Fri, Apr 26, 2024
Volume 4, Issue 3 (Summer 2018)
Caspian J Neurol Sci 2018, 4(3): 108-113 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sedighi B, Ghaseminejad A, Abna Z, Hassani B. Optical Coherence Tomography and Corpus Callosum Index in Cognitive Assessment of Multiple Sclerosis Patients. Caspian J Neurol Sci 2018; 4 (3) :108-113
URL: http://cjns.gums.ac.ir/article-1-220-en.html
URL: http://cjns.gums.ac.ir/article-1-220-en.html
1- Associate Professor of Neurology, Neurological Diseases Research Center, Kerman University of Medical Sciences, Kerman, Iran
2- Assistant Professor of Ophthalmology, Ophthalmology Research Center, Kerman University of Medical Sciences, Kerman, Iran
3- Neurologist, Private Practitioner, Kerman, Iran , abna.zohreh90@yahoo.com
4- Neuroscientist and Neurocognitive Psychologist, Neurological Diseases Research Center, Kerman University of Medical Sciences, Kerman, Iran
2- Assistant Professor of Ophthalmology, Ophthalmology Research Center, Kerman University of Medical Sciences, Kerman, Iran
3- Neurologist, Private Practitioner, Kerman, Iran , abna.zohreh90@yahoo.com
4- Neuroscientist and Neurocognitive Psychologist, Neurological Diseases Research Center, Kerman University of Medical Sciences, Kerman, Iran
Full-Text [PDF 1181 kb]
(958 Downloads)
| Abstract (HTML) (2840 Views)
Full-Text: (759 Views)
Introduction
Multiple Sclerosis (MS) is an immune-mediated disease of the Central Nervous System (CNS) with both inflammatory and degenerative components [1]. Axonal and neuronal degeneration seem to be the pathological processes responsible for permanent disability in this disease [2]. Cognitive dysfunction is among major aspects of MS neurodegeneration which involves recent memory, attention, information processing speed, and executive functions [3, 4]. As the disease progresses, patients may experience greater difficulties at work, in social interactions and daily activities, regardless of physical defects.
Different cognitive tests are used in the screening or evaluation of cognitive function in MS; however, the use of such tests are limited due to being time consuming and costly, and disregarding domain extent/specificity. Physical disability does not accurately predict cognitive dysfunction in these patients [3, 5]. Thus, there is a great need to identify objective and quantitative methods with high sensitivity and specificity for the early evaluation of axonal degeneration, including cognitive disorders in patients with MS.
Various studies highlighted the role of neuroimaging in the evaluation of neuronal degeneration and cognitive dysfunction in MS [6, 9]. Axonal loss has been reported in the Corpus Callosum (CC) of MS patients, a major white matter bundle, connecting the cortical and subcortical regions of both hemispheres [10, 11]. Corpus Callosum Index (CCI) reflects the extent of axonal loss in MS patients [10].
In a study by Carolina et al. cognitive dysfunction was concomitant with changes in the CC; however, cognitive decline is not directly related to macroscopic lesion load [11]. Therefore, the quantitative MRI abnormalities in the CC-especially the portions outside T2 lesions- partially account for cognitive dysfunction in MS [12]. Other imaging technique to identify the axonal degeneration in MS is the analysis of Retinal Nerve Fiber Layer (RNFL) thickness that is measured by Optic Coherence Tomography (OCT) [2].
The measurement of RNFL thickness by OCT may be a better way than brain MRI to detect and monitor axonal loss in MS [13]. There is also evidence that OCT changes are correlated with an increased level of cognitive impairment [14]. According to Toledo et al. the degree of RNFL atrophy was correlated with cognitive disability [15]. Therefore, this study aimed to compare OCT with CCI, to evaluate cognitive dysfunction in MS patients. This would help to identify new methods for monitoring the disease course and early detection of axonal damages like cognitive dysfunction. Another objective of this study was to explore whether OCT or CCI can be used as predictive methods for cognitive impairments in MS patients, in other words, which method is more accurate.
Materials and Methods
This study was conducted in 5 months, in 2016. A total of 30 diagnosed patients with relapsing-remitting MS according to 2017 revised McDonald criteria referring to outpatient clinic of a university hospital (Shafa Hospital in Kerman, Iran) were selected. To improve the accuracy of the study, the patients with a history of optic neuritis, also the patients with ophthalmic disorders such as glaucoma, cataract and severe refractive error, were excluded from this study. Clinical and demographic information including age, sex and the duration of disease were collected from the patients. The degree of disability was assessed by a neurologist using the Expanded Disability Status Scale (EDSS).
Multiple Sclerosis (MS) is an immune-mediated disease of the Central Nervous System (CNS) with both inflammatory and degenerative components [1]. Axonal and neuronal degeneration seem to be the pathological processes responsible for permanent disability in this disease [2]. Cognitive dysfunction is among major aspects of MS neurodegeneration which involves recent memory, attention, information processing speed, and executive functions [3, 4]. As the disease progresses, patients may experience greater difficulties at work, in social interactions and daily activities, regardless of physical defects.
Different cognitive tests are used in the screening or evaluation of cognitive function in MS; however, the use of such tests are limited due to being time consuming and costly, and disregarding domain extent/specificity. Physical disability does not accurately predict cognitive dysfunction in these patients [3, 5]. Thus, there is a great need to identify objective and quantitative methods with high sensitivity and specificity for the early evaluation of axonal degeneration, including cognitive disorders in patients with MS.
Various studies highlighted the role of neuroimaging in the evaluation of neuronal degeneration and cognitive dysfunction in MS [6, 9]. Axonal loss has been reported in the Corpus Callosum (CC) of MS patients, a major white matter bundle, connecting the cortical and subcortical regions of both hemispheres [10, 11]. Corpus Callosum Index (CCI) reflects the extent of axonal loss in MS patients [10].
In a study by Carolina et al. cognitive dysfunction was concomitant with changes in the CC; however, cognitive decline is not directly related to macroscopic lesion load [11]. Therefore, the quantitative MRI abnormalities in the CC-especially the portions outside T2 lesions- partially account for cognitive dysfunction in MS [12]. Other imaging technique to identify the axonal degeneration in MS is the analysis of Retinal Nerve Fiber Layer (RNFL) thickness that is measured by Optic Coherence Tomography (OCT) [2].
The measurement of RNFL thickness by OCT may be a better way than brain MRI to detect and monitor axonal loss in MS [13]. There is also evidence that OCT changes are correlated with an increased level of cognitive impairment [14]. According to Toledo et al. the degree of RNFL atrophy was correlated with cognitive disability [15]. Therefore, this study aimed to compare OCT with CCI, to evaluate cognitive dysfunction in MS patients. This would help to identify new methods for monitoring the disease course and early detection of axonal damages like cognitive dysfunction. Another objective of this study was to explore whether OCT or CCI can be used as predictive methods for cognitive impairments in MS patients, in other words, which method is more accurate.
Materials and Methods
This study was conducted in 5 months, in 2016. A total of 30 diagnosed patients with relapsing-remitting MS according to 2017 revised McDonald criteria referring to outpatient clinic of a university hospital (Shafa Hospital in Kerman, Iran) were selected. To improve the accuracy of the study, the patients with a history of optic neuritis, also the patients with ophthalmic disorders such as glaucoma, cataract and severe refractive error, were excluded from this study. Clinical and demographic information including age, sex and the duration of disease were collected from the patients. The degree of disability was assessed by a neurologist using the Expanded Disability Status Scale (EDSS).
MRI procedure
MRI Siemens, 1.5 Tesla was used in the current study. CCI was calculated by a neurologist based on the previous studies. A conventional mid-sagittal T1W image was used. A straight line was drawn at the greatest anteroposterior diameter of CC and a perpendicular line was drawn to its midline. Anterior, posterior, and medium segments of CC were measured and normalized to its greatest anteroposterior diameter (Figure 1) [16]. With respect to differences on the quantitative analysis of CC due to racial/ethnic factors [17], we have selected healthy subjects matched on age, gender, and race to the cases, as the controls to obtain the normal range of CCI. The mean score of CCI was 0.384 cm in the control group.
Optical Coherence Tomography (OCT)
OCT for assessing optical nerve thickness was interpreted by an ophthalmologist, with the use of Optovue device (RTvue, USA) [18]. The cutoff point for the thinning of RNFL was 90 μm.
Cognitive assessment
Cognitive assessment of MS patients was performed by a neurocognitive psychologist via Brief International Cognitive Assessment for MS (BICAMS). This is a brief cognitive assessment for MS patients, optimized for small centers and is a subset of the Minimal Assessment of Cognitive Function in MS battery (MACFIMS) [19]. The test consists of 3 components: Brief Visuospatial Memory Test-Revised (BVMT-R), California Verbal Learning Test-Second edition (CVLT2), and Symbol Digit Modality Test (SDMT). The raw score of 9 was considered as the cutoff point.
Data analysis
All data were analyzed using SPSS. The Chi-square test was used to compare the categorical variables. P<0.05 were regarded as statistically significant.
Results
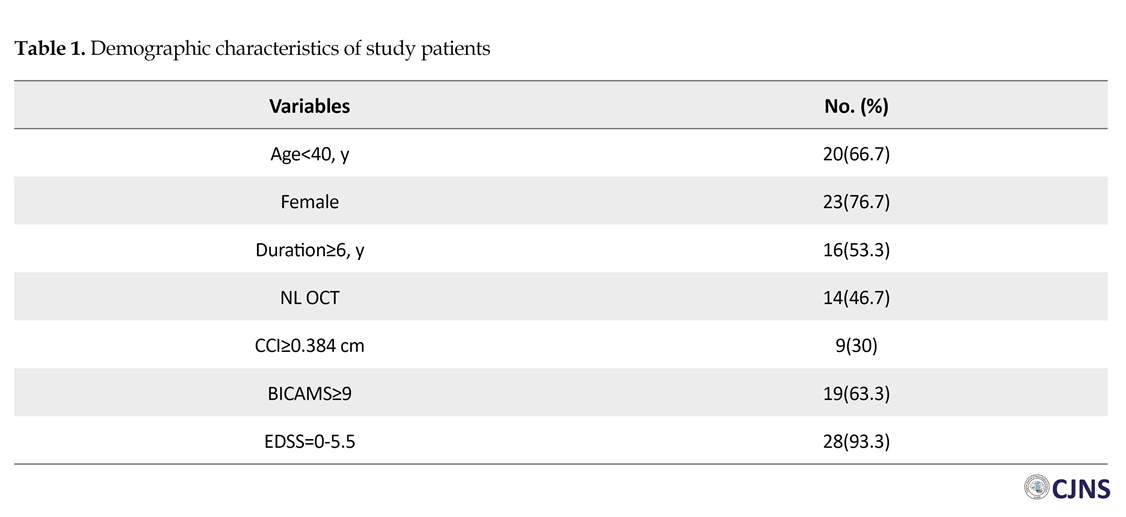
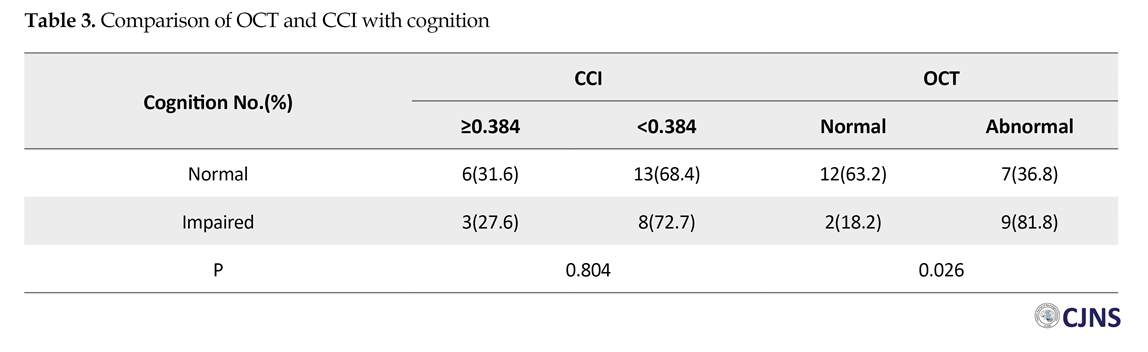
The numbers (%) of all variables are presented in Table 1. A total of 11 (36.7%) patients had an impaired cognitive function as per BICAMS. Among all patients, 21 (70%) subjects had CCI less than 0.384 cm, and 16 (53.3%) had abnormal OCT in one or both eyes. Table 2 represents age, sex, the duration of disease and EDSS of the patients, according to their cognitive status. There was no significant difference among these subjects (P>0.05). Table 3 demonstrates the correlation between OCT and CCI, in respect with cognition. According to Table 3, there was a significant difference between cognitive status and OCT in MS patients (P=0.026).
Discussion
Results of the current study revealed a significant correlation between OCT and cognitive dysfunction in relapsing-remitting MS patients, but there was no such relation with respect of CCI in them. Therefore, OCT could be used as a diagnostic tool for the early assessment of cognitive dysfunction in relapsing-remitting MS patients. The earliest research regarding the use of OCT in the evaluation of MS progress was performed by Parisi and associates. They suggested that OCT is a precise tool for the assessment of MS progress, because it demonstrates the earliest neurodegenerative changes occurring in the CNS [20].
In a systematic review by Petzold et al., the evidence for the use of OCT in MS was reviewed. Retinal thinning in the absence of optical neuritis could be explained through the retrograde trans-synaptic degeneration and the progressive loss of retinal ganglion cells. They suggested OCT as a new and promising evaluation tool for studying and the evaluation of MS [21].
Previous studies have evaluated structural changes in brain MRI of MS patients. The results of a 17-year longitudinal study by Granberg suggested that corpus
MRI Siemens, 1.5 Tesla was used in the current study. CCI was calculated by a neurologist based on the previous studies. A conventional mid-sagittal T1W image was used. A straight line was drawn at the greatest anteroposterior diameter of CC and a perpendicular line was drawn to its midline. Anterior, posterior, and medium segments of CC were measured and normalized to its greatest anteroposterior diameter (Figure 1) [16]. With respect to differences on the quantitative analysis of CC due to racial/ethnic factors [17], we have selected healthy subjects matched on age, gender, and race to the cases, as the controls to obtain the normal range of CCI. The mean score of CCI was 0.384 cm in the control group.
Optical Coherence Tomography (OCT)
OCT for assessing optical nerve thickness was interpreted by an ophthalmologist, with the use of Optovue device (RTvue, USA) [18]. The cutoff point for the thinning of RNFL was 90 μm.
Cognitive assessment
Cognitive assessment of MS patients was performed by a neurocognitive psychologist via Brief International Cognitive Assessment for MS (BICAMS). This is a brief cognitive assessment for MS patients, optimized for small centers and is a subset of the Minimal Assessment of Cognitive Function in MS battery (MACFIMS) [19]. The test consists of 3 components: Brief Visuospatial Memory Test-Revised (BVMT-R), California Verbal Learning Test-Second edition (CVLT2), and Symbol Digit Modality Test (SDMT). The raw score of 9 was considered as the cutoff point.
Data analysis
All data were analyzed using SPSS. The Chi-square test was used to compare the categorical variables. P<0.05 were regarded as statistically significant.
Results
The numbers (%) of all variables are presented in Table 1. A total of 11 (36.7%) patients had an impaired cognitive function as per BICAMS. Among all patients, 21 (70%) subjects had CCI less than 0.384 cm, and 16 (53.3%) had abnormal OCT in one or both eyes. Table 2 represents age, sex, the duration of disease and EDSS of the patients, according to their cognitive status. There was no significant difference among these subjects (P>0.05). Table 3 demonstrates the correlation between OCT and CCI, in respect with cognition. According to Table 3, there was a significant difference between cognitive status and OCT in MS patients (P=0.026).
Discussion
Results of the current study revealed a significant correlation between OCT and cognitive dysfunction in relapsing-remitting MS patients, but there was no such relation with respect of CCI in them. Therefore, OCT could be used as a diagnostic tool for the early assessment of cognitive dysfunction in relapsing-remitting MS patients. The earliest research regarding the use of OCT in the evaluation of MS progress was performed by Parisi and associates. They suggested that OCT is a precise tool for the assessment of MS progress, because it demonstrates the earliest neurodegenerative changes occurring in the CNS [20].
In a systematic review by Petzold et al., the evidence for the use of OCT in MS was reviewed. Retinal thinning in the absence of optical neuritis could be explained through the retrograde trans-synaptic degeneration and the progressive loss of retinal ganglion cells. They suggested OCT as a new and promising evaluation tool for studying and the evaluation of MS [21].
Previous studies have evaluated structural changes in brain MRI of MS patients. The results of a 17-year longitudinal study by Granberg suggested that corpus
callosum area was closely associated with cognitive decline in MS patients [22], nonetheless brain imaging has sophisticated methodological requirements, not always practical and accessible to most centers [10]. It is proved to have a potential for long-term follow-up of patients with MS [17].
Ozturk et al. demonstrated that quantitative MRI abnormalities in the CC, partially account for cognitive dysfunction in MS. Since cognitive dysfunction is particularly difficult to measure at the bedside, the imaging data may be useful for assessing these disabilities [12]. In a follow-up study by Faria et al. CCI scoring analysis showed 2 distinct patterns between relapsing-remitting MS and secondary-progressive MS patients. According to their study, CCI had a potential to be used for a long-term follow-up and mostly in secondary-progressive MS patients [17].
Neurodegeneration occurs in the CNS and could explain cognitive dysfunctions observed in MS patients and as the disease progresses, such dysfunctions are more pronounced. Based on the current findings, OCT as a non-invasive, easy-to-perform, accessible and relatively inexpensive method, has the potential to assess the axonal degeneration and cognitive dysfunction in
Ozturk et al. demonstrated that quantitative MRI abnormalities in the CC, partially account for cognitive dysfunction in MS. Since cognitive dysfunction is particularly difficult to measure at the bedside, the imaging data may be useful for assessing these disabilities [12]. In a follow-up study by Faria et al. CCI scoring analysis showed 2 distinct patterns between relapsing-remitting MS and secondary-progressive MS patients. According to their study, CCI had a potential to be used for a long-term follow-up and mostly in secondary-progressive MS patients [17].
Neurodegeneration occurs in the CNS and could explain cognitive dysfunctions observed in MS patients and as the disease progresses, such dysfunctions are more pronounced. Based on the current findings, OCT as a non-invasive, easy-to-perform, accessible and relatively inexpensive method, has the potential to assess the axonal degeneration and cognitive dysfunction in
MS. Therefore, changes in OCT might predict the alterations in cognitive function that might occur later in the progress of the disease. Based on this study, OCT is a useful method in the evaluation of axonal loss and predicting cognitive dysfunction in MS patients, compared to CCI or other measures.
Conclusion
The current study approved OCT as an appropriate tool for the evaluation of axonal loss and MS progression. Indeed, the accuracy of OCT in the prediction of cognitive dysfunction in MS was greater than other methods like brain imaging. Considering the small sample size of the present investigation, further studies with larger sample size and longer duration of MS disease are suggested to offset the limitations of the current study. Clinicians are suggested to use OCT as the initial screening tool for the evaluation of neurodegeneration, cognitive changes, and brain atrophy in MS.
Ethical Considerations
Compliance with ethical guidelines
This cross-sectional study was performed after receiving the approval from the Ethics Committee of Kerman University of Medical Sciences (code: K/92/67).
Funding
This work was supported by Research Center of Kerman University of Medical Sciences.
Conflict of interest
The authors certify that they have no affiliation with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials dismissed in this manuscript.
Acknowledgements
Authors would like to thank the research deputy of Kerman University of Medical Sciences for the financial support and all the MS patients who kindly participated in this study.
References
Conclusion
The current study approved OCT as an appropriate tool for the evaluation of axonal loss and MS progression. Indeed, the accuracy of OCT in the prediction of cognitive dysfunction in MS was greater than other methods like brain imaging. Considering the small sample size of the present investigation, further studies with larger sample size and longer duration of MS disease are suggested to offset the limitations of the current study. Clinicians are suggested to use OCT as the initial screening tool for the evaluation of neurodegeneration, cognitive changes, and brain atrophy in MS.
Ethical Considerations
Compliance with ethical guidelines
This cross-sectional study was performed after receiving the approval from the Ethics Committee of Kerman University of Medical Sciences (code: K/92/67).
Funding
This work was supported by Research Center of Kerman University of Medical Sciences.
Conflict of interest
The authors certify that they have no affiliation with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials dismissed in this manuscript.
Acknowledgements
Authors would like to thank the research deputy of Kerman University of Medical Sciences for the financial support and all the MS patients who kindly participated in this study.
References
- Fjeldstad C, Bemben M, Pardo G. Reduced retinal nerve fiber layer and macular thickness in patients with multiple sclerosis with no history of optic neuritis identified by the use of spectral domain high-definition optical coherence tomography. J Clin Neurosci. 2011; 18(11):1469-72. [DOI:10.1016/j.jocn.2011.04.008] [PMID]
- Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ. Optical coherence tomography: A window into the mechanisms of multiple sclerosis. Natl Clin Pract Neurol. 2008; 4(12):664-75. [DOI:10.1038/ncpneuro0950] [PMID] [PMCID]
- Wallin MT, Wilken JA, Kane R. Cognitive dysfunction in multiple sclerosis: Assessment, imaging, and risk factors. J Rehabil Res Dev. 2006; 43(1):63-72. [DOI:10.1682/JRRD.2004.09.0120] [PMID]
- Sartori E, Edan G. Assessment of cognitive dysfunction in multiple sclerosis. J Neurol Sci. 2006; 245(1-2):169-75. [DOI:10.1016/j.jns.2005.07.016] [PMID]
- Achiron A, Barak Y. Cognitive changes in early MS: A call for a common framework. J Neurol Sci. 2006; 245(1-2):47-51. [DOI:10.1016/j.jns.2005.05.019]
- Guenter W, Jablonska J, Bielinski M, Borkowska A. Neuroimaging and genetic correlates of cognitive dysfunction in multiple sclerosis. Psychiatr Pol J. 2015; 49(5):897-910. [DOI:10.12740/PP/32182] [PMID]
- Dobryakova E, Wylie GR, DeLuca J, Chiaravalloti ND. A pilot study examining functional brain activity 6 months after memory retraining in MS: the MEMREHAB trial. Brain Imaging Behav. 2014; 8(3):403-6. [DOI:10.1007/s11682-014-9309-9] [PMID] [PMCID]
- Rocca MA, Valsasina P, Hulst HE, Abdel-Aziz K, Enzinger C, Gallo A, et al. Functional correlates of cognitive dysfunction in multiple sclerosis: A multicenter fMRI Study. Hum Brain Mapp. 2014; 35(12):5799-814. [DOI:10.1002/hbm.22586] [PMID]
- Wojtowicz MA, Ishigami Y, Mazerolle EL, Fisk JD. Stability of intraindividual variability as a marker of neurologic dysfunction in relapsing remitting multiple sclerosis. J Clin Exp Neuropsychol. 2014; 36(5):455-63. [DOI:10.1080/13803395.2014.903898] [PMID]
- Yaldizli O, Atefy R, Gass A, Sturm D, Glassl S, Tettenborn B, et al. Corpus Callosum Index and long-term disability in multiple sclerosis patients. J Neurol. 2010; 257(8):1256-64. [DOI:10.1007/s00415-010-5503-x] [PMID]
- Rimkus Cde M, Junqueira Tde F, Lyra KP, Jackowski MP, Machado MA, Miotto EC, et al. Corpus callosum microstructural changes correlate with cognitive dysfunction in early stages of relapsing-remitting multiple sclerosis: Axial and radial diffusivities approach. Mult Scler Int. 2011; 2011:1-7. [DOI:10.1155/2011/304875]
- Ozturk A, Smith SA, Gordon-Lipkin EM, Harrison DM, Shiee N, Pham DL, et al. MRI of the corpus callosum in multiple sclerosis: association with disability. Mult Scler J Exp Transl Clin. 2010; 16(2):166-77. [DOI:10.1177/1352458509353649] [PMID] [PMCID]
- Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmol J. 2006; 113(2):324-32. [DOI:10.1016/j.ophtha.2005.10.040] [PMID]
- Yeh EA, Weinstock-Guttman B, Lincoff N, Reynolds J, Weinstock A, Madurai N, et al. Retinal nerve fiber thickness in inflammatory demyelinating diseases of childhood onset. Mult Scler J. 2009; 15(7):802-10. [DOI:10.1177/1352458509104586] [PMID]
- Toledo J, Sepulcre J, Salinas-Alaman A, Garcia-Layana A, Murie-Fernandez M, Bejarano B, et al. Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler J. 2008; 14(7):906-12. [DOI:10.1177/1352458508090221] [PMID]
- Figueira FF, Santos VS, Figueira GM, Silva AC. Corpus Callosum Index: A practical method for long-term follow-up in multiple sclerosis. Arq Neuropsiquiatr. 2007; 65(4a):931-5. [DOI:10.1590/S0004-282X2007000600001] [PMID]
- Mohammadi MR, Zhand P, Mortazavi Moghadam B, Golalipour MJ. Measurement of the corpus callosum using magnetic resonance imaging in the north of iran. Iran J Radiol. 2011; 8(4):218-23. [DOI:10.5812/iranjradiol.4495] [PMID] [PMCID]
- Sedighi B, Shafa MA, Abna Z, Ghaseminejad AK, Farahat R, Nakhaee N, et al. Association of cognitive deficits with optical coherence tomography changes in multiple sclerosis patients. J Mult Scler. 2013; 1(2):117. [DOI:10.4172/jmso]
- Benedict RH, Amato MP, Boringa J, Brochet B, Foley F, Fredrikson S, et al. Brief International Cognitive Assessment for MS (BICAMS): International standards for validation. BMC Neurol J. 2012; 12:55. [DOI:10.1186/1471-2377-12-55] [PMID] [PMCID]
- Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999; 40(11):2520-7. [PMID]
- Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, et al. Optical coherence tomography in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2010; 9(9):921-32. [DOI:10.1016/S1474-4422(10)70168]
- Granberg T, Martola J, Bergendal G, Shams S, Damangir S, Aspelin P, et al. Corpus callosum atrophy is strongly associated with cognitive impairment in multiple sclerosis: Results of a 17-year longitudinal study. Mult Scler J. 2015; 21(9):1151-8. [DOI:10.1177/1352458514560928] [PMID]
Type of Study: Applicable |
Subject:
Special
Received: 2017/12/10 | Accepted: 2018/04/1 | Published: 2018/07/1
Received: 2017/12/10 | Accepted: 2018/04/1 | Published: 2018/07/1
References
1. Fjeldstad C, Bemben M, Pardo G. Reduced retinal nerve fiber layer and macular thickness in patients with multiple sclerosis with no history of optic neuritis identified by the use of spectral domain high-definition optical coherence tomography. J Clin Neurosci. 2011; 18(11):1469-72. [DOI:10.1016/j.jocn.2011.04.008] [PMID] [DOI:10.1016/j.jocn.2011.04.008]
2. Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ. Optical coherence tomography: A window into the mechanisms of multiple sclerosis. Natl Clin Pract Neurol. 2008; 4(12):664-75. [DOI:10.1038/ncpneuro0950] [PMID] [PMCID] [DOI:10.1038/ncpneuro0950]
3. Wallin MT, Wilken JA, Kane R. Cognitive dysfunction in multiple sclerosis: Assessment, imaging, and risk factors. J Rehabil Res Dev. 2006; 43(1):63-72. [DOI:10.1682/JRRD.2004.09.0120] [PMID] [DOI:10.1682/JRRD.2004.09.0120]
4. Sartori E, Edan G. Assessment of cognitive dysfunction in multiple sclerosis. J Neurol Sci. 2006; 245(1-2):169-75. [DOI:10.1016/j.jns.2005.07.016] [PMID] [DOI:10.1016/j.jns.2005.07.016]
5. Achiron A, Barak Y. Cognitive changes in early MS: A call for a common framework. J Neurol Sci. 2006; 245(1-2):47-51. [DOI:10.1016/j.jns.2005.05.019] [DOI:10.1016/j.jns.2005.05.019]
6. Guenter W, Jablonska J, Bielinski M, Borkowska A. Neuroimaging and genetic correlates of cognitive dysfunction in multiple sclerosis. Psychiatr Pol J. 2015; 49(5):897-910. [DOI:10.12740/PP/32182] [PMID] [DOI:10.12740/PP/32182]
7. Dobryakova E, Wylie GR, DeLuca J, Chiaravalloti ND. A pilot study examining functional brain activity 6 months after memory retraining in MS: the MEMREHAB trial. Brain Imaging Behav. 2014; 8(3):403-6. [DOI:10.1007/s11682-014-9309-9] [PMID] [PMCID] [DOI:10.1007/s11682-014-9309-9]
8. Rocca MA, Valsasina P, Hulst HE, Abdel-Aziz K, Enzinger C, Gallo A, et al. Functional correlates of cognitive dysfunction in multiple sclerosis: A multicenter fMRI Study. Hum Brain Mapp. 2014; 35(12):5799-814. [DOI:10.1002/hbm.22586] [PMID] [DOI:10.1002/hbm.22586]
9. Wojtowicz MA, Ishigami Y, Mazerolle EL, Fisk JD. Stability of intraindividual variability as a marker of neurologic dysfunction in relapsing remitting multiple sclerosis. J Clin Exp Neuropsychol. 2014; 36(5):455-63. [DOI:10.1080/13803395.2014.903898] [PMID] [DOI:10.1080/13803395.2014.903898]
10. Yaldizli O, Atefy R, Gass A, Sturm D, Glassl S, Tettenborn B, et al. Corpus Callosum Index and long-term disability in multiple sclerosis patients. J Neurol. 2010; 257(8):1256-64. [DOI:10.1007/s00415-010-5503-x] [PMID] [DOI:10.1007/s00415-010-5503-x]
11. Rimkus Cde M, Junqueira Tde F, Lyra KP, Jackowski MP, Machado MA, Miotto EC, et al. Corpus callosum microstructural changes correlate with cognitive dysfunction in early stages of relapsing-remitting multiple sclerosis: Axial and radial diffusivities approach. Mult Scler Int. 2011; 2011:1-7. [DOI:10.1155/2011/304875] [DOI:10.1155/2011/304875]
12. Ozturk A, Smith SA, Gordon-Lipkin EM, Harrison DM, Shiee N, Pham DL, et al. MRI of the corpus callosum in multiple sclerosis: association with disability. Mult Scler J Exp Transl Clin. 2010; 16(2):166-77. [DOI:10.1177/1352458509353649] [PMID] [PMCID] [DOI:10.1177/1352458509353649]
13. Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmol J. 2006; 113(2):324-32. [DOI:10.1016/j.ophtha.2005.10.040] [PMID] [DOI:10.1016/j.ophtha.2005.10.040]
14. Yeh EA, Weinstock-Guttman B, Lincoff N, Reynolds J, Weinstock A, Madurai N, et al. Retinal nerve fiber thickness in inflammatory demyelinating diseases of childhood onset. Mult Scler J. 2009; 15(7):802-10. [DOI:10.1177/1352458509104586] [PMID] [DOI:10.1177/1352458509104586]
15. Toledo J, Sepulcre J, Salinas-Alaman A, Garcia-Layana A, Murie-Fernandez M, Bejarano B, et al. Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler J. 2008; 14(7):906-12. [DOI:10.1177/1352458508090221] [PMID] [DOI:10.1177/1352458508090221]
16. Figueira FF, Santos VS, Figueira GM, Silva AC. Corpus Callosum Index: A practical method for long-term follow-up in multiple sclerosis. Arq Neuropsiquiatr. 2007; 65(4a):931-5. [DOI:10.1590/S0004-282X2007000600001] [PMID] [DOI:10.1590/S0004-282X2007000600001]
17. Mohammadi MR, Zhand P, Mortazavi Moghadam B, Golalipour MJ. Measurement of the corpus callosum using magnetic resonance imaging in the north of iran. Iran J Radiol. 2011; 8(4):218-23. [DOI:10.5812/iranjradiol.4495] [PMID] [PMCID] [DOI:10.5812/iranjradiol.4495]
18. Sedighi B, Shafa MA, Abna Z, Ghaseminejad AK, Farahat R, Nakhaee N, et al. Association of cognitive deficits with optical coherence tomography changes in multiple sclerosis patients. J Mult Scler. 2013; 1(2):117. [DOI:10.4172/jmso] [DOI:10.4172/jmso]
19. Benedict RH, Amato MP, Boringa J, Brochet B, Foley F, Fredrikson S, et al. Brief International Cognitive Assessment for MS (BICAMS): International standards for validation. BMC Neurol J. 2012; 12:55. [DOI:10.1186/1471-2377-12-55] [PMID] [PMCID] [DOI:10.1186/1471-2377-12-55]
20. Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999; 40(11):2520-7. [PMID] [PMID]
21. Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, et al. Optical coherence tomography in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2010; 9(9):921-32. [DOI:10.1016/S1474-4422(10)70168] [DOI:10.1016/S1474-4422(10)70168-X]
22. Granberg T, Martola J, Bergendal G, Shams S, Damangir S, Aspelin P, et al. Corpus callosum atrophy is strongly associated with cognitive impairment in multiple sclerosis: Results of a 17-year longitudinal study. Mult Scler J. 2015; 21(9):1151-8. [DOI:10.1177/1352458514560928] [PMID] [DOI:10.1177/1352458514560928]
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |