Fri, Apr 26, 2024
Volume 3, Issue 4 (Autumn 2017)
Caspian J Neurol Sci 2017, 3(4): 206-213 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shoeibi A, Razmi N, Ghabeli Juibary A, Hashemy S I. The Evaluation and Comparison of Oxidative Stress in Hemorrhagic and Ischemic Stroke. Caspian J Neurol Sci 2017; 3 (4) :206-213
URL: http://cjns.gums.ac.ir/article-1-203-en.html
URL: http://cjns.gums.ac.ir/article-1-203-en.html
1- Department of Neurology, Faculty of medicine, Mashhad University of Medical Sciences, Mashhad, Iran
2- Surgical Oncology Research Center, Mashhad University of Medical Sciences, Mashhad, Iran; hashemyi@mums.ac.ir
2- Surgical Oncology Research Center, Mashhad University of Medical Sciences, Mashhad, Iran; hashemyi@mums.ac.ir
Full-Text [PDF 689 kb]
(1182 Downloads)
| Abstract (HTML) (5745 Views)
Discussion
In the present study we have focused on the relationship between serum oxidative profile and various prognostic factors of ischemic and hemorrhagic stroke. Throughout the past decades, there has been accumulating evidence of the association between oxidative stress and stroke injury, and several studies have proved oxidative damage as a mechanism underlying neuronal damage in different stages after stroke (27). Free radicals generated in the affected area of brain can exert their effect by lipid peroxidation leading to membrane changes and apoptosis, protein oxidation and impaired enzymatic functions and DNA oxidation that can eventually lead to cell death. While these factors are present in the brain in normal state and they have many important roles in signaling and as a defense mechanism against infections, they usually cannot cross the blood brain barrier
and their half-life is not long enough to reach significant concentrations in serum. However following a vascular attack with or without reperfusion their modifications in plasma can reflect brain damage. Various oxidative stress markers have been previously shown to be higher in ischemic stroke patients compared to control. However, until now none of these results could suggest a different diagnostic or therapeutic approach for clinicians in practice (28-30). In an attempt to find a path between the biochemistry of stroke and its clinical management, we measured the plasma concentrations of two markers in a group of stoke patients. Malonyldealdehyde, a breakdown product of lipid peroxidation and total antioxidant capacity were chosen as indicators of the oxidant and antioxidant balance state.
This study included patients with a recent cerebrovascular accident. We assigned different prognostic factors for the two groups based on what had been previously associated with higher mortality and morbidity rate in each type. The majority of ischemic strokes are due to a thrombosis or an embolus. Previous studies have shown MDA levels are higher in ischemic patients in comparison to general population. However, few studies have focused on the correlation between stroke severity, its outcome and MDA or other markers of oxidative state. Ozkul et al. previously reported an association between MDA and Canadian Neurological Scale Scores (CNS). In another study, Leinonen et al. observed an inverse correlation of plasma total peroxyl radical–trapping potential (TRAP) and lesion volume and a significant or inverse correlation with the scores in NIHSS and HMS and a direct correlation with BI score at all-time points after the stroke in ischemic stroke (31). We could not detect any significant correlation between levels of MDA or TAC and NIHSS or consciousness. However, these levels were measured only at the initial presentation, and follow-up measurements could help to clarify MDA and TAC changes and ischemic stroke outcomes.
Stroke is a disease of different pathophysiology and hence different subtypes. It seems that hemorrhagic patients have been under represented in the literature. One reason could be its lower incidence compared to ischemic stroke. Only 15 percent of all strokes happen because of an intracranial hemorrhage (ICH). However hemorrhagic strokes are generally associated with greater severity and higher mortality after age, gender and relevant risk factors adjustments, and they are a major healthcare burden. Patients with hemorrhagic stroke are
shown to have higher levels of oxidative biomarkers than control population (32). Among our 24 hemorrhagic patients 11 had lesion in putamen which is the most common location, 7 in thalamus, 1 had pontine lesion and 3 had lobar hemorrhage (1 in frontal, 2 in parietal lobe). No association between MDA levels or TAC and the location of ICH was observed. We found lesion volume to be significantly correlated with MDA levels and negatively correlated with TAC.
Comparison of MDA levels and TAC between the two major groups of stroke did not yield significant difference which is consistent with other similar studies (12). However our sample study was larger than previous studies with higher mean of NIHSS (NIHSS mean of our population in total was 21, IS=20, HS=23) and generally more severe cases.
Conclusion
This data suggested that oxidative stress is associated with lesion volume and therefore severity of hemorrhagic stroke; however, no correlation was observed between the serum level of MDA/TAC and the consciousness level as well as NIHSS in ischemic stroke. Although there is strong evidence supporting the role of oxidative stress in brain injury following a cerebrovascular attack, further research is needed to show, how it is involved in the evolution of clinical manifestations of stroke patients and its application in predicting patients’ outcome.
Acknowledgment
This study was financially supported by
the Research Council of Mashhad University
of Medical Sciences, Mashhad, Iran. Conflict of Interest
The authors have no conflict of interest.
References
1. Rodrigo R, Fernández-Gajardo R, Gutiérrez
R, Matamala JM, Carrasco R, Miranda-
Merchak A, et al. Oxidative Stress and
Pathophysiology of Ischemic Stroke: Novel
Therapeutic Opportunities. CNS Neurol
Disord Drug Targets 2013;12(5):698-714.
2. Roger VL, Go AS, Lloyd-Jones DM, Adams
RJ, Berry JD, Brown TM, et al. American
Heart Association. Heart Disease and Stroke
Statistics. 2011 update. Circulation 2011;
123(4):e18-e209.
3. Cherubini A, Polidori MC, Bregnocchi M,
Pezzuto S, Cecchetti R, Ingegni T.
Antioxidant Profile and Early Outcome in
Stroke Patients. Stroke 2000; 31(10):2295-
300.
4. Andersen KK, Olsen TS, Dehlendorff C,
Kammersgaard LP. Hemorrhagic and
Ischemic Strokes Compared: Stroke Severity,
Mortality, and Risk Factors. Stroke 2009;
40(6):2068-72.
5. Pradeep H, Diya JB, Shashikumar S,
Rajanikant GK. Oxidative Stress – assassin
behind the Ischemic Stroke. Folia
Neuropathol 2012;50(3):219-30.
6. Yang TH, Chang CY, Hu ML. Various Forms
of Homocysteine and Oxidative Status in the
Plasma of Ischemic-Stroke Patients as
Compared to Healthy Controls. Clin Biochem
2004;37(6):494-9.
7. Spranger M, Krempien S, Schwab S,
Donneberg S, Hacke W. Superoxide
Dismutase Activity in Serum of Patients with
Acute Cerebral Ischemic Injury. Stroke 1997;
28(12):2425-8.
8. Loh KP, Huang SH, De Silva R, Tan BK, Zhu
YZ. Oxidative Stress: Apoptosis in Neuronal
injury. Curr Alzheimer Res 2006;3(4):327-37.
9. Facchinetti F, Dawson VL, Dawson TM. Free
Radicals as Mediators of Neuronal Injury.
Cell Mol Neurobiol 1998;18(6):667-82.
10. Shi H, Liu KJ. Cerebral Tissue Oxygenation
and Oxidative Brain Injury During Ischemia
and Reperfusion. Front Biosci 2007;12:1318-
28.
11. Rehncrona S, Mela L, Siesjo BK. Recovery of
Brain Mitochondrial Function in the Rat After
Complete Cerebral Ischaemia. Stroke
1979;10(4): 437-46.
12. Kaur J, Sarika A, Bhawna S, Thakur LC,
Gambhir J , Prabhu KM. Role of Oxidative
Stress in Pathophysiology of Transient
Ischemic Attack and Stroke. Int J Biol Med
Res 2011;2(3):611-5.
13. Aygul R, Demircan B, Erdem F, Ulvi H,
Yildirim A, Demirbas F. Plasma Values of
Oxidants and Antioxidants in Acute Brain
Hemorrhage. Biol Trace Elem Res 2005;
108(1-3):43-52.
14. Kelly PJ, Morrow JD, Ning M, Koroshetz W,
Lo EH, Terry E, et al. Oxidative Stress and
Matrix Metalloproteinase-9 in Acute Ischemic
Stroke: the Biomarker Evaluation for
Antioxidant Therapies in Stroke (BEATStroke)
Study. Stroke 2008;39(1):100-4.
15. Domínguez C, Delgado P, Vilches A, Martín-
Gallán P, Ribó M, Santamarina E, et al.
Oxidative Stress After Thrombolysis-induced
Reperfusion in Human Stroke. Stroke
2010;41(4):653-60.
16. Cano CP, Bermúdez VP, Atencio HE, Medina
MT, Anilsa A, Souki A, et al. Increased
Serum Malondialdehyde and Decreased Nitric
Oxide within 24 hours of Thrombotic Stroke
Onset. Am J Ther 2003;10(6):473-6.
17. Seet RC, Lee CY, Chan BP, Sharma VK,
Teoh HL, Venketasubramanian N, et al.
Oxidative Damage in Ischemic Stroke
Revealed Using Multiple Biomarkers. Stroke
2011;42(8):2326-9.
18. Ullegaddi R, Powers HJ, Gariballa SE.
Antioxidant Supplementation with or Without
Bgroup Vitamins After Acute Ischemic
Stroke: a Randomized Controlled Trial. JPEN
J Parenter Enteral Nutr 2006;30(2):108-14.
19. Polidori MC, Praticó D, Ingegni T, Mariani E,
Spazzafumo L, Del Sindaco P, et al. Effects of
Vitamin C and Aspirin in Ischemic Strokerelated
Lipid Peroxidation: Results of the
AVASAS (Aspirin Versus Ascorbic acid plus
Aspirin in Stroke) Study. Biofactors 2005;
24(1-4):265-74.
20. Asplund K. Antioxidant Vitamins in the
Prevention of Cardiovascular Disease: aSystematic Review. J Int Med 2002;
251(5):372-92.
21. Valenzuela A. The Biological Significance of
Malondialdehyde Determination in
Assessment of Tissue Oxidative Stress. Life
Sci 1991;48(4):301-9.
22. Appelros P, Terént A. Characteristics of the
NIHSS results: Results from a Populationbased
Stroke Cohort at Baseline and After
One Year. Cerebrovasc Dis 2004; 17(1):21-7.
23. Kothari RU, Brott T, Broderick JP, Barsan
WG, Sauerbeck LR, Zuccarello M. The ABCs
of Measuring Intracerebral Hemorrhage
Volumes. Stroke 1996;27(8):1304-5.
24. Miller NJ, Rice-Evans C, Davies MJ,
Gopinathan V, Milner A. A Novel Method for
Measuring Antioxidant Capacity and Its
Application to Monitoring the Antioxidant
Status in Premature Neonates. Clin Sci (Lond)
1993; 84(4):407-12.
25. Miller NJ, Rice-Evans CA. Factors
Influencing the Antioxidant Activity
Determined by the ABTS.+ Radical Cation
Assay. Free Radic Res 1997; 26(3):195-9.
26. Armstrong D, Browne R. The Analysis of
Free Radicals, Lipid Peroxides, Antioxidant
Enzymes and Compounds to Oxidative Stress
as Applied to the Clinical Chemistry
Laboratory. Adv Exp Med Biol 1994;366:43-
58.
27. Allen CL, Bayraktutan U. Oxidative Stress
and Its Role in the Pathogenesis of Ischaemic
Stroke. Int J Stroke 2009;4(6):461-70.
28. Santos MT, Valles J, Aznar J, Vilches J.
Determination of plasma malondialdehyde
like material and its clinical application in
stroke patients. J Clin Pathol.
1980;33(10):973-6.
29. Srikrishna R, Suresh DR. Biochemical Study
of Antioxidant Profile in Acute Ischemic
Stroke. Stroke BJMP 2009;2(1):35-7.
30. Zimmermann C, Winnefeld K, Streck S,
Roskos M, Haberl RL. Antioxidant Status in
Acute Stroke Patients and Patients at Stroke
Risk. Eur Neurol 2004; 51(3):157-61.
31. Leinonen JS, Ahonen JP, Lönnrot K,
Jehkonen M, Dastidar P, Molnár G, et al. Low
Plasma Antioxidant Activity Is Associated
with High Lesion Volume and Neurological
Impairment in Stroke. Stroke 2000;31(1):33-
9.
32. Gonullu H, Aslan M, Karadas S, Kati C,
Duran L, Milanlioglu A, et al. Serum
Prolidase Enzyme Activity and Oxidative
Stress levels in Patients with Acute
Hemorrhagic Stroke. Scand J Clin Lab Invest
2014 74(3):199-205.
Full-Text: (1032 Views)
Introduction
Introduction
Introduction
According to WHO report, 17 million people die annually because of vascular diseases. Among them, cerebrovascular accidents are a leading cause
of mortality and long-term disability (1,2). Even though 85% of all strokes are ischemic in which an artery is blocked by a clot or a plaque (3), hemorrhagic strokes (HS) are associated with a higher rate of mortality and are generally more severe than ischemic strokes (IS) even after adjustment for relevant risk factors (4).
of mortality and long-term disability (1,2). Even though 85% of all strokes are ischemic in which an artery is blocked by a clot or a plaque (3), hemorrhagic strokes (HS) are associated with a higher rate of mortality and are generally more severe than ischemic strokes (IS) even after adjustment for relevant risk factors (4).
Several attempts have been made to understand the molecular basis of tissue damage in stroke patients (5). Special attention has been paid to reactive oxygen spices (ROS) which are involved in physiological processes like aging as well as many pathologic states such as atherosclerosis, cancer, neurodegenerative diseases, and etc (6,7). It is evident from both animal and human studies that the oxidative damage of membrane lipids and cellular proteins increases during cerebral ischemia and reperfusion (8-11).
In the ischemic process, the circulatory arrest to brain cells can cause uncontrolled activation of calcium dependent enzymes such as phospholipase A2, cyclooxygenase and neuronal nitric oxide synthase which is followed by excessive radical production (3). Although less studied, ROS and lipid peroxidation might also have a role in brain injury following HS (12,13).
Several studies in the past decade have focused on measuring oxidative biomarkers in stroke patients during the acute phase after the events and in the subsequent recovery time (14-16). Detailed biomarkers’ profiles of serum, urine and CSF of stroke patients have shown a statistically significant rise in markers of oxidative damage in comparison to the controls (17).
These studies can contribute to understanding the cellular factors involved in initiation and development of stroke. Also, the possible protective role of antioxidants against brain injury can lead to new therapeutic strategies in the future for reducing mortality and morbidity among both
ischemic and hemorrhagic stroke patients (18-20).
The present study was designed to investigate the correlation of prognostic factors in stroke patients with serum levels of oxidative stress biomarkers. Until now there have been few studies that compared the differences between two types of strokes. We aimed to measure serum malondialdehyde (MDA) (21), the main byproduct of lipid peroxidation, and total antioxidant capacity (TAC) in the first 24 hours following the stroke, and to obtain evidence for their possible relationship to prognostic factors in patients with ischemic or hemorrhagic strokes.
Materials and Methods
Subjects:
A group of 24 patients with acute ischemic stroke and an equal number of patients with hemorrhagic stroke were recruited within the first 24 hours of their attack who were hospitalized at the neurological emergency ward of Ghaem hospital, Mashhad in 2014. If a patient was excluded from the study, he or she was replaced by another patient who met the inclusion criteria to reach to the determined sample volume. The diagnosis was made by the clinical examination and brain CT scan. Our excluding criteria were a previous history of a cerebrovascular event, history of a recent infectious or inflammatory disease, cancer, autoimmune disorder, hematological disorder, renal or hepatic disease, or use of immune-suppressive or anti-inflammatory drugs in the previous two months. Venous blood samples were obtained on admission. A written informed consent was provided by each patient or their relatives, and the study was approved by the Ethics committee of Mashhad University of Medical Sciences (Ethical code: 900398)
Method:
Recorded variables included modified NIHSS (NIH Stroke Scale) (23) and consciousness on admission in all patients, as well as hematoma volume or ischemic lesion size and location in the first CT Scan (22). All examinations were conducted by the same neurologist. Lesion volume was manually estimated from digital CT images with the conventional ABC/2 formula explained by Kothari et al. (23) (A: the greatest hemorrhage diameter by CT, B: The diameter 90 to A, and C: the approximate number of CT slices with hemorrhage multiplied by the slice thickness).
Blood samples were centrifuged at 4°C at 3000 rpm for 10 min; and after sera were separated, they were kept at -80°C until analysis.
TAC was measured by a chemical colorimetric method using the Antioxidant Assay Kit purchased from Cayman Chemical Company, USA (Item Number 709001). This method relies on the ability of antioxidants in the sample to inhibit the oxidation of ABTS® (2,2'-azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS® •+ by metmyoglobin (24,25).
Serum MDA was measured using a colorimetric method based on the formation of adduct between MDA and thiobarbituric acid (TBA) under high temperature (90-100°C) and acidic conditions that is detectable colorimetrically at 530-540 nm. For this purpose, TBARS Assay kit was obtained from Cayman Chemical Company, USA (Item Number 10009055) (26).
Statistics:
The Statistical Package for the Social Sciences (SPSS) 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis and statistical significance was defined as p<0.05. Comparisons were performed using the Mann–Whitney U-test or Student’s t-test. Kruskal-Wallis Test was used for analyzing categorical variables. Pearson correlation analysis and Spearman’s test were used for correlation analysis of variables with normal distribution and abnormal distribution, respectively.
Results
Our study included 24 ischemic and 24 hemorrhagic stroke patients. Each group consisted of 11 males (46%) and 13 females (54%). Their age varied between 41 and 91 (72.5±15.5 and 69.3±13.2 in IS and HS, respectively) and there was no significant difference of age between two groups.
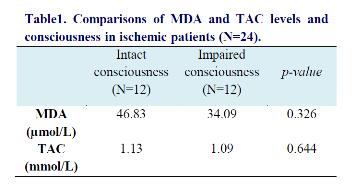
Serum total antioxidant level was 1.11±0.11 mmol/L in the ischemic patients and 1.14±0.14 in hemorrhagic patients; the difference was not significant (95% CI: -0.07 - 0.52, p=0.45).
The serum levels of MDA were 40.46±6.42 µmol/L in ischemic patients and 52.25±5.41 µmol/L in hemorrhagic patients (95% CI: -32.9 -70.8); this difference was not significant either (p=0.24).
Ischemic Group:
Among IS patients, the mean NIHSS was 20. There was a non-significant correlation between NIHSS and MDA (r=0.03, p=0.791) and no significant correlation between NIHSS and TAC (β= -0.272, p=0.211) .We found no significant correlation between consciousness and TAC and MDA levels (table 1).
In the ischemic process, the circulatory arrest to brain cells can cause uncontrolled activation of calcium dependent enzymes such as phospholipase A2, cyclooxygenase and neuronal nitric oxide synthase which is followed by excessive radical production (3). Although less studied, ROS and lipid peroxidation might also have a role in brain injury following HS (12,13).
Several studies in the past decade have focused on measuring oxidative biomarkers in stroke patients during the acute phase after the events and in the subsequent recovery time (14-16). Detailed biomarkers’ profiles of serum, urine and CSF of stroke patients have shown a statistically significant rise in markers of oxidative damage in comparison to the controls (17).
These studies can contribute to understanding the cellular factors involved in initiation and development of stroke. Also, the possible protective role of antioxidants against brain injury can lead to new therapeutic strategies in the future for reducing mortality and morbidity among both
ischemic and hemorrhagic stroke patients (18-20).
The present study was designed to investigate the correlation of prognostic factors in stroke patients with serum levels of oxidative stress biomarkers. Until now there have been few studies that compared the differences between two types of strokes. We aimed to measure serum malondialdehyde (MDA) (21), the main byproduct of lipid peroxidation, and total antioxidant capacity (TAC) in the first 24 hours following the stroke, and to obtain evidence for their possible relationship to prognostic factors in patients with ischemic or hemorrhagic strokes.
Materials and Methods
Subjects:
A group of 24 patients with acute ischemic stroke and an equal number of patients with hemorrhagic stroke were recruited within the first 24 hours of their attack who were hospitalized at the neurological emergency ward of Ghaem hospital, Mashhad in 2014. If a patient was excluded from the study, he or she was replaced by another patient who met the inclusion criteria to reach to the determined sample volume. The diagnosis was made by the clinical examination and brain CT scan. Our excluding criteria were a previous history of a cerebrovascular event, history of a recent infectious or inflammatory disease, cancer, autoimmune disorder, hematological disorder, renal or hepatic disease, or use of immune-suppressive or anti-inflammatory drugs in the previous two months. Venous blood samples were obtained on admission. A written informed consent was provided by each patient or their relatives, and the study was approved by the Ethics committee of Mashhad University of Medical Sciences (Ethical code: 900398)
Method:
Recorded variables included modified NIHSS (NIH Stroke Scale) (23) and consciousness on admission in all patients, as well as hematoma volume or ischemic lesion size and location in the first CT Scan (22). All examinations were conducted by the same neurologist. Lesion volume was manually estimated from digital CT images with the conventional ABC/2 formula explained by Kothari et al. (23) (A: the greatest hemorrhage diameter by CT, B: The diameter 90 to A, and C: the approximate number of CT slices with hemorrhage multiplied by the slice thickness).
Blood samples were centrifuged at 4°C at 3000 rpm for 10 min; and after sera were separated, they were kept at -80°C until analysis.
TAC was measured by a chemical colorimetric method using the Antioxidant Assay Kit purchased from Cayman Chemical Company, USA (Item Number 709001). This method relies on the ability of antioxidants in the sample to inhibit the oxidation of ABTS® (2,2'-azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS® •+ by metmyoglobin (24,25).
Serum MDA was measured using a colorimetric method based on the formation of adduct between MDA and thiobarbituric acid (TBA) under high temperature (90-100°C) and acidic conditions that is detectable colorimetrically at 530-540 nm. For this purpose, TBARS Assay kit was obtained from Cayman Chemical Company, USA (Item Number 10009055) (26).
Statistics:
The Statistical Package for the Social Sciences (SPSS) 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis and statistical significance was defined as p<0.05. Comparisons were performed using the Mann–Whitney U-test or Student’s t-test. Kruskal-Wallis Test was used for analyzing categorical variables. Pearson correlation analysis and Spearman’s test were used for correlation analysis of variables with normal distribution and abnormal distribution, respectively.
Results
Our study included 24 ischemic and 24 hemorrhagic stroke patients. Each group consisted of 11 males (46%) and 13 females (54%). Their age varied between 41 and 91 (72.5±15.5 and 69.3±13.2 in IS and HS, respectively) and there was no significant difference of age between two groups.
Serum total antioxidant level was 1.11±0.11 mmol/L in the ischemic patients and 1.14±0.14 in hemorrhagic patients; the difference was not significant (95% CI: -0.07 - 0.52, p=0.45).
The serum levels of MDA were 40.46±6.42 µmol/L in ischemic patients and 52.25±5.41 µmol/L in hemorrhagic patients (95% CI: -32.9 -70.8); this difference was not significant either (p=0.24).
Ischemic Group:
Among IS patients, the mean NIHSS was 20. There was a non-significant correlation between NIHSS and MDA (r=0.03, p=0.791) and no significant correlation between NIHSS and TAC (β= -0.272, p=0.211) .We found no significant correlation between consciousness and TAC and MDA levels (table 1).
Hemorrhagic Group:
Among HS patients, 11 had hemorrhage in putamen, 7 in thalamus, 1 in pons and 3 had lobar hemorrhage. The size of hemorrhage was between 3-160 cm3 with a mean of 46 cm3.
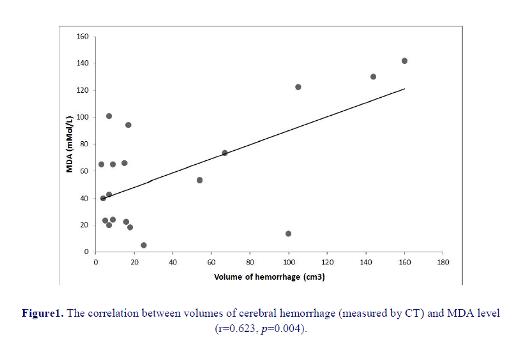
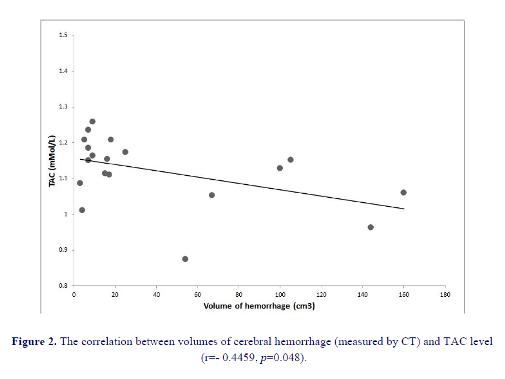
We found a significant negative correlation between TAC and lesion volume (figure 1) and a significant positive correlation between MDA levels and lesion volume in HS patients (figure 2). Patients with a higher lesion volume had a lower level of TAC (p=0.048) and higher mean level of MDA (p=0.004) than patients with lower lesion volume. We could not find any statistically significant difference between lesion location and TAC and MDA levels (p>0.05).
Among HS patients, 11 had hemorrhage in putamen, 7 in thalamus, 1 in pons and 3 had lobar hemorrhage. The size of hemorrhage was between 3-160 cm3 with a mean of 46 cm3.
We found a significant negative correlation between TAC and lesion volume (figure 1) and a significant positive correlation between MDA levels and lesion volume in HS patients (figure 2). Patients with a higher lesion volume had a lower level of TAC (p=0.048) and higher mean level of MDA (p=0.004) than patients with lower lesion volume. We could not find any statistically significant difference between lesion location and TAC and MDA levels (p>0.05).
Discussion
In the present study we have focused on the relationship between serum oxidative profile and various prognostic factors of ischemic and hemorrhagic stroke. Throughout the past decades, there has been accumulating evidence of the association between oxidative stress and stroke injury, and several studies have proved oxidative damage as a mechanism underlying neuronal damage in different stages after stroke (27). Free radicals generated in the affected area of brain can exert their effect by lipid peroxidation leading to membrane changes and apoptosis, protein oxidation and impaired enzymatic functions and DNA oxidation that can eventually lead to cell death. While these factors are present in the brain in normal state and they have many important roles in signaling and as a defense mechanism against infections, they usually cannot cross the blood brain barrier
and their half-life is not long enough to reach significant concentrations in serum. However following a vascular attack with or without reperfusion their modifications in plasma can reflect brain damage. Various oxidative stress markers have been previously shown to be higher in ischemic stroke patients compared to control. However, until now none of these results could suggest a different diagnostic or therapeutic approach for clinicians in practice (28-30). In an attempt to find a path between the biochemistry of stroke and its clinical management, we measured the plasma concentrations of two markers in a group of stoke patients. Malonyldealdehyde, a breakdown product of lipid peroxidation and total antioxidant capacity were chosen as indicators of the oxidant and antioxidant balance state.
This study included patients with a recent cerebrovascular accident. We assigned different prognostic factors for the two groups based on what had been previously associated with higher mortality and morbidity rate in each type. The majority of ischemic strokes are due to a thrombosis or an embolus. Previous studies have shown MDA levels are higher in ischemic patients in comparison to general population. However, few studies have focused on the correlation between stroke severity, its outcome and MDA or other markers of oxidative state. Ozkul et al. previously reported an association between MDA and Canadian Neurological Scale Scores (CNS). In another study, Leinonen et al. observed an inverse correlation of plasma total peroxyl radical–trapping potential (TRAP) and lesion volume and a significant or inverse correlation with the scores in NIHSS and HMS and a direct correlation with BI score at all-time points after the stroke in ischemic stroke (31). We could not detect any significant correlation between levels of MDA or TAC and NIHSS or consciousness. However, these levels were measured only at the initial presentation, and follow-up measurements could help to clarify MDA and TAC changes and ischemic stroke outcomes.
Stroke is a disease of different pathophysiology and hence different subtypes. It seems that hemorrhagic patients have been under represented in the literature. One reason could be its lower incidence compared to ischemic stroke. Only 15 percent of all strokes happen because of an intracranial hemorrhage (ICH). However hemorrhagic strokes are generally associated with greater severity and higher mortality after age, gender and relevant risk factors adjustments, and they are a major healthcare burden. Patients with hemorrhagic stroke are
shown to have higher levels of oxidative biomarkers than control population (32). Among our 24 hemorrhagic patients 11 had lesion in putamen which is the most common location, 7 in thalamus, 1 had pontine lesion and 3 had lobar hemorrhage (1 in frontal, 2 in parietal lobe). No association between MDA levels or TAC and the location of ICH was observed. We found lesion volume to be significantly correlated with MDA levels and negatively correlated with TAC.
Comparison of MDA levels and TAC between the two major groups of stroke did not yield significant difference which is consistent with other similar studies (12). However our sample study was larger than previous studies with higher mean of NIHSS (NIHSS mean of our population in total was 21, IS=20, HS=23) and generally more severe cases.
Conclusion
This data suggested that oxidative stress is associated with lesion volume and therefore severity of hemorrhagic stroke; however, no correlation was observed between the serum level of MDA/TAC and the consciousness level as well as NIHSS in ischemic stroke. Although there is strong evidence supporting the role of oxidative stress in brain injury following a cerebrovascular attack, further research is needed to show, how it is involved in the evolution of clinical manifestations of stroke patients and its application in predicting patients’ outcome.
Acknowledgment
This study was financially supported by
the Research Council of Mashhad University
of Medical Sciences, Mashhad, Iran. Conflict of Interest
The authors have no conflict of interest.
References
1. Rodrigo R, Fernández-Gajardo R, Gutiérrez
R, Matamala JM, Carrasco R, Miranda-
Merchak A, et al. Oxidative Stress and
Pathophysiology of Ischemic Stroke: Novel
Therapeutic Opportunities. CNS Neurol
Disord Drug Targets 2013;12(5):698-714.
2. Roger VL, Go AS, Lloyd-Jones DM, Adams
RJ, Berry JD, Brown TM, et al. American
Heart Association. Heart Disease and Stroke
Statistics. 2011 update. Circulation 2011;
123(4):e18-e209.
3. Cherubini A, Polidori MC, Bregnocchi M,
Pezzuto S, Cecchetti R, Ingegni T.
Antioxidant Profile and Early Outcome in
Stroke Patients. Stroke 2000; 31(10):2295-
300.
4. Andersen KK, Olsen TS, Dehlendorff C,
Kammersgaard LP. Hemorrhagic and
Ischemic Strokes Compared: Stroke Severity,
Mortality, and Risk Factors. Stroke 2009;
40(6):2068-72.
5. Pradeep H, Diya JB, Shashikumar S,
Rajanikant GK. Oxidative Stress – assassin
behind the Ischemic Stroke. Folia
Neuropathol 2012;50(3):219-30.
6. Yang TH, Chang CY, Hu ML. Various Forms
of Homocysteine and Oxidative Status in the
Plasma of Ischemic-Stroke Patients as
Compared to Healthy Controls. Clin Biochem
2004;37(6):494-9.
7. Spranger M, Krempien S, Schwab S,
Donneberg S, Hacke W. Superoxide
Dismutase Activity in Serum of Patients with
Acute Cerebral Ischemic Injury. Stroke 1997;
28(12):2425-8.
8. Loh KP, Huang SH, De Silva R, Tan BK, Zhu
YZ. Oxidative Stress: Apoptosis in Neuronal
injury. Curr Alzheimer Res 2006;3(4):327-37.
9. Facchinetti F, Dawson VL, Dawson TM. Free
Radicals as Mediators of Neuronal Injury.
Cell Mol Neurobiol 1998;18(6):667-82.
10. Shi H, Liu KJ. Cerebral Tissue Oxygenation
and Oxidative Brain Injury During Ischemia
and Reperfusion. Front Biosci 2007;12:1318-
28.
11. Rehncrona S, Mela L, Siesjo BK. Recovery of
Brain Mitochondrial Function in the Rat After
Complete Cerebral Ischaemia. Stroke
1979;10(4): 437-46.
12. Kaur J, Sarika A, Bhawna S, Thakur LC,
Gambhir J , Prabhu KM. Role of Oxidative
Stress in Pathophysiology of Transient
Ischemic Attack and Stroke. Int J Biol Med
Res 2011;2(3):611-5.
13. Aygul R, Demircan B, Erdem F, Ulvi H,
Yildirim A, Demirbas F. Plasma Values of
Oxidants and Antioxidants in Acute Brain
Hemorrhage. Biol Trace Elem Res 2005;
108(1-3):43-52.
14. Kelly PJ, Morrow JD, Ning M, Koroshetz W,
Lo EH, Terry E, et al. Oxidative Stress and
Matrix Metalloproteinase-9 in Acute Ischemic
Stroke: the Biomarker Evaluation for
Antioxidant Therapies in Stroke (BEATStroke)
Study. Stroke 2008;39(1):100-4.
15. Domínguez C, Delgado P, Vilches A, Martín-
Gallán P, Ribó M, Santamarina E, et al.
Oxidative Stress After Thrombolysis-induced
Reperfusion in Human Stroke. Stroke
2010;41(4):653-60.
16. Cano CP, Bermúdez VP, Atencio HE, Medina
MT, Anilsa A, Souki A, et al. Increased
Serum Malondialdehyde and Decreased Nitric
Oxide within 24 hours of Thrombotic Stroke
Onset. Am J Ther 2003;10(6):473-6.
17. Seet RC, Lee CY, Chan BP, Sharma VK,
Teoh HL, Venketasubramanian N, et al.
Oxidative Damage in Ischemic Stroke
Revealed Using Multiple Biomarkers. Stroke
2011;42(8):2326-9.
18. Ullegaddi R, Powers HJ, Gariballa SE.
Antioxidant Supplementation with or Without
Bgroup Vitamins After Acute Ischemic
Stroke: a Randomized Controlled Trial. JPEN
J Parenter Enteral Nutr 2006;30(2):108-14.
19. Polidori MC, Praticó D, Ingegni T, Mariani E,
Spazzafumo L, Del Sindaco P, et al. Effects of
Vitamin C and Aspirin in Ischemic Strokerelated
Lipid Peroxidation: Results of the
AVASAS (Aspirin Versus Ascorbic acid plus
Aspirin in Stroke) Study. Biofactors 2005;
24(1-4):265-74.
20. Asplund K. Antioxidant Vitamins in the
Prevention of Cardiovascular Disease: aSystematic Review. J Int Med 2002;
251(5):372-92.
21. Valenzuela A. The Biological Significance of
Malondialdehyde Determination in
Assessment of Tissue Oxidative Stress. Life
Sci 1991;48(4):301-9.
22. Appelros P, Terént A. Characteristics of the
NIHSS results: Results from a Populationbased
Stroke Cohort at Baseline and After
One Year. Cerebrovasc Dis 2004; 17(1):21-7.
23. Kothari RU, Brott T, Broderick JP, Barsan
WG, Sauerbeck LR, Zuccarello M. The ABCs
of Measuring Intracerebral Hemorrhage
Volumes. Stroke 1996;27(8):1304-5.
24. Miller NJ, Rice-Evans C, Davies MJ,
Gopinathan V, Milner A. A Novel Method for
Measuring Antioxidant Capacity and Its
Application to Monitoring the Antioxidant
Status in Premature Neonates. Clin Sci (Lond)
1993; 84(4):407-12.
25. Miller NJ, Rice-Evans CA. Factors
Influencing the Antioxidant Activity
Determined by the ABTS.+ Radical Cation
Assay. Free Radic Res 1997; 26(3):195-9.
26. Armstrong D, Browne R. The Analysis of
Free Radicals, Lipid Peroxides, Antioxidant
Enzymes and Compounds to Oxidative Stress
as Applied to the Clinical Chemistry
Laboratory. Adv Exp Med Biol 1994;366:43-
58.
27. Allen CL, Bayraktutan U. Oxidative Stress
and Its Role in the Pathogenesis of Ischaemic
Stroke. Int J Stroke 2009;4(6):461-70.
28. Santos MT, Valles J, Aznar J, Vilches J.
Determination of plasma malondialdehyde
like material and its clinical application in
stroke patients. J Clin Pathol.
1980;33(10):973-6.
29. Srikrishna R, Suresh DR. Biochemical Study
of Antioxidant Profile in Acute Ischemic
Stroke. Stroke BJMP 2009;2(1):35-7.
30. Zimmermann C, Winnefeld K, Streck S,
Roskos M, Haberl RL. Antioxidant Status in
Acute Stroke Patients and Patients at Stroke
Risk. Eur Neurol 2004; 51(3):157-61.
31. Leinonen JS, Ahonen JP, Lönnrot K,
Jehkonen M, Dastidar P, Molnár G, et al. Low
Plasma Antioxidant Activity Is Associated
with High Lesion Volume and Neurological
Impairment in Stroke. Stroke 2000;31(1):33-
9.
32. Gonullu H, Aslan M, Karadas S, Kati C,
Duran L, Milanlioglu A, et al. Serum
Prolidase Enzyme Activity and Oxidative
Stress levels in Patients with Acute
Hemorrhagic Stroke. Scand J Clin Lab Invest
2014 74(3):199-205.
Type of Study: Research |
Subject:
Special
Received: 2017/11/23 | Accepted: 2017/11/23 | Published: 2017/11/23
Received: 2017/11/23 | Accepted: 2017/11/23 | Published: 2017/11/23
References
1. Rodrigo R, Fernández-Gajardo R, Gutiérrez R, Matamala JM, Carrasco R, Miranda-Merchak A, et al. Oxidative Stress and Pathophysiology of Ischemic Stroke: Novel Therapeutic Opportunities. CNS Neurol Disord Drug Targets 2013;12(5):698-714. [DOI:10.2174/1871527311312050015] [PMID]
2. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. American Heart Association. Heart Disease and Stroke Statistics. 2011 update. Circulation 2011; 123(4):e18-e209. [DOI:10.1161/CIR.0b013e3182009701] [PMID] [PMCID]
3. Cherubini A, Polidori MC, Bregnocchi M, Pezzuto S, Cecchetti R, Ingegni T. Antioxidant Profile and Early Outcome in Stroke Patients. Stroke 2000; 31(10):2295-300. [DOI:10.1161/01.STR.31.10.2295] [PMID]
4. Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and Ischemic Strokes Compared: Stroke Severity, Mortality, and Risk Factors. Stroke 2009; 40(6):2068-72. [DOI:10.1161/STROKEAHA.108.540112] [PMID]
5. Pradeep H, Diya JB, Shashikumar S, Rajanikant GK. Oxidative Stress – assassin behind the Ischemic Stroke. Folia Neuropathol 2012;50(3):219-30. [DOI:10.5114/fn.2012.30522] [PMID]
6. Yang TH, Chang CY, Hu ML. Various Forms of Homocysteine and Oxidative Status in the Plasma of Ischemic-Stroke Patients as Compared to Healthy Controls. Clin Biochem 2004;37(6):494-9. [DOI:10.1016/j.clinbiochem.2004.02.006] [PMID]
7. Spranger M, Krempien S, Schwab S, Donneberg S, Hacke W. Superoxide Dismutase Activity in Serum of Patients with Acute Cerebral Ischemic Injury. Stroke 1997; 28(12):2425-8. [DOI:10.1161/01.STR.28.12.2425] [PMID]
8. Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ. Oxidative Stress: Apoptosis in Neuronal injury. Curr Alzheimer Res 2006;3(4):327-37. [DOI:10.2174/156720506778249515] [PMID]
9. Facchinetti F, Dawson VL, Dawson TM. Free Radicals as Mediators of Neuronal Injury. Cell Mol Neurobiol 1998;18(6):667-82. [DOI:10.1023/A:1020221919154] [PMID]
10. Shi H, Liu KJ. Cerebral Tissue Oxygenation and Oxidative Brain Injury During Ischemia and Reperfusion. Front Biosci 2007;12:1318-28. [DOI:10.2741/2150] [PMID]
11. Rehncrona S, Mela L, Siesjo BK. Recovery of Brain Mitochondrial Function in the Rat After Complete Cerebral Ischaemia. Stroke 1979;10(4): 437-46. [DOI:10.1161/01.STR.10.4.437] [PMID]
12. Kaur J, Sarika A, Bhawna S, Thakur LC, Gambhir J, Prabhu KM. Role of Oxidative Stress in Pathophysiology of Transient Ischemic Attack and Stroke. Int J Biol Med Res 2011;2(3):611-5.
13. Aygul R, Demircan B, Erdem F, Ulvi H, Yildirim A, Demirbas F. Plasma Values of Oxidants and Antioxidants in Acute Brain Hemorrhage. Biol Trace Elem Res 2005; 108(1-3):43-52. [DOI:10.1385/BTER:108:1-3:043]
14. Kelly PJ, Morrow JD, Ning M, Koroshetz W, Lo EH, Terry E, et al. Oxidative Stress and Matrix Metalloproteinase-9 in Acute Ischemic Stroke: the Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) Study. Stroke 2008;39(1):100-4. [DOI:10.1161/STROKEAHA.107.488189] [PMID]
15. Domínguez C, Delgado P, Vilches A, Martín-Gallán P, Ribó M, Santamarina E, et al. Oxidative Stress After Thrombolysis-induced Reperfusion in Human Stroke. Stroke 2010;41(4):653-60. [DOI:10.1161/STROKEAHA.109.571935] [PMID]
16. Cano CP, Bermúdez VP, Atencio HE, Medina MT, Anilsa A, Souki A, et al. Increased Serum Malondialdehyde and Decreased Nitric Oxide within 24 hours of Thrombotic Stroke Onset. Am J Ther 2003;10(6):473-6. [DOI:10.1097/00045391-200311000-00018] [PMID]
17. Seet RC, Lee CY, Chan BP, Sharma VK, Teoh HL, Venketasubramanian N, et al. Oxidative Damage in Ischemic Stroke Revealed Using Multiple Biomarkers. Stroke 2011;42(8):2326-9. [DOI:10.1161/STROKEAHA.111.618835] [PMID]
18. Ullegaddi R, Powers HJ, Gariballa SE. Antioxidant Supplementation with or Without Bgroup Vitamins After Acute Ischemic Stroke: a Randomized Controlled Trial. JPEN J Parenter Enteral Nutr 2006;30(2):108-14. [DOI:10.1177/0148607106030002108] [PMID]
19. Polidori MC, Praticó D, Ingegni T, Mariani E, Spazzafumo L, Del Sindaco P, et al. Effects of Vitamin C and Aspirin in Ischemic Stroke-related Lipid Peroxidation: Results of the AVASAS (Aspirin Versus Ascorbic acid plus Aspirin in Stroke) Study. Biofactors 2005; 24(1-4):265-74. [DOI:10.1002/biof.5520240131] [PMID]
20. Asplund K. Antioxidant Vitamins in the Prevention of Cardiovascular Disease: a Systematic Review. J Int Med 2002; 251(5):372-92. [DOI:10.1046/j.1365-2796.2002.00973.x] [PMID]
21. Valenzuela A. The Biological Significance of Malondialdehyde Determination in Assessment of Tissue Oxidative Stress. Life Sci 1991;48(4):301-9. [DOI:10.1016/0024-3205(91)90550-U]
22. Appelros P, Terént A. Characteristics of the NIHSS results: Results from a Population-based Stroke Cohort at Baseline and After One Year. Cerebrovasc Dis 2004; 17(1):21-7. [DOI:10.1159/000073894] [PMID]
23. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M. The ABCs of Measuring Intracerebral Hemorrhage Volumes. Stroke 1996;27(8):1304-5. [DOI:10.1161/01.STR.27.8.1304] [PMID]
24. Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A Novel Method for Measuring Antioxidant Capacity and Its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin Sci (Lond) 1993; 84(4):407-12. [DOI:10.1042/cs0840407]
25. Miller NJ, Rice-Evans CA. Factors Influencing the Antioxidant Activity Determined by the ABTS.+ Radical Cation Assay. Free Radic Res 1997; 26(3):195-9. [DOI:10.3109/10715769709097799] [PMID]
26. Armstrong D, Browne R. The Analysis of Free Radicals, Lipid Peroxides, Antioxidant Enzymes and Compounds to Oxidative Stress as Applied to the Clinical Chemistry Laboratory. Adv Exp Med Biol 1994;366:43-58.
_4 [DOI:10.1007/978-1-4615-1833-4] [PMID]
27. Allen CL, Bayraktutan U. Oxidative Stress and Its Role in the Pathogenesis of Ischaemic Stroke. Int J Stroke 2009;4(6):461-70. [DOI:10.1111/j.1747-4949.2009.00387.x] [PMID]
28. Santos MT, Valles J, Aznar J, Vilches J. Determination of plasma malondialdehyde like material and its clinical application in stroke patients. J Clin Pathol. 1980;33(10):973-6. [DOI:10.1136/jcp.33.10.973] [PMID] [PMCID]
29. Srikrishna R, Suresh DR. Biochemical Study of Antioxidant Profile in Acute Ischemic Stroke. Stroke BJMP 2009;2(1):35-7.
30. Zimmermann C, Winnefeld K, Streck S, Roskos M, Haberl RL. Antioxidant Status in Acute Stroke Patients and Patients at Stroke Risk. Eur Neurol 2004; 51(3):157-61. [DOI:10.1159/000077662] [PMID]
31. Leinonen JS, Ahonen JP, Lönnrot K, Jehkonen M, Dastidar P, Molnár G, et al. Low Plasma Antioxidant Activity Is Associated with High Lesion Volume and Neurological Impairment in Stroke. Stroke 2000;31(1):33-9. [DOI:10.1161/01.STR.31.1.33] [PMID]
32. Gonullu H, Aslan M, Karadas S, Kati C, Duran L, Milanlioglu A, et al. Serum Prolidase Enzyme Activity and Oxidative Stress levels in Patients with Acute Hemorrhagic Stroke. Scand J Clin Lab Invest 2014 74(3):199-205. [DOI:10.3109/00365513.2013.873949] [PMID]
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |