells 5), which enhances the expression of sodium sensing serine/threonine kinase named SGK1 (Serum Glucocorticoid Kinase 1). SGK1 has been shown to be highly expressed in CD4+ T cells and has a pivotal role in Th 17 differentiation and induction of IL-17A production [8, 15]. Based on these studies, gene silencing or chemical inhibition of SGK1, p38/MAPK or NFAT5 can prevent the development of high-salt induced Th 17 cell [16].
Multiple Sclerosis (MS) is a chronic inflammatory disorder of the central nervous system leading to both axonal damage and demyelination. This disease is more common in young adults [1, 2]. Common presentations of MS include sensory disturbance, visual loss, diplopia, limb weakness, and vertigo [3]. The number of patients with MS is increasing in developing countries such as Iran [4-6]. It seems that MS usually develops in genetically susceptible people as a result of environmental exposure. Several environmental factors, such as viral infection, vitamin D deficiency, smoking, obesity, sunlight exposure, and diet have been postulated as potential risk factors for the increasing incidence of autoimmune diseases such as MS [7-9].
The mean sodium intake in most Asian countries is over 100 mmol/d, while the recommended salt intake value by WHO is 80-90 mmol/d [10] and in many societies the mean potassium consumption is below 70-80 mmol/d, which is the value recommended by the WHO (1988, 2003). Previous research studies have shown that high salt intake is associated with the development of autoimmune diseases and aggravations of MS symptoms in patients, while potassium supplementation has a diverse effect [11, 12].
T helper 17 (Th 17) cells produce Interleukin (IL)-17, which is a pro-inflammatory cytokine. It plays a critical role in the pathogenesis of autoimmune diseases. High physiological salt concentrations have a direct effect on the induction of Th 17 cells and IL-17, which initiate pro-inflammatory signatures. However, potassium supplements block IL-17 production in T lymphocytes [11-14]. In this research, we performed a case-control study to investigate whether NaCl uptake in MS patients is one of the underlying factors of the disease.
Materials and Methods
A total of 23 patients with Relapsing-Remitting Multiple Sclerosis (RRMS) were randomly selected from Alzahra Clinic of MS, Isfahan, Iran. Then, 23 healthy controls matched with the cases based on gender and age were selected, too. The participants were recruited between October and June 2016, and all of them signed informed consent forms. Furthermore, the Ethics Committee of Isfahan Research Committee of Multiple Sclerosis approved this project.
Study samples
The MS patients, aged 15-46 years, would be included in this study if they were clinically diagnosed with MS with a relapsing-remitting course for more than 1 year. Exclusion criteria were the presence of renal disease, diabetes insipidus, and adrenal disorders. In addition, the patients using diuretics were excluded from this study.
Sodium chloride (NaCl) and Potassium measurements
The levels of sodium chloride (NaCl) and potassium were measured in 24 hours of urine. To confirm that the high level of sodium in urine is not due to the elevated level of sodium in the blood, we also measured the level of blood sodium. In addition, the blood creatinine level and Glomerular Filtration Rate (GFR) were also measured in all cases and controls to rule out kidney diseases. All the measurements were performed in one laboratory and by the same technicians.
Statistical analysis
All analyses were performed in SPSS (version 23.0). We used the Chi-square test to compare NaCl and potassium level in 24 hours of urine between cases and controls. Conditional logistic regression was performed to determine the effect of daily sodium and potassium intake on the development of MS. The level of significance was set at P<0.050 in all analysis.
Results
In this study, a total of 46 subjects (23 cases and 23 matched controls for sex and age) were included. The Mean±SD age of cases and controls was 27±7 years. In each group, 5 (22%) males and 18 (78%) females were studied. Serum sodium concentrations were in the normal range, and none of the cases or controls had hyper- or hypo-natremia (Mean±SD: 139±2 mEq/L vs. 139±2 mEq/L, respectively) (P=0.262). Urine sodium level was relatively higher in cases compared to controls (Interquartile Range [IQR]; 160 [140-211] mEq/24 h vs. 128 [83-166] mEq/24 h), which is probably due to higher sodium intake in the patients. The difference in the level of urine sodium between the two groups of cases and controls was significant (P=0.027). Urine potassium concentration was lower in cases compared to controls (IQR; 47[27-70] mEq/24 h vs. 50[29-56] mEq/24 h]. However, this difference was not significant (P=0.807). Table 1 presents the level of the measured variables and the significant differences between the two groups.
Discussion
There are several reasons to postulate that sodium and potassium intake is involved in MS. Recent studies have shown the complex effects of sodium on different cellular components which are involved in central nervous system autoimmunity [15].
First of all, the high salt condition can activate the MAPK (p38/Mitogen-Activated Protein Kinase) pathway leading to upregulation of the transcriptional factor NFAT5 (Nuclear Factor of Activated T-cells 5), which enhances the expression of sodium sensing serine/threonine kinase named SGK1 (Serum Glucocorticoid Kinase 1). SGK1 has been shown to be highly expressed in CD4+ T cells and has a pivotal role in Th 17 differentiation and induction of IL-17A production [8, 15]. Based on these studies, gene silencing or chemical inhibition of SGK1, p38/MAPK or NFAT5 can prevent the development of high-salt induced Th 17 cell [16].
Th 17 bias and IL-17A are involved in the pathogenesis of autoimmune diseases [17]. However, studies have shown that potassium is able to reduce IL-17 expression that has increased due to high levels of NaCl. In a recent study performed by Wen et al. it is demonstrated that high potassium intake can block the effects of sodium on p38/MAPK-SGK1 signaling pathway. In addition, some research suggests that potassium supplements antagonize pathogenicity of salt by their antioxidant effects. Moreover, it has been hypothesized that elevated level of extracellular potassium can increase the activity of membrane sodium pump and results in hyperpolarization of the cell membrane and decreases oxidase activity [12].
The differentiation of Th 17 is crucial since these cells are the first ones that cross the blood-brain barrier [18] and upon interaction with antigen-presenting microglial cells in the CNS, a chemokine cascade is activated that recruits further immune cells, such as other T cells and inflammatory monocytes [15]. However, in addition to the increased production of Th 17 cells and IL-17A, excess dietary sodium intake can lead to autoimmunity by inhibiting the function of FOXP3+ Tregs (regulatory T-cells) that are involved in the maintenance of self-tolerance. People who frequently use processed food, characterized by high levels of salt, have an increased ratio of proinflammatory IL-17+/anti-inflammatory FOXP3+ T cells in comparison to individuals who have a low intake of processed food [19].
High-salt diets induce a proinflammatory Treg phenotype associated with an SGK1-dependent increase in IFNγ (Interferon gamma) secretion which has been seen in patients with relapsing-remitting MS [11, 20]. Hernandez et al. have shown that under high-salt in vitro conditions, IFNγ blockade by either monoclonal antibodies or shRNA-mediated knockdown can restore Treg function. Furthermore, shRNA-mediated knockdown or chemical inhibition of SGK1 in human Tregs inhibits IFNγ secretion while restoring suppressive function [11]. In addition to the mentioned effects of sodium on autoimmunity, proinflammatory myeloid cells, including monocytes, macrophages, and microglial cells, also play a crucial role in inflammation of CNS [21].
High concentrations of NaCl induce myeloid cell activation and promote the phosphorylation of members of the MAPK pathway in macrophages. Macrophages also produce high levels of chemokines, such as MIP (macrophage inflammatory protein-2) and CCL2 (C-C motif Ligand 2) which are strongly related to salt intake. CCL2 plays a pivotal role in the migration of inflammatory monocytes to the CNS during Experimental Allergic Encephalomyelitis (EAE, an animal model of MS) [15]. Moreover, studies have shown that intestinal epithelial cells play a significant role in the maintenance of self-tolerance.
In vitro studies have demonstrated that in high salt condition, induction of Na+-glucose co-transport in colon epithelial cells activates the Na+/H+ exchanger 3. As these events occur, the intestinal permeability increases, leading to the entry of foreign immunogenic antigens that can induce autoimmune processes [15]. In addition, hyperosmolaric treatment of epithelial cells promotes the production of proinflammatory cytokines mediated by the MAPK pathway [22]. Furthermore, endothelial cells, which are in contact with plasma sodium, can respond to small increases in sodium level. Under in vitro shear stress conditions, high-salt condition promotes responsiveness to TNFα (tumor necrosis factor-alpha) in endothelial cells, which is characterized by enhanced expression of adhesion molecules such as E-selectin and VCAM-1 (vascular cell adhesion molecule 1) [11].
Under saltwater treatment, CCL2 is highly upregulated which promotes the adhesion of leukocytes to the brain microvasculature [23]. Therefore, sodium changes the phenotype of endothelial cells and results in immune cell infiltration to CNS which can lead to autoimmunity. Finally, recent studies have shown that high-salt diets can affect gut microbiota and gut flora might influence Th 17 cells and Tregs [11]. Thus, reducing salt intake and consumption of probiotic foods may have beneficial effects on autoimmune diseases.
Conclusion
In this study, for the first time, we have shown that high levels of sodium intake are associated with the development of Multiple Sclerosis. Therefore, it can be considered as one of the risk factors for MS. However, while there are some hypothesis showing that potassium also plays a role in the development of MS, but we did not observe any significant correlation between potassium intake and the risk of MS. Additional studies are needed to investigate whether an alternation in dietary salt and potassium intake proves beneficial in MS.
Ethical Considerations
Compliance with ethical guidelines
All the study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki (1957 Draft).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
Initial draft: Sarah Ghorbani; Writing, revision, and editing the manuscript: All authors; Funding acquisition: Sarah Ghorbani, Amir Hossain Mahmoudzadeh, Sina Raeisi; Providing resources: All authors; and Study supervision: Masoud Etemadifar.
Conflict of interest
The authors declared no conflict of interest.
References
Gustavsen MW, Page CM, Moen SM, Bjølgerudl A, Berg-Hansen P, Nygaard GO, et al. Environmental exposures and the risk of Multiple Sclerosis investigated in a Norwegian case-control study. BMC Neurol. 2014; 14:196. [DOI:10.1186/s12883-014-0196-x] [PMID] [PMCID]
Yadav SK, Mindur JE, Ito K, Dhib-Jalbut S. Advances in the immunopathogenesis of Multiple Sclerosis. Curr Opin Neurol. 2015; 28(3):206-19. [DOI:10.1097/WCO.0000000000000205] [PMID]
Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006; 354(6):610-21. [DOI:10.1056/NEJMra052723] [PMID]
Harandi AA, Shahbeigi S, Pakdaman H, Fereshtehnejad SM, Nikravesh E, Jalilzadeh R. Association of serum 25 (OH) vitamin D3 concentration with severity of Multiple Sclerosis. Iran J Neurol. 2012; 11(2):54-8. [PMID] [PMCID]
Eskandarieh S, Heydarpour P, Elhami SR, Sahraian MA. Prevalence and incidence of Multiple Sclerosis in Tehran, Iran. Iran J Public Health. 2017; 46(5):699-704. [PMID] [PMCID]
Etemadifar M, Mehrabi B, Kiani‐Peykani R, Abtahi SH, Nekouie‐Isfahani K, Ramagopalan SV, et al. Soil heavy metals are associated with the distribution of Multiple Sclerosis in Isfahan, Iran. Acta Neurol Scand. 2016; 134(4):292-9. [DOI:10.1111/ane.12543] [PMID]
Ramagopalan SV, Handel AE, Giovannoni G, Siegel SR, Ebers GC, Chaplin G. Relationship of UV exposure to prevalence of Multiple Sclerosis in England. Neurol. 2011; 76(16):1410-4. [DOI:10.1212/WNL.0b013e318216715e] [PMID] [PMCID]
Takeuchi H. Does salt exacerbate Multiple Sclerosis? Clin Exp Neuroimmunol. 2013; 4(1):5-6. [DOI:10.1111/cen3.12016]
Zostawa J, Adamczyk J, Sowa P, Adamczyk-Sowa M. The influence of sodium on pathophysiology of Multiple Sclerosis. Neurol Sci. 2017; 38(3):389-98. [DOI:10.1007/s10072-016-2802-8] [PMID] [PMCID]
Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: Implications for public health. Int J Epidemiol. 2009; 38(3):791-813. [DOI:10.1093/ije/dyp139] [PMID]
Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015; 125(11):4212-22. [DOI:10.1172/JCI81151] [PMID] [PMCID]
Wen W, Wan Z, Ren K, Zhou D, Gao Q, Wu Y, et al. Potassium supplementation inhibits IL-17A production induced by salt loading in human T lymphocytes via p38/MAPK-SGK1 pathway. Exp Mol Pathol Suppl. 2016; 100(3):370-7. [DOI:10.1016/j.yexmp.2016.03.009] [PMID]
Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T. The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediators Inflamm. 2017; 2017. [DOI:10.1155/2017/3908061.] [PMID] [PMCID]
Cortese M, Yuan C, Chitnis T, Ascherio A, Munger KL. No association between dietary sodium intake and the risk of Multiple Sclerosis. Neurol. 2017; 89(13):1322-9. [DOI:10.1212/WNL.0000000000004417] [PMID] [PMCID]
Hucke S, Wiendl H, Klotz L. Implications of dietary salt intake for Multiple Sclerosis pathogenesis. Mult Scler J. 2016; 22(2):133-9. [DOI:10.1177/1352458515609431] [PMID]
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic T H 17 cells. Nat. 2013; 496(7446):518-22. [DOI:10.1038/nature11868] [PMID] [PMCID]
Yamaguchi Y, Fujio K, Shoda H, Okamoto A, Tsuno NH, Takahashi K, et al. IL-17B and IL-17C are associated with TNF-α production and contribute to the exacerbation of inflammatory arthritis. J Immunol. 2007; 179(10):7128-36. [DOI:10.4049/jimmunol.179.10.7128] [PMID]
Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, et al. CC chemokine receptor 6–regulated entry of T H-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009; 10(5):514-23. [DOI:10.1038/ni.1716] [PMID]
Poutahidis T, Kleinewietfeld M, Smillie C, Levkovich T, Perrotta A, Bhela S, et al. Microbial reprogramming inhibits Western diet-associated obesity. PloS one. 2013; 8(7):e68596. [DOI:10.1371/journal.pone.0068596] [PMID] [PMCID]
Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1–like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011; 17(6):673-5. [DOI:10.1038/nm.2389] [PMID] [PMCID]
Mildner A, Mack M, Schmidt H, Brück W, Djukic M, Zabel MD, Hille A, Priller J, Prinz M. CCR2+ Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009; 132(9):2487-500. [DOI:10.1093/brain/awp144] [PMID]
Wang C, Shi X, Chen X, Wu H, Zhang H, Xie J, et al. 17-β-estradiol inhibits hyperosmolarity-induced proinflammatory cytokine elevation via the p38 MAPK pathway in human corneal epithelial cells. Mol Vis. 2012; 18:1115-22. [PMID] [PMCID]
dos Santos AC, Barsante MM, Arantes RM, Bernard CC, Teixeira MM, Carvalho-Tavares J. CCL2 and CCL5 mediate leukocyte adhesion in experimental autoimmune encephalomyelitis—an intravital microscopy study. J Neuroimmunol. 2005; 162(1-2):122-9. [DOI:10.1016/j.jneuroim.2005.01.020] [PMID]
The differentiation of Th 17 is crucial since these cells are the first ones that cross the blood-brain barrier [18] and upon interaction with antigen-presenting microglial cells in the CNS, a chemokine cascade is activated that recruits further immune cells, such as other T cells and inflammatory monocytes [15]. However, in addition to the increased production of Th 17 cells and IL-17A, excess dietary sodium intake can lead to autoimmunity by inhibiting the function of FOXP3+ Tregs (regulatory T-cells) that are involved in the maintenance of self-tolerance. People who frequently use processed food, characterized by high levels of salt, have an increased ratio of proinflammatory IL-17+/anti-inflammatory FOXP3+ T cells in comparison to individuals who have a low intake of processed food [19].
High-salt diets induce a proinflammatory Treg phenotype associated with an SGK1-dependent increase in IFNγ (Interferon gamma) secretion which has been seen in patients with relapsing-remitting MS [11, 20]. Hernandez et al. have shown that under high-salt in vitro conditions, IFNγ blockade by either monoclonal antibodies or shRNA-mediated knockdown can restore Treg function. Furthermore, shRNA-mediated knockdown or chemical inhibition of SGK1 in human Tregs inhibits IFNγ secretion while restoring suppressive function [11]. In addition to the mentioned effects of sodium on autoimmunity, proinflammatory myeloid cells, including monocytes, macrophages, and microglial cells, also play a crucial role in inflammation of CNS [21].
High concentrations of NaCl induce myeloid cell activation and promote the phosphorylation of members of the MAPK pathway in macrophages. Macrophages also produce high levels of chemokines, such as MIP (macrophage inflammatory protein-2) and CCL2 (C-C motif Ligand 2) which are strongly related to salt intake. CCL2 plays a pivotal role in the migration of inflammatory monocytes to the CNS during Experimental Allergic Encephalomyelitis (EAE, an animal model of MS) [15]. Moreover, studies have shown that intestinal epithelial cells play a significant role in the maintenance of self-tolerance.
In vitro studies have demonstrated that in high salt condition, induction of Na+-glucose co-transport in colon epithelial cells activates the Na+/H+ exchanger 3. As these events occur, the intestinal permeability increases, leading to the entry of foreign immunogenic antigens that can induce autoimmune processes [15]. In addition, hyperosmolaric treatment of epithelial cells promotes the production of proinflammatory cytokines mediated by the MAPK pathway [22]. Furthermore, endothelial cells, which are in contact with plasma sodium, can respond to small increases in sodium level. Under in vitro shear stress conditions, high-salt condition promotes responsiveness to TNFα (tumor necrosis factor-alpha) in endothelial cells, which is characterized by enhanced expression of adhesion molecules such as E-selectin and VCAM-1 (vascular cell adhesion molecule 1) [11].
Under saltwater treatment, CCL2 is highly upregulated which promotes the adhesion of leukocytes to the brain microvasculature [23]. Therefore, sodium changes the phenotype of endothelial cells and results in immune cell infiltration to CNS which can lead to autoimmunity. Finally, recent studies have shown that high-salt diets can affect gut microbiota and gut flora might influence Th 17 cells and Tregs [11]. Thus, reducing salt intake and consumption of probiotic foods may have beneficial effects on autoimmune diseases.
Conclusion
In this study, for the first time, we have shown that high levels of sodium intake are associated with the development of Multiple Sclerosis. Therefore, it can be considered as one of the risk factors for MS. However, while there are some hypothesis showing that potassium also plays a role in the development of MS, but we did not observe any significant correlation between potassium intake and the risk of MS. Additional studies are needed to investigate whether an alternation in dietary salt and potassium intake proves beneficial in MS.
Ethical Considerations
Compliance with ethical guidelines
All the study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki (1957 Draft).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
Initial draft: Sarah Ghorbani; Writing, revision, and editing the manuscript: All authors; Funding acquisition: Sarah Ghorbani, Amir Hossain Mahmoudzadeh, Sina Raeisi; Providing resources: All authors; and Study supervision: Masoud Etemadifar.
Conflict of interest
The authors declared no conflict of interest.
References
Gustavsen MW, Page CM, Moen SM, Bjølgerudl A, Berg-Hansen P, Nygaard GO, et al. Environmental exposures and the risk of Multiple Sclerosis investigated in a Norwegian case-control study. BMC Neurol. 2014; 14:196. [DOI:10.1186/s12883-014-0196-x] [PMID] [PMCID]
Yadav SK, Mindur JE, Ito K, Dhib-Jalbut S. Advances in the immunopathogenesis of Multiple Sclerosis. Curr Opin Neurol. 2015; 28(3):206-19. [DOI:10.1097/WCO.0000000000000205] [PMID]
Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006; 354(6):610-21. [DOI:10.1056/NEJMra052723] [PMID]
Harandi AA, Shahbeigi S, Pakdaman H, Fereshtehnejad SM, Nikravesh E, Jalilzadeh R. Association of serum 25 (OH) vitamin D3 concentration with severity of Multiple Sclerosis. Iran J Neurol. 2012; 11(2):54-8. [PMID] [PMCID]
Eskandarieh S, Heydarpour P, Elhami SR, Sahraian MA. Prevalence and incidence of Multiple Sclerosis in Tehran, Iran. Iran J Public Health. 2017; 46(5):699-704. [PMID] [PMCID]
Etemadifar M, Mehrabi B, Kiani‐Peykani R, Abtahi SH, Nekouie‐Isfahani K, Ramagopalan SV, et al. Soil heavy metals are associated with the distribution of Multiple Sclerosis in Isfahan, Iran. Acta Neurol Scand. 2016; 134(4):292-9. [DOI:10.1111/ane.12543] [PMID]
Ramagopalan SV, Handel AE, Giovannoni G, Siegel SR, Ebers GC, Chaplin G. Relationship of UV exposure to prevalence of Multiple Sclerosis in England. Neurol. 2011; 76(16):1410-4. [DOI:10.1212/WNL.0b013e318216715e] [PMID] [PMCID]
Takeuchi H. Does salt exacerbate Multiple Sclerosis? Clin Exp Neuroimmunol. 2013; 4(1):5-6. [DOI:10.1111/cen3.12016]
Zostawa J, Adamczyk J, Sowa P, Adamczyk-Sowa M. The influence of sodium on pathophysiology of Multiple Sclerosis. Neurol Sci. 2017; 38(3):389-98. [DOI:10.1007/s10072-016-2802-8] [PMID] [PMCID]
Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: Implications for public health. Int J Epidemiol. 2009; 38(3):791-813. [DOI:10.1093/ije/dyp139] [PMID]
Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015; 125(11):4212-22. [DOI:10.1172/JCI81151] [PMID] [PMCID]
Wen W, Wan Z, Ren K, Zhou D, Gao Q, Wu Y, et al. Potassium supplementation inhibits IL-17A production induced by salt loading in human T lymphocytes via p38/MAPK-SGK1 pathway. Exp Mol Pathol Suppl. 2016; 100(3):370-7. [DOI:10.1016/j.yexmp.2016.03.009] [PMID]
Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T. The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediators Inflamm. 2017; 2017. [DOI:10.1155/2017/3908061.] [PMID] [PMCID]
Cortese M, Yuan C, Chitnis T, Ascherio A, Munger KL. No association between dietary sodium intake and the risk of Multiple Sclerosis. Neurol. 2017; 89(13):1322-9. [DOI:10.1212/WNL.0000000000004417] [PMID] [PMCID]
Hucke S, Wiendl H, Klotz L. Implications of dietary salt intake for Multiple Sclerosis pathogenesis. Mult Scler J. 2016; 22(2):133-9. [DOI:10.1177/1352458515609431] [PMID]
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic T H 17 cells. Nat. 2013; 496(7446):518-22. [DOI:10.1038/nature11868] [PMID] [PMCID]
Yamaguchi Y, Fujio K, Shoda H, Okamoto A, Tsuno NH, Takahashi K, et al. IL-17B and IL-17C are associated with TNF-α production and contribute to the exacerbation of inflammatory arthritis. J Immunol. 2007; 179(10):7128-36. [DOI:10.4049/jimmunol.179.10.7128] [PMID]
Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, et al. CC chemokine receptor 6–regulated entry of T H-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009; 10(5):514-23. [DOI:10.1038/ni.1716] [PMID]
Poutahidis T, Kleinewietfeld M, Smillie C, Levkovich T, Perrotta A, Bhela S, et al. Microbial reprogramming inhibits Western diet-associated obesity. PloS one. 2013; 8(7):e68596. [DOI:10.1371/journal.pone.0068596] [PMID] [PMCID]
Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1–like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011; 17(6):673-5. [DOI:10.1038/nm.2389] [PMID] [PMCID]
Mildner A, Mack M, Schmidt H, Brück W, Djukic M, Zabel MD, Hille A, Priller J, Prinz M. CCR2+ Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009; 132(9):2487-500. [DOI:10.1093/brain/awp144] [PMID]
Wang C, Shi X, Chen X, Wu H, Zhang H, Xie J, et al. 17-β-estradiol inhibits hyperosmolarity-induced proinflammatory cytokine elevation via the p38 MAPK pathway in human corneal epithelial cells. Mol Vis. 2012; 18:1115-22. [PMID] [PMCID]
dos Santos AC, Barsante MM, Arantes RM, Bernard CC, Teixeira MM, Carvalho-Tavares J. CCL2 and CCL5 mediate leukocyte adhesion in experimental autoimmune encephalomyelitis—an intravital microscopy study. J Neuroimmunol. 2005; 162(1-2):122-9. [DOI:10.1016/j.jneuroim.2005.01.020] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2018/04/29 | Accepted: 2018/10/5 | Published: 2019/01/1
Received: 2018/04/29 | Accepted: 2018/10/5 | Published: 2019/01/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |